Abstract
The decrease in water resources due to the excessive use of water for irrigation purpose and climatic changes represents a serious world-wide threat to food security. In this regards, 50 wheat accessions were analyzed, using completely random factorial design at the seedlings stage under normal and drought stress conditions. Significant variation was detected among all accessions under both conditions. All characters studied showed variations in the mean values in water deficit environments in studied gemplasm at seedling stage. As seedling fresh weight, dry weight, relative water content, cell membrane thermo-stability, chlorophyll a & b were positively associated among themselves under drought conditions which showed the significance of these attribute for water deficit areas in future wheat breeding programs. Based on their performance, five accessions namely Aas-11, Chakwal-86, Pasban-90, Chakwal-97 and Kohistan-97 were selected as drought tolerant and three accessions namely Mairaj-08, Lasani-2008 and Gomal-2008 were selected as drought susceptible genotypes. The choice of wheat accessions based on the characteristics of the seedlings is informal, low-priced and less hassle. Likewise, the seedlings attributes exhibit moderate to high variation with an additive genetics effects on the environments. Best performance accessions under water deficit environment will be beneficial in future wheat breeding schemes and early screening for the attributes suggested in current experiment will be useful for producing best-yielded and drought-tolerance wheat genotypes to sustainable food security.
Keywords: Chlorophyll, Photosynthesis, Wheat, Seedling, Drought
1. Introduction
In the early 1960′s improved conventional wheat breeding adopted with better cultural practices led to a generous wheat production in the world known as “green revolution”. According to (Dixon 2009), wheat demand is increasing faster and it is expected that will be achieve to 40 %percent in one decade. Therefore, it is necessary to enhance wheat yield to sustainable food security. Several problems exists which are accountable for lower wheat production, as well as low quality of seed, using improper broadcasting methods for sowing, late cultivation, worst soil, uneven fertilizer doses, unsuitable weed eradicating, disease and less supply of water and heat as the results of climate changes (Ahmed et al., 2019a). Among cereals crops, wheat crop status is important due to the nutritional values and more feeding. Massive growth in populations and the liberated life style has directed to emerging issues/problems for wheat scientists to create new genotypes having prominent yield in water deficit areas and improved quality seed (Mujtaba et al., 2016).
Crop productivity faces many challenges, one of them the biggest challenge is drought, which is mainly due to changes in the pattern of precipitation and inadequate rainfall pattern (Faisal et al., 2017). Different mechanisms involved in plant body to manage the drought stress. In the previous years, numerous restrictions stuck about the drought mechanisms because poor concept of developmental and physiological basis for yield contribution attributes under drought environments due to polygenic inheritance pattern of drought tolerance associated characters (Khan et al., 2018). Drought stress is a complex mechanism so, number of genes/QTLs having trivial effects which regulate this mechanism (Ahmed et al., 2017a).
To create tolerance against water deficit, it is very important to primarily understand the mechanism and behavior of the plant under drought stress conditions. To stand against the water shortage conditions plant has various developmental phases like, morphologically, physiologically, biochemically, anatomically and at molecular level. Drought tolerance mechanism is complex at a cellular and molecular level as well as whole plant body level. Several details contributes concern the complication of drought tolerant system like, crops species, intensities, duration of stresses and plants development stages (Saeidi and Abdoli, 2018). Survivals of plants in drought may be adoptive several tolerance mechanisms operative simultaneously. Mainly three basics mechanism, a plant can familiarize to face in water deficit conditions. (i) Escape (ii) avoidance or tolerance (iii) resistance mechanism. In first mechanism, plant completes the life cycles before shortage of water. In second mechanism, plant take step to face in the condition of less supply of water, e.g. close of stomatal opening, decrease the rates of transpirations. In third mechanism, plants take step at the cell levels against water deficit conditions through developing antioxidant which maintain of osmotic adjustments and at tissue level (Wang et al., 2013, Shahinnia et al., 2016).
Various morphological modifications occur in plants body under drought environment like leaf area shrinkage, stomata frequency reduce, cell walls thickening of leaf, epicuticles waxes deposition, and conductive system development, large vessels frequency increase, senescence before maturity and formation of leaves in cereals like tubes structure (Ahmed et al., 2017b). Drought is also occurs due to high temperature of climate, which disturbs the photosynthesis process by raising the evapo-transpiration rate. Among different abiotic stresses restricting the productivity of crop, the most difficult and complex to breeding is drought due to polygenic in nature (Zhu et al., 2016).
Plant and water relation is badly affected by drought stress, resultantly total water contents reduced and altered the turgor of cells. It also induces stomata closure, reduces the rate of transpiration, restrictions in gases exchanges and inhibits photosynthetic activity (Kosar et al., 2015). Due to drought stress, several structure and function alterations in photosynthesis machineries such as modifications in the photosynthetic pigments (chlorophyll a & b), slow down the CO2 uptake process due to closure of stomata and shortage of photosynthates assimilation because inhibit the chloroplast activity (Liu et al., 2016). One of the main causes of photosynthetic suppression is the formations of (ROS) like super oxides and hydroxyl radicals, which impaired the photosynthetic machinery. Under water deficient condition the synthesis of chlorophyll is inhibited. Minerals uptake and transport process are badly affected and ultimately reduced the leaf area, changed assimilate partitioning and finally decrease the yield of wheat plant. Wheat production decreased from 50 to 90 % of their irrigation potentials in the progressing areas by drought (Li et al., 2017).
In plants cell, photosynthetic is an important mechanisms and regulate under low concentration of water culture medium. If chlorophylls pigment concentrations increase, then photosynthetic systems will be great effectual. The chlorophylls content causes greater decrease in wheat with increased amount of drought, as the thylakoids membrane disintegrates with cell dehydrations (Maghsoudi et al., 2015). The amount of leaf chlorophyll contents are the indicator of the photosynthetic capacity of plant tissue. Decreased or unchanged levels of chlorophylls in drought conditions previously, stated in several crops, as of water shortage occurred. The amount of chlorophyll content changes in cereal crops especially in wheat under water deficit environments (Barutçular et al., 2016).
The choice of wheat accessions based on the characteristics of the seedlings is informal, low-priced and less hassle. Likewise, the seedlings attributes were exhibit moderates to high variation with an additives genetics effects on the environments (Rehman et al., 2016). The current experiment was performed for the selection of fifty different wheat accessions for drought tolerance on the performance of seedlings characters to regulate appropriate choice principles non-stressed and water deficit environments. This will offer the source of drought-tolerant for dry land cultivation in semiarid and rain-fed areas to fulfill the wheat demand to overcome the food security.
2. Material and methods
The current experiment was conceded out to determine the behaviors of drought and photosynthetic related attributes in wheat seedling for breeding drought tolerant-genotypes. The seeds of 50 wheat accessions were received from the seed bank of Department of Plant Breeding and Genetics, University of Agriculture, Faisalabad (PBG-UAF)-Pakistan. The accessions code, names and their pedigrees are presented in Table 1. Experiment was planted using sands filled polythene bags followed factorial complete randomize design in 3 replication under normal and drought conditions. One polythene bag contained 2 seeds of each genotype, allowed to emergences and thinned to 1 seedling/bag after germination. Each accession was sown in different five bags for each replications (Ahmed et al., 2019a). One set of accessions was regular irrigation (100% of field capacity). Second set of similar accessions were reserved in water deficit stresses (at 50% field capacity) afterward applications of irrigation when sown. The fields’ capacity (FC) of the sand calculated with pressures membranes apparatus (Gugino et al., 2009, Moebius-Clune et al., 2016). Data of drought and photosynthetic related attributes, like seedling fresh weights, seedling dry weights, relatives water content, cell membranes thermo-stability, chlorophyll a and chlorophyll b contents were recorded from 3 weeks old wheat seedling under non-stress and water deficit conditions. Relative water content was calculated using the following formula (Barrs and Weatherley, 1962): RWC = [(Fresh weight–Dry weights)/(Turgid weights–Dry weights)] × 100
Table 1.
Genotypes code, name and pedigree of 105 spring wheat genotypes.
| Code | Name | Pedigree |
|---|---|---|
| G1 | Aas-2011 | PRL/PASTOR//2236 |
| G2 | Parwaz-94 | V.5648/PARULA or V.5648/PRL |
| G3 | Mairaj-08 | SPARROW/INIA//V.7394/WL711/3/BAUS |
| G4 | Margalla-99 | OPATA/BOW'S' |
| G5 | NARC-2009 | INQALAB 91*2/TUKURU |
| G6 | Chakwal-86 | FORLANI/ACC//ANA or Fln/ACS//ANA |
| G7 | Pak-81 | VEERY. |
| G8 | BARS-2009 | PFAU/SERI//BOW |
| G9 | DPW-621–50 | KAUZ//ALTAR-84/(AOS)AWNED-ONAS/3/MILAN/KAUZ/4/HUITES |
| G10 | Moomal-2002 | BUC or BUCS/4/TZPP/IRN46 |
| G11 | Pasban-90 | INIA F66/TH.DISTICHUM//INIAF66/3/GENARO T81 or INIA F66/A.DISTCHUM//INIA66/3/GEN |
| G12 | Shafaq-2006 | LU 26/HD 2179/ 2*INQALAB 91 |
| G13 | Marvi-2000 | CMH-77A917/PKV 1600//RL6010/6*SKA |
| G14 | Fakhar-e-Sarhad | NORD-DESPREZ(ND)/VG-9144//KALYANSONA/BLUEBIRD/3/YACO/4/VEERY-5 |
| G15 | Lasani-2008 | LUAN/KOH-97 |
| G16 | Chakwal-97 | BUC'S'/FCT'S' |
| G17 | AARI-2011 | SH-88/90A-204//MH97 |
| G18 | Abadgar-93 | PSN/BOW |
| G19 | Anmol-91 | KVZ/TRM//PTM/ANA |
| G20 | Uqab-2000 | CROW'S'/NAC//BOW'S' |
| G21 | Kohistan-97 | V-1562//CHRC'S'/HORK/3/KUFRA-I/4/CARP'S'/BJY'S' |
| G22 | Bahawal-97 | PFAU'S'/SERI |
| G23 | Ufaq-2002 | V.84133/V83150 |
| G24 | Bwp-2000 | AU/UP301//GLL/Sx/3/PEW S/4/MAI S/MAY A S//PEWS |
| G25 | Gomal-2008 | Attila |
| G26 | Bakhtawar-94 | Mentana/Mayo//4–11 |
| G27 | Bakhar-2002 | P102/PIMA//F371/TTR/BOW/3/PVN |
| G28 | Bakhtawar-93 | AU/UP301//GLL/SX/3/PEW/4/MAI/MAYA//PEW |
| G29 | Bathoor-2008 | URES/JUN//KAUZ |
| G30 | Chakwal-50 | ATTILA/3/HUI/CARC//CHEN/CHTO/4/ATTILA |
| G31 | AS-2002 | KHP/D31708//CMH74A370/3/ENO79/4/R26043/*4NAC |
| G32 | Fareed-2006 | PT'S'/3/TOB/LFN//BB/4/BB/HD-832–5//ON/5/G-V/ALD'S'//HPO |
| G33 | FSD-2008 | PBW65/2*Pastor |
| G34 | Millat-2011 | CHENAB2000/INQ-91 |
| G35 | FSD-85 | MAYA/MON//KVZ/TRM |
| G36 | GA 2002 | DWL5023/SNB//SNB |
| G37 | Galaxy-2013 | Pb96/Watan/MH-97 |
| G38 | Hashim-2008 | JUP/ALD'S'//KLT'S'/3/VEE'S'/6/BEZ//TOB/8156/4/ON/3/6*TH/KF//6*LEE/KF/--------- |
| G39 | Inq-91 | WL 711/CROW “S” |
| G40 | Iqbal-2000 | BURGUS/SORT 12–13//KAL/BB/3/PAK 81 |
| G41 | Kaghan-93 | TTR/JUN |
| G42 | Khyber-87 | KVZ/TRM//PTM/ANA |
| G43 | Kohinoor-83 | ORE F1 158/FDL//MFN/2*TIBA63/3/COC |
| G44 | Kohsar-95 | PSN/BOW |
| G45 | Ufaq-2002 | V.84133/V83150 |
| G46 | Maxi-Pak 65 | PJ/GB55 |
| G47 | Mehran-89 | KVZ/BUHO//KAL/BB |
| G48 | FSD-83 | FURY//KAL/BB |
| G49 | Nifa-barsat 2010 | FRET2 |
| G50 | Nowshera-96 | BUC/FLK//MYNA/VUL |
Cell membrane thermal stability was estimated using two different equations (Blum and Ebercon, 1981), Chlorophyll contents a & b were noted according to the method of (Arnon, 1949, Wellburn and Lichtenthaler, 1984). The chlorophyll a & b was calculated by following formulas.
where, V = Volume of Extract, W = weights of Fresh leaves, OD = optimal Density
2.1. Statistical analysis
Collected data of seedling traits were analyzed to ANOVA procedure (Steel 1997) using the GenStat® version 17, VSN, International (Payne 2008). Wheat seedlings attribute exhibiting significant variations among studied genotypes considered as significance. RADAR-graph were developed using Excel-Stat (Ahmed et al., 2019a) in which display values relative to a center point for examined traits under both environments. Pearson's correlation coefficients (r) were performed to conclude the association among studied seedling traits in normal and drought conditions using SPSS version 23 (Spss 2012). For correlation analysis significance levels, α = 0.01 was used for highly significant effects and α = 0.05 was used for significant effects. Based on the results derived from above mentioned analysis, the drought-tolerant genotypes and favorable morpho-physiological and chlorophyll related seedling traits were selected to conferring the drought-tolerant wheat genotypes.
3. Results and discussions
Studied germplasm, total 50 wheat genotypes were selected in green house through factorial complete randomize design. There is highly significant variations were showed among genotypes in both environments for all examined attributes (Table 2). The descriptive data of six wheat seedlings attributes in studied environments are presented in Table 3. All studied characters showed fluctuations in the mean value in drought conditions for most genotypes. In RADAR graph (Fig. 1, Fig. 2, Fig. 3) the average values of seedling attributes were mentioned and showed decreasing trends of mean value in drought as compared to normal conditions. Similar findings were reported by Khan et al., (2018) in wheat plant against drought tolerance. Those accessions resist in the variation of performance of the studied characters in drought environments that were considered drought tolerant.
Table 2.
Mean sum of squares of 50 wheat accessions of studied attributes.
| Source | DF | FWT | DWT | RWC | CMT | Chla | Chlb |
|---|---|---|---|---|---|---|---|
| Replication | 2 | 0.004 | 0.000 | 1.380 | 1.690 | 0.016 | 0.013 |
| Genotypes (G) | 49 | 0.020 | 0.010 | 108.880 | 104.519 | 0.022 | 0.004 |
| Environments (E) | 1 | 3.087 | 3.456 | 7845.900 | 119.511 | 6.965 | 2.071 |
| G*E | 49 | 0.016 | 0.007 | 91.290 | 74.323 | 0.008 | 0.003 |
| Error | 198 | 0.001 | 0.001 | 4.690 | 4.293 | 0.002 | 0.001 |
| Total | 299 |
FWT = Fresh weight, DWT = Dry weight, RWC = Relative Water Contents, CMT = Cell membrane thermostability, Chla = Chlorophyll a, Chlb = Chlorphyll b
Table 3.
Descriptive statistics of six wheat seedlings attributes of 50 wheat accessions under non-stress and water deficit conditions.
| FWT |
DWT |
RWC |
CMT |
Chl a |
Chl b |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Normal | Drought | Normal | Drought | Normal | Drought | Normal | Drought | Normal | Drought | Normal | Drought | |
| Mean | 0.96 | 0.62 | 0.29 | 0.16 | 72.40 | 62.85 | 66.12 | 55.59 | 1.52 | 1.50 | 0.55 | 0.48 |
| SD | 0.05 | 0.08 | 0.07 | 0.04 | 4.76 | 4.76 | 3.90 | 3.90 | 0.07 | 0.07 | 0.04 | 0.04 |
| SE Mean | 0.01 | 0.01 | 0.01 | 0.00 | 0.67 | 0.67 | 0.55 | 0.55 | 0.01 | 0.01 | 0.01 | 0.01 |
| C.V. | 5.61 | 13.02 | 9.77 | 10.13 | 6.57 | 7.57 | 5.89 | 7.01 | 4.82 | 4.86 | 7.41 | 8.48 |
| Minimum | 0.91 | 0.56 | 0.22 | 0.15 | 65.69 | 56.14 | 55.05 | 45.51 | 1.41 | 1.42 | 0.52 | 0.45 |
| Maximum | 1.16 | 0.96 | 0.53 | 0.31 | 83.35 | 73.80 | 75.05 | 64.51 | 1.67 | 1.41 | 0.67 | 0.60 |
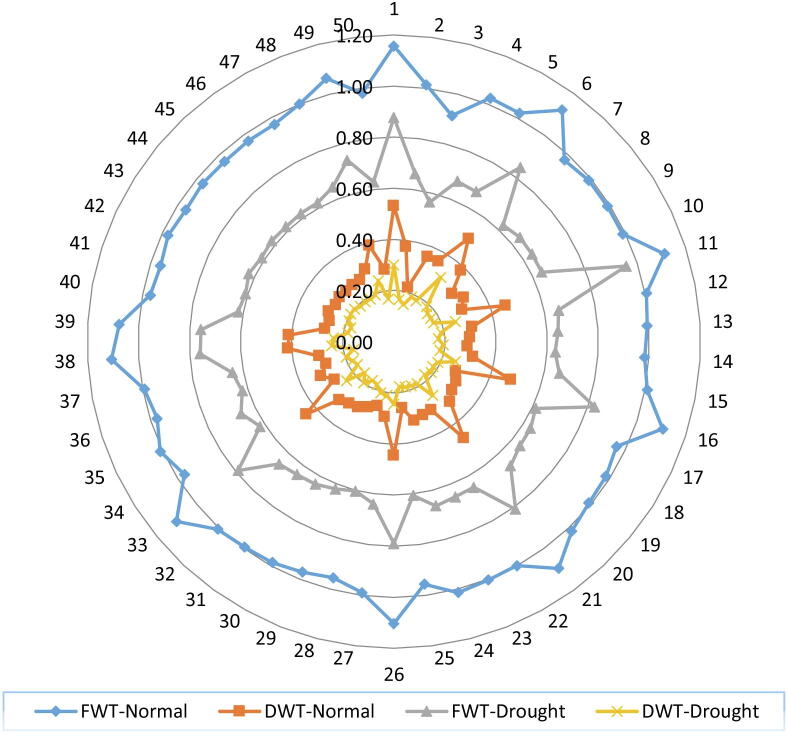
Fig. 1.
Behaviors of fresh and dry seedling weight of 50 wheat accessions under non-stress and water deficit conditions, FWT = Fresh weight, DWT = Dry weight.
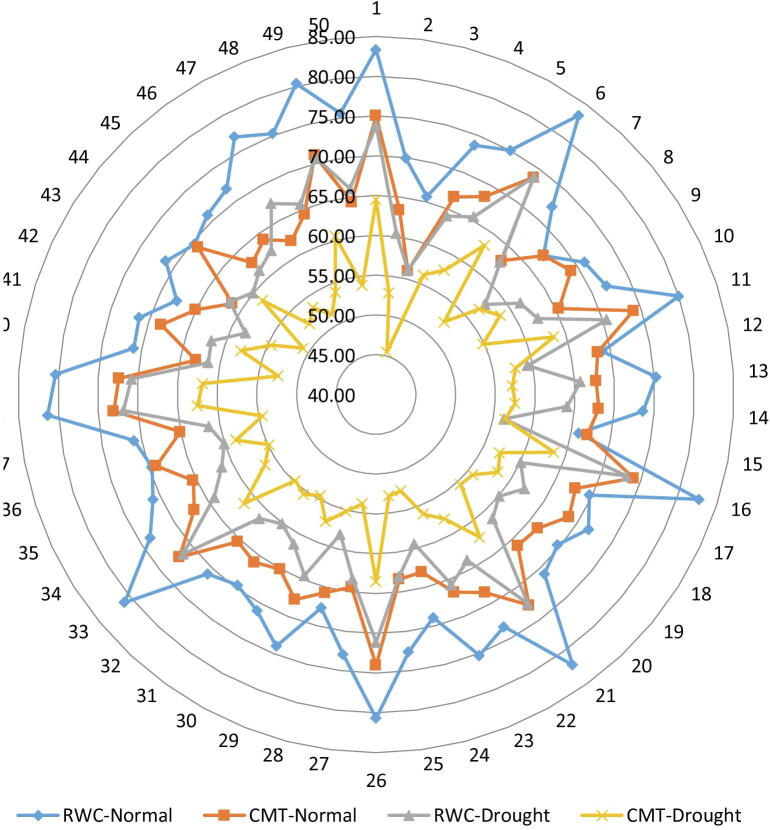
Fig. 2.
Behaviors of relative water contents and cell membrane thermo-stability at seedling stage of 50 wheat accessions under non-stress and water deficit conditions RWC = Relative Water Contents, CMT = Cell membrane thermostability.
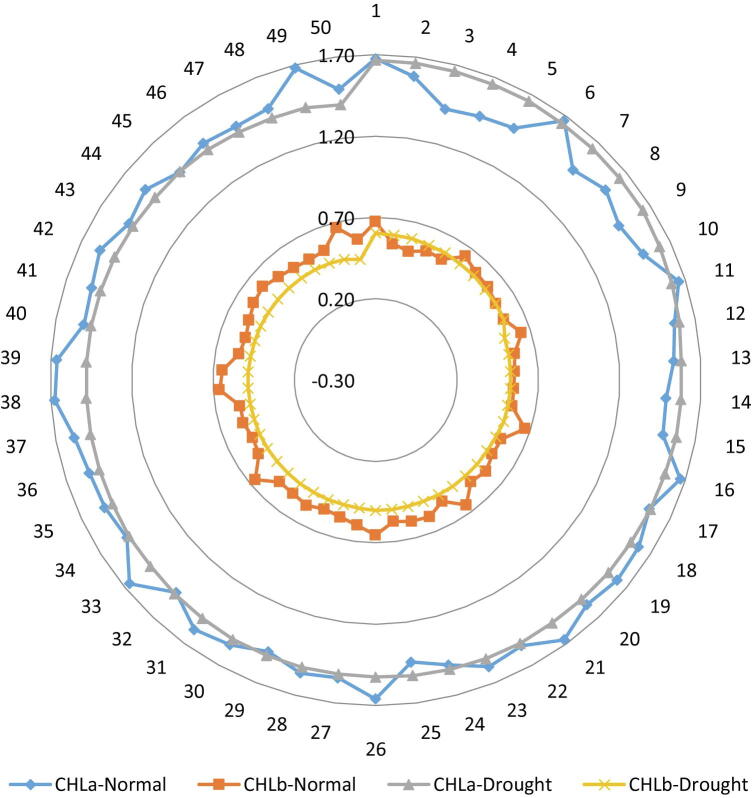
Fig. 3.
Behaviors of chlorophyll a and chlorophyll b at seedling stage of 50 wheat accessions under non-stress and water deficit conditions, Chla = Chlorophyll a, Chlb = Chlorphyll b.
3.1. Fresh weight
Data collected for fresh weight of wheat seedling for all accessions varied significantly ranging from 0.91 to 1.16 g under normal condition and in water stress condition ranging from 0.56 to 0.96 g (Table 3). The G-1 and G-11 had maximum fresh weight with the values of 1.16 and 0.96 g under normal condition and stress condition respectively, while lowest fresh weight with the values of 0.91 and 0.75 g observed in G-3 under both conditions respectively (Fig. 1) Wheat scientists (Chachar et al., 2016, Ahmed et al., 2019b) reported fresh weight of wheat seedling under drought environment, they categorized as minimum and maximum reduction in drought tolerant and susceptible wheat genotypes. In our experiment fresh weight of wheat seedling also decreased under drought stress in all studied genotypes and the genotype G-3 indicating highest decreased in fresh seedling weight among all studied accessions and not recommended as drought tolerant genotypes while G-11 considered as drought tolerant genotype on the basis of seedling fresh weight trait. In this experiment, the best performance of accessions G-1 followed by G-6, G-11 and G-16 were considered (Table 4) as drought tolerant while worst performer accessions G-3 followed by G-15 and G-25 suggested as drought susceptible accessions for this trait.
Table 4.
Trait-wise best performance and worst performance of wheat accessions under both environments.
| Seedling Traits | Best performer genotypes/Drought tolerant | worst performer genotypes/Drought Susceptible |
|---|---|---|
| Fresh Weight (g) | G-1 followed by G-6, G-11, G-16 and G-21 | G-3 followed by G-15 and G-25 |
| Dry Weight (g) | G-1 followed by G-6, G-11, G-21 and G-16 | G-3 followed by G-15 and G-25 |
| Relative Water Contents (%) | G-6 followed by G-1, G-16, G-21 &G-11 | G-3 followed by G-25 and G-15 |
| Cell Membrane Thermo-stability (%) | G-1 followed by G-11, G-16, G-21 and G-6 | G-3 followed by G-15 and G-25 |
| Chlorophyll a (mg/g Fw) | G-1 followed by G-16, G-11, G-21 and G-6 | G-3 followed by G-25 and G-15 |
| Chlorophyll b (mg/g Fw) | G-1 followed by G-16, G-11, G-6 and G-21 | G-3 followed by G-25 and G-15 |
3.2. Dry weight
Dry weight of wheat seedling is an important attribute and is also affected by water deficient stress. By it we come to know how much biomass is gained by the seedling other than the water. Dry weight for all accessions recorded as shown in Table 3 varied significantly ranging from 0.22 to 0.53 g under normal condition and in water stress condition ranging from 0.15 to 0.31 g. The G-1 and G-6 had maximum dry weight with the values of 0.53 and 0.31 g under normal condition and drought stress condition respectively, while lowest dry weight with the values of 0.22 and 0.15 g observed in G-3 under both conditions (Fig. 1). The superior seedling mass under drought stress have been proposed as reliable drought selection criteria for different plant species, including wheat crop (Chachar et al., 2016). In this experiment, the best performance genotypes G-1 followed by G-6, G-11, G-21 and G-16 under both conditions for this trait, were considered (Table 4) as drought tolerant accessions. Many scientists reported that drought resistance is considered by small reduction of dry weights under water stress environments (Ahmed et al., 2019b). Seedling biomass is very important attribute while selecting drought tolerant accessions. The decreasing trend in dry seedling weight was also reported by other researchers (Mujtaba et al., 2016) who found that water stress had a significant effect on dry matter production of seedling.
3.3. Relative water content
Soil moisture deficit as a major adverse factor, in arid and semi-arid zones, can lower leaf water potential, leading to reduced turgor and ultimately lower crop productivity. In this study, data collected for relative water content for all accessions varied significantly ranging from 65.69 to 83.35 under normal condition while, in water deficit stress condition (Table 3) ranging from 56.14 to 73.80. The G-1 had maximum relative water content with the values of 830.35 and 73.80 under normal condition and drought stress condition respectively. The lowest value for relative water content 65.69 and 56.14 observed in G-3 under normal and drought stress condition, respectively (Fig. 2). Drought induce reduction in the relative water content has been reported in many crops including wheat (Keyvan, 2010, Ahmed et al., 2019b). Similarly, a decline in relative water contents under water deficit stress in wheat seedlings was also observed in present study. Similar higher reduction in relative water content in drought susceptible wheat accessions as compared to tolerant ones has been observed earlier (Van Heerden and De Villiers, 1996, Ahmed et al., 2019a). This physiological character has great importance while screening wheat accessions for drought tolerance. In this experiment, the best performance of accessions G-1 followed by G-6, G-16, G-21 and G-38 were considered (Table 4) as drought tolerant accessions under both conditions, while worst performer accessions G-3 followed by G-25 and G-15 were suggested as drought susceptible accessions for this trait. In particular, the higher water potential RWC (%) is an indicator of tolerance to drought by osmoregulation with higher RWC under higher external osmotic potentials. For the tolerant variety, an active accumulation of solutes (osmoregulation) occurs. This is the only adaptive and positive response beneficial to the plant under water stress conditions (Khan et al., 2018). Osmoregulation allows the plant to maintain high turgor pressure and also to survive under stress conditions. Osmotic potential is considered an important selection criterion for wheat against drought tolerance. Among cereals crops, wheat crop status is important due to the nutritional values and more feeding. Massive growth in populations and the liberated life style has directed to emerging issues/problems for wheat scientists to create new accessions having prominent yield and improved quality seed (Ahmed et al., 2019a).
3.4. Cell membrane thermo-stability
In the current experiment, data collected for cell membrane thermo-stability for all accessions varied significantly ranging from 55.05 to 75.05 under normal condition and in water stress condition ranging from 45.51 to 64.51 (Table 3). The G-1 had maximum cell membrane thermo-stability with the values of 75.05 and 64.51 under normal condition and stress condition respectively, while lowest mean values of cell membrane thermo-stability 55.05 and 45.51 observed in G-3 under normal and stress conditions, respectively (Fig. 2). The positive relationship of electrolyte leakage with drought was also reported (Ahmadizadeh, 2013). Effect of drought was also evident as increase in relative cell injury percentage as compared to the controlled condition. Cell membrane thermo-stability (CMT) also evinced as relative cell injury percentage (RCI %) in terms of electrolyte leakage, is an efficient physiological criterion while studying drought tolerance. The tolerant accessions showed minimal reduction in CMT values under drought stress and higher temperatures environments. The decrease in cell viability due to high temperature treatment can be attributed to the rupture of the internal mitochondrial membrane that resulted in the decoupling of the electron transport chain and inactivation of various airway enzymes (Hussain et al., 2013, Bala and Sikder, 2017, Khan et al., 2018). In this experiment, the best performance of accessions G-1 followed by G-11, G-16, G-26 and G-6 were considered as drought tolerant while worst performer accessions G-3 followed by G-15 and G-25 were suggested (Table 4) as drought susceptible accessions for this trait. From these results, it was concluded that drought and heat tolerant accessions had greater cell membrane thermo-stability, which ultimately increased the survival potential of accessions under drought and heat shock treatments. So, CMT trait could be used as a selection criterion for drought stress tolerance at the seedlings stage, thus reduce the time and cost needed for the field experiments (Ahmadizadeh, 2013).
3.5. Photosynthetic pigments
3.5.1. Chlorophyll a and b
All wheat accessions behaved diversely in terms of seedling’s photosynthetic pigments against drought induced by water deficient condition in this study. Chlorophyll a for studied accessions varied significantly ranging from 1.42 (mg/g Fw) to 1.68 (mg/g Fw) under normal condition and in water deficit stress condition ranging from 1.41 (mg/g Fw) to 1.67 (mg/g Fw) (Table 3). The G-38 had maximum chlorophyll a with the values of 1.68 (mg/g Fw) and 1.67 (mg/g Fw) under normal conditions and drought stress condition, respectively. The lowest mean values of chlorophyll a 1.42 (mg/g Fw) and 1.41 (mg/g Fw) observed in G-3 under normal and drought stress conditions, respectively (Fig. 3). In this experiment, the best performance of accessions G-49 followed by G-38, G-1, G-21 and G-6 were considered as drought tolerant while worst performer accessions G-3 followed by G-25 and G-15 were suggested (Table 4) as drought susceptible accessions for this trait. Data collected for chlorophyll b for all accessions (Table 3) varied significantly ranging from 0.52 (mg/g Fw) to 0.67 (mg/g Fw) under normal conditions and in water deficit stress conditions ranging from 0.45 (mg/g Fw) to 0.60 (mg/g Fw). The G-1 had maximum chlorophyll b with the value of 0.67 (mg/g Fw) and 0.60 (mg/g Fw) under normal condition and stress condition respectively, while lowest mean values of chlorophyll b 0.52 (mg/g Fw) and 0.45 (mg/g Fw) observed in G-3 under normal and drought stress conditions, respectively (Fig. 3). In plant cells, photosynthesis is a key process which regulated under low concentration of water culture medium (Rehman et al., 2016).
If chlorophyll pigments concentration increase than photosynthesis system will be more efficient (Faisal et al., 2017). In this experiment, the best performance of accessions G-1 followed by G-16, G-49, G-33 and G-38 were considered (Table 4) as drought tolerant while worst performer accessions G-3 followed by G25 and G-15 were suggested as drought susceptible accessions for this trait. The chlorophyll content performed more reduction in all wheat accessions with increased levels of water stress, because the thylakoid membranes disintegrate with the dehydration of the cells (Kalaji et al., 2016). The maintenance of chlorophyll is essential for photosynthesis under water stress. Photosynthetic pigment contents of wheat genotypes were affected by drought. Higher chlorophyll content and lower percentage of reduction under drought stress in the tolerant wheat genotype have also been reported by many researchers (Keyvan, 2010, Faisal et al., 2017, Ahmed et al., 2019a).
3.6. Correlation analysis
Correlation coefficient describes the degree of association between two variables. It is also valuable in plant sciences since it can show a foretelling association that can be exploited in practice. It provide evidence about the relationship between several preferred traits. It offers a core concept of the association among various yield-contributing traits, which is beneficial for plant breeders in choosing varieties having desired attributes (Ghafoor et al., 2013). In the current study, information about the association of seedling traits under normal and drought conditions may further help to develop the strategies for indirect selection (Table 5). Evidence of the correlation in current study of seedling traits in non-stress and stress conditions may help advance strategies for the assortment of required varieties with preferred traits.
Table 5.
Correlation analysis among wheat seedling traits under both environments.
| Variables | Environments | FWT | DWT | RWC | CMT | Chl a |
|---|---|---|---|---|---|---|
| DWT | Normal | 0.55** | ||||
| Drought | 0.90** | |||||
| RWC | Normal | −0.68** | −0.35** | |||
| Drought | 0.83** | 0.93** | ||||
| CMT | Normal | 0.45** | 0.70** | −0.49** | ||
| Drought | 0.88** | 0.90** | 0.92** | |||
| Chl a | Normal | 0.34* | 0.71** | −0.14 ns | 0.67** | |
| Drought | 0.52** | 0.68** | 0.65** | 0.70** | ||
| Chl b | Normal | 0.15 ns | 0.45** | −0.29* | 0.40* | 0.5** |
| Drought | 0.53** | 0.66** | 0.59** | 0.60** | 0.84** |
** Highly significant (0.01); * significant (0.05): ns non-significant.
In the current experiment, simple correlation coefficients of seedling fresh weight exhibited positively strong association with seedling dry weight, relative water contents, cell membrane thermo-stability and chlorophyll a under both conditions, while non-significant association with chlorophyll a under normal conditions. Seedling dry weight positively correlated with all studied traits under both environments except RWC which showed negative correlation under normal conditions. Relative water contents showed positive association with all studied traits only under drought conditions and negatively correlated with studied traits under normal conditions. Cell membrane thermo-stability exhibited significant and positive association with examined attributes under both conditions except relative water contents which showed non-significant association only under normal conditions (Table 5).
Both physiological trait, RWC and CMT are useful indices for rapid evaluation of drought response in wheat breeding. Chlorophyll a had positive and significant association under drought conditions in all studied traits but showed non-signification association with relative water contents under normal conditions. Chlorophyll b showed negative association with relative water contents under drought and strong association with chlorophyll b under drought conditions. In this study, relationship between chlorophyll a and chlorophyll b was positive and highly significant. In present experiment as fresh weight, dry weight, relative water contents, cell membrane thermostability, chlorophyll a & b were positively correlated among themselves under drought conditions, therefore selection of anyone of these traits enhances the performance of other traits.
Rehman et al. (2016) obtained the results he stated that dry seedling weight showed positive association with other seedling traits under normal and drought condition. Higher chlorophyll contents in tolerant genotypes have also been reported earlier findings similar with Kalaji et al. (2016). Chlorophyll destruction was revealed to be accompanied by the injury of mesophyll chloroplasts, which led to a lesser photosynthetic rate. In current experiment highly significant association exist of cell membrane thermostability with chlorophyll a and chlorophyll b. Furthermore, the reduction in the level chlorophyll contents in crop plants under water-deficient environments was deliberated as a typical symptom of oxidative stress and may be the result of pigment photo-oxidation and chlorophyll degradation. In this experiment strong association exists of chlorophyll a with chlorophyll b. Present results supported with the findings of Ahmed et al. (2019b) they stated that Chlorophyll b negative association with relative water contents. Relative water content was positive associated with fresh weight, dry weight, cell membrane thermostabilty, chlorophyll a and chlorophyll b in this study. Ahmed et al. (2017b) investigated six wheat genotypes for its capacity to stand in drought conditions. Cell membrane thermostability was positively associated with fresh weight, dry weight, relative water content, chlorophyll a and chlorophyll b. (Keyvan, 2010, Epée Missé, 2018) findings supported the current results that correlation studies indicated the cell membrane thermo-stability of wheat seedling was the most important trait, followed by fresh weight and dry weight on the basis of their relationships with other traits. This indicated that these seedling traits of wheat plant play an important role under drought stress conditions to determine the response of drought. Faisal et al. (2017) evaluated physiological traits as indicators of drought tolerance in wheat and concluded that those genotypes which possess high percentage of relative water contents and cell membrane thermostability resist more against drought were considered drought-tolerant genotypes. Those genotypes which possess low percentage of relative water contents in wheat were suggested as drought-susceptible genotypes. It was also stated that maximum RWC and CMT is a resistance mechanism against water-deficient stress; it is the result of high osmotic regulation or a low elasticity of cell-wall tissue (Keyvan, 2010, Faisal et al., 2017, Ahmed et al., 2019a). The different behavior of the indices in various conditions and their association may be due to an altered behavior of varieties under different environments.
4. Conclusions
In this study, 50 wheat genotypes were screened at the seedling stage against normal and drought stress under factorial CRD using, seedling fresh weight, seedling dry weight, relative water contents, cell membrane thermo-stability, chlorophyll a and chlorophyll b as drought indices. Strong association among the photosynthetic and drought related attributes under drought condition which indicated the importance of these traits for future wheat breeding scheme for drought stressed areas. Therefore, selection of any one of these traits enhances the performance of other traits. On the basis of behavior, 50 wheat accessions under studied environments were categories as drought tolerant and susceptible genotypes. Those accessions behaved superior were classified as drought tolerant and such accessions showed worst behavior suggested as drought susceptible wheat genotypes. So using this criterion, 5 genotypes were selected as drought tolerant genotypes namely Aas-11, Chakwal-86, Pasban-90, Chakwal-97 and Kohistan-97 and drought susceptible genotypes namely Mairaj-08, Lasani-2008 and Gomal-2008 in this study. Furthermore, these accessions would be beneficial to develop best-yielded and drought tolerance wheat varieties to fulfill the wheat demand and sustainable food security.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This work was funded by the China Agriculture Research System (CARS-05-01A-04), China.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Hafiz Ghulam Muhu-Din Ahmed, Email: ahmedbreeder@gmail.com.
Yawen Zeng, Email: zengyw1967@126.com.
References
- Ahmadizadeh M. Physiological and agro-morphological response to drought stress. Middle-East J. Sci. Res. 2013;13(8):998–1009. [Google Scholar]
- Ahmed H.G.M.-D., Khan A.S., Kashif M., Khan S.H. Genetic mechanism of leaf venation and stomatal traits for breeding drought tolerant lines in wheat. Bangladesh J. Botany. 2017;46(1):35–41. [Google Scholar]
- Ahmed, H.G.M.-D., LI, M.-j., Khan, S.H., Kashif, M., 2019a. Early selection of bread wheat genotypes using morphological and photosynthetic attributes conferring drought tolerance. Journal of Integrative Agriculture. 18 (11), 2483-2491.
- Ahmed H., Khan A.S., Khan S.H., Kashif M. Genome wide allelic pattern and genetic diversity of spring wheat genotypes through SSR markers. Int. J. Agric. Biol. 2017;19:1559–1565. [Google Scholar]
- Ahmed H.G.M.-D., Sajjad M., Li M., Azmat M.A., Rizwan M., Maqsood R.H., Khan S.H. Selection criteria for drought-tolerant bread wheat genotypes at seedling stage. Sustainability. 2019;11(9):2584. [Google Scholar]
- Arnon D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant physiol. 1949;24(1):1. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bala P., Sikder S. Evaluation of heat tolerance of wheat genotypes through membrane thermostability test. MAYFEB J. Agri. Sci. 2017;2:1–6. [Google Scholar]
- Barrs H., Weatherley P. A re-examination of the relative turgidity technique for estimating water deficits in leaves. Australian J. Biolog. Sci. 1962;15(3):413–428. [Google Scholar]
- Barutçular C., Yıldırım M., Koc M., Akıncı C., Toptaş I., Albayrak O., Tanrıkulu A., El Sabagh A. Evaluation of SPAD chlorophyll in spring wheat genotypes under different environments. Fresen. Environ. Bull. 2016;25(4):1258–1266. [Google Scholar]
- Blum A., Ebercon A. Cell membrane stability as a measure of drought and heat tolerance in wheat 1. Crop Sci. 1981;21(1):43–47. [Google Scholar]
- Chachar Z., Chachar N., Chachar Q., Mujtaba S., Chachar G., Chachar S. Identification of drought tolerant wheat genotypes under water deficit conditions. Int. J. Res. granthaalayah. 2016;4(2):206–214. [Google Scholar]
- Dixon J. What causes civil wars? Integrating quantitative research findings. Int. Stud. Rev. 2009;11(4):707–735. [Google Scholar]
- Epee Misse, P.T., 2018. Wheat Seedling Physiological Adaptation to Overcome Water Stress. Available at SSRN 3307427.
- Faisal S., Mujtaba S., Khan M., Mahboob W. Morpho-physiological assessment of wheat (Triticum aestivum L.) genotypes for drought stress tolerance at seedling stage. Pak. J. Bot. 2017;49(2):445–452. [Google Scholar]
- Ghafoor G., Hassan G., Ahmad I., Khan S.N., Suliman S. Correlation analysis for different parameters of F2 bread wheat population. Pure Appl. Biol. 2013;2(1):28. [Google Scholar]
- Gugino, B., Abawi, G., Idowu, O., Schindelbeck, R., Smith, L., Thies, J. & Van Es, HM., 2009. Cornell soil health assessment training manual.
- Hussain M., Askandar H.S., Hassan Z.A. Selecting high yielding wheat hybrids from a restricted factorial mating design. Sarhad J. Agric. 2013;29(2):173–179. [Google Scholar]
- Kalaji H.M., Jajoo A., Oukarroum A., Brestic M., Zivcak M., Samborska I.A., Cetner M.D., Łukasik I., Goltsev V., Ladle R.J. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta physiologiae plantarum. 2016;38(4):102. [Google Scholar]
- Keyvan S. The effects of drought stress on yield, relative water content, proline, soluble carbohydrates and chlorophyll of bread wheat cultivars. J. Anim. Plant Sci. 2010;8(3):1051–1060. [Google Scholar]
- Khan A., Khaliq I., Ahmad M., Ahmed H., Khan A.G., Farooq M.S. Comparative performance of spring wheat (Triticum aestivum L.) through heat stress indices. Pak. J. Bot. 2018;50(2):481–488. [Google Scholar]
- Kosar F., Akram N., Ashraf M. Exogenously-applied 5-aminolevulinic acid modulates some key physiological characteristics and antioxidative defense system in spring wheat (Triticum aestivum L.) seedlings under water stress. S. Afr. J. Bot. 2015;96:71–77. [Google Scholar]
- Li Y., Li H., Li Y., Zhang S. Improving water-use efficiency by decreasing stomatal conductance and transpiration rate to maintain higher ear photosynthetic rate in drought-resistant wheat. The Crop Journal. 2017;5(3):231–239. [Google Scholar]
- Liu E., Mei X., Yan C., Gong D., Zhang Y. Effects of water stress on photosynthetic characteristics, dry matter translocation and WUE in two winter wheat genotypes. Agric. Water Manag. 2016;167:75–85. [Google Scholar]
- Maghsoudi K., Emam Y., Ashraf M. Influence of foliar application of silicon on chlorophyll fluorescence, photosynthetic pigments, and growth in water-stressed wheat cultivars differing in drought tolerance. Turk. J. Bot. 2015;39(4):625–634. [Google Scholar]
- Moebius-Clune, B., Moebius-Clune, D., Gugino, B., Idowu, O., Schindelbeck, R., Ristow, A., Van Es, H., Thies, J., Shayler, H., McBride, M., 2016. Comprehensive assessment of soil health. The Cornell Framework Manual.
- Mujtaba S., Faisal S., Khan M., Mumtaz S., Khanzada B. Physiological studies on six wheat (Triticum aestivum L.) genotypes for drought stress tolerance at seedling stage. Agri. Res. Technol. Open Access J. 2016;1(2):1–6. [Google Scholar]
- Payne R. VSN International; Hempstead, UK: 2008. A Guide to ANOVA and Design in GenStat. [Google Scholar]
- Rehman S.U., Bilal M., Rana R.M., Tahir M.N., Shah M.K.N., Ayalew H., Yan G. Cell membrane stability and chlorophyll content variation in wheat (Triticum aestivum) genotypes under conditions of heat and drought. Crop Pasture Sci. 2016;67(7):712–718. [Google Scholar]
- Saeidi, M., Abdoli, M., 2018. Effect of drought stress during grain filling on yield and its components, gas exchange variables, and some physiological traits of wheat cultivars.
- Shahinnia F., Le Roy J., Laborde B., Sznajder B., Kalambettu P., Mahjourimajd S., Tilbrook J., Fleury D. Genetic association of stomatal traits and yield in wheat grown in low rainfall environments. BMC Plant Biol. 2016;16(1):150. doi: 10.1186/s12870-016-0838-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spss I. International Business Machines Corp; Boston, Mass: 2012. IBM SPSS statistics version 21; p. 126. [Google Scholar]
- Steel, R.G., 1997. Pinciples and procedures of statistics a biometrical approach. 0070610282.
- Van Heerden P., De Villiers O. Evaluation of the relative water content and the reduction of 2, 3, 5-triphenyltetrazoliumchloride as indicators of drought tolerance in spring wheat cultivars. S. Afr. J. Plant Soil. 1996;13(4):131–135. [Google Scholar]
- Wang S., Li Z., Jia S., Sun D., Shi Y., Fan H., Liang Z., Jing R. Relationships of wheat leaf stomatal traits with wheat yield and drought-resistance. Ying yong sheng tai xue bao= The journal of applied ecology. 2013;24(6):1609–1614. [PubMed] [Google Scholar]
- Wellburn A., Lichtenthaler H. Advances in photosynthesis. research. Springer; 1984. Formulae and program to determine total carotenoids and chlorophylls a and b of leaf extracts in different solvents; pp. 9–12. [Google Scholar]
- Zhu M., Shabala S., Shabala L., Fan Y., Zhou M. Evaluating predictive values of various physiological indices for salinity stress tolerance in wheat. J. Agron. Crop Sci. 2016;202(2):115–124. [Google Scholar]