Abstract
Microplastic pollution is ubiquitous in the marine environment and is ingested by numerous marine species. Sharks are an understudied group regarding their susceptibility to microplastic ingestion. Here, we provide evidence of ingestion of microplastic and other anthropogenic fibres in four demersal sharks species found in the waters of the United Kingdom and investigate whether body burdens of contamination vary according to species, sex or size. Sharks were collected from the North-East Atlantic. Stomachs and digestive tracts of 46 sharks of 4 species were examined and 67% of samples contained at least one contaminant particle. Although we acknowledge modest sample size, estimated particle burden increased with body size but did not vary systematically with sex or species. A total of 379 particles were identified, leading to median estimates ranging from 2 to 7.5 ingested contaminants per animal for the 4 species. The majority were fibrous in nature (95%) and blue (88%) or black (9%) in colour. A subsample of contaminants (N = 62) were subject to FT-IR spectroscopy and polymers identified as: synthetic cellulose (33.3%), polypropylene (25%), polyacrylamides (10%) and polyester (8.3%). The level of risk posed to shark species by this level of contamination is unknown. Nevertheless, this study presents the first empirical evidence and an important baseline for ingestion of microplastics and other anthropogenic fibres in native UK shark species and highlights the pervasive nature of these pollutants.
Subject terms: Marine biology, Ocean sciences, Ichthyology, Environmental impact
Introduction
Plastics in the marine environment
Research on plastic in the marine environment has accelerated rapidly in the last decade, with numerous publications describing its impact on ecosystems and marine taxa1–7. It is estimated that between 4.8 and 12.7 million tonnes of plastic enter the oceans every year from a variety of sources6. Plastic is a popular material due to its durability, low production cost and efficiency in its uses8. It is these properties, alongside its often disposable nature that leads to its prevalence in the environment for many years9.
Microplastics (defined as plastic particles < 5 mm)10 are ubiquitous in the marine environment11–13. Despite this knowledge, quantitative assessments of their abundance are still fairly limited14, although some estimates place their abundance at 5.25 trillion particles globally, weighing in at over 250,000 tonnes5. Microplastics, in the form of fibres, fragments or beads/spheres, assimilate in the marine ecosystem via multiple avenues. Larger pieces of plastic can disintegrate over time due to UV radiation exposure, wave action and physical abrasion, eventually fragmenting into microscopic particles15. Microplastics are also found in many everyday items used by humans including cosmetic products and can be produced by clothing wear16–19. These can then reach the oceans via wastewater treatment plants20.
Ingestion of microplastics in marine species
Ingestion of microplastics is reported in many marine species including turtles, marine mammals and fish1,21–25. Alongside these larger species, microplastics have been reported in invertebrates such as zooplankton and crustaceans26–28. Our understanding of the impacts of microplastic ingestion is better understood in the latter group, with reports suggesting dose-dependent detrimental effects on feeding behaviour, development, reproduction and lifespan29–31.
Microplastic ingestion in elasmobranchs
Elasmobranchs are relatively understudied in regards to threats from plastic pollution32,33, nonetheless their susceptibility to microplastic ingestion has been reported in a handful of scientific publications22,34–39. It is thought that some species of elasmobranch may be at higher risk of microplastic ingestion based on their feeding strategies or habitat use35. Filter feeding species (such as whale sharks and basking sharks) that occupy habitats which overlap areas with high densities of plastic pollution have been suggested to be at higher risk of microplastic ingestion35,40,41. Many shark species, however, are non-filter feeders, instead feeding on a range of larger organisms such as fish, crustaceans, marine turtles and marine mammals, all of which have records of microplastic ingestion22–24,27.
North-East Atlantic demersal elasmobranchs
The North-East Atlantic is home to numerous shark and ray species, including small to medium sized demersal sharks. These species can be found at varying depths from 5 to 900 m42,43, most often residing in benthic habitats44,45. They feed on a wide range of small teleost fishes, crustaceans and cephalopods44,46. Due to their habitat choice they are often caught in demersal fisheries as bycatch, however targeted fisheries for these species also exist47,48. The exposure of microplastics to demersal shark species globally, is currently poorly investigated, with only a few reports of plastic ingestion, mostly situated in and around the Mediterranean Sea22,36–38,49,50. There have, however, been multiple studies of plastic ingestion in bony fish in the region, with ingestion rates varying from 1 to 47% across the species22,51–54.
Here we carry out the first detailed comparative study of microplastic ingestion in four shark species in the North-East Atlantic (small-spotted catshark; Scyliorhinus canicula, starry smooth-hound; Mustelus asterias, spiny dogfish; Squalus acanthias and bull huss; Scyliorhinus stellaris). These species were chosen due to their availability as bycatch in local fisheries. Alongside this, all four species are primarily demersal in their habitat choice, therefore studying microplastic ingestion within them may provide insights into contaminant levels for this marine biome and as a result indicate whether these species would be suitable bio-indicators for marine pollution. Given interspecific differences in habitat niche, ontogenetic shifts in diet and sex variation in life history strategies, we hypothesized that there would be differences in contaminant load among species, between sex and among size classes.
Materials and methods
Collection and dissection of shark samples
The study was conducted in Cornwall, UK using sharks caught as bycatch in a demersal hake fishery, fishing in and around the North-East Atlantic and Celtic Sea (ICES rectangles: VIIg, VIIh and VIIf). Four species of sharks were investigated (Total N = 46), including: small-spotted catshark (Scyliorhinus canicula) (n = 12), spiny dogfish (Squalus acanthias) (n = 12), starry smooth-hound (Mustelus asterias) (n = 12) and bull huss (Scyliorhinus stellaris) (n = 10). Standard shark morphometric measurements were taken for each species (for full details see Supplementary Materials).
Necropsy and analysis
Upon dissection, the entire gastrointestinal tracts were removed (stomach and intestines) and 10 ml (20–50% of total volume depending on species) of their contents were removed for analysis and visual inspection of gut contents (see Supplementary Fig. S1). Samples were treated with 20% potassium hydroxide (KOH) as recent studies have highlighted its efficacy at digesting fish ingesta53,55–57 and heated for 48 h at 60 °C to aid digestion of biological materials. Digested samples were filtered and subsequently analysed under a digital stereo microscope (Leica M165C) and classified by type (fibre, fragment or bead) and colour, as well as measured (mm). A subsample of the contaminants identified (including potential fragments and fibres) underwent Fourier Transform Infrared spectroscopy (FT-IR) to gain insights into their polymer make-up and possible origins. Substantial measures were taken to reduce and control for contamination of samples throughout laboratory work, including the running of procedural blanks and air-borne contamination blanks at every stage of the necropsy and subsequent analysis (for full details, including quality control and contamination control measures see Supplementary Materials). All methods were carried out in accordance with relevant guidelines and regulations.
All statistical analyses were conducted on raw data. A negative binomial generalised linear model (GLM) was used to investigate the influence of species, sex and individual length on the estimated number of ingested fibres, using the MASS package58 in R v3.5.1.59 All combinations of terms were examined and ranked by Akaike’s Information Criteria (AIC) using subset selection of the maximal model using the MuMIn package v1.42.1.60 Top ranked models were defined as models ΔAIC ≤ 2 units of the best supported model, after excluding further models where a simpler model attained stronger weighting61.
Particle terminology
Throughout the manuscript a range of terms are used to describe the various identified contaminants. The following terms are hereby explained. Microplastics and/or microplastic fibres refer to traditional petrochemical-derived polymer compounds. Anthropogenic fibres encompass compounds that are naturally occurring, however have been repurposed for human use, this includes the likes of synthetic regenerated cellulose, viscose, rayon and cotton. Contaminants/contaminant particles, in this context, refers to both microplastics and anthropogenic fibres as an umbrella term for compounds not-naturally occurring within these sharks.
Results
Descriptive statistics
In total, 46 individual sharks were analysed, of which 56.5% were male, although proportion varied across species (Proportion male for individual species: small-spotted catshark 66.6%, starry smooth-hound 25%, spiny dogfish 83.3%, bull huss 50%). Overall, 67.4% of sharks were classified as adults although again, the proportion differed among species (Proportion adult for individual species: small-spotted catshark 75%, starry smooth-hound 66.6%, spiny dogfish 58.3%, bull huss 58.3%).
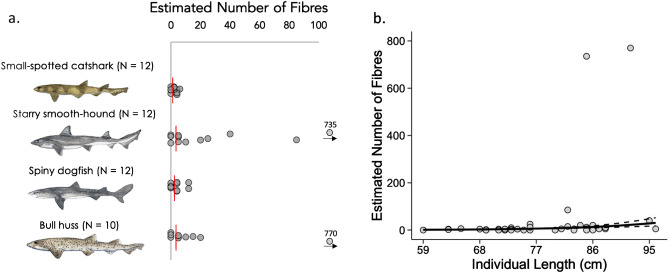
Almost all particles identified in sharks were classified as fibres, with only two fragments identified, and no beads/spheres found. Of the 46 sharks analysed in this study, samples from 67% (31/46) contained at least one contaminant particle and incidence was relatively consistent across species (small-spotted catshark 66.6%, starry smooth-hound 75%, spiny dogfish 58%, bull huss 70%). Estimated number of fibres varied across the four shark species: estimated median fibres (IQ range; range): Overall: 4(0–9; 0–770) (IQ range; range), starry smooth-hound (7.5(3.8–28.75; 0–735), small spotted catshark (2(0–4; 0–6), spiny dogfish (4(0–4; 0–12), bull huss (5(1.3–13.8; 0–770).
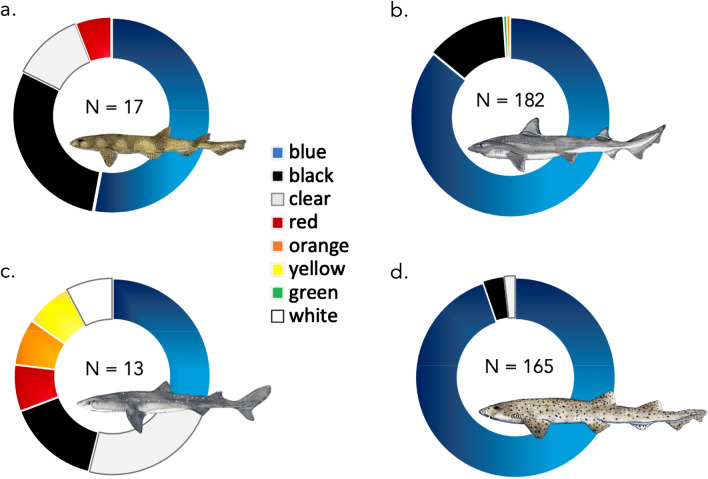
Fibres ranged in length from 0.3 mm to 14.4 mm and had an average length of 2.7 mm ± 2.6 SD (see Fig. 1). The vast majority of fibres were blue (88.0%) or black (8.8%) in colour, with the remaining colours including: red, yellow and other (clear, green and white) each making up 3.8% (see Fig. 2 A-D). The two fragments identified were blue and white in colour. Fibres larger than 5 mm (n = 50) were considered here as macroplastics and were excluded from the analysis, although can be found grouped together in the ≥ 5 mm category on Fig. 1.
Figure 1.
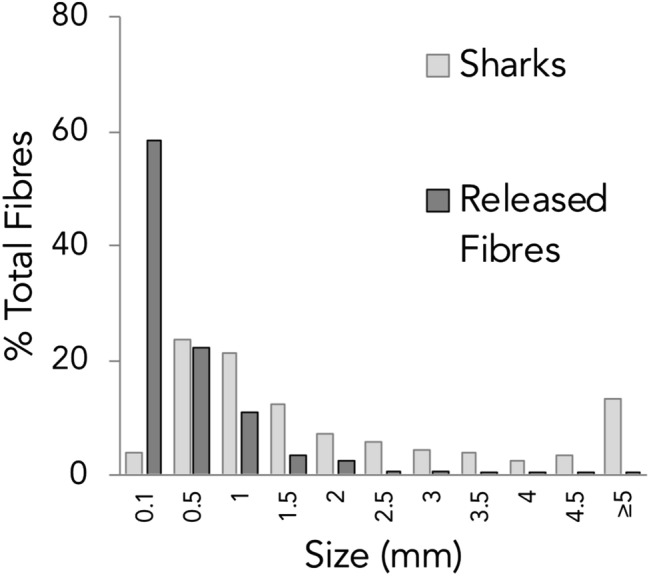
Fibre length distribution. Fibre lengths as a proportion of total fibres for fibres found in shark species (light grey) and fibres released in laboratory conditions after washing of various cotton and polyethylene terephthalate textiles. Palacios Marin AV, (2019) Release of microfibres from comparative common textile structures during laundering (Unpublished Masters dissertation). University of Leeds, UK.
Figure 2.
Composition of colours of ingested fibres, found across both the stomachs and intestines of four species of north-east Atlantic demersal sharks: (a) small-spotted catshark (Scyliorhinus canicula), (b) starry smooth-hound (Mustelus asterias), (c) spiny dogfish (Squalus acanthias) and (d) bull huss (Scyliorhinus stellaris). Total N of coloured fibres identified annotated within figure. Elasmobranch drawings by Lucie Jones.
Differences between species, sex and body size
The estimated number of ingested microfibres was positively influenced by individual shark body length, however it did not differ between species or sex (See Fig. 3A,B, Supplementary Table S2 and Supplementary Fig. S5). It should be noted two individuals in this study (one starry smooth-hound and one bull huss) had much higher levels, with the sample from the former individual containing 147 fibres and the sample from the latter containing 154 fibres. Upon visual examination, these fibres appeared to be strands of blue rope, subsequently confirmed as olefin polypropylene. (Supplementary Figures S4–S8 have been created with these outliers removed/added for comparison).
Figure 3.
Estimated fibre ingestion and relationship with total length (cm). (a) Expected number of fibres based on extrapolation from full stomach/GI tract volumes. Medians marked by red line. N = annotated. Elasmobranch drawings by Lucie Jones. (b) Relationship between the estimated number of ingested fibres and individual length. Lines denote predictions from the top ranked model presented in Supplementary Table S2. Standard errors are shown by the dashed lines.
Polymer identification
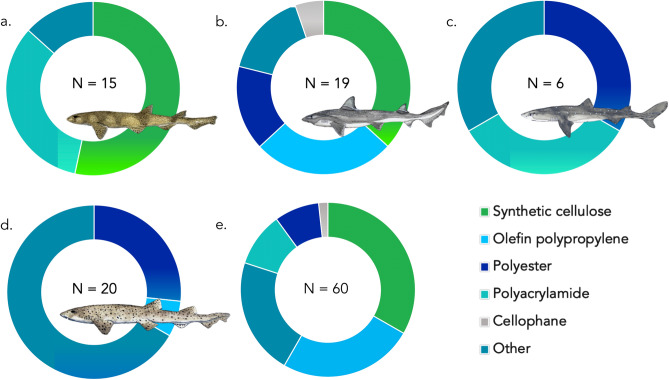
A subsample of contaminant particles (n = 60 fibres, n = 2 fragments) were subject to FT-IR analysis (16% of total contaminants identified). However, when we consider the sample set without the two outliers mentioned above which were olefin polypropylene fibres, the subsample of contaminants that underwent FT-IR spectroscopy equalled 79% of all particles isolated.
Our analysis revealed 33.3% of fibres (n = 20) were cellulose derivatives (Alpha & Ecteola modified), however further analysis by light microscopy revealed these cellulose fibres were anthropogenic in nature due to their uniform diameter distribution across the fibre length and observation of convoluted structure of the fibre; a characteristic of cotton fibres (see Supplementary Fig. S2). Polyacrylamides made up 10% of fibres (n = 6), 8.3% of fibres were polyesters (n = 5) and 1.7% were cellophane (n = 1). Another 25% (n = 15) registered as Olefin polypropylene. Combined with the aforementioned microplastic contaminants (polyester and polyacrylamide), this results in a total of 43.3% of particles being true microplastics.
The remaining 21.6% of fibres (n = 13) were either unidentifiable due to low spectral match scores (n = 7) or returned as biological in nature (n = 6). Biological returns were excluded from broader statistical analysis. See Fig. 4 & Supplementary Table S1.
Figure 4.
Composition of polymer make up of fibres between shark species. N of polymers identified in each species annotated on figure. (a) small-spotted catshark. (b) starry smooth-hound. (c) Spiny dogfish. (d) bull huss. (e) Total polymer percentages for all four species. Other = Biological materials and/or low spectral match scores. Elasmobranch drawings by Lucie Jones.
The two fragments identified returned as polyethylene and polypropylene (see Supplementary Fig. S3).
Discussion
Our study is the first to demonstrate the presence of microplastic and anthropogenic particle contaminants in resident UK shark species in the North-East Atlantic. Despite there being no substantial differences in microplastic uptake among the shark species studied here, the research provides an important empirical baseline for future work investigating contaminant levels in UK sharks. Greater levels of contamination might be expected in animals that inhabit other parts of the UK with lower water quality. Although we have not demonstrated any health impacts on the sharks, the presence of these particle contaminants indicates their pervasiveness in the marine environment. With increasing global plastic production and its prevalence in every day products, the abundance of such marine pollutants is likely set to increase.
Contaminant particle ingestion by species, sex and size
Nearly 70% of all sharks sampled in our study contained at least one contaminant particle in their digestive tracts. Although this is likely to be a conservative estimate of incidence, this number is significantly higher than many other reports for similar shark species around the world22,36,39,50,62,63 see Table 1. Studies by Alomar and Deudero38 and Smith36 revealed ingestion rates of microplastics at 16.8% in blackmouth catsharks sampled in the Mediterranean, and 15% in small-spotted catsharks from the North sea, respectively36,63. Interestingly, the Mediterranean is considered by some to be one of the worst affected oceans with regards to plastic pollution5,64,65, therefore ingestion of contaminant particles may have been expected to have been lower in North-East Atlantic. The only other study to have been conducted on similar species and within a similar ocean area is that of Neves et al.22, which found microplastic ingestion rates of 20% in small-spotted catsharks collected from the North-East Atlantic coast of Portugal, with microplastics being mostly fibrous in nature.
Table 1.
Breakdown of publications on elasmobranchs and microplastics, featuring ocean basin, location, species examined, Number of samples examined, methodology for extraction and percentage of contaminant ingestion for species studied.
| Ocean basin | Species examined | N | Methodology | % Ingestion | References |
|---|---|---|---|---|---|
| Atlantic | |||||
| North-East | Scyliorhinus canicula | 46 | Dissection, 20% KOH digestion, FT-IR | 67% | Parton et al. (in press) |
| Scyliorhinus stellaris | |||||
| Mustelus asterias | |||||
| Squalus acanthias | |||||
| Scyliorhinus canicula | 20 | Dissection, FT-IR | 20% | Neves et al.22 | |
| Raja asterias | 7 | 40% | |||
| North Sea | Scyliorhinus canicula | 20 | Dissection, visual inspection | 15% | Smith36 |
| Mediterranean | |||||
| Balearic Islands | Galeus melastomus | 125 | Dissection, FT-IR | 17% | Alomar and Deudero38 |
| Western Ligurian Sea | Prionace glauca | 95 | Dissection, FT-IR | 25% | Bernardini et al.39 |
| Tyrrenhenian Sea | Galeus melastomus | 96 | Dissection, 10% KOH digestion, FT-IR | 69% | Valente et al.37 |
| Scyliorhinus canicula | |||||
| Etmopterus spinax | |||||
| Ionian Sea | Pteroplatytrygon violacea | 2 | Dissection, Visual inspection | 50% | Anastasopoulou et al.50 |
| Galeus melastomus | 741 | 3% | |||
| Squalus blainville | 75 | 1% | |||
| Etmopterus spinax | 16 | 6% | |||
| Pacific | |||||
| Gulf of California | Rhincodon typus | 12 | Skin biopsy (used to infer contaminant levels) | 8.42 ng/g w.w. PCBs | Fossi et al.40 |
| 1.31 ng/g w.w. DDTs | |||||
| 0.29 ng/g w.w. PBDEs | |||||
| 0.19 ng/g w.w. HCB | |||||
| Indian | |||||
| KwaZulu-Natal, South Africa | 14 species, see study for details | 15,666 | Dissection, Visual inspection | 0.38% (macroplastics) | Cliff et al.62 |
Some figures presented here as reported in their respective study.
The contaminants found within our sharks is consistent with other studies investigating the presence of pollutants in the marine environment23,66–68, and their colours23,66,68,69. Fibres are quickly becoming the most ubiquitous contaminant type in many compartments of marine ecosystems, as well as in the gut contents of numerous marine species including turtles, seals and cetaceans23,24,70,71. Fibres have a number of potential sources, including break-off from fishing and maritime equipment such as nets and ropes72, fibre shedding from automotive tyre wear and the washing of synthetic fabrics in clothing, as well as breakage and release from other textiles16,19,73–75.
We hypothesised that there would be differences in estimated contaminant load among species, between sexes and across size classes. The expected number of ingested fibres was only influenced by individual length (TL cm) with more found in larger sharks. As we were unable to control for location/habitat in this study, this remains to be explored in further detail. While diet could be an additional influencing factor for these shark species, with the current presented data and relatively small sample size we can only speculate as to the factors influencing contaminant burden in these demersal sharks.
Ingestion pathways
There are at least two potential ingestion pathways for contaminant particles by demersal shark species. Firstly, via the presence of contaminants directly in their food source. Microplastics and other anthropogenic materials have been reported in several prey species for these sharks, including crustaceans and molluscs28,51,52,76,77. Some of these prey items have also been to shown to take-up and translocate microplastics around their bodies in laboratory conditions30,78, as well as transfer microplastics up the food-web79. The species in this study show some variation in their published dietary strategies with starry smooth-hounds and spiny dogfish having fairly specialist diets, compared to small-spotted catsharks and bull huss which are more generalist44,46,80,81. We may have expected the generalist feeding sharks to have more contaminants due to feeding on a wider range of prey items, however this was not evident.
The second pathway for exposure could be through direct engulfment alongside target prey species. Habitat use has been identified as a potential driver of plastic ingestion for other elasmobranch species, including whale sharks and manta rays41, as well as bony fish species82. The sharks analysed in this study all display similar strategies while feeding in their demersal habitat, in that to swallow their prey, they engulf it whole using suction feeding83,84. In doing so, many of these species will ingest large quantities of sediment alongside their prey. Although the majority of this is immediately expelled from the mouth, some makes its way to the gut85,86. Numerous studies have revealed that microplastics eventually sink to the seafloor and rest in the sediment87–90. Naturally occurring sediment particles occur quite regularly in the guts of these shark species and it is highly likely that many of the ingested microfibres will be excreted alongside natural sediment particles. The potential for either plastic or natural particles to cause internal damage before excretion remains to be tested.
Existing studies have attempted to analyse environmental microplastic contamination in the North-East Atlantic, both on the sea surface and the sediment68,90. Lusher et al.68 found that 94% of samples from surface waters in the North-East Atlantic contained what they believed to be potential microplastics, although after further analysis 63% of these appeared to be matt black anthropogenic fibres and not true microplastics. These matt black fibres are similar in description to many of the fibres found in our current study. When analysed under Raman FT-IR, Lusher et al.68 found they were matched closely with cellulose and rayon, again similar to the cellulose fibres found in this study. In a separate study, Maes et al.90 identified microplastic particles in 89% of sediment samples from the North Sea and English channel, with most of the plastics considered spheres (microbeads) and fibres90, however these authors do not allude to regenerated cellulose fibres in their samples, which may have been present, but not recorded. Given these environmental levels, it should, therefore, be no surprise that approximately 70% of the sharks in this study contained at least 1 contaminant particle.
Polymer identification
Analysing the polymer make-up of marine plastics can reveal potential sources, fate and causes for ingestion23,24. The use of FT-IR spectrometry to analyse environmental samples is a reliable method of determining their polymer make-up91–93 and should be fundamental to any future study. The polymers we identified largely reflect the results of similar studies in the marine environment23,94–96 and are also similar to polymer diversity of microplastics globally23,66,97, with polypropylene being one of the most widely abundant polymers identified worldwide23.
Synthetic cellulose fibres are being recorded in environmental samples across multiple studies23,66,88,98, although currently their diverse origins remains somewhat understudied. These anthropogenic fibres made up a third of the analysed contaminants and were identified as regenerated cellulose, such as viscose and rayon, as well as lyocell and cotton, with the likely source of such fibres being textiles or personal hygiene items19,73,74. Spectral libraries for FT-IR set-ups must continue to expand moving forwards, in order to develop reliable databases that are capable of accurately identifying regenerated cellulose fibres within environmental samples.
Estimates show that an average clothes wash of 6 kg can release more than 700,000 fibres into waste water facilities and although some will be retained by these facilities, many will inevitably make their way into the marine environment, often via river systems19. These released fibres such as polyester and cotton are globally in-demand between 24–46 million tonnes per year99. Interestingly, the fibre lengths identified in the digestive tracts of sharks were similar to that of fibres released upon washing of various textiles under laboratory conditions (see Fig. 1), highlighting the washing of clothes as a major route for fibres to enter the environment.
Potential implications
As we only tested a sub-sample of gut content for each animal (20 ml), the proportional incidence of anthropogenic contaminants we report is a conservative estimate. Due to the microscopic size of these synthetic fibres, direct internal organ damage is unlikely, when compared to ingestion of larger macro-plastics, although the ability of small fibres to cause inflammatory damage is acknowledged in other contexts100–102. Translocation of relatively large (150 µm) particles can occur across the vertebrate gut via persorption (the passage of particles through the epithelial layers of the gastro-intestinal tract), whilst smaller particles are taken up through normal digestive processes such as pinocytosis and phagocytosis, circulating through the blood and lymph vessels. Thus, there is the opportunity for such circulating particles to enter cells and induce inflammatory damage before being excreted103. Fibres of 100–1,000 µm will most likely pass straight through the digestive tract and be excreted with other waste products23.
Future research could aim to assess whether certain fibres present exposure risks of associated contaminants and/or persistent organic pollutants23,24. Certain textiles may contain toxic chemicals such as BPA (bisphenol A) and BPS (bisphenol S)104, with both chemicals capable of causing disruption to reproductive and endocrine systems as well as growth suppression in marine taxa, at relatively low doses105–108. Other studies have shown different associated contaminants can present inherent biological risks to various species, including elasmobranchs34,35,40,109.
Research has revealed that spiny dogfish and small-spotted catshark are regularly sold in fish and chip shops under pseudonyms such as “Rock”, “Rock salmon” and “Murgey”110. If contaminants are able to pass from the digestive tract to the muscle tissue of these shark species, then humans may inadvertently be consuming these pollutants. Although currently there is no conclusive evidence to suggest these pollutants present inherent health risks to humans, we recommend further research to investigate the presence or absence of these contaminant particles in the muscle tissues of these shark species and other fish consumed across the world.
Conclusions
This study presents the first evidence of microplastics and anthropogenic fibre contaminants in a range of native UK demersal shark species. Although not occurring in as high levels as in other marine megafauna, the presence of anthropogenic particles in these marine species highlights their ubiquitous nature. Although highly unlikely, neither individual nor population level effects of this level of contamination are known. Due to these low levels of ingestion, these species are perhaps not ideal candidates to be used as bio-indicators for marine pollution in demersal habitats when compared to other bony fish species. Nonetheless, if inorganic pollutants can attach to these microfibres, alongside a future increase in their prevalence throughout the marine environment, biological side-effects may occur. Further research on the sources and pathways of anthropogenic fibres may inform policy to reduce their overall prevalence in the environment. By limiting their production in everyday products (through supporting reduction, reuse and replacement of fibre-generating materials from the resource flow) and implementing strategies to prevent their initial entry into the oceans there lies the potential to dramatically reduce the occurrence of microfibres in the marine environment and across food webs.
Supplementary information
Acknowledgements
The authors thank Dr Sarah Nelms and Dr Emily Duncan for their help with laboratory analysis and general advice, illustrator Lucie Jones for her elasmobranch drawings and the anonymous reviewers and the editor, whose comments greatly improved the manuscript. This work was undertaken with approval of the University of Exeter Animal Ethics Committee. NERC Grant NE/S003975/1.
Author contributions
T.G. oversaw training of K.P. in lab skills required for analysis. K.P. conducted processing of samples and led data collection, D.S. helped conduct analysis of samples using FT-IR, M.T. analysed fibres to elucidate their origins and helped create Fig. 1. B.G. oversaw creation of figures and tables. L.O. conducted statistical analysis. K.P. B.G., T.G. and M.T. wrote the main manuscript text. All authors reviewed the manuscript.
Data availability
Data is available from the British Oceanographic Centre (Marine).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-68680-1.
References
- 1.Denuncio P, et al. Plastic ingestion in Franciscana dolphins, Pontoporia blainvillei (Gervais and d’Orbigny, 1844), from Argentina. Mar. Pollut. Bull. 2011;62:1836–1841. doi: 10.1016/j.marpolbul.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 2.Ryan, P. G., Shomura, R. S. & Godfrey, M. L. The marine plastic debris problem of Southern Africa: types of debris, their environmental effects, and control measures. Proc. Second Int. Conf. Mar. debris 623–634 (1989). doi:NOAA-TM-NMFS-SWFSC-15
- 3.Cole M, Lindeque P, Halsband C, Galloway TS. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 2011;62:2588–2597. doi: 10.1016/j.marpolbul.2011.09.025. [DOI] [PubMed] [Google Scholar]
- 4.Galloway TS, Lewis CN. Marine microplastics spell big problems for future generations. Proc. Natl. Acad. Sci. 2016;113:2331–2333. doi: 10.1073/pnas.1600715113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eriksen M, et al. Plastic Pollution in the World’s Oceans: More than 5 Trillion Plastic Pieces Weighing over 250,000 Tons Afloat at Sea. PLoS ONE. 2014;9:e111913. doi: 10.1371/journal.pone.0111913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jambeck JR, et al. Marine pollution. Plastic waste inputs from land into the ocean. Science. 2015;347:768–71. doi: 10.1126/science.1260352. [DOI] [PubMed] [Google Scholar]
- 7.Bucci K, Tulio M, Rochman C. What is known and unknown about the effects of plastic pollution: a meta-analysis and systematic review. Ecol. Appl. 2019 doi: 10.1002/eap.2044. [DOI] [PubMed] [Google Scholar]
- 8.Ryan PG, Moore CJ, Van Franeker JA, Moloney CL. Monitoring the abundance of plastic debris in the marine environment. Philos. Trans. R. Soc. B Biol. Sci. 2009;364:1999–2012. doi: 10.1098/rstb.2008.0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barnes DKA, Galgani F, Thompson RC, Barlaz M. Accumulation and fragmentation of plastic debris in global environments. Philos. Trans. R. Soc. B Biol. Sci. 2009;364:1985–1998. doi: 10.1098/rstb.2008.0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arthur, C., Baker, J. E. & Bamford, H. A. Proceedings of the International Research Workshop on the Occurrence, Effects, and Fate of Microplastic Marine Debris, September 9–11, 2008, University of Washington Tacoma, Tacoma, WA, USA. in (2009).
- 11.Sussarellu R, et al. Oyster reproduction is affected by exposure to polystyrene microplastics. Proc. Natl. Acad. Sci. USA. 2016;113:2430–2435. doi: 10.1073/pnas.1519019113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galloway TS, Cole M, Lewis C. Interactions of microplastic debris throughout the marine ecosystem. Nat. Ecol. Evol. 2017;1:0116. doi: 10.1038/s41559-017-0116. [DOI] [PubMed] [Google Scholar]
- 13.Koelmans AA, Besseling E, Foekema EM. Leaching of plastic additives to marine organisms. Environ. Pollut. 2014;187:49–54. doi: 10.1016/j.envpol.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 14.Avio CG, Gorbi S, Regoli F. Plastics and microplastics in the oceans: From emerging pollutants to emerged threat. Mar. Environ. Res. 2017;128:2–11. doi: 10.1016/j.marenvres.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 15.Browne MA, Galloway T, Thompson R. Microplastic-an emerging contaminant of potential concern? Integr. Environ. Assess. Manag. 2007;3:559–561. doi: 10.1002/ieam.5630030412. [DOI] [PubMed] [Google Scholar]
- 16.De Falco F, et al. Evaluation of microplastic release caused by textile washing processes of synthetic fabrics. Environ. Pollut. 2018;236:916–925. doi: 10.1016/j.envpol.2017.10.057. [DOI] [PubMed] [Google Scholar]
- 17.Carney Almroth BM, et al. Quantifying shedding of synthetic fibers from textiles; a source of microplastics released into the environment. Environ. Sci. Pollut. Res. 2018;25:1191–1199. doi: 10.1007/s11356-017-0528-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hernandez E, Nowack B, Mitrano DM. Polyester textiles as a source of microplastics from households: a mechanistic study to understand microfiber release during washing. Environ. Sci. Technol. 2017;51:7036–7046. doi: 10.1021/acs.est.7b01750. [DOI] [PubMed] [Google Scholar]
- 19.Napper IE, Thompson RC. Release of synthetic microplastic plastic fibres from domestic washing machines: Effects of fabric type and washing conditions. Mar. Pollut. Bull. 2016;112:39–45. doi: 10.1016/j.marpolbul.2016.09.025. [DOI] [PubMed] [Google Scholar]
- 20.Murphy F, Ewins C, Carbonnier F, Quinn B. Wastewater treatment works (WwTW) as a source of microplastics in the aquatic environment. Environ. Sci. Technol. 2016;70:1–5. doi: 10.1021/acs.est.5b05416. [DOI] [PubMed] [Google Scholar]
- 21.Nadal MA, Alomar C, Deudero S. High levels of microplastic ingestion by the semipelagic fish bogue Boops boops (L.) around the Balearic Islands. Environ. Pollut. 2016;214:517–523. doi: 10.1016/j.envpol.2016.04.054. [DOI] [PubMed] [Google Scholar]
- 22.Neves D, Sobral P, Ferreira JL, Pereira T. Ingestion of microplastics by commercial fish off the Portuguese coast. Mar. Pollut. Bull. 2015;101:119–126. doi: 10.1016/j.marpolbul.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 23.Duncan EM, et al. Microplastic ingestion ubiquitous in marine turtles. Glob. Chang. Biol. 2018;25:744–752. doi: 10.1111/gcb.14519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nelms SE, Galloway TS, Godley BJ, Jarvis DS, Lindeque PK. Investigating microplastic trophic transfer in marine top predators. Environ. Pollut. 2018;238:999–1007. doi: 10.1016/j.envpol.2018.02.016. [DOI] [PubMed] [Google Scholar]
- 25.Schuyler Q, Hardesty BD, Wilcox C, Townsend K. Global analysis of anthropogenic debris ingestion by sea turtles. Conserv. Biol. 2014;28:129–139. doi: 10.1111/cobi.12126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Setälä O, Fleming-Lehtinen V, Lehtiniemi M. Ingestion and transfer of microplastics in the planktonic food web. Environ. Pollut. 2014;185:77–83. doi: 10.1016/j.envpol.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 27.Murray F, Cowie PR. Plastic contamination in the decapod crustacean Nephrops norvegicus (Linnaeus, 1758) Mar. Pollut. Bull. 2011;62:1207–1217. doi: 10.1016/j.marpolbul.2011.03.032. [DOI] [PubMed] [Google Scholar]
- 28.Devriese LI, et al. Microplastic contamination in brown shrimp (Crangon crangon, Linnaeus 1758) from coastal waters of the Southern North Sea and Channel area. Mar. Pollut. Bull. 2015;98:179–187. doi: 10.1016/j.marpolbul.2015.06.051. [DOI] [PubMed] [Google Scholar]
- 29.Botterell ZLR, et al. Bioavailability and effects of microplastics on marine zooplankton: a review. Environ. Pollut. 2019;245:98–110. doi: 10.1016/j.envpol.2018.10.065. [DOI] [PubMed] [Google Scholar]
- 30.Gandara e Silva, P. P., Nobre, C. R., Resaffe, P., Pereira, C. D. S. & Gusmão, F. Leachate from microplastics impairs larval development in brown mussels. Water Res.106, 364–370 (2016). [DOI] [PubMed]
- 31.Cole M, Lindeque P, Fileman E, Halsband C, Galloway TS. The impact of polystyrene microplastics on feeding, function and fecundity in the marine copepod Calanus helgolandicus. Environ. Sci. Technol. 2015;49:1130–1137. doi: 10.1021/es504525u. [DOI] [PubMed] [Google Scholar]
- 32.Stelfox M, Hudgins J, Sweet M. A review of ghost gear entanglement amongst marine mammals, reptiles and elasmobranchs. Mar. Pollut. Bull. 2016;111:6–17. doi: 10.1016/j.marpolbul.2016.06.034. [DOI] [PubMed] [Google Scholar]
- 33.Parton K, Galloway T, Godley B. Global review of shark and ray entanglement in anthropogenic marine debris. Endanger. Species Res. 2019;39:173–190. [Google Scholar]
- 34.Fossi, M. C. et al. Are whale sharks exposed to persistent organic pollutants and plastic pollution in the Gulf of California (Mexico)? First ecotoxicological investigation using skin biopsies. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol.199, 48–58 (2017). [DOI] [PubMed]
- 35.Germanov ES, Marshall AD, Bejder L, Fossi MC, Loneragan NR. Microplastics: no small problem for filter-feeding megafauna. Trends Ecol. Evol. 2018;33:227–232. doi: 10.1016/j.tree.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 36.Smith LE. Plastic ingestion by Scyliorhinus canicula trawl captured in the North Sea. Mar. Pollut. Bull. 2018;130:6–7. doi: 10.1016/j.marpolbul.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 37.Valente T, et al. Exploring microplastic ingestion by three deep-water elasmobranch species: a case study from the Tyrrhenian Sea. Environ. Pollut. 2019;253:342–350. doi: 10.1016/j.envpol.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 38.Alomar C, Deudero S. Evidence of microplastic ingestion in the shark Galeus melastomus Rafinesque, 1810 in the continental shelf off the western Mediterranean Sea. Environ. Pollut. 2017;223:223–229. doi: 10.1016/j.envpol.2017.01.015. [DOI] [PubMed] [Google Scholar]
- 39.Bernardini I, Garibaldi F, Canesi L, Fossi MC, Baini M. First data on plastic ingestion by blue sharks (Prionace glauca) from the Ligurian Sea (North-Western Mediterranean Sea) Mar. Pollut. Bull. 2018;135:303–310. doi: 10.1016/j.marpolbul.2018.07.022. [DOI] [PubMed] [Google Scholar]
- 40.Fossi MC, et al. Large filter feeding marine organisms as indicators of microplastic in the pelagic environment: the case studies of the Mediterranean basking shark (Cetorhinus maximus) and fin whale (Balaenoptera physalus) Mar. Environ. Res. 2014;100:17–24. doi: 10.1016/j.marenvres.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 41.Germanov ES, et al. Microplastics on the menu: plastics pollute indonesian manta ray and whale shark feeding grounds. Front. Mar. Sci. 2019;6:679. [Google Scholar]
- 42.Sulikowski J, et al. Use of satellite tags to reveal the movements of spiny dogfish Squalus acanthias in the western North Atlantic Ocean. Mar. Ecol. Prog. Ser. 2010;418:249–254. [Google Scholar]
- 43.Sims D, Nash J, Morritt D. Movements and activity of male and female dogfish in a tidal sea lough: alternative behavioural strategies and apparent sexual segregation. Mar. Biol. 2001;139:1165–1175. [Google Scholar]
- 44.Ellis JR, Pawson MG, Shackley SE. The comparative feeding ecology of six species of shark and four species of ray (Elasmobranchii) In The North-East Atlantic. J. Mar. Biol. Assoc. UK. 2009;76:89. [Google Scholar]
- 45.Fordham, S., Fowler, S. L., Coelho, R. P., Goldman, K. & Francis, M. P. Squalus acanthias. The IUCN Red List of Threatened Species 2016 (2016). Available at: https://www.iucnredlist.org/species/91209505/2898271. (Accessed: 2nd December 2019)
- 46.Domi N, Bouquegneau JM, Das K. Feeding ecology of five commercial shark species of the Celtic Sea through stable isotope and trace metal analysis. Mar. Environ. Res. 2005;60:551–569. doi: 10.1016/j.marenvres.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 47.Hammond TR, Ellis JR. Bayesian assessment of northeast atlantic spurdog using a stock production model & with prior for intrinsic population growth rate set by demographic methods. J. Northw. Atl. Fish. Sci. 2004;35:299–308. [Google Scholar]
- 48.Revill AS, Dulvy NK, Holst R. The survival of discarded lesser-spotted dogfish (Scyliorhinus canicula) in the Western English Channel beam trawl fishery. Fish. Res. 2005;71:121–124. [Google Scholar]
- 49.Bellas J, Martínez-Armental J, Martínez-Cámara A, Besada V, Martínez-Gómez C. Ingestion of microplastics by demersal fish from the Spanish Atlantic and Mediterranean coasts. Mar. Pollut. Bull. 2016;109:55–60. doi: 10.1016/j.marpolbul.2016.06.026. [DOI] [PubMed] [Google Scholar]
- 50.Anastasopoulou, A., Mytilineou, C., Smith, C. J. & Papadopoulou, K. N. Plastic debris ingested by deep-water fish of the Ionian Sea (Eastern Mediterranean). Deep Sea Res. Part I Oceanogr. Res. Pap.74, 11–13 (2013).
- 51.Murphy F, Russell M, Ewins C, Quinn B. The uptake of macroplastic & microplastic by demersal & pelagic fish in the Northeast Atlantic around Scotland. Mar. Pollut. Bull. 2017;122:353–359. doi: 10.1016/j.marpolbul.2017.06.073. [DOI] [PubMed] [Google Scholar]
- 52.Rummel CD, et al. Plastic ingestion by pelagic and demersal fish from the North Sea and Baltic Sea. Mar. Pollut. Bull. 2016;102:134–141. doi: 10.1016/j.marpolbul.2015.11.043. [DOI] [PubMed] [Google Scholar]
- 53.Foekema EM, et al. Plastic in north sea fish. Environ. Sci. Technol. 2013;47:8818–8824. doi: 10.1021/es400931b. [DOI] [PubMed] [Google Scholar]
- 54.Lusher, A. L., O’Donnell, C., Officer, R. & O’Connor, I. Microplastic interactions with North Atlantic mesopelagic fish. ICES J. Mar. Sci. J. du Cons.73, 1214–1225 (2016).
- 55.Dehaut A, et al. Microplastics in seafood: Benchmark protocol for their extraction and characterization. Environ. Pollut. 2016;215:223–233. doi: 10.1016/j.envpol.2016.05.018. [DOI] [PubMed] [Google Scholar]
- 56.Kühn S, et al. The use of potassium hydroxide (KOH) solution as a suitable approach to isolate plastics ingested by marine organisms. Mar. Pollut. Bull. 2017;115:86–90. doi: 10.1016/j.marpolbul.2016.11.034. [DOI] [PubMed] [Google Scholar]
- 57.Bessa F, et al. Occurrence of microplastics in commercial fish from a natural estuarine environment. Mar. Pollut. Bull. 2018;128:575–584. doi: 10.1016/j.marpolbul.2018.01.044. [DOI] [PubMed] [Google Scholar]
- 58.Venables W, Ripley B. Modern applied statistics with S. 4. Berlin: Springer; 2002. [Google Scholar]
- 59.R Core Team. R: A language and environment for statistical computing. (2018).
- 60.Barton, K. MuMIn: Multi-model inference. (2015).
- 61.Richards SA, Whittingham MJ, Stephens PA. Model selection and model averaging in behavioural ecology: the utility of the IT-AIC framework. Behav. Ecol. Sociobiol. 2011;65:77–89. [Google Scholar]
- 62.Cliff G, Dudley SFJ, Ryan PG, Singletonc N. Large sharks and plastic debris in KwaZulu-Natal, South Africa. Mar. Freshw. Res. 2002;53:575–581. [Google Scholar]
- 63.Deudero S, Alomar C. Mediterranean marine biodiversity under threat: Reviewing influence of marine litter on species. Mar. Pollut. Bull. 2015;98:58–68. doi: 10.1016/j.marpolbul.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 64.Cózar A, et al. Plastic accumulation in the mediterranean sea. PLoS ONE. 2015;10:1–12. doi: 10.1371/journal.pone.0121762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Suaria G, et al. The Mediterranean Plastic Soup: synthetic polymers in Mediterranean surface waters. Sci. Rep. 2016;6:37551. doi: 10.1038/srep37551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gago J, Carretero O, Filgueiras AV, Viñas L. Synthetic microfibers in the marine environment: A review on their occurrence in seawater and sediments. Mar. Pollut. Bull. 2018;127:365–376. doi: 10.1016/j.marpolbul.2017.11.070. [DOI] [PubMed] [Google Scholar]
- 67.Compa M, Ventero A, Iglesias M, Deudero S. Ingestion of microplastics and natural fibres in Sardina pilchardus (Walbaum, 1792) and Engraulis encrasicolus (Linnaeus, 1758) along the Spanish Mediterranean coast. Mar. Pollut. Bull. 2018;128:89–96. doi: 10.1016/j.marpolbul.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 68.Lusher AL, Burke A, O’Connor I, Officer R. Microplastic pollution in the Northeast Atlantic Ocean: Validated and opportunistic sampling. Mar. Pollut. Bull. 2014;88:325–333. doi: 10.1016/j.marpolbul.2014.08.023. [DOI] [PubMed] [Google Scholar]
- 69.Steer M, Cole M, Thompson RC, Lindeque PK. Microplastic ingestion in fish larvae in the western English Channel. Environ. Pollut. 2017;226:250–259. doi: 10.1016/j.envpol.2017.03.062. [DOI] [PubMed] [Google Scholar]
- 70.Lusher, A. Microplastics in the Marine Environment: Distribution, Interactions and Effects. in Marine Anthropogenic Litter 245–307 (Springer International Publishing, 2015). 10.1007/978-3-319-16510-3_10.
- 71.Zhu J, et al. Cetaceans and microplastics: First report of microplastic ingestion by a coastal delphinid Sousa chinensis. Sci. Total Environ. 2019;659:649–654. doi: 10.1016/j.scitotenv.2018.12.389. [DOI] [PubMed] [Google Scholar]
- 72.Welden NA, Cowie PR. Degradation of common polymer ropes in a sublittoral marine environment. Mar. Pollut. Bull. 2017;118:248–253. doi: 10.1016/j.marpolbul.2017.02.072. [DOI] [PubMed] [Google Scholar]
- 73.Hartline NL, et al. Microfiber Masses Recovered from Conventional Machine Washing of New or Aged Garments. Environ. Sci. Technol. 2016;50:11532–11538. doi: 10.1021/acs.est.6b03045. [DOI] [PubMed] [Google Scholar]
- 74.Salvador Cesa, F., Turra, A. & Baruque-Ramos, J. Synthetic fibers as microplastics in the marine environment: a review from textile perspective with a focus on domestic washings. Sci. Total Environ.598, 1116–1129 (2017). [DOI] [PubMed]
- 75.Wagner S, et al. Tire wear particles in the aquatic environment—a review on generation, analysis, occurrence, fate and effects. Water Res. 2018;139:83–100. doi: 10.1016/j.watres.2018.03.051. [DOI] [PubMed] [Google Scholar]
- 76.Van Cauwenberghe L, Janssen CR. Microplastics in bivalves cultured for human consumption. Environ. Pollut. 2014;193:65–70. doi: 10.1016/j.envpol.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 77.Watts AJR, et al. Uptake and retention of microplastics by the shore crab Carcinus maenas. Environ. Sci. Technol. 2014;48:8823–8830. doi: 10.1021/es501090e. [DOI] [PubMed] [Google Scholar]
- 78.Browne, M. A., Dissanayake, A., Galloway, T. S., Lowe, D. M. & Thompson, R. C. Ingested Microscopic Plastic Translocates to the Circulatory System of the Mussel, Mytilus edulis (L.). Environ. Sci. Technol.42, 5026–5031 (2008). [DOI] [PubMed]
- 79.Farrell, P. & Nelson, K. Trophic level transfer of microplastic: Mytilus edulis (L.) to Carcinus maenas (L.). Environ. Pollut.177, 1–3 (2013). [DOI] [PubMed]
- 80.Saldanha, L., Almeida, A. J., Andrade, F. & Guerreiro, J. Observations on the Diet of Some Slope Dwelling Fishes of Southern Portugal. Int. Rev. der gesamten Hydrobiol. und Hydrogr.80, 217–234 (1995).
- 81.Martinho F, Sá C, Falcão J, Cabral HN, Pardal MÂ. Comparative feeding ecology of two elasmobranch species, Squalus blainville and Scyliorhinus canicula, off the coast of Portugal. Fish. Bull. 2012;110:71–84. [Google Scholar]
- 82.Ogonowski M, et al. Microplastic Intake, Its Biotic Drivers, and Hydrophobic Organic Contaminant Levels in the Baltic Herring. Front. Environ. Sci. 2019;7:134. [Google Scholar]
- 83.Wilga, C. & Motta, P. Conservation and variation in the feeding mechanism of the spiny dogfish squalus acanthias. J. Exp. Biol.201, (1998). [DOI] [PubMed]
- 84.Huber DR, Motta PJ. Comparative analysis of methods for determining bite force in the spiny dogfishSqualus acanthias. J. Exp. Zool. 2004;301A:26–37. doi: 10.1002/jez.a.20003. [DOI] [PubMed] [Google Scholar]
- 85.Kalmijn, A. J. The Electric Sense of Sharks and Rays. J. Exp. Biol.55, (1971). [DOI] [PubMed]
- 86.Smith JW, Merriner JV. Food habits and feeding behavior of the Cownose Ray, Rhinoptera bonasus, in Lower Chesapeake Bay. Estuaries. 1985;8:305. [Google Scholar]
- 87.Martin J, Lusher A, Thompson RC, Morley A. The deposition and accumulation of microplastics in marine sediments and bottom water from the Irish continental shelf. Sci. Rep. 2017;7:10772. doi: 10.1038/s41598-017-11079-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Woodall LC, et al. The deep sea is a major sink for microplastic debris. R. Soc. Open Sci. 2014;1:140317. doi: 10.1098/rsos.140317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ling SD, Sinclair M, Levi CJ, Reeves SE, Edgar GJ. Ubiquity of microplastics in coastal seafloor sediments. Mar. Pollut. Bull. 2017;121:104–110. doi: 10.1016/j.marpolbul.2017.05.038. [DOI] [PubMed] [Google Scholar]
- 90.Maes T, et al. Microplastics baseline surveys at the water surface and in sediments of the North-East Atlantic. Front. Mar. Sci. 2017;4:135. [Google Scholar]
- 91.Shim WJ, Hong SH, Eo SE. Identification methods in microplastic analysis: a review. Anal. Methods. 2017;9:1384–1391. [Google Scholar]
- 92.Jung MR, et al. Validation of ATR FT-IR to identify polymers of plastic marine debris, including those ingested by marine organisms. Mar. Pollut. Bull. 2018;127:704–716. doi: 10.1016/j.marpolbul.2017.12.061. [DOI] [PubMed] [Google Scholar]
- 93.Hidalgo-Ruz V, Gutow L, Thompson RC, Thiel M. Microplastics in the marine environment: a review of the methods used for identification and quantification. Environ. Sci. Technol. 2012;46:3060–3075. doi: 10.1021/es2031505. [DOI] [PubMed] [Google Scholar]
- 94.Remy F, et al. When microplastic is not plastic: the ingestion of artificial cellulose fibers by macrofauna living in seagrass macrophytodetritus. Environ. Sci. Technol. 2015;49:11158–11166. doi: 10.1021/acs.est.5b02005. [DOI] [PubMed] [Google Scholar]
- 95.Cai L, et al. Characteristic of microplastics in the atmospheric fallout from Dongguan city, China: preliminary research and first evidence. Environ. Sci. Pollut. Res. 2017;24:24928–24935. doi: 10.1007/s11356-017-0116-x. [DOI] [PubMed] [Google Scholar]
- 96.Comnea-Stancu IR, Wieland K, Ramer G, Schwaighofer A, Lendl B. On the identification of rayon/viscose as a major fraction of microplastics in the marine environment: discrimination between natural and manmade cellulosic fibers using fourier transform infrared spectroscopy. Appl. Spectrosc. 2017;71:939–950. doi: 10.1177/0003702816660725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.White EM, et al. Ingested micronizing plastic particle compositions and size distributions within stranded post-hatchling sea turtles. Environ. Sci. Technol. 2018;52:10307–10316. doi: 10.1021/acs.est.8b02776. [DOI] [PubMed] [Google Scholar]
- 98.Lusher AL, McHugh M, Thompson RC. Occurrence of microplastics in the gastrointestinal tract of pelagic and demersal fish from the English Channel. Mar. Pollut. Bull. 2013;67:94–99. doi: 10.1016/j.marpolbul.2012.11.028. [DOI] [PubMed] [Google Scholar]
- 99.Ladewig SM, Bao S, Chow AT. Natural fibers: a missing link to chemical pollution dispersion in aquatic environments. Environ. Sci. Technol. 2015;49:12609–12610. doi: 10.1021/acs.est.5b04754. [DOI] [PubMed] [Google Scholar]
- 100.Pham CK, et al. Plastic ingestion in oceanic-stage loggerhead sea turtles (Caretta caretta) off the North Atlantic subtropical gyre. Mar. Pollut. Bull. 2017;121:222–229. doi: 10.1016/j.marpolbul.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 101.Ivar do Sul, J. A. & Costa, M. F. The present and future of microplastic pollution in the marine environment. Environ. Pollut.185, 352–364 (2014). [DOI] [PubMed]
- 102.Ryan PG, et al. Impacts of plastic ingestion on post-hatchling loggerhead turtles off South Africa. Mar. Pollut. Bull. 2016;107:155–160. doi: 10.1016/j.marpolbul.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 103.Galloway, T. S. Micro- and Nano-plastics and Human Health. in Marine Anthropogenic Litter 343–366 (Springer International Publishing, 2015). 10.1007/978-3-319-16510-3_13
- 104.Xue J, Liu W, Kannan K. Bisphenols, benzophenones, and bisphenol a diglycidyl ethers in textiles and infant clothing. Environ. Sci. Technol. 2017;51:5279–5286. doi: 10.1021/acs.est.7b00701. [DOI] [PubMed] [Google Scholar]
- 105.Park S, Hong Y, Lee J, Kho Y, Ji K. Chronic effects of bisphenol S and bisphenol SIP on freshwater waterflea and ecological risk assessment. Ecotoxicol. Environ. Saf. 2019;185:109694. doi: 10.1016/j.ecoenv.2019.109694. [DOI] [PubMed] [Google Scholar]
- 106.Huang Q, et al. Embryonic exposure to low concentration of bisphenol A affects the development of Oryzias melastigma larvae. Environ. Sci. Pollut. Res. 2012;19:2506–2514. doi: 10.1007/s11356-012-1034-6. [DOI] [PubMed] [Google Scholar]
- 107.Huang Q, et al. New insights into the metabolism and toxicity of bisphenol A on marine fish under long-term exposure. Environ. Pollut. 2018;242:914–921. doi: 10.1016/j.envpol.2018.07.048. [DOI] [PubMed] [Google Scholar]
- 108.Aluru N, Leatherland JF, Vijayan MM. Bisphenol A in oocytes leads to growth suppression and altered stress performance in juvenile rainbow trout. PLoS ONE. 2010;5:e10741. doi: 10.1371/journal.pone.0010741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rochman CM, Kurobe T, Flores I, Teh SJ. Early warning signs of endocrine disruption in adult fish from the ingestion of polyethylene with and without sorbed chemical pollutants from the marine environment. Sci. Total Environ. 2014;493:656–661. doi: 10.1016/j.scitotenv.2014.06.051. [DOI] [PubMed] [Google Scholar]
- 110.Hobbs CAD, Potts RWA, Bjerregaard Walsh M, Usher J, Griffiths AM. Using DNA Barcoding to Investigate Patterns of Species Utilisation in UK Shark Products Reveals Threatened Species on Sale. Sci. Rep. 2019;9:1028. doi: 10.1038/s41598-018-38270-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is available from the British Oceanographic Centre (Marine).