Graphical abstract
Keywords: Melia azedarach, Zinc oxide nanoparticles, Soybean, Seed-borne fungi, C. cladosporioides, F. oxysporum
Abstract
Present study, report the biofabrication of zinc oxide nanoparticles from aqueous leaf extract of Melia azedarach (MaZnO-NPs) through solution combustion method and their novel application in preventing the growth of seed-borne fungal pathogens of soybean (Cladosporium cladosporioides and Fusarium oxysporum). The standard blotter method was employed to isolate fungi and was identified through molecular techniques. The characterization of MaZnO-NPs was carried out by UV–Vis spectroscopy, Fourier Transform Infrared Spectroscopy (FT-IR), X-Ray Diffraction (XRD), Scanning Electron Microscopy (SEM) equipped with Energy Dispersive Spectroscopy (EDS) and Transmission Electron Microscopy (TEM). The physicochemical characterization confirmed the particles were of high purity and nano size (30–40 nm) with a hexagonal shape. The synthesized MaZnO-NPs inhibited the growth of C. cladosporioides and F. oxysporum in a dose dependent manner. Biomass, ergosterol, lipid peroxidation, intracellular reactive oxygen species and membrane integrity determination upon MaZnO-NPs treatment offered significant activities there by confirming the mechanism of action against the test pathogens. In conclusion, due to the effectiveness of MaZnO-NPs in controlling the growth of C. cladosporioides and F. oxysporum, the synthesized MaZnO-NPs provides insight towards their potential application in agriculture and food industries.
1. Introduction
Food shortage and nourishment deficiency are the major issues of developing as well as developed countries worldwide (Friedmann, 1993, Koffi et al., 2017). To full fill the ever-escalating food demands of the speedily growing world population, agriculture products need to upsurge by an additional 50% by 2025 (Mccalla, 1998). In developing countries, mainly, the cultivation of legumes is the paramount and quickest way to boost protein products (Mark, 1999). Soybean [Glycine max (L.) Merrill] a legume crop, owing to its high protein and vegetable oil content is cultivated all over the world (Van and McHale, 2017). In India, the more significant part of the harvest yield is decreased because of seed-borne mycoflora. Seed-borne fungi are the substantial detritivores of seeds and seed products, making them unfit for human utilization by hindering their nutritional value by producing toxins (Christensen and Kaufmann, 2003). The grains stored for consumption purposes cannot be treated with fungicides, pesticides, bactericides, etc., due to the chemicals used are of non-biodegradable and extremely toxic in nature. It is, in this way, necessary to look for novel antifungal specialists for control measures that are financially savvy, non-lethal, eco-accommodating and which also reduce the rate of critical pathogens, to avoid bio-deterioration of soybean seeds.
Nanotechnology is one of the state-of-the-art technologies among the most exciting forefront fields in the 21st century (Tanaka, 1999). Studies and progression in this field are developing rapidly throughout the world. A significant commitment of this field is the creation of functional materials, gadgets, and frameworks in the nanometre scale. These usually are particulate materials with a measurement of 1–100 nm (nm). Nanoparticles display new or enhanced properties because of certain qualities (size, distribution, morphology, phase, etc.) compared with larger particles (Kittelson, 1998, Mohanraj and Chen, 2006, Murali et al., 2017, Mahendra et al., 2017, Lakshmeesha et al., 2014). It has been observed that a decrease in size and morphology in these particles results in the higher surface area to volume ratio (Eastman, 1995). Inferable to their size, nanoparticles exhibit various characteristic activities such as optical, magnetic, electrocatalytic, etc. (Luo et al., 2006, Ito et al., 2005). Apart from these physical properties, nanoparticles are also extensively used in the fields of diagnostics, cancer therapy, biosensing, drug delivery systems, antimicrobial agents, pharmaceutical applications, etc. (Slowing et al., 2007, Ferrari, 2005).
Zinc oxide nanoparticles (ZnO-NPs) standout amongst the most nonmaterial with properties like semi conducting and adaptable applications. ZnO is a stable wurtzite structure comprising of various planes made of tetrahedral planned Zn2+ and O2− ions, which are stacked alternately along the c-axis (Kong et al., 2004). ZnO is a natural well-disposed material and biocompatible, which is alluring, particularly for biomedical applications. Additional it has a wide band-gap semiconductor (3.36 eV) with large exciton binding energy 60 meV at room temperature; therefore it has received increased attention in the field of self-fuelled nanosystems, short-wavelength optoelectronic gadgets, bright lasers, sensors, field-emanation gadgets, antibacterial activity, sunscreen products, artworks, mechanical coatings, etc., (Petkova et al., 2016, Smijs and Pavel, 2011, Ebert and Bhushan, 2012, Zhang et al., 2007, Kalyanasundaram and Grätzel, 1998).
The biological method for ZnO-NPs synthesis using microorganisms and plant extracts has turned out to be vastly improved than that of synthetic techniques due to more ecological cordial and also financially savvy (Kharissova et al., 2013). The plant facilitated the green synthesis of nanoparticles is favorable over chemical and physical strategies (Raveendran et al., 2003). Different parts of plants, for example, leaf, stem, flower, seed have been utilized to synthesize nanoparticles (Kumar and Yadav, 2009). Plants give a superior stage to nanoparticles synthesis as they are free from toxic substances and also will act as natural capping agents. Moreover, the utilization of plant products lessens the cost of microorganism isolation and culture media, improving the cost-focused plausibility over nanoparticle synthesis by microorganisms (Mittal et al., 2013). The usage of plants for the union of nanoparticles is a fast, minimal effort, eco-accommodating and single-step strategy, which can be straightforwardly used for drug delivery and other comparative application without any coating or core–shell (Thakkar et al., 2010).
Melia azedarach Linn. is a large indigenous tree belonging to the family Meliaceae, a native of India, China, and Persia. It is commonly known as Chinaberry tree or Indian Lilac (Wandscheer et al., 2004). Leaves and fruit extracts of the plant are traditionally used for antibacterial (Sen and Batra, 2012), antifungal (Carpinella et al., 2003), antimalarial activity (Bickii et al., 2000), etc. Hence, the present study was aimed at the objectives: (i) Green synthesis of ZnO-NPs using an aqueous leaf extract of M. azedarach as bioreductant and stabilizing agents. (ii) Characterization of green-synthesized MaZnO-NPs by UV–Vis spectrophotometer, FT-IR, XRD, SEM, EDS, and TEM. (iii) Isolation of fungi from soybean seeds by Standard blotter method and characterization by molecular techniques. (iv) Evaluation of the antifungal potential of MaZnO-NPs against seed-borne pathogenic fungi.
2. Materials and methods
2.1. Materials
Czapek dox broth (CZB), Czapek dox agar (CZA), potato dextrose broth (PDB), potato dextrose agar (PDA), blotting paper, double-layered muslin cloth, Whatman No.1 filter paper, and high-grade AR zinc nitrate hexahydrate (Zn(NO3)2·6H2O) were purchased from Sigma-Aldrich, India. The other chemicals, reagents, and solvents of fine grade were from Merck Millipore, India. The glassware was obtained from Borosil, India and was sterilized before use.
2.2. Biofabrication and characterization of zinc oxide nanoparticles
M. azedarach plant was chosen in light of an ethnobotanical review. The fresh leaves were collected from the local surroundings of the University of Mysore, Mysuru, India. Collected leaves were air-dried at room temperature and powdered utilizing a sterile electric blender and blended in nano-pure water (1:10, w/v). The blend was sonicated and the supernatant was passed through two layers of muslin cloth and centrifuged at 4000 rpm for 30 min and the supernatant was used for further studies. Zinc nitrate hexahydrate [Zn(NO3)2·6H2O] was used as a precursor material and plant extract has fuel for MaZnO-NPs synthesis. A stoichiometric amount of Zn(NO3)2·6H2O was dissolved in the plant extract. This reaction mixture was stirred well using a magnetic stirrer for 10 min and then placed in a pre-heated muffle furnace maintained at 300 ± 10 °C. The final product was subjected to physicochemical characterization.
The synthesized M. azedarach zinc oxide nanoparticle (MaZnO-NPs) was characterized by UV–Vis absorption Spectrophotometer (DU739, Beckman Coulter, Germany) operated at a resolution of 1 nm at room temperature. The presence of nanoparticles was confirmed by obtaining a spectrum in the visible range of 200–800 nm. The specific functional groups present in the MaZnO-NPs were carried out in FTIR spectroscopy (Perkin Elmer Spectrum 1000) between 400 and 4000 cm−1. The crystallinity and phase purity of MaZnO-NPs were characterized by X-Ray diffraction (XRD) using a Rigaku Desktop Miniflex II X-Ray powder diffractometer with Cu kα radiation with an angle 2θ (λ = 0.15418 nm). The particle size was determined by the intercept and slope calculated by the XRD values (often referred to as a Williamson–Hall plot) and Scherrer’s equation:
where λ is the wavelength (Cu Kα) of X-Rays, β is the full width at half- maximum (FWHM) of the peak, and θ is the diffraction angle. The XRD pattern of MaZnO-NPs was analyzed with the ICDD Powder Diffraction File database (International Centre for Diffraction Data) using Crystallographica Search-Match Version 2, 1, 1, 1. SEM analysis was used to study the surface morphology and images were taken using an HR-FESEM SU-70 Hitachi instrument and Energy Dispersive Spectroscopy (EDS) using HITACHI (Noran System 7, USA) to find an elemental composition in the reaction mixture. To determine the size of the MaZnO-NPs, TEM analysis was performed on Philips model CM 200 instrument.
2.3. Isolation and molecular characterization soybean seed-borne fungi
Freshly harvested and locally available two kilograms of soybean seeds (JS-335 variety) were collected from the agricultural field, Bugudanahalli, Tumkur, Karnataka, India. The presence or absence of seed-borne fungi was tested by blotter plate methods for the isolation of seed-borne fungi according to the International Seed Testing Association (ISTA), 2006 (Sadeghi et al., 2011). Three layers of blotting paper (9 cm diameter) were placed at the bottom and moistened with sterile distilled water (SDW). Excess water was drained-off and Petri plates were autoclaved for 121 °C for 20 min at 15 psi. Soybean seeds (400) were surface disinfected with 1% sodium hypochlorite solution for about 2 min and repeatedly washed (with sterile distilled water) to remove the remnants. Each Petri plate was placed with 10 seeds equidistantly and incubated for seven days at room temperature in alternating cycles of 12 h darkness and 12 h light. After incubation, each Petri plate was observed under a stereo binocular microscope for the presence of seed-borne fungi. The most frequently observed fungi in the incubation test were selected for molecular characterization. Liquid cultures of fungal isolates were prepared using 50 mL of PDB in 250 mL Erlenmeyer flasks to obtain fresh mycelia for DNA extraction. The fungal mycelia mat was separated by centrifugation at 8,000 rpm at 4 °C for 10 min. The obtained mycelia were crushed in liquid nitrogen and about 100 mg was used for genomic DNA extraction. Polymerase chain reaction (PCR) was used to amplify the internal transcribed spacer (ITS) region of ribosomal DNA, which encompasses the 5.8S gene and the ITS-1 (5′ TCC GTA GGT GAA CCT GCG G 3′) and ITS-4 (5′ TCC TCCGCT TATTGATAT GC 3′) regions. A contiguous sequence out of two sequences was generated using CAP3 sequence assembly program and submitted to the GenBank, National Center for Biotechnology Information (NCBI) (White et al., 1990).
2.4. Fungicidal activity of MaZnO-NPs on seed-borne fungi
Fungicidal activity of MaZnO-NPs against isolated soybean seed-borne fungi, C. cladosporioides and F. oxysporum were determined by micro-well dilution method as per guidelines of Clinical & Laboratory Standards Institute (CLSI, 2012). Fungicidal mechanism of MaZnO-NPs was demonstrated by determining fungal growth (biomass), ergosterol content, lipid peroxidation, intracellular reactive oxygen species (ROS), and membrane integrity (Naveen Kumar et al., 2016, Kalagatur et al., 2018). The fungi, C. cladosporioides and F. oxysporum were grown on PDA for 7 days at 25 ± 2 °C and spores were collected in peptone solution (0.001% Tween 80). The spore count was done using hemocytometer, and count was adjusted to 106 mL−1 and used in the further studies.
2.4.1. Micro-well dilution assay
The minimum inhibitory concentration (MIC) and minimum fungicidal concentration (MFC) of MaZnO-NPs were determined from micro-well dilution assay (Kalagatur et al., 2015). A quantity of 10 µL of fungal spore suspension (106 mL−1) and different concentrations of MaZnO-NPs were added in 96-well microtiter plate, and the total volume was made to 100 µL with CZB. Nystatin was used as positive control and fungal spore suspension alone with CZB served as negative control. The test samples were incubated at 25 ± 2 °C for 3 days and the concentration of MaZnO-NPs at which visible fungal growth is absent is referred to as MIC. Next, 10 µL was collected from test samples and inoculated on CZA Petri plates and the concentration of MaZnO-NPs at which no evident fungal growth was referred to as MFC.
2.4.2. Assessment of fungal biomass and ergosterol content
Two experimental sets were distinctly prepared for estimation on the effect of MaZnO-NPs on fungal biomass and ergosterol content. Briefly, 10 µL of fungal spore suspension (106 mL−1) and different concentrations of MaZnO-NPs (up to 250 µg mL−1) were added to 100 mL of CZB in 250 mL Erlenmeyer flasks and incubated at 25 ± 2 °C for 14 days with a duration of 12 h under rotary shaker (140 rpm). The flasks with fungal spore suspension and without MaZnO-NPs were considered as control. Subsequently, mycelia were separated from broth filtering through Whatman No.1 filter paper. One set of mycelia were dried at 60 °C using hot-air oven and used for estimation of fungal biomass. The other set of mycelia was used for HPLC quantification of ergosterol as per the methodology of Sellamani et al. (2016).
2.4.3. Assessment of membrane integrity
Briefly, 1 mL of fresh fungal spore suspension (106 mL−1) was treated with different concentrations of MaZnO-NPs (up to 200 µg mL−1) and incubated under rotary shaker (140 rpm) for 24 h with 12 h light at 25 ± 2 °C. The samples without MaZnO-NPs served as negative control. Each sample was treated with 5 µM of propidium iodide for 15 min and subjected to wash twice with phosphate-buffer saline (pH 7.4). The propidium iodide stained spores were counted at 490/635 nm using flow cytometry (Beckman Coulter). A minimum of 1000 spores was counted in each sample, and results were expressed as number of propidium iodide stained spores per 100 spores.
2.4.4. Assessment of ROS and MDA levels
The oxidative stress induced fungicidal activity by MaZnO-NPs was studied over assessing ROS and malondialdehyde (MDA) levels. Two experimental sets were separately considered for ROS and MDA analysis. Briefly, 10 µL of fungal spore suspension (106 mL−1) was added to 1 mL of CZB in a 12-well culture plate and incubated at 25 ± 2 °C for 7 days with a duration of 12 h light/day under rotary shaker (140 rpm). Following, different concentrations of MaZnO-NPs (up to 200 µg mL−1) were added and again incubated for 24 h at the above conditions. The samples not treated with MaZnO-NPs were considered as control. Subsequently, one set of samples was stained with 5 µM of DCFH-DA for 15 min and washed twice with phosphate-buffer saline (pH 7.4). The optical density was measured at 495/550 nm using the plate reader (Synergy H1, BioTek, USA) and results stated with respect to control (100%). The other set of samples was used for quantification of MDA levels using lipid peroxidation assay kit as per instructions from the manufacturer.
2.5. Statistical analysis
The experiments were executed in 6 replicates and observations were expressed as a mean ± standard deviation. Means were separated by one-way ANOVA test at 0.5 significant (P < 0.05) using GraphPad Prism Version 7. The statistical difference between test samples were assessed following Tukey’s multiple comparison test.
3. Results
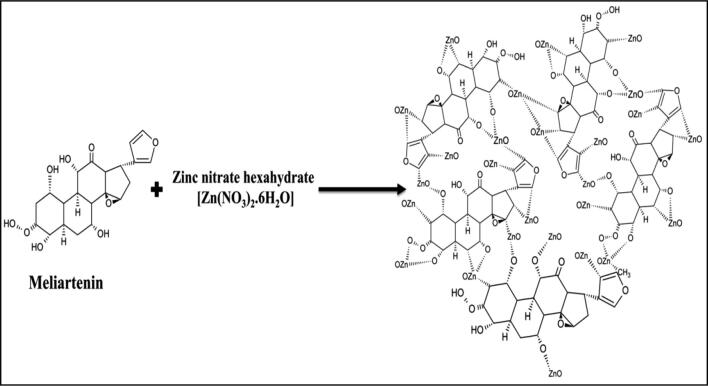
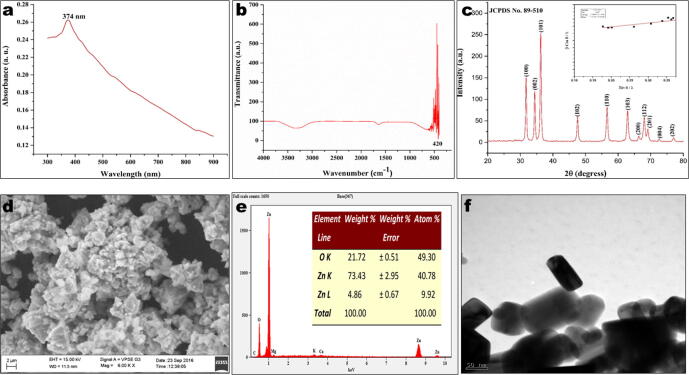
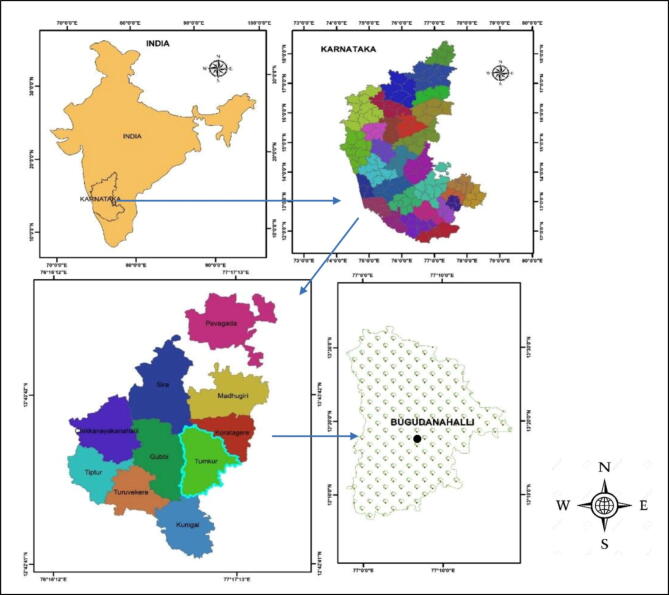
The major compound present in Melia azedarach extract was reported to be meliartenin (Coria et al., 2008). The egg box model has been used to predict the possible mechanism involved in the formation of MaZnO-NPs (Fig. 1). Fig. 2a, shows UV–Vis absorption spectrum of as-synthesized MaZnO-NPs. An absorption peak obtained at 374 nm is well-matched with literature. The synthesized MaZnO-NPs were subjected to FT-IR analysis to detect the presence of metal oxides (Fig. 2b). Metal oxide absorption bands are observed in the region below 600 cm−1. The major peak at 551 cm−1 can be attributed to the Zn-O stretching of ZnO lattice. The powdered sample was subjected to X-Ray Diffractometer for confirming the presence of synthesized MaZnO-NPs. Fig. 2c, shows XRD pattern of as-prepared MaZnO-NPs. The location of the peaks as compared to literature values and the presence of ZnO-NPs were confirmed. XRD spectrum shows characteristic peaks corresponding to (1 0 0), (0 0 2), (1 0 1), (1 0 2), (1 1 0), (1 0 3), (2 0 0), (1 1 2) and (2 0 1) planes that are in good agreement with the hexagonal wurtzite ZnO phase (JCPDS No. 89–510). The crystallite size of MaZnO-NPs calculated by Sherrer’s equation and Williamson-Hall (W − H) plot method (Fig. 2c) was found to be 23.72 nm and 30.95 nm, respectively. SEM and TEM analysis were employed to investigate the morphology of MaZnO-NPs. SEM images showed that the particles are of hexagonal in shape which is upward-aligned to form an aggregation of bundles (Fig. 2d). Fig. 2e, depicts that the synthesized ZnO NPs were of pure form. EDS and TEM analysis confirmed that the shape of the synthesized MaZnO NPs was hexagonal and sizes were in the range of 30–40 nm. Bugudanahalli lies between 130 10l 0ll N and 130 30l 0llN latitude and lies between 770 0l 0ll E and 770 10l 0ll E longitude. The area was marked using the survey of India toposheet 57G/3 (Tumkur), the scale of the topo sheet is 1:50,000 and using Arc GIS (9.3) software. The details of the above location are marked in map (Fig. 3.).
Fig. 1.
Egg-box model for the interaction of meliartenin during the formation of MaZnO-NPs – A schematic representation.
Fig. 2.
(a) UV–visible spectrum; (b) FT-IR spectrum; (c) of X-Ray diffraction pattern; (d) SEM; (e) EDS and (c) TEM image of MaZnO-NPs.
Fig. 3.
Geographical location of Bugudanahalli.
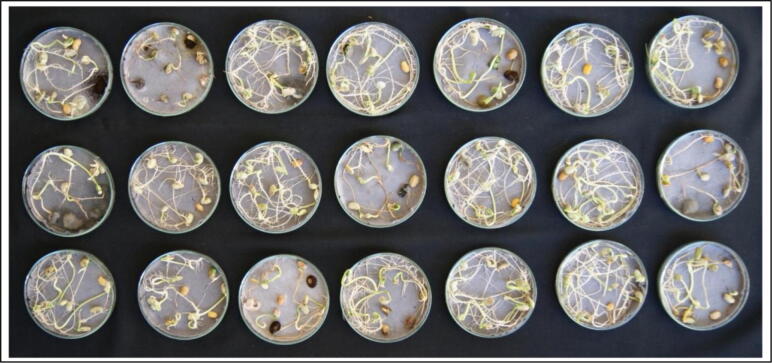
The Standard blotter method results showed that soybean (JS 335) variety harboured a diverse fungal species (Fig. 4.). In the present investigation, the most frequently occurred soybean seed-borne fungi (C. cladosporioides and F. oxysporum) were identified by rDNA sequencing analysis. The DNA fragments were sequenced and deposited in the GenBank, NCBI under the accession number KF849291 (C. cladosporioides) and KM921744 (F. oxysporum).
Fig. 4.
Soybean seed showing diverse fungal species after seven days of incubation by Standard blotter method (SBM).
The preliminary fungicidal activity of MaZnO-NPs on seed-borne fungi was determined by micro-well dilution method. The MaZnO-NPs presented potent fungicidal activity, and the MIC and MFC values were 81.67 and 178.3 µg mL−1, and 93.33 and 208.3 µg mL−1 for C. cladosporioides and F. oxysporum, respectively. Whereas, synthetic fungicidal agent Amphotericin B exhibited MIC and MFC values of 203.3 and 326.7 µg mL−1 and 236.7 and 381.7 µg mL−1 against C. cladosporioides and F. oxysporum, respectively. The detected fungicidal activity of MaZnO-NPs was found to be much potent contrasted to synthetic fungicidal agent Amphotericin B.
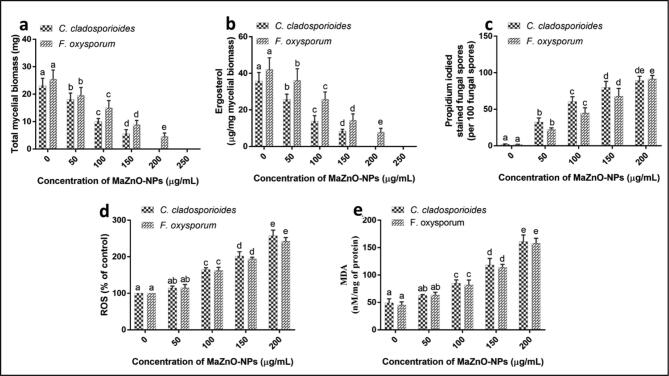
Succeeding, fungal biomass, ergosterol, membrane integrity, ROS, and MDA were contemplated for revealing detailed fungicidal action of MaZnO-NPs (Fig. 5a–e). The fungal biomass and ergosterol were studied as indicator for fungal growth. Particularly, ergosterol is vital component in membrane of fungi and it serves same functions of cholesterol like in animals. Therefore, till date, ergosterol has become primary target for discovery of novel fungicidal agents. Interestingly, in the present study, MaZnO-NPs have effectively inhibited fungal growth (biomass) by way of reducing ergosterol content. The total fungal biomass (mg) and ergosterol content (µg mg−1 mycelial biomass) in control was noticed as 23.09 and 35.84, and 25.47 and 42.12 for C. cladosporioides and F. oxysporum, respectively. Whereas, fungal biomass and ergosterol content were significantly reduced in MaZnO-NPs treated fungi related to control and it was observed as dose-dependent. The growth of C. cladosporioides and F. oxysporum were correspondingly absent at 200 and 250 µg mL−1 of MaZnO-NPs and found in accordance with results of micro-well dilution assay (Fig. 5a and b). The study evidently showed that MaZnO-NPs have reduced the fungal growth through depleting ergosterol content. Further, the effect of MaZnO-NPs on membrane integrity of fungal spores was investigated by propidium iodide (PI) staining assay. The results showed that MaZnO-NPs were detrimentally affected the membrane integrity of fungal spores in a dose-dependent manner (Fig. 5c). Thereafter, oxidative stress induced fungicidal action of MaZnO-NPs was concluded by estimating ROS and MDA levels. The ROS levels in MaZnO-NPs treated fungal samples were estimated with respect to control (100%). The ROS levels were enhanced in MaZnO-NPs treated fungal samples in a dose-dependent manner (Fig. 5d). The MDA is measured as an indicator for tissue and cellular damage and result from lipid peroxidation of polyunsaturated fatty acids by detrimental action of ROS. In the present study, MDA levels were significantly greater in MaZnO-NPs treated fungal samples compared to control and exhibited dose-dependently (Fig. 5e). Overall, the study unveiled that MaZnO-NPs shown potent fungicidal activity on seed-borne fungi through elevating the ROS and MDA levels, adversely affecting membrane integrity, and depleting ergosterol synthesis.
Fig. 5.
Fungicidal activity of MaZnO-NPs. Dose-dependent effect of MaZnO-NPs on (a) fungal growth; (b) ergosterol content; (c) membrane integrity; (d) intracellular ROS; (e) MDA of seed-borne fungi, C. cladosporioides and F. oxysporum. The experiments were performed in 6 replicates and results were expressed as mean ± standard deviation. The data was processed by one-way ANOVA and statistical difference between the tests samples were analyzed by Tukey’s multiple comparison tests. The bars with different alphabetic letters were significant (p ≤ 0.05) in the respective study.
4. Discussion
Since the past few decades, nanotechnology has been most frequently used in various fields, including agriculture and food industry. Most of the stored seeds are contaminated by seed-borne fungi leading to the deterioration of nutritive value of seeds. Hence, the developments of an effective antifungal agent that can be used for seed coating and give protection against seed-borne fungi have gained significant attention during recent decades. Soybean seed-borne fungi were isolated by following the method given by ISTA and identified by molecular methods. Fungal diagnostics have increased dramatically with the introduction of molecular tools, in particular, that of PCR. Molecular methods involving the use of ITS region were studied by various workers to identify the fungal genera and species (Das et al., 2010, Gherbawy and Voigt, 2016). Nanoparticles have been known to have antifungal activity during recent studies.
In the present study, MaZnO-NPs have been synthesized using M. azedarach leaf extract, which was of highly simplistic, inexpensive and non-toxic approach. M. azedarach leaf extract was used as a fuel for the synthesis of MaZnO-NPs. Meliartenin has been reported to be one of the few major compounds present in M. azedarach leaf extract (Kistler, 1997). The synthesized MaZnO-NPs were characterized, which were in line with the previous reports (Isman, 2006, Suresh et al., 2015). Our results revealed that MaZnO-NPs possessed antifungal activity against soybean seed-borne fungi and showed a higher concentration of MaZnO-NPs had higher inhibition of fungi. Antifungal results were in line with the previous reports (Sangeetha et al., 2011, He et al., 2011). The antifungal efficacy possessed by MaZnO-NPs could efficiently restrain the growth of soybean seed-borne fungi and could be a potent substitute for synthetic fungicides used in the agriculture and food industry.
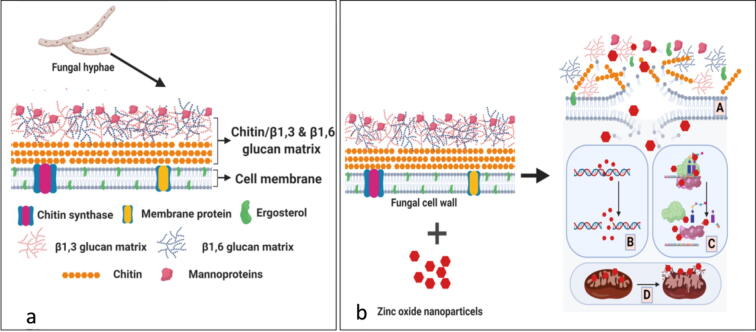
Ergosterol is a major sterol present in the cell membranes of fungi, which play a vital role in cell membrane integrity and function similar to that of cholesterol in animals (Tutaj et al., 2016). Hence, several antifungal drugs are prepared to target enzymes involved in ergosterol synthesis. In our study, it was read that the ergosterol content was decreased as the concentration of MaZnO-NPs increases which were similar to the previous reports (Sellamani et al., 2016, Lipovsky et al., 2011). In the present study, it has been investigated that the antifungal activity may be attributed due to the generation of ROS by MaZnO-NPs coming in contact with fungal cell wall. The ROS generated results in elevated stress, leading to oxidative damage to the fungal cell wall and cell components. Previous reports suggested that the antifungal activity of ZnO NPs was mediated through ROS production (Fig. 6) (Lipovsky et al., 2011, Ansari et al., 2020, Jalal et al., 2018).
Fig. 6.
Mechanism of antifungal activity of ZnO NPs. (a) Fungal cell wall; (b) Mechanism of action; (A) Disruption of fungal cell wall; (B) DNA damage; (C) Inhibition of protein synthesis; (D) Mitochondria damage.
5. Conclusions
In conclusion, MaZnO-NPs were prepared by solution combustion technique and studied for their antifungal activity against soybean seed-borne fungi. XRD and TEM results confirmed that the synthesized crystallites size of MaZnO-NPs was 23.72 nm to 32.95 nm. Egg box model was used to predict the possible mechanism for the formation of ZnO NPs. Antifungal activity, MIC showed the lowest concentration at which the fungi were inhibited. The results affirm that the synthesized nanoparticles had antifungal activity, which can be used against the soybean seed-borne fungi.
Declaration of Competing Interest
The authors declare that there is no conflict of interests regarding the publications of this paper.
Acknowledgment
The authors LTR and MM like to acknowledge the University Grants Commission (UGC), New Delhi, India for providing the financial support under UGC Post-Doctoral Fellowship for SC/ST Candidates (No. F/PDFSS-2015-17-KAR-11458 and No. F/PDFSS-2015-17-KAR-11846).
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Mohammad Azam Ansari, Email: maansari@iau.edu.sa.
S.R. Niranjana, Email: niranjanasr@rediffmail.com.
References
- Ansari M.A., Murali M., Prasad D., Alzohairy M.A., Almatroudi A., Alomary M.N., Udayashankar A.C., Singh S.B., Asiri S.M.M., Ashwini B.S., Gowtham H.G. Cinnamomum verum Bark extract mediated green synthesis of ZnO nanoparticles and their antibacterial potentiality. Biomolecules. 2020;10(2):336. doi: 10.3390/biom10020336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickii J., Njifutie N., Ayafor Foyere J., Basco L.K., Ringwald P. In vitro antimalarial activity of limonoids from Khaya grandifoliola C.D.C. (Meliaceae) J. Ethnopharmacol. 2000;69:27–33. doi: 10.1016/S0378-8741(99)00117-8. [DOI] [PubMed] [Google Scholar]
- Carpinella M.C., Giorda L.M., Ferrayoli C.G., Palacios S.M. Antifungal effects of different organic extracts from Melia azedarach L. on phytopathogenic fungi and their isolated active components. J. Agric. Food Chem. 2003;51:2506–2511. doi: 10.1021/jf026083f. [DOI] [PubMed] [Google Scholar]
- Christensen C.M., Kaufmann H.H. Deterioration of stored grains by fungi. Annu. Rev. Phytopathol. 2003;3:69–84. doi: 10.1146/annurev.py.03.090165.000441. [DOI] [Google Scholar]
- CLSI, 2012. M27-S4 Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts. Fourth Informational Supplement (ISBN 1562388649).
- Coria C., Almiron W., Valladares G., Carpinella C., Ludueña F., Defago M., Palacios S. Larvicide and oviposition deterrent effects of fruit and leaf extracts from Melia azedarach L. on Aedes aegypti (L.) (Diptera: Culicidae) Bioresour. Technol. 2008;99:3066–3070. doi: 10.1016/j.biortech.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Das K., Tiwari R.K.S., Shrivastava D.K. Techniques for evaluation of medicinal plant products as antimicrobial agent: Current methods and future trends. J. Med. Plants Res. 2010;4:104–111. doi: 10.5897/JMPR09.030. [DOI] [Google Scholar]
- Eastman, J.A., 1995. Enhancing thermal conductivity of fluids with nanoparticles A metal-organic vapor phase epitaxy system for advanced in situ x-ray studies of III-nitride growth View project in situ oxide growth by radio frequency-magnetron sputtering View project.
- Ebert D., Bhushan B. Transparent, superhydrophobic, and wear-resistant coatings on glass and polymer substrates using SiO2, ZnO, and ITO nanoparticles. Langmuir. 2012;28:11391–11399. doi: 10.1021/la301479c. [DOI] [PubMed] [Google Scholar]
- Ferrari M. Cancer nanotechnology: opportunities and challenges. Nat. Rev. Cancer. 2005;5:161–171. doi: 10.1038/nrc1566. [DOI] [PubMed] [Google Scholar]
- Friedmann H. The political economy of food: a global crisis. New Left Rev. 1993;197:29–57. [Google Scholar]
- Gherbawy Y., Voigt K. Molecular identification of fungi. Mol. Mycorrhizal Symbios. 2016:301–322. [Google Scholar]
- He L., Liu Y., Mustapha A., Lin M. Antifungal activity of zinc oxide nanoparticles against Botrytis cinerea and Penicillium expansum. Microbiol. Res. 2011;166:207–215. doi: 10.1016/j.micres.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Isman M.B. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol. 2006;51:45–66. doi: 10.1146/annurev.ento.51.110104.151146. [DOI] [PubMed] [Google Scholar]
- Ito A., Shinkai M., Honda H., Kobayashi T. Medical application of functionalized magnetic nanoparticles. J. Biosci. Bioeng. 2005 doi: 10.1263/jbb.100.1. [DOI] [PubMed] [Google Scholar]
- Jalal M., Ansari M.A., Ali S.G., Khan H.M., Rehman S. Anticandidal activity of bioinspired ZnO NPs: effect on growth, cell morphology and key virulence attributes of Candida species. Artif. Cells Nanomed. Biotechnol. 2018;46:912–925. doi: 10.1080/21691401.2018.1439837. [DOI] [PubMed] [Google Scholar]
- Kalagatur N.K., Mudili V., Siddaiah C., Gupta V.K., Natarajan G., Sreepathi M.H., Vardhan B.H., Putcha V.L.R. Antagonistic activity of Ocimum sanctum L. essential oil on growth and zearalenone production by Fusarium graminearum in maize grains. Front. Microbiol. 2015;6:892. doi: 10.3389/fmicb.2015.00892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalagatur N.K., Nirmal Ghosh O.S., Sundararaj N., Mudili V. Antifungal activity of chitosan nanoparticles encapsulated with Cymbopogon martinii essential oil on plant pathogenic fungi Fusarium graminearum. Front. Pharmacol. 2018;9:610. doi: 10.3389/fphar.2018.00610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyanasundaram K., Grätzel M. Applications of functionalized transition metal complexes in photonic and optoelectronic devices. Coord. Chem. Rev. 1998;177:347–414. doi: 10.1016/s0010-8545(98)00189-1. [DOI] [Google Scholar]
- Kharissova O.V., Dias H.V.R., Kharisov B.I., Pérez B.O., Pérez V.M.J. The greener synthesis of nanoparticles. Trends Biotechnol. 2013;31:240–248. doi: 10.1016/j.tibtech.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Kistler H.C. Population genetics of soilborne fungal plant pathogens genetic diversity in the plant-pathogenic fungus Fusarium oxysporum. Phytopathology. 1997;87:474–479. doi: 10.1094/PHYTO.1997.87.4.474. [DOI] [PubMed] [Google Scholar]
- Kittelson D.B. Engines and nanoparticles: a review. J. Aerosol Sci. 1998;29 [Google Scholar]
- Koffi C.K., Djoudi H., Gautier D. Landscape diversity and associated coping strategies during food shortage periods: evidence from the Sudano-Sahelian region of Burkina Faso. Reg. Environ. Chang. 2017;17:1369–1380. doi: 10.1007/s10113-016-0945-z. [DOI] [Google Scholar]
- Kong X.Y., Ding Y., Yang R., Wang Z.L. Single-crystal nanorings formed by epitaxial self-coiling of polar nanobelts. Science. 2004;303(5662):1348–1351. doi: 10.1126/science:1092356. [DOI] [PubMed] [Google Scholar]
- Kumar V., Yadav S.K. Plant-mediated synthesis of silver and gold nanoparticles and their applications. J. Chem. Technol. Biotechnol. 2009;84:151–157. [Google Scholar]
- Lakshmeesha T.R., Sateesh M.K., Prasad B.D., Sharma S.C., Kavyashree D., Chandrasekhar M., Nagabhushana H. Reactivity of crystalline ZnO superstructures against fungi and bacterial pathogens: Synthesized using nerium oleander leaf extract. Cryst. Growth Des. 2014;14:4068–4079. doi: 10.1021/cg500699z. [DOI] [Google Scholar]
- Lipovsky A., Nitzan Y., Gedanken A., Lubart R. Antifungal activity of ZnO nanoparticles-the role of ROS mediated cell injury. Nanotechnology. 2011;22:105101. doi: 10.1088/0957-4484/22/10/105101. [DOI] [PubMed] [Google Scholar]
- Luo X., Morrin A., Killard A.J., Smyth M.R. Application of nanoparticles in electrochemical sensors and biosensors. Electroanalysis. 2006;18:319–326. doi: 10.1002/elan.200503415. [DOI] [Google Scholar]
- Mahendra C., Murali M., Manasa G., Ponnamma P., Abhilash M.R., Lakshmeesha T.R., Satish A., Amruthesh K.N., Sudarshana M.S. Antibacterial and antimitotic potential of bio-fabricated zinc oxide nanoparticles of Cochlospermum religiosum (L.) Microb. Pathog. 2017;110:620–629. doi: 10.1016/j.micpath.2017.07.051. [DOI] [PubMed] [Google Scholar]
- Mark J. Messina Legumes and soybeans: overview of their nutritional profiles and health effects. Am. J. Clin. Nutr. 1999;70:439–450. doi: 10.1093/ajcn/70.3.439s. [DOI] [PubMed] [Google Scholar]
- Mccalla A.F. Agriculture and food needs to 2025. Int. Agric. Dev. 1998:39–54. [Google Scholar]
- Mittal A.K., Chisti Y., Banerjee U.C. Synthesis of metallic nanoparticles using plant extracts. Biotechnol. Adv. 2013;31:346–356. doi: 10.1016/j.biotechadv.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Mohanraj V.J., Chen Y. Nanoparticles – a review. Trop. J. Pharmaceut. Res. 2006;5:5. [Google Scholar]
- Murali M., Mahendra C., Nagabhushan, Rajashekar N., Sudarshana M.S., Raveesha K.A., Amruthesh K.N. Antibacterial and antioxidant properties of biosynthesized zinc oxide nanoparticles from Ceropegia candelabrum L. – An endemic species. Spectrochim. Acta – Part A Mol. Biomol. Spectrosc. 2017;179:104–109. doi: 10.1016/j.saa.2017.02.027. [DOI] [PubMed] [Google Scholar]
- Naveen Kumar K., Venkataramana M., Allen J.A., Chandranayaka S., Murali H.S., Batra H.V. Role of Curcuma longa L. essential oil in controlling the growth and zearalenone production of Fusarium graminearum. LWT – Food Sci. Technol. 2016;69:522–528. doi: 10.1016/j.lwt.2016.02.005. [DOI] [Google Scholar]
- Petkova P., Francesko A., Perelshtein I., Gedanken A., Tzanov T. Simultaneous sonochemical-enzymatic coating of medical textiles with antibacterial ZnO nanoparticles. Ultrason. Sonochem. 2016;29:244–250. doi: 10.1016/j.ultsonch.2015.09.021. [DOI] [PubMed] [Google Scholar]
- Raveendran P., Fu J., Wallen S.L. Completely, “Green” Synthesis and Stabilization of Metal Nanoparticles. J. Am. Chem. Soc. 2003;125:13940–13941. doi: 10.1021/ja029267j. [DOI] [PubMed] [Google Scholar]
- Sadeghi H., Khazaei F., Yari L., Sheidaei S. Effect of seed osmopriming on seed germination behavior and vigor of soybean (Glycine max L.) ARPN J. Agric. Biol. Sci. 2011;6:39–43. [Google Scholar]
- Sangeetha G., Rajeshwari S., Venckatesh R. Green synthesis of zinc oxide nanoparticles by aloe barbadensis miller leaf extract: Structure and optical properties. Mater. Res. Bull. 2011;46:2560–2566. doi: 10.1016/j.materresbull.2011.07.046. [DOI] [Google Scholar]
- Sellamani M., Kalagatur N.K., Siddaiah C., Mudili V., Krishna K., Natarajan G., Rao Putcha V.L. Antifungal and zearalenone inhibitory activity of Pediococcus pentosaceus isolated from dairy products on Fusarium graminearum. Front. Microbiol. 2016;7:890. doi: 10.3389/fmicb.2016.00890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen A., Batra A. Evaluation of antimicrobial activity of different solvent extracts of medicinal plant: Melia Azedarach L. Int. J. Curr. Pharm. Res. 2012;4:67–73. [Google Scholar]
- Slowing I.I., Trewyn B.G., Giri S., Lin V.S.Y. Mesoporous silica nanoparticles for drug delivery and biosensing applications. Adv. Funct. Mater. 2007;17:1225–1236. doi: 10.1002/adfm.200601191. [DOI] [Google Scholar]
- Smijs T.G., Pavel S. Titanium dioxide and zinc oxide nanoparticles in sunscreens: Focus on their safety and effectiveness. Nanotechnol. Sci. Appl. 2011;4:95–135. doi: 10.2147/nsa.s19419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suresh D., Nethravathi P.C., Udayabhanu, Rajanaika H., Nagabhushana H., Sharma S.C. Green synthesis of multifunctional zinc oxide (ZnO) nanoparticles using Cassia fistula plant extract and their photodegradative, antioxidant and antibacterial activities. Mater. Sci. Semicond. Process. 2015;31:446–454. doi: 10.1016/j.mssp.2014.12.023. [DOI] [Google Scholar]
- Tanaka K. Nanotechnology towards the 21st century. Thin Solid Films. 1999;341:120–125. doi: 10.1016/S0040-6090(98)01508-9. [DOI] [Google Scholar]
- Thakkar K.N., Mhatre S.S., Parikh R.Y. Biological synthesis of metallic nanoparticles. Nanomedicine Nanotechnology. Biol. Med. 2010;6:257–262. doi: 10.1016/j.nano.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Tutaj K., Szlazak R., Szalapata K., Starzyk J., Luchowski R., Grudzinski W., Osinska-Jaroszuk M., Jarosz-Wilkolazka A., Szuster-Ciesielska A., Gruszecki W.I. Amphotericin B-silver hybrid nanoparticles: Synthesis, properties and antifungal activity. Nanomed. Nanotechnol. Biol. Med. 2016;12:1095–1103. doi: 10.1016/j.nano.2015.12.378. [DOI] [PubMed] [Google Scholar]
- Van K., McHale L.K. Meta-Analyses of QTLs associated with protein and oil contents and compositions in soybean [Glycine max (L.) Merr.] Seed. Int. J. Mol. Sci. 2017;18 doi: 10.3390/ijms18061180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandscheer C.B., Duque J.E., Da Silva M.A.N., Fukuyama Y., Wohlke J.L., Adelmann J., Fontana J.D. Larvicidal action of ethanolic extracts from fruit endocarps of Melia azedarach and Azadirachta indica against the dengue mosquito Aedes aegypti. Toxicon. 2004 doi: 10.1016/j.toxicon.2004.07.009. [DOI] [PubMed] [Google Scholar]
- White T.J., Bruns T., Lee S., Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc. Guid. Methods Appl. 1990;18:315–322. doi: 10.1016/b978-0-12-372180-8.50042-1. [DOI] [Google Scholar]
- Zhang L., Jiang Y., Ding Y., Povey M., York D. Investigation into the antibacterial behaviour of suspensions of ZnO nanoparticles (ZnO nanofluids) J. Nanopart. Res. 2007;9:479–489. doi: 10.1007/s11051-006-9150-1. [DOI] [Google Scholar]