Abstract
The aim of our study was to estimate regional and global cataract prevalence, its prevalence in different age groups, and the determinants of heterogeneity and its prevalence. For that, we used international databases such as PubMed, Web of Science, Scopus, Embase, and other sources of information to conduct a systematic search for all articles concerning the prevalence of age-related cataract and its types in different age groups. Of the 9922 identified articles, 45 studies with a sample size of 161,947 were included in the analysis, and most of them were from the Office for the Western Pacific Region (19 studies). Age- standardized pooled prevalence estimate (ASPPE) and 95% confidence interval (95% CI) of any cataract, cortical cataract, nuclear cataract, and posterior subcapsular (PSC) cataract were 17.20% (13.39–21.01), 8.05% (4.79–11.31), 8.22% (4.93–11.52), and 2.24% (1.41–3.07), respectively. Significant effects on heterogeneity were observed for the WHO region in the prevalence of any cataract (b: 6.30; p: 0.005) and study year in the prevalence of nuclear cataract (b: −0.66, p: 0.042). In general, the prevalence of cataract not only varies by region but also by age group, and most cases are over the age of 60 years. We examined the sources of variance in the prevalence of cataract and its different types, and identified age as a responsible factor in the prevalence of any cataract, cortical cataract, nuclear cataract, and PSC of cataract, WHO region in the prevalence of any cataract, and study year in the prevalence of nuclear cataract.
Subject terms: Epidemiology, Vision disorders
摘要
本文旨在评估区域性和全球性白内障患病率, 不同年龄组白内障患病率, 以及遗传异质性在白内障患病率中的作用。通过PubMed、Web of Science、Scopus、Embase等国际数据库检索, 我们对所有年龄相关性白内障的患病率及其在不同年龄组不同类型的相关文章进行了分析。在9922篇文章中, 共纳入45项研究, 总样本量为161947例, 其中大部分来自西太平洋地区 (19项研究) 。年龄标准化患病率 (Age standardized pooled prevalence estimate, ASPPE) 和95%置信区间 (Confidence interval, CI) (95% CI:任何类型的白内障、皮质性白内障、核性白内障和后发性白内障 (Posterior sub-capsular, PSC) 分别占17.20% (13.39–21.01) 、8.05% (4.79–11.31) 、8.22% (4.93–11.52) 和2.24% (1.41–3.07) 。我们在世界卫生组织的分区内观察到, 在纳入研究的一年中, 遗传异质性在白内障的患病率 (b: 6.30;p: 0.005) 以及核性白内障的患病率 (b: −0.66, p: 0.042) 中起到了重要作用。研究发现, 白内障患病率不仅因地区而异, 且因年龄而异, 多数病例年龄患病年龄在60岁以上。我们研究了导致白内障及不同类型的白内障的患病率差异的原因。研究发现, 在白内障, 皮质性白内障, 核性白内障及后发性白内障的患病率中, 年龄可作为一个决定性的影响因素。此外, 我们还研究了世界卫生组织不同区域的不同类型的白内障及研究年中核性白内障的患病率. 关键字: 全球流行;区域流行;年龄相关性白内障;皮质性白内障;核性白内障;后发性白内障;荟萃分析
Introduction
Although cataract is almost always a curable disease [1], it is still one of the most common causes of visual impairment around the world [2–4]. This disease, which can significantly reduce patients’ quality of life [5], is still one of the main ophthalmological public health problems in developed and developing countries [2], and it is known as the main cause of blindness in many countries [2–4, 6–8]. Studies indicate that 36 million people are blind worldwide, and over 12 million of them are due to cataract [4, 8]. It is projected that this estimate will reach 13.5 million people in 2020 [8]. The importance of cataract blindness is that more than 90% of the total disability-adjusted life years lost due to cataract is in developing countries [4].
Cataract is usually an inevitable side effect of aging [2]. However, it should be noted that some genetic and environmental factors such as smoking cigarettes, ultraviolet light exposure, and certain diseases, such as diabetes, uveitis, IOP-lowering medications/surgery, trauma, steroid usage, and certain occupations, increase the risk of developing cataract [4, 7, 9–18]. Hence, various population-based studies have been carried out over the past three decades to provide information on its prevalence and risk factors in different ethnic groups and regions around the world [14, 17–24]. Knowledge of cataract prevalence can offer information on the extent and burden of the disease [2], be used for planning and providing the infrastructure for disease control [6], and shed light on the natural evolution of the disease [2].
Despite the availability of information about cataract prevalence in different ethnicities and regions around the world, to our knowledge, only one study has combined data from eye cohorts, and estimated the pooled prevalence estimate (PPE) of cataract in western countries [25]. However, this study has extensive methodological limitations, such as lack of systematic search, statistical methods for estimating the PPE, and data from other countries, as well as including studies using different cataract- grading systems. Therefore, we were prompted to implement a systematic review and meta-analysis study with a sound methodological approach to determine the PPE of cataract and its types by age, gender, and geographical area, as well as its trend of changes over the last three decades. The information generated from this study may certainly be useful for public health policy makers for planning interventions and health policies.
Methods
Search strategy and selection of studies
The search strategy is described below that is applied based on PICOTS for MEDLINE (MeSH, Medical Subject Headings) and then used in other databases:
Cataract [text word] OR Cataract [Mesh term].
Lens opacity [text word] OR lens opacity [Mesh term].
1 OR 2.
Prevalence [text word] OR Prevalence [Mesh term].
Frequency [text word] OR Frequency [Mesh term].
Incidence [text word] OR incidence [Mesh term].
7: 4 OR 5 OR 6.
8: Cross-sectional studies [text word] OR Cross-sectional studies [Mesh term].
9: cohort studies [text word] OR cohort studies [Mesh term].
10: observational studies [text word] OR observational studies [Mesh term].
11: 8 OR 9 OR 10.
12: 3 AND 7 AND 11.
Also the Google Scholar was used to access gray literature [26]. In addition, a cataract expert was consulted to identify important articles.
Then all the extracted articles from each database were entered in Endnote X6, and screening was done after removing duplicates. The screening was done in three steps. In the first step, the titles were reviewed, and if the article was relevant, then the abstract and then the full text of the article was reviewed. For articles that lacked enough raw data, an email was sent to the corresponding author. The three steps were followed independently by two raters (RP and MKH). Inter-rater discrepancies were resolved based on the third person’s opinion (HH). Blinding and task separation were applied in the study selection procedure. The inter-rater agreement was 89%.
Exclusion criteria
In this study, we only reviewed age-related cataract in normal populations; therefore, studies on specific groups such as those in hospitals, nursing home residents, people working in certain professions (e.g., welding), and those with specific ocular or systemic diseases (e.g., Down syndrome, glaucoma, arthritis, and diabetes) were not included. Exclusions were also applied to other types of cataract, including acquired cataracts due to trauma or medications, congenital cataracts, and other types of secondary cataract due to certain diseases such as diabetes. Publications such as letters, conference papers and abstracts, reviews, notes, editorials, clinical studies, retrospective studies, follow-up, and longitudinal studies were also excluded.
In addition, since several cataract-grading systems are available, only studies using Lens Opacity Classification Systems (LOCS) 2 OR 3 were included to allow for data pooling. All studies using other grading systems, those that did not mention their methods, and studies that used self-report or a questionnaire for the diagnosis of cataract, were excluded.
Data extraction and quality assessment
In this study, we closely reviewed all articles on the prevalence of age-related cataract and its different types that reached the final step of screening. Article information such as the name of the author, the publication year, the country of the study, the study design, the characteristics of the participants (including age and gender), sample size, number of cataract cases, and the prevalence of cataract (regardless of aphakia and pseudophakia), and the diagnostic criteria were extracted and entered into the database.
The Newcastle–Ottawa Scale adapted for cross-sectional studies [27] was applied to evaluate the quality of the studies. This scale has three sections: 1—selection (3 items, maximum score: 3 points), 2—comparability (1 item, maximum score: 2 points), and 3—outcome (2 items, maximum score: 3 points). The studies were evaluated by two raters (RP and MKH) independently, and a total score was calculated for each study. The studies were then assigned to one of the following categories accordingly: very good studies: 7–8 scores; good studies: 5–6 scores; satisfactory studies: 3–2 scores; unsatisfactory studies: 0–1 score.
Definition of variables
For age classification, we considered three categories 20–39, 40–59, and ≥60 years. Countries were categorized based on the latest WHO definition that includes the following six regions: Regional Office for Africa (AFRO), Regional Office of Americas (AMRO), Regional Office for the Eastern Mediterranean (EMRO), Regional Office for Europe (EURO), Regional Office for South-East Asia (SEARO), and the Regional Office for the Western Pacific (WPRO).
Statistical analysis
All analyses were conducted with Stata software version 14.0 (College Station, Texas). The number of cases, the prevalence of cataract, and its different types were extracted. If a study did not report the prevalence, it was calculated using a binomial distribution of the sample size, and the number of cataract cases were available. Pooled prevalence was calculated using the “metaprop” command, and presented by a forest plot [28]. We use Freeman–Tukey double-arcsine transformation as a variance- stabilizing meta-analysis technique. Heterogeneity was determined using Cochran’s Q test of heterogeneity, and the I2 index was used to quantify heterogeneity. In accordance with Higgins classification approach, I2 values above 0.7 were considered as high heterogeneity. To estimate the PPE for subgroup analysis, the fixed-effect model was used, and when the heterogeneity was greater than 0.7, the random effects model was used. We also calculated age-standardized pooled prevalence estimate (ASPPE) of cataract and its subtypes in total by direct standardization and using world health organization population [29] to adjust the structural age between different age groups and regions.
The meta-regression analysis was used to examine the effect of age, gender, sample size, publication date, study quality, and geographical area as factors affecting heterogeneity among studies. The Metabias command was used to check for publication bias [30], and if there was any publication bias, the prevalence rate was adjusted with the Metatrim command using the trim-and- fill method. In all analyses, a significance level of 0.05 was considered.
Method of literature search
All steps in this systematic review and meta-analysis study were registered in the International Prospective Register of Systemic Reviews with CRD42018097105 code [31] based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [32]. For this purpose, a complete and comprehensive search without any restriction was conducted in international databases, including Web of Science, PubMed, Scopus, and Embase to identify articles on age-related cataract prevalence and types including cortical cataract, nuclear cataract, and posterior subscapular (PSC) cataract published by 13th August 2019. Searches were done using text words and MESH terms. The PICOTS explored in this study were
Population: None
Intervention: None
Comparison: None
Outcome: Prevalence of cataract or lens opacity
Time: None
Study design: Observational study
Results
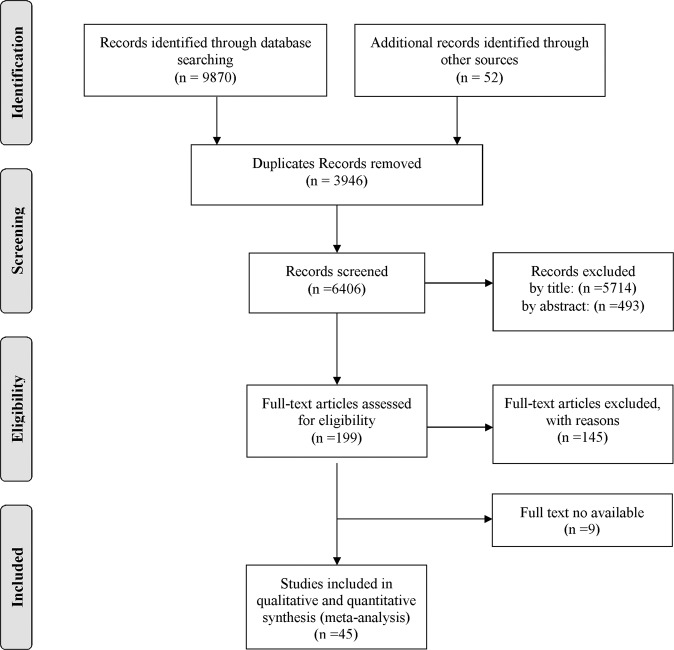
Overall, 9870 studies were found through databases, and 52 studies were identified through other sources. After excluding redundant papers, 6406 studies remained. Screening was done in three steps. In the first step, 5714 studies were excluded after reviewing the titles, and 692 articles remained. After reading abstracts, 493 studies were excluded from the list. Then, the full text of the remaining 199 studies was reviewed, and 145 studies were excluded.
Access to full texts and complete data extraction were not possible for 9 out of the remaining 54 studies, which were excluded from analysis [33–41]. Finally, 45 studies [2, 9, 13, 14, 16–24, 42–61] with a total sample size of 161,947 were included in the analysis (Table 1). The flowchart of this selection process is shown in Fig. 1. WPRO had the highest number of studies (19 studies) and AFRO had the lowest number (1 study). The oldest studies were published in 1994 [44], and the most recent study was published in 2019 [57, 61]. The minimum age range of the subjects was 19–29 years, and the maximum age range was 90–99 years. Four studies provided the prevalence of cataract in the 20–39-year age group, 24 studies reported the prevalence in the 40–59-year age group, and 40 studies in the over-60-year age group. Twenty-eight studies were very good quality, 14 studies were good quality, and 3 studies were found to be satisfactory quality (Table 1).
Table 1.
Summary of the studies included in this meta-analysis.
| Author | Country | Design | Publication year | Age | Sample size | Any cataract, N (%) | Cortical cataract, N (%) | Nuclear cataract, N (%) | PSC cataract, N (%) | Diagnostic criteria based on LOCSa | QAS | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cortical | Nuclear | PSC | |||||||||||
| Husain et al. [62] | Indonesia | PBCSS | 2005 | 20–39 | 500 | 21 (4.20) | 19 (3.80) | 9 (1.80) | 5 (1.00) | ≥4.0 | ≥4.0 | ≥2.0 | 7 |
| 40–59 | 298 | 84 (28.48) | 66 (22.14) | 76 (25.50) | 19 (6.37) | ||||||||
| ≥60 | 116 | 96 (82.75) | 58 (50.00) | 69 (59.48) | 45 (38.79) | ||||||||
| Male | 420 | 88 (20.95) | 65 (15.48) | 67 (15.95) | 31 (7.38) | ||||||||
| Female | 494 | 113 (22.87) | 78 (15.79) | 87 (17.61) | 39 (7.89) | ||||||||
| Stocks et al. [63] | United Kingdom | PBCSS | 2002 | ≥60 | 903 | 398 (44.07) | 75 (8.30) | 470 (52.04) | 15 (1.66) | >1.9 | >2.9 | >1.9 | 7 |
| Vashist et al. [64] | India | PBCSS | 2011 | ≥60 | 5871 | 3241 (55.20) | 198 (3.37) | 1559 (26.55) | 242 (4.12) | ≥3 | ≥4 | ≥2 | 7 |
| Varma et al. [6] | United States | PBCSS | 2004 | 40–59 | 4216 | NA | 194 (4.60) | 54 (1.28) | 39 (0.92) | ≥2 | ≥2 | ≥2 | 6 |
| ≥60 | 1926 | NA | 606 (31.46) | 483 (25.07) | 148 (7.68) | ||||||||
| Male | 2559 | NA | 320 (12.50) | 203 (7.93) | 79 (3.08) | ||||||||
| Female | 3583 | NA | 480 (13.40) | 334 (9.32) | 108 (3.01) | ||||||||
| Yu et al. [7] | China | PBCSS | 2016 | 40–59 | 458 | 41 (8.95) | 12 (2.62) | 5 (1.10) | 9 (1.96) | ≥2 | ≥2 | ≥2 | 6 |
| ≥60 | 355 | 199 (56.05) | 92 (25.91) | 45 (12.67) | 7 (1.97) | ||||||||
| Li et al. [3] | China | PBCSS | 2009 | 40–59 | 572 | 75 (13.11) | 51 (8.91) | 37 (6.46) | 9 (1.57) | ≥1 | ≥1 | ≥1 | 7 |
| ≥60 | 672 | 331 (49.25) | 230 (34.22) | 261 (38.83) | 56 (8.33) | ||||||||
| Male | 433 | 109 (25.17) | 70 (16.17) | 76 (17.55) | 21 (4.85) | ||||||||
| Female | 811 | 297 (36.62) | 211 (26.02) | 222 (27.37) | 44 (5.42) | ||||||||
| Nam et al. [4] | Korea | NWCSS | 2015 | Male | 6833 | 3351 (49.04) | 736 (10.77) | 1754 (25.67) | 37 (0.54) | ≥2 | ≥2 | ≥1 | 7 |
| Female | 9033 | 4431 (49.05) | 877 (9.70) | 2205 (24.41) | 51 (0.56) | ||||||||
| Hashemi et al. [65] | Iran | PBCSS | 2017 | 40–59 | 292 | 69 (23.63) | 10 (3.42) | 23 (7.87) | 17 (5.82) | ≥2 | ≥2 | ≥2 | 8 |
| ≥60 | 645 | 208 (32.24) | 85 (13.17) | 40 (6.20) | 30 (4.65) | ||||||||
| Male | 435 | 133 (30.57) | 51 (11.72) | 33 (7.58) | 22 (5.05) | ||||||||
| Female | 502 | 144 (28.69) | 44 (8.76) | 30 (5.97) | 25 (4.98) | ||||||||
| Hashemi et al. [1] | Iran | PBCSS | 2009 | 40–59 | 1003 | 93 (9.27) | NA | NA | NA | ≥3 | ≥3 | ≥2 | 8 |
| ≥60 | 330 | 155 (46.96) | NA | NA | NA | ||||||||
| Male | 634 | 111 (17.51) | NA | NA | NA | ||||||||
| Female | 656 | 137 (20.88) | NA | NA | NA | ||||||||
| Rim et al. [66] | Korea | NWCSS | 2014 | 40–59 | 6372 | 1348 (21.15) | NA | NA | NA | ≥3 | ≥4 | ≥2 | 8 |
| ≥60 | 5219 | 4175 (79.99) | NA | NA | NA | ||||||||
| Athanasiov et al. [10] | Myanmar | PBCSS | 2008 | 40–59 | 1258 | 187 (14.86) | 32 (2.54) | 76 (6.04) | 21 (1.66) | ≥2 | ≥4 | ≥2 | 8 |
| ≥60 | 777 | 547 (70.39) | 44 (5.66) | 254 (32.68) | 19 (2.44) | ||||||||
| Male | 829 | 298 (35.95) | 28 (3.37) | 145 (17.49) | 18 (2.17) | ||||||||
| Female | 1215 | 436 (35.88) | 48 (3.95) | 185 (15.23) | 22 (1.81) | ||||||||
| Athanasiov et al. [11] | Sri Lanka | PBCSS | 2009 | 40–59 | 801 | 146 (18.22) | NA | NA | NA | ≥2 | ≥4 | ≥2 | 8 |
| ≥60 | 501 | 290 (57.88) | NA | NA | NA | ||||||||
| Male | 528 | 241 (45.64) | 202 (38.26) | 39 (7.38) | 62 (11.74) | ||||||||
| Female | 777 | 169 (21.75) | 143 (18.40) | 21 (2.70) | 40 (5.14) | ||||||||
| Wang et al. [42] | China | NWCSS | 2016 | ≥60 | 4369 | 2121 (48.54) | 1776 (40.65) | 1349 (30.87) | 414 (9.47) | ≥2 | ≥4 | ≥2 | 6 |
| Cristina Leske et al. [43] | Barbados | PBCSS | 1997 | 40–59 | 2325 | NA | 292 (12.56) | 48 (2.06) | 22 (0.94) | ≥2 | ≥2 | ≥2 | 8 |
| ≥60 | 1806 | NA | 1012 (56.03) | 638 (35.32) | 101 (5.59) | ||||||||
| Male | 1902 | NA | 562 (29.55) | 336 (17.67) | 72 (3.78) | ||||||||
| Female | 2457 | NA | 895 (36.43) | 502 (20.43) | 99 (4.02) | ||||||||
| Giuffre et al. [44] | Italy | PBCSS | 1995 | 40––59 | 576 | 41 (7.11) | 30 (5.20) | 33 (5.72) | 18 (3.12) | ≥2 | ≥2 | ≥2 | 6 |
| ≥60 | 492 | 237 (48.17) | 132 (26.82) | 209 (42.47) | 115 (23.37) | ||||||||
| Male | 479 | 118 (24.63) | NA | NA | NA | ||||||||
| Female | 589 | 156 (26.49) | NA | NA | NA | ||||||||
| Lee et al. [45] | Korea | NWCSS | 2015 | 40–59 | 4305 | 945 (21.95) | NA | NA | NA | ≥2 | ≥2 | ≥2 | 8 |
| ≥60 | 609 | 364 (59.77) | NA | NA | NA | ||||||||
| Male | 2134 | 551 (25.82) | 137 (6.42) | 308 (14.43) | NA | ||||||||
| Female | 2780 | 690 (24.82) | 171 (6.15) | 413 (14.86) | NA | ||||||||
| Nirmalan et al. [46] | India | PBCSS | 2003 | 40–59 | 3532 | 1142 (32.33) | NA | NA | NA | ≥3 | ≥3 | ≥2 | 7 |
| ≥60 | 1618 | 1307 (80.77) | NA | NA | NA | ||||||||
| Male | 2314 | NA | 34 (1.46) | 521 (22.52) | 30 (1.29) | ||||||||
| Female | 2836 | NA | 31 (1.09) | 628 (22.14) | 38 (1.34) | ||||||||
| Tsai et al. [47] | Taiwan | PBCSS | 2002 | ≥60 | 1361 | 631 (46.36) | 298 (21.89) | 530 (38.94) | 125 (9.18) | ≥3 | ≥3 | ≥3 | 6 |
| Male | 822 | NA | 159 (19.34) | 300 (36.50) | 63 (11.5) | ||||||||
| Female | 539 | NA | 139 (25.79) | 230 (42.67) | 62 (7.02) | ||||||||
| Hirvelii et al. [14] | Finland | PBCSS | 1995 | ≥60 | 500 | 293 (58.60) | NA | NA | NA | ≥2 | ≥2 | ≥2 | 6 |
| Male | 179 | 85 (47.49) | NA | NA | NA | ||||||||
| Female | 321 | 208 (64.80) | NA | NA | NA | ||||||||
| Kim et al. [17] | Korea | PBCSS | 2014 | 20–39 | 3415 | 60 (1.75) | NA | NA | NA | ≥3 | ≥4 | ≥2 | 8 |
| 40–59 | 4659 | 1351 (29.00) | NA | NA | NA | ||||||||
| ≥60 | 2174 | 1917 (88.17) | NA | NA | NA | ||||||||
| Male | 4397 | 1404 (31.93) | NA | NA | NA | ||||||||
| Female | 5851 | 1924 (32.88) | NA | NA | NA | ||||||||
| Ostberg et al. [19] | Sweden | NWCSS | 2006 | ≥60 | 495 | 102 (20.60) | 29 (5.85) | 56 (11.31) | 33 (6.66) | >3 | ≥4 | ≥2 | 7 |
| Male | 251 | 35 (13.94) | NA | NA | NA | ||||||||
| Female | 262 | 47 (17.94) | NA | NA | NA | ||||||||
| Paunksnis et al. [22] | Lithuania | NWCSS | 2007 | 20–39 | 403 | 17 (4.21) | 4 (0.99) | 10 (2.48) | 3 (0.74) | ≥2 | ≥2 | ≥1 | 7 |
| 40–59 | 435 | 49 (11.26) | 15 (3.44) | 28 (6.43) | 6 (1.37) | ||||||||
| ≥60 | 444 | 170 (38.28) | 58 (13.06) | 106 (23.87) | 6 (1.35) | ||||||||
| Male | 573 | 101 (17.63) | 30 (5.23) | 65 (11.34) | 6 (1.04) | ||||||||
| Female | 709 | 135 (19.04) | 47 (6.62) | 79 (11.14) | 9 (1.26) | ||||||||
| Park et al. [21] | Korea | PBCSS | 2014 | 40–59 | 7487 | 3778 (50.46) | NA | NA | NA | ≥3 | ≥2 | ≥3 | 7 |
| ≥60 | 7297 | 3588 (49.17) | NA | NA | NA | ||||||||
| Male | 4811 | 2199 (45.71) | NA | NA | NA | ||||||||
| Female | 6265 | 3402 (54.30) | NA | NA | NA | ||||||||
| Richter et al. [23] | United States | PBCSS | 2012 | Male | 1610 | 452 (28.07) | 197 (12.24) | 88 (5.46) | 13 (0.80) | ≥2 | ≥2 | ≥2 | 6 |
| Female | 3222 | 643 (19.96) | 271 (8.41) | 129 (4.01) | 14 (0.43) | ||||||||
| Park et al. [20] | Korea | NWCSS | 2013 | Male | 1421 | 892 (62.77) | 185 (13.02) | 490 (34.48) | 7 (0.49) | ≥4 | ≥4 | ≥2 | 8 |
| Female | 1827 | 1147 (62.78) | 594 (32.51) | 15 (0.82) | NA | ||||||||
| Seah et al. [24] | Singapore | PBCSS | 2002 | 40–59 | 565 | 132 (23.36) | 87 (15.39) | 61 (10.79) | 20 (3.53) | ≥2 | ≥4 | ≥2 | 7 |
| ≥60 | 557 | 391 (70.19) | 344 (61.75) | 373 (66.96) | 110 (19.74) | ||||||||
| Male | 503 | 329 (65.41) | 194 (38.57) | 214 (42.54) | 58 (11.53) | ||||||||
| Female | 619 | 360 (58.16) | 237 (38.29) | 236 (38.13) | 72 (11.63) | ||||||||
| Krishnaiah et al. [18] | India | PBCSS | 2005 | 40–59 | 2420 | NA | NA | 267 (11.03) | NA | NA | ≥3 | NA | 6 |
| ≥60 | 1137 | NA | NA | 628 (55.23) | NA | ||||||||
| Male | 3319 | NA | NA | 405 (12.2) | NA | ||||||||
| Female | 3929 | NA | NA | 496 (12.62) | NA | ||||||||
| Cedrone et al. [2] | Italy | PBCSS | 1999 | 40–59 | 498 | 5 (1.00) | NA | NA | NA | ≥2 | ≥4 | ≥2 | 6 |
| ≥60 | 362 | 27 (5.42) | NA | NA | NA | ||||||||
| Male | 376 | 15 (3.01) | NA | NA | NA | ||||||||
| Female | 484 | 17 (3.41) | NA | NA | NA | ||||||||
| Mahdi et al. [9] | Nigeria | PBCSS | 2014 | 40–59 | 1008 | 54 (5.35) | 35 (3.47) | 14 (1.38) | 10 (0.99) | ≥2 | ≥4 | ≥2 | 8 |
| ≥60 | 629 | 270 (42.92) | 156 (24.80) | 130 (20.66) | 38 (6.04) | ||||||||
| Female | 872 | 198 (22.70) | 119 (13.64) | 90 (10.32) | 27 (3.09) | ||||||||
| Male | 765 | 126 (16.47) | 72 (9.41) | 54 (7.05) | 21 (2.75) | ||||||||
| Pan et al. [48] | Singapore | PBCSS | 2012 | ≥60 | 5768 | NA | 1486 (25.76) | 620 (10.40) | 173 (2.99) | ≥2 | ≥4 | ≥2 | 7 |
| Rauf et al. [49] | United Kingdom | PBCSS | 2013 | ≥60 | 922 | 651 (70.60) | NA | NA | NA | ≥2 | ≥3 | ≥2 | 6 |
| Hashemi et al. [50] | Iran | PBCSS | 2011 | 40–59 | 1316 | 222 (16.86) | 29 (2.20) | 171 (12.99) | 76 (5.77) | ≥3 | ≥3 | ≥2 | 7 |
| Wong et al. [51] | Singapore | PBCSS | 2003 | ≥60 | 989 | NA | 324 (32.76) | 338 (34.17) | 98 (9.90) | NA | NA | NA | 6 |
| Tang et al. [52] | China | PBCSS | 2015 | ≥60 | 2006 | 883 (44.01) | 827 (41.22) | 607 (30.25) | 29 (1.44) | ≥2 | ≥2 | ≥2 | 8 |
| Delcourt et al. [13] | France | PBCSS | 2000 | ≥60 | 2468 | NA | 89 (3.60) | 134 (5.42) | 138 (5.59) | ≥4 | ≥4 | ≥2 | 6 |
| Female | 1386 | NA | 59 (4.25) | 81 (5.84) | 93 (6.71) | ||||||||
| Male | 1082 | NA | 30 (2.77) | 53 (4.89) | 76 (7.02) | ||||||||
| Duan et al. [53] | China | PBCSS | 2013 | 20–39 | 1192 | 34 (2.85) | 25 (2.09) | 1 (0.08) | 2 (0.16) | ≥2 | ≥4 | ≥2 | 8 |
| 40–59 | 3717 | 433 (11.64) | 403 (10.84) | 20 (0.53) | 28 (0.75) | ||||||||
| ≥60 | 1614 | 895 (55.45) | 768 (47.58) | 313 (19.39) | 66 (4.08) | ||||||||
| Female | 3506 | 829 (23.64) | 751 (21.42) | 201 (5.73) | 58 (1.65) | ||||||||
| Male | 3017 | 533 (17.66) | 445 (14.74) | 133 (4.40) | 38 (1.25) | ||||||||
| Nirmalan et al. [16] | India | PBCSS | 2004 | Female | 1314 | NA | 1249 (95.05) | 407 (30.97) | 604 (45.96) | ≥3 | ≥3 | ≥2 | 7 |
| Male | 1135 | NA | 986 (86.87) | 309 (27.22) | 519 (45.72) | ||||||||
| Carlos et al. [54] | Brazil | PBCSS | 2009 | ≥60 | 4229 | 209 (4.94) | NA | NA | NA | NA | NA | NA | 6 |
| Germano et al. [55] | Brazil | PBCSS | 2017 | ≥60 | 377 | 50 (13.26) | NA | NA | NA | NA | NA | NA | 4 |
| Huang et al. [56] | Taiwan | PBCSS | 2010 | ≥60 | 2316 | 1913 (82.59) | NA | NA | NA | ≥2 | ≥3 | ≥2 | 6 |
| Female | 1390 | 1146 (82.44) | NA | NA | NA | ||||||||
| Male | 927 | 767 (82.74) | NA | NA | NA | ||||||||
| Singh et al. [57] | India | PBCSS | 2019 | ≥60 | 4331 | 2588 (59.75) | 376 (8.68) | 221 (5.10) | 466 (10.75) | ≥2 | ≥4 | ≥2 | 8 |
| Female | 2399 | 986 (41.10) | NA | NA | NA | ||||||||
| Male | 1932 | 757 (39.18) | NA | NA | NA | ||||||||
| Tang et al. [58] | China | PBCSS | 2016 | 40–59 | 5303 | 964 (18.17) | 533 (10.05) | 669 (12.61) | 98 (1.84) | ≥2 | ≥2 | ≥2 | 7 |
| ≥60 | 4931 | 3296 (66.84) | 2123 (43.05) | 2501 (50.71) | 370 (7.50) | ||||||||
| Female | 6073 | 2571 (42.33) | 1962 (32.30) | 1568 (25.81) | 286 (4.70) | ||||||||
| Male | 4160 | 1689 (40.60) | 1208 (29.03) | 1088 (26.15) | 182 (4.37) | ||||||||
| Bojarskien et al. [59] | Lithuania | PBCSS | 2005 | 40–59 | 2708 | 247 (9.12) | NA | NA | NA | ≥2 | ≥2 | ≥1 | 7 |
| Female | 596 | 106 (17.78) | NA | NA | NA | ||||||||
| Male | 759 | 141 (18.57) | NA | NA | NA | ||||||||
| Xu et al. [60] | China | PBCSS | 1996 | ≥60 | 1817 | NA | 550 (30.26) | 520 (28.61) | 158 (8.69) | NA | NA | NA | |
| Yoshikawa et al. [61] | Japan | PBCSS | 2019 | ≥60 | 490 | NA | 106 (21.63) | 66 (13.46) | 28 (5.71) | ≥2 | ≥3 | ≥2 | 3 |
PBCSS Population-Based Cross-Sectional Study, NWCSS Nation-Wide Cross-Sectional Survey, NA not available, LOCS Lens Opacity Classification System, QAS Quality Assessment Score, PSC posterior subcapsular cataract. Any cataract was defined as the presence, in either eye, of any cataract (nuclear, cortical, or posterior subcapsular cataract).
Italic numbers are based on LOCS II.
aLens grading is based on LOCS III.
Fig. 1.
Flowchart of the study selection process.
The minimum and maximum reported prevalence was 1% in the 20–39-years age group [2] and 88.17% in the over-60 age group for any cataract [17], 0.99% in the under-40 age group [22] and 61.75% in the over-60 age group for cortical cataract [24], 0.08% in the 20–39-years age group [53], and 66.96% in the over-60 age group for nuclear cataract [24], and 0.16% in the 20–39-years age group [53] and 38.79% in the over-60 age groups for PSC cataract [62].
The ASPPE and PPE of cataract and its types based on age, gender, and WHO region
The ASPPE of any cataract was 17.20% (95% CI: 13.39–21.01). The SPPE of cortical, nuclear, and PSC cataract was 8.05% (95% CI: 4.79–11.31), 8.22% (95% CI: 4.93–11.52), and 2.24% (95% CI: 1.41–3.07), respectively. The PPE of cataract and its types by age is illustrated in Fig. 2. As demonstrated, the PPE of any cataract in the 20–39-year, 40–59-year, and over-60-year age groups was 3.01 (95% CI: 1.68–4.34), 16.97% (95% CI: 11.36–22.57), and 54.38% (95% CI: 47.57–61.18), respectively. These values were respectively 2.18% (95% CI: 0.82–3.54), 7.26% (95% CI: 4.95–9.57), and 24.78% (95% CI: 14.84–34.73) for cortical cataract, 1.12% (95% CI: 0.70–2.94), 5.77% (95% CI: 2.58–8.96), and 31.19% (95% CI: 23.88–38.50) for nuclear cataract, and 0.52% (95% CI: 0.07–1.13), 1.91% (95% CI: 1.31–2.50), and 7.29% (95% CI: 5.50–9.07) for PSC cataract. These results indicated an age-related increase in the prevalence of cataract and its different types.
Fig. 2. Age-standardized pooled prevalence estimate (ASPPE) of any cataract, cortical, nuclear, and posterior subcapsular (PSC) cataract based on the random effects model in total and pooled prevalence estimate (PPE) in sex and different age subgroups.
The diamond mark illustrates the pooled estimate.
Figure 2 also shows the PPE of cataract and its types by gender. Accordingly, the prevalence in females and males was respectively 33.67% (95% CI: 25.90–41.44) and 32.57% (95% CI: 26.29–38.85) for any cataract, 15.22% (95% CI: 9.79–20.65) and 13.64% (95% CI: 9.17–18.11) for cortical cataract, 14.09% (95% CI: 9.67–18.51) and 15.63% (95% CI: 11.44–20.33) for nuclear cataract, and 3.66% (95% CI: 3.34–4.98) and 3.70% (95% CI: 2.35–5.05) for PSC cataract.
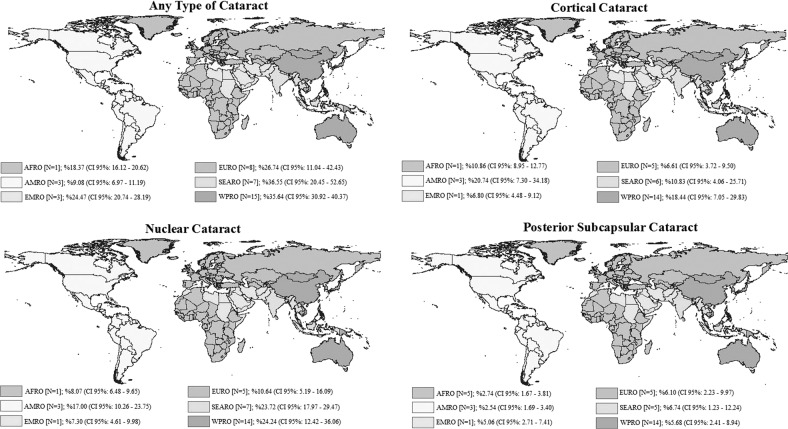
Figure 3 illustrates the ASPPE of cataract and its types in the six WHO regions. Accordingly, the ASPPE of any cataract, cortical cataract, nuclear cataract, and PSC cataract was the highest in SEARO, WPRO, and WPRO and SEARO, respectively.
Fig. 3.
Age-standardized pooled prevalence estimate (ASPPE) and 95% confidence interval of any cataract, cortical, nuclear, and posterior subcapsular (PSC) cataract based on WHO regions.
Heterogeneity and meta-regression
According to Cochran’s Q test of heterogeneity, there was significant heterogeneity among studies (all p < 0.001). The heterogeneity for cataract and its types was higher than 97% based on the I2 index, which indicates high heterogeneity. Table 2 presents the results of the univariate meta-regression; age had a significant and direct relationship with any cataract (b: 29.83; p < 0.001), cortical cataract (b: 15.06; p < 0.001), nuclear cataract (b: 19.78; p < 0.001), and PSC cataract (b: 4.54; p < 0.006). There was also a significant difference in the prevalence of any cataract in the six WHO regions (b: 6.30; p: 0.005); as such, the average prevalence of cataract varied by 6.30% among the six WHO regions. In other words, the prevalence of any cataract in EMRO was 6.30% higher compared with AMRO or SERO in contrast to EURO. This finding is demonstrated in Fig. 3, which is the ASPPE of any cataract based on the WHO-Region subgroup analysis. There was also an inverse relationship between the study year and the prevalence of nuclear cataract, but the significance level was borderline (b: −0.66, p: 0.042). Variables of gender, sample size, and quality assessment had no significant effect on the variation in the prevalence of cataract and its types (heterogeneity) (Table 2).
Table 2.
Results of the univariate meta-regression analysis on the heterogeneity of the determinants.
| Variables | Any cataract | Cortical cataract | Nuclear cataract | PSC cataract | ||||
|---|---|---|---|---|---|---|---|---|
| Coefficient (95% CI) | p value | Coefficient (95% CI) | p value | Coefficient (95% CI) | p value | Coefficient (95% CI) | p value | |
| Age group | 29.83 (22.57–37.09) | <0.001* | 15.06 (7.36–22.75) | <0.001* | 19.78 (12.32–27.23) | <0.001* | 4.54 (1.37–7.70) | 0.006* |
| Sex | 1.03 (−9.88 to 11.95) | 0.850 | 1.75 (−5.78 to 9.30) | 0.640 | −1.12 (−8.38 to 6.13) | 0.755 | −0.03 (−2.29 to 2.21) | 0.974 |
| WHO Regional Office | 6.30 (2.02–10.59) | 0.005* | 3.19 (−1.63 to 8.03) | 0.187 | −2.58 (−0.80 to 5.97) | 0.130 | 0.37 (−0.65 to 1.40) | 0.463 |
| Year | 0.43 (−0.60 to 1.47) | 0.400 | −0.20 (−1.17 to 0.76) | 0.670 | −0.66 (−1.30 to 0.02) | 0.042* | −0.16 (−0.36 to 0.02) | 0.092 |
| Sample size | 0.01 (−0.01 to 0.02) | 0.160 | −0.01 (−0.01 to 0.01) | 0.740 | −0.01 (0.01 to 0.02) | 0.726 | −0.01 (−0.01 to 0.01) | 0.067 |
| Quality assessment | −2.71 (−10.01 to 6.01) | 0.488 | −3.02 (−12.01 to 3.99) | 0.392 | −1.02 (−9.13 to 8.03) | 0.886 | −2.13 (−3.85 to 1.86) | 0.159 |
*Significance.
Coding of age group: 20–40 years old = 1; 40–60 years old = 2; ≥60 years old = 3.
Coding of WHO Regional Office: AMRO = 1; EMRO = 2; EURO = 3; SERO = 4; WPRO = 5; there is no study in AFRO.
PSC posterior subcapsular.
Publication bias
Based on the results of Begg’s test, significant publication bias was observed for PSC cataract (Z score: 2.67; p: 0.009). Therefore, the fill- and trim-adjusted ASPPE of PSC cataract (2.20%, 95% CI: 1.45–3.20) was generated, which was not significantly different from the original ASPPE (2.24%, 95% CI: 1.41–3.07). The results of the publication bias analyses, based on Begg’s test, indicated no publication bias for any cataract (Z score: −0.41, p: 0.899), cortical cataract (Z score: 1.42, p: 0.091), or nuclear cataract (Z score: 1.23, p: 0.29).
Discussion
Our study is the first meta-analysis that provides comprehensive information on the global prevalence of age-related cataract and its types based on LOCS in different age groups. Accordingly, the ASPPE of cataract was 17.20%. In other words, of every 1000 people who were selected randomly from all over the world, with 95% confidence, we expected to find 133–210 people to have cataract, especially the elderly. This prevalence, which was extracted from all studies without considering aphakic and pseudophakic status, indicates that untreated cataract is still one of the major unsolved ophthalmology problems in the world [63], which affects a large percentage of the global population, and despite being easily treatable [1], no measure has been taken to do so.
Unfortunately, to date, there has been no systematic study on the global prevalence of cataract, and only one study in the United States [25] attempted to estimate the PPE of cataract, with no systematic search strategy, and by integrating data from several large eye cohort studies that applied different lens-grading systems, and any comparison with this study should be done with caution. Accordingly, the PPE of cataract in the mentioned study [25] was 17.20%, which was similar to our study (17.20%). It should be noted that due to the increasing trend of population aging in the world, it is expected that the ASPPE of cataract will increase in the future; although effective surgical methods are available [1], it should receive more attention from health policy makers as one of the reasons for blindness [6].
That is, while people with cataracts usually depend on others for their daily tasks due to poor vision, this is accompanied by a decline in the quality of life [5]. On the other hand, the PPE of any cataract ranged between 3% in the 20- to 39-year age group and 54% in the over-60 age group. The ascending trend of age-related cataract was seen for other types of cataract, including nuclear, cortical, and PSC cataract, and these changes were significant even in the meta-regression analysis (Table 2). In other words, age directly correlated with cataract and its types. An increase in the prevalence of cataract with age has also been observed in other studies [1, 2, 10, 11, 14, 17, 18, 22, 44–46, 53, 62, 64, 65], and is often considered a normal part of the aging process [23]. However, some scholars disagree with this theory and do not consider the relationship between age and cataract a completely causal one. They believe that this is a cumulative effect of certain risk factors [66] such as ultraviolet radiation or oxidative damage [7, 9–14, 16, 23, 65]. The ASPPE of any cataract was significantly different in the six geographical regions; the highest rate was 36.55% in the SEARO region, and the lowest prevalence was 9.08% in the AMRO region. The difference in cataract prevalence in different races and regions has been reported previously [17, 24]. However, it should be noted that the comparison of prevalence rates based on geographical areas should be done with caution, because inter-study differences can be due to different methodologies and diagnostic criteria. Regardless of these issues, many studies consider environmental factors, ethnic and racial differences, and UV radiation to be strong determinants [4, 7, 9, 15, 16, 62, 67–70], such that the high prevalence of cataract in the SEARO region can be attributed to countries being underdeveloped in this region. The prevalence of cataract is higher in lesser-developed societies due to the low economic status [4], low literacy [7, 15, 62, 69, 70], high rate of outdoor activity [62], and less access to cataract surgery services [9, 16]. The low prevalence of cataract in the study that was conducted by integrating studies conducted in developed Western countries [25] confirms this hypothesis. Of course, the role of environmental and other risk factors such as UV radiation [67, 68], high rates of smoking [13], and certain diseases associated with cataract such as diabetes, hypertriglyceridemia [71], and genetic factors [72] should not be overlooked.
Different studies have reported conflicting results regarding the most common type of cataract. Studies in Nigeria [9], Barbados [15], Tanzania [25], Sri Lanka [11], and the United States [71] reported the most common type to be cortical cataract, followed by nuclear and PSC cataract. In contrast, other studies in India [46, 73–75], Australia [76], Taiwan [12], Finland [14], China [77], and Myanmar [10] reported nuclear cataract as the most common type followed by cortical and PSC cataract. This difference was attributed to differences in the prevalence of risk factors such as UV radiation, smoking, and the different prevalence of cataract-related diseases such as pterygium, diabetes, and radiation [9, 24, 78]. However, in our review of 45 studies, the calculated ASPPE showed that the most common type of cataract was nuclear cataract, followed by cortical and PSC cataract, which was in line with some studies [10, 12, 14, 46, 74–77].
According to available evidence on cataract, there is still no agreement on the inter-gender differences. Some studies have suggested that female gender is a risk factor for cataract, and they have attributed this to hormonal changes, especially at older ages, higher exposure to biomass-cooking fuels, lack of access to reproductive health services, and genetic variations [9, 16, 19, 22–24, 66, 73]. Nonetheless, some other studies have reported a reverse trend, and suggested male gender as a risk factor due to higher exposure to UV rays, cigarette smoking, and other known risk factors [16]. The results in our study and the report by Munoz et al. [79] were different in that the overall prevalence of cataract and its types was not much different between the two genders, and the difference was not significant in the meta-regression analysis. In other words, gender does not seem to be related to cataract, and the differences observed in previous studies can be due to selection bias or methodological limitations.
We expected to find a higher cataract prevalence over the past few years on account of changes in lifestyle [80], increased exposure to known risk factors [81], and an increase in the prevalence of diseases associated with cataract such as diabetes [82], and improved diagnostic methods. However, our study did not show such increase, and there was no significant change in the trend of any cataract, nuclear cataract, or cortical cataract, except that the exception was PSC cataract, which showed a very slight decrease during this period. It seems that many patients have managed to treat their cataract due to better access to surgical services, improved surgical procedures, and better distribution of surgical facilities. As such, an ascending trend in cataract surgery, which is one of the goals of the Vision 2020: Right to Sight Initiative [83], has been observed in many countries including Iran [84, 85], Australia [86], America [87], Singapore [88], England [89], and Canada [90].
In light of the numerous exclusion criteria applied in this study, our team was concerned about possible bias; therefore, we examined the reported prevalence of cataract and its types in terms of publication bias. The results indicated that there was no publication bias for any cataract, nuclear cataract, or cortical cataract, and significant publication bias was only observed for PSC cataract. Next, we used the trim-and-fill approach to adjust this bias, and we observed that the publication bias had very little effect (less than 0.04%) on the ASPPE.
Although we made every effort to conduct a flawless study, there were certain limitations that should be mentioned. First, we aimed to include all studies reporting cataract prevalence into the analyses, there were large differences in cataract-grading methods, and since the results could not be converted, we had to exclude many studies. Second, there were very few studies from certain continents, and thus, it was not possible to get a more robust estimate based on WHO region.
However, our study had several strengths, including the fact that it is the first to estimate the prevalence of age-related cataract and its types globally and in each WHO region. The extensive search allowed us to retrieve a large number of articles, and finally 45 studies with a sample size of 161,947 were included in the analyses that support a sufficient statistical power. Moreover, direct standardization was used to estimate the pooled prevalence, and neutralize different age structures, which made comparison possible. We also calculated the cataract prevalence and its types, particularly in different age groups, including the 20- to 39-year age group, which is usually neglected in most of ophthalmologic studies; this is being done for the first time in the past three decades. By including studies that had implemented LOCS, we were able to calculate the pooled cataract prevalence, and this can be the most important strength of our study.
Conclusion
From the public health point of view, cataract is still a global challenge, especially in Western Pacific countries. Despite the lack of inter-gender differences, cataract prevalence increases with age, especially after the age of 60 years. Knowledge about cataract prevalence can inform health-care planners in planning and prioritizing resource allocation.
Acknowledgments
Financial support
This project was supported by Noor Research Center for Ophthalmic Epidemiology.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hashemi H, Hatef E, Fotouhi A, Feizzadeh A, Mohammad K. The prevalence of lens opacities in Tehran: the Tehran Eye Study. Ophthalmic Epidemiol. 2009;16:187–92. doi: 10.1080/09286580902863031. [DOI] [PubMed] [Google Scholar]
- 2.Cedrone C, Culasso F, Cesareo M, Mancino R, Ricci F, Cupo G, et al. Prevalence and incidence of age-related cataract in a population sample from Priverno, Italy. Ophthalmic Epidemiol. 1999;6:95–103. doi: 10.1076/opep.6.2.95.1562. [DOI] [PubMed] [Google Scholar]
- 3.Li T, He T, Tan X, Yang S, Li J, Peng Z, et al. Prevalence of age-related cataract in high-selenium areas of China. Biol Trace Elem Res. 2009;128:1–7. doi: 10.1007/s12011-008-8248-y. [DOI] [PubMed] [Google Scholar]
- 4.Nam GE, Han K, Ha SG, Han BD, Kim DH, Kim YH, et al. Relationship between socioeconomic and lifestyle factors and cataracts in Koreans: the Korea National Health and Nutrition Examination Survey 2008-2011. Eye. 2015;29:913–20. doi: 10.1038/eye.2015.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mangione CM, Phillips RS, Lawrence MG, Seddon JM, Orav EJ, Goldman L. Improved visual function and attenuation of declines in health-related quality of life after cataract extraction. Arch Ophthalmol. 1994;112:1419–25. doi: 10.1001/archopht.1994.01090230033017. [DOI] [PubMed] [Google Scholar]
- 6.Varma R, Torres M. Prevalence of lens opacities in Latinos: the Los Angeles Latino Eye Study. Ophthalmology. 2004;111:1449–56. doi: 10.1016/j.ophtha.2004.01.024. [DOI] [PubMed] [Google Scholar]
- 7.Yu JM, Yang DQ, Wang H, Xu J, Gao Q, Hu LW, et al. Prevalence and risk factors of lens opacities in rural populations living at two different altitudes in China. Int J Ophthalmol. 2016;9:610–6. doi: 10.18240/ijo.2016.04.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flaxman SR, Bourne RR, Resnikoff S, Ackland P, Braithwaite T, Cicinelli MV, et al. Global causes of blindness and distance vision impairment 1990–2020: a systematic review and meta-analysis. Lancet Glob Health. 2017;5:e1221–e34. doi: 10.1016/S2214-109X(17)30393-5. [DOI] [PubMed] [Google Scholar]
- 9.Mahdi AM, Rabiu M, Gilbert C, Sivasubramaniam S, Murthy GVS, Ezelum C, et al. Prevalence and risk factors for lens opacities in Nigeria: results of the National Blindness and Low Vision Survey. Invest. Ophthalmol Vis Sci. 2014;55:2642–51. doi: 10.1167/iovs.12-10303. [DOI] [PubMed] [Google Scholar]
- 10.Athanasiov PA, Casson RJ, Sullivan T, Newland HS, Shein WK, Muecke JS, et al. Cataract in rural Myanmar: prevalence and risk factors from the Meiktila Eye Study. Br J Ophthalmol. 2008;92:1169–74. doi: 10.1136/bjo.2008.139725. [DOI] [PubMed] [Google Scholar]
- 11.Athanasiov PA, Edussuriya K, Senaratne T, Sennanayake S, Sullivan T, Selva D, et al. Cataract in central Sri Lanka: prevalence and risk factors from the Kandy Eye Study. Ophthalmic Epidemiol. 2010;17:34–40. doi: 10.3109/09286580903324900. [DOI] [PubMed] [Google Scholar]
- 12.Cheng C, Liu J, Chen S, Lee F. Population-based study on prevalence and risk factors of age-related cataracts in Peitou, Taiwan. Chin Med J. 2000;63:641–8. [PubMed] [Google Scholar]
- 13.Delcourt C, Cristol JP, Tessier F, Leger CL, Michel F, Papoz L. Risk factors for cortical, nuclear, and posterior subcapsular cataracts: the POLA study. Pathologies Oculaires Liees a l’Age. Am J Epidemiol. 2000;151:497–504. doi: 10.1093/oxfordjournals.aje.a010235. [DOI] [PubMed] [Google Scholar]
- 14.Hirvelä H, Luukinen H, Laatikainen L. Prevalence and risk factors of lens opacities in the elderly in Finland: a population-based study. Ophthalmology. 1995;102:108–17. doi: 10.1016/s0161-6420(95)31072-x. [DOI] [PubMed] [Google Scholar]
- 15.Leske MC, Chylack LT, Wu S-Y. The lens opacities case-control study: risk factors for cataract. Arch Ophthalmol. 1991;109:244–51. doi: 10.1001/archopht.1991.01080020090051. [DOI] [PubMed] [Google Scholar]
- 16.Nirmalan PK, Robin AL, Katz J, Tielsch JM, Thulasiraj RD, Krishnadas R, et al. Risk factors for age related cataract in a rural population of southern India: the Aravind Comprehensive Eye Study. Br J Ophthalmol. 2004;88:989–94. doi: 10.1136/bjo.2003.038380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim TN, Lee JE, Lee EJ, Won JC, Noh JH, Ko KS, et al. Prevalence of and factors associated with lens opacities in a Korean adult population with and without diabetes: the 2008-2009 Korea National Health and Nutrition Examination Survey. PLoS One. 2014;9:e94189. doi: 10.1371/journal.pone.0094189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krishnaiah S, Vilas K, Shamanna BR, Rao GN, Thomas R, Balasubramanian D. Smoking and its association with cataract: results of the Andhra Pradesh eye disease study from India. Invest Ophthalmol Vis Sci. 2005;46:58–65. doi: 10.1167/iovs.04-0089. [DOI] [PubMed] [Google Scholar]
- 19.Östberg A, Löth A, Gustafson D, Lindblom B. Skövde Cataract Study. I. Prevalence of lens opacities in a swedish community. Ophthalmology. 2006;113:970–5. doi: 10.1016/j.ophtha.2006.01.067. [DOI] [PubMed] [Google Scholar]
- 20.Park S, Kim T, Cho SI, Lee EH. Association between cataract and the degree of obesity. Optom Vis Sci. 2013;90:1019–27. doi: 10.1097/OPX.0b013e31829cae62. [DOI] [PubMed] [Google Scholar]
- 21.Park YH, Shin JA, Han K, Yim HW, Lee WC, Park YM. Gender difference in the association of metabolic syndrome and its components with age-related cataract: The Korea National Health and Nutrition Examination Survey 2008-2010. PLoS One. 2014;9:e85068. doi: 10.1371/journal.pone.0085068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paunksnis A, Bojarskiene F, Cimbalas A, Cerniauskiene LR, Luksiene DI, Tamosiunas A. Relation between cataract and metabolic syndrome and its components. Eur J Ophthalmol. 2007;17:605–14. doi: 10.1177/112067210701700420. [DOI] [PubMed] [Google Scholar]
- 23.Richter GM, Torres M, Choudhury F, Azen SP, Varma R. Risk factors for cortical, nuclear, posterior subcapsular, and mixed lens opacities: the Los Angeles Latino Eye Study. Ophthalmology. 2012;119:547–54. doi: 10.1016/j.ophtha.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seah SK, Wong TY, Foster PJ, Ng TP, Johnson GJ. Prevalence of lens opacity in Chinese residents of Singapore: The Tanjong Pagar Survey. Ophthalmology. 2002;109:2058–64. doi: 10.1016/s0161-6420(02)01221-6. [DOI] [PubMed] [Google Scholar]
- 25.Congdon N, Vingerling J, Klein B, West S, Friedman D, Kempen J, et al. Prevalence of cataract and pseudophakia/aphakia among adults in the United States. Arch Ophthalmol. 2004;122:487–94. doi: 10.1001/archopht.122.4.487. [DOI] [PubMed] [Google Scholar]
- 26.Haddaway NR, Collins AM, Coughlin D, Kirk S. The role of Google Scholar in evidence reviews and its applicability to grey literature searching. PLoS One. 2015;10:e0138237. doi: 10.1371/journal.pone.0138237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analysis. 2011. www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 13 Nov 2019.
- 28.Nyaga V, Arbyn M, Aerts M. METAPROP: Stata module to perform fixed and random effects meta-analysis of proportions. 2017. https://EconPapers.repec.org/RePEc:boc:bocode:s457781.
- 29.Ahmad OB, Boschi-Pinto C, Lopez AD, Murray CJ, Lozano R, Inoue M. Age standardization of rates: a new WHO standard. Vol. 9. Geneva: World Health Organ; 2001. p. 1–14.
- 30.Harbord R, Harris RJ, Sterne J, Steichen T. METABIAS: Stata module to test for small-study effects in meta-analysis. 2009. https://EconPapers.repec.org/RePEc:boc:bocode:s404901.
- 31.Worldwide and regional prevalence of age-related cataract: a comprehensive systematic review and meta-analysis of observational studies. International prospective register of systematic reviews (PROSPERO). https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=97105
- 32.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–9. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 33.Bao YZ, Cao XG, Li XX, Chen J, Hu JX, Zhu T. Prevalence of age-related cataract among adults aged 50 and above in four rural areas in Western China. Chin Med J. 2008;88:1697–702. [PubMed] [Google Scholar]
- 34.Guan HJ, Yao Y, Liang CK, Zhu RR, Liu BH, Qi YX, et al. Prevalence and surgical status of cataract among adults aged 50 years or above in rural Jiangsu Province. Chin Med J. 2013;93:330–5. [PubMed] [Google Scholar]
- 35.Hu C. An epidemiologic survey of cataract in Shunyi County, Beijing. Chin J Ophthalmol. 1989;25:360–4. [PubMed] [Google Scholar]
- 36.Jiao WZ, Zhou CC, Wang LH. Prevalence and surgery status of cataract in adults aged 50 years or above in rural Shandong Province. Ophthalmol Chin. 2013;22:234–9. [Google Scholar]
- 37.Luan L, Yao Y, Fu D, Zhu J, Xie T, Yin L, et al. Survey of the cataract prevalence and surgical coverage rate among 50 or above in Wuxi City. Chin J Exp Ophthalmol. 2014;32:551–5. [Google Scholar]
- 38.Peng YS, Zhou AY, Chen L, He Y, Ren BC. Prevalence of age related cataract and blindness in rural areas of Shaanxi province. Int J Ophthalmol. 2007;7:220–3. [Google Scholar]
- 39.Quan YL, Yang JG, Ren BC. Epidemic survey for cataract in Yang County, Shaanxi Province. Int J Ophthalmol. 2006;6:1464–7. [Google Scholar]
- 40.Zhong YH, Lin BJ, Wei DL, Zhang ZH, Xie WX. Epidemiology investigation and intervention strategies of age-related cataract in Zhongshan City. Int Eye Sci. 2012;12:1753–5. [Google Scholar]
- 41.Zhou J, Yuan Y, Zhang X, Yang M, Guan HJ. [Prevalence and surgery status of cataract among adults aged 60 years or above in two villages of Nantong] Chin J Ophthalmol. 2017;53:514–21. doi: 10.3760/cma.j.issn.0412-4081.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 42.Wang H, Sun HP, Wang P, Xu Y, Pan CW. Cataract and depressive symptoms among older chinese adults. Optom Vis Sc. 2016;93:1479–84. doi: 10.1097/OPX.0000000000000960. [DOI] [PubMed] [Google Scholar]
- 43.Cristina Leske M. Prevalence of lens opacities in the Barbados Eye Study. Arch Ophthalmol. 1997;115:105–11. doi: 10.1001/archopht.1997.01100150107018. [DOI] [PubMed] [Google Scholar]
- 44.Giuffrè G, Giammanco R, Di Pace F, Ponte F. Casteldaccia eye study: prevalence of cataract in the adult and elderly population of a Mediterranean town. Int Ophthalmol. 1994;18:363–71. doi: 10.1007/BF00930317. [DOI] [PubMed] [Google Scholar]
- 45.Lee DS, Han K, Kim HA, Lee SY, Park YH, Yim HW, et al. The gender-dependent association between obesity and age-related cataracts in middle-aged Korean adults. PLoS One. 2015;10:e0124262. doi: 10.1371/journal.pone.0124262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nirmalan PK, Krishnadas R, Ramakrishnan R, Thulasiraj RD, Katz J, Tielsch JM, et al. Lens opacities in a rural population of Southern India: The Aravind Comprehensive Eye Study. Invest Ophthalmol Vis Sci. 2003;44:4639–43. doi: 10.1167/iovs.03-0011. [DOI] [PubMed] [Google Scholar]
- 47.Tsai SY, Hsu WM, Cheng CY, Liu JH, Chou P. Epidemiologic study of age-related cataracts among an elderly Chinese population in Shih-Pai, Taiwan. Ophthalmology. 2003;110:1089–95. doi: 10.1016/S0161-6420(03)00243-4. [DOI] [PubMed] [Google Scholar]
- 48.Pan CW, Cheung CY, Aung T, Cheung CM, Zheng YF, Wu RY, et al. Differential associations of myopia with major age-related eye diseases: the Singapore Indian Eye Study. Ophthalmology. 2013;120:284–91. doi: 10.1016/j.ophtha.2012.07.065. [DOI] [PubMed] [Google Scholar]
- 49.Rauf A, Malik R, Bunce C, Wormald R. The British Asian community eye study: outline of results on the prevalence of eye disease in British Asians with origins from the Indian subcontinent. Indian J Ophthalmol. 2013;61:53–8. doi: 10.4103/0301-4738.107191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hashemi H, Khabazkhoob M, Miraftab M, Mohammad K, Fotouhi A. The association between refractive errors and cataract: The Tehran eye study. Middle East Afr J Ophthalmol. 2011;18:154–8. doi: 10.4103/0974-9233.80705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wong TY, Foster PJ, Johnson GJ, Seah SKL. Refractive errors, axial ocular dimensions, and age-related cataracts: The Tanjong Pagar Survey. Invest Ophthalmol Vis Sci. 2003;44:1479–85. doi: 10.1167/iovs.02-0526. [DOI] [PubMed] [Google Scholar]
- 52.Tang Y, Ji Y, Ye X, Wang X, Cai L, Xu J, et al. The association of outdoor activity and age-related cataract in a rural population of Taizhou Eye Study: Phase 1 report. PLoS One. 2015;10:e0135870. doi: 10.1371/journal.pone.0135870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Duan XR, Liang YB, Wang NL, Wong TY, Sun LP, Yang XH, et al. Prevalence and associations of cataract in a rural Chinese adult population: The Handan Eye Study. Graefes Arch Clin Exp Ophthalmol = Albrecht von Graefes Arch fur klinische und experimentelle Ophthalmologie. 2013;251:203–12. doi: 10.1007/s00417-012-2012-x. [DOI] [PubMed] [Google Scholar]
- 54.Carlos GA, Schellini SA, Espíndola RF, Lana FP, Rodrigues AC, Padovani CR. Cataract prevalence in Central-West region of São Paulo State, Brazil. Arq Bras Oftalmol. 2009;72:375–9. doi: 10.1590/s0004-27492009000300018. [DOI] [PubMed] [Google Scholar]
- 55.Germano RAS, Kawai RM, Souza BL, Germano FAS, Germano CS, Germano JE. Frequency of ocular conditions in native Brazilians from Avaí City, São Paulo State. Rev Bras Oftalmol. 2017;76:227–31. [Google Scholar]
- 56.Huang TL, Hsu SY, Tsai RK, Sheu MM. Etiology of ocular diseases in elderly Amis aborigines in Eastern Taiwan (The Amis Eye Study) Jpn J Ophthalmol. 2010;54:266–71. doi: 10.1007/s10384-010-0817-x. [DOI] [PubMed] [Google Scholar]
- 57.Singh S, Pardhan S, Kulothungan V, Swaminathan G, Ravichandran JS, Ganesan S, et al. The prevalence and risk factors for cataract in rural and urban India. Indian J Ophthalmol. 2019;67:477–83. doi: 10.4103/ijo.IJO_1127_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tang Y, Wang X, Wang J, Huang W, Gao Y, Luo Y, et al. Prevalence of age-related cataract and cataract surgery in a Chinese adult population: The Taizhou Eye Study. Invest Ophthalmol Vis Sci. 2016;57:1193–200. doi: 10.1167/iovs.15-18380. [DOI] [PubMed] [Google Scholar]
- 59.Bojarskiene F, Paunksnis A. Prevalence of cataract in 35-64 years old Kaunas city population. Medicine (Kaunas, Lith) 2005;41:774–80. [PubMed] [Google Scholar]
- 60.Xu J, Yu Q, Zhu S, Liu Q. A population-based study of lens opactities. Eye Sci. 1996;12:115–7. [PubMed] [Google Scholar]
- 61.Yoshikawa T, Obayashi K, Miyata K, Nishi T, Ueda T, Kurumatani N, et al. Diminished circadian blood pressure variability in elderly individuals with nuclear cataracts: cross-sectional analysis in the HEIJO-KYO cohort. Hypertens Res. 2019;42:204–10. doi: 10.1038/s41440-018-0140-3. [DOI] [PubMed] [Google Scholar]
- 62.Husain R, Tong L, Fong A, Cheng JF, How A, Chua WH, et al. Prevalence of cataract in rural Indonesia. Ophthalmology. 2005;112:1255–62. doi: 10.1016/j.ophtha.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 63.Gillies M, Brian G, La Nauze J, Le Mesurier R, Moran D, Taylor H, et al. Modern surgery for global cataract blindness: preliminary considerations. Arch Ophthalmol. 1998;116:90–2. doi: 10.1001/archopht.116.1.90. [DOI] [PubMed] [Google Scholar]
- 64.Hashemi H, Khabazkhoob M, Nabovati P, Ostadimoghaddam H, Shafaee S, Doostdar A, et al. The prevalence of age-related eye disease in an elderly population. Ophthalmic Epidemiol. 2017;24:222–8. doi: 10.1080/09286586.2016.1270335. [DOI] [PubMed] [Google Scholar]
- 65.Rim THT, Kim MH, Kim WC, Kim TI, Kim EK. Cataract subtype risk factors identified from the Korea National Health and Nutrition Examination survey 2008-2010. BMC Ophthalmol. 2014;14:1–15. doi: 10.1186/1471-2415-14-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McCarty C, Taylor H. A review of the epidemiologic evidence linking ultraviolet radiation and cataracts. Dev Ophthalmol. 2002;35:21–31. doi: 10.1159/000060807. [DOI] [PubMed] [Google Scholar]
- 67.Taylor HR, West SK, Rosenthal FS, Muñoz B, Newland HS, Abbey H, et al. Effect of ultraviolet radiation on cataract formation. N. Engl J Med. 1988;319:1429–33. doi: 10.1056/NEJM198812013192201. [DOI] [PubMed] [Google Scholar]
- 68.Pasquale LR, Jiwani AZ, Zehavi-Dorin T, Majd A, Rhee DJ, Chen T, et al. Solar exposure and residential geographic history in relation to exfoliation syndrome in the United States and Israel. AMA Ophthalmol. 2014;132:1439–45. doi: 10.1001/jamaophthalmol.2014.3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mohan M, Sperduto RD, Angra SK, Milton RC, Mathur RL, Underwood BA, et al. India-US case-control study of age-related cataracts. Arch Ophthalmol. 1989;107:670–6. doi: 10.1001/archopht.1989.01070010688028. [DOI] [PubMed] [Google Scholar]
- 70.Attebo K, Mitchell P, Cumming R, BMath WS. Knowledge and beliefs about common eye diseases. Aust NZ J Ophthalmol. 1997;25:283–7. doi: 10.1111/j.1442-9071.1997.tb01516.x. [DOI] [PubMed] [Google Scholar]
- 71.Stocks N, Patel R, Sparrow J, Davey-Smith G. Prevalence of cataract in the Speedwell Cardiovascular Study: a cross-sectional survey of men aged 65-83. Eye. 2002;16:275–80. doi: 10.1038/sj.eye.6700106. [DOI] [PubMed] [Google Scholar]
- 72.Hammond CJ, Duncan DD, Snieder H, de Lange M, West SK, Spector TD, et al. The heritability of age-related cortical cataract: the twin eye study. Invest Ophthalmol Vis Sci. 2001;42:601–5. [PubMed] [Google Scholar]
- 73.Vashist P, Talwar B, Gogoi M, Maraini G, Camparini M, Ravindran RD, et al. Prevalence of cataract in an older population in India: the India study of age-related eye disease. Ophthalmology. 2011;118:272–8 e1-2. doi: 10.1016/j.ophtha.2010.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Murthy GV, Gupta SK, Maraini G, Camparini M, Price GM, Dherani M, et al. Prevalence of lens opacities in North India: the INDEYE feasibility study. Invest Ophthalmol Vis Sci. 2007;48:88–95. doi: 10.1167/iovs.06-0284. [DOI] [PubMed] [Google Scholar]
- 75.Srinivasan M, Rahmathullah R, Blair CR, Murphy AC, Beck RW, Wilkins JH, et al. Cataract progression in India. Br J Ophthalmol. 1997;81:896–900. doi: 10.1136/bjo.81.10.896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Landers J, Henderson T, Craig J. Prevalence and associations of refractive error in indigenous Australians within central Australia: the Central Australian Ocular Health Study. Clin Exp Ophthalmol. 2010;38:381–6. doi: 10.1111/j.1442-9071.2010.02258.x. [DOI] [PubMed] [Google Scholar]
- 77.Xu L, Cui T, Zhang S, Sun B, Zheng Y, Hu A, et al. Prevalence and risk factors of lens opacities in urban and rural Chinese in Beijing. Ophthalmology. 2006;113:747–55. doi: 10.1016/j.ophtha.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 78.West S. Ocular ultraviolet B exposure and lens opacities: a review. J Epidemiol. 1999;9:97–101. doi: 10.2188/jea.9.6sup_97. [DOI] [PubMed] [Google Scholar]
- 79.Munoz B, West SK, Rubin GS, Schein OD, Quigley HA, Bressler SB, et al. Causes of blindness and visual impairment in a population of older Americans: The Salisbury Eye Evaluation Study. Arch Ophthalmol. 2000;118:819–25. doi: 10.1001/archopht.118.6.819. [DOI] [PubMed] [Google Scholar]
- 80.Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006;367:1747–57. doi: 10.1016/S0140-6736(06)68770-9. [DOI] [PubMed] [Google Scholar]
- 81.Molarius A, Parsons RW, Dobson AJ, Evans A, Fortmann SP, Jamrozik K, et al. Trends in cigarette smoking in 36 populations from the early 1980s to the mid-1990s: findings from the WHO MONICA Project. Am J Public Health. 2001;91:206–12. doi: 10.2105/ajph.91.2.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Komlos J, Brabec M. The trend of BMI values of US adults by deciles, birth cohorts 1882–1986 stratified by gender and ethnicity. Econ Hum Biol. 2011;9:234–50. doi: 10.1016/j.ehb.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 83.Foster A. Cataract and “Vision 2020—the right to sight” initiative. Br J Ophthalmol. 2001;85:635–7. doi: 10.1136/bjo.85.6.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hashemi H, Alipour F, Mehravaran S, Rezvan F, Fotouhi A, Alaedini F. Five year cataract surgical rate in Iran. Optom Vis Sci. 2009;86:890–4. doi: 10.1097/OPX.0b013e3181ae1cc6. [DOI] [PubMed] [Google Scholar]
- 85.Hashemi H, Fotouhi A, Rezvan F, Etemad K, Gilasi H, Asgari S, et al. Cataract surgical rate in Iran: 2006 to 2010. Optom Vis Sci. 2014;91:1355–9. doi: 10.1097/OPX.0000000000000389. [DOI] [PubMed] [Google Scholar]
- 86.Keeffe JE, Taylor HR. Cataract surgery in Australia 1985–94. Aust N Z J Ophthalmol. 1996;24:313–7. doi: 10.1111/j.1442-9071.1996.tb01601.x. [DOI] [PubMed] [Google Scholar]
- 87.Lansingh VC, Resnikoff S, Tingley-Kelley K, Nano ME, Martens M, Silva JC, et al. Cataract surgery rates in Latin America: a four-year longitudinal study of 19 countries. Ophthalmic Epidemiol. 2010;17:75–81. doi: 10.3109/09286581003624962. [DOI] [PubMed] [Google Scholar]
- 88.Wong TY. Cataract extraction rates among Chinese, Malays, and Indians in Singapore: a population-based analysis. Arch Ophthalmol. 2001;119:727–32. doi: 10.1001/archopht.119.5.727. [DOI] [PubMed] [Google Scholar]
- 89.Keenan TD, Salmon JF, Yeates D, Goldacre M. Trends in rates of primary angle closure glaucoma and cataract surgery in England from 1968 to 2004. J Glaucoma. 2009;18:201–5. doi: 10.1097/IJG.0b013e318181540a. [DOI] [PubMed] [Google Scholar]
- 90.Hatch WV, Campbell EdL, Bell CM, El-Defrawy SR, Campbell RJ. Projecting the growth of cataract surgery during the next 25 years. Arch Ophthalmol. 2012;130:1479–81. doi: 10.1001/archophthalmol.2012.838. [DOI] [PubMed] [Google Scholar]