Abstract
One of the pathways to reduce cholesterol production in the liver is through the inhibition of HMG-Coa reductase (HMGCR) by current drugs, statins. However, these have side effects if consumed in prolonged periods. Tangeretin and trans-ethyl caffeate as alternative drugs in reducing hypercholesterolemia and preventing atherosclerosis have never been reported. Their effects on inhibiting HMGCR activity were investigated through enzymatic method (in vitro and in vivo). The toxicity property was analyzed on the Serum Glutamate Oxalate Transaminase (SGOT)/Serum Glutamate Piruvate Transaminase (SGPT) levels and rat liver histology. The results showed that both compounds inhibited HMGCR activity significantly compare to the control simvastatin (p < 0.05). Tangeretin which showed very good activity in inhibiting HMGCR (83.8 of % inhibition, equal to simvastatin) was selected and used for anti-hypercholesterolemia in vivo assessment. Furthermore, tangeretin was shown to effectively reduced Total Cholesterol (TC) and Low Density Lipoprotein (LDL), and increased High Density Lipoprotein (HDL) levels significantly compared to the simvastatin group (p < 0.05). Tangeretin group was also proven to inhibit HMGCR rat liver activity significantly compare to the control simvastatin (p < 0.05). The toxicity study on the SGOT/SGPT levels and liver histology revealed that there were no side effects after administration by tangeretin. Results found that both tangeretin and trans-ethyl caffeate are potent candidates as anti-hypercholesterolemia agent in vitro. In addition, tangeretin was also shown to be safe and suitable as an alternative treatment for controlling hypercholesterolemia in vivo as well as have potency for preventing atherosclerosis.
Keywords: Tangeretin, Trans-ethyl caffeate, HMG-CoA reductase, Hypercholesterolemia
1. Introduction
Hypercholesterolemia condition is a high-risk factor for coronary heart disease (CHD) that may predispose to atherosclerosis because of narrowing and hardening of the arterial wall by cholesterol. Atherosclerosis can trigger cardiovascular disease (CVD) which is the main cause of death worldwide (WHO, 2011). This condition is an essential reason for conducting studies related to alternative medicines from natural resources to reduce plasma cholesterol levels. One of the pathways for lowering cholesterol levels is through the inhibition of HMGCR enzyme-like statin drugs. Nowadays, a widely-used treatment to reduce high cholesterol levels utilizes statins (3-hydroxy-3-methylglutaryl). It is a HMGCR inhibitor drug which is used to decrease cholesterol levels and also to prevent atherosclerosis, but it has some adverse side effects. Therefore, other alternative compounds that are more effective and safer than statin to patients who are resistant to or intolerant of conventional pharmacotherapy is required. One of the promising and underutilized sources is Pandan laut (Pandanus tectorius) fruits, a coastal plant, as an alternative natural HMGCR inhibitor (Andriani et al., 2019). Anti-hypercholesterolemia potency trials of P. tectorius fruits extract rich in tangeretin, ethyl caffeate (also known as trans-ethyl caffeate), and coffeic guinic acid has been carried out by Zhang et al (2012). Besides, Andriani et al. (2019) also reported that tangeretin and ethyl caffeate contents in methanol extract of the P. tectorius fruits could be correlated to their potency as anti-hypercholesterolemia and anti-atherosclerosis activity. Thus, two compounds, namely tangeretin and ethyl caffeate were selected in our study to investigate their strength in reducing cholesterol via HMGCR activity.
The HMGCR is a crucial enzyme for the cholesterol biosynthetic pathway in the liver. The activity of the HMGCR can be modulated by HDL particles. To reduce the risk of atherosclerosis, the HDL particles function through several pathways such as SR-B1 (Li et al., 2016). Extracts or compounds which are potent in increasing the scavenger receptor class B type I (SR-B1) gene (HDL receptor) expression could also be influential in inhibiting HMGCR action in the cholesterol biosynthetic pathway. Our screening study showed that tangeretin and ethyl caffeate (Fig. 1a and b), two bioactive compounds found in P. tectorius fruits are highly potent increasing the SR-B1 gene expression (data not shown). The HMGCR enzymes are abundant in the liver cells, hence the cytotoxicity property of both compounds on HepG2 cells were important to be known first before they used for further investigation. Cytotoxicity study of the tangeretin and ethyl caffeate on HepG2 cells has been studied (Andriani et al., 2019). They stated that both compounds did not show any cytotoxic activity against HepG2 cells compared to the negative control and drug (simvastatin).
Fig. 1.

Chemical structure of tangeretin (a) and ethyl caffeate (b) (Kuroskwa and Manthey, 2004, PubChem, 2005).
It is well known that tangeretin is a citrus flavonoid and have been reported to have anticancer and antitumor activities on Human mammary cells (Depypere et al., 2000), human breast and colon cancer cells, leukaemia cancer cells HL60 (Morley et al., 2007) and human gastric cancer AGS (Dong et al., 2014). On the other hand, ethyl caffeate was reported to have anti-cancer activity against human ovarian cancer SKOV-3 cells (Lee et al., 2014), human skin cancer (Lim et al., 2014) and anti-inflammatory activity (Chiang et al., 2005). Tangeretin has potency in anti-hypercholesterolemia activity (Kuroskwa and Manthey, 2004). However there is not much research on this aspect. High cholesterol level in hypercholesterolemia condition is an essential factor causing atherosclerosis (Andriani et al., 2019). Compounds with anti-hypercholesterolemia activity have potential activity in preventing atherosclerosis.
The potency of tangeretin and ethyl caffeate compounds as anti-hypercholesterolemia as well as anti-atherosclerosis agents via the mechanism of HMGCR inhibition has never been investigated. Hence, this study was conducted to determine their potency in inhibiting the activity of HMGCR by enzymatic analysis. To support this potency, the effects of tangeretin (it was selected since it has been shown to have a good ability to inhibit HMGCR in vitro) on plasma cholesterol levels in the rats fed by a high cholesterol diet, and its mechanism of action in inhibiting of HMGCR rat liver activity were explored in this study. Subsequently, the safety property observed via analysis of SGOT/SGPT blood levels were examined, and histological observation of the rat liver conducted.
2. Material and methods
All of organic solvent and other reagents were of analytical grade and purchased from Sigma aldric. While, the rats were male Spraque dawley purchased from Takrif Bistari Enterprise, Kuala Lumpur, Malaysia.
2.1. HMGCR enzymatic assay (In Vitro)
This assay was conducted to determine the inhibitory effects of trans-ethyl caffeate and tangeretin on HMGCR activity by comparing to pravastatin (kit inhibitor, 100 µM) and simvastatin (commercial inhibitor, 200 µg/mL) as references. HMGCR in vitro assay kit (Sigma Aldrich) was used for detecting the activity of HMGCR enzyme, especially to screen for different inhibitors of this enzyme, which might play a crucial role in therapeutics. This assay was based on the spectrophotometric measurement of absorbance at 340 nm. Reducing absorbance represented the oxidation of NADPH by the catalytic subunit of HMGCR in the presence of the substrate HMG-CoA. In the assay, trans-ethyl caffeate and tangeretin were tested at three concentrations; 400, 200, and 100 µg/mL. Concentrations of samples were chosen according to the concentration of tangeretin (Vanhoecke et al., 2005) with some modifications because tangeretin would be used for further investigation (in vivo study). Based on Vanhoecke et al. (2005), the daily intake of tangeretin by a single mouse for oral administration was 0.22 mg (~200 µg/mL). Simvastatin was also tested as a controlled drug at a concentration of 200 µg/mL. The reaction assays were prepared by mixing HMGCR, HMG-CoA, and 1X assay buffer in the presence or absence of extracts/compounds/control drugs as shown in Table 1.
Table 1.
The volume of constituents of a reaction mixture for each well in a 96-well plate. Samples were ethyl caffeate, tangeretin, with controlled drug (simvastatin) as reference.
| 1x Assay buffer | Control drug | Sample | NADPH | HMG-CoA | HMGCR | |
|---|---|---|---|---|---|---|
| Blank | 184 µL | – | – | 4 µL | 12 µL | 2 µL |
| Activity | 182 µL | – | – | 4 µL | 12 µL | 2 µL |
| Inhibition | 181 µL | 1 µL | – | 4 µL | 12 µL | 2 µL |
| Sample | 181 µL | – | 1 µL | 4 µL | 12 µL | 2 µL |
After all samples (at various concentrations) were included in reactions into a 96-well plate, the samples were thoroughly mixed in the wells. The activity of HMGCR enzyme was then determined by reading the colour produced by the reaction using ELISA reader in the kinetics program. The A340 reduced due to the decrease in NADPH concentration. The inhibition of HMGCR was calculated using the equation below as described by the manufacturer.
| (1) |
where
12.44= - the extinction coefficient for NADPH at 340 nm is 6.22
12.44 represents the 2 NADPH consumed in the reaction.
TV = Total volume of the reaction in mL (0.2 mL for plates)
V = Volume of enzyme used in the assay (mL)
0.6 = Enzyme concentration in mg-protein (mgP)/mL (0.50–0.70 mgP/mL)
LP = Light path in cm (0.55 for plates).
2.2. In vivo study: The anti-hypercholesterolemia properties on plasma cholesterol and the inhibitory effect of tangeretin on HMGCR rat liver
These series of studies were based on an experimental animal model to determine the total cholesterol, LDL, and HDL levels, SGOT and SGPT levels in the blood plasma, to assess HMGCR in vivo activity in the liver, and to analyze the liver histology on hypercholesterolemia-induced rat. The samples used for treatment on rats were tangeretin and simvastatin. The experiment was carried out in the Laboratory of Animal House at the Institute of Marine Biotechnology, Universiti Malaysia Terengganu from July to August 2018. All necessary permits were obtained for the described field studies. The animal study was approved by the Ethics Committee of Universiti Malaysia Terengganu with the number of UMT/RMIC/2-2/1/23 (59) and in accordance to the National Institute of Health Regulation for the care and use of animals in this research. All efforts were made to minimize animal suffering
The chemical reagents in this experiment were cholesterol (Nacalai Tesque), cholic acid (Nacalai Tesque), 0.9% NaCl solution, kethamil, xylazine, dolethal (200 mg/L solution of pentobarbitone sodium), tangeretin (Sigma Aldrich), simvastatin, distilled water, a standard diet of the rats (Gold Coin), and vegetable oil. The instruments of this experiment were 27G × ½ (0.40 × 13 mm) needles (Terumo), 1 mL syringes (Terumo), alcohol swabs (BD), tissues, ball-tipped gavage needles of size 16 × 3″ (HARVARDTM, U.S.A), rat cages (including drinking bottles and pallet plates), the bedding, a knife, an oven, a blender, a mixer, scalpel blades, forceps, surgical scissors, microcentrifuge tubes (Eppendorf), and a centrifuge (Eppendorf).
2.2.1. Experimental animal method
The rats were male Spraque dawley purchased from Takrif Bistari Enterprise, Kuala Lumpur, Malaysia. The rats used were approximately 6–8 weeks old, healthy body, and weighing ~150–200 g. The animals were placed in a clean-grade animal room at 20–24 °C temperature and controlled humidity. The light and dark cycles were 12 h. The rats were fed a standard diet and water made available ad libitum for this experiment. The number of animals used was 32 male rats (Sprague dawley). Detail of rat groups was shown in Table 2. The rats were divided into 4 groups (n = 8 rats/group) namely group A, B, C, and D for the animal experimental model during the 42 days (six weeks) experimental duration (Figs. 2 and 3).
Table 2.
Detail of rat groups for 42 experimental days (n = 8 rats/group).
| Group | Detail of Rat Groups |
|---|---|
| A | The rats were fed with a standard diet for 28 days as well as in the following 14 days of the recovery period. Group A was a negative control. |
| B | The rats were fed with a high cholesterol diet for 28 days and a standard diet for 14 days of the recovery period. Group B was a positive control. |
| C | The rats were fed with a high cholesterol diet for 14 days, treated with simvastatin (10 mg/Kg body weight) as a treatment for 14 days, and fed by a standard diet for 14 days of the recovery period. Group C was a controlled drug |
| D | The rats were fed with a high cholesterol diet for 14 days, treated with tangeretin (7 mg/Kg body weight) as a treatment for 14 days, and fed with a standard diet for 14 days of the recovery period. Group D was a treatment sample. |
Fig. 2.
Animal treatment design.
Fig. 3.
Animal treatment schedule.
The experimental animals were fed a dose of the food and drug according to the OECD guidelines 425 (OECD, 2008). A dose of simvastatin (10 mg/Kg body weight) and tangeretin (7 mg/Kg body weight) was used based on Vanhoecke et al., 2005, Chan et al., 2018, respectively in C and D. The high cholesterol diet was made at the Fish Diet Laboratory in PPSPA, Universiti Malaysia Terengganu. The cholesterol-enriched food was made by combining cholesterol 1% (w/w), cholic acid 0.1% (w/w), vegetable oil 8.5%, and standard food until 100% (modified by Andriani et al., 2015, Gilat et al., 2003, AlSharari et al., 2016). All ingredients were mixed and made into pellets, which were dried in an oven at 50 °C overnight. A high cholesterol diet was given to each rat at 50 g per day starting from day 1 to day 14 (group C and group D) and on day 1 to day 28 (group B). The rats had free access to water ad libitum during this intervention. After feeding a high cholesterol diet (for 14 days), the hypercholesterolemic rats were treated by simvastatin for group C and by tangeretin for group D starting from day 15 to day 28 (for 14 days). Group C and D were given simvastatin and tangeretin, respectively once daily by oral administration using sterile ball-tipped gavage needle.
The food consumption of the rats was recorded every day and calculated every week during 6 weeks (42 days). The bodyweight of the rats was measured every week at day 0 (baseline), 7, 14, 21, 28, 35, and 42, and determination of total cholesterol, LDL, and HDL levels, and also SGOT/SGPT activities was done on the blood samples. Their blood was withdrawn for the blood plasma analysis once a week for 42 experimental days from the tail vein using a sterile needle. After the treatment, the rats were closely observed in the first four hours to assess any adverse side effect symptoms such as abnormal behaviour, abnormal posture, blood in urine, and increasing heartbeat caused by tangeretin or simvastatin. Only half of the rats (n = 4) in each group were analyzed for abnormalities and toxicological sign via histological examination. Afterwards, the other half, the surviving rats (n = 4) were returned to their cage from the outside and kept for 14 days for an observation period which is known as the washout or recovery period. The rats were given a standard diet and water ad libitum freely accessed for this period. At the end of the whole treatment (after 42 days), all rats were sacrificed to obtain the liver for further tests. The abnormalities and toxicological signs of the rat liver were analyzed via histological observation. The rat liver was further analyzed for fatty liver progression. The protocol for the histological preparation and processing was carried out according to Zhang (2008) with some modifications. The sample section for the toxicity and challenge study was examined under a light microscope for any effects and changes and photomicrograph was taken using an image analyzer microscope (Leica) at 20x magnification.
2.2.2. Oral feeding administration technique for chemical delivery
Hypercholesterolemia-induced rats were were injected with simvastatin (for group C) or tangeretin (for group D) once daily by oral administration using a sterile ball-tipped gavage needles for 14 days. Each selected rat for the treatment was restrained with its shoulders locked up with the thumb and the index finger, and lower limbs supported by the right hand. The rat was then left to lie down on the left palm. After the rat became calm, a sterile ball-tipped feeding needle was used to administer samples mixed with distilled water as a substance vehicle. The feeding needle was introduced into the rat from the pharynx into the oesophagus when the rat would be in the act of swallowing. Smooth entering of feeding needle indicated the correct oesophagus pathway. Samples were immediately injected into the rat’s throat when the entire feeding needle entered. After the oral administration, the rats were returned to their cage, and any adverse symptoms such as passive or active behaviour and breathing difficulty observed at the first four hours due to the forced simvastatin and tangeretin. The rats had free access to water ad libitum and regular diet during this treatment.
2.2.3. Anesthesia and euthanasia methods
The animals were individually housed in cages under a 12-h light and 12-h dark cycle at a temperature-controlled room (25–27 °C). Prior to the experiments, the rats were allowed free access to the standard laboratory animal diet with water ad libitum for a week in order to adapt to the laboratory condition. Blood was withdrawn every week from each tail vein for determining the antibody production of the rats. A gauge needle was used to collect the blood. All rats were anaesthesised via intramuscular injection utilizing a mixture of ketamine-xylazine-distilled water with a volume of 1 mL (0.1 mL ketamine-xylazine in 8.9 mL distilled water, respectively). The rat liver were collected for HMGCR in vivo assay and histological observation on day 35 and day 42 of the experiment. The rats were killed by euthanasia method using dolethal at a dose of 1 mL/Kg bodyweight based on the protocol in the laboratory. The animals were killed by piercing into the heart using a sharp needle. The rats were then observed for such signs as eye colour turning from red into white, no heartbeat, and no whisker movement. These conditions indicated that the rats had died.
2.2.4. Blood plasma collection method
Blood sample was collected in a microcentrifuge tube until a minimum volume of 0.5 mL was obtained. After harvesting, the whole blood was allowed to clot by leaving it standing at room temperature for 10 min. Subsequently, the blood clot was removed by centrifuging at 3000x g at 4 °C for 10 min in a microcentrifuge. The resulting supernatant was designated the serum. The serum was placed into a clean tube for the blood plasma analyses and maintained at 2–8 °C while handling. The serum was apportioned into 0.5 mL aliquots and stored at −20 °C until use for blood plasma assays.
2.2.5. Blood plasma assay
In this part of the study, blood plasma analyses were carried out for total, LDL, HDL cholesterol levels, and SGOT/SGPT activity. Measurement of the blood plasma analyses was conducted in BP Clinical Lab, Kuala Terengganu, Malaysia, according to the standard protocol in the laboratory.
-
•
Total cholesterol assay
This analysis was carried out to determine total cholesterol levels in the blood after the rats had been fed a high cholesterol diet and treated with simvastatin (commercial inhibitor) or tangeretin. The analysis was conducted using an enzymatic-colourimetric method. Briefly, 10 µL of blood serum and 1000 μL of reagent were added into a reaction tube. All contents were mixed and incubated at room temperature for 10 min. This resulted in the production of free cholesterol and fatty acids by CHE (cholesterol esterase). Subsequently, the cholesterol oxidized into cholest-4-ene-3-one and hydrogen peroxide in the reaction catalyzed by CHOD (cholesterol oxidase). Hydrogen peroxide combined with HBA (hydroxybenzoic acid) and 4-aminoantipyrine form a chromophore (quinonemine dye) which could be quantitated at a 500 nm wavelength using a spectrophotometer. The measurement of the cholesterol standard is based on the reaction between 10 μL of cholesterol and 1000 μL of reagent that results in the absorbance at 500 nm. In order to construct a standard curve, 10 µL of a serially diluted cholesterol standard (0; 40; 80; 100; 120; 160; 200 mg/dL) were mixed with 1000 μL of reagent and subjected to spectrophotometric reading. A 1000 μL volume of reagent was used as a blank. The concentration of cholesterol from plasma samples was determined by the standard curve.
-
•
LDL cholesterol assay
This analysis was to determine LDL levels in the rat blood after feeding a high cholesterol diet followed by treatment with simvastatin or tangeretin. LDL levels of the intervention groups were compared to the normal group. This assay used the enzymatic method and the determination of LDL levels were calculated from the HDL assay as described by BP Clinical Lab using the standard protocol.
-
•
HDL cholesterol assay
This analysis was conducted to determine HDL cholesterol levels in the rat blood after hypercholesterolemia-induced rats had been treated using simvastatin or tangeretin. The analysis was also to compare HDL cholesterol levels of intervention groups to the normal group. This assay was conducted using the enzymatic method by eliminating CM (chylomicron), LDL, and VLDL (very low density lipoprotein) by CHE, CHOD, and catalase, followed by specific reaction of HDL cholesterol after the release of HDL cholesterol. Briefly, 10 µL of blood serum and the 100 µL of reagent was added into a reaction tube. All contents were mixed well and incubated at room temperature for 10 min. Subsequently, the mixture was read by spectronic 20D (Milton Roy Company) at 500 nm.
-
•
SGOT/SGPT assay
This assay was carried out to investigate the damage level of the rat liver over a period of 42 experimental days. The measurement of SGOT and SGPT levels was carried using NADH reagent using the International Federation of Clinical Chemistry (IFCC) method. The determination of SGOT levels was conducted through a combined mixture of reagent I (contains tris, L-aspartic, MDH, and LDH), and reagent II (contains 2-oxoglutarate and NADH). The determination of SGPT levels was carried out in a combined mixture of reagent I (contains tris, L-alanine, and LDH) and reagent II (contains 2-oxoglutarate and NADH). Reagent I and reagent II for each test were mixed well. Subsequently, 50 µL of serum sample was added to 1000 µL of the working reagent, and the absorbance read at 340 nm against a water blank. Readings of the absorbance were repeated every minute for the next two minutes. The data were calculated using this formula:
| (2) |
2.2.6. HMGCR in vivo assay
-
•
HMGCR in vivo assay
This assay was intended to evaluate the inhibitory effect of tangeretin and simvastatin (as a reference material) on HMGCR in hypercholesterolemia-induced rats. Subsequently, the HMGCR enzyme activity of the liver was determined by colourimetric method as described by Venugopala and Ramakrishnan (1975) based on HMG-CoA/mevalonate ratio, used as an index of the HMGCR activity. HMG-CoA was determined by a reaction with hydroxylamine at pH 5.5 detected by the colourimetric measurement of the resulting hydroxamic acid due to the formation of complexes with ferric salts. The mevalonate content was determined by a reaction with hydroxylamine at pH 2.1, a value to represent the reaction between the lactone form of mevalonate and hydroxylamine to form hydroxamate (Navarro-Gonzàlez et al., 2014). After the treatment, the rat liver was immediately excised and chilled in ice-cold saline to clean the liver of the blood. Subsequently, the liver was blotted, weighed, and stored at −80 °C for further analysis. A liver tissue of approximately 0.5 g was first homogenized with 5 mL of saline arsenate solution. Then equal volumes of 10% tissue homogenate and 5% perchloric acid solution were mixed well, and gently shaken for 5 min at room temperature. The mixture was centrifuged at 2000 rpm for 10 min. A supernatant volume of 2 mL was treated separately in two test tubes with 0.5 mL of freshly prepared hydroxylamine reagent (alkaline hydroxylamine reagent to quantify the content of HMG-CoA and aqueous hydroxylamine hydrochloride reagent to quantify the mevalonate) and gently mixed. After an incubation period of 5 min at room temperature, 5.2 g of TCA and 10 g of ferric chloride was dissolved in 50 mL of 0.65 mol/L HCL and diluted to 100 mL with the latter. Subsequently, the mixture was incubated for 10 min at room temperature. The absorbance was then read at 540 nm on a UV–Vis spectrophotometer. Saline arsenate solution was similarly treated as a blank. The HMGCR activity was evaluated as the ratio between the absorbance readings of the HMG-CoA and mevalonate in the test tube following this formula:
| (3) |
-
•
Western blot
Western blot analysis for HMGCR was done in liver microsomes. Tissue homogenates (10% w/v) were prepared in 20 mM Tris–HCl buffer (pH 7.4) at 4 °C and centrifuged at 3000 × g for 10 min. The supernatants were collected and used in the assay. Protein samples (30 g) from each treatment group were separated on 7.5% SDS-polyacrylamide gel electrophoresis (BIORAD, UK). The separated proteins were electrophoretically transferred to PVDF membrane (Immobilon-P, Millipore, USA). Anti-β-actin (Cell Signaling Technology Inc.) was used as the loading control.
2.2.7. Histological observation
Histological analysis on the rat liver was conducted to evaluate the effect of organ structure after 35 and 42 experimental days. It was also conducted to investigate the toxic effects on the liver using the hematoxyline-eosin staining method. The rats were euthanized by dolethal to dissect out the liver. To confirm that rats had been completely euthanized certain indications were observed; such as eye colour turning from red into white, no heartbeat, and no whisker movement. After collecting, the organ was fixed and soaked into 10% buffered formalin for 48 h.
The preparation of slides began by the fixation of tissue specimens. It was carried out to prevent tissue samples from undergoing autolysis and putrefaction. The organs were excised into cross-sections (not>10 mm of thickness) at different lobes and immersed into 10% buffered formalin for 24 h before proceeding to tissue processing for further histological procedures. After fixation, specimens were transferred to cassettes and labeled. The filled tissue cassettes were then stored in 10% buffered formalin until processing begins. Tissue processing into microscopic sections was carried out using a paraffin block. This section was conducted using a tissue processor machine. The process involved dehydration through a graded alcohol series (from 5% to 100%) to remove the water and formalin from the tissue, clearing in toluene to remove the alcohol, allowing infiltration by paraffin wax, and impregnation. Dehydrated tissues were embedded in plastic cassettes using paraffin wax in an embedding machine. Before sectioning, the blocks were trimmed to remove excessive paraffin wax. Each sample was sectioned into 4 µm thickness by a rotary microtome machine. The samples were then immersed into a water bath at 38 °C and floated for 3 min. Floated samples were fished out and fixed on clean glass slides coated with Mayers’s albumin as a tissue adhesive. Subsequently, the slides were dried in the oven at 38 °C for 24 h. The rat liver was stained using Harris haematoxylin and eosin to clarify the tissue structures and for easier evaluation. The specimen slides were placed in a staining jar, deparaffinized by submerging into two series of absolute xylene for 5 min and followed by 100%, 90%, 80%, 70%, and 50% alcohol for 3 min at each concentration. Then, the slides were submerged into the haematoxylin solution for 15 min. Subsequently, the slides were washed under running tap water for 10 min, differentiated with 1% acid alcohol for 3 to 5 dips, and washed under running tap water for 3 to 5 min. The slides were soaked into the eosin solution for 3 s. The slides were re-submerged into an alcohol series followed by 95% and 100% alcohol for 3 min and then re-soaked into a series of xylene for 5 min. Subsequently, the slides were mounted with DPX and left to dry overnight. The tissue specimens on the slides were observed under a light microscope and evaluated for toxicity effects. The photomicrograph of each sample was recorded using an image analyzer microscope at 20x. The specimens were analyzed for the shape of hepatocytes and the presence of fatty degradation in the liver cells. The liver cells of the intervention groups were compared to the normal group.
2.3. Statistical analysis
The data obtained from this study were statistically analyzed using SPSS software version 20. The analytical measurements of the blood plasma and the liver were carried out in triplicates and expressed as mean ± SD (standard deviation). The determination of significant differences for all analyzed parameters was performed by one-way ANOVA (analysis of variance) and followed by Duncan or LSD tests at a significant level of (α = 0.05). The results are deemed statistically significant if the p-value was 0.05 or less among the groups.
3. Results
3.1. The in vitro HMGCR inhibitory effect of tangeretin and ethyl caffeate compounds
The study was carried out to assess the effects of trans-ethyl caffeate and tangeretin on HMGCR activity using in vitro cell-free assay. This assay was the first step to determine the inhibitory effect of samples on HMGCR activity before in vivo study using rat models. The activity of HMGCR after the inclusion of samples in the reaction is shown in Fig. 4. This figure shows HMGCR activity after treatment with trans-ethyl caffeate and tangeretin at concentration of 100 to 400 µg/mL. The graph exhibited that trans-ethyl caffeate (100 µg/mL) and tangeretin (400 µg/mL) produced low HMGCR activity. The percentage inhibition of HMGCR activity by P. tectorius fruit extracts as compared to untreated control is shown in Table 3. The table shows that trans-ethyl caffeate (100 µg/mL) and tangeretin (400 µg/mL) produced the highest % inhibition compared to other concentrations of the respective compounds, similar to % inhibition of pravastatin and simvastatin (reference controlled drugs). Overall, trans-ethyl caffeate and tangeretin used in this study exhibited significant (p < 0.05) inhibition activity on HMGCR compared to the control (the untreated HMGCR) which indicated all samples have the potential to be categorized as an inhibitor of HMGCR via a similar mechanism of action on pravastatin and simvastatin.
Fig. 4.
Enzyme activity of HMGCR (units/mgP) after treating by samples: pravastatin (a kit inhibitor, 100 µM), simvastatin (a controlled drug, 200 µg/mL), tangeretin and trans-ethyl caffeate (treatment samples) at concentrations of 400, 200, and 100 µg/mL. Data expressed as mean as ± SD. The different word on the graph shows the different significance at the 0.05 level.
Table 3.
Inhibition activity of compounds against the HMGCR enzyme (%).
| Sample | % Inhibition |
|---|---|
| Enzyme | 0 |
| Simvastatin | 86.5 |
| Tangeretin at concentration of 400 µg/mL | 83.8 |
| Tangeretin at concentration of 200 µg/mL | 81.1 |
| Tangeretin at concentration of 100 µg/mL | 70.2 |
| Trans-ethyl caffeate at concentration of 400 µg/mL | 81.1 |
| Trans-ethyl caffeate at concentration of 200 µg/mL | 86.5 |
| Trans-ethyl caffeate at concentration of 100 µg/mL | 94.6 |
3.2. In vivo study: Anti-hypercholesterolemia properties of tangeretin
These experiments were conducted to determine the efficacy and safety of tangeretin treatment on hypercholesterolemia-induced rats and to investigate the mechanism of HMGCR inhibition by the compound via in vivo study. Data were collected from 32 Sprague dawley male rats subject to four different treatments during 42 experimental days. The blood was tested at the beginning of the experiment as a baseline and subsequently, rats were tested every week. The intervention was conducted to determine total cholesterol, LDL, HDL, SGPT, and SGOT levels. The diet food intake and the body weight of rats were also recorded. All investigations were recorded and analyzed every week (7 days).
3.2.1. Blood plasma analysis
The efficacy of simvastatin (10 mg/Kg body weight) and tangeretin (7 mg/Kg body weight) was elucidated in high cholesterol diet-induced hypercholesterolemia rats after 42 days of the intervention. Blood serum was tested every week during the experimental period to collect the blood serum. Before conducting this analysis, the rats have fasted for 12 h. The serum was collected to analyze the plasma cholesterol levels and SGOT/SGPT activity.
-
•
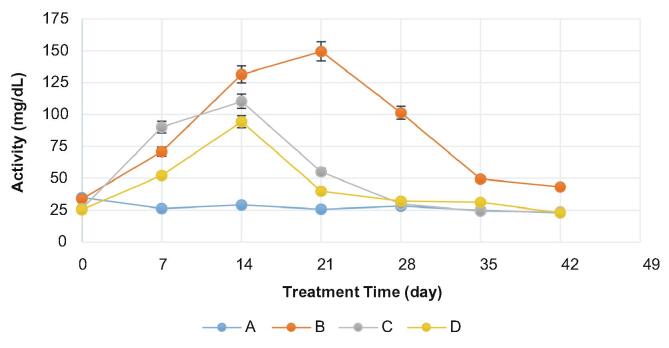
The analysis of total cholesterol levels
Fig. 5 shows that the average total cholesterol levels for all groups on day 0 (baseline) was 65.7 ± 7.4 mg/dL (in normal condition). After feeding a high cholesterol diet for 14 days (on day 14), total cholesterol levels of group B, C, and D increased by 132.3%, 138.6%, and 126.0%, respectively. On day 28, total cholesterol levels for group B, C, and D decreased by 21.8%, 54.8%, and 48.1%, respectively. Hypercholesterolemic rats of group C and D on day 28 were treated by simvastatin and tangeretin for 14 days, respectively, and exhibited the decrease in total cholesterol levels. At the recovery period (on day 42), total cholesterol levels of group B, C, and D further decreased by 44.9%, 24.5%, and 11.6%, respectively. Group A (as a normal group) showed no significant (p > 0.05) changes in total cholesterol levels during the whole experimental period.
Fig. 5.
Total cholesterol levels (n = 8 rats/group) for 42 experimental days in group A (a negative control using a standard diet), B (a positive control using a high cholesterol diet), C (a controlled drug by simvastatin), and D (a treatment sample by tangeretin). The data represent means ± SD.
Fig. 6 shows that the average LDL levels for all groups on day 0 (baseline) was 30 ± 4.9 mg/dL (normal condition). After feeding a high cholesterol diet for 14 days (on day 14), LDL levels of group B, C, and D increased by 289.3%, 322.1%, and 271.1%, respectively. On day 28, LDL levels for group B, C, and D decreased by 22.9%, 72.9%, and 66.1%, respectively. Hypercholesterolemic rats of group C and D on day 28 which had been treated with simvastatin and tangeretin for 14 days, respectively, exhibited the higher decrease in LDL levels. At the end recovery period (on day 42), LDL levels of group B, C, and D further decreased by 57.2%, 20.6%, and 28.3%, respectively. Group A (as a normal group) showed no significant (p > 0.05) changes in LDL levels throughout the experimental period.
-
•
The analysis of LDL cholesterol levels
Fig. 6.
LDL cholesterol levels (n = 8 rats/group) for 42 experimental days in group A (a negative control using a standard diet), B (a positive control using a high cholesterol diet), C (a controlled drug treatment by simvastatin), and D (a treatment sample by tangeretin). The data represent means ± SD.
Fig. 6 shows that the average LDL levels for all groups on day 0 (baseline) was 30 ± 4.9 mg/dL (normal condition). After feeding a high cholesterol diet for 14 days (on day 14), LDL levels of group B, C, and D increased by 289.3%, 322.1%, and 271.1%, respectively. On day 28, LDL levels for group B, C, and D decreased by 22.9%, 72.9%, and 66.1%, respectively. Hypercholesterolemic rats of group C and D on day 28 which had been treated with simvastatin and tangeretin for 14 days, respectively, exhibited the higher decrease in LDL levels. At the end recovery period (on day 42), LDL levels of group B, C, and D further decreased by 57.2%, 20.6%, and 28.3%, respectively. Group A (as a normal group) showed no significant (p > 0.05) changes in LDL levels throughout the experimental period.
-
•
The analysis of HDL cholesterol levels
Fig. 7 shows that the average HDL levels for all groups on day 0 (baseline) was 23.3 ± 2.4 mg/dL (in normal condition). After feeding a high cholesterol diet for 14 days (on day 14), HDL levels of group B, C, and D decreased by 27.1%, 38.3%, and 24.4%, respectively. On day 28, HDL levels for group B further decreased by 11.7%, but HDL levels for group C and D increased by 74.9% and 53.8%, respectively. Hypercholesterolemic rats of group C and D on day 28, treated by simvastatin and tangeretin for 14 days, respectively, exhibited increase in HDL levels. At the end of the recovery period (on day 42), HDL levels of group B and D increased by 77.1% and 18.1%, respectively, but HDL levels of group C decreased by 14.2%. Group A (as a normal group) showed no significant (p > 0.05) changes in HDL levels throughout the experimental period.
-
•
The analysis of SGOT Activity
Fig.7.
HDL cholesterol levels (n = 8 rats/group) for 42 experimental days in group A (a negative control using a standard diet), B (a positive control using a high cholesterol diet), C (a controlled drug by simvastatin), and D (a treatment sample by tangeretin). Data represent means ± SD.
Table 4 shows that on average normal SGOT levels were between the range of around 94.4–116.9 U/L on day 0 (baseline), which is still the range reported under normal conditions based on the literature (Rajendran et al., 2009, Karwani and Sosodia, 2011). The table also exhibited SGOT levels of cholesterol groups (group B, C, and D) from day 7 to day 14 ranged from 80.6 to 110.4 U/L. These are still within the normal limit for healthy liver. Subsequently, this observation was continued until day 42, but for feeding a high cholesterol diet, only group B (as a negative control) was fed by the diet from day 7 to day 28. In this period, the results showed that the range of SGOT levels in group B was from 95.8 to 115.0 U/L and this is still categorized within the normal limit according to Rajendran et al., 2009, Karwani and Sosodia, 2011. The treatment on hypercholesterolemia-induced rat using simvastatin or tangeretin performed from day 21 to day 28 showed SGOT levels for group C and D of 99.0–101.5 U/L and 83.3–94.9 U/L. These values are within the SGOT limit for normal condition. Until the end of the experiment (on day 42), the observation of simvastatin (group C) and tangeretin (group D) toxicity in the rat liver was still recorded in the recovery period. Group C and D exhibited SGOT levels ranging from 80.6 to 110.4 U/L and from 83.3 to 101.8 U/L, respectively. The SGOT levels for group B, C, and D during this experiment from day 7 to day 42 were not significant different (p > 0.05) from group A as a normal group.
-
•
The analysis of SGPT activity
Table 4.
SGOT activity (U/L) of rat groups (n = 8 rats/group) during 42 experimental days.
| Group | Treatment Time (Day) |
||||||
|---|---|---|---|---|---|---|---|
| 0 | 7 | 14 | 21 | 28 | 35 | 42 | |
| A | 116.9 ± 26.8 | 90.1 ± 10.9 | 84.0 ± 9.3 | 91.0 ± 15.3 | 107.5 ± 18.9 | 94.3 ± 18.4 | 93.0 ± 8.5 |
| B | 108.3 ± 19.4 | 90.9 ± 20.0 | 99.3 ± 18.7 | 95.8 ± 12.8 | 102.1 ± 19.5 | 97.3 ± 6.4 | 115.0 ± 13.2 |
| C | 97.5 ± 14.1 | 110.4 ± 36.1 | 80.6 ± 21.1 | 101.5 ± 41.6 | 99.0 ± 14.2 | 100.5 ± 19.9 | 97.8 ± 27.5 |
| D | 94.4 ± 12.4* | 89.3 ± 14.9 | 98.4 ± 22.1 | 83.3 ± 12.8 | 94.9 ± 16.8 | 93.0 ± 5.7 | 101.8 ± 6.8 |
Note: Day 0 is baseline; day 1–14 is feeding a high cholesterol diet; day 15–28 is injecting simvastatin and tangeretin; day 29–42 is recovery period. A = rats fed by a standard diet; B = rats fed by a high cholesterol diet; C = rats treated by simvastatin; and D = rats treated by tangeretin. Data expressed as mean ± SD and *, significantly different compared to group A (p < 0.05).
Table 5 showed the toxicity effect on SGPT levels for all groups during 42 experimental days. The values of all groups on day 0 (baseline) were from 38.9 to 46.8 U/L. The levels were still within normal limits based on Nelson and Michael (2000). After feeding a high cholesterol diet, the range of SGPT levels for group B, C, and D was around 34.5–51.6 U/L. The values also exhibited in normal limits, according to Nelson and Michael (2000). The influence of sample administration using simvastatin and tangeretin for group C and D, respectively, was observed from day 21 to day 42. Group C treated simvastatin exhibited SGPT activity which was no significant difference (p > 0.05) from group A (a normal group). The range of its SGPT levels was around 33.8–64.8 U/L. The levels were still within normal limits based on Ravikumar and Gnanadesign (2011). Group D treated by tangeretin and its range of SGPT levels was around 39.6–60.0 U/L. The levels were still categorized in normal limits and not significantly different (p > 0.05) from group A.
Table 5.
SGPT activity (U/L) of rat groups (n = 8 rats/group) during 42 experimental days.
| Group | Treatment Time (Day |
||||||
|---|---|---|---|---|---|---|---|
| 0 | 7 | 14 | 21 | 28 | 35 | 42 | |
| A | 42.6 ± 8.3 | 46.1 ± 8.6 | 46.9 ± 7.7 | 53.0 ± 11.0 | 41.1 ± 7.6 | 46.5 ± 8.8 | 43.8 ± 13.7 |
| B | 40.5 ± 7.2 | 34.5 ± 8.3* | 51.6 ± 7.9 | 48.9 ± 7.7 | 51.0 ± 12.5 | 38.0 ± 12.5 | 47.3 ± 19.8 |
| C | 38.9 ± 8.6 | 39.1 ± 10.0 | 48.6 ± 7.2 | 64.8 ± 34.4 | 45.4 ± 12.3 | 33.8 ± 9.0 | 35.3 ± 6.4 |
| D | 46.8 ± 9.3 | 37.9 ± 9.3 | 50.0 ± 7.6 | 41.9 ± 8.4 | 39.6 ± 7.1 | 44.5 ± 2.1 | 60.0 ± 7.1 |
Note: Day 0 is baseline; day 1–14 is feeding a high cholesterol diet; day 15–28 is injecting simvastatin or tangeretin; day 29–42 is recovery period. A = rats fed by a standard diet; B = rats fed by a high cholesterol diet; C = rats treated by simvastatin; and D = rats treated by tangeretin. Data expressed as mean ± SD and *, p > 0.05.
3.2.2. HMGCR in vivo activity
Fig. 8 shows that a significant decrease (p < 0.05) in the activity of the enzyme was observed in liver samples of the intervention group, as reflected by the increase in HMG-CoA/mevalonate ratio. The principle of this assay is that the higher the HMG-CoA/mevalonate ratio, the lower is the HMGCR activity and vice versa. The graph exhibited that among all groups, group D produced the highest ratio on day 28 and day 42 in the experiment. The highest HMG-CoA/mevalonate ratio in group D shows that HMGCR activity in the liver was the lowest among all groups. Group C also produced high HMG-CoA/mevalonate ratio. It was higher than group A and B, but it was lower than group D. It means the enzyme activity in group C was lower than the enzyme activity in group A and B. As expected, the highest HMGCR activity was produced by group B because it was the controlled positive group for hypercholesterolemic rat model which was fed with a high cholesterol pellet for 28 experimental days, so expected increased cholesterol production in the liver.
Fig. 8.
Average of HMG-CoA/mevalonate ratio (n = 8 rats/group). HMG-CoA/mevalonate ratio in the liver tissue of male rats after 28 and 42 intervention days with 4 groups: Group A (a negative control using a standard diet); Group B (a positive control using a high cholesterol diet); Group C (a controlled drug by simvastatin); Group D (a treatment sample by tangeretin). Data represent means ± SD, and the mean difference is significant at the 0.05 level. Note: *, p < 0.05 compared to group B and #, p < 0.05 compared to group A.
As observed, treating with simvastatin and tangeretin for group C and D, respectively, produced positive responses in which the HMGCR activity for both groups was lower than the controlled group (group A and B). This study confirmed that tangeretin and simvastatin could inhibit the function of HMGCR enzyme and cause a decrease in the HMGCR activity. Taking into consideration that the liver is involved in lipid metabolism, the administration of tangeretin and subsequent results could be correlated with its cholesterol-lowering effect observed in rats that were fed with a high cholesterol diet and treated with tangeretin for 14 experimental days.
Treating with simvastatin and tangeretin for group C and D, respectively, produced positive responses in which the HMGCR activity for both groups was lower than the controlled group (group A and B). Furthermore, Fig. 9 shows the protein expression of HMGCR in the liver tissue which normalized by β-Actin, the molecular weight of β-actin and HMGCR were demonstrated at 51 kDa and 91 kDa, respectively. Fig. 9 revealed that the expression of HMGCR of group C, and D that were treated by simvastatin drug and tangeretin compound were significantly lower compared to group B (high-cholesterol diet group). HMGCR expression reduced by 35.4%, and 32.2% in animals with treated simvastatin drug and tangeretin compound compared to cholesterol group. This result proved that tangeretin compound has proven in decreasing of HMGCR expression at protein levels by western blotting analysis.
Fig. 9.
The protein expression of HMGCR in the liver tissue was normalized by β-Actin. Group A = non-cholesterol, group B = high-cholesterol (non-treated), group C = high-cholesterol treated with simvastatin, group D = high-cholesterol treated with tangeretin. Value is expressed as means ± SD (n = 3). Note: # significant differences p < 0.05 compared to group B.
3.2.3. Histological analysis
The histological observation of the rat liver in this study on day 28 and on day 42 is shown in Fig. 10. This section describes the difference in histological hepatocyte cells among normal rats (group A), hypercholesterolemic rats without treatment (group B), hypercholesterolemic rats with treatment using simvastatin (group C) and tangeretin (group D) on day 28 (after intervention time) and on day 42 (after recovery period). Fig. 10 (on day 28) showed that group A has normal liver cells, there is no fatty degeneration, as a standard comparison. Group B has many fatty degenerations in mild forms that indicated the effect of feeding a high cholesterol diet without treatment for 28 days. Group C and group D have few fatty degenerations which indicated that the treatment with simvastatin and tangeretin reduced fatty degenerations on hypercholesterolemic rats. The amount of fatty degenerations of group C and group D were less than in group B. Fig. 10 (on day 42) shows that group A has normal liver cells, there is no fatty degeneration, as a standard comparison. Group B has a few fatty degenerations but the amount of fatty degenerations for this group on day 42 is less than on day 28. In this case, it was caused by rats not being fed a high cholesterol during the recovery period but a standard diet, instead. The figure also exhibited that group C and group D have normal liver cells similar to group A. The fatty liver degenerations on both groups in the recovery period were no longer visible compared to group B. The results of this study concluded that tangeretin and simvastatin could lower fatty degenerations in hepatocyte cells in the rat liver.
Fig. 10.
Histological observation of the rat liver with HE staining (magnification 20x) is on day 28 and day 42. Group A = a negative control using a standard diet; Group B = a positive control using a high cholesterol diet; Group C = a controlled drug by simvastatin, and Group D = a treatment sample by tangeretin. CV = Central Vein; H = Hepatocyte; S = Sinusoid; FD = Fatty Degeneration.
4. Discussion
4.1. The HMGCR inhibitory effect of tangeretin and ethyl caffeate compounds
This assay was the first step to determine the inhibitory effect of trans-ethyl caffeate and tangeretin against HMGCR enzyme prior to the in vivo study using rats as the animal model experiment. Trans-ethyl caffeate at 100 µg/mL and tangeretin at 400 µg/mL were shown to have the lowest activity of HMGCR among other concentrations because these samples were most efficient (within their respective groups) in inhibiting HMGCR in this assay through the reduced conversion of mevalonate acid from HMG-CoA (a substrate) by HMGCR. The samples interfere with the enzymic activity of HMGCR to convert mevalonate through competitive activity with HMG-CoA to bind the active site of the HMGCR. When the samples successfully bound with HMGCR, the formation of mevalonate is disrupted, thus leads to decrease in mevalonate concentration due to the lowering of HMGCR activity. Overall, trans-ethyl caffeate and tangeretin have good ability to inhibit HMGCR as displayed by the lowering of HMGCR activity compared to the control (HMGCR). The results showed that the system assay worked well. As reference drugs, pravastatin and simvastatin also lowered activity of HMGCR being cholesterol-lowering drugs via the mechanism of HMGCR inhibition.
However, it was also shown unexpectedly, that trans-ethyl caffeate at low concentration (100 µg/mL) produced the highest % inhibition on HMGCR and at high concentration (400 µg/mL) produced the lowest % inhibition on HMGCR. This could be explained as a result of the antagonistic characteristics of PHK, PMK, and trans-ethyl caffeate which have components in the sample that cause interference in the activity to inhibit HMGCR in this assay. In the low concentration compounds, the lower number of components reduced interference in the function of the samples to inhibit HMGCR. On the other hand, the high concentration compounds contain many components that interfere with each other, thus reducing inhibition of the HMGCR. Therefore, the antagonistic characteristic of samples in this assay permits the less concentrated samples to exhibit higher percentage inhibition against HMGCR.
In contrast to the above, tangeretin at high concentration (400 µg/mL) produced the highest % inhibition on HMGCR and at low concentration (100 µg/mL) produced the lowest % inhibition on HMGCR. This is because both samples have protagonistic characteristics where all components in the sample function in tandem to inhibit HMGCR in this assay. These high concentration compound contain many components which more effectively inhibit HMGCR, meaning that there is no interference in this activity. The lower concentration compounds have fewer components so inhibition of HMGCR is less effective. Therefore, due to the protagonistic characteristic of the samples in this assay, the higher the concentration of samples, the higher is the % inhibition of samples against HMGCR. The % inhibition of samples against HMGCR reflects the ability of the sample in inhibiting HMGCR to convert mevalonate from HMG-CoA. High % inhibition of samples with values similar or greater than pravastatin/simvastatin means effective ability in the inhibition of HMGCR.
Tangeretin was selected compound for further investigation in the in vivo study because it has been shown to have a good ability to inhibit HMGCR. Previous studies by Kuroskwa and Manthey, 2004, Roza et al., 2007 reported that tangeretin reduced plasma cholesterol levels but the mechanism of action of tangeretin in lowering cholesterol had not been studied further, previously. Hence, this study investigated the mechanism of action of tangeretin in reducing cholesterol through the mechanism of HMGCR inhibition. Although both tangeretin and trans-ethyl caffeate was shown to inhibit HMGCR in in vitro studies, only tangeretin was investigated. As the amount of isolated tangeretin was insufficient to complete the in vivo studies, the compound was purchased from Sigma Aldrich. Due to limited funds, trans-ethyl caffeate which is more costly was not tested. Moreover, previous studies have mainly focused on the anti-inflammatory property of trans-ethyl caffeate (Chiang et al., 2005) and its effects in lowering cholesterol levels have not been documented in the literature.
Furthermore, the in vivo study showed that feeding a high cholesterol diet on the experimental rats produced low HMG-CoA/mevalonate ratio. It indicated high HMGCR activity leading to increased mevalonate concentration for the biosynthesis of cholesterol in the liver. The treatment of hypercholesterolemia rats with tangeretin and simvastatin (as a reference) produced high HMG-CoA/mevalonate ratio that resulted in low activity of HMGCR due to its inhibitory function to convert mevalonate from HMG-CoA. This study confirmed that tangeretin and simvastatin could inhibit the function of HMGCR and cause a decrease in HMGCR activity. The results are consistent with findings by Navarro-Gonzàlez et al., 2014, Iqbal et al., 2014 who revealed that tomato juice and Ficus virens Ait rich in bioactive compounds such as phenolics and flavonoids, respectively, can act as natural inhibitor of HMGCR to regulate plasma cholesterol. In this study, tangeretin also showed inhibitory effect on HMGCR via in vitro and in vivo studies, and it also exhibited reduction in the effect of LDL and total cholesterol levels in hypercholesterolemic rats via in vivo study. Moreover, plant extracts containing phenolic compounds and flavonoids have also been shown to inhibit HMG-CoA reductase via ex vivo study (Kwon et al., 2010, Reddy et al., 2014) and via in vivo study (Qinna et al., 2012).
HMGCR is one of the most clinically important enzymes involved in the cholesterol biosynthetic pathway. This enzyme catalyzes the 4-electron reduction of HMG-CoA to mevalonate and CoA. It is the rate-limiting step in sterol synthesis (Murray et al., 2009). When hypercholesterolemic animal models were treated using an inhibitor, the initial reduction in the cholesterol biosynthesis leads to compensatory responses that start with the activation of sterol regulatory element-binding protein-2 (SRBEP-2) because SRBEP-2 is a key regulator of cholesterol (Miserez et al., 2002). As a result, plasma cholesterol decrease and HMGCR activity also decrease. This results in normal levels of cholesterol biosynthesis after intervention due to the presence of tangeretin and simvastatin in which both samples compensated for high levels of HMGCR. The higherHMG-CoA/mevalonate ratio is produced in samples, the lower HMGCR activity occurs in the individuals.
Changes in the HMGCR activity are closely related to changes in the overall rate of cholesterol biosynthesis. This suggests that the HMGCR inhibition would be an effective means to reduce plasma cholesterol (Visavadiya and Narasimhacharya, 2007). If the function of HMGCR to convert mevalonate from HMG-CoA is inhibited, the concentration of mevalonate is also reduced and causes lowering of cholesterol production in the liver. Hence, HMGCR is a target of the widely available plasma cholesterol-lowering drugs known, collectively, as statin drugs (Da Silva et al., 2013) but the drugs have some adverse side effects if they are consumed in prolonged periods. Therefore, finding a natural HMGCR inhibitor has great potential in the treatment and management of hypercholesterolemia. Nowadays, medicinal plants are viewed as a potent source of the drug discovery program. This study investigated tangeretin that is a natural compound abundant in P. tectorius fruits to be used as an alternative medicine to lower plasma cholesterol levels in the blood through the mechanism of action of HMGCR inhibition in the liver. Taking into consideration that the liver is involved in lipid metabolism, tangeretin could correlate with the cholesterol lowering effect and could prevent a high cholesterol diet-enhanced HMGCR activity.
4.2. Anti-hypercholesterolemia properties of tangeretin
4.2.1. The analysis of plasma cholesterol levels
The increase in cholesterol levels (total cholesterol and LDL) after feeding a high cholesterol diet is concordant with previous findings by Wang et al. (2010) where feeding a high cholesterol diet containing 1% cholesterol (w/w) can significantly increase total cholesterol levels as much as 115% compared to the non-cholesterol group. Another study by AlSharari et al. (2016) revealed that high cholesterol diet significantly increased plasma levels of total cholesterol and LDL compared to control group. The high cholesterol diet utilized in the current study constitutes 1% cholesterol, 0.1% cholic acid, and 8.5% vegetable oil. Gilat et al., 2003, AlSharari et al., 2016 observed that a mixture of pellet composed of cholesterol and cholic acid could induce hypercholesterolemia in rats. The addition of cholic acid in the pellet enhances cholesterol absorption (Woollett et al. 2014). Hence, the diet supplemented with cholesterol and cholic acid has been used in many hypercholesterolemic experiments (Sudhahar et al., 2007). Moreover, the addition of palm oil can increase blood cholesterol levels because it contains many saturated fatty acids. The increase in LDL levels is caused by the liver producing excess cholesterol. It will be transported by LDL to be carried to other parts of the body. The more cholesterol produced, the more LDL is needed to transport cholesterol to the blood tissue, thus causing an increase in the cholesterol levels in the blood. Cholesterol carried by LDL can stick and accumulate in the blood vessels (artery), leading to atherosclerosis.
Feeding a high cholesterol diet for 14 days in this study also significantly decreased HDL levels. This is consistent with Olivia and Agustini (2019) who reported that a high cholesterol diet significantly decreased HDL levels compared to the control normal group in Wistar rats. The significant decrease in HDL levels occurs due to the administration of a high cholesterol diet which is shown by the occurrence of hypercholesterolemia. According to Suyatna (2007), the induction of cholesterol in a high cholesterol diet for an extended period of the certain time will progressively decrease HDL levels. A high cholesterol diet reduces HDL levels by increasing the intake and absorption of lipids resulting in a high amount of lipid (including cholesterol and triglycerides) in lipoproteins and peripheral cells. This is followed by an increase in reverse cholesterol transport activity, thus decreasing HDL levels (Mayes, 2003). In dyslipidemia resulting from feeding with a high cholesterol diet, synthesis of LDL will be more easily oxidized. This can increase the destruction of HDL synthesis in the liver. Besides that, feeding a high cholesterol diet can also increase hepatic lipase enzyme activity, a lipolytic enzyme synthesized by hepatocyte cells. This will catalize dietary fats into smaller molecules such as fatty acids and glycerol. These small molecules contribute towards the formation of LDL particles. Hence, the enhancement of hepatic lipase activity in rats can cause an increase of LDL levels and reduction in HDL levels in the blood (Octifani, 2007).
The decrease in plasma cholesterol levels (such as total cholesterol and LDL) in this study after treating with simvastatin and tangeretin for group C and D, respectively, for 14 days on hypercholesterolemia-induced rat is also correlated with reduced incident of cardiovascular disease (CVD) especially atherosclerosis. The reduction of 2 mg/dL of LDL could lower to as much as 1% the risk of CVD (Akpanabiatu et al., 2005). The role of tangeretin in this study is to inhibit HMGCR from converting mevalonate from HMG-CoA in the cholesterol biosynthesis. Therefore, the treatment with tangeretin on hypercholesterolemia-induced rat reduced plasma cholesterol levels (total cholesterol and LDL) and reverted the levels to normal levels similar to simvastatin treatment (as controlled drug). The results demonstrated that tangeretin was almost equally effective in ameliorating plasma lipid levels like simvastatin which was used as a reference. Tangeretin effectively blocked the increase in total cholesterol and LDL levels. Thus, it revealed a comparable effect with simvastatin.
Kuroskwa and Manthey (2004) reported that formulations containing citrus polymethoxylated flavones (PMFs), mainly tangeretin, or citrus flavone glucosides, hesperidin and naringenin, are flavonoids with the potential of lowering plasma cholesterol levels in hamsters with diet-induced hypercholesterolemia. This is in agreement with this study that shows tangeretin can reduce plasma cholesterol levels as tangeretin includes a polymethoxylated flavonoid where it can reduce LDL in the body through its anti-hypercholesterolemic activity. Flavonoids can also decrease the density of LDL receptors in the liver and bind apolipoprotein B as reported by Radhika et al. (2011). Other studies by Casaschi et al., 2004, Ogawa et al., 2005 revealed that flavonoids can reduce cholesterol levels from the blood by inhibiting the action of HMGCR. The mechanism of flavonoids is similar to the mechanism of anti-hyperlipidemic statin drug (such as simvastatin) or several other HMGCR inhibitors. Compared to previous studies, pure tangeretin (not a mixture of formulation) was used to investigate the effects of tangeretin in lowering cholesterol levels via the mechanism of HMGCR. Although previous studies had investigated P. tectorius fruit extracts or fractions which were rich in tangeritin, to assess their effects in lowering cholesterol but these did not explain the specific mechanisms in reducing cholesterol levels (such as the mechanism of HMGCR, SR-B1, PCSK9, or the others).
Lowering in cholesterol levels later can decrease the incidence of hypercholesterolemia leading to atherosclerosis through reverse cholesterol transport pathway. Increase in HDL levels was followed by decreasing LDL levels after hypercholesterolemia-induced rats were treated by simvastatin and tangeretin for group C and D, respectively. Tangeretin could elevate HDL levels because tangeretin as flavonoids play a role in lowering LDL and raising HDL levels. It is consistent with the findings by Kuroskwa and Manthey (2004) which have reported tangeretin as a cholesterol lowering agent. Another report that tangeretin is also rich in P. tectorius fruits (Zhang et al., 2012) that is polymethoxylated flavones (flavonoid subclass), in which flavonoids can inhibit the activity of HMGCR (Kuang et al., 2017). Moreover, polymethoxylated flavones are a subclass of bioflavonoids, such as tangeretin and nobiletin found in many citrus peels that are suggested to have the most potent cholesterol-lowering effect than other citrus flavonoids (Kuroskwa and Manthey, 2004). This study proved that P. tectorius fruit extracts and tangeretin (from the flavonoid group) could be utilized as an alternative natural agent in lowering cholesterol levels and recommend increased utilization of the fruits. Consistent with the in vitro results, tangeretin could also lower the in vivo HMGCR activity.
4.2.2. The analysis SGOT/SGPT
In general, enzymes are extremely good indicators of tissue damage. Organ or tissue damage triggers a rise in the number of enzymes circulated in the bloodstream (Maron et al., 2003). The measurements of SGOT and SGPT levels are two of the parameters used to detect liver damage. The analysis of SGOT was used to assess the toxicity effect of tangeretin consumption. Besides, this section was also carried out to see the effects in the rat liver, on the utilization of cholesterol and cholic acid which was added to a high cholesterol diet. Previous studies by Rajendran et al., 2009, Karwani and Sosodia, 2011 reported that normal SGOT levels on rats is around 105.5 ± 2.8 U/L and 124.8 ± 23.7 U/L, respectively. In this study, SGOT levels on rats after feeding a high cholesterol diet were still within the normal range (80.6–116.9 U/L). SGPT levels in this study were also in the normal limits (33.8–64.8 U/L) based on Nelson and Michael (2000) who revealed that the range of normal SGPT levels is 30.0–60.0 U/L. Both cases showed that the usage of 1% cholesterol and 0.1% cholic acid in the high cholesterol diet is safe on rats or still in non-toxic categories. This finding is consistent with the study by Jeong et al. (2005) who revealed that the utilization of cholesterol dosage of more than 3% in a high cholesterol diet can cause toxicity in the rat liver. A previous study by Lassoued et al. (2014) reported that a high cholesterol diet can increase SGOT and SGPT levels which significantly reflect hepatocyte lysis. SGOT and SGPT coexist in a direct -proportional manner where the increase in SGPT levels will be accompanied by increase in SGOT levels (Rajasekaran et al., 2006).
The treatment of tangeretin and simvastatin (as a reference drug) for 14 days on hypercholesterolemia-induced rat in this study showed normal SGPT and SGOT levels according to the literatures by Rajendran et al., 2009, Nelson and Michael, 2000, respectively. Thus, the administration of tangeretin (7 mg/Kg body weight) and simvastatin (10 mg/Kg body weight) was safe for utilization and did not trigger any disturbances to the function of the rat liver. Both tangeretin and simvastatin in this study were injected to rats in normal dose based on Vanhoecke et al., 2005, Chan et al., 2018. In this study, the dose of simvastatin used is consistent with its application as a commercial drug to treat hypercholesterolemia. Based on this study, it is concluded that tangeretin is considered safe to be used as an alternative medicine because the treatment of hypercholesterolemic rats with tangeretin did not cause heart inflammation or heart damage. If the liver cells had been damaged, the SGPT and SGOT enzymes in the liver cells would enter the bloodstream so that the amount of both enzymes in the blood increases. This increase in enzymes can be caused by the toxic effects of compounds or drugs used. In extensive acute liver necrosis, SGOT and SGPT levels could indicate three categories of necrosis severity. Both the SGOT and SGPT level for high necrosis is 1000–3000 U/L, less severe necrosis is 500–1000 U/L, and mild liver disease is 50–200 U/L (Wahyuni, 2005). High levels of SGPT indicate that there are hepatocellular and liver injury, where the injured cells release SGPT enzyme. Several forms of liver injuries which can be associated with elevated SGPT levels are inflammation, necrosis, and hepatic steatosis.
4.2.3. Histological analysis
The main stuctural components of the liver are hepatocytes (liver cells), ito cells (fat-storing cells), and kupffer cells (stellate macrophages). Hepatocytes are groups of epithelial cells which form interconnected plates. The liver divides into several hexagonal lobules, with the central vein in the centre. Hepatocytes around the central vein are also called centrilobular hepatocytes and near the periportal area are known as periportal hepatocytes (Gartner and Hiatt, 2001). Approximately 80% of the liver population is hepatocytes that contain small amounts of lipids but may increase when an abnormality occurs. Each hepatocyte contains blood capillaries (Bloom and Fawcett, 2002). The effect of a high cholesterol diet is hepatocyte damage in the form of fatty degenerations found in the central venous areas. This damage is caused by the blood from the intestine which contains cholesterol and cholic acid (as a toxin in the rat body). The initial damage is found in hepatocytes of the portal veins which extend towards the central veins carrying the metabolic products (Paderi, 2007). After treatment with tangeretin and simvastatin for 14 days, fatty degenerations in the hepatocytes of the intervention group were reduced compared to the high cholesterol diet group without treatment. Tangeretin caused the decrease in fatty liver degenerations since it has an active compound, flavonoid group, which could lower plasma cholesteol levels in the liver via the mechanism of HMGCR inhibition and via increasing LDL receptors. Simvastatin can decrease fatty degenerations because it is a commercial drug to treat hypercholesterolemia in lowering cholesterol levels and it is an inhibitor of HMGCR enzyme in the cholesterol biosynthesis in the liver.
5. Conclusion
This study has revealed the mechanism of tangeretin in lowering the plasma cholesterol levels by the inhibition of HMGCR activity. Two selected compounds (tangeretin and ethyl caffeate) in the in vitro study were as shown very good activity in inhibiting HMGCR compared to controlled drugs (simvastatin). The in vivo study has demonstrated that tangeretin could reduce hypercholesterolemia in male Spraque dawley rats fed by a high cholesterol diet. Tangeretin significantly decreased (p < 0.05) total cholesterol and LDL cholesterol levels, and also significantly increased (p < 0.05) HDL cholesterol levels after injecting the compound for 14 days. The toxicity study showed that the oral administration of tangeretin for 14 days did not produce significant alteration SGOT/SGPT activity. Histological analysis also exhibited no significant side effects in the liver compared to control normal group (after treatment with tangeretin). The reducing of plasma cholesterol levels shown in this study could be correlated to the inhibition of HMGCR activity by tangeretin. The reducing of cholesterol levels will prevent the formation of atherosclerosis. Thus, results concluded that tangeretin is suitable and safe to suggest as an alternative control for reducing cholesterol levels and also could have potency as an anti-atherosclerotic potential agent. Further study on the molecular interactions between HMGCR and tangeretin such as docking simulations could be performed to ascertain the binding capacity of HMGCR to tangeretin.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
The authors wish to thank the Ministry of Higher Education (MOHE) – Malaysia for research funding under the Fundamental Research Grant Scheme (FRGS) Fasa I/2017 (Vote No 59477) .
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Inten Pangestika, Email: pangestika.intancarbon0607@gmail.com.
Efriyana Oksal, Email: efriyana.oksal18@gmail.com.
Tengku Sifzizul Tengku Muhammad, Email: sifzizul@umt.edu.my.
Hermansyah Amir, Email: hermansyah1962@gmail.com.
Desy Fitrya Syamsumir, Email: desy@umt.edu.my.
Mohd Effendy Abdul Wahid, Email: effendy@umt.edu.my.com.
Yosie Andriani, Email: yosie.hs@umt.edu.my.
References
- Akpanabiatu M.I., Umoh I.B., Udosen E.O., Udoh A.E., Edet E.E. Rat serum electrolytes, lipid profile, and cardiovascular activity on Nuclea latifolia leaf extract administration. Indian J. Clin. Biochem. 2005;20(2):29–34. doi: 10.1007/BF02867397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AlSharari S.D., Al-Rejaie S.S., Abuohashish H.M., Ahmed M.M., Hafez M.M. Rutin attenuates hepatoxicity in high-cholesterol-diet-fed-rats. Oxid. Med. Cell. Longev. 2016;2016:1–11. doi: 10.1155/2016/5436745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andriani, Y., Pangestika, I., Oksal, E., Mohammad, H., Amir, H., Tengku Muhammad, T.S., Effendy, E.W.M., 2019. Anti-Atherosclerosis potency of Pandanus tectorius fruit rich by tangeretin and ethyl trans-caffeate, and their cytotoxicity against HepG2 cell line. In: IOP Conf. Series: Materials Science and Engineering, Proceedings of 13th Joint Conference on Chemistry (13th JCC). IOP Publishing, 509 (2019) 012155, 1-8. doi:10.1088/1757-899X/509/1/012155.
- Andriani Y., Ramli N.M., Syamsumir D.F., Kassim M.N.I., Jaafar J., Azis N.A., Marlina L., Musa N.S., Mohamad H. Phytochemical analysis, antioxidant, antibacteria and cytotoxicity activities of keys and cores part of Pandanus tectorius fruits. Arabian J. Chem. 2015 doi: 10.1016/j.arabjc.2015.11.003. [DOI] [Google Scholar]
- Bloom, W. and Fawcett, D.W. 2002. Buku Ajar Histologi. Edisi 12. Terjemahan Jan Tambayong. Jakarta: EGC.
- Casaschi A., Maiyoh G.K., Rubio B.K., Li R.W., Adeli K., Theriault A.G. The chalcone xanthohumol inhibits triglyceride and apoliproptein B secretion in HepG2 cells. J. Nutr. 2004;134:1340–1346. doi: 10.1093/jn/134.6.1340. [DOI] [PubMed] [Google Scholar]
- Chan K.M., Ismail M., Esa N.M., Alitheen N.B.M., Imam M.U., Ooi D.J., Khong N.M.H. Defatted kenaf (Hibiscus cannabinus L.) seed meal and its phenolic-saponin-rich extract protect hypercholesterolemic rats against oxidative stress and systemic inflammation via transcriptional modulation of hepatic antioxidant genes. Oxid. Med. Cell. 2018;Longev:1–18. doi: 10.1155/2018/6742571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang Y.M., Lo C.P., Chen Y.P., Wang S.Y., Yang N.S., Kuo Y.H., Shyur L.F. Ethyl caffeate suppresses NF-kB activation and its downstream inflammatory mediators, iNOS, COX-2, and PGE2 in vitro or in mouse skin. Br. J. Pharmacol. 2005;146:352–363. doi: 10.1038/sj.bjp.0706343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva J.B., Temponi V.S., Gasparetto C.M., Fabri R.L., Aragão D.M.O., Pinto N.C.C., Ribeiro A., Scio E., Del-Vechio-Vieira G., Sousa O.V., Alves M.S. Vernoniacondensata Baker (Asteraceae): a promising source of antioxidants. Oxid. Med. Cell. Longev. 2013;2013:1–10. doi: 10.1155/2013/698018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depypere H.T., Bracke M.E., Boterberg T., Mareel M.M., Nuytinck M., Vennekens K., Serreyn R. Inhibition of tamoxifen’s therapeutic benefit by tangeretin in mammary cancer. Eur. J. Cancer. 2000;36:73. doi: 10.1016/s0959-8049(00)00234-3. [DOI] [PubMed] [Google Scholar]
- Dong Y., Cao A., Shi J., Yin P., Wang L., Ji G., Xie J., Wu D. Tangeretin, a citrus polymethoxyflavonoid, induces apoptosis of human gastric cancer AGS cells through extrinsic and intrinsic signaling pathways. Oncol. Rep. 2014;31:1788–1794. doi: 10.3892/or.2014.3034. Epub 2014 Feb 19. [DOI] [PubMed] [Google Scholar]
- Gartner L., Hiatt J. second ed. WB Saunders Company; Philadepia, United States: 2001. Color Textbook of Histology. [Google Scholar]
- Gilat T., Leikin-Frenkel A., Goldiner I., Juhel C., Lafont H., Gobbi D., Konikof F.M. Prevention of diet-induced fatty liver in experimental animals by the oral administration of a fatty acid bile acid conjugate (FABAC) Hepatology. 2003;38:436–442. doi: 10.1053/jhep.2003.50348. [DOI] [PubMed] [Google Scholar]
- Iqbal D., Khan M.S., Khan A., Ahmad S., Srivastava A.K., Bagga P. In vitro screening for β-hydroxy-β-methyl glutaryl-coa reductase inhibitory and antioxidant activity of sequentially extracted fractions of Ficus palmata Forsk. Biomed Res. Int. 2014;1–10 doi: 10.1155/2014/762620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong W.I., Jeong D.H., Do S.H., Kim Y.K., Park H.Y., Kwon O.D., Kim T.H., Jeong K.S. Mild hepatic fibrosis in cholesterol and sodium cholate diet-feed rats. J. Vet. Med. Sci. 2005;67:235–242. doi: 10.1292/jvms.67.235. [DOI] [PubMed] [Google Scholar]
- Karwani G., Sosodia S. Hepatoprotective activity of Mimosa pudica Linn. In carbon tetrachloride induce hepatoxicity in rats. J. Herbal Med. Toxicol. 2011;5:27–32. [Google Scholar]
- Kuroskwa E.M., Manthey J.A. Hypolipidemic effects and absorption of citrus polymethoxylated flavonos in hamsters with diet-induced hypercholesterolemia. J. Agric. Food Chem. 2004;52(10):2879–2886. doi: 10.1021/jf035354z. [DOI] [PubMed] [Google Scholar]
- Kuang W., Zhang X., Lan Z. Flavonoids extracted from Linaria vulgaris protect against hyperlipidemia and hepatic steatosis induced by western-type diet in mice. Achives Pharmacal Res. 2017;41:1190–1198. doi: 10.1007/s12272-017-0941-y. [DOI] [PubMed] [Google Scholar]
- Kwon E.K., Lee D.Y., Lee H.J., Kim D.O., Baek N.I., Kim Y.E., Kim H.Y. Flavonoids from the buds of Rosa damascena inhibit the activity of 3-hydroxy-3-methylglutaryl-coenzyme a reductase and angiotensin I-converting enzyme. J. Agric. Food Chem. 2010;58:882–886. doi: 10.1021/jf903515f. [DOI] [PubMed] [Google Scholar]
- Lassoued I., Trigui M., Ghlissi Z., Nasri R., Jamoussi K., Kessis M., Barkia A. Evaluation of hypercholesterolemic effect and antioxidant activity of boops boops proteins in cholesterol-fed rats. Food Funct. 2014;5(6):1224–1231. doi: 10.1039/c3fo60705d. [DOI] [PubMed] [Google Scholar]
- Lee H.N., Kim J.K., Kim J.H., Lee S.J., Ahn E.K., Oh J.S., Seo D.W. A mechanistic study on the anti-cancer activity of ethyl caffeate in human ovarian cancer SKOV-3 cells. Chem-Bio. Interact. 2014;219:151–158. doi: 10.1016/j.cbi.2014.05.017. [DOI] [PubMed] [Google Scholar]
- Li M., Diao Y., Liu Y., Huang H., Li Y., Tan P., Liang H., He Q., Nie J., Dong X., Wang Y., Zhou L., Gao X. Chronic moderate alcohol intakes accelerate SR-B1 mediated reverse cholesterol transport. Sci. Rep. 2016;6:1–11. doi: 10.1038/srep33032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim D.Y., Lee M.H., Shin S.H., Chen H., Ryu J., Shan L., Li H., Bode A.M., Zhang W.D., Dong Z. (+)-2-(1-Hydroxyl-4-oxocyclohexyl) ethyl caffeate suppresses solar UV-induced skin carcinogenesis by targeting PI3K, ERK1/2, and p38. Cancer Prev. Res. 2014;7:856–865. doi: 10.1158/1940-6207.CAPR-13-0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maron D.J., Lu G.P., Cai N.S., Wu Z.G., Li Y.H., Chen H., Zhu J.Q., Jin X.J., Wouters B.C., Zhao J. Cholesterol-lowering effect of a theaflavin-enriched green tea extract: a randomized controlled trial. Arch. Int. Med. 2003;163:448–1453. doi: 10.1001/archinte.163.12.1448. [DOI] [PubMed] [Google Scholar]
- Mayes, P.A. 2003. Sintesis, Pengangkutan, dan Ekstresi Kolesterol. Biokimia Harper. Edisi 25. EGC, Jakarta.
- Miserez A.R., Muller P.Y., Barella L., Staehelin H.B., Leitersdorf E., Kark J.D., Friedlander Y. Sterol-regulatory element-binding protein (SREBP)-2 contributes to polygenic hypercholesterolemia. Atherosclerosis. 2002;164:15–26. doi: 10.1016/s0021-9150(01)00762-6. [DOI] [PubMed] [Google Scholar]
- Morley K.L., Ferguson P.J., Koropatnick J. Tangeretin and nobiletin induce G1 cell cycle arrest butnot apoptosis in human breast and colon cancer cells. Cancer Lett. 2007;251:168–178. doi: 10.1016/j.canlet.2006.11.016. [DOI] [PubMed] [Google Scholar]
- Murray, R.K., Bender, D.A., Botham, K.M., Kennely, P.J., Rodwell, V.W., Anthony, P., 2009. Harper’s Biochemistry, 28th ed. McGraw Hill Lange, United States.
- Navarro-Gonzàlez I., Pérez-Sàchez H., Martín-Pazuelo G., Garcià-Alonso J., Jesús-Periago M. The inhibitory effects of bioactive compounds of tomato juice binding to hepatic HMGCR: In vivo study and moleculear modeling. PLoS ONE. 2014;9:1–11. doi: 10.1371/journal.pone.0083968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D.L., Michael M.C. 3rd Edition. New York, United States; Worth Publisher: 2000. Lehninger Principles of Biochemistry. [Google Scholar]
- Octifani S. Universitas Diponegoro, Indonesia; Skipsi: 2007. Pengaruh Pemberian Margarin terhadap Rasio Kolesterol LDL/HDL Tikus Sprague dawley. [Google Scholar]
- OECD, 2008. 425 OECD Guidelines for testing of chemicals: Acute oral toxicity-up-and-down-procedure (UDP). The Organization for Economic Co-operation and Development, pp. 1–27.
- Ogawa H., Ohno M., Baba K. Hypotensive and lipid regulatory actions of 4-hydroxyderricin, a chalcone from Angelica keiskei, in stroke-prone spontaneously hypertensive rats. Clin. Exp. Pharmacol. Physiol. 2005;32(1-2):19–23. doi: 10.1111/j.1440-1681.2005.04147.x. [DOI] [PubMed] [Google Scholar]
- Olivia Z., Agustini R. Pengaruh pemberian sekam Psyllium (Psyllium Husk) terhadap kadar LDL dan kadar HDL tikus putih (Rattus Norvegicus) galur Wistar hiperkolesterolemia. Jurnal Kesehatan. 2019;7(2):75–81. [Google Scholar]
- Paderi A.Z. Fakultas Kedokteran Hewan; Institut Pertanian Bogor: 2007. Kajian perubahan jaringan uji khasiat buah merah (Pandanus conoideus) sebagai bahan penghambat kerusakan hati. [Google Scholar]
- PubChem. Tangeretin. 2005. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Tangeretin#section=Top (accessed on 11 September 2018).
- Qinna N.A., Kamona B.S., Alhussainy T.M., Taha H., Badwan A.A., Matalka K.Z. Effects of prickly pear dried leaves, artichoke leaves, tumeric, and garlic extracts, and their combinations on preventing dyslipidemia in rats. ISRN Pharmacol. 2012;1–7 doi: 10.5402/2012/167979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasekaran S., Ravi K., Sivagnanam K., Subramanian S. Beneficial effects of Aloe vera leaf gel extract on lipid profile status in rats with streptozotocin diabetes. Clin. Exp. Pharmacol. Physiol. 2006;33:232–237. doi: 10.1111/j.1440-1681.2006.04351.x. [DOI] [PubMed] [Google Scholar]
- Rajendran, R., Hemalatha, S., Akasakalai, K., Madhu-Khrisna, C.H., Sohil, B., Vittal, Sundaram, M., 2009. Hepatoprotective activity of Mimosa pudica leaves against carbon tetrachloride induced toxicity. J. Nat. Prod. 2, 116–122. WWW. JournalofNaturalProducts.com.
- Radhika S., Smila K.H., Muthezhilan R. Antidiabetic and hypolipidemic activity of Punica granatum Linn on alloxan induced rats. World J. Med. Sci. 2011;6:178–182. [Google Scholar]
- Ravikumar S., Gnanadesign M. Hepatoprotective and antioxidant activity of a mangrove plant Lumnitzera recemosa. Asian Pac. J. Trop. Biomed. 2011;1:348–352. doi: 10.1016/S2221-1691(11)60078-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy P.V., Sarkar L., Urooj A. Inhibition of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase (ex vivo) by medicinal plants from Western Ghats. AP. 2014;3:56–61. doi: 10.1155/2014/318561. [DOI] [Google Scholar]
- Roza J.M., Xian-Liu Z., Guthrie N. Effect of citrus flavonoids and tocotrienols on serum cholesterol levels in hypercholesterolemic subjects. Alternative Therapies. 2007;13(6):44–48. [PubMed] [Google Scholar]
- Sudhahar V., Kumar S.A., Mythili Y., Varalaksmi P. Remedial effect of lupeol and its ester derivative on hypercholesterolemia-induced oxidative and inflammatory stresses. Nutr. Res. 2007;27(778):787. doi: 10.1007/s11010-008-9786-5. [DOI] [Google Scholar]
- Suyatna F.D. Departemen Farmakologi dan Terapeutik Fakultas Kedokteran UI; Jakarta: 2007. Farmakologi dan Terapi, Edisi Kelima. [Google Scholar]
- Vanhoecke B.W., Delporte F., Braeckel E.V., Heyerick A., Depypere H.T., Nuytinck M., Keukeleire D.D., Bracke M.E. A safety study of oral tangeretin and Xanthohumol administration to laboratory mice. Vivo. 2005;19:103–108. PMID:15796161. [PubMed] [Google Scholar]
- Visavadiya N.P., Narasimhacharya A.V.R.L. Sesame as a hypocholesteraemic and antioxidant dietary component. Food Chem. Toxicol. 2007;46(6):1889–1895. doi: 10.1016/j.fct.2008.01.012. [DOI] [PubMed] [Google Scholar]
- Venugopala R.A., Ramakrishnan S. Indirect assessment of hydroxymethylglutaryl-CoA reductase (NADPH) activity in liver tissue. Clin. Chem. 1975;21:1523–1525. PMID:1157326. [PubMed] [Google Scholar]
- Wahyuni S. Pengaruh daun sambiloto (Andrographis paniculata, nees) terhadap kadar SGPT dan SGOT tikus putih. GAMMA. 2005;1(1):45–53. [Google Scholar]
- Wang Y.M., Zhang B., Xue Y., Li Z.J., Wang J.F., Xue C.H., Yanagita T. The mechanism of dietary cholesterol effects on lipids metabolism in rats. Lipids Health Dis. 2010;9:1–6. doi: 10.1186/1476-511X-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woollett L.A., Buckley D.D., Yao L., Jones P.J.H., Granholm N.A., Tolley E.A., Tso P., Heubi J.E. Cholic acid supplementation enhances cholesterol absorption in humans. Gastroenterology. 2014;126:724–731. doi: 10.1053/j.gastro.2003.11.058. [DOI] [PubMed] [Google Scholar]
- WHO, 2011. Global Atlas on Cardiovascular Disease Prevention and Control 2011, 2-8
- Zhang J.Z. Drexel University, USA; 2008. An approach to analyzing histology segmentations using shape distributions. Mater Thesis. [Google Scholar]
- Zhang X., Guo P., Sun G., Chen S., Yang M., Fu N., Wu H., Xu X. Phenolic compounds and flavonoids from the fruits of Pandanus tectorius Soland. J. Med. Res. 2012;6:2622–2626. [Google Scholar]