Abstract
An outbreak of coronavirus disease 2019 (COVID-19), a disease caused by a novel pneumonia virus, has affected over 200 countries and regions worldwide. With the increasing number of patients and deaths, WHO have declared it as a global pandemic currently, indicating a third large-scale epidemic coronavirus has appeared since the emergence of severe acute respiratory syndrome coronavirus (SARS) and Middle-East respiratory syndrome (MERS) in the twenty-first century. Considering the great harm it has caused, researchers throughout the world have been chasing to exploit the pathophysiology, characteristics, and potential remedies for COVID-19 to better battle the outbreak. Therefore, the current study revisits advances of the virology, epidemiology, clinical features, therapeutic options, and prevention of COVID-19. The features of asymptomatic carriers are also been explored.
Keywords: coronavirus disease 2019, COVID-19, epidemiology, clinical features, treatment
Introduction
At the end of 2019, a sudden outbreak of pneumonia occurred in Wuhan, China, which was later confirmed to be caused by a coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).1,2 Soon after, the virus spread rapidly around the world and affected human health as well as causing huge economic losses to society.3–5 On 12 February 2020, this novel disease was named coronavirus disease 2019 (COVID-19) by WHO.6 Since then, medical experts around the world have been trying to find the control measures and treatment for it.
Because COVID-19 is highly contagious, the number of patients has increased rapidly. Thus, realizing its basic characteristics and finding appropriate methods to detect and cure COVID-19 is necessary for outbreak control. The detection and rehabilitation judgment of COVID-19 are mainly through reverse transcription-polymerase chain reaction (RT-PCR),7 but experts have pointed out the low sensitivity will lead to missed diagnosis. So a combination of RT-PCR and other clinical features of COVID-19 is important for diagnosis.8 The treatments for COVID-19 are still unclear and mainly contain administration of oxygen, drug therapy, and emergency treatments such as ECMO.9, 10 As we know, few studies have summarized and classified therapies for COVID-19 systematically.
Therefore, we review basic characteristics such as virology and epidemiology of COVID-19 and classified explore antiviral therapy, symptomatic treatment, and traditional Chinese medicines for curing COVID-19. Considering no licensed vaccine exists for COVID-19, we also summarize the research and development of its vaccine. Notice that the recent emergence of asymptomatic carriers has brought great difficulties for prevention and control of this disease,11 we highlight the characteristics of asymptomatic carriers and how to reduce their impact on the development of the COVID-19 pandemic.
Virology
In January 2020, after the outbreak of COVID-19, scientists identified its pathogen and immediately confirmed it to be a novel coronavirus, which is found to be a β coronavirus of group 2B and was named 2019-nCoV by WHO initially.2 Because 70% of its genetic sequences are similar to the SARS-CoV, it was later renamed SARS-CoV-2.12 The origin of SARS-CoV-2 is unclear and is thought to be related to bat coronaviruses. Zhou et al. compared gene sequence of SARS-CoV-2 with RaTG13, a bat coronavirus, and found the whole-genome sequence of SARS-CoV-2 is 96.2% similar to RaTG13, indicating that SARS-CoV-2 may originate from a bat coronavirus.13 At the same time, Wu and colleagues reported that the genome sequence of SARS-CoV-2 was closely linked with SL-CoVZC45, another bat coronavirus, with 82.3% similarity.14 However, despite the high similarity of SARS-CoV-2 to the virus from bats, the genetic differences will take at least a few decades of evolution to make up.15 Thus, bats are unlikely to be the definitive hosts of it, and there may exist other intermediate hosts which brought the virus into humans.16 The relative synonymous codon usage analysis of viruses found SARS-CoV-2 used the translation machinery of several snakes effectively. Therefore, snakes are a potential reservoir of the novel virus.17 Apart from this, pangolins are thought to be the potential intermediate host of SARS-CoV-2 due to the sequence similarity of their spike receptor binding domains.18 Furthermore, Liu et al. declared that turtles were another possible host of SARS-CoV-2.19 Thus, more studies are necessary for confirming the intermediate host of SARS-CoV-2.
The pathogenic mechanism of SARS-CoV-2 is still unclear and thought to be closely related to viral sepsis,20 but structural biology has explained how viruses enter cells. Angiotensin converting enzyme II (ACE2) has been demonstrated to be the only receptor for SARS-CoV-2.13 The S protein on the surface of coronavirus was confirmed to mediate virus entry,21 which contains proteins S1 and S2 subunits. The receptor-binding domain (RBD) of S1 is responsible for the recognition of ACE2 on the surface of human cells to complete the binding of viruses to cells. The S2 subunit mediates the fusion of the virus envelope and human cell membrane to complete the invasion process. There are two important conserved repeated amino acid sequences, HR1 and HR2, in the S2 subunit of SARS-CoV-2. Together, they form a spiral structure called 6-HB, which is the key for the fusion of a coronavirus envelope with cell membrane.22 The serine protease TMPRSS2 triggers the S protein and promotes its fusion with the cell membrane.23 After that, the RNA of the virus is released into the cytoplasm to complete its biosynthesis. SARS-CoV-2 is more contagious, although it has a similar structure of S protein to SARS-CoV, so virus-receptor-binding affinity is now being conducted in further studies. Goh et al. found the rigidity and stability of SARS-CoV-2 shell are apparently higher than SARS-CoV,24 which increase its adaption to the environment. Moreover, the structural difference of HR1 contributes to the higher structural stability for the 6-HB of SARS-CoV-2, which has caused the better membrane fusion capacity of SARS-CoV-2.22 Thus, SARS-CoV-2 has a stronger infectious ability. It is worth noting that the expressions of ACE2 in patients with some diseases such as diabetes are notably higher than those in healthy people,25 indicating that people with underlying diseases are more likely to be infected.
Epidemiology
On 31 December 2019, Wuhan Municipal Health Commission reported that 27 pneumonia patients were associated with a South China seafood market, which was the earliest discovery of COVID-19.26 Then, the quantity of COVID-19 patients increased rapidly after Chinese New Year. Up to 16 May 2020, COVID-19 had affected more than 200 countries and regions, and the quantity of confirmed patients had reached 4 425 485 globally.27 After the breakthrough of COVID-19, experts began to predict the incubation stage of the SARS-CoV-2. According to the data collected in January 2020, the incubation stage of COVID-19 patients ranges between 2 and 14 days, and its average is about 5 days (95% credible interval: 4.2–6.0) when implementing the best-fit lognormal distribution. Thus, experts recommended that suspected patients are quarantined for at least 14 days.28 The mode of SARS-CoV-2 transmission is also an important part of epidemiological investigation. It is clear that human-to-human transmission of COVID-19 occurs mainly via droplet respiratory particles,29 but whether SARS-CoV-2 can be transmitted via the eyes is still unclear.30 It should be noted that several studies have showed that chief complaint of some COVID-19 patients were digestive symptoms, and nucleic acids of SARS-CoV-2 were even found in fecal samples or anal swabs of some patients, demonstrating the possibility of an oral–fecal route.31–33 Furthermore, mother-to-child transmission is also a potential route of COVID-19.34,35 Luckily, infection rates of children are relatively low, and most inpatient children only have mild symptoms even if they are infected,36–38 thus less damage would occur to them.
Up until now, the largest case series data of China has shown that 86.6% of COVID-19 patients were aged 30–79 years, 1% were aged under 1 year old, 1% aged 10–19 years, and 3% were aged above 80 years. Among all patients, males account for 51%, and females account for 49%. Furthermore, 4% of them are medical workers.39 In the United States, the elderly (aged over 65) account for 31% of total COVID-19 cases, 45% of inpatients, 53% of ICU patients, and 80% of death cases.40
In terms of epidemiology, the basic reproductive number and mortality rate are indispensable. Basic reproductive number (R0) is thought to be an important index for predicting the development of an outbreak. The main method of calculating R0 is using the data of infected people, such as serial interval distribution and latent period, to build a proper mathematical model for predicting the trend of the epidemic. A big epidemiological data of 425 confirmed COVID-19 patients reported that the basic reproductive number of SARS-CoV-2 was 2.2, which means each patient could infect 2.2 other people.1 However, more recent research estimated that basic reproductive number was 5.8.41 It is obvious that distinctions of R0 exist in these studies, and they result in data and model differences. The incubation period and serial interval distribution of former research are calculated only through 10 confirmed cases and six pairs of cases in clusters, respectively.1 Furthermore, the model of the former research is made merely on the basis of one region,1 thus its R0 distinctly lacks universality. By contrast, the latter study gathered the information of incubation period, serial interval, and infectious period from the individual cases reported throughout China and designed two models to infer the growth rate of the outbreak in different perspectives.41 So, its R0 better reflects the epidemic situation of China. Similarly, the R0 of around 3.87 reported by Imperial College COVID-19 Response Team only represented the situation in Europe.42 According to the data analysis of the Chinese Center for Disease Control and Prevention, the overall mortality of COVID-19 patients was approximately 2.3%,43 which is obviously lower than Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERS).44 However, because of the lager quantity of COVID-19 patients, the number of COVID-19 deaths is still high. Furthermore, the data from the epidemic area indicated that people aged 30–59 years had the highest death rate of any age group, and the risk of symptomatic infection increased year by year.45
Clinical features and diagnosis (Table 1)
Table 1.
Typical clinical features of COVID-19.
| Clinical symptoms | Fever |
| Common respiratory symptoms such as cough and dyspnea | |
| Non-respiratory symptoms such as fatigue, myalgia, headache, and digestive symptoms | |
| Laboratory examination | Decrease: lymphocytes and eosinophils |
| Increase: C-reactive protein, prothrombin time, and procalcitonin | |
| CT features | GGO, consolidation, and interlobular septal thickening |
Typical clinical features of COVID-19
Like most respiratory illnesses, COVID-19 has many typical respiratory clinical symptoms. Data from 1099 confirmed COVID-19 patients have shown that 88.7% had a fever, 67.8% developed a cough, and 2.3% received invasive mechanical ventilation,46 which is consistent with other studies.47 For some severe patients, acute respiratory distress syndrome (ARDS), shock, and arrhythmia are common complications.48,49 Apart from these symptoms, COVID-19 patients sometimes have other non-respiratory symptoms such as fatigue, myalgia, headache, and digestive tract symptoms.48,50 Moreover, the main changes in laboratory results of COVID-19 are decreases in lymphocytes and eosinophils as well as increases in D-dimer, C-reactive protein, prothrombin time, and procalcitonin.48,51
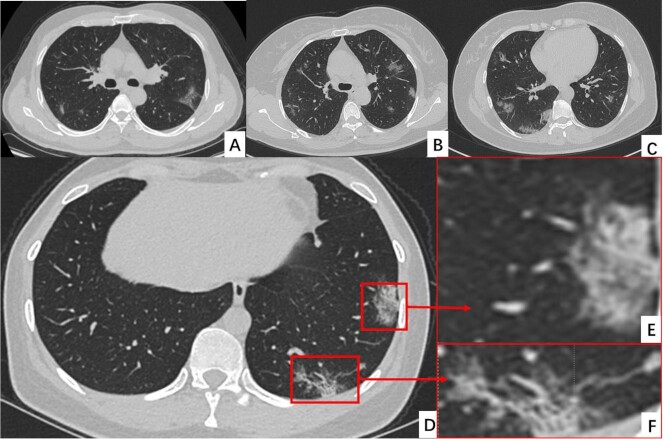
Computed tomography (CT) is one of the most efficient techniques for evaluating severity and differential diagnosis of pulmonary diseases, thus the CT characteristics (Fig. 1) of COVID-19 are another important clinical feature. This clinical evidence indicates that ground glass opacity (GGO), consolidation, and interlobular septal thickening are the most common CT characteristics of COVID-19, which occurs mainly in the posterior lung area and middle and lower lung regions.52,53 Compared to patients with mild symptoms, signs such as consolidation, thickened bronchial wall, linear opacities, extrapulmonary lesions, and higher CT scores are marked CT features of critically ill patients.54 Therefore, CT is potentially used to diagnose and predict the prognosis of COVID-19 patients.
Figure 1.
The typical CT images of the COVID-19 patients.
Clinical diagnosis of COVID-19
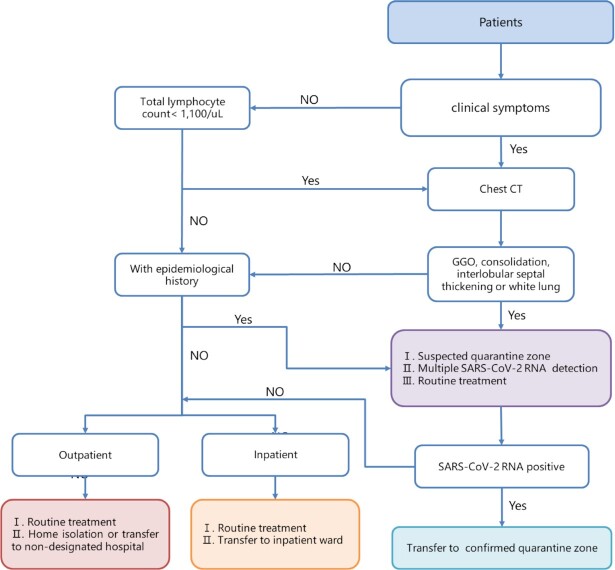
Clinical symptoms are often used as indicators of clinically suspected cases. Thus, when it comes to the clinical features above, it is easy to think about clinical diagnosis of COVID-19 (Table 2 and Fig. 2). According to the Diagnosis and Treatment Program of Novel Coronavirus Pneumonia, only a suspected case has one of the pieces of evidence of etiology or serology, such as positive nucleic acid, confirmation of gene sequencing, and virus specific antibody, to be confirmed to be COVID-19 patient,55 and the suspected cases were identified by a comprehensive analysis of epidemiological history and clinical manifestations.56 However, there are also many problems with this program. The most obvious problem is that RT-PCR is the most common method for nucleic acid testing, but its sensitivity is not as high as expected,57 thus it possibly causes misdiagnosis of COVID-19. Furthermore, although viral gene sequencing is an accurate diagnostic method and used on a small scale,58 its complex operation and time-consuming process make it difficult for wider clinical use. SARS-CoV-2 specific antibody detection is a simple detection method with higher sensitivity than RT-PCR,59 but its high sensitivity is only reflected after 5.5 days of symptom onset,60 so it is just a supplementary means to RT-PCR. Luckily, the fast and convenient method, CT, was found to have a higher diagnostic sensitivity for COVID-19 than other diagnostic methods such as RT-PCR and can predict the prognosis of patients,57,61 thus it is meaningful for the initial screening. Zhang et al. developed a deep learning system for image recognition of COVID-19 patients. They collected CT images from 4154 patients for validation and found this system precisely identified the COVID-19 and other forms of pneumonia, and it also divided these patients into high- and low-risk groups correctly,62 so rational use of artificial intelligence such as this may be better able to screen COVID-19 patients and give them timely interventions to ensure a better prognosis. In addition, a CRISPR-Cas12-based technique was confirmed to be a faster test for COVID-19 with higher sensitivity,63 thus it is possibly a replacement for the RT-PCR assay.
Table 2.
Diagnostic criteria of COVID-19 (Data from Diagnosis and Treatment Program of Novel Coronavirus Pneumonia (trial seventh version)).55
| Suspected cases |
| Patients have any epidemiological history and conform to at least two clinical manifestations, or patients without clear epidemiological history conform to three clinical manifestations. |
| Epidemiological history: |
| Ⅰ. Travel or residence history of an affected area or close contact with a suspected or confirmed case within 14 days before onset |
| Ⅱ. Have contact with COVID-19 cases (nucleic acid positive) within 14 days prior to onset |
| Ⅲ. Have contact with patients with fever or respiratory symptoms from an affected area, or from communities with COVID-19 cases |
| Ⅳ. Cluster onset (two or more cases of fever and/or respiratory symptoms within 2 weeks in a small area such as home, office, school, and class) |
| Clinical manifestations: |
| Ⅰ. Fever and/or respiratory symptoms |
| Ⅱ. With COVID-19 imaging characteristics |
| Ⅲ. Normal or reduced number of white blood cells and/or lymphocytes in early COVID-19 |
| Confirmed cases |
| The suspected cases have at least one of the following etiological or serological evidences. |
| Ⅰ. The real-time fluorescent RT-PCR for specimens with a positive result of SARS-CoV-2 RNA |
| Ⅱ. Virus gene sequences are highly homologous to SARS-CoV-2 |
| Ⅲ. Positive SARS-CoV-2 specific IgM antibody and IgG antibody in serum; the serum SARS-CoV-2 specific IgG antibody changes from negative to positive or is four times higher in the recovery period than in the acute phase |
Figure 2.
The screening process of COVID-19 in clinic and inpatient departments.
Remedies
The therapeutic schedule issued by the General Office of National Health Committee of China on 3 March has shown that therapy of COVID-19 should be combined with etiological treatment and symptomatic treatment, and symptomatic treatment is especially important for severe and critical patients. Furthermore, traditional Chinese medicine can be also used for the whole treatment cycle of COVID-19 patients.55 In the processes of COVID-19 therapy, drugs and bioproducts play important roles (Table 3). Here, we summarize the main treatments of novel pneumonia.
Table 3.
Main antiviral medicine and bioproducts for COVID-19.
| Items | Clinical function | Mechanism/Principle |
|---|---|---|
| Chloroquine | Penetration and uncoating inhibitors | Interfering the glycosylation of ACE2 or alkalizing the phagolysosome of SARS-CoV-2 to inhibit the penetration of virus. |
| Arbidol | Penetration and uncoating inhibitors | Suppressing the fusion of virus lipid membrane and host cells. |
| Camostat mesylate | Penetration and uncoating inhibitors | Interfering the S protein priming to inhibit the penetration of virus. |
| Remdesivir | biosynthesis inhibitors | Inhibiting the RNA-dependent RNA polymerase activity of SARS-CoV-2. |
| Sofosbuvir | biosynthesis inhibitors | Inhibiting the RNA-dependent RNA polymerase activity of SARS-CoV-2. |
| Ribavirin | biosynthesis inhibitors | Inhibiting the RNA-dependent RNA polymerase activity of SARS-CoV-2. |
| Lopinavir/Ritonavir | biosynthesis inhibitors | Inhibiting the 3-chymotrypsin-like protease activity of SARS-CoV-2. |
| Convalescent plasma | Virus neutralizer | Neutralizing the virus in body fluids to decrease the quantity of SARS-CoV-2. |
| Monoclonal antibody | Virus neutralizer | Neutralizing the virus in body fluids to decrease the quantity of SARS-CoV-2. |
| IFNs | Antiviral therapy and improving immunity | Inducing cells to synthesize antiviral proteins and activating natural killer cells, T cells, and macrophages to inhibit viruses. |
Etiological treatment for COVID-19
The etiological treatment for COVID-19 means antiviral therapy for it, which depends on antiviral drugs and bioproducts. The processes of virus infection could be described as attachment, penetration, uncoating, biosynthesis, assembly, and release. After that, the virus complete its proliferation and causes damage to host cells. Similarly, the mechanism of antiviral therapy is through disturbing these processes. Thus, the medicines for antiviral treatment of COVID-19 are divided into penetration and uncoating inhibitors, biosynthesis inhibitors, and assembly inhibitors.
Antiviral medicines for COVID-19
Penetration and uncoating inhibitors of SARS-Cov-2 mainly include chloroquine, arbidol, and camostat mesilate. Chloroquine is an efficient drug for treatment of malaria as well as autoimmune rheumatic diseases. Wang and colleagues treated Vero E6 cells that were infected by SARS-CoV-2 with several drugs in vitro and found a low half-maximal effective concentration at 1.13 μmol and a high selectivity index of chloroquine, indicating that chloroquine probably inhibits the viral infection.64 There have been tens of clinical trials to confirm the safety and efficiency of chloroquine in treating COVID-19 patients, and its mechanism can be described as interfering with the glycosylation of ACE2 or alkalizing the phagolysosome to inhibit viral replication,65, 66 which prevents the SARS-Cov-2 entering the host cells. The in vitro study indicated that chloroquine only had an antiviral effect under high doses. However, the clinical data of chloroquine showed the mortality of high-dosage group was apparently higher than the low-dosage group, but there was no significant difference in efficacy,67 and the QT interval of some COVID-19 patients was prolonged after hydroxychloroquine/azithromycin treatment,68 thus it might not be an effective medicine for COVID-19. Arbidol is the other penetration inhibitor that suppresses the fusion of the virus lipid membrane and host cells to block the replication of the virus. Arbidol also has the function of interferon (IFN) inducement, thus it has been widely used to treat A and B influenza viruses. It is promising that several studies of arbidol have shown a significant therapeutic effect for curing COVID-19 patients. Wang and colleagues found arbidol reduced the mortality and improved discharging rate by analysis of 67 discharged COVID-19 patients.69 Furthermore, the other study compared the treatment effects between combination of arbidol and lopinavir/ritonavir, and simple lopinavir/ritonavir, finding that their CT scan improvement rates are 69% and 29%, and viral RNA decrement rates are 75% and 35%, respectively.70 Thus, arbidol perhaps serves as a specific treatment for COVID-19. However, another clinical report pointed out arbidol could not improve the symptoms or accelerate the clearance of SARS-CoV-2.71 Thus, further studies are needed to validate its antiviral efficiency. Camostat mesylate is a specific drug for inhibiting TMPRSS2, a protease used for S protein priming to promote the entry of SARS-CoV-2, thus it is another potential penetration inhibitor.23 However, there is still lack of clinical evidence to prove its effectiveness.
The biosynthesis inhibitors for SARS-Cov-2 chiefly contain remdesivir, sofosbuvir, ribavirin, and lopinavir/ritonavir. Remdesivir is as kind of broad spectrum antiviral medicine that suppresses the virus replication through inhibiting the activity of RNA-dependent RNA polymerase,72, 73 and its efficiency has been widely confirmed in coronaviruses such as SARS-CoV and MERS-CoV. Recently, remdesivir has been found to be a potential drug to treat COVID-19. A previous study showed that half-maximal effective concentration of remdesivir was 0.77 in an in vitro test treating COVID-19 with a high selectivity index, demonstrating its therapeutic effect.64 There, the first COVID-19 case of America was reported to be cured completely after intravenous injection of remdesivir,74 and clinical data showed that remdesivir dramatically alleviated clinical symptoms and reduced mortality for severe patients,75 which demonstrated it is a potential drug for treating novel pneumonia. However, a patient died of hypotension and cardiac arrest after remdesivir treatment in a randomized controlled trial of Ebola virus (EBOV) treatment,76 and 60% COVID-19 patients had side effects after using remdesivir,75 which indicated its security is worthy of further confirmation. Up to now, several agencies have published conflicting clinical results from remdesivir.77, 78 Thus, more research should be conducted to verify its curative efficacy. Furthermore, in vitro tests showed that other broad-spectrum antiviral medicines such as sofosbuvir and ribavirin interacted with RNA-dependent RNA polymerase of SARS-CoV-2, which prevents the replication of virus, thus they can also serve as antiviral drugs for COVID-19.79 Lopinavir and ritonavir are suppressors of 3-chymotrypsin-like protease, a protease of coronavirus. Furthermore, ritonavir suppresses the activity of cytochrome P450 isoenzymes and thus elevates plasma concentration of other medicines. Therefore, combining lopinavir and ritonavir has a good inhibitory effect on virus biosynthesis, which was confirmed in the treatment of SARS-CoV and MERS-CoV.80 Recently, many COVID-19 patients have received lopinavir/ritonavir therapy and to good effect.81 Moreover, the first report of the lopinavir/ritonavir clinical trial results have been published on 19 March 2020. In that study, 99 patients were assigned to the lopinavir/ritonavir group and 100 patients were treated with the routine therapy, and the median time of clinical improvement was advanced by 1 day when given the lopinavir/ritonavir treatment.82 But it failed to accelerate clinical improvement significantly, and reduce mortality and viral RNA detected in the throat.82 Similarly, the clinical trial in severe COVID-19 patients indicated lopinavir/ritonavir was of no benefit compared to standard care.83 Therefore, further studies are needed to identify or exclude the possible benefits of lopinavir/ritonavir-based therapies.
Antiviral biological products for COVID-19
The most common antiviral products are IFNs, which induce cells to synthesize antiviral proteins and thus inhibit all processes of the viral replication cycle. Furthermore, it could also enhance immunity of patients, so it is widely used for therapy for multiple viruses such as MERS-CoV.84 In this outbreak, IFNs combined with antiviral drugs were recommended to treat COVID-19,85 which has achieved good clinical therapeutic effect. Xu et al. reported that the combination of IFNs and arbidol or lopinavir/ritonavir cured 62 COVID-19 patients in the Zhejiang province.50 In addition, a combination use of IFN beta-1b, ribavirin, and lopinavir-ritonavir was found to be more effective than pure lopinavir-ritonavir.86 These therapies have been initiated into multiple clinical trials.
Convalescent plasma therapy (CPT) is based on the principle of using a certain titer of viral-specific antibodies in the recovered plasma to obtain passive immunity, neutralize specific pathogens, and eventually clear the pathogens in blood circulation, thus achieving the treatment expectation.87 Luckily, key indicators of laboratory testing, clinical signs, and symptoms of several COVID-19 patients were confirmed to improve significantly after CPT,88 so CPT is recommended for COVID-19 treatment. Currently, clinical trials are under way to further evaluate the efficiency and safety of CPT to COVID-19. The monoclonal antibody is a highly uniform antibody that is produced by a single B cell and specific to target the antigen epitopes, which have been confirmed to suppress viruses entering host cells extracellularly for many coronaviruses including SARS-CoV-2.89, 90 Tian et al. reported that monoclonal antibody CR3022 bound stably to receptor-binding domain of SARS-CoV-2 (KD of 6.3 nM),91 thus it potentially cures COVID-19. Nevertheless, the development of monoclonal antibodies takes a certain period of time. For new pathogens, monoclonal antibodies are a research direction, but it is difficult to achieve clinical application in a short time.
Complications treatment of COVID-19
Like most diseases, the main treatments of the complications of COVID-19 are to strengthen supportive treatment, ensure adequate energy, and pay attention to water and electrolyte balance to maintain internal environment homeostasis.55 For COVID-19, hypoxia is a typical clinical symptom of COVID-19, thus oxygen inhalation is the essential treatment for both mild and severe patients.92, 93
It is worth noting that severe cases of COVID-19 often develop severe inflammation, shock, combination of bacterial infection, and severe kidney damage as well as acute respiratory distress syndrome (ARDS), which often result in death.94 Thus, timely complications treatment is necessary for critical patients. Glucocorticoids such as methylprednisolone and dexamethasone have strong anti-inflammatory as well as antishock effects, so they are usually used to save critical patients, especially patients with ARDS.95 However, they suppress immunity, cause femoral head necrosis of patients, and cannot save patients with shock who have increased intrathoracic pressure, thus WHO did not recommend using glucocorticoids initially.96 For these situations, physicians suggested that short-term administration of glucocorticoids should be adopted to decrease the side effects.97 Moreover, considering the immunosuppressive action, immune boosters such as α-IFN and thymosin are important for use in avoiding adverse events. For severe cytokine storms, simple glucocorticoids cannot suppress the inflammation efficiently, so potent blood purification measures such as an artificial liver system are essential.98 The processes of urgent antishock therapy mainly contain complements of blood volume, improving cardiac contractility and vascular activity, thus vasoactive agents and positive inotropic drugs such as epinephrine, dopamine, and norepinephrine99 are necessary for first aid of critical COVID-19 patients. For patients with severe kidney damage, we should actively search for the primary cause and pay attention to the water, electrolyte, and acid-base balance. When homeostasis is decompensated and/or multiple organ failure occurs, continuous renal replacement therapy (CRRT) should be adopted for treatment.100 ARDS is one of most critical complications for patients, which is known for its high mortality rate, especially for pregnant patients.101 For its therapy, ECMO is usually recommended and has a certain curative effect.102 However, its complex operation and low cure rate make it difficult to perform in a primary hospital. Luckily, stem cells and their extracellular vesicles are able to repair the damage and relieve lung symptoms, thus they have been used to cure ARDS.103 Currently, some COVID-19 patients have been treated with umbilical cord mesenchymal stem cells and achieved good effects.104 Therefore, stem cells may be the hope for the treatment of severe COVID-19 patients. Tens of clinical trials of stem cell treatment have been registered in Chinese Clinical Trial Registry. Recently, many COVID-19 patients were found to have thrombotic risk,105 therefore, antithrombotic treatments have potential application value in the treatment of COVID-19.
Furthermore, patients with underlying diseases such as hypertension, diabetes, and cardiovascular disease have a potential bad prognosis,106–108 so care should be taken to cure these basic diseases during treatment. COVID-19 patients with low immunity are susceptible to hospital-acquired bacterial infections,109 therefore, antibiotics such as amoxicillin are another symptomatic treatment medicine for COVID-19.
Traditional Chinese medicine for treatment of COVID-19
Traditional Chinese medicine has been confirmed to play a vital role in treating many respiratory viruses such as SARS-CoV. Furthermore, the General Office of National Health Committee pointed out that Chinese medicines such as qingfei paidu decoction, qingfei touxie fuzheng recipe, and sheganmahuang decoction could be used for the whole therapeutic process of COVID-19,55 thus they are widely used for COVID-19 treatment currently. The present clinical evidence has shown that a combination of western and Chinese medicines apparently improved curative effect.110 For example, Wan et al. treated 124 patients with multiple traditional Chinese medicines such as Suhuang Zhike capsule and Xuebijing, and found their respiratory symptoms were significantly improved in a shorter time.109 Furthermore, four cases treated with antiviral drugs and Shufeng Jiedu Capsule had an obvious improvement both in symptoms and blood biochemical indexes.111 However, lack of a control group means these results are not reliable enough.
Although treatment based on syndrome differentiation and lack of scientific evidence on mechanisms are typical characteristics of traditional Chinese medicines, many of them are gradually found to have antiviral potential for SARS-CoV-2. Theaflavin is conventional ingredient of Chinese medicine, which was found to have hydrogen bonds to RNA-dependent RNA polymerase with a binding energy of −8.8 kcal/mol,112 thus it possibly inhibits the proliferation of SARS-CoV-2. Lianhuaqingwen was also confirmed to significantly inhibit the replication of SARS-CoV-2 and suppress the inflammatory factors TNF-α, IL-6, CCL-2/MCP-1, and CXCL-10/IP-10 in Vero E6 cells,113 so it is potentially a specific medicine for COVID-19 both etiologically and symptomatically.
Prevention and control of COVID-19
The prevention and control of COVID-19 can be divided into controlling the source of infection, cutting off the transmission route, and protecting susceptible people. Controlling the source of infection in this outbreak mainly refers to timely isolation of suspected and confirmed cases, which greatly depends on wireless communication data.114 Isolation of convalescent patients is also necessary, because some of them have a positive RT-PCR test even after 13 days of recovery.115 Cutting off the transmission route includes sterilizing and wearing suitable masks such as N95, KN95, and medical surgical masks.116 Protecting susceptible people means self-segregation, improving constitution, and prophylactic vaccination.
Vaccine
Considering the strong infectivity of COVID-19, the secondary outbreak of COVID-19 can be prevented only if the public has immunity to SARS-CoV-2, thus vaccine is the key area among all prevention measures. Therefore, scientists all over the world are working on the vaccine for SARS-CoV-2, aiming to completely control the outbreak. After a long period of effort, the vaccine targets of the virus had been found and shared with the world.117, 118 Soon afterward, medical institutions around the world adapted joint research and identified five development approaches, including a live attenuated vaccine, inactivated whole-virus vaccine, nucleic acid vaccine, protein vaccine, and viral vector-based vaccine (Table 4),119 and most of them used the S protein of SARS-CoV-2 as the main inducer of neutralizing antibodies directly, or inducing the expression of the whole S protein or receptor-binding domain of the S protein indirectly.120 By interaction of the S protein and ACE2 of host cells, uncoating and penetration of SARS-CoV-2 are induced.23 Thus, S-protein-based vaccines potentially inhibit the uncoating and penetration of the virus.
Table 4.
The major COVID-19 vaccines entering clinical trials worldwide.
| Sponsor | Candidate vaccine | Registration ID | ETC |
|---|---|---|---|
| Henan Provincial Center for Disease Control and Prevention | Inactivated vaccine | ChiCTR2000031809 | 10 November 2021 |
| Henan Provincial Center for Disease Control and Prevention | Inactivated vaccine | ChiCTR2000032459 | 28 November 2021 |
| Sinovac Biotech Co., Ltd | Inactivated vaccine | NCT04352608 | 13 December 2020 |
| Shenzhen Geno-Immune Medical Institute | Lentiviral vector vaccine | NCT04299724, NCT04276896 | 31 July 2023 |
| Insitute of Biotechnology, Academy of Military Medical Sciences, PLA of China | Adenovirus vector vaccine | NCT04341389 | 31 January 2021 |
| CanSino Biologics Inc. | Adenovirus vector vaccine (Adenovirus Type 5 Vector) | NCT04313127 | 30 December 2020 |
| Inovio Pharmaceuticals | DNA vaccine (INO-4800) | NCT04336410 | November 2020 |
| Symvivo Corporation | DNA vaccine | NCT04334980 | 31 August 2021 |
| National Institute of Allergy and Infectious Diseases (NIAID) | mRNA vaccine (mRNA-1273) | NCT04283461 | 1 June 2021 |
| Biontech SE | 4 RNA vaccines | NCT04368728 | 27 January 2023 |
| Novavax | Recombinant Spike Protein Nanoparticle Vaccine | NCT04368988 | 31 July 2021 |
ECT: Estimated time of completion.
Live attenuated vaccine and inactivated whole-virus vaccine
Whole-virus vaccines are conventional strategies to develop a vaccine for most viruses. By injecting artificially attenuated or inactivated viruses, pathogens trigger the body's immune response without causing disease. Compared to other vaccines, these keep the inherent immunogenicity of SARS-CoV-2 and stimulate the body's innate immunity such as releasing toll-like receptors. However, live viruses have the potential to cause disease. Furthermore, it has been reported that patients were more easily infected by SARS-CoV after immunization with a live attenuated vaccine or inactivated whole-virus vaccine.121 Thus, sufficient experiments are necessary to verify their safety. Currently, three inactivated novel coronavirus pneumonia vaccines have entered the phase I/II clinical trials.122–124
Nucleic acid vaccine
Nucleic acid vaccines, including DNA vaccines and mRNA vaccines, are to directly deliver foreign genes encoding antigenic proteins into host cells and synthesize the antigenic proteins by host cells to induce the immunity of hosts. Nucleic acid vaccines of COVID-19 mainly aim to induce the whole S protein or receptor-binding domain of S protein expression, and several of them have entered clinical trials.125 For example, researchers of Inovio Pharmaceuticals are conducting the clinical trial of a DNA vaccine.126 In the meantime, the National Institute of Allergy and Infectious Diseases have developed a mRNA vaccine, mRNA-1273, which uses lipid nanoparticles to encapsulate the mRNA encoding S protein of SARS-CoV-2.127 Interestingly, Symvivo Corporation have developed an oral DNA vaccine to avoid invasive injection. It consists of live Bifidobacterium longum, which contain the synthetic DNA encoding S protein.128
Protein vaccine
The protein vaccines of SARS-CoV-2 mainly include the whole S protein, recombinant S protein, and receptor-binding domain of the S protein, aiming to stimulate the body to produce corresponding neutralizing antibodies directly. Up to now, several subunit vaccines have been developed. For instance, Vaxart developed an oral recombinant protein vaccine to stimulate the mucosal immune responses for COVID-19.129 Up to now, some protein vaccines have entered clinical trials.130
Viral vector-based vaccine
Viral vector-based vaccine is a kind of carrier vaccine that induces an immune response by inserting an exogenous antigen gene into the viral genome to obtain a recombinant virus, and then express the corresponding target protein after vaccination. The viral vector-based vaccines of COVID-19 are engineered with the insertion of genes encoding the full length S protein or S protein receptor-binding domain, which include the lentiviral vector vaccine and adenovirus vector vaccine.131–134 Shenzhen Geno-Immune Medical Institute developed a lentiviral vector system (NHP/TYF) for expression of the S protein in human body to activate T cells.133, 134 Furthermore, Adenovirus vector vaccine (Adenovirus Type 5 Vector) sponsored by Insitute of Biotechnology, Academy of Military Medical Sciences, PLA of China, has entered phase II clinical trials.132
Asymptomatic patients of COVID-19
Although patients with typical symptoms are easily diagnosed and isolated, we still need to pay attention to asymptomatic carriers.135 In January 2020, a family cluster of three COVID-19 patients with one symptomatic and two asymptomatic carriers drew great attention.11 Because any one of them could have the first one to transmit the virus to others, there may exist asymptomatic patients with high infectivity who have not been detected. At the same time, another family cluster of five patients was found to have contact with an asymptomatic carrier before onset of their symptoms.136 According to time sequence, it is obvious that the virus was transmitted by an asymptomatic carrier. Hereafter, the phenomenon of asymptomatic infection has been reported in several articles.137, 138 On the basis of virology of SARS-CoV-2, quantity of shedding SARS-CoV-2 from pharyngeal sources is very high in the first week of symptoms, and they also replicate actively during this time, thus the first week after onset of symptoms is when the patients have the highest infectivity.139 But in this time period, some patients only have mild symptoms or even no symptoms, so they might be invisible sources of infection. Therefore, people who have traveled to endemic areas or had contact with patients should be isolated, monitored, and screened strictly, even if they are asymptomatic, to rule out the possibility of infection.
Conclusions and future perspectives
COVID-19, a disease that seriously endanger the safety of human life and properties, is caused by SARS-CoV-2, which attacks host cells through ACE2. The spread of COVID-19 mainly depends on airborne and droplet transmission. Some researchers pointed out the possibility of fecal-oral transmission and vertical transmission, but these opinions need further confirmation. An accurate diagnosis of COVID-19 is essential for containing disease, thus combnation of RT-PCR and clinical characteristics is widely used for its detection currently. The typical clinical features of COVID-19 patients include fever, cough, and dyspnea. Moreover, GGO, consolidation, and interlobular septal thickening are also typical CT signals of COVID-19. Using artificial intelligence to screen COVID-19 early on is likely to be the future diagnostic directions of it.
Good treatment is necessary to reduce mortality, and drug therapy is the main treatment of COVID-19. Thus, we summarize the chief medicines and bioproducts for its treatment. According to the current reports, combined use of the drugs and bioproducts to treat COVID-19 both etiologically and symptomatically has received great clinical effect. However, not all antiviral drugs for COVID-19 have passed the clinical trials. A few clinical trial reports demonstrated that lopinavir-ritonavir, chloroquine, and remdesivir did not work as well as expected,67, 75, 82 which reflects the fact that many antiviral drugs have gone through many extensive clinical trials with currently only modest effect. Instead, some bioproducts such as monoclonal antibodies and stem cells are more likely to play important roles in treatment of severely affected patients in the future. Furthermore, we should also focus on vaccine research and artificial intelligence-based studies for fighting the outbreak, both of which play vital roles in controlling the it.
Although antiviral effects of many drugs and vaccines have been obtained in vitro, it remains to be seen as to whether they have comparable activity in vivo. Therefore, establishing efficient animal models as soon as possible is important for virology research, drug, and vaccine development. Currently, rhesus macaques models have been established to study the transmission and distribution of SARS-CoV-2 as well as effects of viral infection on host prognosis,140, 141 which is meaningful for virology study. Furthermore, hACE2 transgenic mice have been found to own typical COVID-19 clinical symptoms, which could not be found in wild-type mice,142 indicating this model may be used for development of therapeutic drugs and vaccines. Thus, perhaps further researching effective animal models and making full use of them shows the future direction to develop drugs and vaccines with high specificity as soon as possible.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grants No. 81871890 and 91859203)and the Science and Technology Project of Chengdu for Precision Medicine Research (grant No. 2017-CY02-00030-GX).
Contributor Information
Jingwei Li, Department of Respiratory and Critical Care Medicine, West China Medical School/West China Hospital, Sichuan University, Chengdu 610041, China.
Jun Shao, Department of Respiratory and Critical Care Medicine, West China Medical School/West China Hospital, Sichuan University, Chengdu 610041, China.
Chengdi Wang, Department of Respiratory and Critical Care Medicine, West China Medical School/West China Hospital, Sichuan University, Chengdu 610041, China.
Weimin Li, Department of Respiratory and Critical Care Medicine, West China Medical School/West China Hospital, Sichuan University, Chengdu 610041, China.
Author contributions
J.L., J.S., and C.W. wrote the initial draft of the manuscript. J.L. and C.W. gathered important information. C.W. and W.L. provided critical revision of the manuscript.
Conflict of interests
All authors declared that they had no conflict of interest.
References
- 1. Li Q, Guan X, Wu P, et al.. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–207. doi:10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhu N, Zhang D, Wang W, et al.. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–33. doi:10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020. doi:10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 4. Spellberg B, Haddix M, Lee R, et al.. Community prevalence of SARS-CoV-2 among patients with influenzalike illnesses presenting to a Los Angeles Medical Center in March 2020. JAMA. 2020. doi:10.1001/jama.2020.4958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Phan LT, Nguyen TV, Luong QC, et al.. Importation and human-to-human transmission of a novel coronavirus in Vietnam. N Engl J Med. 2020;382:872–4. doi:10.1056/NEJMc2001272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McMichael TM, Currie DW, Clark S, et al.. Epidemiology of Covid-19 in a long-term care facility in King County, Washington. N Engl J Med. 2020;382:2005–11. doi:10.1056/NEJMoa2005412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lim J, Jeon S, Shin HY, et al.. The author's response: case of the index patient who caused tertiary transmission of coronavirus disease 2019 in Korea: the application of Lopinavir/Ritonavir for the treatment of COVID-19 pneumonia monitored by quantitative RT-PCR. J Korean Med Sci. 2020;35:e89. doi:10.3346/jkms.2020.35.e89. [DOI] [PubMed] [Google Scholar]
- 8. Wang Y, Kang H, Liu X, et al.. Combination of RT-qPCR testing and clinical features for diagnosis of COVID-19 facilitates management of SARS-CoV-2 outbreak. J Med Virol. 2020. doi:10.1002/jmv.25721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ramanathan K, Antognini D, Combes A, et al.. Planning and provision of ECMO services for severe ARDS during the COVID-19 pandemic and other outbreaks of emerging infectious diseases. Lancet Resp Med. 2020;8:518–26. doi:10.1016/S2213-2600(20)30121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Matthay MA, Aldrich JM, Gotts JE. Treatment for severe acute respiratory distress syndrome from COVID-19. Lancet Resp Med. 2020;8:433–4. doi:10.1016/S2213-2600(20)30127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pan X, Chen D, Xia Y, et al.. Asymptomatic cases in a family cluster with SARS-CoV-2 infection. Lancet Infectious Dis. 2020;20:410–1. doi:10.1016/S1473-3099(20)30114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nature Microbiol. 2020;5:536–44. doi:10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhou P, Yang XL, Wang XGet al.. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–3. doi:10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wu F, Zhao S, Yu B, et al.. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–9. doi:10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang YZ, Holmes EC. A genomic perspective on the origin and emergence of SARS-CoV-2. Cell. 2020;181:223–7. doi:10.1016/j.cell.2020.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lu R, Zhao X, Li J, et al.. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–74. doi:10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ji W, Wang W, Zhao X, et al.. Cross-species transmission of the newly identified coronavirus 2019-nCoV. J Med Virol. 2020;92:433–40. doi:10.1002/jmv.25682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chaw SM, Tai JH, Chen SL, et al.. The origin and underlying driving forces of the SARS-CoV-2 outbreak. J Biomed Sci. 2020;27:73. 10.1186/s12929-020-00665-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu Z, Xiao X, Wei X, et al.. Composition and divergence of coronavirus spike proteins and host ACE2 receptors predict potential intermediate hosts of SARS-CoV-2. J Med Virol. 2020. doi:10.1002/jmv.25726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li H, Liu L, Zhang D, et al.. SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet North Am Ed. 2020;395:1517–20. doi:10.1016/s0140-6736(20)30920-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tortorici MA, Veesler D. Structural insights into coronavirus entry. Adv Virus Res. 2019;105:93–116. doi:10.1016/bs.aivir.2019.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xia S, Zhu Y, Liu M, et al.. Fusion mechanism of 2019-nCoV and fusion inhibitors targeting HR1 domain in spike protein. Cell Mol Immunol. 2020. doi:10.1038/s41423-020-0374-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hoffmann M, Kleine-Weber H, Schroeder S, et al.. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–80. doi:10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Goh GK, Dunker AK, Foster JA, et al.. Rigidity of the outer shell predicted by a protein intrinsic disorder model sheds light on the COVID-19 (Wuhan-2019-nCoV) infectivity. Biomolecules. 2020;10:331. doi:10.3390/biom10020331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Choi JY, Lee HK, Park JH, et al.. Altered COVID-19 receptor ACE2 expression in a higher risk group for cerebrovascular disease and ischemic stroke. Biochem Biophys Res Commun. 2020; . doi:10.1016/j.bbrc.2020.05.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wuhan Municipal Health Commission. Information about the current situation of pneumonia in Wuhan, 2019, wjw.wuhan.gov.cn/front/web/showDetail/2019123108989, accessed date: February, 29, 2020. [Google Scholar]
- 27. WHO. Coronavirus disease 2019 (COVID-19) Situation Report – 117, 2020, https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200516-covid-19-sitrep-117.pdf?sfvrsn = 8f562cc_2, accessed date: May 17, 2020. [Google Scholar]
- 28. Linton NM, Kobayashi T, Yang Y, et al.. Incubation period and other epidemiological characteristics of 2019 novel coronavirus infections with right truncation: a statistical analysis of publicly available case data. J Clin Med. 2020;9:538. doi:10.3390/jcm9020538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chan JF, Yuan S, Kok KHet al.. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–23. doi:10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wu P, Duan F, Luo C, et al.. Characteristics of ocular findings of patients with coronavirus disease 2019 (COVID-19) in Hubei Province, China. JAMA Ophthalmol. 2020. doi:10.1001/jamaophthalmol.2020.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yeo C, Kaushal S, Yeo D. Enteric involvement of coronaviruses: is faecal–oral transmission of SARS-CoV-2 possible?. Lancet Gastroenterol Hepatol. 2020;5:335–7. doi:10.1016/s2468-1253(20)30048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Xu Y, Li X, Zhu B, et al.. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat Med. 2020;26:502–5. doi:10.1038/s41591-020-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang W, Xu Y, Gao R, et al.. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020. doi:10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dong L, Tian J, He S, et al.. Possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn. JAMA. 2020. doi:10.1001/jama.2020.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen H, Guo J, Wang C, et al.. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395:809–15. doi:10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wei M, Yuan J, Liu Y, et al.. Novel coronavirus infection in hospitalized infants under 1 year of age in China. JAMA. 2020. doi:10.1001/jama.2020.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zeng H, Xu C, Fan J, et al.. Antibodies in infants born to mothers with COVID-19 pneumonia. JAMA. 2020. doi:10.1001/jama.2020.4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Liu W, Zhang Q, Chen J, et al.. Detection of Covid-19 in children in early january 2020 in Wuhan, China. N Engl J Med. 2020;382:1370–1. doi:10.1056/NEJMc2003717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. The Novel Coronavirus Pneumonia Emergency Response Epidemiology. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19)—China, 2020. China CDC Weekly. 2020;2:113–22. doi:10.3760/cma.j.issn.0254-6450.2020.02.003. [PMC free article] [PubMed] [Google Scholar]
- 40. Team C. C.-R. Severe outcomes among patients with coronavirus disease 2019 (COVID-19) - United States, February 12-March 16, 2020. MMWR Morbidity and Mortality Weekly Rep. 2020;69:343–6. doi:10.15585/mmwr.mm6912e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sanche S, Lin YT, Xu C, et al.. High contagiousness and rapid spread of severe acute respiratory syndrome coronavirus 2. Emerg Infect Dis. 2020;26. doi:10.3201/eid2607.200282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Team IC. C.-R. Report 13 - Estimating the number of infections and the impact of non-pharmaceutical interventions on COVID-19 in 11 European countries, 2020, https://www.imperial.ac.uk/mrc-global-infectious-disease-analysis/covid-19/report-13-europe-npi-impact/, accessed date: April, 16, 2020. [Google Scholar]
- 43. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020. doi:10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 44. Chang D, Lin M, Wei L, et al.. Epidemiologic and clinical characteristics of novel coronavirus infections involving 13 patients outside Wuhan, China. JAMA. 2020;323:1092–3. doi:10.1001/jama.2020.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wu JT, Leung K, Bushman M, et al.. Estimating clinical severity of COVID-19 from the transmission dynamics in Wuhan, China. Nat Med. 2020;26:506–10. doi:10.1038/s41591-020-0822-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Guan WJ, Ni ZY, Hu Y, et al.. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–20. doi:10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chen N, Zhou M, Dong X, et al.. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–13. doi:10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang D, Hu B, Hu C, et al.. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020. doi:10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Arentz M, Yim E, Klaff L, et al.. Characteristics and outcomes of 21 critically Ill patients with COVID-19 in Washington State. JAMA. 2020. doi:10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Xu XW, Wu XX, Chen KD, et al.. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368:m606. doi:10.1136/bmj.m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhang JJ, Dong X, Cao YY, et al.. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020. doi:10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 52. Wei J, Xu H, Xiong J, et al.. 2019 novel coronavirus (COVID-19) pneumonia: serial computed tomography findings. Korean J Radiol. 2020;21:501–4. doi:10.3348/kjr.2020.0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Guan WJ, Ni ZY, Hu Y, et al.. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–20. doi:10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Li K, Wu J, Wu F, et al.. The clinical and chest CT features associated with severe and critical COVID-19 pneumonia. Invest Radiol. 2020; 55:327–31. doi:10.1097/RLI.0000000000000672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Committee, G. O. o. N. H. Diagnosis and Treatment Program of Novel Coronavirus Pneumonia (trial seventh version), 2020, http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989.shtml, accessed date: March 4, 2020. [Google Scholar]
- 56. Zhang J, Zhou L, Yang Y, et al.. Therapeutic and triage strategies for 2019 novel coronavirus disease in fever clinics. Lancet Resp Med. 2020;8:e11–2. doi:10.1016/S2213-2600(20)30071-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ai T, Yang Z, Hou H, et al.. Correlation of chest CT and RT-PCR testing in Coronavirus Disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020;200642. doi:10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Huang C, Wang Y, Li X, et al.. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi:10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. To KK, Tsang OT, Leung WS, et al.. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infectious Dis. 2020;20:565–74. doi:10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Guo L, Ren L, Yang S, et al.. Profiling early humoral response to diagnose novel coronavirus disease (COVID-19). Clin Infect Dis. 2020. doi:10.1093/cid/ciaa310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Pan F, Ye T, Sun P, et al.. Time course of lung changes on chest CT during recovery from 2019 novel coronavirus (COVID-19) pneumonia. Radiology. 2020;295:715–21. doi:10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zhang K, Liu X, Shen J, et al.. Clinically applicable AI system for accurate diagnosis, quantitative measurements, and prognosis of COVID-19 pneumonia using computed tomography. Cell. 2020. doi:10.1016/j.cell.2020.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Broughton JP, Deng X, Yu G, et al.. CRISPR-Cas12-based detection of SARS-CoV-2. Nat Biotechnol. 2020. doi:10.1038/s41587-020-0513-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wang M, Cao R, Zhang L, et al.. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–71. doi:10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Gao J, Tian Z, Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Bioscience Trends. 2020;14:72–3. doi:10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 66. Colson P, Rolain JM, Raoult D. Chloroquine for the 2019 novel coronavirus SARS-CoV-2. Int J Antimicrob Agents. 2020;55:105923. doi:10.1016/j.ijantimicag.2020.105923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Borba MGS, Val FFA, Sampaio VS, et al.. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: a randomized clinical trial. JAMA Netw Open. 2020;3:e208857. doi:10.1001/jamanetworkopen.2020.8857. [DOI] [PubMed] [Google Scholar]
- 68. Chorin E, Dai M, Shulman E, et al.. The QT interval in patients with COVID-19 treated with hydroxychloroquine and azithromycin. Nat Med. 2020. doi:10.1038/s41591-020-0888-2. [DOI] [PubMed] [Google Scholar]
- 69. Wang Z, Yang B, Li Q, et al.. Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin Infectious Dis. 2020. doi:10.1093/cid/ciaa272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Deng L, Li C, Zeng Q, et al.. Arbidol combined with LPV/r versus LPV/r alone against corona virus disease 2019: a retrospective cohort study. J Infect. 2020. doi:10.1016/j.jinf.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lian N, Xie H, Lin S, et al.. Umifenovir treatment is not associated with improved outcomes in patients with coronavirus disease 2019: a retrospective study. Clin Microbiol Infect. 2020. doi:10.1016/j.cmi.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Gao Y, Yan L, Huang Y, et al.. Structure of the RNA-dependent RNA polymerase from COVID-19 virus. Science. 2020;368:779–82. doi:10.1126/science.abb7498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Yin W, Mao C, Luan X, et al.. Structural basis for inhibition of the RNA-dependent RNA polymerase from SARS-CoV-2 by remdesivir. Science. 2020. doi:10.1126/science.abc1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Holshue ML, DeBolt C, Lindquist S, et al.. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929–36. doi:10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Grein J, Ohmagari N, Shin D, et al.. Compassionate use of remdesivir for patients with severe Covid-19. N Engl J Med. 2020. doi:10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Mulangu S, Dodd LE, Davey RT Jr, et al.. A randomized, controlled trial of ebola virus disease therapeutics. N Engl J Med. 2019;381:2293–303. doi:10.1056/NEJMoa1910993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wang Y, Zhang D, Du G, et al.. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet North Am Ed. 2020;395:1569–78. doi:10.1016/s0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. National Institutes of Health. NIH Clinical Trial Shows Remdesivir Accelerates Recovery from Advanced COVID-19, 2020, https://www.niaid.nih.gov/news-events/nih-clinical-trial-shows-remdesivir-accelerates-recovery-advanced-covid-19, accessed date: May, 4, 2020. [Google Scholar]
- 79. Elfiky AA. Anti-HCV, nucleotide inhibitors, repurposing against COVID-19. Life Sci. 2020;248:117477. doi:10.1016/j.lfs.2020.117477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Maxmen A. More than 80 clinical trials launch to test coronavirus treatments. Nature. 2020;578:347–8. doi:10.1038/d41586-020-00444-3. [DOI] [PubMed] [Google Scholar]
- 81. Young BE, Ong SWX, Kalimuddin S, et al.. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA. 2020. doi:10.1001/jama.2020.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Cao B, Wang Y, Wen D, et al.. A trial of Lopinavir-Ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382:1787–99. doi:10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Cao B, Wang Y, Wen D, et al.. A trial of Lopinavir-Ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382:1787–99. doi:10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Li HS, Kuok DIT, Cheung MC, et al.. Effect of interferon alpha and cyclosporine treatment separately and in combination on Middle East Respiratory Syndrome Coronavirus (MERS-CoV) replication in a human in-vitro and ex-vivo culture model. Antiviral Res. 2018;155:89–96. doi:10.1016/j.antiviral.2018.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Li G, De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV). Nat Rev Drug Discov. 2020;19:149–50. doi:10.1038/d41573-020-00016-0. [DOI] [PubMed] [Google Scholar]
- 86. Hung IF, Lung KC, Tso EY, et al.. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020;395:1695–1704. doi:10.1016/S0140-6736(20)31042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Chen L, Xiong J, Bao L, et al.. Convalescent plasma as a potential therapy for COVID-19. Lancet Infect Dis. 2020;20:398–400. doi:10.1016/s1473-3099(20)30141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Shen C, Wang Z, Zhao F, et al.. Treatment of 5 critically Ill patients with COVID-19 with convalescent plasma. JAMA. 2020. doi:10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Wang C, Li W, Drabek D, et al.. A human monoclonal antibody blocking SARS-CoV-2 infection. Nat Commun. 2020;11:2251. doi:10.1038/s41467-020-16256-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Wu Y, Wang F, Shen C, et al.. A noncompeting pair of human neutralizing antibodies block COVID-19 virus binding to its receptor ACE2. Science. 2020. doi:10.1126/science.abc2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Tian X, Li C, Huang A, et al.. Potent binding of 2019 novel coronavirus spike protein by a SARS coronavirus-specific human monoclonal antibody. Emerg Microbes Infect. 2020;9:382–5. doi:10.1080/22221751.2020.1729069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Qiu H, Wu J, Hong L, et al.. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: an observational cohort study. Lancet Infectious Dis. 2020;20:689–96. doi:10.1016/S1473-3099(20)30198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Sorbello M, El-Boghdadly K, Di Giacinto I, et al.. The Italian coronavirus disease 2019 outbreak: recommendations from clinical practice. Anaesthesia. 2020;75:724–32. doi:10.1111/anae.15049. [DOI] [PubMed] [Google Scholar]
- 94. Chen T, Wu D, Chen H, et al.. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi:10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Villar J, Ferrando C, Martínez D, et al.. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. The Lancet Respiratory Medicine. 2020;8:267–76. doi:10.1016/S2213-2600(19)30417-5. [DOI] [PubMed] [Google Scholar]
- 96. Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet North Am Ed. 2020;395:473–5. doi:10.1016/s0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Stebbing J, Phelan A, Griffin I, et al.. COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infectious Dis. 2020;20:400–2. doi:10.1016/S1473-3099(20)30132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Zhang C, Huang S, Zheng F, et al.. Controversial treatments: an updated understanding of the Coronavirus Disease 2019. J Med Virol. 2020. doi:10.1002/jmv.25788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Udesen NLJ, Helgestad OKL, Banke ABS, et al.. Impact of concomitant vasoactive treatment and mechanical left ventricular unloading in a porcine model of profound cardiogenic shock. Crit Care. 2020;24:95. doi:10.1186/s13054-020-2816-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Wang L, , Li X, Chen H, et al.. Coronavirus disease 19 infection does not result in acute kidney injury: An analysis of 116 hospitalized patients from Wuhan, China. Am J Nephrol. 2020;; 51:343–8. doi:10.1159/000507471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Pacheco LD, Saad AF, Saade G. Early acute respiratory support for pregnant patients with coronavirus disease 2019 (COVID-19) infection. Obstet Gynecol. 2020. doi:10.1097/AOG.0000000000003929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. MacLaren G, Fisher D, Brodie D. Preparing for the most critically Ill patients with COVID-19: the potential role of extracorporeal membrane oxygenation. JAMA. 2020. doi:10.1001/jama.2020.2342. [DOI] [PubMed] [Google Scholar]
- 103. Worthington EN, Hagood JS. Therapeutic use of extracellular vesicles for acute and chronic lung disease. Int J Mol Sci. 2020;21:2318. doi:10.3390/ijms21072318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Atluri S, Manchikanti L, Hirsch JA. Expanded umbilical cord mesenchymal stem cells (UC-MSCs) as a therapeutic strategy in managing critically Ill COVID-19 patients: the case for compassionate use. Pain Physician. 2020;23:E71–83. [PubMed] [Google Scholar]
- 105. Violi F, Pastori D, Cangemi R, et al.. Hypercoagulation and antithrombotic treatment in coronavirus 2019: a new challenge. Thromb Haemost. 2020;120:949–56. doi:10.1055/s-0040-1710317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Bonow RO, Fonarow GC, O'Gara PT, et al.. Association of coronavirus disease 2019 (COVID-19) with myocardial injury and mortality. JAMA Cardiol. 2020. doi:10.1001/jamacardio.2020.1105. [DOI] [PubMed] [Google Scholar]
- 107. Zhou F, Yu T, Du R, et al.. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–62. doi:10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Chen CF, Chien CH, Yang YP, et al.. Role of dipeptidyl peptidase 4 inhibitors in diabetic patients with Coronavirus-19 infection. J Chin Med Assoc. 2020. doi:10.1097/JCMA.0000000000000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Wan S, Xiang Y, Fang W, et al.. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J Med Virol. 2020;92:797–806. doi:10.1002/jmv.25783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Ren JL, Zhang AH, Wang XJ. Traditional Chinese medicine for COVID-19 treatment. Pharmacol Res. 2020;155:104743. doi:10.1016/j.phrs.2020.104743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Wang Z, Chen X, Lu Y, et al.. Clinical characteristics and therapeutic procedure for four cases with 2019 novel coronavirus pneumonia receiving combined Chinese and Western medicine treatment. Bioscience Trends. 2020;14:64–8. doi:10.5582/bst.2020.01030. [DOI] [PubMed] [Google Scholar]
- 112. Lung J, Lin YS, Yang YH, et al.. The potential chemical structure of anti-SARS-CoV-2 RNA-dependent RNA polymerase. J Med Virol. 2020. doi:10.1002/jmv.25761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Runfeng L, Yunlong H, Jicheng H, et al.. Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2). Pharmacol Res. 2020:156:104761. doi:10.1016/j.phrs.2020.104761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Kamel Boulos MN, Geraghty EM. Geographical tracking and mapping of coronavirus disease COVID-19/severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) epidemic and associated events around the world: how 21st century GIS technologies are supporting the global fight against outbreaks and epidemics. Int J Health Geog. 2020;19:8. doi:10.1186/s12942-020-00202-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Lan L, Xu D, Ye G, et al.. Positive RT-PCR test results in patients recovered from COVID-19. JAMA. 2020. doi:10.1001/jama.2020.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Pompeii LA, Kraft CS, Brownsword EA, et al.. Training and fit testing of health care personnel for reusable elastomeric half-mask respirators compared with disposable N95 respirators. JAMA. 2020. doi:10.1001/jama.2020.4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Grifoni A, Sidney J, Zhang Y, et al.. A sequence homology and bioinformatic approach can predict candidate targets for immune responses to SARS-CoV-2. Cell Host Microbe. 2020;27:671–680 e2. doi:10.1016/j.chom.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Walls AC, Park YJ, Tortorici MA, et al.. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020:181:281–292 e6. doi:10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Pang J, Wang MX, Ang IYH, et al.. Potential rapid diagnostics, vaccine and therapeutics for 2019 novel coronavirus (2019-nCoV): a systematic review. J Clin Med. 2020;9:623. doi:10.3390/jcm9030623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Ahmed SF, Quadeer AA, McKay MR. Preliminary identification of potential vaccine targets for the COVID-19 coronavirus (SARS-CoV-2) based on SARS-CoV immunological studies. Viruses. 2020;12:254. doi:10.3390/v12030254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Jiang S, Bottazzi ME, Du L, et al.. Roadmap to developing a recombinant coronavirus S protein receptor-binding domain vaccine for severe acute respiratory syndrome. Expert Rev Vaccines. 2012;11:1405–13. doi:10.1586/erv.12.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Henan Provincial Center for Disease Control and Prevention. A randomized, double-blind, placebo parallel-controlled phase I/II clinical trial for inactivated Novel Coronavirus Pneumonia vaccine (Vero cells), 2020, http://www.chictr.org.cn/showproj.aspx?proj=52227, accessed date: May 4, 2020. [Google Scholar]
- 123. Sinovac Biotech Co., L. Safety and Immunogenicity Study of 2019-nCoV Vaccine (Inactivated) for Prophylaxis SARS CoV-2 Infection (COVID-19), 2020, https://clinicaltrials.gov/ct2/show/NCT04352608?term = vaccine&cond = COVID-19&draw = 2&rank = 3, accessed date: April, 26, 2020. [Google Scholar]
- 124. Henan Provincial Center for Disease Control and Prevention. A phase I/II clinical trial for inactivated novel coronavirus (2019-CoV) vaccine (Vero cells), 2020, http://www.chictr.org.cn/showproj.aspx?proj=53003, accessed date: May 4, 2020. [Google Scholar]
- 125. Biontech SE. Study to Describe the Safety, Tolerability, Immunogenicity, and Potential Efficacy of RNA Vaccine Candidates Against COVID-19 in Healthy Adults, 2020, https://clinicaltrials.gov/ct2/show/NCT04368728?term = vaccine&cond = COVID-19&draw = 1&rank = 12, accessed date: May 4, 2020. [Google Scholar]
- 126. Pharmaceuticals, I. Safety, Tolerability and Immunogenicity of INO-4800 for COVID-19 in Healthy Volunteers, 2020, https://clinicaltrials.gov/ct2/show/NCT04336410?term = vaccine&cond = COVID-19&draw = 2&rank = 10, accessed date: April 14, 2020. [Google Scholar]
- 127. National Institute of Allergy and Infectious Diseases (NIAID). Safety and Immunogenicity Study of 2019-nCoV Vaccine (mRNA-1273) for Prophylaxis SARS CoV-2 Infection (COVID-19), 2020, https://clinicaltrials.gov/ct2/show/NCT04283461?term = vaccine&cond = COVID-19&draw = 2&rank = 9, accessed date: April 14, 2020. [Google Scholar]
- 128. Corporation, S. Evaluating the Safety, Tolerability and Immunogenicity of bacTRL-Spike Vaccine for Prevention of COVID-19, 2020, https://clinicaltrials.gov/ct2/show/NCT04334980?term = vaccine&cond = COVID-19&draw = 2&rank = 7, accessed date: April 14, 2020. [Google Scholar]
- 129. Vaxart. Vaxart Announces Initiation of Coronavirus Vaccine Program, 2020; https://pipelinereview.com/index.php/2020020273689/Vaccines/Vaxart-Announces-Initiation-of-Coronavirus-Vaccine-Program.html, accessed date: April 14, 2020. [Google Scholar]
- 130. Novavax. Evaluation of the Safety and Immunogenicity of a SARS-CoV-2 rS (COVID-19) Nanoparticle Vaccine With/Without Matrix-M Adjuvant, 2020; https://clinicaltrials.gov/ct2/show/NCT04368988?term = vaccine&cond = COVID-19&draw = 2&rank = 13, accessed date: May 4, 2020. [Google Scholar]
- 131. CanSino Biologics Inc. A Phase I Clinical Trial in 18–60 Adults (APICTH), 2020; https://clinicaltrials.gov/ct2/show/record/NCT04313127?term = vaccine&cond = COVID-19&draw = 1&rank = 8, accessed date: May 4, 2020. [Google Scholar]
- 132. Insitute of Biotechnology, Academy of Military Medical Sciences, PLA of China. A Phase II Clinical Trial to Evaluate the Recombinant Novel Coronavirus Vaccine (Adenovirus Vector) (CTII-nCoV), https://clinicaltrials.gov/ct2/show/record/NCT04341389?term = vaccine&cond = COVID-19&draw = 1&rank = 6&view = record, accessed date: May 4, 2020. [Google Scholar]
- 133. Shenzhen Geno-Immune Medical Institute. Safety and Immunity of Covid-19 aAPC Vaccine, 2020; https://clinicaltrials.gov/ct2/show/record/NCT04299724?term = vaccine&cond = COVID-19&draw = 1&rank = 2, accessed date: April 14, 2020. [Google Scholar]
- 134. Shenzhen Geno-Immune Medical Institute. Immunity and Safety of Covid-19 Synthetic Minigene Vaccine, 2020; https://clinicaltrials.gov/ct2/show/record/NCT04276896?term = vaccine&cond = COVID-19&draw = 1&rank = 3, accessed date: April 14, 2020. [Google Scholar]
- 135. Day M. Covid-19: four fifths of cases are asymptomatic, China figures indicate. BMJ. 2020;369:m1375. doi:10.1136/bmj.m1375. [DOI] [PubMed] [Google Scholar]
- 136. Bai Y, Yao L, Wei T, et al.. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020. doi:10.1001/jama.2020.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Rothe C, Schunk M, Sothmann P, et al.. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med. 2020;382:970–1. doi:10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Hoehl S, Rabenau H, Berger A, et al.. Evidence of SARS-CoV-2 infection in returning travelers from Wuhan, China. N Engl J Med. 2020;382:1278–80. doi:10.1056/NEJMc2001899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Wolfel R, Corman VM, Guggemos W, et al.. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–9. doi:10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 140. Munster VJ, Feldmann F, Williamson BN, et al.. Respiratory disease in rhesus macaques inoculated with SARS-CoV-2. Nature. 2020. doi:10.1038/s41586-020-2324-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Ziegler, C. G. K, Allon, S. J, Nyquist, S. K, et al.. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell. 2020;181:1016–1035 e19. doi:10.1101/2020.03.13.990226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Bao L, Deng W, Huang B, et al.. The pathogenicity of SARS-CoV-2 in hACE2 transgenic mice. Nature. 2020. doi:10.1038/s41586-020-2312-y. [DOI] [PubMed] [Google Scholar]