Abstract
Luspatercept is a recombinant fusion protein that enhances late‐stage erythroid maturation. This report describes the population pharmacokinetics and exposure–response relationship of luspatercept in 260 patients with anemia due to myelodysplastic syndromes. Luspatercept displayed linear and time‐invariant pharmacokinetics over a dose range of 0.125–1.75 mg/kg administered subcutaneously once every 3 weeks. Body weight was the only clinically relevant covariate of luspatercept exposure, supporting the weight‐based dosing. The probability of achieving transfusion independence ≥ 8 weeks increased with time‐averaged luspatercept serum exposure, reaching the plateau at doses 1.0–1.75 mg/kg. The probability of achieving multiple efficacy end points increased with slower luspatercept clearance, independent of effects of luspatercept exposure or disease characteristics. The probability of experiencing severe treatment‐emergent adverse events decreased with increasing luspatercept exposure, especially during long‐term treatment. These results provide a positive benefit–risk profile for the titration‐to‐response dose regimen (1.0–1.75 mg/kg) recommended for this population.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
☑ Luspatercept, a recombinant fusion protein, has demonstrated erythroid improvement in patients with anemia associated with ineffective erythropoiesis.
WHAT QUESTION DID THIS STUDY ADDRESS?
☑ What is the dose–exposure–response relationship of luspatercept in patients with myelodysplastic syndromes under a dose‐titration regimen (1.0–1.75 mg/kg)?
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
☑ The pharmacokinetic are linear and time‐invariant, with moderate variability. Erythroid response was positively correlated with luspatercept serum exposure, although the correlation was partially obscured by dose escalation. Slower luspatercept clearance was strongly associated with increased probability of efficacy. Dose escalation to 1.75 mg/kg was safe; incidence of severe treatment‐emergent adverse events decreased at higher luspatercept exposure.
HOW MIGHT THIS CHANGE DRUG DISCOVERY, DEVELOPMENT, AND/OR THERAPEUTICS?
☑ The titration‐to‐response regimen has a positive benefit–risk profile and luspatercept clearance may be an early marker of efficacy; there may also be a benefit to symptom improvement with long‐term luspatercept treatment. The impact of dose escalation and baseline luspatercept clearance should be considered when evaluating dose appropriateness by exposure–response analysis.
Myelodysplastic syndromes (MDS) are a heterogeneous group of clonal disorders of hematopoietic stem cells characterized by ineffective hematopoiesis and progressive cytopenias. Anemia is the most common symptom in patients with MDS, often resulting in red blood cell (RBC) transfusion‐dependence. 1 , 2 Upregulated Smad2/3, downstream effector proteins of the transforming growth factor beta (TGF‐β) superfamily pathway, has been linked to ineffective erythropoiesis in MDS. 3 , 4 , 5
Luspatercept is a recombinant fusion protein consisting of a modified form of the extracellular domain of human activin receptor type IIB linked to the human fragment crystallizable (Fc) domain of human immunoglobin G1. The activin receptor type IIB receptor and its ligands are members of the TGF‐β superfamily. 6 By binding several endogenous TGF‐β superfamily ligands, luspatercept led to diminished Smad2/3 signaling and enhanced late‐stage erythroid maturation in the bone marrow. 5 In clinical trials for MDS, luspatercept treatment led to sustained increases in hemoglobin (Hb) levels as well as reduced RBC transfusion frequency. 7 , 8 , 9 , 10 Luspatercept was well‐tolerated in these studies, with the maximum tolerated dose not reached at the highest clinical dose evaluated (1.75 mg/kg). 8
Here, we evaluate the population pharmacokinetics (PKs) and exposure–response relationship for luspatercept in patients with MDS under a titration‐to‐response dosing regimen. These findings provided support for benefit–risk assessments of the proposed dosing regimen for the treatment of MDS.
METHODS
Studies and treatment
This analysis was based on data from patients with MDS in three studies: A536‐03, A536‐05, and ACE‐536‐MDS‐001. Institutional review boards or ethics committees at each site approved the protocols; all patients provided written informed consent. More details about these studies are summarized in Table S1 . Luspatercept was administered subcutaneously once every 3 weeks (q3w). In A536‐03 dose escalation cohorts, the dose level ranged from 0.125 mg/kg to 1.75 mg/kg and each patient received only one dose level. In A536‐03 expansion cohorts, A536‐05, and ACE‐536‐MDS‐001, the starting dose was generally 1.0 mg/kg and the dose could be increased in a step‐wise manner (from 1.0 mg/kg to 1.33 mg/kg, and then to 1.75 mg/kg) if patients had RBC transfusions or undesirable Hb response during the two most recent prior treatment cycles at the same dose level. Patients in A536‐03 received luspatercept for up to five doses, whereas patients in A536‐05 and ACE‐536‐MDS‐001 could receive luspatercept for up to 5 years.
Population PK analysis
A fully validated enzyme‐linked immunosorbent assay was used to quantify luspatercept concentration in serum. The range of this assay was 50–600 ng/mL in 100% human serum with the standard curve fitted through 8 calibration standards using a 5‐parameter logistics fit. The inter‐run coefficient of variation was ≤ 12.0% and the inter‐run accuracy was 97.7–107.6% of the nominal concentration.
The population PK model was developed with nonlinear mixed‐effects modeling software in three stages: structural model selection, covariate analysis, and model evaluation. A small number (0.6%) of postdosing concentrations were below the limit of quantitation and excluded from the analysis. Luspatercept concentrations were natural logarithm‐transformed prior to the analysis. One‐compartment and two‐compartment models as well as nonlinear models were tested to identify the structural model. Model selection was based on the objective function (change > 10 for one additional parameter), goodness‐of‐fit plots, and scientific plausibility. Interindividual variability was modeled using an exponential error model. Residual variability was modeled using an additive error model. The continuous and categorical candidate covariates tested are summarized in Table 1 . In addition, effects of antidrug antibodies (positive and negative) and subcutaneous injection location (upper arm, thigh, and abdomen) were tested as a time‐varying covariate. The full‐model approach 11 was used in the covariate analysis, in which all covariate–parameter relationships of interest were simultaneously incorporated into the model. The final model was derived from the full model by dropping statistically insignificant (95% confidence intervals (CIs) of the covariate effect parameter included the null value) or clinically unimportant (95% CIs of the covariate effect within 25% of the null value) covariates. The final model was evaluated using the nonparametric bootstrap approach (1,000 replicates) and visual predictive check (VPC; 1,000 simulations).
Table 1.
Summary of patient characteristics in the population PK analysis
| Characteristic | Total (N = 260) |
|---|---|
| Sex, n (%) | |
| Female | 101 (38.8) |
| Male | 159 (61.2) |
| Ring sideroblasts, n (%) | |
| Positive | 216 (83.1) |
| Negative | 30 (11.5) |
| Unknown | 14 (5.4) |
| IPSS‐R risk, n (%) | |
| Very‐low/Low | 189 (72.7) |
| Intermediate | 60 (23.1) |
| High/Very‐high | 11 (4.2) |
| Renal impairment category, n (%) | |
| No | 70 (26.9) |
| Mild (eGFR 60–89 mL/minute/1.73 m2) | 134 (51.5) |
| Moderate (eGFR 30–59 mL/minute/1.73 m2) | 56 (21.5) |
| Hepatic impairment category, n (%) | |
| No | 154 (59.2) |
| Mild (BIL > 1–1.5 × ULN; ALT or AST > ULN) | 82 (31.5) |
| Moderate (BIL > 1.5–3 × ULN; any ALT or AST) | 23 (8.8) |
| Severe (BIL > 3 × ULN; any ALT or AST) | 1 (0.4) |
| Concurrent use of ICT, n (%) | |
| Yes | 100 (38.5) |
| No | 160 (61.5) |
| Age, median (range), years | 72.0 (27.0–95.0) |
| Weight, median (range), kg | 76.3 (46.0–124) |
| Erythropoietin, median (range), U/L | 138 (9.80–2,450) |
| Transfusion burden, median (range), units/24 weeks | 15.1 (0.00–43.4) |
| BIL, median (range), μmol/L | 14.0 (4.00–68.0) |
| Albumin, median (range), g/L | 44.0 (31.0–52.6) |
| AST, median (range), U/L | 21.0 (7.00–96.0) |
| eGFR, median (range), mL/min/1.73 m2 | 73.1 (29.6–150) |
ALT, alanine transaminase; AST, aspartate transaminase; BIL, total bilirubin; eGFR, estimated glomerular filtration rate; ICT, iron chelation therapy; IPSS‐R, International Prognostic Scoring System‐Revised; PK, pharmacokinetic; ULN, upper limit of normal.
Monte Carlo simulations were performed using the final model to evaluate the clinical relevance of significant covariates. One hundred clinical trials, with each trial having the same number of patients and the same distribution of covariates as in three clinical studies, were simulated for fixed (133 mg) and weight‐based dose (1.75 mg/kg). Patients were grouped into three subpopulations according to the distribution of their covariates: normal (10th–90th percentiles), low (< 10th percentile), and high (> 90th percentile). Individual values of area under the concentration–time curve (AUC) at steady state (AUCss) and maximum concentration at steady state (C max.ss) were derived from the simulation. The percentage difference in the median exposure at low or high covariate values relative to median exposure at normal covariate values was computed using the following equation:
where EXP was steady‐state exposure (AUCss or C max.ss) and extreme was either the low or high covariate values.
Exposure–response analysis
Individual measures of luspatercept serum exposure for exposure–response analyses were estimated based on empirical Bayes estimates of luspatercept apparent clearance (CL/F) from the final population PK model and actual dosing records.
Efficacy data for up to 1 year were included in the analysis. The efficacy end point for the pooled analysis was RBC transfusion independence (RBC‐TI) ≥ 8 weeks in weeks 1–15. The efficacy end points for the phase III study included RBC‐TI ≥ 8 weeks in weeks 1–24 (primary), RBC‐TI ≥ 12 weeks in weeks 1–24 (key secondary), and modified hematologic improvement–erythroid (mHI‐E) in weeks 1–24. The mHI‐E was defined by the International Working Group as mean Hb increase ≥ 1.5 g/dL from baseline in any 8‐week interval in patients with baseline transfusion burden < 4 RBC units/8 weeks or decrease of ≥ 4 RBC units from baseline in any 8‐week interval in patients with baseline transfusion burden ≥ 4 RBC units/8 weeks. 12 The exposure end point was average AUC (AUCavg) during a given evaluation period (weeks 1–15 or weeks 1–24), calculated as (cumulative dose/(CL/F)/treatment days·21 days). AUCavg was selected for exposure–efficacy analyses because it better reflected the exposure associated with efficacy (RBC‐TI often lasted through week 15 or 24) and considered dose modifications.
The treatment‐emergent adverse event (TEAE) records up to 60 days after the last dose as the cutoff date were included in the analysis. The safety end points included the incidence of serious TEAEs, ≥ grade 3 TEAEs, ≥ grade 1 asthenia, bone pain, bone pain‐like events, dizziness, hypertension, and myalgia. Selection of these TEAEs was based on the severity of TEAEs, imbalance in the incidence between active and placebo arms, and biologic consideration. The exposure end point was AUCss during the dosing interval when the TEAEs occurred (AUCTEAE) at the first event, calculated as (actual dose/(CL/F)). The actual dose was the last luspatercept dose administered prior to or on the start day of the first event for patients who had the specified TEAEs, or the last dose during the evaluation period for patients who did not have any specified TEAE. It was assumed that TEAEs were more likely to be associated with the most recent exposure level, as the frequency or severity of most TEAEs did not increase with administration of each higher dose.
Exposure–response modeling was conducted using logistic regression in R version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria) or higher. Both linear and maximum exposure (Emax) models were initially explored for drug effect in exposure–efficacy analyses. The linear model was selected for the final exposure–efficacy analysis because there were insufficient data at low exposure ranges to characterize the full shape of the relationship and the model discriminatory performance (area under the receiver operating characteristic curves) was similar for linear and Emax models. Model fitting was performed by first fitting a univariate base model with the luspatercept exposure as the only covariate. The impact of risk factors and CL/F was examined by adding the candidate covariate one by one to the base model and then in a full covariate model, including all potential factors. The final model was derived from the full model by dropping statistically insignificant factors. The likelihood ratio test was used to assess the significance of the covariate effect. In addition, the exposure–safety relationship over time was explored by Kaplan–Meier curves stratified by luspatercept AUCTEAE groups, followed by Cox proportional hazards regression analysis. The efficacy and safety data from 76 patients who received placebo were included in the graphs for visual comparison, but they were excluded from the exposure–response modeling.
RESULTS
Analysis population
The PK population included 260 patients: 107 from a phase II dose‐finding/expansion study (A536‐03 (“PACE‐MDS”); NCT01749514 8 ) and 153 from the pivotal phase III study (ACE‐536‐MDS‐001 (“MEDALIST”); NCT02631070 10 ). The patients were primarily white (82.3%) and male (61.2%), with a median age of 72 years (Table 1 ). Most patients (91.0%) received a starting dose of 1.0 mg/kg and 69.0% of them had their dose escalated to a maximum 1.33 mg/kg (24.1%) or 1.75 mg/kg (44.9%) during the first year of treatment. The remaining patients (9.0%) received a constant dose of 0.125–0.75 mg/kg. The dosing schedule was q3w for all patients. There were 2,403 quantifiable luspatercept serum concentration records collected at 4–784 days following the first dose.
The efficacy population included 226 patients from the above 2 studies who required RBC transfusions, defined as average transfusion burden ≥ 2 RBC units/8 weeks at baseline. The safety population included all patients (N = 260) from the above two studies and the safety data also included records from a phase II extension study (A536‐05; NCT02268383) for patients rolled over from study A536‐03.
Luspatercept population PK model
A one‐compartment model with first‐order absorption and elimination best described the concentration–time profiles of luspatercept after subcutaneous injection. The model was parameterized in terms of the absorption rate constant, CL/F, and apparent volume of distribution (V1/F). The interindividual variability (IIV) was determined for CL/F and V1/F (Table 2 ). Inclusion of IIV for absorption rate constant led to large shrinkage, indicating insufficient data to inform the numerical estimation of this variable. The PK of luspatercept was linear over the studied dose range, as dose did not have a significant effect on CL/F and a model in which luspatercept elimination described by a combination of linear and nonlinear (Michaelis–Menten) terms did not converge. A time‐varying CL/F model 13 was explored but this model led to an extremely large value (~ 108 days) for the time at which 50% of maximum changes would occur, suggesting a most probable time‐invariant CL/F. The mean elimination half‐life of luspatercept was ~ 13 days. The IIV for AUCss was 38.0%.
Table 2.
Parameter estimates of final population PK model
| Parameter (unit) | NONMEM estimate | Bootstrap estimates a | |
|---|---|---|---|
| Median | 95% CI | ||
| Fixed effect | |||
| CL/F, L/day | 0.469 | 0.469 | 0.449, 0.489 |
| V1/F, L | 9.22 | 9.20 | 8.88, 9.52 |
| K a, 1/day | 0.456 | 0.456 | 0.383, 0.652 |
| Weight, kg, on CL/F | 0.769 | 0.768 | 0.561, 0.986 |
| Age, years, on CL/F | −0.534 | −0.534 | −0.764, −0.315 |
| Albumin, g/L, on CL/F | −1.17 | −1.18 | −1.61, −0.726 |
| Weight, kg, on V1/F | 0.877 | 0.878 | 0.709, 1.05 |
| Albumin, g/L, on V1/F | −0.610 | −0.609 | −1.01, −0.216 |
| Random effect, % | |||
| Interindividual variability of CL/F | 36.4 | 36.0 | 31.1, 40.9 |
| Interindividual variability of V1/F | 22.5 | 22.3 | 17.0, 27.6 |
| Residual variability | 22.4 | 22.3 | 17.8, 27.5 |
CI, confidence interval; CL/F, apparent clearance; K a, absorption rate constant; NONMEM, nonlinear mixed‐effects modeling; PK, pharmacokinetics; V1/F, apparent volume of distribution.
Estimated from nonparametric bootstrap procedure (1,000 successful replicates).
There was no obvious bias in the prediction of luspatercept concentrations at the population and individual levels or at any specific time point (Figure S1 ). Relative differences in parameters were < 1% between the final model and bootstrap estimates (Table 2 ). The VPC plot (Figure 1a ) showed that the observed concentration–time course of luspatercept at the 5th, 50th, and 95th percentiles fell within the corresponding 95% CI of simulated data, indicating that the model adequately characterized the main trend and associated variability of observed data.
Figure 1.
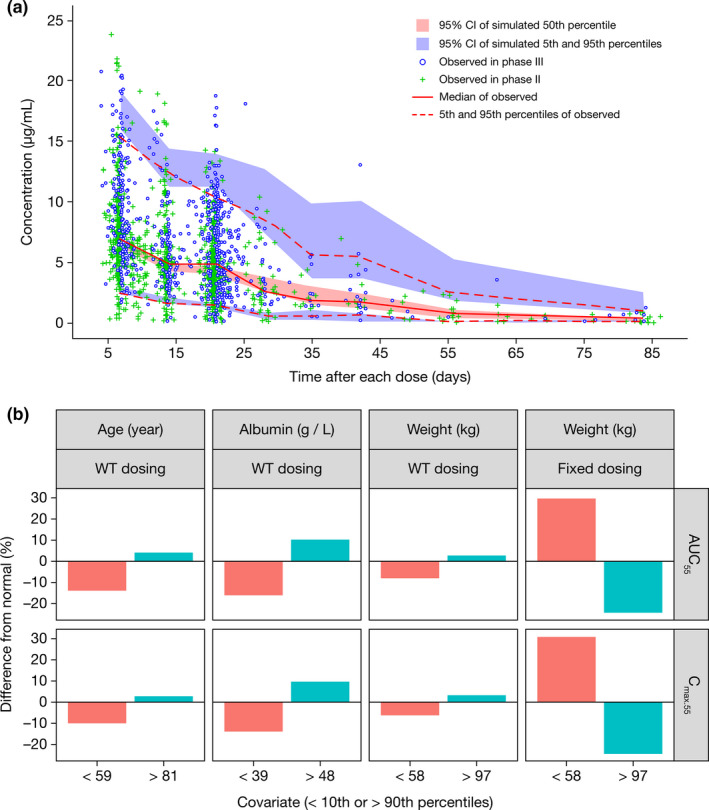
Luspatercept population PK modeling and simulation. (a) Visual predictive check for the final population PK model of luspatercept and (b) clinical relevance of statistically significant covariates. % Difference from normal, % difference in median exposure at the low or high covariate values relative to the normal covariate values; AUCss, area under the concentration–time curve at steady state; Cmax.ss, maximum concentration at steady state; CI, confidence interval; PK, pharmacokinetics; WT, body weight.
Body weight, age, and baseline albumin were identified as statistically significant covariates of CL/F. Inclusion of these covariates reduced the IIV of CL/F from 41.5% in the base model to 36.4% in the final model. The final covariate model for CL/F at the population level is described as follows:
Body weight and baseline albumin were statistically significant covariates of V1/F. Inclusion of the 2 covariates reduced the IIV of V1/F from 29.8% in the base model to 22.5% in the final model. The final covariate model for V1/F at the population level is described as follows:
The clinical relevance of these covariates was evaluated by PK simulation. The exposure difference between light or heavy patients and normal weight patients was predicted to be < 10% for weight‐based dosing but 25–30% for fixed dosing (Figure 1b ). With weight‐based dosing, the exposure difference between patients with extreme values of age or albumin and patients with normal values of age or albumin was predicted to be < 20% (Figure 1b ).
Effects of other baseline characteristics of patients or MDS disease, such as sex, mild‐to‐moderate renal impairment, mild‐to‐moderate hepatic impairment, liver enzymes (alanine transaminase and aspartate transaminase), total bilirubin, RBC transfusion burden, positive ring sideroblasts, serum erythropoietin (EPO), and MDS risk score on CL/F or exposure were either insignificant or of low clinical relevance. Locations of subcutaneous injection and concurrent use of iron chelation therapy also had no effect on luspatercept PK. The effect of antidrug antibodies on CL/F did not reach statistical significance in the covariate analysis.
Exposure–response of efficacy
The analysis was conducted in patients pooled from studies A536‐03 and ACE‐536‐MDS‐001 (N = 226) to allow a broader dose range (0.125–1.75 mg/kg) for a better description of the exposure–efficacy relationship. It was also conducted specifically for study ACE‐536‐MDS‐001 (N = 153) to assess the adequacy of the phase III doses (1.0–1.75 mg/kg) for efficacy. Stratification of the exposure–response curve for RBC‐TI ≥ 8 weeks by dose escalation status (Figure 2a ) showed a greater response and a better exposure–response relationship in patients who had no dose escalation than those with dose escalation. Therefore, two approaches of analyses were used: one included only the patients without dose escalation and the other included all luspatercept‐treated patients.
Figure 2.
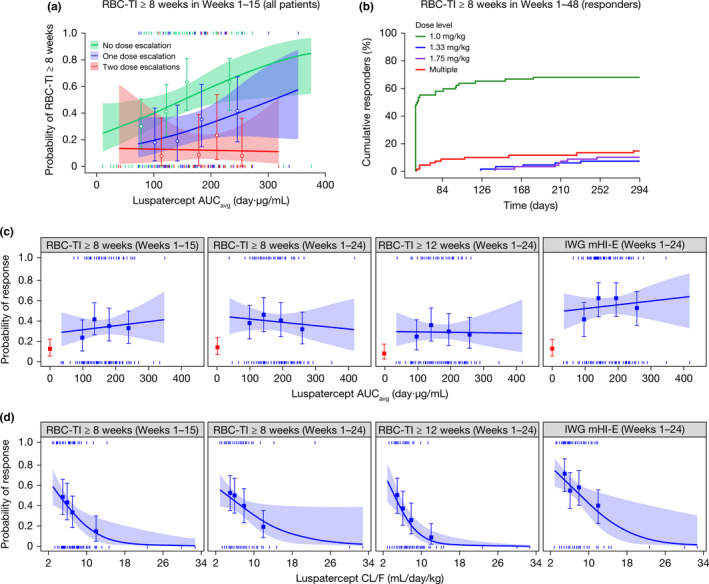
Association of luspatercept exposure and clearance with erythroid responses. (a) Logistic regression analysis of the relationship between RBC‐TI and luspatercept serum exposure by dose escalation status (data pooled from phase II and III studies). (b) Cumulative response over time for the first event of RBC‐TI in responders by dose levels (phase III study only). (c) Logistic regression analysis of the relationship between erythroid response and luspatercept serum exposure (phase III study only). (d) Logistic regression analysis of the relationship between erythroid response and luspatercept clearance (phase III study only). Observed proportions (circles or squares) and 95% CIs (error bars) are presented along with the predicted logistic regression fits (slanting lines) and 95% CIs (shaded area). Red square and error bar are data from placebo‐treated patients. Vertical ticks at individual values of AUCavg or CL/F represent whether the patient achieved a response (at 1) or not (at 0). AUCavg, average area under the concentration–time curve during the specified evaluation period; CI, confidence interval; CL/F, apparent clearance; IWG, International Working Group; mHI‐E, modified hematologic improvement–erythroid; RBC‐TI, red blood cell transfusion independence.
With both approaches for the pooled analysis, higher luspatercept AUCavg during the evaluation period was associated with increased probability of achieving RBC‐TI ≥ 8 weeks in weeks 1–15 after accounting for the effect of baseline risk factors (Table 3 ). The effect of luspatercept AUCavg on efficacy was more pronounced in patients without a dose escalation (odds ratio [OR] = 1.936) than in all patients (OR = 1.338). The near‐maximal response was associated with AUCavg values ≥ 150 day·μg/mL regardless of dose escalation status (Figure 2a ), corresponding to the mean AUCss values predicted for the 1.0–1.75 mg/kg dose (151–264 day·μg/mL).
Table 3.
Effect of luspatercept exposure or clearance on the probability of achieving erythroid response after adjusting for significant baseline covariates
| Study | Patients | Efficacy end point | Covariate adjusted a | PK measure b | OR (95% CI) | P value |
|---|---|---|---|---|---|---|
| Phase II + III | No dose escalation | RBC‐TI ≥ 8 weeks in weeks 1–15 | RBCT, EPO | AUCavg | 1.936 (1.27, 3.105) | 0.0017 |
| Phase II + III | No dose escalation | RBC‐TI ≥ 8 weeks in weeks 1–15 | RBCT, EPO, AUCavg | CL/F | 0.699 (0.547, 0.863) | 0.0003 |
| Phase II + III | All | RBC‐TI ≥ 8 weeks in weeks 1–15 | RBCT, EPO, BIL | AUCavg | 1.348 (1.017, 1.809) | 0.0382 |
| Phase II + III | All | RBC‐TI ≥ 8 weeks in weeks 1–15 | RBCT, EPO, BIL, AUCavg | CL/F | 0.712 (0.558, 0.874) | 0.0003 |
| Phase III only | All | RBC‐TI ≥ 8 weeks in weeks 1–15 | RBCT, BIL | AUCavg | 1.167 (0.810, 1.695) | 0.4058 |
| Phase III only | All | RBC‐TI ≥ 8 weeks in weeks 1–15 | RBCT, BIL | CL/F | 0.755 (0.605, 0.891) | 0.0001 |
| Phase III only | All | RBC‐TI ≥ 8 weeks in weeks 1–24 | RBCT, EPO, age | AUCavg | 0.993 (0.731, 1.346) | 0.9628 |
| Phase III only | All | RBC‐TI ≥ 8 weeks in weeks 1–24 | RBCT, EPO, age | CL/F | 0.833 (0.695, 0.961) | 0.0082 |
| Phase III only | All | RBC‐TI ≥ 12 weeks in weeks 1–24 | RBCT, age, BIL | AUCavg | 1.103 (0.797, 1.527) | 0.5505 |
| Phase III only | All | RBC‐TI ≥ 12 weeks in weeks 1–24 | RBCT, age, BIL | CL/F | 0.620 (0.463, 0.790) | < 0.0001 |
| Phase III only | All | IWG mHI‐E in weeks 1–24 | BIL | AUCavg | 1.101 (0.857, 1.422) | 0.4505 |
| Phase III only | All | IWG mHI‐E in weeks 1–24 | BIL | CL/F | 0.818 (0.710, 0.919) | 0.0003 |
AUCavg, average area under the concentration–time curve during the evaluation period; BIL, baseline total bilirubin; CI, confidence interval; CL/F, apparent clearance; EPO, baseline serum erythropoietin; IWG, International Working Group; mHI‐E, modified hematologic improvement–erythroid; OR, odds ratio for PK measure; PK, pharmacokinetics; RBCT, red blood cell transfusion burden; RBC‐TI, red blood cell transfusion independence.
Only statistically significant covariates are included, tested by the likelihood ratio test in the same order as shown.
As the last covariate added in the likelihood ratio test.
In the analysis including all patients from study ACE‐536‐MDS‐001, the exposure–response curves for all tested efficacy end points were nearly flat (Figure 2c ), whereas the proportion of responders was considerably greater at all luspatercept AUCavg quantiles compared with that of placebo. In the multivariate analysis, the effect of AUCavg was statistically insignificant after adjusting for effects of baseline risk factors (Table 3 ).
High baseline transfusion burden (≥ 6 units/8 weeks) and EPO levels (> 500 U/L) were frequently associated with decreased probability of achieving RBC‐TI (Table 3 ). These two factors were also associated with dose escalation (Table S2 ).
In the dose assessment for responders during the entire treatment period of study ACE‐536‐MDS‐001 (Figure 2b ), 68.1% (47/69) of responders achieved their first event of RBC‐TI ≥ 8 weeks at 1.0 mg/kg; 7.2% (5/69) of responders required dose escalation to 1.33 mg/kg, and 10.1% (7/69) of responders required dose escalation to 1.75 mg/kg to achieve their first response.
A greater probability of achieving erythroid responses was observed consistently at slower CL/F (Figure 2d ). Although CL/F was correlated with body weight, age, and albumin in the population PK analysis, a separate effect of body weight and albumin on efficacy was insignificant and the occasional association of age with efficacy was weaker. Furthermore, the effect of CL/F remained highly significant after accounting for the effects of serum exposure and/or baseline risk factors, including age (Table 3 ).
Exposure–response of safety
For all TEAEs tested, higher luspatercept AUCTEAE was numerically or statistically associated with decreased probability of experiencing TEAEs in a univariate logistic regression analysis (Table S3 ). The relationship between luspatercept AUCTEAE and TEAEs ≥ grade 3 was further assessed. A flat relationship (Figure 3a ) was observed during the first two treatment cycles when no dose escalation was allowed, whereas an inverse relationship (Figure 3b ) was observed during long‐term treatment allowing dose escalations. An exposure‐dependent reduction in TEAEs ≥ grade 3 was more apparent in the Kaplan–Meier analysis (Figure 3c ). As such, the highest AUCTEAE group was separated completely from the placebo group or the lowest AUCTEAE group. Older age was identified as the only baseline risk factor contributing to ≥ grade 3 TEAEs. The exposure‐dependent reduction in TEAEs ≥ grade 3 remained highly significant (P < 0.0001) after accounting for age effect in the Cox proportional hazards regression analysis. Luspatercept CL/F was not found to be associated with any TEAEs.
Figure 3.
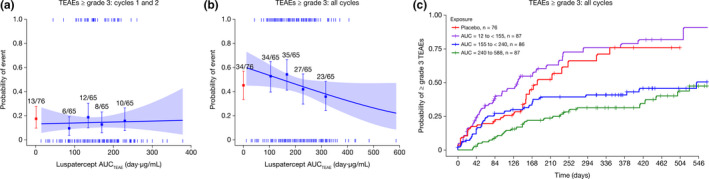
Association of luspatercept exposure with TEAEs ≥ grade 3. Analysis of luspatercept exposure and TEAEs ≥ grade 3 in (a) the first two treatment cycles and in (b) all available treatment cycles. Observed proportions (squares) and 95% CIs (error bars) are presented along with the predicted logistic regression fits (slanting lines) and 95% CIs (shaded area). Red square and error bar are data from placebo‐treated patients. Vertical ticks at individual values of AUCTEAE represent whether the patient achieved an event (at 1) or not (at 0). (c) Kaplan–Meier curve of the time to the first event of severe TEAEs. AUC or AUCTEAE, area under the concentration–time curve at steady state during the dosing period when the event occurred; CI, confidence interval; TEAE, treatment‐emergent adverse event.
Therapeutic margin of luspatercept under the titration dosing regimen
The therapeutic margin under the titration dosing regimen was evaluated by combining exposure–efficacy and exposure–safety analyses and adjusting for placebo effect. As illustrated in Figure 4 , the probability of achieving RBC‐TI ≥ 8 weeks reached the plateau (predicted ~ 25% at 1.0 mg/kg) and the probability of experiencing TEAEs ≥ grade 3 was low (predicted < 5% at 1.0 mg/kg) and decreased at higher luspatercept AUC (up to 1.75 mg/kg).
Figure 4.
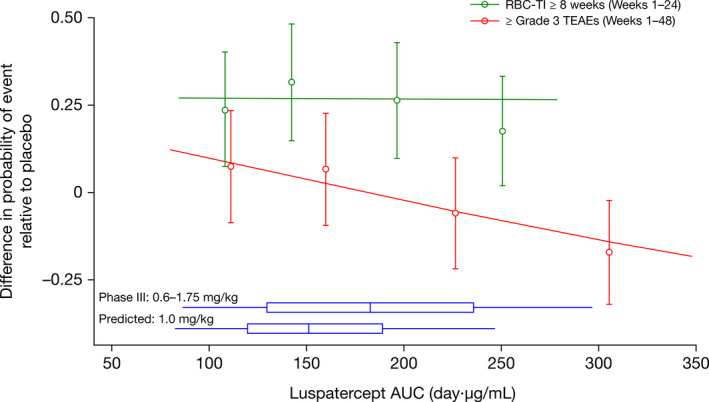
Observed and predicted therapeutic margin of luspatercept under titration dosing regimen in patients with MDS requiring RBC transfusions at baseline. The symbols and error bars represent the estimated difference in proportion relative to placebo (90% CI) of patients who experienced the event, grouped by quartiles of AUCss of luspatercept in serum and plotted at the median for each AUC quartile group (average AUCss in weeks 1–24 is used for efficacy and AUCss during the dosing interval when the event occurred is used for safety). The lines represent the predicted placebo‐adjusted probabilities from the final exposure–response models. Where the models include categorical covariates, the prediction is taken as the weighted average of the predictions for each combination of the categorical covariates, weighted by the relative frequency of each combination in the study population; where the models include continuous covariates, the prediction is taken at the median of the covariates. The horizontal box shows the distribution of the observed AUCss in weeks 1–48 in the pivotal phase III study or predicted AUCss at the starting dose of 1.0 mg/kg. The interior bar represents the median, the ends of the box represent the 25th and 75th percentiles, and the whiskers represent the 5th and 95th percentiles. AUC, area under the concentration–time curve; AUCss, area under concentration–time curve at steady state; CI, confidence interval; MDS, myelodysplastic syndromes; RBC, red blood cell; RBC‐TI, red blood cell transfusion independence; TEAE, treatment‐emergent adverse event.
DISCUSSION
The PK of luspatercept in patients with MDS was best described by a one‐compartment model with first‐order absorption and elimination and time‐invariant CL/F. Individual concentration–time profiles from 0.125 mg/kg to 1.75 mg/kg administered q3w for > 1 year were well‐described by the current model. Model evaluation with goodness‐of‐fit plots, bootstrap procedures, and VPC demonstrated robust stability and predictive performance of the final PK model.
Both CL/F and V1/F increased with heavier body weight according to an allometric relationship, with the exponent value > 0.75. As suggested by PK simulation, the body weight‐based dosing would perform better than the fixed dosing by limiting overexposing or underexposing light or heavy patients, respectively. Thus, the effect of weight is considered clinically relevant. Both CL/F and V1/F increased with decreasing albumin. Hypoalbuminemia could be an indication of decreased efficiency of neonatal Fc receptor 14 or elevated protein catabolism due to other mechanisms, 15 leading to faster clearance of luspatercept and lower exposure (contributing to lower V1/F). Luspatercept CL/F also decreased slightly with increasing age. The effect of albumin and age on luspatercept serum exposure appeared less clinically relevant as < 20% difference in luspatercept exposure was predicted for patients with extreme values of albumin or age for weight‐based dosing.
In clinical studies, most patients were eligible for two levels of dose escalation (1.33 and 1.75 mg/kg) if the response at the initial dose was not desirable. Such a dosing regimen better mimicked the real‐world clinical practice and better reflected benefit–risk considerations. It did, however, introduce selection bias into the exposure–efficacy relationship in univariate analyses, because patients who received higher dose levels were more likely to be nonresponders compared with patients who stayed at the lower dose levels. The analysis with patients who did not have any dose escalations was more sensitive to exposure‐dependent events, but this might inflate the response rate by excluding nonresponders who had dose escalation. The analysis with all patients provided the real‐world exposure–efficacy relationship, which might be obscured by the selection bias due to dose escalation. This bias was largely corrected by multivariate analyses, where the effect of exposure was assessed after considering the factors associated with dose escalation, mainly higher transfusion burden, and, to a less degree, higher EPO at baseline.
Using the above approaches and the pooled data over the entire clinical dose range (0.125–1.75 mg/kg), a positive correlation was observed between the probability of achieving RBC‐TI ≥ 8 weeks and luspatercept AUCavg after accounting for effects of baseline risk factors. The near‐maximal response was seen at AUCavg values ≥ 150 day·μg/mL, the mean AUC range predicted for the phase III doses (1.0–1.75 mg/kg). In the phase III population over the more effective dose range (1.0–1.75 mg/kg), the effect of exposure on any tested efficacy end point was no longer significant after adjusting for effects of baseline risk factors, thus confirming the maximum effective exposure was reached in most patients under the phase III regimen. The two factors associated with dose escalation, transfusion burden ≥ 6 units/8 weeks and EPO > 500 U/L, were also the key baseline risk factors associated with lack of achievement of RBC‐TI, thereby explaining the individual variations in the response to luspatercept in the MDS population.
To understand the contribution of each dose level to efficacy, luspatercept dose associated with the first response event was assessed for patients who achieved RBC‐TI ≥ 8 weeks in weeks 1–48. The 1.0 mg/kg starting dose was sufficient for most early responders (~ 68%); dose escalation increased the responders by at least 17%. These observations confirmed the appropriateness of the 1.0 mg/kg starting dose and suggested dose escalation to 1.75 mg/kg may improve response.
Luspatercept CL/F was strongly associated with efficacy; patients who had a slower CL/F were more likely to achieve erythroid responses. This effect was independent of luspatercept AUCavg, covariates of CL/F, or MDS disease characteristics. Possible hypotheses for the clearance‐associated efficacy are consumption of therapeutic proteins or proteolytic cleavage of the hinge region of Fc fusion proteins by tumors. 16 , 17 , 18 , 19 , 20 Thus, luspatercept CL/F may be a good estimate of catabolic activity, reflecting severity of the disease or anemia that impact the response to treatment. Similar hypotheses have been used to explain the association of slower clearance of several antitumor monoclonal antibodies with better antitumor efficacy. 13 , 21 , 22 , 23 , 24 Our findings demonstrate that clearance‐associated efficacy is not limited to monoclonal antibodies targeting tumors. Luspatercept is the first therapeutic biologic identified with a clearance‐associated erythroid response.
During the first two cycles, when no dose modifications occurred and all patients had the same treatment duration, the exposure–TEAE relationship was flat. During the entire study, the incidence of TEAEs in the higher AUCTEAE groups decreased compared with that in lower AUCTEAE groups, leading to an inverse exposure–TEAE relationship. The exposure–TEAE relationship for the entire study could be confounded by dose increase over time and by individual variations in the treatment duration during which certain TEAEs manifest. Additionally, patients who were tolerant to treatment and experienced clinical benefit continued treatment beyond 24 weeks. As indicated by a similar incidence in the placebo cohort, TEAEs ≥ grade 3 observed in luspatercept‐treated patients were more likely to be associated with disease, not drug exposure. Thus, the possibility that long‐term luspatercept treatment reduced TEAEs associated with patients’ disease (e.g., anemia, comorbidities worsened by anemia, and RBC transfusions) cannot be ruled out. Overall, these data suggest that increasing luspatercept exposure or dose level does not increase the incidence and severity of TEAEs.
Collectively, the exposure–response analyses demonstrated a wide therapeutic margin for luspatercept under the phase III dosing titration regimen for patients with MDS, as evidenced by the saturated probability of achieving RBC‐TI ≥ 8 weeks and the reduced probability of experiencing TEAEs ≥ grade 3 at higher luspatercept AUC after adjusting for placebo effect. The favorable benefit–risk profile in combination with moderate variability (~ 38%) in serum exposure and individual variations in erythroid response support the titration‐to‐response regimen to maximize the efficacy potential of luspatercept. Starting at a lower effective dose followed by dose escalation depending on the patient’s condition would also limit rapid Hb rise and unnecessary exposure to high drug levels.
In summary, luspatercept PK was well‐described by a linear population model with time‐invariant clearance. Body weight was the only clinically relevant covariate of luspatercept PK, supporting weight‐based dosing. No other patient characteristics were found to warrant dosing modifications. Increasing luspatercept serum exposure was associated with an increased probability of achieving erythroid response, which plateaued at the phase III dose levels. A slower luspatercept CL/F was strongly associated with an increased probability of achieving erythroid response, making it a potential early marker for efficacy. On the contrary, the probability of experiencing ≥ grade 3 TEAEs decreased with increasing luspatercept AUCTEAE, especially during long‐term treatment. These analyses provide a positive benefit–risk profile for the recommended therapeutic doses (1.0–1.75 mg/kg, q3w).
Funding
All clinical data were generated in studies sponsored by Bristol Myers Squibb or Acceleron Pharma.
Conflict of Interest
N.C., A.L., S.E.M., P.S., A.C.G., S.R., S.Z., and M.P. are employees of Bristol Myers Squibb. N.K. is an employee of Certara Strategic Consulting and a paid consultant. P.G.L., B.B., and J.G.R. are employees of Acceleron Pharma.
Author Contributions
N.C., S.Z., and M.P., wrote the manuscript. N.C., N.K., A.L., A.C.G., S.R., P.G.L., and J.G.R. designed the research. N.C., A.L., A.C.G., P.G.L., B.B., and J.G.R. performed the research. N.C., N.K., S.E.M., and P.S. analyzed the data.
Data Sharing
Data requests may be submitted to Celgene, a Bristol Myers Squibb Company, at https://vivli.org/ourmember/celgene/ and must include a description of the research proposal.
Supporting information
Figure S1
Table S1
Table S2
Table S3
Acknowledgments
The authors thank all patients and their families, and investigators and site staff who participated in these studies. This study was sponsored by Bristol Myers Squibb, Princeton, NJ, USA, in collaboration with Acceleron Pharma, Cambridge, MA, USA. The authors received editorial and writing support from Jacqueline Moy, PhD, from Excerpta Medica, funded by the sponsor. The authors are fully responsible for all content and editorial decisions for this paper.
References
- 1. Adès, L. , Itzykson, R. & Fenaux, P. Myelodysplastic syndromes. Lancet 383, 2239–2252 (2014). [DOI] [PubMed] [Google Scholar]
- 2. Foran, J.M. & Shammo, J.M. Clinical presentation, diagnosis, and prognosis of myelodysplastic syndromes. Am. J. Med. 125 (suppl. 7), S6–S13 (2012). [DOI] [PubMed] [Google Scholar]
- 3. Zhou, L. et al Inhibition of the TGF‐beta receptor I kinase promotes hematopoiesis in MDS. Blood 112, 3434–3443 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhou, L. et al Reduced SMAD7 leads to overactivation of TGF‐beta signaling in MDS that can be reversed by a specific inhibitor of TGF‐beta receptor I kinase. Cancer Res. 71, 955–963 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Suragani, R.N.V.S. et al Transforming growth factor‐β superfamily ligand trap ACE‐536 corrects anemia by promoting late‐stage erythropoiesis. Nat. Med. 20, 408–414 (2014). [DOI] [PubMed] [Google Scholar]
- 6. Schmierer, B. & Hill, C.S. TGFβ‐SMAD signal transduction: molecular specificity and functional flexibility. Nat. Rev. Mol. Cell Biol. 8, 970–982 (2007). [DOI] [PubMed] [Google Scholar]
- 7. Komrokji, R.S. Activin receptor II ligand traps: new treatment paradigm for low‐risk MDS. Curr. Hematol. Malig. Rep. 14, 346–351 (2019). [DOI] [PubMed] [Google Scholar]
- 8. Platzbecker, U. et al Luspatercept for the treatment of anaemia in patients with lower‐risk myelodysplastic syndromes (PACE‐MDS): a multicentre, open‐label phase 2 dose‐finding study with long‐term extension study. Lancet Oncol. 18, 1338–1347 (2017). [DOI] [PubMed] [Google Scholar]
- 9. Fenaux, P. , Kiladjian, J.J. & Platzbecker, U. Luspatercept for the treatment of anemia in myelodysplastic syndromes and primary myelofibrosis. Blood 133, 790–794 (2019). [DOI] [PubMed] [Google Scholar]
- 10. Fenaux, P. et al Luspatercept in patients with lower‐risk myelodysplastic syndromes. N. Engl. J. Med. 382, 140–151 (2020). [DOI] [PubMed] [Google Scholar]
- 11. Byon, W. Establishing best practices and guidance in population modeling: an experience with an internal population pharmacokinetic analysis guidance. CPT Pharmacometrics Syst. Pharmacol. 2, e51 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cheson, B.D. et al Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood 108, 419–425 (2006). [DOI] [PubMed] [Google Scholar]
- 13. Liu, C. et al Association of time‐varying clearance of nivolumab with disease dynamics and its implications on exposure response analysis. Clin. Pharmacol. Ther. 101, 657–666 (2017). [DOI] [PubMed] [Google Scholar]
- 14. Andersen, J.T. & Sandlie, I. The versatile MHC class I‐related FcRn protects IgG and albumin from degradation: implications for development of new diagnostics and therapeutics. Drug Metab. Pharmacokinet. 24, 318–332 (2009). [DOI] [PubMed] [Google Scholar]
- 15. Gupta, D. & Lis, C.G. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr. J. 9, 69 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Porporato, P.E. Understanding cachexia as a cancer metabolism syndrome. Oncogenesis 22, e200 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lelbach, A. , Muzes, G. & Feher, J. Current perspectives of catabolic mediators of cancer cachexia. Med. Sci. Monit. 13, Ra168–Ra173 (2007). [PubMed] [Google Scholar]
- 18. Brezski, R.J. & Jordan, R.E. Cleavage of IgGs by proteases associated with invasive diseases: an evasion tactic against host immunity? MAbs 2, 212–220 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jordan, R.E. , Fan, X. , Salazar, G. , Zhang, N. & An, Z. Proteinase‐nicked IgGs: an unanticipated target for tumor immunotherapy. Oncoimmunology 7, e1480300 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Flint, T.R. et al Tumor‐induced IL‐6 reprograms host metabolism to suppress anti‐tumor immunity. Cell Metabol. 24, 672–684 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li, H. et al Time dependent pharmacokinetics of pembrolizumab in patients with solid tumor and its correlation with best overall response. J. Pharmacokinet. Pharmacodyn. 44, 403–414 (2017). [DOI] [PubMed] [Google Scholar]
- 22. Wilkins, J.J. Time‐varying clearance and impact of disease state on the pharmacokinetics of avelumab in Merkel cell carcinoma and urothelial carcinoma. CPT Pharmacometrics Syst. Pharmacol. 8, 415–427 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Turner, D.C. et al Pembrolizumab exposure‐response assessments challenged by association of cancer cachexia and catabolic clearance. Clin. Cancer Res. 24, 5841–5849 (2018). [DOI] [PubMed] [Google Scholar]
- 24. Bajaj, G. et al Model‐based population pharmacokinetic analysis of nivolumab in patients with solid tumors. CPT Pharmacometrics Syst. Pharmacol. 6, 58–66 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Table S1
Table S2
Table S3


