Abstract
Evidence suggests that effects of interleukin‐6 pathway inhibitors sarilumab, tocilizumab, and sirukumab on absolute neutrophil count (ANC) are due to margination of circulating neutrophils into rapidly mobilizable noncirculating pools. We developed a population pharmacodynamic model using compartments for neutrophil margination and ANC‐specific tolerance to describe rapid, transient ANC changes in blood following administration of subcutaneous sarilumab and intravenous/subcutaneous tocilizumab based on data from 322 patients with rheumatoid arthritis in two single‐dose (NCT02097524 and NCT02404558) and one multiple‐dose (NCT01768572) trials. The model incorporated a tolerance compartment to account for ANC nadir and beginning of recovery before maximal drug concentration after subcutaneous dosing, and absence of a nadir plateau when the ANC response is saturated after subcutaneous or intravenous dosing. The model effectively describes the ANC changes and supports neutrophil margination and tolerance as an explanation for the absence of increased infection risk associated with low ANC due to interleukin‐6 pathway inhibitor treatment.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
☑ Decreased absolute neutrophil count (ANC) is observed within hours following administration of interleukin‐6 receptor (IL‐6R) inhibitors sarilumab and tocilizumab. This decrease is not associated with increased risk of infection. Emerging evidence suggests the effects of IL‐6R inhibitors on ANC are due to margination of circulating neutrophils into a rapidly mobilizable noncirculating pool without loss of function, rather than a decreased overall number of neutrophils in the body.
WHAT QUESTION DID THIS STUDY ADDRESS?
☑ Does a margination–tolerance model adequately predict neutrophil profile after treatment with IL‐6R inhibitors?
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
☑ This study adds a population pharmacodynamic model that describes the ANC kinetics and incorporates neutrophil circulation, margination, and ANC‐specific tolerance, and is consistent with the safety profile of IL‐6R inhibitors.
HOW MIGHT THIS CHANGE DRUG DISCOVERY, DEVELOPMENT, AND/OR THERAPEUTICS?
☑ The application of pharmacometric modeling provides additional support and insight for the hypothesis that neutrophil margination and tolerance underlie the dissociation between low ANC and infection risk during treatment with a major class of immunomodulators targeting IL‐6R.
Interleukin‐6 (IL‐6) is a multifunctional cytokine that plays a key role in the pathophysiology of rheumatoid arthritis (RA). 1 , 2 , 3 Sarilumab (KEVZARA®) and tocilizumab (ACTEMRA®) are monoclonal antibodies (human and humanized, respectively) approved for the treatment of RA that bind membrane‐bound and soluble IL‐6 receptors (IL‐6R). 4 , 5 Treatment with these IL‐6R inhibitors is associated with transient decreases in circulating absolute neutrophil count (ANC). 6 , 7 , 8 An integrated analysis of long‐term treatment with sarilumab in combination with conventional synthetic disease‐modifying antirheumatic drugs, such as methotrexate, found maximum neutropenia grades of 2, 3, and 4 in 21%, 14%, and 1% of patients, respectively. 9 In contrast to the drug‐induced neutropenia seen with chemotherapy, 10 the neutropenia seen with sarilumab and tocilizumab is not associated with an increased risk of infection. 6 , 9 , 11 A decrease in ANC is evident within 4 hours after administration of a single dose of sarilumab with nadir reached by a median 3–5 days, 7 whereas typical ANC decreases with chemotherapy are not observed until 5 days after administration with nadir occurring at 7 days, even in the presence of marrow‐stimulating therapy. 12
Neutrophil margination and demargination are physiological processes that maintain a reservoir of neutrophils that can be rapidly mobilized for return to the circulation in response to inflammatory stimuli. 13 The process of margination due to a decrease in inflammatory stimuli after treatment with IL‐6R blockers is distinct from the infiltration/recruitment of neutrophils into infected and inflamed tissues. 14 , 15 IL‐6 appears to promote demargination: administration of recombinant IL‐6 produces a biphasic blood neutrophilia in rabbits due to rapid mobilization of neutrophils from a noncirculating marginated pool (2–6 hours) followed by accelerated release of neutrophils from the bone marrow (12–24 hours). 16 The question arises whether inhibition of IL‐6 signaling produces an opposite effect on the neutrophil margination–demargination equilibrium resulting in increased margination into a noncirculating pool, which includes attachment to blood vessel walls. The rapid kinetics of the decrease in circulating ANC seen with IL‐6R inhibition and the absence of increased infection support a hypothesis that the effects of IL‐6R inhibition on ANC are due to neutrophil margination rather than accelerated apoptosis or a suppression of hematopoiesis. Consistent with this hypothesis, an investigation of neutrophil function and distribution in healthy volunteers using radiolabeled autologous neutrophils found a trend for greater retention of neutrophils in the liver and spleen, balanced by lower retention in the bone marrow, following IL‐6R inhibition with tocilizumab compared with placebo. 17
The primary modeling mechanism‐of‐action hypothesis was that the profile of a decrease in ANC after treatment with IL‐6R inhibitors is explained by margination (followed by quick partial tolerance), rather than by accelerated death of neutrophils; the tolerance is a pharmacologic concept describing reduced reaction to a drug over time. This hypothesis has not been investigated in previous models of ANC kinetics. The Friberg model of chemotherapy‐induced myelosuppression includes a compensatory increase in production rate of neutrophils after their eradication by chemotherapies, which can explain tolerance‐like patterns, but was not previously explored to address a potential increase in production rate of neutrophils after their decrease in the circulation pool due to margination or in relation to IL‐6R inhibition. 18 Subsequent publications have mentioned margination of neutrophils after blocking IL‐6R as a potential mechanism‐of‐action but they model a decrease in half‐life of neutrophils. 19 , 20 Previously modeled neutrophil data did not show development of neutrophil‐specific tolerance due to the sparsity of laboratory samples. 19 , 20 This is the first paper to describe a model of neutrophil margination followed by quick onset of neutrophil‐specific tolerance after introduction of IL‐6R blockers. The goal was to complement recent biological findings using a modeling approach applied to a new dataset.
Methods
Study designs
The dataset comprised data from adult patients with RA treated with sarilumab or tocilizumab in three clinical studies: study 1309 (NCT02097524), a phase I randomized, open‐label study in which 102 patients received a single dose of s.c. sarilumab 150 or 200 mg, or i.v. tocilizumab 4 or 8 mg/kg 7 ; study PDY14191 (NCT02404558), a phase I randomized, open‐label study in which 30 Japanese patients received a single dose of s.c. sarilumab 150 mg or s.c. tocilizumab 162 mg 21 ; and ASCERTAIN (NCT01768572), a phase III randomized, double‐blind, double‐dummy study in which 202 patients received multiple doses of s.c. sarilumab 150 or 200 mg every 2 weeks (q2w), or i.v. tocilizumab every 4 weeks, starting at 4 mg/kg and increased to 8 mg/kg based on clinical response, for 24 weeks. 7 The studies were conducted in accordance with good clinical practice and with the principles laid down in the Declaration of Helsinki. The protocols and patient information were approved by the relevant ethical review boards, and all patients gave written informed consent. Information on sampling and data handling is provided in Supplementary Section S1 .
Population pharmacodynamic model
Sarilumab and tocilizumab concentrations were modeled using a two‐compartment pharmacokinetic (PK) model with parallel linear and Michaelis–Menten elimination. 22 The population pharmacodynamic (PD) model was characterized by a nonlinear mixed‐effects model using Monolix version 2010R2 and NONMEM version 7.4.1 (ICON Development Solutions, Dublin, Ireland). Data were analyzed using stochastic approximation expectation–maximization and importance sampling methods. Sequential modeling was conducted (i.e., individual PK parameters were fixed in the PD model). Starting base PD models were selected based on biological consideration as explained in the Results and Discussion sections. The primary/final PD model was a margination–tolerance (MT) model represented by blood and margination compartments, a tolerance compartment, and a transit margination compartment (Figure 1 ).
Figure 1.
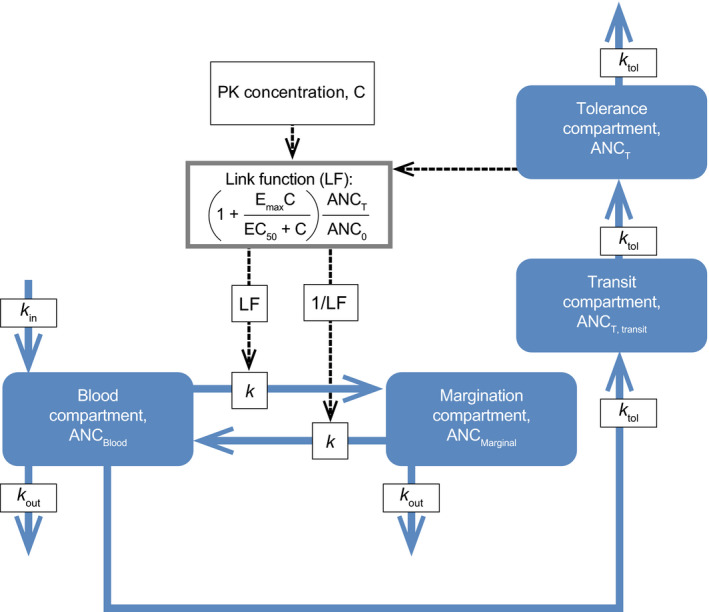
Structure of the MT model. ANC0, estimated baseline absolute neutrophil count; ANCBlood, absolute neutrophil count in circulation; ANCMarginal, absolute neutrophil counts not in circulation; ANCT, absolute neutrophil count in tolerance compartment; ANCT, transit, absolute neutrophil count in tolerance transit compartment; C, concentration of IL‐6R inhibitor; EC50, concentration of IL‐6R inhibitor causing half‐maximal effect; Emax, maximal effect of IL‐6R inhibitor on intercompartmental rates; IL‐6R, interleukin‐6 receptor; k, intercompartmental rate; k in, calculated production rate of neutrophils; k out, elimination rate of neutrophils based on published estimates of half‐life; k tol, tolerance rate; LF, link function; MT, margination–tolerance; PK, pharmacokinetic.
Forward inclusion and backward elimination were utilized to build both base and covariate models. A covariate or parameter was retained in the model when addition resulted in α ≤ 0.01 and removal of the covariate resulted in α ≤ 0.001. The following multiplicative models were used to test for covariates:
for continuous covariates where Y(λi) is a population PD parameter adjusted for covariate, λi is an individual covariate value, i is a subject number, Y is a population PD parameter at a median or predefined value, and θ is a parameter describing the effect of a covariate on the population PD parameter; and
for dichotomous covariates where Y is a population PD parameter when λi = 0; λi is equal to 0 or 1. Drug (sarilumab vs. tocilizumab) and baseline ANC were tested as covariates.
The model was evaluated through bootstrapping (repeated resampling of subject data from the analysis dataset with replacement) 23 to obtain bootstrap confidence intervals of parameter estimates; 100 bootstraps each followed by a model run were executed for each model. Empirical assessment of stability (randomly changing initial PD parameters) and visual predictive checks were also utilized. Log‐normally distributed additive error of ANC was compared with normally distributed additive, proportional, or combined (additive and proportional) errors. An addition of power parameter ϒ was tested in the tolerance models with tolerance feedback expressed as: (ANC(t)/ANC0)ϒ, in which, depending on the model, ANC(t) represents ANC count in a tolerance or blood compartment.
Models demonstrating signs of instability or lack of structural identifiability were excluded from further consideration and are not presented.
The objective function value (OFV) was used to obtain P values when nested models were compared. In cases requiring comparison of different structural models, Bayesian information criterion (BIC) was applied. A substantial advantage of BIC is that it can be used to compare non‐nested models. 24
Results
Patient population
In the PD analysis dataset, patient mean (SD) age was 53.4 years (12.3), 262 patients were female (81.4%), and mean baseline ANC was 5.13 (2.33) ×109/L. The PD analysis dataset comprised 4,592 ANC observations collected from 322 patients: 101 patients and 1,883 samples from study 1309, 30 patients and 409 samples from study PDY14191, and 191 patients and 2,300 samples from ASCERTAIN. Eleven patients were excluded from the analysis due to early termination and insufficient numbers of ANC values (n = 1), or < 1 PK post‐treatment measures above the limit of quantification after removal of outliers (n = 10).
Structural model
The structure of an MT model that described the ANC decrease, tolerance, and return to baseline after administration of IL‐6R inhibitors sarilumab and tocilizumab is depicted in Figure 1 . The MT model is represented by a blood and a margination compartment. The addition of a tolerance compartment was tested in response to observations in study 1309 that (i) s.c. administration of sarilumab induced ANC nadir and the beginning of recovery before maximal drug concentration, and (ii) after s.c. dosing of sarilumab or i.v. dosing of tocilizumab a plateau in the nadir was absent when the ANC response was saturated. 25 , 26 These tolerance patterns closely resembled those described and modeled by Gabrielsson and Weiner, 25 and their model of tolerance was used. Implementation of a tolerance compartment led to a substantial improvement of the model fit based on stability, OFV, and diagnostic plots. In addition, different numbers of transit compartments for tolerance were tested to further explore the development of tolerance, with optimal improvement associated with one tolerance transit compartment (OFV improved by 144). The tolerance compartment models a delay between the decrease in blood ANC and the decrease in drug effect on neutrophils. The MT model was parameterized with estimated baseline ANC (ANC0), intercompartmental rate (k), maximum drug‐induced effect on k (Emax), concentration of drug causing half‐maximal effect on k (EC50), tolerance rate (k tol), and rate of neutrophil elimination (k out; Table 1 ). Log‐normal distributed additive error of ANC was selected. Ordinary differential equations are provided in Supplementary Section S3 .
Table 1.
Final base MT model with PD effect on margination and demargination rates: population PD parameters and bootstrap CIs
| Parameter value (SE) | Bootstrap median (95% CI) | |
|---|---|---|
| Parameters | ||
| ANC0, ×109/L | 4.53 (0.0986) | 4.53 (4.34–4.68) |
| k, 1/day | 1.60 (0.0915) | 1.59 (1.18–2.22) |
| Emax, unitless | 1.65 (0.0980) | 1.64 (1.36–1.94) |
| EC50, mg/L | 0.797 (0.0812) | 0.769 (0.376–1.72) |
| k tol, 1/day | 0.291 (0.0104) | 0.288 (0.247–0.354) |
| k out, 1/day | 0.877 Fixed | 0.877 Fixed |
| Omegas | ||
| ω, ANC0 | 0.347 (0.0161) | 0.348 (0.319–0.374) |
| ω, Emax | 0.717 (0.0421) | 0.723 (0.625–0.809) |
| SD of residual error | ||
| σexponential | 0.253 (0.00156) | 0.253 (0.245–0.264) |
| Log‐likelihood estimation | ||
| −2 log‐likelihood | 12,617.692 | – |
| BIC | 12,693.581 | – |
ANC, absolute neutrophil count; ANC0, estimated baseline ANC; BIC, Bayesian information criterion; CI, confidence interval; EC50, concentration of drug causing half‐maximal effect; Emax, maximum drug‐induced effect; k, intercompartmental rate; k out, neutrophil elimination rate; k tol, tolerance rate; MT, margination–tolerance; ω, between‐subject SD in parameters; PD, pharmacodynamic; SE, standard error; σ, SD.
In the primary covariate MT model, which can also be called final, drug (sarilumab vs. tocilizumab) was a significant covariate on EC50, consistent with sarilumab having higher affinity for the IL‐6R than tocilizumab, 27 and baseline ANC was a significant covariate on Emax and ANC0 (Table 2 ).
Table 2.
Final covariate MT model with PD effect on margination and demargination rates: population PD parameters and bootstrap CIs
| Parameter value (SE) | Bootstrap median (95% CI) | |
|---|---|---|
| Parameters | ||
| ANC0, ×109/L | 4.57 (0.0572) | 4.56 (4.49–4.63) |
| k, 1/day | 1.31 (0.0771) | 1.32 (0.954–1.69) |
| Emax, unitless | 1.75 (0.131) | 1.74 (1.51–2.07) |
| EC50, mg/L: sarilumab | 0.137 (0.0187) | 0.131 (0.0612–0.440) |
| EC50, mg/L: tocilizumab | 1.78 | – |
| k tol, 1/day | 0.281 (0.00948) | 0.277 (0.255–0.328) |
| k out, 1/day | 0.887 Fixed | 0.877 Fixed |
| Covariates | ||
| β, ANCB on ANC0 | 0.887 (0.0263) | 0.889 (0.855–0.930) |
| β, ANCB on Emax | 0.549 (0.147) | 0.587 (0.247–0.847) |
| β, drug on EC50 | 2.56 (0.168) | 2.65 (1.11–3.77) |
| Omegas | ||
| ω, ANC0 | 0.102 (0.00737) | 0.101 (0.0804–0.119) |
| ω, Emax | 0.803 (0.0440) | 0.800 (0.713–0.887) |
| SD of residual error | ||
| σexponential | 0.249 (0.00156) | 0.249 (0.240–0.257) |
| Log‐likelihood estimation | ||
| −2 log‐likelihood | 11,963.699 | – |
| BIC | 12,064.88 | – |
ANC, absolute neutrophil count; ANC0, estimated baseline ANC; ANCB, ANC values observed before the first dose; β, covariate coefficient; BIC, Bayesian information criteria; CI, confidence interval; EC50, concentration of drug causing half‐maximal effect; Emax, maximum drug‐induced effect; k, intercompartmental rate; k out, neutrophil elimination rate; k tol, tolerance rate; MT, margination–tolerance; ω, between‐subject SD in parameters; PD, pharmacodynamic; SE, standard error; σ, SD.
To model changes in demargination as well as margination when IL‐6R inhibitors are introduced, a link function and an inverse of the link function were imposed on the margination and demargination rates, respectively. As this change was physiologically meaningful and led to a statistically significant improvement in OFV, the model implementing the bidirectional impact of IL‐6R inhibitors was selected. Chemotaxis is a known mechanism of neutrophil attraction. Consistent with the bidirectional impact, chemotaxis causes neutrophils to favor the margination space rather than move between blood and margination compartments. PD parameters of the model with a link function imposed only on margination rate are presented in Tables S1 and S2 .
MT models with and without production/elimination of neutrophils were tested; production and elimination of neutrophils were added as fixed parameters to the model for standardization with existing perceptions of how neutrophil kinetics should be modeled even though it did not improve the model based on OFV and diagnostic plots. The neutrophil elimination rate was fixed based on the published half‐life of neutrophils (~ 19 hours 28 ), and the production rate was calculated as a function of baseline ANC and k out. The total ANC (ANCblood + ANCmarginal) in the blood and margination compartments of the model remained constant (flat horizontal line) throughout the baseline and treatment periods.
In the MT model, the ratio of neutrophils in the blood and margination compartments at baseline was 63%/37%, whereas previously reported ratios for granulocytes in healthy subjects vary between experiments, and include 60%/40% before prednisone treatment and 46%/54% before bacterial endotoxin treatment; 29 neutrophils are the most abundant of the granulocytes. When it is known from an experiment, this ratio can be altered in the model by changing the ratio between baseline margination and demargination rates or by assuming that newborn neutrophils enter not only the circulation but also the margination compartment (Figure 1 ). The latter change in the model was tested (analysis not shown) and somewhat worsened the OFV without a noticeable impact on diagnostic plots. The same baseline equality can be assured by assuming that neutrophils are deleted only from the blood compartment. Quantitative information about the circulating:marginated ratio in RA was not found. It is expected though that due to an increased IL‐6 concentration in RA at baseline, ANC in circulation can be higher than in a marginated pool due to IL‐6‐induced demargination. 16
The MT model was used to simulate the ANC–time course after multiple doses of sarilumab s.c. and tocilizumab i.v. tested in the ASCERTAIN study (Figure 2a ). Tocilizumab s.c. treatment was not simulated because there were only 15 subjects from the single‐dose study PDY14191 and no multiple‐dose data were available. The simulations showed a similar decrease and partial recovery in ANC after the first dose, disregarding the drug and dosing regimen considered, followed by different amplitudes of saw‐tooth patterns on repeated dosing. Tocilizumab 4 mg/kg i.v. showed the most pronounced fluctuations in ANC levels; sarilumab 150 and 200 mg, and tocilizumab 8 mg i.v. fluctuated within a comparatively narrow range.
Figure 2.
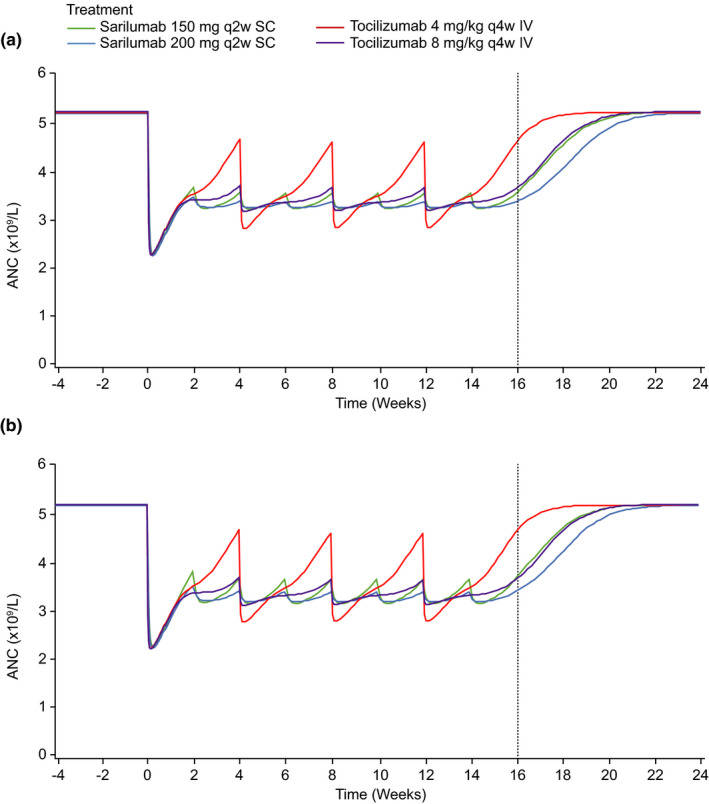
Median simulated ANC over time from covariate MT model. (a) Model training dataset and (b) sensitivity analysis dataset. The vertical dotted line indicates the last trough day. ANC, absolute neutrophil count; MT, margination–tolerance.
Two alternative structural models were evaluated to explore other plausible mechanisms of generating tolerance‐like ANC profiles. These models are considered hypothesis‐generating as they do not have direct quantitative support based on biological experiments. The first exploratory model was named the margination‐increase‐in‐turnover (MIT) model and implemented a proportional increase in the production and elimination of neutrophils as a function of drug concentration (Figure S1a ). This model predicted a similar tolerance pattern (Figure S2a ), but the BIC substantially worsened by 223 (unitless), suggesting an inferior model fit. This MIT model predicted a small, short, temporary decrease in total (circulating and marginated) ANC after treatment (Figure S3a ). In treatment regimens with saturating effect on ANC at the end of the treatment intervals at steady‐state, this decrease mostly disappeared after the second dose. The second exploratory model was named the margination‐increase‐in‐production (MIP) model and implemented a compensatory increase in the production rate of neutrophils in response to margination (Figure S1b ). The feedback signal imposed on k in was a function of circulating ANC. This model also predicted a similar tolerance pattern to that of the MT model, but the BIC substantially worsened by 128. The tolerance‐like pattern in this model is caused by the delay between an increase in k in and the appearance of the neutrophils in the blood. The MIP model predicted an increase in combined circulating and marginated neutrophil quantity during treatment due to the increase in the number of neutrophils produced (Figure S3b ). A distinct property of the MT compared with the MIP model is a somewhat sharper nadir (Figure 2a and Figure S2b , respectively), which is more aligned with the observed data. This difference is presumably due to demargination occurring faster than the increase in the production rate. An implication of the predicted increase in total (circulating and marginal) ANC is that this prediction can be tested using biological experiments.
It appeared the part of the MIP model explaining tolerance patterns resembles the Friberg model of compensatory increase in production rate of neutrophils after chemotherapies. 18 The optimal number of compartments describing production of neutrophils in the MIP model was three, whereas four compartments were used in the Friberg model.
Model evaluation
An empirical assessment of stability demonstrated good stability. The bootstrap confidence intervals were satisfactory and consistent with the parameter estimates (Tables 1 and 2 ). Diagnostic plots showed a good model fit: there was good agreement between individual‐predicted and population‐predicted vs. observed ANC (Figure 3a ), and individual weighted residuals showed a balanced distribution around zero across the range of predicted values (Figure 3c ). Visual predictive checks showed that the 95% prediction intervals contained nearly all the percentiles of observed data across the doses of sarilumab and tocilizumab studied (Figure 4 ). Simulations for the margination model in which tolerance was not implemented are shown in Figure S2c and demonstrate profound differences in ANC profile when compared with the MT model (Figure 2a ). Examples of individual observed and modeled ANC vs. time plots illustrate the individual model fit (Figure S4 ).
Figure 3.
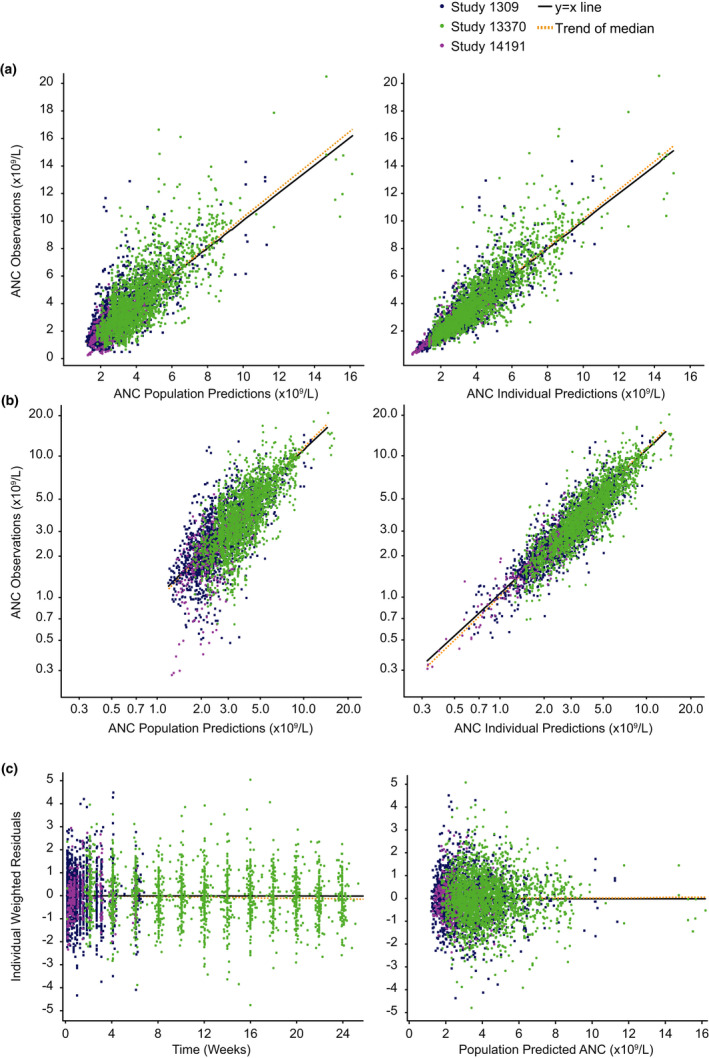
Diagnostic plots. (a) Population and individual observed vs. predicted ANC, (b) log‐scaled population and individual observed vs. predicted ANC, and (c) IWRES vs. time and population‐predicted ANC. ANC, absolute neutrophil count; IWRES, individual weighted residuals.
Figure 4.
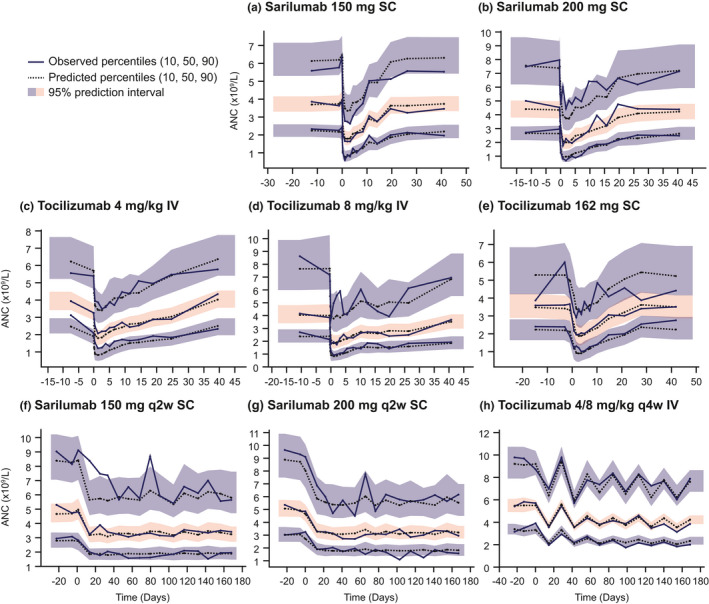
Visual predictive checks. After single doses of s.c. sarilumab (a, b), i.v. tocilizumab (c, d), and s.c. tocilizumab (e), and multiple doses of s.c. sarilumab (f, g) and i.v. tocilizumab (h). ANC, absolute neutrophil count.
Discussion
This study found that ANC changes over time seen with IL‐6R inhibitors were well described by the MT model, which incorporates concepts of neutrophil margination and tolerance. The proposed property of tolerance is an ANC‐specific effect, and there is no evidence of a tolerance effect on the reduction of C‐reactive protein or other PD markers, or on improvement in symptoms or suppression of bone destruction in RA. 30 The MT model supports an effect of sarilumab and tocilizumab on promoting the migration of neutrophils from the vascular circulation into a rapidly mobilizable margination compartment. This compartment can be identified physiologically through the process of neutrophil adhesion to blood vessel walls, particularly the capillary endothelium in the liver, spleen, and lungs. 13 , 31 Notably, the ANC level at nadir was similar for tocilizumab and sarilumab, reflecting saturation of the margination effect regardless of the drugs’ different affinities for the IL‐6R. 7 , 27 An assumption in the MT model was that neither the production nor the elimination of neutrophils changes after dosing, and, therefore, the total quantity of neutrophils stays constant. As well as fitting the ANC observations, the decrease in circulating rather than total neutrophils implied by margination in the MT model is supported by the absence of association between drug‐induced neutropenia and an increased risk of infection seen in clinical trials of sarilumab and tocilizumab. 9 , 11
The imposition of a link function on rates is an approach used in some quantitative systems pharmacology models, 32 and was applied to the rate of margination and demargination, or unidirectionally on margination rate only. The rationale for changing both rates was to accommodate for potential chemotaxis implying that not only margination but also slowing of demargination may occur. The testing and inclusion of a tolerance compartment, informed by the ANC kinetics observed in sarilumab studies with frequent assessment schedules, 7 resulted in acceptable stability of the model and substantially improved model fit.
The development of the MT model follows an evolution of previous models of ANC kinetics with IL‐6R inhibition. Previous models did not allow for ANC‐specific tolerance because the published ANC data were mostly sparse. ANC was modeled by Gibiansky and Frey in a model in which ANC was directly dependent on the concentrations of unbound soluble IL‐6R, and subsequently in a less complex indirect‐response (IR) model in which effects on ANC were mediated indirectly though effects of tocilizumab on the rate of neutrophil elimination from the circulation. 19 , 20 Although Gibiansky and Frey's approximation of the target‐mediated model is innovative and has merit, it is less suitable for our study due to assumptions that sIL‐6R and mIL‐6R have the same occupancy and are distributed solely in circulation. One can hypothesize that accumulation of IL‐6 after blockade of IL‐6R can lead to tolerance‐like patterns in ANC, but predicted ANC did not demonstrate such a pattern. 19 In addition, such a hypothesis suggests that the development of tolerance is not specific to neutrophils and should affect efficacy. However, the tolerance was specific to ANC. Here, it is important to remember that although IL‐6R inhibitors increase the level of IL‐6 in circulation by delaying IL‐6 clearance, IL‐6 signaling is inhibited as long as IL‐6R is inhibited. 33 The authors postulated margination as a possible explanation for neutrophil elimination from the circulation, but did not include a dedicated compartment for margination. As part of the current work, we modeled the effect of including a tolerance compartment in the previously published IR model (Figure S1c ), and found that similarly to the MT model, addition of a tolerance compartment together with a single transit compartment to account for any tolerance lag time resulted in acceptable stability and substantially improved model fit. The adapted IR model accounting for tolerance was named the IR‐tolerance (IRT) model.
The base MT model was superior to the IRT model based on BIC but had similar diagnostic plots. In these circumstances, biologic plausibility offers means of discrimination. The half‐life of neutrophils in the MT model was fixed to 19 hours, as observed in healthy subjects. 28 Furthermore, there is evidence that inflammatory conditions, such as RA, may lead to some prolongation of neutrophil half‐life. 34 , 35 In the IRT model, the half‐life was estimated as 6.4 hours with maximal decrease to 2.7 hours after drug administration; both half‐lives are substantially lower than the estimate of 19 hours in healthy subjects and, therefore, are lower than a potentially prolonged half‐life in patients with RA. 34 , 35 This attribute of the IRT model is inconsistent with the prolongation of neutrophil half‐life in patients with RA, as fixing the half‐life at baseline to 19 hours in the IRT model led to substantial worsening of model fit (analysis not shown). Additionally, the increase in elimination rate or decrease in production rate of neutrophils in the IRT model is inconsistent with the absence of an impact on the rate of infections. The OFV of the MT model had low sensitivity to large changes in k out; as an example, decreasing the half‐life from 19 to 6.4 hours led to statistically insignificant change in OFV and did not affect the remaining parameters. This occurs because when k out is fixed to a different value, the model‐estimated baseline ANC remains similar; therefore. k in, which is not estimated in the model but can be calculated, increases with k out.
A possible physiologic explanation of the tolerance component is compensatory feedback on margination as a function of the change in circulating ANC; that is, margination decreases over time. However, as different assumptions can lead to the same equations, other interpretations of the tolerance model are possible. Two model modifications exploring hypotheses of tolerance evaluated a proportional increase in ANC production and elimination rate, or an increase in production rate as possible compensatory mechanisms of margination‐like patterns. The models are not presented in detail because they were substantially statistically inferior to the primary/final MT model based on BIC and because additional biologic evidence is needed to support the underlying hypotheses.
The values of Emax and EC50 from the MT model should not be directly compared with published IR model counterparts due to different physiologic and mathematical meaning. If in the IR model, a fold‐change in k out leads to the corresponding reverse fold‐change in steady‐state ANC, the addition of the tolerance part of the model complicates this relationship. Furthermore, the same values of EC50 in the MT and IR models relate to different changes in ANC. Similarly, the same fold‐changes in Emax in the MT and IR models lead to different maximal changes in ANC.
Overall, the MT (primary) model explains tolerance by a compensatory mechanism leading to quick partial demargination of neutrophils back from the margination pool. Based on BIC, this model was the best of the models tested. The MIT model is based on the observation of prolonged half‐life of neutrophils in RA, 34 , 35 and an assumption that treatment changes half‐life back to normal; as the ANC value in RA may only be slightly higher than in healthy subjects, it follows that the production rate of neutrophils increases after treatment. The MIP model was based on the assumption that the decrease in circulation of neutrophils leads to a compensatory increase in production of neutrophils and this is consistent with the Friberg model of compensatory increase in production rate of neutrophils after eradicating them by chemotherapies 18 ; the Friberg model predicts an increase in total (circulating and marginated) ANC (Figure S3b ). Biological differentiation of the MT, MIT, and MIP models will require quantitative measurement of total and/or marginated neutrophils. The IRT model was inconsistent with recent clinical research demonstrating margination and recently published half‐life of neutrophils. 17 , 28 Similar tolerance patterns were observed across models (Figure 2a and Figure S2a,b ) with some differences in patterns, which may explain the large differences in the BIC between the primary MT and supportive models.
In conclusion, the observed rapid and transient decrease in ANC following administration of IL‐6R inhibitors is effectively described by a model that incorporates neutrophil margination together with ANC‐specific tolerance. The MT model was superior to the other models based on BIC suggesting a compensatory mechanism leading to quick demargination of neutrophils back to circulation from the margination pool. The hypothesis of tolerance was supported by ensuring stability, improving diagnostic plots, and demonstrating high statistical significance of the tolerance part. A deeper understanding of properties of neutrophils through such models supports better understanding of the safety profile of a major class of immunomodulators targeting IL‐6R inhibitors. These findings are consistent with the observed absence of association between low ANC and infection or serious infection in sarilumab and tocilizumab studies.
Funding
Study funding and medical writing support (Matt Lewis, PhD, of Adelphi Communications Ltd, Macclesfield, UK) were provided by Sanofi Genzyme (Cambridge, MA, USA) and Regeneron Pharmaceuticals, Inc. (Tarrytown, NY, USA) in accordance with GPP3 guidelines.
Conflicts of Interest
P.K., A.P., A.B., M.C.N., J.D.D., and A.T.D. are employees of Regeneron Pharmaceuticals, Inc., and may hold stock and/or stock options in the company. C.X. is an employee of Sanofi Genzyme and may hold stock and/or stock options in the company. G.S.J. and R.R. were employees of Regeneron Pharmaceuticals, Inc. at the time of this work, and may hold stock and/or stock options in the company.
Author Contributions
P.K. drafted the manuscript. P.K. designed and performed the research. P.K., A.P., A.B., C.X., G.S.J., M.C.N., J.D.D., R.R., and A.T.D. analyzed the data.
Supporting information
Data S1
Data S2
Data S3
Data S4
Acknowledgments
The authors thank Ching‐Ha (Vicki) Lai, Matthew Andisik, Lisa DeStefano, Colin Stefan, Kevin Laurino, and Stephen Okaine for generating the PK data, and Matt Lewis, PhD, of Adelphi Communications Ltd (Macclesfield, UK) for medical writing support, which was funded by Sanofi Genzyme (Cambridge, MA, USA) and Regeneron Pharmaceuticals, Inc. (Tarrytown, NY, USA) in accordance with Good Publication Practice (GPP3) guidelines.
References
- 1. McInnes, I.B. , Buckley, C.D. & Isaacs, J.D. Cytokines in rheumatoid arthritis – shaping the immunological landscape. Nat. Rev. Rheumatol. 12, 63–68 (2016). [DOI] [PubMed] [Google Scholar]
- 2. Choy, E. Understanding the dynamics: pathways involved in the pathogenesis of rheumatoid arthritis. Rheumatology (Oxford) 51 (suppl. 5), v3–v11 (2012). [DOI] [PubMed] [Google Scholar]
- 3. Dayer, J.M. & Choy, E. Therapeutic targets in rheumatoid arthritis: the interleukin‐6 receptor. Rheumatology (Oxford) 49, 15–24 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lamb, Y.N. & Deeks, E.D. Sarilumab: a review in moderate to severe rheumatoid arthritis. Drugs 78, 929–940 (2018). [DOI] [PubMed] [Google Scholar]
- 5. Scott, L.J. Tocilizumab: a review in rheumatoid arthritis. Drugs 77, 1865–1879 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schiff, M.H. et al Integrated safety in tocilizumab clinical trials. Arthritis Res. Ther. 13, R141 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Emery, P. et al Safety and tolerability of subcutaneous sarilumab and intravenous tocilizumab in patients with rheumatoid arthritis. Rheumatology (Oxford) 58, 849–858 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wright, H.L. , Cross, A.L. , Edwards, S.W. & Moots, R.J. Effects of IL‐6 and IL‐6 blockade on neutrophil function in vitro and in vivo. Rheumatology (Oxford) 53, 1321–1331 (2014). [DOI] [PubMed] [Google Scholar]
- 9. Fleischmann, R. et al Long‐term safety of sarilumab in rheumatoid arthritis: an integrated analysis with up to 7 years' follow‐up. Rheumatology (Oxford) 59, 292–302 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Crawford, J. , Dale, D.C. & Lyman, G.H. Chemotherapy‐induced neutropenia: risks, consequences, and new directions for its management. Cancer 100, 228–237 (2004). [DOI] [PubMed] [Google Scholar]
- 11. Moots, R.J. et al Effect of tocilizumab on neutrophils in adult patients with rheumatoid arthritis: pooled analysis of data from phase 3 and 4 clinical trials. Rheumatology (Oxford) 56, 541–549 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li, Y. et al Trajectory of absolute neutrophil counts in patients treated with pegfilgrastim on the day of chemotherapy versus the day after chemotherapy. Cancer Chemother. Pharmacol. 77, 703–712 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Scheiermann, C. , Frenette, P.S. & Hidalgo, A. Regulation of leucocyte homeostasis in the circulation. Cardiovasc. Res. 107, 340–351 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kolaczkowska, E. & Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 13, 159–175 (2013). [DOI] [PubMed] [Google Scholar]
- 15. Deniset, J.F. & Kubes, P. Recent advances in understanding neutrophils. F1000Res 5, 2912 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Suwa, T. , Hogg, J.C. , English, D. & Van Eeden, S.F. Interleukin‐6 induces demargination of intravascular neutrophils and shortens their transit in marrow. Am. J. Physiol. Heart Circ. Physiol. 279, H2954–H2960 (2000). [DOI] [PubMed] [Google Scholar]
- 17. Lok, L.S.C. et al Effects of tocilizumab on neutrophil function and kinetics. Eur. J. Clin. Invest. 47, 736–745 (2017). [DOI] [PubMed] [Google Scholar]
- 18. Friberg, L.E. , Henningsson, A. , Maas, H. , Nguyen, L. & Karlsson, M.O. Model of chemotherapy‐induced myelosuppression with parameter consistency across drugs. J. Clin. Oncol. 20, 4713–4721 (2002). [DOI] [PubMed] [Google Scholar]
- 19. Gibiansky, L. & Frey, N. Linking interleukin‐6 receptor blockade with tocilizumab and its hematological effects using a modeling approach. J. Pharmacokinet. Pharmacodyn. 39, 5–16 (2012). [DOI] [PubMed] [Google Scholar]
- 20. Gibiansky, L. , Frey, N. & Hsu, J.C. Effects of tocilizumab on neutrophil counts in patients with rheumatoid arthritis (RA). J. Pharmacokinet. Pharmacodyn. 41, S85–S86 (2014). [Google Scholar]
- 21. Ishii, T. et al AB0472 Pharmacodynamic effect and safety of single‐dose sarilumab sc or tocilizumab iv or sc in patients with rheumatoid arthritis (RA). Ann. Rheum. Dis. 2018, 1397–1398 (2018). [Google Scholar]
- 22. Xu, C. , Su, Y. , Paccaly, A. & Kanamaluru, V. Population pharmacokinetics of sarilumab in patients with rheumatoid arthritis. Clin. Pharmacokinet. 58, 1455–1467 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Thai, H.T. , Mentre, F. , Holford, N.H. , Veyrat‐Follet, C. & Comets, E. Evaluation of bootstrap methods for estimating uncertainty of parameters in nonlinear mixed‐effects models: a simulation study in population pharmacokinetics. J. Pharmacokinet. Pharmacodyn. 41, 15–33 (2014). [DOI] [PubMed] [Google Scholar]
- 24. Burnham, K. & Anderson, D. Model Selection and Multi‐Model Inference. (Springer, New York, NY, 2002). [Google Scholar]
- 25. Gabrielsson, J. & Weiner, D. Pharmacokinetic and Pharmacodynamic Data Analysis: Concepts and Applications, 4th edn. (Swedish Pharmaceutical Press, Stockholm, 2007). [Google Scholar]
- 26. Kovalenko, P. et al Pharmacodynamic (PD) model of neutrophil margination to describe transient effect of sarilumab on absolute neutrophil count (ANC) in patients with rheumatoid arthritis (RA) after single‐dose administration. 26th Population Approach Group in Europe Meeting. Budapest, Hungary: (2017). [Google Scholar]
- 27. Rafique, A. et al AB0037 Evaluation of the binding kinetics and functional bioassay activity of sarilumab and tocilizumab to the human IL‐6 receptor (IL‐6R) alpha. Ann. Rheum. Dis. 72 (suppl. 3), 797 [Abstract No. AB0037]. (2013).23291386 [Google Scholar]
- 28. Lahoz‐Beneytez, J. et al Human neutrophil kinetics: modeling of stable isotope labeling data supports short blood neutrophil half‐lives. Blood 127, 3431–3438 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Athens, J.W. et al Leukokinetic studies. IV. The total blood, circulating and marginal granulocyte pools and the granulocyte turnover rate in normal subjects. J. Clin. Invest. 40, 989–995 (1961). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Genovese, M.C. et al Long‐term safety and efficacy of sarilumab plus methotrexate on disease activity, physical function and radiographic progression: 5 years of sarilumab plus methotrexate treatment. RMD Open 5, e000887 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Christoffersson, G. & Phillipson, M. The neutrophil: one cell on many missions or many cells with different agendas? Cell Tissue Res. 371, 415–423 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Karelina, T. , Voronova, V. , Demin, O. , Colice, G. & Agoram, B.M. A mathematical modeling approach to understanding the effect of anti‐interleukin therapy on eosinophils. CPT Pharmacometrics Syst. Pharmacol. 5, 608–616 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nishimoto, N. et al Mechanisms and pathologic significances in increase in serum interleukin‐6 (IL‐6) and soluble IL‐6 receptor after administration of an anti‐IL‐6 receptor antibody, tocilizumab, in patients with rheumatoid arthritis and Castleman disease. Blood 112, 3959–3964 (2008). [DOI] [PubMed] [Google Scholar]
- 34. Grieshaber‐Bouyer, R. & Nigrovic, P.A. Neutrophil heterogeneity as therapeutic opportunity in immune‐mediated disease. Front. Immunol. 10, 346 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen, W. , Wang, Q. , Ke, Y. & Lin, J. Neutrophil function in an inflammatory milieu of rheumatoid arthritis. J. Immunol. Res. 2018, 1–12 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Data S2
Data S3
Data S4


