Abstract
Background:
Insufficient data are available to support the routine use of tranexamic acid (TXA) in anterior cruciate ligament (ACL) surgeries with respect to administration method and frequency, exposure duration, dose, and adverse effects.
Purpose:
To investigate whether intra-articular (IA) administration of TXA could reduce hemarthrosis and postoperative pain in patients after ACL reconstruction.
Study Design:
Randomized controlled trial; Level of evidence, 1.
Methods:
A total of 47 patients were included in this study, which was performed between July 2017 and May 2019. Single-bundle reconstructions using autologous hamstring tendon grafts were performed in all patients. The patients were randomized into 2 groups: the TXA group (received the index procedure with 100-mL IA injection of TXA [30 mg/mL]) and a control group (did not receive IA injection of TXA). No patients received a drain. Blood loss was calculated on the basis of hemoglobin balance at postoperative day (PD) 2. The visual analog scale (VAS) for pain score was assessed at PD 3. The midpatellar circumference was measured at PD 2 and PD 5. Knee range of motion (ROM) was evaluated 6 weeks after surgery.
Results:
The mean ± SD blood loss was 467 ± 242 mL in the TXA group and 558 ± 236 mL in the control group. No significant differences were found for blood loss (P = .20), VAS pain scores (P = .28), ROM at postoperative week 6 (P = .61), or patellar circumference at PD 2 (P = .75) and PD 5 (P = .84).
Conclusion:
This study showed that IA administration of 3.0 g of TXA had no effect in reducing blood loss and postoperative pain after primary anatomic single-bundle ACL reconstruction using quadruple hamstring autografts.
Registration:
NCT04042688 (ClinicalTrials.gov identifier).
Keywords: anterior cruciate ligament, hemarthrosis, randomized controlled trial, tranexamic acid
Tranexamic acid (TXA) has an antifibrinolytic effect via competitive blockage of lysine-binding sites in plasminogen molecules and inhibition of fibrinolysis.6 Antifibrinolytic agents potentially reduce blood loss during surgery, with decreased intraoperative bleeding and complications in relation to transfusion.12 TXA can be administered via various methods during surgery: intravenous (IV), oral, and topical. Current evidence supports that TXA reduces blood loss and the need for allogeneic blood transfusion, without an apparent increase in the risk of deep vein thrombosis, pulmonary embolism, or other thromboembolic complications after total knee replacement or total hip replacement.22,25 However, the best administration method, dose, and timing and the number of administrations required have not been established.
Hemarthrosis after anterior cruciate ligament (ACL) reconstruction leads to postoperative pain and limits rehabilitation.6 Recent studies have shown that IV or intra-articular (IA) TXA administration may reduce postoperative hemarthrosis, thereby decreasing pain, promoting better rehabilitation, and improving functional outcomes in the short-term period after ACL reconstruction.5,15 However, data supporting the routine use of TXA in ACL surgeries with respect to the administration method and frequency, exposure duration, dose, and adverse effects are insufficient compared with data regarding arthroplasty.
The purpose of this study was to investigate whether IA administration of TXA could reduce hemarthrosis and postoperative pain in patients undergoing ACL reconstruction. The hypothesis of the study was that patients who received IA TXA treatment would have significantly less postoperative hemarthrosis, less pain in the early phase of rehabilitation, and more range of motion compared with those who did not receive IA TXA treatment.
Methods
The study was approved by an institutional review board and registered at ClinicalTrials.gov. All patients provided informed consent.
Patient Demographics
Patients who were scheduled for arthroscopic ACL reconstruction at our institution were screened prospectively between July 2017 and May 2019. The inclusion criterion was primary single-bundle ACL reconstruction using a hamstring autograft with or without meniscal surgeries. The exclusion criteria were use of other grafts (eg, allograft and bone–patellar tendon–bone autograft), revision ACL reconstruction, concomitant knee cartilage or collateral ligament surgeries, history of knee surgery on the affected knee, concomitant fracture, significant preoperative pain (visual analog scale [VAS] score >5), coagulation or bleeding disorders, and preoperative anticoagulation treatment. During the study period, a total of 106 patients were screened, and 60 patients (60 knees) were enrolled on the basis of the sample size calculated and the inclusion and exclusion criteria. The patients were randomly allocated to 1 of 2 groups during preoperative preparation through use of computer-generated randomization: the TXA group (IA administration of TXA) and a control group (no administration of TXA). The list resulting from this process was accessible to only the independent orthopaedic residents who participated in the surgeries. The patients and orthopaedic surgeons involved in this study were blinded to the group allocation.
ACL Reconstruction
Patients received prophylactic antibiotic treatment (a single injection of second-generation cephalosporin [1 g]) 30 minutes before surgery. Anatomic single-bundle ACL reconstruction with a quadruple hamstring tendon autograft was performed by a senior surgeon (J.-H.B.) with the same technique under tourniquet control (300 mm Hg), as follows: (1) arthroscopic examination, (2) gracilis and semitendinosus tendon harvest and graft preparation, (3) femoral tunnel preparation using the 2-incision (outside-in) technique, (4) tibial tunnel preparation, and (5) graft fixation. For graft fixation, the surgeon performed (1) fixation of the femoral tunnel first using bioabsorbable interference screws (Biosure Regenesorb; Smith & Nephew), (2) fixation of the tibial tunnel next using bioabsorbable interference screws, and (3) back-up fixation using 6.5-mm cancellous screws and spike washers (JMT). Meniscal repair or partial meniscectomy was performed according to the tissue quality, tissue repairability, and patient factors such as age in case of unstable meniscal lesions.
Interventions
Based on previous meta-analysis findings, TXA was administered via the IA route at a concentration of 30 mg/mL.2,27 The operating room nurses prepared 3 g of TXA in 100 mL of saline solution during surgery. After the surgical wound was sutured and the orthopaedic surgeons left the operating room, the orthopaedic residents who were not involved in the postoperative evaluation administered the TXA solution into the suprapatellar pouch via a superolateral approach before tourniquet release in the patients in the TXA group. The control group did not receive any solution. No patients received a drain.
Postoperative Regimen
The same postoperative analgesic protocol was followed for all patients, including IV administration of nefopam hydrochloride with a standard fluid resuscitation protocol (35 mL/kg per 24 hours of lactated Ringer solution) until postoperative day (PD) 2 and oral administration of medications (acetaminophen 325 mg and tramadol hydrochloride 37.5 mg) twice daily until PD 5. Based on internationally accepted and approved conversion values, each patient’s morphine consumption level was 65 morphine milligram equivalents (MME) per day; therefore, the total consumption was 325 MME for postoperative pain control.10 A compression stocking and a mechanical air cuff were routinely applied to prevent deep vein thrombosis; however, no pharmacologic intervention was used. Furthermore, no ice or cryotherapy was used routinely. The patients were discharged on PD 5.
After ACL reconstruction, the same rehabilitation protocol was followed for all patients. Range of motion (ROM) and isometric quadriceps exercises were started 1 or 2 days after surgery, and the intensities were gradually increased. Gait training, balance exercise, and proprioceptive exercise were started 3 to 4 weeks postoperatively. A brace was used for the first 4 to 6 weeks during outdoor activities. Patients were allowed partial weightbearing using crutches for 6 to 8 weeks. Full weightbearing was allowed 6 to 8 weeks after surgery.
Outcomes
Demographic variables (ie, age, sex, body mass index, and time from injury to surgery), clinical outcomes, and surgical records including surgical time, intraoperative blood loss, and meniscal and cartilage lesions were collected by an independent orthopaedic resident. The primary outcome was blood loss calculated on the basis of hemoglobin (Hb) balance. We assumed that the blood volume (BV; mL) at PD 2 was the same as that before surgery.16 We estimated BV using the method described by Nadler and colleagues,19 taking into account sex, body mass index, and height. We then estimated Hb loss (g) using the following formula16:
where Hb loss (g) is the amount of Hb lost; Hbi (g L ± 1) is the Hb concentration before surgery; Hbe (g L ± 1) is the Hb concentration at PD 2; and Hbt (g) is the total amount of allogeneic Hb transfused. The blood loss amount (mL) was calculated in relation to a patient’s preoperative Hb value (g L-1):
The secondary outcome was the extent of pain on PD 3 measured by use of the VAS. We documented the VAS pain score consistently on the afternoon of PD 1 and for the next 4 days (until PD 5). The VAS is a numeric response scale with scores ranging from 0 to 10 points (0 points, no pain; 10 points, severe pain). The other secondary outcomes included the midpatellar circumference at PD 2 and PD 5 and the knee ROM at postoperative week 6, which were measured by an independent orthopaedic fellow (J.-W.L.) who was blinded to the details of this study. The patellar circumference was measured at the transverse axis of the patellar center to evaluate IA effusion. To minimize interobserver variance in the patellar circumference, we considered the difference between the preoperative and postoperative results. The ROM was documented through use of an orthopaedic goniometer.
Statistical Analysis
The sample size was determined via equality power calculation. The power analysis was performed by use of GPower 3.1.7 According to a previous study,15 a difference of 90 (effect size, d = 1.14) was considered significant, and the 2-group independent-sample t test revealed a sample size of 23 considering a loss to follow-up of 20% in each group and an accepted alpha error of .05 with a power value of 0.90.
Absolute values and differences between the preoperative and postoperative data were evaluated. The Student t test was used for normally distributed continuous variables between the TXA and control groups. The nonparametric Wilcoxon signed rank test was used to compare matched groups of data. For dichotomous variables, the Fisher exact test was used. Differences were considered statistically significant at P values less than .05.
Results
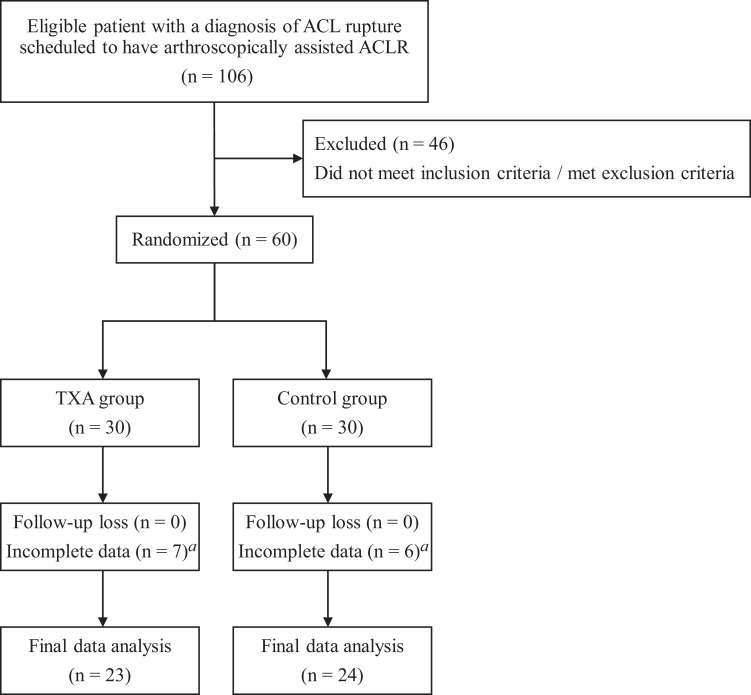
We excluded 13 patients after randomization as a result of loss of clinical data. Complete data were available for 23 patients in the TXA group and 24 patients in the control group (Figure 1). No differences in the demographic and intraoperative variables between the 2 groups were found, except for age (older in the TXA group) and meniscal repair (more common in the TXA group). The preoperative patient evaluation data and intraoperative clinical data are presented in Tables 1 and 2, respectively.
Figure 1.
Flowchart of patient inclusion. ACL, anterior cruciate ligament; ACLR, ACL reconstruction; TXA, tranexamic acid. aDischarge earlier than postoperative 5 days.
TABLE 1.
Preoperative Patient Evaluation Dataa
| Variables | Control Group (n = 24) |
TXA Group (n = 23) |
P Value |
|---|---|---|---|
| Demographic data | |||
| Age, y | 25.1 ± 8.1 (15-49) | 30.3 ± 9.0 (17-47) | .04 |
| Sex, male/female | 21 (87.5)/3 (12.5) | 20 (87.0)/3 (13.0) | .96 |
| Height, cm | 173.4 ± 6.4 (160-186) | 172.0 ± 9.8 (153-185) | .55 |
| Weight, kg | 76.9 ± 12.2 (52-103) | 78.7 ± 9.6 (63-100) | .60 |
| Body mass index, kg/m2 | 25.5 ± 3.3 (19.1-31.9) | 26.5 ± 2.8 (20.8-33.7) | .99 |
| Time to surgery, mo | 3.4 ± 5.7 (0.3-24.0) | 2.2 ± 2.8 (0.3-12.0) | .34 |
| Preoperative clinical data | |||
| VAS score | 1.4 ± 1.4 (0-5) | 1.6 ± 0.9 (0-3) | .59 |
| Range of motion, deg | 128 ± 18 (90-155) | 122 ± 13 (100-150) | .39 |
| Patellar circumference, cm | 37.8 ± 2.3 (34.0-42.7) | 39.1 ± 3.9 (34.8-46.1) | .09 |
| Lysholm score | 54.2 ± 28.5 (2-90) | 42.0 ± 24.4 (4-81) | .12 |
aData are presented as mean ± SD (range) or as number (%) of patients. Boldface indicates a significant difference between groups (P < .05). TXA, tranexamic acid; VAS, visual analog scale.
TABLE 2.
Intraoperative Clinical Dataa
| Variables | Control Group (n = 24) |
TXA Group (n = 23) |
P |
|---|---|---|---|
| Meniscal repair | 6 (25) | 13 (57) | .03 |
| Partial meniscectomy | 5 (21) | 2 (9) | .25 |
| Tunnel diameter, cm | 7.9 ± 0.5 (7.0-8.5) | 7.9 ± 0.3 (7.0-8.0) | .63 |
aData are presented as mean ± SD (range) or as number (%) of patients. Boldface indicates a significant difference between groups (P < .05). TXA, tranexamic acid.
The primary and secondary outcomes are described in Table 3. We found no significant difference in the amount of blood loss, VAS pain score, patellar circumference at PD 2 and PD 5, and ROM at postoperative week 6 between the 2 groups. No systemic adverse effects or need for aspiration were noted in either group during the follow-up period.
TABLE 3.
Study Resultsa
| Variables | Control Group (n = 24) |
TXA Group (n = 23) |
P Value | Between-Group Difference (95% CI) |
|---|---|---|---|---|
| Estimated blood loss, mL | 558 ± 236 (136-1088) | 467 ± 242 (179-1127) | .20 | –90.8 (–231.1 to 49.6) |
| VAS score | ||||
| Postoperative day 1 | 4.2 ± 1.8 (1-7) | 3.3 ± 1.3 (2-5) | .07 | –0.9 (–1.8 to 0.1) |
| Postoperative day 2 | 3.3 ± 1.7 (0-7) | 3.1 ± 1.3 (2-7) | .64 | –0.2 (–1.1 to 0.7) |
| Postoperative day 3 | 3.0 ± 1.3 (0-6) | 2.6 ± 1.2 (1-6) | .28 | –0.4 (–1.1 to 0.3) |
| Postoperative day 4 | 2.9 ± 1.3 (0-6) | 2.3 ± 1.1 (1-6) | .08 | –0.6 (–1.3 to 0.1) |
| Postoperative day 5 | 2.6 ± 1.2 (0-6) | 2.1 ± 1.1 (1-5) | .18 | –0.5 (–1.1 to 0.2) |
| Range of motion, degb | ||||
| Postoperative week 6 | 111.3 ± 19.4 (90-155) | 108.3 ± 20.8 (70-150) | .61 | –3.0 (–14.8 to 8.8) |
| Patellar circumference, cm | ||||
| Postoperative day 2 | 41.2 ± 2.9 (36.5-47.2) | 40.9 ± 2.9 (35.8-49.3) | .75 | –0.3 (–2.0 to 1.4) |
| Postoperative day 5 | 40.7 ± 2.4 (36.1-45.9) | 40.8 ± 3.0 (36.5-50.1) | .84 | 0.1 (–1.4 to 1.7) |
| Hemoglobin level, g/dL | ||||
| Preoperative period | 15.4 ± 0.9 (13.4-16.9) | 14.9 ± 1.1 (12.4-16.7) | .17 | –0.5 (–1.0 to 0.2) |
| Postoperative day 1 | 13.9 ± 1.1 (12.1-15.7) | 13.8 ± 1.1 (11.2-15.6) | .72 | –0.1 (–0.7 to 0.5) |
| Postoperative day 2 | 13.5 ± 1.2 (11.3-15.9) | 13.6 ± 1.1 (10.2-15.0) | .89 | 0.1 (–0.6 to 0.7) |
| Postoperative day 5 | 13.6 ± 1.2 (10.8-15.9) | 13.9 ± 1.0 (11.4-15.3) | .32 | 0.3 (–0.3 to 1.0) |
aData are presented as mean ± SD (range) or as number (%) of patients. TXA, tranexamic acid; VAS, visual analog scale.
bSum of the degrees of flexion and extension.
Discussion
This study investigated whether IA administration of TXA could reduce blood loss and postoperative pain in patients after ACL reconstruction. In contrast to our hypothesis, IA TXA administration in patients undergoing ACL reconstruction did not significantly reduce postoperative blood loss and pain in the early phase of the rehabilitation process.
In contrast to our study, previous studies have reported positive effects of TXA in reducing hemarthrosis after ACL reconstruction despite the different amounts of TXA administered and different administration methods. Karaaslan et al15 reported that the risk of hemarthrosis decreased and the functional outcomes after ACL reconstruction improved by IV administration of TXA. Those investigators administered an IV infusion of 15 mg/kg TXA in 100 mL of saline solution 20 minutes before tourniquet release in the patients in their treatment group, and their control group received the same volume of saline solution without TXA. The investigators did not mention their postoperative analgesic protocol. Felli et al8 described that a single IV administration of TXA improved early-phase clinical outcomes after ACL reconstruction by reducing the amount of BV drainage and ROM and reducing fever episodes. Those investigators administered TXA at 10 mg/kg/h; it was continued for the next 3 hours after completion of surgery in the patients in the treatment group, and the control group received the same volume of saline solution without TXA. The postoperative analgesic protocol consisted of IV administration of ketorolac 30 mg every 8 hours for the first day and dexibuprofen 400 mg twice daily for the next 5 days. Both of these previous studies had 2 major differences with our study: method of TXA administration and measurement of blood loss. Although using different administration protocols, both previous studies selected IV administration taking into consideration the potential chondrotoxic effect of topical administration. Moreover, we used the Hb-balance method to calculate blood loss instead of using a drainage system. In addition to these differences, we did not administer normal saline intra-articularly to the patients in the control group, and we used different analgesic protocols.
Recently, Chiang et al5 reported results similar to those of previous studies. These authors demonstrated that IA injection of TXA could reduce postoperative bleeding in the first 24 hours and decrease pain.5 Their study design was similar to that of our study in terms of the administration method (IA TXA injection), group of patients investigated, and outcome measurement, although the study by Chiang et al used a drainage system. However, many factors limit comparisons of our study with previous studies, owing to differences in the route of administration, dose of TXA, analgesic protocol, methods of outcome measurement, and timing of outcome evaluation.
Previous studies reported different amounts of hemarthrosis. Felli et al8 reported a drainage amount of 59.3 ± 29.5 mL in their IV TXA group and 133.3 ± 56.1 mL in their control group (P < .001). Karaaslan et al15 reported a drainage amount of 60 mL (range, 20-150 mL) in their IV TXA group and 150 mL (range 75-500 mL) in their control group (P < .001). Chiang et al5 observed a significant decrease in the amount of drainage in patients receiving IA injections in their TXA group (56.1 ± 34.1 mL) compared with their control group (80.1 ± 48 mL; P < .05). Conversely, we reported a blood loss larger than those in previous studies. We used an indirect method to measure blood loss instead of using a drainage system; thus, the blood loss that we found included bleeding that occurs outside of the knee joint and might have been overestimated.
Comparing the current study with previous studies with regard to the VAS score entails limitations due to differences in pain control protocols and surgical techniques; nonetheless, we found that our results were similar to those of previous studies,4,8,15 except for the study by Chiang et al.5 That study reported that the difference in the VAS pain score between their 2 groups was 3.5 points (3.2 ± 1.0 points in the TXA group and 6.7 ± 2.5 points in the control group at PD 3), with 24 mL of mean difference in the drainage amount. The mean difference in the drainage output was only 24 mL, which can be considered a small amount of difference compared with findings in previous studies. Felli et al8 reported a larger difference in the amount of drainage (70 mL) but no difference in the VAS pain score between their 2 groups, compared with the findings by Chiang et al.5 These serial results show that the VAS pain score of 6.7 points in the control group reported by Chiang et al was likely to be exaggerated and that the effectiveness of IA TXA administration in pain control by reducing hemarthrosis is uncertain. Thus, further studies are needed.
The chondrotoxic effect after local administration of TXA is important but also controversial. Only a few studies have investigated the effect of topical administration of TXA in minimally invasive joint surgery in which the articular cartilage is preserved. Some studies demonstrated that topical exposure of TXA had no effect on the articular cartilage; however, concern arises regarding prolonged exposure to TXA.3,11 Indeed, administration of high-dose TXA (100 mg/mL) into tissues or a closed joint space may result in concentrations greater than 1 mg/mL remaining within the synovial fluid and tissue from 18 to 24.5 hours.1 Previous studies have also indicated that toxicity may occur between concentrations of 25 and 50 mg/mL in bovine and murine articular cartilage.23 A previous study using human articular cartilage suggested that increasing concentrations above 20 mg/mL resulted in an atypical morphologic status and reduced cellular adhesion and metabolic activity associated with increased in vitro chondrocyte death. The investigators reported that this effect was dose-dependent for concentrations greater than 20 mg/mL.20 A recent study provided evidence of TXA toxicity to human periarticular tissue at concentrations of 50 or 100 mg/mL and with durations of treatment routinely used in a clinical environment.17 In our study, we administered IA TXA at a concentration of 30 mg/mL, regarded as the minimum dose for reducing blood loss and the need for blood transfusions without increasing the risk of complications.2,27 We believe that this dose was relatively lower than the concentration routinely used; however, the safe range of TXA concentration has not been identified. Our intervention might have caused cartilage toxicity in our study group; however, we could not evaluate the change in cartilage status. Thus, longer follow-up is needed to identify whether IA TXA administration is safe regarding cartilage injury.
Regarding complications of hemarthrosis, Felli et al8 noted 13 fever episodes in their control group, and Karaaslan et al15 identified 19 cases of aspiration in their control group. However, Chiang et al5 reported that no patients exhibited complications, such as infection and arthrofibrosis, or needed aspiration procedures. We did not use a drainage system and noted no complications in relation to hemarthrosis. We also found no difference in ROM between the 2 groups. Prevention of hemarthrosis can be helpful in minimizing joint fibrosis after an injury, thus preserving motion within the joint and maintaining short-term function.24,26 However, we believe that acute hemarthrosis and contained growth factors contribute to ligament or meniscal healing, as supported by the literature.13,14,18 It is questionable whether it is essential to administer TXA by the IA route for arthroscopic ACL reconstruction, because regardless of TXA administration, we noted minimal complications in relation to hemarthrosis, and the effectiveness of IA TXA administration was not proven sufficiently. Thus, further studies are needed.
This study had several limitations. First, we used an indirect method to measure the amount of blood loss. We considered a difference of 90 mL as significant based on previous study findings. In our study, the mean difference in blood loss was 91 mL; however, it was not a significant difference. Among the 4 common calculation methods, the Hb-balance method is regarded as the most reliable and scientifically logical method and is widely applied to evaluate postoperative blood loss.9 Second, we did not administer normal saline via the IA route to the patients in the control group. Ryu et al21 reported that drain clamping with a 50-mL saline infusion containing epinephrine could effectively control postoperative bleeding after total knee arthroplasty. However, no studies have yet demonstrated the amount of saline needed for hemostasis in arthroscopic procedures. If we had shown our hypothesis to be true, we could posit that the results were affected by the lack of injection of saline via an increase in blood loss in the control group. However, we found no differences in any outcomes in our study; thus, it is difficult to judge whether our intervention affected the results. Third, the proportion of meniscal repair differed between the 2 groups. There were 7 more cases of meniscal repair in the TXA group; the number of cases had a significant difference between the 2 groups. Most repaired meniscal lesions were medial and lateral meniscal posterior horn longitudinal tears; thus, we used the all-inside technique in many cases. Meniscal repair might increase blood loss and induce pain. However, despite the biased distribution, we found no significant differences in blood loss and VAS pain score between the 2 groups. Fourth, the time interval from injury to surgery varied among patients. It was difficult to establish consistent surgical timing because of the diversity among patients regarding timing of diagnosis, extent of ROM, and swelling.
Conclusion
This study showed that IA administration of 3.0 g of TXA had no effect in reducing neither blood loss nor postoperative pain after primary anatomic single-bundle ACL reconstruction using quadruple hamstring autografts.
Acknowledgment
The authors acknowledge the Kora University Department of Biostatistics for statistics consultation. We also thank Editage (www.editage.co.kr) for English-language editing.
Footnotes
Final revision submitted February 10, 2020; accepted March 1, 2020.
The authors declared that there are no conflicts of interest in the authorship and publication of this contribution. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was obtained from Korea University Guro Hospital (study No. 2018GR0011).
References
- 1. Ahlberg A, Eriksson O, Kjellman H. Diffusion of tranexamic acid to the joint. Acta Orthop Scand. 1976;47(5):486–488. [DOI] [PubMed] [Google Scholar]
- 2. Alshryda S, Sukeik M, Sarda P, Blenkinsopp J, Haddad F, Mason J. A systematic review and meta-analysis of the topical administration of tranexamic acid in total hip and knee replacement. Bone Joint J. 2014;96(8):1005–1015. [DOI] [PubMed] [Google Scholar]
- 3. Ambra LF, de Girolamo L, Niu W, Phan A, Spector M, Gomoll AH. No effect of topical application of tranexamic acid on articular cartilage. Knee Surg Sports Traumatol Arthrosc. 2019;27(3):931–935. [DOI] [PubMed] [Google Scholar]
- 4. Bahl V, Goyal A, Jain V, Joshi D, Chaudhary D. Effect of haemarthrosis on the rehabilitation of anterior cruciate ligament reconstruction—single bundle versus double bundle. J Orthop Surg Res. 2013;8(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chiang E-R, Chen K-H, Wang S-T, et al. Intra-articular injection of tranexamic acid reduced postoperative hemarthrosis in arthroscopic anterior cruciate ligament reconstruction: a prospective randomized study. Arthroscopy. 2019;35(7):2127–2132. [DOI] [PubMed] [Google Scholar]
- 6. Faught C, Wells P, Fergusson D, Laupacis A. Adverse effects of methods for minimizing perioperative allogeneic transfusion: a critical review of the literature. Transfus Med Rev. 1998;12(3):206–225. [DOI] [PubMed] [Google Scholar]
- 7. Faul F, Erdfelder E, Lang AG, et al. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–191. [DOI] [PubMed] [Google Scholar]
- 8. Felli L, Revello S, Burastero G, et al. Single intravenous administration of tranexamic acid in anterior cruciate ligament reconstruction to reduce postoperative hemarthrosis and increase functional outcomes in the early phase of postoperative rehabilitation: a randomized controlled trial. Arthroscopy. 2019;35(1):149–157. [DOI] [PubMed] [Google Scholar]
- 9. Gao F-Q, Li Z-J, Zhang K, Sun W, Zhang H. Four methods for calculating blood-loss after total knee arthroplasty. Chin Med J (Engl). 2015;128(21):2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gilson AM, Maurer MA, Ryan KM, Rathouz PJ, Cleary JF. Using a morphine equivalence metric to quantify opioid consumption: examining the capacity to provide effective treatment of debilitating pain at the global, regional, and country levels. J Pain Symptom Manage. 2013;45(4):681–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Goderecci R, Giusti I, Necozione S, et al. Short exposure to tranexamic acid does not affect, in vitro, the viability of human chondrocytes. Eur J Med Res. 2019;24(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Henry DA, Carless PA, Moxey AJ, et al. Anti-fibrinolytic use for minimising perioperative allogeneic blood transfusion. Cochrane Database Syst Rev. 2011;3:CD001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ishizue K, Lyon R, Amiel D, et al. Hemarthrosis: a biochemical and mechanical evaluation of effects on the anterior cruciate ligament and menisci. Trans Orthop Res Soc. 1988;13:55. [Google Scholar]
- 14. Ishizue KK, Lyon RM, Amiel D, Woo SLY. Acute hemarthrosis: a histological, biochemical, and biomechanical correlation of early effects on the anterior cruciate ligament in a rabbit model. J Orthop Res. 1990;8(4):548–554. [DOI] [PubMed] [Google Scholar]
- 15. Karaaslan F, Karaoğlu S, Yurdakul E. Reducing intra-articular hemarthrosis after arthroscopic anterior cruciate ligament reconstruction by the administration of intravenous tranexamic acid: a prospective, randomized controlled trial. Am J Sports Med. 2015;43(11):2720–2726. [DOI] [PubMed] [Google Scholar]
- 16. Lisander B, Ivarsson I, Jacobsson SA. Intraoperative autotransfusion is associated with modest reduction of allogeneic transfusion in prosthetic hip surgery. Acta Anaesthesiol Scand. 1998;42(6):707–712. [DOI] [PubMed] [Google Scholar]
- 17. McLean M, McCall K, Smith I, et al. Tranexamic acid toxicity in human periarticular tissues. Bone Joint Res. 2019;8(1):11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Molloy T, Wang Y, Murrell GA. The roles of growth factors in tendon and ligament healing. Sports Med. 2003;33(5):381–394. [DOI] [PubMed] [Google Scholar]
- 19. Nadler SB, Hidalgo JU, Bloch T. Prediction of blood volume in normal human adults. Surgery. 1962;51(2):224–232. [PubMed] [Google Scholar]
- 20. Parker J, Lim K, Kieser D, Woodfield T, Hooper G. Is tranexamic acid toxic to articular cartilage when administered topically? What is the safe dose? Bone Joint J. 2018;100(3):404–412. [DOI] [PubMed] [Google Scholar]
- 21. Ryu J, Sakamoto A, Honda T, Saito S. The postoperative drain-clamping method for hemostasis in total knee arthroplasty: reducing postoperative bleeding in total knee arthroplasty. Bull Hosp Jt Dis. 1997;56(4):251–254. [PubMed] [Google Scholar]
- 22. Sabatini L, Atzori F, Revello S, Scotti L, Debiasi F, Masse A. Intravenous use of tranexamic acid reduces postoperative blood loss in total knee arthroplasty. Arch Orthop Trauma Surg. 2014;134(11):1609–1614. [DOI] [PubMed] [Google Scholar]
- 23. Tuttle J, Feltman P, Ritterman S, Ehrlich M. Effects of tranexamic acid cytotoxicity on in vitro chondrocytes. Am J Orthop (Belle Mead NJ). 2015;44(12):e497–e502. [PubMed] [Google Scholar]
- 24. Von Porat A, Roos EM, Roos H. High prevalence of osteoarthritis 14 years after an anterior cruciate ligament tear in male soccer players: a study of radiographic and patient relevant outcomes. Ann Rheum Dis. 2004;63(3):269–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wei Z, Liu M. The effectiveness and safety of tranexamic acid in total hip or knee arthroplasty: a meta-analysis of 2720 cases. Transfus Med. 2015;25(3):151–162. [DOI] [PubMed] [Google Scholar]
- 26. Wiig ME, Amiel D, Vandeberg J, Kitabayashi L, Harwood FL, Arfors KE. The early effect of high molecular weight hyaluronan (hyaluronic acid) on anterior cruciate ligament healing: an experimental study in rabbits. J Orthop Res. 1990;8(3):425–434. [DOI] [PubMed] [Google Scholar]
- 27. Zhao-yu C, Yan G, Wei C, Yuejv L, Ying-ze Z. Reduced blood loss after intra-articular tranexamic acid injection during total knee arthroplasty: a meta-analysis of the literature. Knee Surg Sports Traumatol Arthrosc. 2014;22(12):3181–3190. [DOI] [PubMed] [Google Scholar]