Abstract
Aims
To evaluate graft healing of decellularized porcine superflexor tendon (pSFT) xenograft in an ovine anterior cruciate ligament (ACL) reconstruction model using two femoral fixation devices. Also, to determine if pSFT allows functional recovery of gait as compared with the preoperative measurements.
Methods
A total of 12 sheep underwent unilateral single-bundle ACL reconstruction using pSFT. Two femoral fixation devices were investigated: Group 1 (n = 6) used cortical suspensory fixation (Endobutton CL) and Group 2 (n = 6) used cross-pin fixation (Stratis ST). A soft screw was used for tibial fixation. Functional recovery was quantified using force plate analysis at weeks 5, 8, and 11. The sheep were euthanized after 12 weeks and comprehensive histological analysis characterized graft healing at the graft-bone interface and the intra-articular graft (ligamentization).
Results
The pSFT remodelled into a ligament-like structure and no adverse inflammatory reaction was seen. The ground reaction force in the operated leg of the Endobutton group was higher at 11 weeks (p < 0.05). An indirect insertion was seen at the graft-bone interface characterized by Sharpey-like fibres. Qualitative differences in tendon remodelling were seen between the two groups, with greater crimp-like organization and more aligned collagen fibres seen with Endobutton fixation. One graft rupture occurred in the cross-pin group, which histologically showed low collagen organization.
Conclusion
Decellularized pSFT xenograft remodels into a ligament-like structure after 12 weeks and regenerates an indirect-type insertion with Sharpey-like fibres. No adverse inflammatory reaction was observed. Cortical suspensory femoral fixation was associated with more enhanced graft remodelling and earlier functional recovery when compared with the stiffer cross-pin fixation.
Keywords: Anterior cruciate ligament, Reconstruction, Porcine xenograft
Article focus
To investigate a decellularized xenograft as graft option for anterior cruciate ligament (ACL) reconstruction that avoids limitations associated with current autograft and allograft options.
To understand the graft healing process of implanted decellularized porcine tissue at the 12-week timepoint, the point when the tendon-bone interface is the ‘weak link’ in ACL reconstruction when using tendon autografts and allografts.
To compare cortical suspensory femoral fixation (Endobutton CL) and cross-pin fixation (Stratis ST) in terms of graft healing and functional recovery.
Key messages
Porcine superflexor tendon (pSFT) xenograft was tolerated by the host and allowed return to functional recovery at the 11-week timepoint as compared with preoperative ground reaction force levels.
Decellularized pSFT grafts regenerated an indirect-type insertion characterized by Sharpey-like fibres, which is similar to currently available ACL grafts. The femoral fixation influences graft healing in the femoral tunnel, with cortical suspensory fixation associated with superior graft healing to that of cross-pin fixation.
Strengths and limitations
There is a wealth of preclinical research reporting the in vitro biomechanical properties of pSFT but no study has reported the in vivo performance.
A key observation was that the stiffer cross-pin femoral device was associated with impaired graft remodelling compared with cortical suspensory fixation.
Further research is needed that correlates the biomechanical properties and histological findings of pSFT grafts at different timepoints in the postoperative course.
Introduction
Rupture of the anterior cruciate ligament (ACL) is a common sports injury associated with knee joint instability.1,2 The ACL has limited capacity to heal, and to restore joint stability the ruptured ligament is routinely surgically replaced in the ACL reconstruction procedure.3 In the USA, approximately 125,000 ACL reconstructions are performed annually but the optimum graft choice remains a matter of controversy.4 Most commonly, the ligament is reconstructed using autogenic grafts, such as four-strand semitendinosus/gracilis hamstring, bone-patellar tendon-bone, and quadriceps tendon.4 Autografts can produce good clinical outcomes but are limited by donor site morbidity and, in the case of hamstring grafts, unpredictable graft size.5 The main alternative is tendon allografts but these are limited by restricted availability, the risk of immune reaction,6 slower biological incorporation,7 and higher clinical failure in young patients.8 Synthetic grafts have fallen out of favour due to inferior wear characteristics and resulting synovitis.9
A promising alternative is an ‘off-the-shelf’ biological scaffold, such as decellularized porcine superflexor tendon (pSFT).10 Traditionally, a challenge of xenotransplantation is immunological rejection by the host.11 However, decellularization processes are now established that can produce a sterile pSFT scaffold with depleted levels of α-Gal epitope, which is a xenoantigen associated with rejection in pig-to-human xenotransplantation.12,13 The pSFT has been shown in vitro to possess the adequate mechanical properties required of an ACL graft and has macroscopic dimensions that enable use with suspensory and cross-pin femoral fixation devices.14 However, to our knowledge the in vivo graft healing of pSFT has not been reported, and it is unknown if decellularized porcine scaffolds regenerate the indirect insertion seen with autogenic and allogenic tendon grafts.
In this study, we aimed to evaluate the in vivo performance of pSFT in an ovine model of ACL reconstruction, and to determine if the femoral fixation device influences graft healing. First, we hypothesized that pSFT xenograft will remodel into a ligament-like tissue with an indirect-type insertion. Second, we hypothesized that femoral fixation would influence graft healing when comparing suspensory with rigid cross-pin fixation. Third, we hypothesized that pSFT will be tolerated by the host and allow return to functional recovery comparable with preoperative ground reaction force levels.
Methods
Experimental design
This research was undertaken in accordance with a project licence under the UK Animals (Scientific Procedures) Act 1986. Sheep are a recognized model for ACL reconstruction due to similarities between the stifle joint and the human knee,15-17 and were chosen because their size and tibial plateau width allow human-sized tendons and fixation devices to be used.18 A total of 12 skeletally mature female mule sheep were included, aged between two and three years old and weighing between 75 kg and 85 kg. Unilateral single-bundle ACL reconstruction was performed using pSFT scaffolds manufactured using previously described methods.10,12,19 The animals were randomized into two groups with different femoral fixation systems. Six animals used the Endobutton CL femoral suspension system (Smith & Nephew, Andover, Massachusetts, USA) and a soft screw (Arthrex, Naples, Florida, USA) for tibial fixation, and six animals used a cross-pin femoral device (Stratis ST; Scandius BioMedical, Inc, Littleton, Massachusetts, USA) and a soft screw for tibial fixation. The use of different devices for the femoral and tibial bone tunnels was chosen to reflect current clinical practice in the UK.20 The animals mobilized postoperatively without restriction, and functional recovery was assessed using force plate gait analysis. Joint range of movement (ROM) was evaluated immediately after euthanasia. A histological evaluation of the graft-bone interface and intra-articular graft was undertaken (Figure 1). Animal studies show that at 12 weeks the tendon-bone interface is the ‘weakest link’ in ACL reconstruction,16,21 hence the animals were euthanized at this timepoint. All assessors were blind to the treatment received. A sample size of n = 6 per group was chosen based on previous large animal ACL studies.22
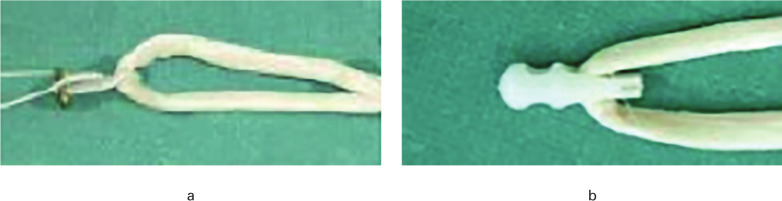
Fig. 1.
The appearance of the decellularized porcine xenograft with two femoral fixation devices: a) Endobutton; and b) Stratis ST.
Surgical procedure
Anaesthesia was induced with intravenous midazolam (5 mg/ml) and ketamine hydrochloride (100 mg/ml), and maintained using 2% isoflurane mixed with oxygen, via an endotracheal tube. The right hind limb was approached through an anteromedial incision. After lateral displacement of the patella, the fat pad was resected, and the ACL was excised leaving no remnant tissue. Bone tunnels were drilled in the femoral and tibial ACL footprints using an outside-in technique. The mean length of the femoral tunnel was 29.3 mm (SD 1.3) in the Endobutton group, and the femoral tunnel had a diameter of 7.0 mm for between 10 mm and 15 mm distally and 4.5 mm diameter for the remaining tunnel. A 15 mm Endobutton CL system meant 15 mm of graft was held within the tunnel. In the cross-pin group, a 7.0 mm diameter tunnel was drilled to accommodate the Stratis block, and a further cross tunnel of 4.5 mm diameter was made for the fixation pin. The Stratis fixation pin was used in a lateral-to-medial fashion and the mean length of the cross-pin was 34.3 mm (SD 4.3). In both groups, 7 mm tibial tunnels were drilled with a mean length of 32.7 mm (SD 2.3).
The pSFT grafts were stored at -20 °C and left to thaw overnight prior to use. The pSFT was trimmed so that the tendon graft occupied the full length and width of the bone tunnels. The length of the double-strand construct was approximately between 55 mm and 60 mm, and the diameter was between 6 mm and 7 mm. The free ends of the graft were secured with a whipstitch suture using No. 2 Ethibond sutures (Ethicon, Somerville, New Jersey, USA). After repeated passive cycles of the joint, a 7 mm × 25 mm soft screw was inserted with the graft at 40 N of tension, a level used in previous studies.17,23 After fixation, the stability and ROM of the stifle joint was checked, and routine layer closure performed. Postoperatively, animals mobilized without restriction in individual pens with natural light and fed on hay. Analgesia was administered in the form of fentanyl transdermal patches. Radiographs were taken immediately postoperatively and at 12 weeks.
Gait analysis
Force plate analysis of the loads passing through both operated and nonoperated limbs was used to monitor functional recovery using our previously published technique.24,25 Functional recovery of the reconstructed limb was assessed by measuring the ground reaction force of the two hind limbs preoperatively and postoperatively at five, eight, and 11 weeks. In total, 12 readings of each limb were obtained by walking over a force plate (Kistler Biomechanics, Alton, UK). The mean peak vertical component of the ground reaction force was determined for each limb and normalized for weight. Functional weight-bearing was expressed as the mean ground reaction force of the operated limb as a percentage of the control limb. No difference was observed in the preoperative ground reaction force (GRF) data recorded for left and right hind limbs for any sheep.
Range of motion assessment
The stifle joint ROM was evaluated immediately after euthanasia using a goniometer.
Histological assessment
After 12 weeks, the femur-pSFT-tibia construct was harvested and fixed in 10% formalin. During retrieval, the macroscopic graft appearance was examined for graft integrity and vascularization. The intra-articular graft was processed for paraffin wax-embedded histology. Staining with haematoxylin and eosin (H&E) was used to study graft remodelling and cellular infiltration of the graft to assess immune response. Collagen fibre alignment and ‘crimping’ were assessed using polarized light microscopy. Undecalcified, resin-embedded histology was performed on the femoral and tibial bone tunnels. Sections were stained with Toluidine blue, followed by Paragon, to study the graft-bone interface morphology.
Statistical analysis
The GraphPad Prism v6.0c (GraphPad Software, San Diego, California, USA) software programme was used. The significance level was set at p < 0.05. Non-normality of the data in the groups was demonstrated using the Shapiro-Wilk test and the Kolmogorov-Smirnov test. Mann-Whitney U tests were used to compare data between the groups. The Wilcoxon signed-rank tests were used to assess differences in gait over time.
Results
Postoperative course
All animals were standing within two hours of surgery, and weight-bearing within 48 hours. No animals were withdrawn from the study due to infection or unexpected complication. Generally, no observable differences were seen between the operated and contralateral hind limb by 12 weeks. The exception was one animal in the Stratis group that displayed signs of lameness and a limp associated with the operated leg. This sheep was subsequently shown to have a ruptured graft. Radiographs revealed no acute complications.
Range of motion
No statistically significant differences were seen in the ROM parameters between groups. Also, no statistically significant differences were seen when comparing the operated limb with contralateral limb (Figure 2).
Fig. 2.
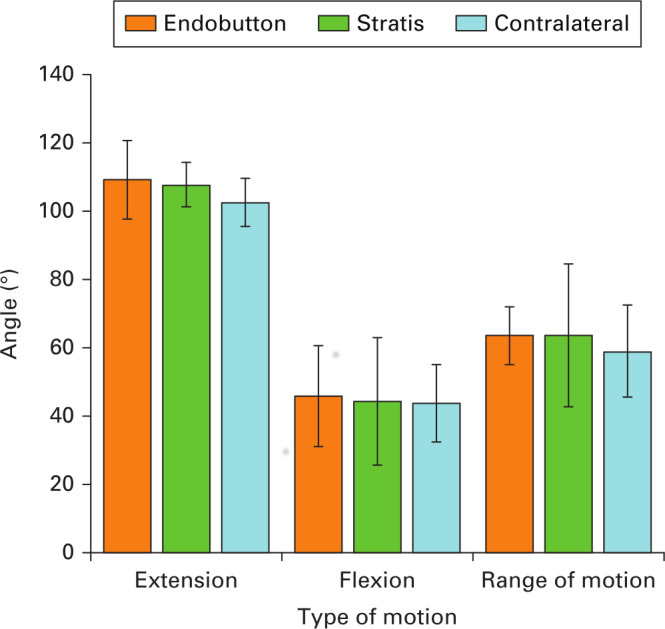
Results of mean range of movement in each group (bars representing SD of the mean).
Gait analysis
The GRF in the operated leg of the Endobutton group was statistically significantly higher at five weeks (p < 0.005) and eight weeks (p < 0.05) compared with the cross-pin group. When comparing with preoperative gait, the relative postoperative GRF on the operated leg of the Endobutton group was significantly lower at five and eight weeks, but no statistical difference was seen at 11 weeks (Figure 3). The relative postoperative GRF of the cross-pin group was significantly lower at five, eight, and 11 weeks compared with preoperative data. When comparing the two groups in terms of the mean relative GRF through the operated limb, there was a significant difference only at the 11-week timepoint.
Fig. 3.
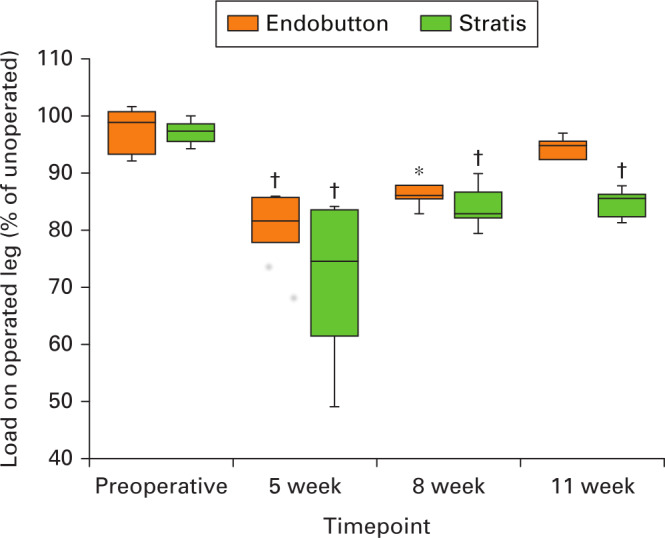
A box and whisker plot (median and interquartile range) showing the functional weight-bearing of the operated leg at weeks 5, 8, and 11 of Endobutton (shown in orange) and cross-pin (shown in green). The statistical significance refers to comparison between preoperative and postoperative data for each group, where *p < 0.05 and †p < 0.005 (Wilcoxon signed-rank tests).
Retrieval findings
No apparent degenerative changes or synovitis were seen. In the Endobutton group, the pSFT crossing the joint had a gross macroscopic appearance that resembled a vascularized ligament-like structure (Figure 4). The same appearance was seen in the cross-pin group, except in one animal where the graft had ruptured.
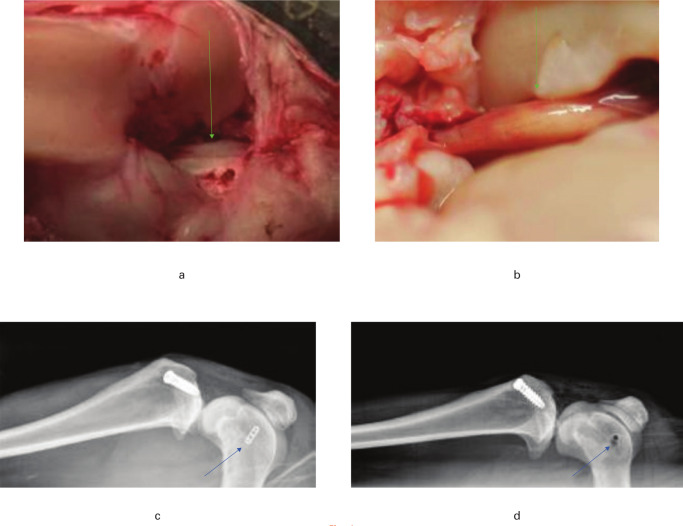
Fig. 4.
Gross and radiological appearances of the anterior cruciate ligament (ACL) reconstruction: a) the double-strand construct of the tendon at implantation (arrow); b) a higher magnification appearance of a single vascularized ligament-like structure (arrow) after 12 weeks; c) a postoperative lateral radiograph of the ACL reconstruction using Endobutton (arrow); d) a postoperative lateral radiograph of the ACL reconstruction using Stratis ST (arrow).
Histology of the graft-bone interface
The decellularized pSFT xenograft was clearly distinguishable from surrounding tissue and the fixation devices in the bone tunnels in the femur (Figures 5a and b) and tibia (Figure 5c). There was no evidence of an adverse inflammatory reaction in the intra-articular space or the bone tunnels.
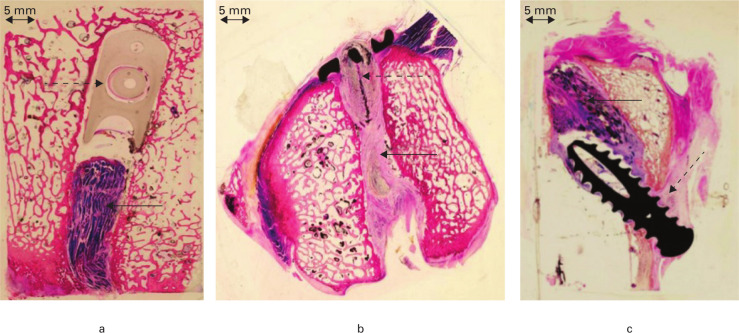
Fig. 5.
Histological sections of the bone tunnels: a) femoral tunnel with Stratis ST cross-pin fixation (dashed arrow) and porcine superflexor tendon (pSFT) xenograft (arrow); b) femoral tunnel with Endobutton fixation (dashed arrow) and pSFT graft (arrow); c) tibial tunnel with soft screw fixation (dashed arrow) and pSFT graft (arrow) (stained with Toluidine Blue and Paragon; magnification ×1.25).
Sharpey-like fibres were seen emanating from the walls of both femoral (Figure 6a) and tibial tunnels (Figure 6b). As the graft entered the intra-articular space, a synovial membrane-like sheath covered the graft (Figure 6c). In the femoral tunnel, the development of highly organized ligamentous-like tissue was more commonly seen in association with Endobutton fixation than with cross-pin tunnels. In the Endobutton group, the little original porcine tendon that remained was surrounded by organized new collagenous tissue. Bone penetration into the Endobutton loop (Figure 6d) was seen although this did not reach the centre of the tunnel. In the cross-pin group, the porcine tendon in the region distal to the Stratis block showed minimal remodelling with large amounts of original porcine tendon remaining. In two sheep, the porcine tendon in the region distal to the Stratis block contained chondroid cells, providing a cartilaginous appearance (Figure 6e). Although direct bone contact was rarely observed with the Stratis blocks, penetration of new bone into the fixation pin tunnel within the block was seen. In terms of the tibial tunnel, no discernible differences were observed across either group. The degree of ossification was greater within tibial bone tunnels than in the femoral bone tunnels. New bone formation was seen at the tunnel walls with acellular mineralization in the centre of the graft (Figure 6f).
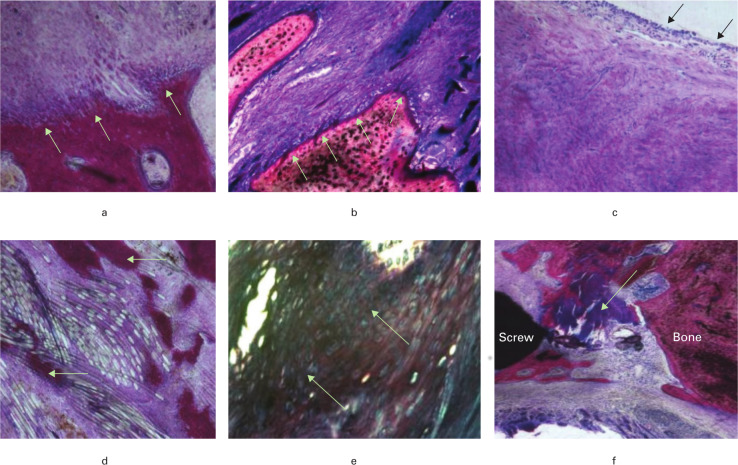
Fig. 6.
Histological findings in the bone tunnels: a) ×100 magnification showing Sharpey's fibres in the femoral tunnel; b) ×100 magnification showing Sharpey's fibres near the tip of tibial screw; c) ×100 magnification showing a synovial membrane-like sheath over remodelled tissue emerging from tibial tunnel; d) ×100 magnification showing bone penetration within Endobutton loop fibres; e) ×200 magnification showing chondrocyte-like cells in the graft distal to the cross-pin device; f) ×100 magnification showing tendon mineralization and ossification between the soft screw and bone tunnel (stained with Toluidine Blue and Paragon).
Histology of the intra-articular graft
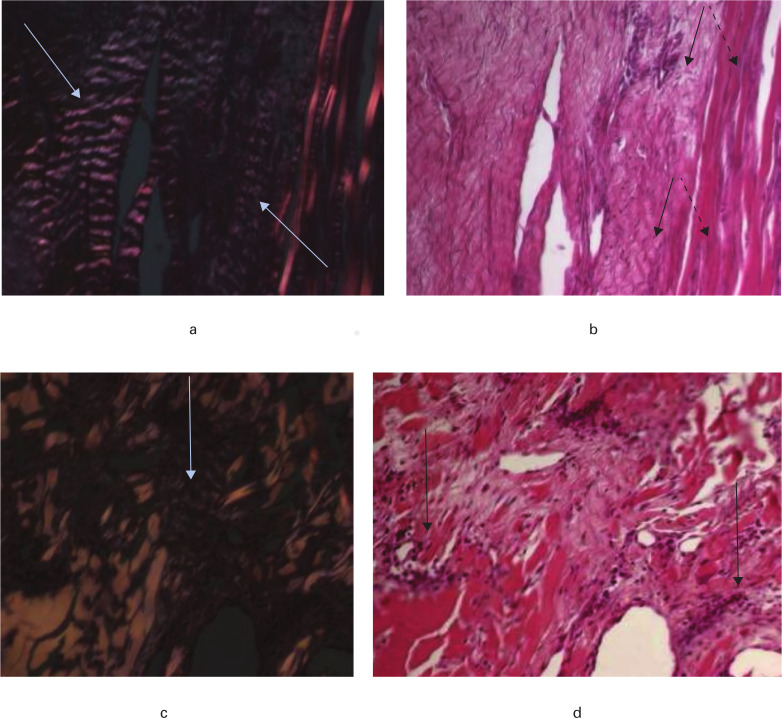
Differences were noted in the pattern of tendon remodelling within the Endobutton and cross-pin groups. The Endobutton group was characterized by an inner core of cellularized but structurally intact graft. The tendon appeared to be undergoing progressive arthroplasty of porcine tissue by new collagenous tissue in a concentric manner, proceeding from an outer sheath into the tendon core. New tissue was at first highly cellular and vascularized, progressing to loosely organized with evidence of crimp, to a more organized tissue with orientated collagen fibres and elongated tenocyte-like cells (Figures 7a and b). Within the cross-pin group, there were regions of clearly elongated tenocyte-like cells but the distribution of these regions was more inconsistent than in the Endobutton group, and was associated with less polarization (Figures 7c and 7d).
Fig. 7.
Histological appearance of the intra-articular graft at 200× magnification. a) In the Endobutton group under polarized light, clear evidence of ligament-like crimped collagenous tissue is seen (arrow); b) in the Endobutton group using light microscopy an intimate association between new remodelled tissue (black arrow) and residual porcine tendon scaffold (dashed arrow) is observed; c) in the cross-pin group under polarized light, relatively little crimp-like organization (arrow) is seen; d) in the cross-pin group using light microscopy, highly cellular regions (arrow) were observed (stained with Toluidine Blue and Paragon).
Comparison of ruptured and structurally intact grafts
In the intra-articular space, the ruptured graft displayed relatively little crimp, low tissue organization on polarized light, and minimal neovascularization. In the femoral tunnel, the ruptured graft showed little remodelling, and large portions of original porcine tendon remained. At the tendon-bone interface, the graft had regenerated a fibrous interface with Sharpey-like fibres, and areas of direct tendon mineralization were seen. In the tibial tunnel, the ruptured graft also contained regions of original porcine tendon but generally appeared well incorporated with an abundance of Sharpey-like fibres and bone on the screw threads. No differences in tunnel position could be seen on postoperative radiographs.
Discussion
An ‘off the shelf’ graft that elicits no immune reaction is an attractive prospect for ACL reconstruction. Decellularized pSFT grafts have advantages over autogenic and allogenic graft options, especially low cost, high availability, and the avoidance of donor site morbidity.12 The advantages of a porcine graft over an allogenic graft are availability and potentially a reduction in the costs. The in vitro mechanical properties of pSFT have been confirmed,19 but ultimately the host tissue response and graft healing in vivo will determine its success as an ACL graft option.
Our first hypothesis that pSFT xenograft will remodel into a ligament-like tissue with an indirect-type insertion was affirmed because, after 12 weeks, the pSFT remodelled into a collagenous tissue displaying crimp with Sharpey-like fibres in both bone tunnels. Our second hypothesis that the choice of femoral fixation device would influence graft remodelling was affirmed because fixation with Endobutton CL was associated with superior graft remodelling. Our third hypothesis that pSFT will be tolerated by the host and allow return to functional recovery was affirmed only with the Endobutton group. The reason for this is because the ground reaction force (GRF) after 12 weeks in the cross-pin group remained significantly lower than the preoperative levels. Beyond 12 weeks, animals with the cross-pin system may have recovered to normal levels but at 12 weeks a more advanced recovery of functional activity was seen in the cortical suspensory device.
Tendon-bone healing in the bone tunnels after ACL reconstruction using soft-tissue grafts is mainly associated with regeneration of an indirect insertion characterized by Sharpey-like fibres.21 Tendon-bone healing in rabbits using adjustable and fixed-loop femoral cortical suspension devices after eight weeks is associated with an indirect-type enthesis characterized by Sharpey-like fibres, which is consistent with what we observed for femoral suspensory fixation.26 With regard to the tibial tunnel, there are conflicting reports depending on fixation technique used, with some studies reporting direct27,28 and some indirect insertions.21,29,30 After 12 weeks in vivo, Weiler et al27 reported formation of a zone of fibrocartilage at the tunnel aperture without a fibrous interzone when using an interference screw fixation in sheep. In this study, we did not observe a direct-type insertion but instead graft incorporation occurred via an indirect insertion. The ossification and graft incorporation process was more advanced in the tibial tunnel, possibly as a consequence of the secure anchorage offered by the soft screw, which is similar to what was reported in a human knee resected for osteosarcoma of the tibia four months after ACL reconstruction.30
Surgical factors have been shown to influence graft healing.31 A major observation was that the stiffer femoral fixation device using cross-pin had inferior graft remodelling and one graft rupture. Graft remodelling appeared less advanced within the cross-pin group, both in the femoral tunnel and in the intra-articular space, which could be due to the more static local environment immediately distal to the Stratis block. This study therefore suggests that the wider use of suspensory femoral fixation over cross-pin fixation may be clinically justified due to superior histological graft remodelling.20 If tissues do not have the proper load because the construct is too stiff, this might negatively influence the graft-healing process and how the tissues behave while remodelling. The rate of graft rupture for the cross-pin group was 1 in 6 (16.67%), and the ruptured graft was associated with low levels of tissue remodelling and collagen organization in the femoral tunnel and intra-articular space. If the graft relies on transformation of the substrate into ligamentous tissue, the cells from the host that are transforming the substrate need to have the appropriate mechanobiological environment. On two occasions, we observed chondroid tissues in the femoral tunnel using cross-pin fixation, perhaps present due to the stiffer construct used.32 Interference screws and cross-pin techniques have been compared in terms of impact on biomechanical properties but no histological comparison has previously been described.33
An important outcome for patients undergoing ACL reconstruction is early return to function.34 Many preclinical studies of ACL reconstruction focus on histological outcomes of graft incorporation and maturation with no clear correlation to what this means in terms of function.22,35-39 Hence in our study we used gait analysis to measure the in vivo functional performance of an ACL graft. Only the Endobutton group achieved statistical equivalence with the preoperative GRF values by 11 weeks. The recovery of functional weight-bearing in the cross-pin group appeared to plateau after eight weeks, whereas functional weight-bearing in the Endobutton group continued to increase towards preoperative values. These observations may reflect the fact that the cross-pin procedure required more tensile drilling and preparation in the femur, and this may have had an effect on progression of graft healing.
This study has a number of limitations. First, a major limitation of this work is that the histological evaluation took place at only one timepoint and the observational claims were not supported with quantitative data. Despite the number of animals used, with n = 6 per group being similar to other studies in the literature,16,17,27 the number of animals remains small. Second, biomechanical properties of the grafts were not measured. Third, joint laxity was not assessed by clinical examination in this study. Fourth, although this was a comparison of two fixation systems with a xenograft tendon, a better evaluation of the pSFT would have been obtained with a control group using tendon allograft or autograft. Fifth, a sheep model does not truly reflect the clinical situation in humans, especially because the sheep stifle joint is kept in a degree of flexion and the reconstruction cannot be protected postoperatively. This could be significant because this would alter the strain through the graft, and tendon-bone healing is influenced by changes in ACL graft force with joint motion.40,41 The decision to evaluate suspensory fixation was made based on its popular usage in the UK, but the fact that suspensory fixation is popular does not necessarily mean it is the correct fixation to use. For instance, the use of interference screw fixation in both the femoral and tibial tunnel has been shown to have good results 15 years postoperatively with respect to objective and subjective outcomes, and to ligamentous stability.29
The process of ligamentization is known to occur over many months, with well documented progression in histological and biomechanical properties of tendon grafts in the years after implantation.16,17 Ovine studies of semitendinosis grafts show intrinsic fibroblast necrosis at 12 weeks, which correlates with a period of low mechanical strength, but this evolves with time and the necrotic lesion disappears at 24 weeks.17 Future research is needed to evaluate in vivo performance of pSFT grafts at multiple timepoints to determine if pSFT behaves the same as autogenic and allogenic tendon grafts over a longer time period. Given that the rate of graft rupture in this study was 1 in 12 (8.33%), such a study should correlate histological findings with biomechanical properties.
In conclusion, pSFT can remodel into a functional ligament-like structure after 12 weeks as evidenced by the regeneration of a ligamentous structure associated with restoration of functional weight-bearing. The femoral fixation systems influenced graft healing, with Endobutton fixation associated with more enhanced graft healing and earlier functional recovery.
Author contributions
A. T. Hexter: Performed the research, Wrote the manuscript.
K. A Hing: Performed the research.
F. S. Haddad: Contributed to writing the manuscript, Reviewed the manuscript prior to submission.
G. Blunn: Supervised the planning and writing of the manuscript, Reviewed the manuscript prior to submission.
Funding statement
A. Hexter reports that the work was funded by a Royal College of Surgeons One-Year Surgical Research Fellowship.
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
ICMJE COI statement
G. Blunn reports an institutional grant from Tissue Regenex (no longer trading) in relation to the work. K. Hing reports an institutional grant from Tissue Science Laboratories Ltd in relation to the work. F. S. Haddad sits on the Editorial Board of The Bone and Joint Journal and the Editorial Board of the Annals of the Royal College of Surgeons. F. S. Haddad reports royalties and lecture and consultancy payments from Smith & Nephew, all unrelated to the work.
Acknowledgements
None declared
Ethical review statement
This study did not require ethical approval. This research was undertaken in accordance with a project licence under the UK Animals (Scientific Procedures) Act 1986.
© 2020 Author(s) et al. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (CC BY-NC-ND 4.0) licence, which permits the copying and redistribution of the work only, and provided the original author and source are credited. See https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- 1.Wright RW, Huston LJ, et al. , MARS Group . Descriptive epidemiology of the multicenter ACL revision study (MARS) cohort. Am J Sports Med. 2010;38(10):1979–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kang K-T, Koh YG, Park KM, et al. . The anterolateral ligament is a secondary stabilizer in the knee joint: A validated computational model of the biomechanical effects of a deficient anterior cruciate ligament and anterolateral ligament on knee joint kinematics. Bone Joint Res. 2019;8(11):509–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murray MM, Martin SD, Martin TL, Spector M. Histological changes in the human anterior cruciate ligament after rupture. J Bone Joint Surg Am. 2000;82-A(10):1387–1397. [DOI] [PubMed] [Google Scholar]
- 4.Mehran N, Moutzouros VB, Bedi A. A Review of Current Graft Options for Anterior Cruciate Ligament Reconstruction. JBJS Rev. 2015;3(11):01874474-201511000-00003 10.2106/JBJS.RVW.O.00009 [DOI] [PubMed] [Google Scholar]
- 5.Barenius B, Nordlander M, Ponzer S, Tidermark J, Eriksson K. Quality of life and clinical outcome after anterior cruciate ligament reconstruction using patellar tendon graft or quadrupled semitendinosus graft: an 8-year follow-up of a randomized controlled trial. Am J Sports Med. 2010;38(8):1533–1541. [DOI] [PubMed] [Google Scholar]
- 6.Dustmann M, Schmidt T, Gangey I, et al. . The extracellular remodeling of free-soft-tissue autografts and allografts for reconstruction of the anterior cruciate ligament: a comparison study in a sheep model. Knee Surg Sports Traumatol Arthrosc. 2008;16(4):360–369. [DOI] [PubMed] [Google Scholar]
- 7.Bhatia S, Bell R, Frank RM, et al. . Bony incorporation of soft tissue anterior cruciate ligament grafts in an animal model: autograft versus allograft with low-dose gamma irradiation. Am J Sports Med. 2012;40(8):1789–1798. [DOI] [PubMed] [Google Scholar]
- 8.Kaeding CC, Aros B, Pedroza A, et al. . Allograft Versus Autograft Anterior Cruciate Ligament Reconstruction: Predictors of Failure From a MOON Prospective Longitudinal Cohort. Sports Health. 2011;3(1):73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tiefenboeck TM, Thurmaier E, Tiefenboeck MM, et al. . Clinical and functional outcome after anterior cruciate ligament reconstruction using the LARS™ system at a minimum follow-up of 10 years. Knee. 2015;22(6):565–568. [DOI] [PubMed] [Google Scholar]
- 10.Edwards JH, Ingham E, Herbert A. Decellularisation affects the strain rate dependent and dynamic mechanical properties of a xenogeneic tendon intended for anterior cruciate ligament replacement. J Mech Behav Biomed Mater. 2019;91:18–23. [DOI] [PubMed] [Google Scholar]
- 11.Stone KR, Abdel-Motal UM, Walgenbach AW, Turek TJ, Galili U. Replacement of human anterior cruciate ligaments with pig ligaments: a model for anti-non-gal antibody response in long-term xenotransplantation. Transplantation. 2007;83(2):211–219. [DOI] [PubMed] [Google Scholar]
- 12.Jones G, Herbert A, Berry H, et al. . Decellularization and characterization of porcine superflexor tendon: a potential anterior cruciate ligament replacement. Tissue Eng Part A. 2017;23(3-4):124–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stone KR, Ayala G, Goldstein J, et al. . Porcine cartilage transplants in the cynomolgus monkey. III. Transplantation of α-galactosidase-treated porcine cartilage. Transplantation. 1998;65(12):1577–1583. [DOI] [PubMed] [Google Scholar]
- 14.Whitaker S, Edwards JH, Guy S, Ingham E, Herbert A. Stratifying the mechanical performance of a decellularized xenogeneic tendon graft for anterior cruciate ligament reconstruction as a function of graft diameter: an animal study. Bone Joint Res. 2019;8(11):518–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allen MJ, Houlton JE, Adams SB, Rushton N. The surgical anatomy of the stifle joint in sheep. Vet Surg. 1998;27(6):596–605. [DOI] [PubMed] [Google Scholar]
- 16.Goradia VK, Rochat MC, Grana WA, Rohrer MD, Prasad HS. Tendon-to-bone healing of a semitendinosus tendon autograft used for ACL reconstruction in a sheep model. Am J Knee Surg. 2000;13(3):143–151. [PubMed] [Google Scholar]
- 17.Kondo E, Yasuda K, Katsura T, et al. . Biomechanical and histological evaluations of the doubled semitendinosus tendon autograft after anterior cruciate ligament reconstruction in sheep. Am J Sports Med. 2012;40(2):315–324. [DOI] [PubMed] [Google Scholar]
- 18.Bascuñán AL, Biedrzycki A, Banks SA, Lewis DD, Kim SE. Large Animal Models for Anterior Cruciate Ligament Research. Front Vet Sci. 2019;6:292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herbert A, Jones GL, Ingham E, Fisher J. A biomechanical characterisation of acellular porcine super flexor tendons for use in anterior cruciate ligament replacement: investigation into the effects of fat reduction and bioburden reduction bioprocesses. J Biomech. 2015;48(1):22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gabr A, De Medici A, Haddad FS, The United Kingdom National Ligament Registry The Fifth Annual Report (2019) https://www.uknlr.co.uk/pdf/annual-report-2019.pdf date last accessed 23 April 2020.
- 21.Rodeo SA, Arnoczky SP, Torzilli PA, Hidaka C, Warren RF. Tendon-healing in a bone tunnel. A biomechanical and histological study in the dog. J Bone Joint Surg Am. 1993;75(12):1795–1803. [DOI] [PubMed] [Google Scholar]
- 22.Hexter AT, Thangarajah T, Blunn G, Haddad FS. Biological augmentation of graft healing in anterior cruciate ligament reconstruction: a systematic review. Bone Joint J. 2018;100-B(3):271–284. [DOI] [PubMed] [Google Scholar]
- 23.Yoshikawa T, Tohyama H, Katsura T, et al. . Effects of local administration of vascular endothelial growth factor on mechanical characteristics of the semitendinosus tendon graft after anterior cruciate ligament reconstruction in sheep. Am J Sports Med. 2006;34(12):1918–1925. [DOI] [PubMed] [Google Scholar]
- 24.Sundar S, Pendegrass CJ, Blunn GW. Tendon bone healing can be enhanced by demineralized bone matrix: a functional and histological study. J Biomed Mater Res B Appl Biomater. 2009;88(1):115–122. [DOI] [PubMed] [Google Scholar]
- 25.Thangarajah T, Shahbazi S, Pendegrass CJ, et al. . Tendon Reattachment to Bone in an Ovine Tendon Defect Model of Retraction Using Allogenic and Xenogenic Demineralised Bone Matrix Incorporated with Mesenchymal Stem Cells. PLoS One. 2016;11(9):e0161473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sato Y, Akagi R, Akatsu Y, et al. . The effect of femoral bone tunnel configuration on tendon-bone healing in an anterior cruciate ligament reconstruction: an animal study. Bone Joint Res. 2018;7(5):327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weiler A, Hoffmann RF, Bail HJ, Rehm O, Südkamp NP. Tendon healing in a bone tunnel. Part II: Histologic analysis after biodegradable interference fit fixation in a model of anterior cruciate ligament reconstruction in sheep. Arthroscopy. 2002;18(2):124–135. [DOI] [PubMed] [Google Scholar]
- 28.Hunt P, Rehm O, Weiler A. Soft tissue graft interference fit fixation: observations on graft insertion site healing and tunnel remodeling 2 years after ACL reconstruction in sheep. Knee Surg Sports Traumatol Arthrosc. 2006;14(12):1245–1251. [DOI] [PubMed] [Google Scholar]
- 29.Pinczewski LA, Clingeleffer AJ, Otto DD, Bonar SF, Corry IS. Integration of hamstring tendon graft with bone in reconstruction of the anterior cruciate ligament. Arthroscopy. 1997;13(5):641–643. [DOI] [PubMed] [Google Scholar]
- 30.Lazarides AL, Eward WC, Green K, et al. . Histological Evaluation of Tendon-Bone Healing of an Anterior Cruciate Ligament Hamstring Graft in a 14-Year-Old Boy. Am J Sports Med. 2015;43(8):1935–1940. [DOI] [PubMed] [Google Scholar]
- 31.Ekdahl M, Wang JHC, Ronga M, Fu FH. Graft healing in anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2008;16(10):935–947. [DOI] [PubMed] [Google Scholar]
- 32.Yamakado K, Kitaoka K, Yamada H, et al. . The influence of mechanical stress on graft healing in a bone tunnel. Arthroscopy. 2002;18(1):82–90. [DOI] [PubMed] [Google Scholar]
- 33.Zantop T, Weimann A, Wolle K, et al. . Initial and 6 weeks postoperative structural properties of soft tissue anterior cruciate ligament reconstructions with cross-pin or interference screw fixation: an in vivo study in sheep. Arthroscopy. 2007;23(1):14–20. [DOI] [PubMed] [Google Scholar]
- 34.Shelbourne KD, Nitz P. Accelerated rehabilitation after anterior cruciate ligament reconstruction. Am J Sports Med. 1990;18(3):292–299. [DOI] [PubMed] [Google Scholar]
- 35.Teuschl A, Heimel P, Nürnberger S, et al. . A Novel Silk Fiber-Based Scaffold for Regeneration of the Anterior Cruciate Ligament: Histological Results From a Study in Sheep. Am J Sports Med. 2016;44(6):1547–1557. [DOI] [PubMed] [Google Scholar]
- 36.Li H, Chen J, Chen S. Remnant Repair-enhanced Polyethylene Terepthalate Artificial Ligament Graft Ligamentization. Int J Sports Med. 2015;36(12):1015–1020. [DOI] [PubMed] [Google Scholar]
- 37.Kuang GM, Yau WP, Lu WW, Chiu KY. Local application of strontium in a calcium phosphate cement system accelerates healing of soft tissue tendon grafts in anterior cruciate ligament reconstruction: experiment using a rabbit model. Am J Sports Med. 2014;42(12):2996–3002. [DOI] [PubMed] [Google Scholar]
- 38.Li F, Jia H, Yu C. ACL reconstruction in a rabbit model using irradiated Achilles allograft seeded with mesenchymal stem cells or PDGF-B gene-transfected mesenchymal stem cells. Knee Surg Sports Traumatol Arthrosc. 2007;15(10):1219–1227. [DOI] [PubMed] [Google Scholar]
- 39.Mutsuzaki H, Sakane M, Nakajima H, et al. . Calcium-phosphate-hybridized tendon directly promotes regeneration of tendon-bone insertion. J Biomed Mater Res A. 2004;70(2):319–327. [DOI] [PubMed] [Google Scholar]
- 40.Ma R, Schär M, Chen T, et al. . Effect of Dynamic Changes in Anterior Cruciate Ligament In Situ Graft Force on the Biological Healing Response of the Graft-Tunnel Interface. Am J Sports Med. 2018;46(4):915–923. [DOI] [PubMed] [Google Scholar]
- 41.Song F, Jiang D, Wang T, et al. . Mechanical Loading Improves Tendon-Bone Healing in a Rabbit Anterior Cruciate Ligament Reconstruction Model by Promoting Proliferation and Matrix Formation of Mesenchymal Stem Cells and Tendon Cells. Cell Physiol Biochem. 2017;41(3):875–889. [DOI] [PubMed] [Google Scholar]