Abstract
The first long-acting formulations of HIV drugs are undergoing regulatory review for use in maintenance of viral suppression in people with HIV. Although these novel drug formulations could contribute greatly to HIV treatment and prevention efforts, their lack of activity against hepatitis B virus (HBV) could limit their global impact, particularly in populations with high burdens of both HIV and HBV. An urgent need for greater investment in research and development of long-acting drugs with dual activity against HIV and HBV exists. Access to long-acting HIV drug formulations with dual activity against HBV would be transformative and have a great impact on efforts to prevent, treat, and eradicate both of these important global epidemics.
Introduction
Important progress has been achieved in the development of long-acting antiviral formulations for the prevention and treatment of HIV. Replacing daily oral regimens with long-acting ones, administered as infrequent injections or implantable devices, could greatly simplify and improve HIV prevention and treatment.1–3 The successful uptake of long-acting injectable formulations for treatment of schizophrenia,4 and the use of implantable formulations of contraceptive medications by millions of women,5,6 suggests that such formulations would be well accepted. Studies have substantiated that people with HIV and people at risk for HIV infection show strong interest and support for the use of long-acting injectable or implantable versions of their medications.7–11
Long-acting formulations of two HIV drugs delivered by intramuscular injection, long-acting cabotegravir and long-acting rilpivirine, have shown promising results in phase 3 trials for HIV treatment.12,13 Cabotegravir is a potent HIV integrase inhibitor. Rilpivirine is a potent second-generation HIV non-nucleoside reverse transcriptase inhibitor with a long half-life and fewer side-effects than other drugs in this class. Multiple studies have shown that people living with HIV who have been taking daily pills that include three or more HIV medications and have achieved viral suppression can stop their pills and safely switch to the two-drug combination of long-acting cabotegravir and long-acting rilpivirine, given as injections every 4 or 8 weeks.9,12,13
People who have stopped oral regimens and switched to injections have few side-effects and can maintain effective HIV viral suppression for at least 48 weeks.12,13 The US Food and Drug Administration (FDA) is reviewing these data and considering approval of injectable formulations of long-acting cabotegravir and rilpivirine for maintenance of viral suppression in HIV-infected people.
Treatment of hepatitis B and HIV co-infection
Long-acting cabotegravir and rilpivirine represent important new tools to support global HIV prevention and treatment efforts, as well as the ambitious public health goal to end the HIV epidemic in the USA.14 However, approximately 7·5% of people living with HIV worldwide are co-infected with hepatitis B virus (HBV), and among some populations the prevalence of co-infection is higher. HBV and HIV share the same modes of transmission; thus, many countries have communities with high burdens of both viruses (figure). For individuals co-infected with HIV and HBV, specific antiretroviral nucleoside analogues that are active against both viruses are recommended.15
Figure: HIV and chronic hepatitis B virus infection prevalence.
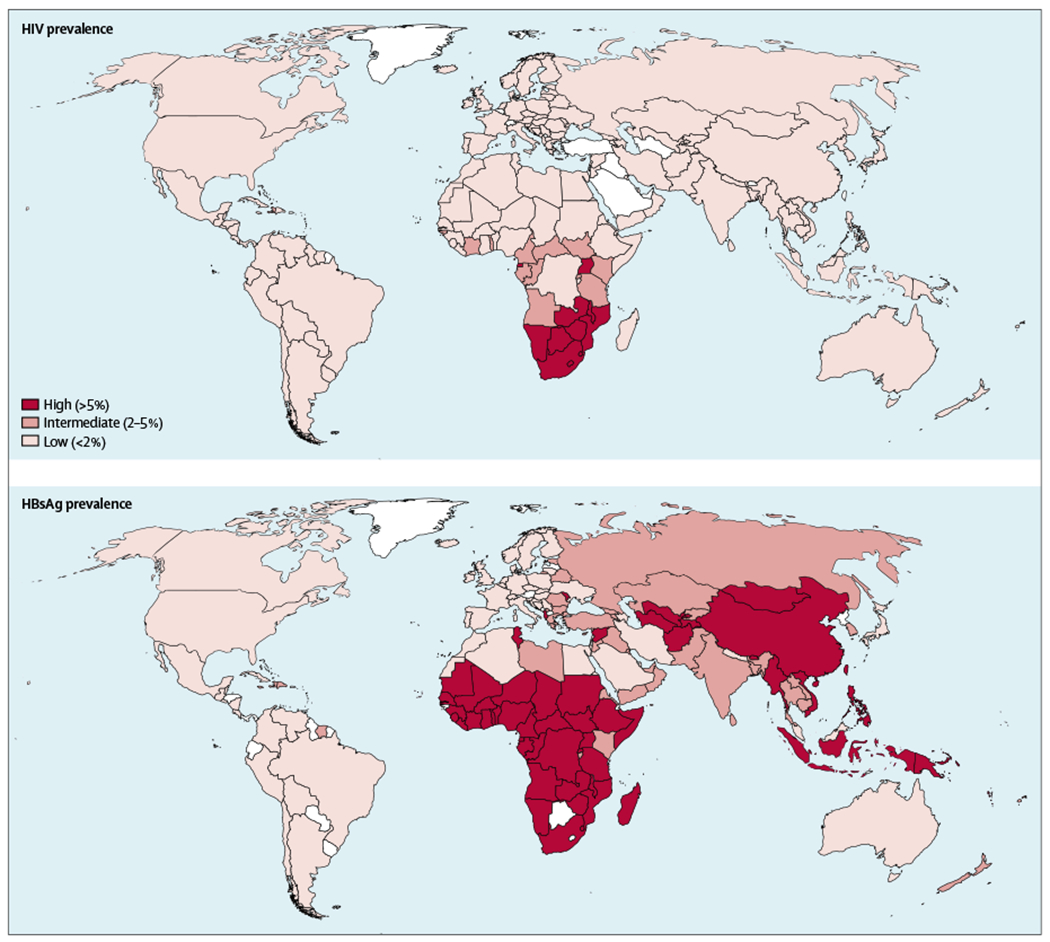
In addition, if those dually active nucleosides are inadvertently discontinued (eg, during a switch to regimens such as long-acting cabotegravir plus long-acting rilpivirine), HBV reactivation and hepatic failure can occur.16 Because people with HBV infection infrequently accomplish a functional cure with current antivirals drugs, therapy is often lifelong. Thus, HIV–HBV co-infected individuals present an important challenge to the effectiveness of the widespread implementation of long-acting cabotegravir and rilpivirine for HIV treatment, since neither has activity against HBV.
Only four FDA-approved drugs for HIV treatment also have activity against HBV. These are all nucleoside reverse transcriptase inhibitors, including lamivudine, emtricitabine, tenofovir disoproxil fumarate, and tenofovir alafenamide.17 All four drugs have been used for the treatment of HBV infection, but tenofovir disoproxil fumarate and tenofovir alafenamide are preferred because they have a higher barrier to HBV resistance than lamivudine and emtricitabine.18–20
Efforts to develop long-acting versions of antiretrovirals with HBV activity are now underway,21,22 but lag far behind the development of long-acting cabotegravir and rilpivirine, and it is too early to speculate how those medications might be integrated into a comprehensive antiviral long-acting regimen.
To optimise strategies for use of long-acting injectable drugs to end the HIV epidemic, a greater investment in research and development of long-acting drugs with dual activity against HIV and HBV is urgently needed. Long-acting formulations of medications with activity against HBV also have great potential to end the HBV epidemic, which is a WHO goal for 2030.23 Although about 40 million people worldwide have HIV, 257 million people have a chronic HBV infection.23,24 Of these HBV-infected individuals, only 4·5 million (<2%) are receiving HBV treatment.25 HBV is the leading global cause of death from chronic liver disease and liver cancer.26 In addition, by 2040, analyses project that HBV will be responsible for more deaths every year than HIV, tuberculosis, or malaria.27 Like HIV, HBV can be transmitted through sexual contact, injections, and blood product transfusions, and from mother to child. Between 6% and 10% of people living with HIV in the USA are also infected with HBV,28 with higher rates among men who have sex with men and people who inject drugs.29,30
The burden of HBV infection worldwide, as with HIV, falls on low-income and middle-income countries. Consequently, rates of HBV co-infection are higher than 10% among many populations of people living with HIV in sub-Saharan Africa, west Africa, east Asia, and India, despite the increasing access to HBV vaccination. 28,31–33 Moreover, HIV mono-infected people are at risk for HBV acquisition even with vaccination since they mount poorer and less durable protective responses against HBV than people without HIV.34,35
HBV co-infection is associated with much higher rates of liver-related mortality among people living with HIV than among people with either HIV or HBV infection alone.36,37 Treatment of HIV–HBV co-infected people with HIV medications that do not also have HBV activity is associated with an increased risk for HBV immune reconstitution inflammatory syndrome.38,39 In addition, discontinuation of HIV medications with anti-HBV activity in co-infected people is associated with HBV reactivation and liver failure.40 Therefore, current US and international guidelines recommend that all people living with HIV should be screened for HBV infection before starting antiretroviral therapy.15,41
HIV drug regimens that include tenofovir disoproxil fumarate or tenofovir alafenamide in combination with emtricitabine or lamivudine are recommended for those with chronic hepatitis B.15 Unfortunately, in practice, most HIV-infected people globally are not prescreened for HBV infection before initiating HIV treatment.
Prescreening for HBV infection is particularly uncommon in sub-Saharan Africa and Asia, as well as among populations of injection drug users, who have higher HBV co-infection rates than the general population. Failing to account for HBV co-infection has not had major public health implications because more than 60% of drug regimens purchased for people living with HIV in sub-Saharan Africa and Asia contain tenofovir disoproxil fumarate.42
However, long-acting cabotegravir and rilpivirine are expected to be available for HIV treatment in high-income countries soon, and could be made available shortly thereafter in low-income and middle-income countries. Given the greater potential adherence, potency, and safety profile of these injectable HIV medications than daily regimens, as well as better protection of health privacy for areas with substantial HIV stigma, many people could benefit from access to these formulations. These medications provide an excellent option for people who have difficulty taking pills every day, including people with injection drug use. However, people with injection drug use have the greatest risk for HBV co-infection of all people living with HIV.
As noted above, people with HIV–HBV co-infection cannot safely transition to a completely injectable regimen of long-acting cabotegravir and rilpivirine because of the risk of HBV reactivation. The much anticipated switch to the initiation of long-acting cabotegravir and rilpivirine will require a strategy of both testing and switching, since most people in high-income countries will require HBV testing before they can stop tenofovir disoproxil fumarate or tenofovir alafenamide. Therefore, people with HIV–HBV co-infection, especially those in low-income and middle-income countries, might not benefit from the availability of the new long-acting antiviral formulations of cabotegravir and rilpivirine.
Long-acting pre-exposure prophylaxis
Long-acting antivirals also have a role in the prevention of HIV and HBV infections. Co-formulated tenofovir disoproxil fumarate and emtricitabine taken once daily is highly protective when taken regularly as pre-exposure prophylaxis (PrEP) for individuals at risk for HIV infection.43,44 However, long-term adherence to oral, daily PrEP is difficult to accomplish and sustain, particularly among women at high risk, injection drug users, and men who have sex with men.45–47 The uptake and access to oral PrEP is suboptimal in many communities, including in people who inject drugs.47,48
The potential of a long-acting injectable formulation of cabotegravir as an alternative to PrEP could be an important new and transformative HIV prevention tool.49 Two important clinical trials are underway through the HIV Prevention Trials Network, comparing the efficacy of injections of long-acting cabotegravir given every 4 or 8 weeks with a daily oral pill of tenofovir disoproxil fumarate and emtricitabine for the prevention of HIV infection among men and women at high risk.50,51 Similar concerns with regards to HBV infection apply to PrEP. If long-acting cabotegravir is regarded as effective, switching from the HBV-active tenofovir disoproxil fumarate and emtricitabine might lead to the outbreak of an underlying HBV infection. In addition, even among HIV mono-infected people, it is now well documented that HBV-active antiretrovirals prevent HBV acquisition.52–54
Although not as advanced in the drug development pipeline as long-acting cabotegravir and rilpivirine, there is progress in the development of long-acting tenofovir alafenamide injectables and implants, which could be valuable for global prevention of both HIV and HBV. The first human clinical trial of a long-acting tenofovir alafenamide formulation (CAPRISA 018), delivered by a non-degradable implant (similar to those used for long-acting hormonal contraceptives), is about to begin in South Africa.55 The safety, pharmacokinetic, and efficacy data from this first human study of a long-acting formulation with activity against both HIV and HBV will be highly important to address the challenges and limitations of the current long-acting HIV pipeline.
Potential applications of long-acting HBV treatments
Many other potentially impactful applications of long-acting HBV treatments also exist. The opportunity to provide pregnant women with a long-acting option for treatment that prevents mother-to-child transmission of both HIV and HBV could be transformative. 90% of all new chronic HBV infections in the world are due to perinatal transmission.56 Although more than 90% of individuals who are infected with HBV as adults recover from their HBV infection, more than 90% of perinatally infected infants develop chronic HBV infection.57 These children contribute to the 700 000 annual deaths from HBV-associated liver disease, cirrhosis, and hepatocellular carcinoma.23,57 High HBV viral load among HIV–HBV co-infected mothers is associated with lower infant birthweight and increased infant mortality.58
Current strategies to prevent perinatal HBV infection include treating pregnant mothers with oral tenofovir disoproxil fumarate, administered daily, and treating their infants at birth with HBV vaccine and immunoglobulin.23,59 Although these strategies can prevent perinatal HBV transmission, less than 40% of infants born to HBV-infected mothers have access to these prevention methods.23,56 The main impediment to global elimination of HBV is the inability to deliver these preventive tools to mothers prenatally and to infants immediately after delivery, which in many regions of the world does not occur in a health-care facility.60 The ability to give long-acting HBV treatment to pregnant women in the second or third trimester could be a major breakthrough in global elimination efforts.
Long-acting HBV treatments could also be used to deliver care to people with chronic hepatitis B without HIV. Approximately 20–30% of the 257 million people with chronic hepatitis B need treatment. However, in 2015, fewer than 9% were diagnosed and less than 10% of those were sustained on treatment.23 Lack of awareness of HBV infection is even a problem in high-income regions of the world, as shown in a community-based study in the USA.61 WHO estimated that HBV elimination will require access to diagnosis to be increased to 90%, and the access to treatment to 80%.23 Long-acting treatments could be very effective in achieving and sustaining this goal.
As we celebrate the inauguration of a generation of long-acting antiviral treatments for HIV, it is essential to consider chronic HBV infection. Governments, industries, and other stakeholders should urgently prioritise the development of long-acting HBV medications, and increase financial support for the necessary additional research and development in accord with the enormous public health potential of such formulations.
Acknowledgments
We thank the following individuals for their valuable input and opinions about this manuscript: Ashwin Balagopal, Chris Hoffman, Charles Holmes, Mina Hosseinipour, Leah M Johnson, and Ariane van der Straten. Support for this work, in part, is from the Long Acting/Extended Release Research Resource Program funded by the US National Institute of Allergy and Infectious Diseases (Grant R24 AI-118397), from the US National Institute of Drug Abuse (Grant R37DA013806), and from the Johns Hopkins University Center for AIDS Research (Grand P30AI094189), funded by multiple US National Institutes of Health Institutes and Centers. All content is solely our own responsibility and does not necessarily represent the official views of the US National Institutes of Health.
Declaration of interests
RCB reports grants from the US National Institutes of Health (NIH), during the conduct of the study, and personal fees from Merck & Co, outside the submitted work. CLT reports grants from NIH during the conduct of the study, and grants from Sanofi outside the submitted work. MSS reports grants and personal fees from Gilead, personal fees from Arbutus, and grants from Assembly Bioscience, during the conduct of the study. CF reports grants from Gilead Sciences, personal fees from Cipla Pharmaceuticals, Merck Laboratories, Mylan Pharmaceuticals, Janssen Pharmaceuticals, and ViiV Healthcare, outside the submitted work. In addition, CF has a patent for semi-solid prodrug nanoparticles pending. JM and DLT declare no competing interests.
References
- 1.Gulick RM, Flexner C. Long-acting HIV drugs for treatment and prevention. Annu Rev Med 2019; 70: 137–50. [DOI] [PubMed] [Google Scholar]
- 2.Flexner C, Thomas DL, Swindells S. Creating demand for long-acting formulations for the treatment and prevention of HIV, tuberculosis, and viral hepatitis. Curr Opin HIV AIDS 2019; 14: 13–20. [DOI] [PubMed] [Google Scholar]
- 3.Benítez-Gutiérrez L, Soriano V, Requena S, Arias A, Barreiro P, de Mendoza C. Treatment and prevention of HIV infection with long-acting antiretrovirals. Expert Rev Clin Pharmacol 2018; 11: 507–17 [DOI] [PubMed] [Google Scholar]
- 4.Brissos S, Veguilla MR, Taylor D, Balanzá-Martinez V. The role of long-acting injectable antipsychotics in schizophrenia: a critical appraisal. Ther Adv Psychopharmacol 2014; 4: 198–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jacobstein R Liftoff: The blossoming of contraceptive implant use in Africa. Glob Health Sci Pract 2018; 6: 17–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tolaymat LL, Kaunitz AM. Long-acting contraceptives in adolescents. Obstet Gynecol 2007; 19: 453–60. [DOI] [PubMed] [Google Scholar]
- 7.Minnis AM, Browne EN, Boeri M, et al. Young women’s stated preferences for biomedical HIV prevention: results of a discrete choice experiment in Kenya and South Africa. J Acquir Immune Defic Syndr 2019; 80: 394–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shapley-Quinn MK, Manenzhe KN, Agot K, Minnis AM, van der Straten A. “We are not the same”: African women’s view of multipurpose prevention products in the TRIO clinical study. Int J Womens Health 2019; 11: 97–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Margolis DA, Gonzalez-Garcia J, Stellbrink HJ, et al. Long-acting intramuscular cabotegravir and rilpivirine in adults with HIV-1 infection (LATTE-2): 96-week results of a randomised, open-label, phase 2b, non-inferiority trial. Lancet 2017; 390: 1499–510. [DOI] [PubMed] [Google Scholar]
- 10.Weld ED, Rana MS, Dallas RH, et al. Interest of youth living with HIV in long-acting antiretrovirals. J Acquir Immune Defic Syndr 2019; 80: 190–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams J, Sayles HR, Meza JL, et al. Long-acting parenteral nanoformulated antiretroviral therapy: interest and attitudes of HIV-infected patients. Nanomedicine (Lond) 2013; 8: 1807–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Swindells S, Andrade-Villanueava JF, Richmond GJ, et al. Long-acting cabotegravir + rilpivirine as maintenance therapy: ATLAS week 48 results. Conference on Retroviruses and Opportunistic Infections; Seattle, WA, USA; March 4–7, 2019. [Google Scholar]
- 13.Orkin CAK, Hernandez-Mora MG, Pokrovsky V, et al. Long-acting cabotegravir + rilpivirine for HIV maintenance: FLAIR week 48 results. Conference on Retroviruses and Opportunistic Infections; Seattle, WA, USA; March 4–7, 2019. [Google Scholar]
- 14.Azar A Ending the HIV epidemic: a plan for America. 2019. https://www.hhs.gov/blog/2019/02/05/ending-the-hiv-epidemic-a-plan-for-america.html (accessed Aug 1, 2019).
- 15.US Department of Health and Human Services. Guidelines for the use of antiretroviral agents in adults and adolescents living with HIV. http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf (accessed Aug 1, 2019).
- 16.Bessesen M, Ives D, Condreay L, Lawrence S, Sherman KE. Chronic active hepatitis B exacerbations in human immunodeficiency virus-infected patients following development of resistance to or withdrawal of lamivudine. Clin Infect Dis 1999; 28: 1032–35. [DOI] [PubMed] [Google Scholar]
- 17.Soriano V, Barreiro P, Benitez L, Peña JM, de Mendoza C. New antivirals for the treatment of chronic hepatitis B. Expert Opin Investig Drugs 2017; 26: 843–51. [DOI] [PubMed] [Google Scholar]
- 18.Stockdale AJ, Phillips RO, Beloukas A, et al. Liver fibrosis by transient elastography and virologic outcomes after introduction of tenofovir in lamivudine-experienced adults with HIV and hepatitis B virus coinfection in Ghana. Clin Infect Dis 2015; 61: 883–91. [DOI] [PubMed] [Google Scholar]
- 19.Matthews GV, Seaberg EC, Avihingsanon A, et al. Patterns and causes of suboptimal response to tenofovir-based therapy in individuals coinfected with HIV and hepatitis B virus. Clin Infect Dis 2013; 56: e87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Terrault NALA, Lok ASF, McMahon BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018; 67: 1560–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flexner C Antiretroviral implants for treatment and prevention of HIV infection. Curr Opin HIV AIDS 2018; 13: 37–80. [DOI] [PubMed] [Google Scholar]
- 22.Johnson LM, Krovi SA, Li L, et al. Characterization of a reservoir-style implant for sustained release of tenofovir alafenamide (TAF) for HIV pre-exposure prophylaxis (PrEP). Pharmaceutics 2019; 11: e315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.WHO. Combating hepatitis B and C to reach elimination by 2030. https://apps.who.int/iris/bitstream/handle/10665/206453/WHO_HIV_2016·04_eng.pdf;jsessionid=6BB43C356FC364B0F095626505D4FB42?sequence=1 (accessed Aug 1, 2019).
- 24.WHO. Guidelines on post-exposure prophylaxis for HIV and the use of co-trimoxazole prophylaxis for HIV-related infections among adults, adolescents, and children: recommendations for a public health approach. 2014. http://www.ncbi.nlm.nih.gov/books/NBK298964/ (accessed Aug 1, 2019). [PubMed]
- 25.Hutin Y, Nasrullah M, Easterbrook P, et al. Access to treatment for hepatitis B virus infection—worldwide, 2016. MMWR Morb Mortal Wkly Rep 2018; 67: 773–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naghavi M, Abajobir AA, Abbafati C, et al. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017; 390: 1151–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Foreman KJ, Marquez N, Dolgert A, et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016–40 for 195 countries and territories. Lancet 2018; 392: 2052–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh KP, Crane M, Audsley J, Avihingsanon A, Sasadeusz J, Lewin SR. HIV-hepatitis B virus coinfection: epidemiology, pathogenesis, and treatment. AIDS 2017; 31: 2035–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kellerman SE, Hanson DL, McNaghten AD, Fleming PL. Prevalence of chronic hepatitis B and incidence of acute hepatitis B infection in human immunodeficiency virus-infected subjects. J Infect Dis 2003; 188: 571–77 [DOI] [PubMed] [Google Scholar]
- 30.Lincoln D, Petoumenos K, Dore GJ. HIV/HBV and HIV/HCV coinfection, and outcomes following highly active antiretroviral therapy. HIV Med 2003; 4: 241–49. [DOI] [PubMed] [Google Scholar]
- 31.Dinesha TR, Boobalan J, Sivamalar S, et al. Occult HBV infection in HIV-infected adults and evaluation of pooled NAT for HBV. J Viral Hepat 2018; 25: 718–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koli S, Girish Kumar CP, Selvaraj V, et al. Profile and prevalence of HBV among HIV affected individuals attending the largest public HIV care center in India. Virusdisease 2016; 27: 215–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Risbud A, Mehendale S, Basu S, et al. Prevalence and incidence of hepatitis B virus infection in STD clinic attendees in Pune, India. Sex Transm Infect 2002; 78: 169–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Irungu E, Mugo N, Ngure K, et al. Immune response to hepatitis B virus vaccination among HIV-1 infected and uninfected adults in Kenya. J Infect Dis 2013; 207: 402–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neukam K, Gutiérrez-Valencia A, Llaves-Flores S, Espinosa N, Viciana P, López-Cortés LF. Response to a reinforced hepatitis B vaccination scheme in HIV-infected patients under real-life conditions. Vaccine 2019; 37: 2758–63. [DOI] [PubMed] [Google Scholar]
- 36.Thio CL, Seaberg EC, Skolasky R Jr, et al. HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter Cohort Study (MACS). Lancet 2002; 360: 1921–26. [DOI] [PubMed] [Google Scholar]
- 37.Kourtis AP, Bulterys M, Hu DJ, Jamieson DJ. HIV-HBV coinfection—a global challenge. N Engl J Med 2012; 366: 1749–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hass M, Hannoun C, Kalinina T, Sommer G, Manegold C, Günther S. Functional analysis of hepatitis B virus reactivating in hepatitis B surface antigen-negative individuals. Hepatology 2005; 42: 93–103. [DOI] [PubMed] [Google Scholar]
- 39.Manegold C, Hannoun C, Wywiol A, et al. Reactivation of hepatitis B virus replication accompanied by acute hepatitis in patients receiving highly active antiretroviral therapy. Clin Infect Dis 2001; 32: 144–48. [DOI] [PubMed] [Google Scholar]
- 40.Dore GJ, Soriano V, Rockstroh J, et al. Frequent hepatitis B virus rebound among HIV-hepatitis B virus-coinfected patients following antiretroviral therapy interruption. AIDS 2010; 24: 857–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saag MS, Benson CA, Gandhi RT, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2018 recommendations of the International Antiviral Society-USA panel. JAMA 2018; 320: 379–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.WHO. Global price reporting mechanism (GPRM). 2017. http://apps.who.int/hiv/amds/price/hdd/Default.aspx (accessed Aug 1, 2019).
- 43.Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med 2012; 367: 399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010; 363: 2587–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Damme L, Corneli A, Ahmed K, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med 2012; 367: 411–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marrazzo JM, Ramjee G, Richardson BA, et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med 2015; 372: 509–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parsons JT, Rendina HJ, Lassiter JM, Whitfield TH, Starks TJ, Grov C. Uptake of HIV pre-exposure prophylaxis (PrEP) in a national cohort of gay and bisexual men in the United States. J Acquir Immune Defic Syndr 2017; 74: 285–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garner W, Wilson BM, Beste L, Maier M, Ohl ME, Van Epps P. Gaps in preexposure prophylaxis uptake for HIV prevention in the Veterans Health Administration. Am J Public Health 2018; 108 (suppl 4): S305–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Biello KB, Edeza A, Salhaney P, et al. A missing perspective: injectable pre-exposure prophylaxis for people who inject drugs. AIDS Care 2019; 31: 1214–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.HIV Prevention Trials Network. HPTN 083. A phase 2b/3 double blind safety and efficacy study of injectable cabotegravir compared to daily oral tenofovir disoproxil fumarate/emtricitabine (TDF/FTC), for pre-exposure prophylaxis in HIV-uninfected cisgender men and transgender women who have sex with men. 2019. https://www.hptn.org/research/studies/hptn083 (accessed Aug 1, 2019).
- 51.HIV Prevention Trials Network. HPTN 084. A phase 3 double blind safety and efficacy study of long-acting injectable cabotegravir compared to daily oral TDF/FTC for pre-exposure prophylaxis in HIV-uninfected women. 2019. https://www.hptn.org/research/studies/hptn084 (accessed Aug 1, 2019).
- 52.Falade-Nwulia O, Seaberg EC, Snider AE, et al. Incident hepatitis B virus infection in HIV-infected and HIV-uninfected men who have sex with men from pre-HAART to HAART periods: a cohort study. Ann Intern Med 2015; 163: 673–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gatanaga H, Hayashida T, Tanuma J, Oka S. Prophylactic effect of antiretroviral therapy on hepatitis B virus infection. Clin Infect Dis 2013; 56: 1812–19. [DOI] [PubMed] [Google Scholar]
- 54.Heuft MM, Houba SM, van den Berk GE, et al. Protective effect of hepatitis B virus-active antiretroviral therapy against primary hepatitis B virus infection. AIDS 2014; 28: 999–1005. [DOI] [PubMed] [Google Scholar]
- 55.Centre for the AIDS Programme of Research in South Africa. CAPRISA 018. A phase I/II trial to assess the safety, acceptability, tolerability and pharmacokinetics of a sustained-release tenofovir alafenamide sub-dermal implant for HIV prevention in women. 2019. https://www.caprisa.org/DBFile/Files/29a84f2d-d267-41c9-8e98-981a4cb9769d/CAPRISA%20018_Study%20protocol%20V1.1_1June18_FINAL.pdf (accessed Aug 1, 2019).
- 56.Nayagam S, Thursz M, Sicuri E, et al. Requirements for global elimination of hepatitis B: a modelling study. Lancet Infect Dis 2016; 16: 1399–408. [DOI] [PubMed] [Google Scholar]
- 57.Edmunds WJ, Medley GF, Nokes DJ, Hall AJ, Whittle HC. The influence of age on the development of the hepatitis B carrier state. Proc Biol Sci 1993; 253: 197–201. [DOI] [PubMed] [Google Scholar]
- 58.Bhattacharya DGR, Tseng C, Emel L, et al. Maternal HBV viremia is associated with adverse infant outcomes in HIV/HBV women. Conferences on Retroviruses and Opportunistic Infections; Seattle, WA, USA; March 4–7, 2019. [Google Scholar]
- 59.US Department of Health and Human Services. Recommendations for the use of antiretroviral drugs in pregnant women with HIV infection and interventions to reduce perinatal HIV transmission in the United States. 2018. https://aidsinfo.nih.gov/guidelines/html/3/perinatal/159/hepatitis-b-virus-hiv-coinfection (accessed Aug 1, 2019).
- 60.Thio CL, Guo N, Xie C, Nelson KE, Ehrhardt S. Global elimination of mother-to-child transmission of hepatitis B: revisiting the current strategy. Lancet Infect Dis 2015; 15: 981–85. [DOI] [PubMed] [Google Scholar]
- 61.Wong RJ, Campbell B, Liu B, Baden R, Bhuket T. Sub-optimal testing and awareness of HCV and HBV among high risk individuals at an underserved safety-net hospital. J Community Health 2018; 43: 65–69. [DOI] [PubMed] [Google Scholar]
- 62.Razavi-Shearer D, Gamkrelidze I, Nguyen MH, et al. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol 2018; 3: 383–403. [DOI] [PubMed] [Google Scholar]
- 63.Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet 2015; 386: 1546–55. [DOI] [PubMed] [Google Scholar]
- 64.UNAIDS. Adult HIV prevalence estimates 2018. http://www.unaids.org/sites/default/files/media_asset/HIV_estimates_from_1990-to-present.xlsx (accessed Aug 1, 2019).
- 65.CIA World Factbook. Country comparison: HIV/AIDS-adult prevalence rate. https://www.cia.gov/library/publications/the-world-factbook/rankorder/2155rank.html (accessed Aug 1, 2019).
- 66.Haddad N, Li JS, Totten S, McGuire M. HIV in Canada-Surveillance Report, 2017. Can Commun Dis Rep 2018; 44: 348–56. [DOI] [PubMed] [Google Scholar]
- 67.Centers for Disease Control and Prevention. Estimated HIV incidence and prevalence in the United States 2010–2016. 2019. https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-supplemental-report-vol-24-1.pdf (accessed Aug 1, 2019).
- 68.China Health and Family Planning Commission. 2015 China AIDS response progress report. 2015. http://www.unaids.org/sites/default/files/country/documents/CHN_narrative_reporlL2015.pdf (accessed Aug 1, 2019).
- 69.National AIDS Centre Agenda of the Ministry of Health. Summary of the HIV/AIDS epidemic in Poland. 2018. https://aids.gov.pl/epidemiology/poland/ (accessed Aug 1, 2019).
- 70.Goliusov AT, Dementyeva LA, Ladnaya NN, et al. Country progress report of the Russian Federation on the implementation of the Declaration of Commitment on HIV/AIDS. 2008. http://data.unaids.org/pub/report/2008/russia_2008_country_progress_report_en.pdf (accessed Aug 1, 2019).


