Abstract
Neural tube defects remain a major problem in developing countries, but there are limited comprehensive national reports to date in Ethiopia. Therefore, this study aimed to assess the prevalence of neural tube defects and associated factors in Ethiopia. Electronic databases and other sources were used to retrieve studies. Fifteen out of 862 studies were included in the final analysis. The estimated pooled prevalence of neural tube defects among children in Ethiopia was 63.3 cases per 10 000 children. The pooled prevalence of spinal bifida, anencephaly, and encephalocele was 41.09, 18.90, and 1.07 per 10 000 children, respectively. Previous family history and unplanned pregnancy were risk factors for neural tube defects. Folic acid supplementation during the first trimester of pregnancy was found to be protective. Neural tube defects are widespread in Ethiopia. Hence, fortification of food with folic acid or folic acid supplementation during childbearing age is recommended.
Keywords: congenital abnormality, spinal bifida, anencephaly, encephalocele, folic acid
Background
Neural tube defects (NTDs) are congenital anomalies resulting from developmental malformation of the central nervous system during the embryonic period. Anencephaly and spina bifida are the most frequent forms of NTDs.1 Since there is a paucity of data on the actual figure of NTDs globally, an estimated 260 100 NTD cases were reported in 2015, which was equivalent to 1.9 per 1000 live births.2 Although the prevalence of NTDs is decreasing in developed countries, it has remained high in developing countries ranging from 1 to 11 per 1000 live births,3 which is around 4 times higher.4 The occurrence of NTDs in Ethiopia may be as high as 13 per 1000 total births.5 This is 6 times more than the lowest rate in the United States where the NTD rate is 0.5 per 1000 births.6
Neural tube defects are associated with multiple medical and socioeconomic consequences. They could lead to about 50% elective terminations of pregnancies fetal malformation or stillbirth. Out of the NTD-affected live births, approximately 75% resulted in under-5 death as well as disabilities globally.2,7 Besides, around 10% of neonatal mortality is also due to malformation of the nervous system in the embryonic period.8 The consequences of NTDs are especially evident in resource-limited countries where preventative measures and long-term quality care for surviving NTD patients are limited.9 Spinal bifida is the most common type of NTD and the patient typically has neurologic deficits, including neurogenic bladder, impaired bowel habits, orthopedic impairments, and pressure ulcers.10
Neural tube defects are associated with numerous factors that could be either genetic or environmental,11 but folic acid deficiency is the predominant preventable contributor of NTDs.12,13 The universal supplementation of folic acid is expected to decrease the incidence of NTDs; however, “folate non-responsive” NTDs continue to occur globally.14 The magnitude is relatively high in Ethiopia where folic acid coverage among reproductive-age women is low and 1 in 3 women have folic acid deficiency.15 Other risk factors such as dietary deficiency, poverty, obesity, diabetes, substance abuse, fever during the preconception period, and medicinal drugs also have contributed to the occurrence of NTDs.16-20 The activation of apoptosis signal-regulating kinase 1 in hyperglycemic conditions, which could lead to activation of the apoptosis mediator caspase 8 by stimulating the FoxO3a transcription factor, is found to have associated with NTDs.21 The recent study finding from Botswana also revealed that exposure to dolutegravir is associated with the incidence of NTDs.22
In Ethiopia, the incidence of NTDs is increasing alarmingly in recent years. Primary studies that were done in different regions of Ethiopia revealed the number of children born with congenital anomalies is increasing in recent years. The burden of NTDs is relatively high in northern Ethiopia as compared with the other regions. A recent finding in Tigray region showed the overall incidence of NTDs was as high as 13 per 1000 births, and the highest burden was reported in the Southern Zone of Tigray, where the incidence was 30 per 1000 births.23 The lowest prevalence was reported from a study conducted in 2 regions (Addis Ababa and Amhara region) with the incidence of 4 per 1000 births.24 It is also stated in other primary surveys in Ethiopia that there are sizeable numbers of pregnancies associated with NTDs.20,25-32 These findings are inconsistent and inconclusive for policy makers and stakeholders. Therefore, the pooled prevalence, patterns, and risk factors for NTDs in Ethiopia were explored in this systematic review and meta-analysis.
Materials and Methods
Search Strategy
Two investigators ZWB (lecturer and researcher) and TW (assistant professor), who are trained in systematic searching methods and comprehensive systematic review and meta-analysis, performed the entire search independently. The searching was done with consultation of senior librarian at St. Paul’s Hospital Millennium Medical College. A systematic search was performed using both electronic and nonelectronic data sources. The electronic databases used were the following: EMBASE (Ovid), EMCare (Ovid), MEDLINE (Ovid), CINAHL (EBSCOhost), SCOPUS, PubMed, CAB Abstracts, Global Health, Web of Science, and Science Direct. Gray literature sources such as World Cat, Google Scholar, and Google were also used for searching unpublished studies. Also, personal email communications with authors regarding unpublished articles and incomplete articles were utilized. Searching was conducted from inception to December 20, 2019. Searching was conducted using indexed terms, combined key terms, text words, and search strings taken from the review questions. The searching terms were prevalence OR magnitude OR “neural tube defects” OR NTDs OR “congenital anomalies” AND neonates OR newborns OR children AND “associated factors” AND Ethiopia. The searching terms were checked for being indexed terms in each electronic database before proceeding to the actual search. The Boolean operators “AND” or “OR” were used accordingly (see Additional File 1, available online). The search was limited to studies conducted in the English language. The method of this systematic review and meta-analysis was developed based on Meta-analysis of Observational Studies in Epidemiology (MOOSE) reporting guideline (see Additional File 2, available online).
Study Selection Procedure
In this study, cross-sectional, cohort, and case-control studies were included in the final analysis. PICOS (participants, interventions, comparison, outcome, and study setting) criteria were used to review the studies. The studies that reported the magnitude or prevalence of NTDs and associated factors that were done in different regions of Ethiopia were selected. First, articles extracted from different sources were exported to EndNote X8 citation manager. Then, duplicate articles were removed. Three authors (ZWB, TW, and AA) screened the titles and abstracts of the studies with predefined inclusion criteria independently. Three authors (ZWB, AA, and AA) also independently collected full texts and evaluated the eligibility for final inclusion by considering study subjects, language, study designs, quality, and outcomes. Finally, 2 authors (ZWB and TW) reviewed the full texts of the selected studies. A total of 862 studies were obtained from the whole search and 120 duplicates were removed. After screening using titles and abstracts, 27 studies were checked for eligibility through full-text assessment. Finally, 15 studies were included in the final analysis. The details are presented using the MOOSE flow diagram (Figure 1).
Figure 1.
The MOOSE flow diagram showing study selection process.
Assessment of Methodological Quality (Risk of Bias Assessment)
Two authors (AA and ZWB) performed the quality assessment (critical appraisal) of studies independently using Joanna Briggs Institute critical appraisal tool for observational studies (cohort, case-control, and cross-sectional studies).33 Tools have Yes/No questions and 1 is given for Yes and 0 for No. The scores were summed up and changed to percentages. Studies with >50% were included in the meta-analysis (see Additional File 3, available online). During critical appraisal and inclusion of the studies, the third author (TW) played a critical role in solving any discrepancies between the 2 authors who did full-text screening. The asymmetry of the funnel plot and/or statistical significance of Egger’s regression test (P < .05)34 was considered as a presence publication bias.
Data Extraction Process
The characteristics of included studies (author, publication year, study area, study period, study design, study population, sample size, the number of NTDs, types of NTDs, and risk factors with OR) were extracted and summarized in Microsoft Word 2016 (Tables 1 and 2). The quantitative data were extracted from the included studies and stored in Microsoft Excel 2016 by 2 authors (TW and ZWB) independently.
Table 1.
Detailed Description of the Included Studies for Computing the Magnitude of Neural Tube Defects (NTDs) in Ethiopia.
| Author, year | Study area | Study design | Study population | Sample size | NTDs/10 000 children | Patterns of NTDs |
Quality score | ||
|---|---|---|---|---|---|---|---|---|---|
| Anencephaly/10 000 children | Spinal bifida/10 000 children | Encephalocele/10 000 children | |||||||
| Berihu et al,23 2018 | Tigray | Cross-sectional | Newborns | 14 903 | 130.8 | 66.4 | 64.4 | — | Medium |
| Seyoum and Adane,31 2018 | Amhara | Cross-sectional | Newborns | 19 650 | 35.6 | 5.1 | 30.5 | — | Medium |
| Gedefaw et al,26 2018 | Addis Ababa | Cross-sectional | Newborns | 8677 | 127.9 | 69.1 | 51.9 | 6.91 | High |
| Sorri and Mesfin,27 2015 | Addis Ababa | Cross-sectional | Newborns | 28 961 | 61.1 | 26.6 | 32.8 | 1.73 | Medium |
| Taye et al,24 2019 | Addis Ababa and Amhara | Cross-sectional | 0-17 years | 76 201 | 47.5 | 4.7 | 42.8 | High | |
| Taye et al,29 2016 | Addis Ababa and Amhara | Cross-sectional | 0-17 years | 319 776 | 37.6 | 5.7 | 31.1 | 0.78 | Medium |
| Mekonen et al,36 2015 | Tigray | Cohort | Newborns | 1516 | 131.9 | 6.6 | 125.3 | — | Medium |
| Abdu and Seyoum,37 2019 | Amhara | Cross-sectional | Newborns | 22 624 | 53.5 | — | 53.5 | — | Medium |
| Abebe et al,38 2019 | Oromia | Cross-sectional | Newborns | 45 951 | 25.7 | 13.7 | 11.1 | 0.87 | Medium |
| Mitiku,39 2017 | Addis Ababa | Cross-sectional | Newborns | 84 | 281.1 | 238.1 | — | — | Medium |
Table 2.
Risk Factors of Neural Tube Defects (NTDs) in Ethiopiaa.
| Author, year | Study area | Study period | Study population |
Total sample size | Study population | Study design | Factors | OR (95% CI) | Quality score | |
|---|---|---|---|---|---|---|---|---|---|---|
| Cases | Controls | |||||||||
| Berihu et al,25 2019 | Tigray | October 2016 to June 2017 | 205 | 412 | 617 | Newborns | Case control | Folic acid | 0.48 (0.23-1.01) | High |
| Residence, urban (reference) | 1.94 (1.37-2.76) | |||||||||
| Age <20 | 2.74 (1.86-4.03) | |||||||||
| Age ≥30 | 6.66 (3.85-11.53) | |||||||||
| History of still birth | 15.02 (3.8-66.77) | |||||||||
| Unplanned pregnancy | 1.97 (1.38-2.81) | |||||||||
| History of BF above 2 years | 15.47 (5.33-44.87) | |||||||||
| Sex of previous child (male-reference) | 0.81 (0.57-1.16) | |||||||||
| Parity ≥3 | 0.59 (0.42-0.83) | Medium | ||||||||
| Aynalem40, 2018 | Addis Ababa | November to May 2018 | 60 | 120 | 180 | <3 years | Case control | Folic acid | 0.03 (0.001-0.55) | |
| Family history of NTD | 13.25 (2.83-61.96) | |||||||||
| Caffeine (reference <3 cups) | 8.14 (4.02-16.48) | |||||||||
| Age <20 | 9.8 (2.64-36.33) | |||||||||
| Age ≥30 | 2.06 (0.914-4.66) | |||||||||
| Preconception fever | 7.93 (3.87-16.25) | |||||||||
| History of antipyretic use | 0.10 (0.013-0.791 | |||||||||
| Oral contraceptive | 2.69-17.74 | |||||||||
| Parity ≥3 | 1.71 (0.88-3.3 | |||||||||
| Male sex | 0.58 (0.31-1.10) | |||||||||
| Gedefaw et al,26 2018 | Addis Ababa | February 1, 2016, to August 30, 2016 | 111 | 222 | 333 | Newborns | Case control | Folic acid | 0.35 (0.19-0.65) | High |
| Unplanned pregnancy | 1.66 (0.95-2.91) | |||||||||
| Male sex | 0.66 (0.42-1.04) | |||||||||
| Low BMI in PX (≥25) | 0.55 (0.31-0.98) | |||||||||
| Oral contraceptive | 0.66 (0.42-1.04) | |||||||||
| Age <20 | 1.37 (0.64-2.96) | |||||||||
| Age ≥30 | 2.08 (0.76-5.69) | |||||||||
| History of still birth | 0.49 (0.10-2.35) | |||||||||
| Atlaw et al,32 2019 | Oromia | October 2017 to February 2018 | 42 | 420 | 462 | Newborns | Case control | Age <20 | 0.18 (0.04-0.74) | Medium |
| Age ≥30 | 1.06 (0.56-2.00) | |||||||||
| Male sex | 1.38 (0.73-2.63) | |||||||||
| Consanguineous marriage | 0.20 (0.06-0.67) | |||||||||
| Passive smoking | 0.16 (0.04-0.69) | |||||||||
| Folic acid | 0.11 (0.04, 0.30) | |||||||||
| Family history of NTDs | 2.57 (0.91-7.20) | |||||||||
| History of still birth | ||||||||||
| Tsehay et al,41 2019 | Amhara | 58 | 298 | 356 | Newborns | Case control | NA | NA | Low | |
| Welderufael et al,20 2019 | Tigray | October 2016 to June 2017 | 205 | 412 | 617 | Newborns | Case control | Low DD score | 1.87 (1.33-2.63) | Medium |
| High DD Score | 0.49 (0.31-0.77) | |||||||||
Abbreviations: OR, odds ratio; CI, confidence interval; BMI, body mass index; NA, not applicable; DD, dietary diversity; BF, breast feeding; PX, pregnancy.
NA because the odds ratios are for all congenital anomalies. Odds are not done specifically for congenital anomalies.
Data Analysis and Assessment of the Certainty of the Findings
The extracted data were exported to STATA Version 15 and Review Manager Software (version 5.3) for analysis of the pooled estimates of NTD prevalence in Ethiopia and to assess the factors associated with NTDs. The meta-analyses results were presented using the forest plot. Heterogeneity among studies was examined by using forest plot and I2 heterogeneity tests. The I2 values of 25%, 50%, and 75% were interpreted as the presence of low, medium, and high heterogeneity, respectively. In this meta-analysis, heterogeneity was declared and justified for I2 ≥50% and a P value of <.05. Also, random-effect and fixed-effect models were used interchangeably in the analysis. Since there is no significant difference in the heterogeneity between the 2 models, the DerSimonian and Laird random-effect model35 was employed in the final analysis. The pooled prevalence of NTDs with 95% confidence interval (CI) and the risk factors based on the odds ratio (OR) with 95% CI was determined by assuming that the true effect size varies between studies. There was remarkable heterogeneity among included studies since the I2 test statistic of the pooled estimates were >75%. Hence, the meta-analyses results of most pooled estimates were presented based on the DerSimonian and Laird random-effect model.
Ethical Approval and Informed Consent
This study is a systematic review and meta-analysis from the original studies, which were conducted in various parts of the country. Ethical approval and informed consent were not applicable to this study since the data were generated from computed pooled estimates. In Ethiopia, most of the research institutions have institutional review boards and therefore the respective studies had prior approval before the actual data collection period.
Results
Study Characteristics
In the final analysis, 10 studies that were done in Tigray region, Amhara region, Addis Ababa, and Oromia regions23,24,26,27,29,31,36-39 were included to estimate the pooled prevalence of NTDs in Ethiopia. Most of the studies were conducted in the central and northern part of the country. All studies were cross-sectional studies23,24,26,27,29,31,37-39 except a cohort study conducted in Tigray.36 All of the studies were conducted in health facilities. In most of the studies, the study populations were newborns.23,26,27,31,36-39 However, 2 studies24,29 assessed NTD prevalence among children 0 to 17 years of age. The study period for 9 of the 10 studies23,24,26,29,31,36-39 was in the past 10 years. The remaining study27 was from 2009 to August 2012 (Tables 1 and 2).
Pooled Prevalence of Neural Tube Defects in Ethiopia
The largest sample size was from a study performed in 2 regions of the country (Addis Ababa and Amhara region),29 using 319 776 children below 17 years of age in selected health facilities. Likewise, the smallest sample size was noted from a study conducted in Addis Ababa using 84 newborns.39 In this study, the prevalence of NTDs was computed per 10 000 children, and 40.5 to 238 NTD cases per 10 000 children were reported in the primary studies.23,24,26,27,29,31,36-39 The largest magnitude was reported from a study done in Addis Ababa39 where 2 out of 84 newborns had NTDs (238 NTDs per 10 000 newborns). The second (145 NTDs per 10 000 newborns) and third (131 NTDs per 10 000) highest rates were reported from studies conducted in the Tigray region.23,36 The prevalence of NTDs was found to be as high as 304 cases per 10 000 newborns in the Southern Zone of Tigray.23 In this systematic review and meta-analysis, the lowest NTD prevalence was reported from a study conducted in Southwest Ethiopia where the rate of NTDs was 40.5 per 10 000 newborns.38
To estimate the pooled prevalence of NTDs per 10 000 populations in Ethiopia, a total of 538 343 newborns and children below 17 years of age were included from the selected studies.23,24,26,27,29,31,36-39 The NTD prevalence based on the pooled estimate was computed from the regions of the country where the studies were conducted. The majority were accounted for by a study done in Amhara region (24.74%)31,37 followed by the studies conducted in Tigray region (14%)23,36 and Oromia region (13.02%).38 Accordingly, the estimated pooled prevalence of NTDs among children below 17 years of age was 63.3 cases per 10 000 children (95% CI = 50.93-75.67) using a random-effect model (I2 = 96.3%, P < .001; Figure 2). Publication bias was not detected, as it was substantiated by Egger’s regression test result (P = .168; Figure 3).
Figure 2.
Forest plot showing the pooled prevalence of neural tube defects in Ethiopia.
Figure 3.
Funnel plot showing the distribution of included studies for prevalence of neural tube defects.
Pooled Prevalence of Spinal Bifida, Anencephaly, and Encephalocele
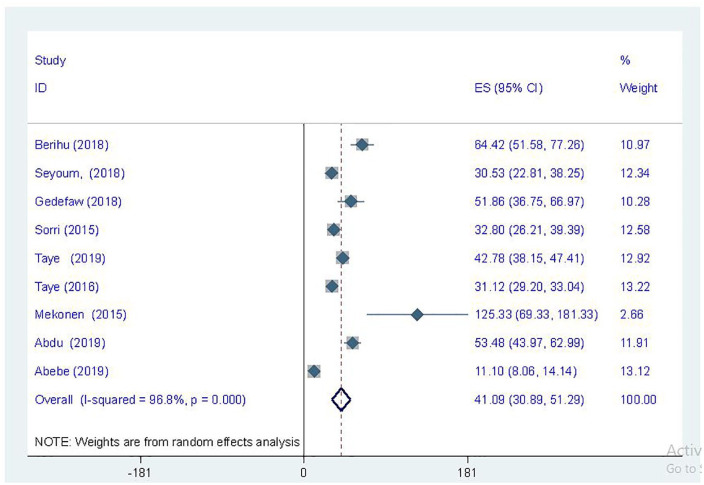
Nine studies included the prevalence of spinal bifida in their report23,24,26,27,29,31,36-38 except one study that was conducted in Addis Ababa.39 The highest prevalence of spinal bifida, which was 125.33 cases per 10 000 newborns, was reported from a study conducted in Tigray region,36 whereas the lowest prevalence reported was 11.10 cases per 10 000 stillbirths and live births.38 A total of 537 449 children was used to compute the pooled estimate of spinal bifida with an estimated pooled prevalence of 41.09 cases per 10 000 children below 17 years of age (95% CI = 30.89-51.29) using the DerSimonian and Laird random-effect model (I2 = 96.8%, P < .001; Figure 4). Similarly, the prevalence of anencephaly was computed using 9 studies.23,24,26,27,29,31,36,38,39 The pooled prevalence of anencephaly among children was 18.90 cases per 10 000 children (95% CI = 13.30-24.49) with DerSimonian and Laird random-effect model (Figure 5). Likewise, the pooled prevalence of encephalocele was computed using 4 eligible articles.26,27,29,38 In the original studies, a total of 40 encephalocele cases were reported, and most of the studies were conducted in Addis Ababa. The pooled prevalence of encephalocele was 1.07 per 10 000 children (95% CI = 0.34-1.81; I2 = 51%; P = .106) using the random-effect model (Figure 6).
Figure 4.
Forest plot showing the prevalence of spinal bifida per 10 000 children in Ethiopia.
Figure 5.
Forest plot showing the prevalence of anencephaly per 10 000 children in Ethiopia.
Figure 6.
Forest plot showing the prevalence of encephalocele per 10 000 children in Ethiopia.
Risk Factors of Neural Tube Defects in Ethiopia
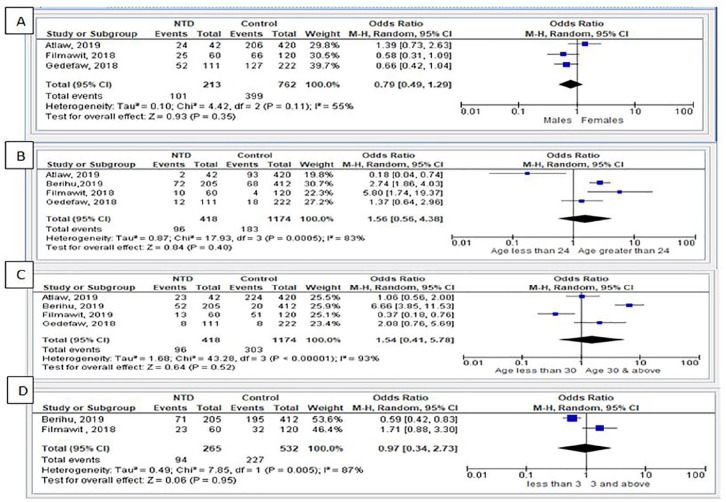
Risk factors for NTDs were reported by 6 case-control studies,20,25,26,32,40,41 which were done in different regions of the country. In the 6 studies, a total of 476 cases (NTDs) and 1472 controls were analyzed. This review assessed socio-demographic and maternal-related factors that were associated with the incidence of NTDs. From these studies, one study from the Tigray region revealed that the incidence of NTDs was related to dietary diversity scores.20 The other case-control study done41 at selected hospitals of East and West Gojjam zones of the Amhara region found 58 NTDs from a total of 100 cases (all forms of congenital anomalies). This study revealed that folic acid supplementation, being alcoholic, and the use of herbal medicine was associated with all forms of congenital malformations. By assessing risk factors using the random-effect model, sex of the child, maternal age during pregnancy, parity >3, and history of stillbirth were found to not be associated risk factors with NTDs (Figure 7).
Figure 7.
Socio-demographic factors: (A) sex of the child; (B) age of the mother during pregnancy (age <24 years); (C) age of the mother during pregnancy (age ≥30); and (D) parity.
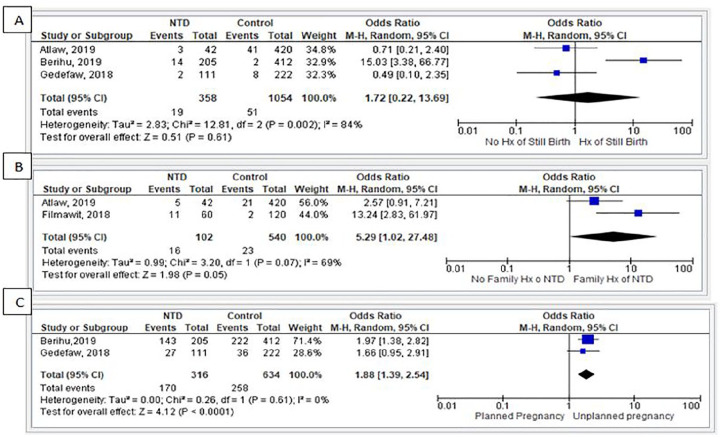
Mothers who took folic acid during the first trimester of pregnancy had a 68% reduced risk of giving birth to a child with NTDs (OR = 0.32, 95% CI = 0.17-0.060, I2 = 36%; Figure 8). Likewise, based on 2 studies,32,40 children from a family with previous history of NTDs had 5.29 times the odds of developing NTDs as compared with children from a family without a previous history of NTDs (OR = 5.29, 95% CI = 1.02-27.48, I2 = 69%). Similarly, from the 2 studies,25,26 children who were born from women with unplanned pregnancies had 1.88 times the odds of developing NTDs as compared with children born from women who had planned pregnancies (OR = 1.88, 95% CI = 1.39-2.54, I2 = 0%; Figure 9).
Figure 8.
Forest plot showing the effect folic acid supplementation during pregnancy.
Figure 9.
Maternal-related factors: (A) history of neural tube defects; (B) family history of neural tube defects; and (C) type of pregnancy.
Discussion
In this systematic review and meta-analysis, the prevalence and possible risk factors of NTDs in Ethiopia were explored and analyzed. To our knowledge, the current work is the first systematic review and meta-analysis that explored the prevalence of NTDs and associated factors among children in Ethiopia. After intense searching, data were explored and analyzed from a total of 15 studies20,23-27,29,31,32,36-41 conducted in different regions of the country. From these studies, 10 studies23,24,26,27,29,31,36-39 were used to compute the pooled prevalence of NTDs and 6 studies20,25,26,32,40,41 were used for identifying the risk factor of NTDs. The studies were done in 4 regions of the country, namely, Tigray, Amhara, Oromia, and Addis Ababa.
The pooled prevalence of NTDs in Ethiopia, which was computed from a total of 538 343 newborns and children below 17 years of age, was found to be 63.3 cases per 10 000 children. Both fixed- and random-effect models were employed to compute the pooled prevalence and the random-effect model was used due to high heterogeneity among the included studies (I2 = 96.3%, P < .001). The final pooled prevalence of NTDs in Ethiopia was reported based on the random-effect model, and a sensitivity analysis was done to explore potential sources of heterogeneity. However, no publication bias was found based on the funnel plot and Egger’s regression test results (P = .168). The current finding implies that the prevalence of NTDs is much higher in Ethiopia as compared with the global estimates where the prevalence is 19 cases per 10 000 children.2 This finding is also much higher than the overall prevalence in Africa (5.2-75.4; 11.7 per 10 000 births)42 and from individual reports from African countries such as Nigeria (27.5 per 10 000 live births)43 and South Africa (25 cases per 10 000 births).44 Similarly, the findings of a review from sub-Saharan Africa were lower compared with the current findings with the pooled prevalence of 15.27 cases per 10 000 births in folic acid non-fortifying countries.2 This could be attributed to the scant reports about the issue and only 17% of African countries have complete reporting on NTDs.42 The pooled prevalence in this study is also higher than the findings of systematic reviews from India (45 NTDs per 10 000 live births)45 and Iran (32 per 10 000 total births).46 An individual study23 conducted in Ethiopia found as high as 304 NTD cases per 10 000 children implying there is much work to be done in this country to decrease this to an acceptable range.
From studies included in this systematic and meta-analysis, spinal bifida and anencephaly were the most common reported congenital abnormalities among children with NTDs. However, encephalocele was the most uncommon type of NTD found in Ethiopia with only 1.07 per 10 000 cases. The prevalence of spinal bifida ranged from 11.1038 to 125.33 cases36 per 10 000 children and the pooled prevalence is 41.09 (95% CI = 30.89-51.29) cases per 10 000 children. This finding is significantly higher as compared with the global prevalence of 3.52 (95% CI = 3.22-3.86) per 10 000 births as well as the prevalence in Africa showing 5.43 (95% CI = 3.67-8.04) per 10 000 births ended up with spinal bifida.47 Even the 11.10 cases per 10 000 children observed as a lower prevalence in an individual study included in this meta-analysis is higher than the prevalence of spinal bifida in areas where folic acid fortification is mandatory.47 Globally, only 59 countries have adopted the mandatory rule of folic acid fortification to prevent this problem, with only 18% of the goal achieved by 2017.48 This implies that the problem is remarkably high in the world and it is worse in Ethiopia where mandatory folic acid fortification is not currently part of the feeding system. Much work is to be done in the future to mitigate the incidence of this preventative public health issue. Likewise, anencephaly is a significant congenital abnormality observed among children in Ethiopia with a pooled prevalence of 18.90 anencephaly cases per 10 000 children. The current finding is significantly lower than the prevalence of spinal bifida, which could be the result of the under-reporting of anencephaly in Ethiopia. This finding is higher than the review finding from the United States where the prevalence of anencephaly was 9.5 per 10 000 live births.49 The study reported from China (4.92/10 000 live births) also revealed lower rates of cases as compared with this finding.50 However, the current finding is lower than the systematic review reported from India with 21.1 cases (95% CI = 16.91-25.29) per 10 000 births.51 As shown with the included studies, the number of encephalocele cases is very limited in Ethiopia. This could be associated with limited access to ultrasound screening during pregnancy, which could lead to under-reporting of these cases.
In the current systematic review and meta-analysis, the reporting of encephalocele was limited with few cases reported by the original studies and only 40 cases were reported from 4 articles,26,27,29,38 mostly all conducted in Addis Ababa. This could be because there could be an improved reporting system of the cases in Addis Ababa since most women give birth in the health institutions. Only 1.07 per 10 000 children found to have encephalocele. This finding is in line with the prevalence of encephalocele in most parts of the world where 0.8 to 5 cases per 10 00052 were born with encephalocele. However, the current finding was significantly lower than a study conducted in Nigeria (66.9 encephaloceles per 10 000 births).53 The disparity could be associated with improved reporting systems in Nigeria as well as the absence of pregnancy scanning services especially in areas outside Addis Ababa that could lead to underreporting of cases. Improving ultrasound screening during pregnancy and institutional deliveries in Ethiopia would likely result in an increasing number of reported encephalocele cases.
In the present meta-analysis, spinal bifida cases were remarkably higher than both anencephaly and encephalocele cases. This could be associated with poor reporting systems by health care providers and some birth attendants may hesitate to report anencephaly and encephalocele due to the normo-centric nature of most Ethiopians. In Ethiopia, only a small number of women have antenatal care follow-up and around 43% of births take place in health institutions.54 Besides, there is very limited access to pregnancy scanning (ultrasound imaging) and no concrete data exists on how many pregnant women have access to pregnancy scanning. In rural areas, most Ethiopian women give birth at health centers where there are no ultrasound machines and there are no current existing national standards for ultrasound screening during pregnancy.55 This is likely a contributing factor to the under-reporting of anencephaly and encephalocele cases in Ethiopia. These findings may imply that pregnancy imaging could help determine the actual figures of NTDs and could provide vital information for policy change for a largely preventable disease.
In this study, we explored data from 6 studies20,25,26,32,40,41 to identify the risk and protective factors associated with NTDs among children by reviewing 476 cases and 1472 controls. Accordingly, sex of the child, age of the mother during pregnancy, parity >3, and a history of stillbirth were found to not be associated with NTDs using random-effect model. In contrast, not taking folic acid supplementation in the first trimester of pregnancy, previous family history of NTDs, and unplanned pregnancy were identified as the risk factors contributing to the occurrence of NTDs among children.
Based on the random-effect model, women who took folic acid during the first trimester of pregnancy were found to have a 68% reduced risk of having a child with NTDs. This is supported by the scientific fact that taking folic acid during pregnancy prevents the occurrence of NTDs by enhancing the closure of neural tubes during the embryogenesis period, which could prevent an estimated 230 000 children from unnecessarily developing folic acid preventable spinal bifida globally.48 The higher prevalence of NTDs observed in Ethiopia could be due to lower folic acid supplementation, as Ethiopia is among the countries with the highest burden of folate deficiency with two thirds of women aged 15 to 49 years exhibiting this nutritional deficit.15 However, increasing periconceptional intake of folic acid through fortification of wheat, flours, and salts appears to have prevented an estimated 41 610 folic acid preventable NTDs.5 The current study revealed that children with a family history of NTDs have a higher risk of developing NTDs compared with their counterparts. This could be associated with a genetic basis for NTDs.56,57 Besides, unplanned pregnancy is one risk factor that contributes to the development of NTDs and could be due to inadequate intake of folic acid secondary to lack of follow-up in an antenatal program in Ethiopia where iron and folate is supplemented. Moreover, one study20 reported that lower dietary diversity score in the mother was a risk factor for the development of NTDs. The evidence for this could be elucidated as folic acid is primarily obtained from diets, and if the dietary diversity of the mother was decreased, there is less chance of obtaining folic acid through dietary intake and thus increased probability of NTDs. Finally, a study done at selected hospitals of the Amhara region reported that being alcoholic and the use of herbal medicine were found to have an association with all forms of congenital malformations.41 This could be due to alcohol-induced abnormal embryogenesis and the teratogenic effect of alcohol, which may cause the abnormal formation of the nervous system.58
Conclusion
Neural tube defects are a neglected public health problem in Ethiopia and widespread throughout the country. Spinal bifida and anencephaly are the most common forms of NTDs. Folic acid intake during pregnancy was found to be the main preventive factor causing NTDs in Ethiopia. The association of family history on the incidence of these anomalies affirms the genetic origins of NTDs. Unplanned pregnancy is a risk for NTDs. In Ethiopia, periconceptional folate supplementation needs to be considered for women during the reproductive age. Food fortification with folic acid could also decrease the prevalence of these preventable malformations. A national surveillance system of congenital anomalies along with prospective longitudinal studies assessing folic acid and NTDs should be considered to identify all the possible factors associated with NTDs.
Strengths and Limitations
The main strength of this systematic review and meta-analysis was that all efforts were made to obtain findings from both electronic and gray literature sources. However, a scarcity of primary studies was the main limitation of this study. Although there were some studies from various regions of the country, we did not find data from all regions of the country, which prohibited us from estimating the geospatial distribution of NTDs in Ethiopia.
Supplemental Material
Supplemental material, Additional_file_1 for Magnitude and Associated Factors of Neural Tube Defects in Ethiopia: A Systematic Review and Meta-Analysis by Zebenay Workneh Bitew, Teshager Worku, Anmut Alebel and Ayinalem Alemu in Global Pediatric Health
Supplemental material, Additional_file_2 for Magnitude and Associated Factors of Neural Tube Defects in Ethiopia: A Systematic Review and Meta-Analysis by Zebenay Workneh Bitew, Teshager Worku, Anmut Alebel and Ayinalem Alemu in Global Pediatric Health
Supplemental material, Additional_file_3 for Magnitude and Associated Factors of Neural Tube Defects in Ethiopia: A Systematic Review and Meta-Analysis by Zebenay Workneh Bitew, Teshager Worku, Anmut Alebel and Ayinalem Alemu in Global Pediatric Health
Acknowledgments
We would like to address our gratitude to the authors of the included studies for this systematic review and meta-analysis and St. Paul’s Hospital Millennium Medical College.
Footnotes
Author Contributions: ZBW, TW, AA, and AA conceived and designed the review. ZWB prepared the draft of the manuscript. The final version of the manuscript was approved by all the authors.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Zebenay Workneh Bitew  https://orcid.org/0000-0001-5695-3896
https://orcid.org/0000-0001-5695-3896
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Botto LD, Moore CA, Khoury MJ, Erickson JD. Neural-tube defects. N Engl J Med. 1999;341:1509-1519. [DOI] [PubMed] [Google Scholar]
- 2. Blencowe H, Kancherla V, Moorthie S, Darlison MW, Modell B. Estimates of global and regional prevalence of neural tube defects for 2015: a systematic analysis. Ann N Y Acad Sci. 2018;1414:31-46. [DOI] [PubMed] [Google Scholar]
- 3. Moore CA, Li S, Li Z, et al. Elevated rates of severe neural tube defects in a high-prevalence area in northern China. Am J Med Genet. 1997;73:113-118. [PubMed] [Google Scholar]
- 4. Lawal TA, Adeleye AO. Determinants of folic acid intake during preconception and in early pregnancy by mothers in Ibadan, Nigeria. Pan Afr Med J. 2014;19:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dixon M, Kancherla V, Magana T, Mulugeta A, Oakley GP., Jr. High potential for reducing folic acid-preventable spina bifida and anencephaly, and related stillbirth and child mortality, in Ethiopia. Birth Defects Res. 2019;111:1513-1519. [DOI] [PubMed] [Google Scholar]
- 6. Williams J, Mai CT, Mulinare J, et al. ; Centers for Disease Control and Prevention. Updated estimates of neural tube defects prevented by mandatory folic acid fortification—United States, 1995-2011. MMWR Morb Mortal Wkly Rep. 2015;64:1-5. [PMC free article] [PubMed] [Google Scholar]
- 7. Dessie MA, Zeleke EG, Workie SB, Berihun AW. Folic acid usage and associated factors in the prevention of neural tube defects among pregnant women in Ethiopia: cross-sectional study. BMC Pregnancy Childbirth. 2017;17:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Safi J, Joyeux L, Chalouhi G. Periconceptional folate deficiency and implications in neural tube defects. J Pregnancy. 2012;2012:295083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lo A, Polšek D, Sidhu S. Estimating the burden of neural tube defects in low–and middle–income countries. J Glob Health. 2014;4(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Holmbeck GN, Zebracki K, Papadakis JL, Driscoll CFB. Spina bifida. In: Roberts MC, Steele RG, eds. Handbook of Pediatric Psychology. 5th ed. Guilford; 2017:312-322. [Google Scholar]
- 11. Padmanabhan R. Etiology, pathogenesis and prevention of neural tube defects. Congenit Anom (Kyoto). 2006;46:55-67. [DOI] [PubMed] [Google Scholar]
- 12. Cuskelly GJ, McNulty H, Scott JM. Effect of increasing dietary folate on red-cell folate: implications for prevention of neural tube defects. Lancet. 1996;347:657-659. [DOI] [PubMed] [Google Scholar]
- 13. Dolin CD, Deierlein AL, Evans MI. Folic acid supplementation to prevent recurrent neural tube defects: 4 milligrams is too much. Fetal Diagn Ther. 2018;44:161-165. [DOI] [PubMed] [Google Scholar]
- 14. Greene ND, Leung KY, Copp AJ. Inositol, neural tube closure and the prevention of neural tube defects. Birth Defects Res. 2017;109:68-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Haidar J, Melaku U, Pobocik R. Folate deficiency in women of reproductive age in nine administrative regions of Ethiopia: an emerging public health problem. South Afr J Clin Nutr. 2010;23:132-137. [Google Scholar]
- 16. Detrait ER, George TM, Etchevers HC, Gilbert JR, Vekemans M, Speer MC. Human neural tube defects: developmental biology, epidemiology, and genetics. Neurotoxicol Teratol. 2005;27:515-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Blatter BM, van der Star M, Roeleveld N. Review of neural tube defects: risk factors in parental occupation and the environment. Environ Health Perspect. 1994;102:140-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kerr SM, Parker SE, Mitchell AA, Tinker SC, Werler MM. Periconceptional maternal fever, folic acid intake, and the risk for neural tube defects. Ann Epidemiol. 2017;27:777-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu S, Evans J, MacFarlane AJ, et al. Association of maternal risk factors with the recent rise of neural tube defects in Canada. Paediatr Perinat Epidemiol. 2019;33:145-153. [DOI] [PubMed] [Google Scholar]
- 20. Welderufael AL, Berihu BA, Berhe Y, et al. Nutritional status among women whose pregnancy outcome was afflicted with neural tube defects in Tigray region of Ethiopia. Brain Dev. 2019;41:406-412. [DOI] [PubMed] [Google Scholar]
- 21. Yang P, Li X, Xu C, et al. Maternal hyperglycemia activates an ASK1-FoxO3a-Caspase 8 pathway that leads to embryonic neural tube defects. Sci Signal. 2013;6:ra74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zash R, Holmes L, Diseko M, et al. Neural-tube defects and antiretroviral treatment regimens in Botswana. N Engl J Med. 2019;381:827-840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Berihu BA, Welderufael AL, Berhe Y, et al. High burden of neural tube defects in Tigray, Northern Ethiopia: hospital-based study. PLoS One. 2018;13:e0206212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Taye M, Afework M, Fantaye W, Diro E, Worku A. Congenital anomalies prevalence in Addis Ababa and the Amhara region, Ethiopia: a descriptive cross-sectional study. BMC Pediatr. 2019;19:234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Berihu BA, Welderufael AL, Berhe Y, et al. Maternal risk factors associated with neural tube defects in Tigray regional state of Ethiopia. Brain Dev. 2019;41:11-18. [DOI] [PubMed] [Google Scholar]
- 26. Gedefaw A, Teklu S, Tadesse BT. Magnitude of neural tube defects and associated risk factors at three teaching hospitals in Addis Ababa, Ethiopia. Biomed Res Int. 2018;2018:4829023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sorri G, Mesfin E. Patterns of neural tube defects at two teaching hospitals in Addis Ababa, Ethiopia a three years retrospective study. Ethiop Med J. 2015;53:119-126. [PubMed] [Google Scholar]
- 28. Taye K, Bedru A. Pattern of neural tube defects at Tikur Anbessa Hospital, Addis Ababa, Ethiopia. Ethiopian Med J. 2009;47:71-76. [PubMed] [Google Scholar]
- 29. Taye M, Afework M, Fantaye W, Diro E, Worku A. Magnitude of birth defects in central and northwest Ethiopia from 2010-2014: a descriptive retrospective study. PLoS One. 2016;11:e0161998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Taye M, Afework M, Fantaye W, Diro E, Worku A. Factors associated with congenital anomalies in Addis Ababa and the Amhara Region, Ethiopia: a case-control study. BMC Pediatr. 2018;18:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Seyoum G, Adane F. Prevalence and associated factors of birth defects among newborns at referral hospitals in Northwest Ethiopia. Ethiopian J Health Dev. 2018;32. [Google Scholar]
- 32. Atlaw D, Worku A, Taye M, Woldeyehonis D, Muche A. Neural tube defect and associated factors in Bale zone hospitals, southeast Ethiopia. J Preg Child Health. 2019;6:412. doi: 10.4172/2376-127X [DOI] [Google Scholar]
- 33. Munn Z, Moola S, Lisy K, Riitano D, Tufanaru C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int J Evid Based Healthc. 2015;13:147-153. [DOI] [PubMed] [Google Scholar]
- 34. Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54:1046-1055. [DOI] [PubMed] [Google Scholar]
- 35. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177-188. [DOI] [PubMed] [Google Scholar]
- 36. Mekonen HK, Nigatu B, Lamers WH. Birth weight by gestational age and congenital malformations in Northern Ethiopia. BMC Pregnancy Childbirth. 2015;15:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Abdu H, Seyoum G. Prevalence and outcomes of birth defects in newborns of South Wollo and Oromia zones of Amhara regional state: a retrospective study. Ethiopian J Health Dev. 2019;33. [Google Scholar]
- 38. Abebe S, Gebru G, Amenu D, Dube L. Prevalence and patterns of birth defects among newborns in Southwestern Ethiopia: retrospective study. Accessed June 22, 2020 https://www.researchsquare.com/article/rs-8191/v2
- 39. Mitiku TM. Pilot Study to Assess the Antenatal Point Prevalence of Neural Tube Defects and Associated Factors in Pregnant Woman Attending ANC in Ghandi Memorial Hospital in Addis Ababa Ethiopia, August 26-September 10 of 2017 [master’s thesis]. Addis Ababa, Ethiopia: Addis Ababa University; 2017. [Google Scholar]
- 40. Aynalem F. Determinants of Neural Tube Defect Among Children, at Zewditu Memorial Hospital, Addis Ababa, Ethiopia, 2018 a Case Control Study [master’s thesis]. Addis Ababa, Ethiopia: Addis Ababa University; 2018. [Google Scholar]
- 41. Tsehay B, Shitie D, Lake A, Abebaw E, Taye A, Essa E. Determinants and seasonality of major structural birth defects among newborns delivered at primary and referral hospital of East and West Gojjam zones, Northwest Ethiopia 2017-2018: case-control study. BMC Res Notes. 2019;12:495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zaganjor I, Sekkarie A, Tsang BL, et al. Describing the prevalence of neural tube defects worldwide: a systematic literature review. PLoS One. 2016;11:e0151586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Anyanwu LJC, Danborno B, Hamman WO. The prevalence of neural tube defects in live born neonates in Kano, North-Western Nigeria. Sub-Saharan Afr J Med. 2015;2:105. [Google Scholar]
- 44. Fieggen KJ, Fieggen AG, eds. Neural Tube Defects and Folic Acid: An Obligation for Prevention. In House Publications; 2018. [Google Scholar]
- 45. Allagh KP, Shamanna BR, Murthy GV, et al. Birth prevalence of neural tube defects and orofacial clefts in India: a systematic review and meta-analysis. PLoS One. 2015;10:e0118961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pasha YZ, Vahedi A, Zamani M, Alizadeh-Navaei R, Pasha EZ. Prevalence of birth defects in Iran: a systematic review and meta-analysis. Arch Iran Med. 2017;20:376-385. [PubMed] [Google Scholar]
- 47. Atta CA, Fiest KM, Frolkis AD, et al. Global birth prevalence of spina bifida by folic acid fortification status: a systematic review and meta-analysis. Am J Public Health. 2016;106:e24-e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kancherla V, Wagh K, Johnson Q, Oakley GP., Jr. A 2017 global update on folic acid-preventable spina bifida and anencephaly. Birth Defects Res. 2018;110:1139-1147. [DOI] [PubMed] [Google Scholar]
- 49. Barron S. Anencephaly: an ongoing investigation in Washington state. Am J Nurs. 2016;116:60-66. [DOI] [PubMed] [Google Scholar]
- 50. Gong TT, Wu QJ, Chen YL, et al. Changing trends in the prevalence of anencephaly in Liaoning province of Northeast China from 2006-2015: data from a population-based birth defects registry. Oncotarget. 2017;8:52846-52853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bhide P, Kar A. A national estimate of the birth prevalence of congenital anomalies in India: systematic review and meta-analysis. BMC Pediatr. 2018;18:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ghritlaharey RK. A brief review of giant occipital encephalocele. J Neurosci Rural Pract. 2018;9:455-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Toma BO, Shilong DJ, Shwe DD, et al. The prevalence and pattern of central nervous system anomalies in a neonatal unit in a tertiary hospital in Jos, north-central Nigeria. J Med Tropics. 2018;20:63-67. [Google Scholar]
- 54. Federal Democratic Republic of Ethiopia. Ethiopia: key indicators of mini demographic and health survey. Accessed June 22, 2020 https://dhsprogram.com/pubs/pdf/PR120/PR120.pdf
- 55. Brooks D, Asta K, Sturza J, et al. Patient preferences for prenatal testing and termination of pregnancy for congenital anomalies and genetic diseases in Ethiopia. Prenat Diagn. 2019;39:595-602. [DOI] [PubMed] [Google Scholar]
- 56. Copp AJ, Stanier P, Greene ND. Genetic basis of neural tube defects. In: Di Rocco C, Pang D, Rutka JT, eds. Textbook of Pediatric Neurosurgery. Springer; 2017:1-28. [Google Scholar]
- 57. Molloy AM, Brody LC, Mills JL, Scott JM, Kirke PN. The search for genetic polymorphisms in the homocysteine/folate pathway that contribute to the etiology of human neural tube defects. Birth Defects Res A Clin Mol Teratol. 2009;85:285-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sulik KK. Prenatal alcohol exposure and abnormal brain development—findings from basic research. In: Jonsson E, Clarren S, Binnie I, eds. Ethical and Legal Perspectives in Fetal Alcohol Spectrum Disorders: Foundational Issues. Springer; 2018:37-48. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Additional_file_1 for Magnitude and Associated Factors of Neural Tube Defects in Ethiopia: A Systematic Review and Meta-Analysis by Zebenay Workneh Bitew, Teshager Worku, Anmut Alebel and Ayinalem Alemu in Global Pediatric Health
Supplemental material, Additional_file_2 for Magnitude and Associated Factors of Neural Tube Defects in Ethiopia: A Systematic Review and Meta-Analysis by Zebenay Workneh Bitew, Teshager Worku, Anmut Alebel and Ayinalem Alemu in Global Pediatric Health
Supplemental material, Additional_file_3 for Magnitude and Associated Factors of Neural Tube Defects in Ethiopia: A Systematic Review and Meta-Analysis by Zebenay Workneh Bitew, Teshager Worku, Anmut Alebel and Ayinalem Alemu in Global Pediatric Health