Abstract
The use of decellularised matrices as scaffolds offers the advantage of great similarity with the tissue to be replaced. Moreover, decellularised tissues and organs can be repopulated with the patient’s own cells to produce bespoke therapies. Great progress has been made in research and development of decellularised scaffolds, and more recently, these materials are being used in exciting new areas like hydrogels and bioinks. However, much effort is still needed towards preserving the original extracellular matrix composition, especially its minor components, assessing its functionality and scaling up for large tissues and organs. Emphasis should also be placed on developing new decellularisation methods and establishing minimal criteria for assessing the success of the decellularisation process. The aim of this review is to critically review the existing literature on decellularised scaffolds, especially on the preparation of these matrices, and point out areas for improvement, finishing with alternative uses of decellularised scaffolds other than tissue and organ reconstruction. Such uses include three-dimensional ex vivo platforms for idiopathic diseases and cancer modelling.
Keywords: Decellularised scaffolds, decellularisation, acellular matrices, tissue engineering, ECM, cell seeding
Introduction
Tissue engineering was elegantly defined in 1993 by Langer and Vacanti as ‘an interdisciplinary field of research that applies the principles of engineering and life sciences towards the development of biological substitutes that restore, maintain, or improve tissue function’.1 In other words, tissue engineering works on understanding how tissue formation occurs in our bodies in order to develop new functional tissues in the laboratory. Since an increase in life expectancy and organ shortage for transplantation have become global issues, tissue engineering became more relevant than ever.
Scaffolds, cells and molecular cues are the golden triad of tissue engineering being instrumental in developing functional tissues and organs: relevant cells attach to a scaffold, infiltrate it and proliferate to form the new tissue, and molecular cues (such as growth factors, therapeutic ions or cytokines) are often needed to direct cells towards the formation of the desired tissue.1 Tissue engineers and biomaterial scientists use polymers (both natural and synthetic), ceramics or most commonly a combination of them (composites) to develop degradable, functional and/or smart scaffolds to act as a temporary extracellular matrix (ECM) for tissue formation. The ECM is the non-cellular component found in all tissues and organs of our body and provides physical scaffolding support for cells.2–4 It also provides essential biochemical, biophysical and biomechanical signals necessary for tissue morphogenesis, differentiation and homeostasis.2,4 The variety of signals provided by the ECM are detected by a myriad of cell surface receptors, triggering intracellular signalling cascades that result in a number of responses including the expression of relevant genes for the regulation of cellular events such as apoptosis, proliferation or differentiation.4 The molecules that comprise the ECM are secreted by the resident cell types throughout life in both healthy and diseased states, as cells modify the secreted ECM components in response to stimuli such as oxygen and nutrient availability, or mechanical cues.4 The fundamental composition of the ECM is water and proteins, of which collagen is the most abundant, and polysaccharides.2 However, each tissue and organ present an ECM with a distinct composition and topology. For instance, the ECM of tendons mainly comprises collagen type I as well as elastin, glycosaminoglycans (GAGs), and collagen type III in smaller amounts, while 90%–95% of the ECM in cartilage is collagen type II, which also has elastin fibres and proteoglycans.3
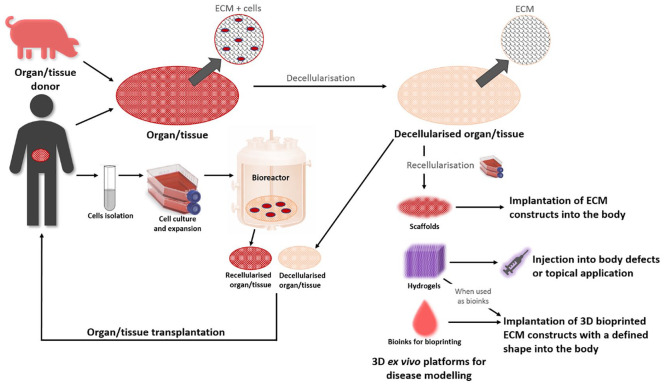
The abovementioned materials have been used to develop scaffolds with a great deal of success but with some limitations. The lack of structural support and composition similar to the ECM of the tissue or organ to be replaced is the main drawback. Therefore, a common strategy among tissue engineers is to use decellularised matrices as scaffolds that offer the advantage of great similarity with the tissue to be replaced.4,5 Decellularisation methods aim to eliminate native cells and genetic material from the ECM while maintaining its structural, biochemical and biomechanical properties. Decellularised tissues and organs can then be repopulated with the patient’s own cells to produce a bespoke therapy4 (Figure 1).
Figure 1.
Conceptual diagram showing the notion behind decellularisation. Decellularised organs or tissues (from human or animal origin, represented by a porcine icon although there are other sources) can be recellularised, for example, with the patient’s own cells to create personalised autologous therapies for organ/tissue transplantation. Recellularised tissue matrices (using various cell sources) can be used for other alternative applications such as three-dimensional (3D) ex vivo platforms for disease modelling. Furthermore, decellularised matrices can be used in bioinks for 3D printing, in hydrogels or in scaffolds to create tissue-engineered constructs.
Although great progress has been made in research and development of decellularised scaffolds, in the authors’ view, much effort is still needed towards preserving the original ECM composition, especially its minor components, assessing its functionality and scaling up for large tissue and organ replacement. Therefore, the aim of this article is to critically review the existing literature on decellularised scaffolds, especially on the preparation of these matrices, and point out areas for improvement, finishing with alternative uses of decellularised scaffolds other than tissue and organ reconstruction.
Criteria and assessment of decellularisation
The major concerns of all decellularisation protocols remain immunogenicity, thrombogenicity and ECM alteration. Moreover, more investigation is needed towards the establishment of the criteria that define successful decellularisation, meaning the achievement of both removal of cellular material and retention of scaffold functionality.
Immunogenicity
The ability of a particular substance or component to elicit an immune response in the human body is defined as immunogenicity. As mentioned, decellularisation aims the complete removal of the cell material of a tissue or organ, leaving behind a structurally and mechanically intact ECM scaffold. The original tissue or organ to be decellularised can be of human or animal (xenogeneic) origin.5,6 In reality, no decellularisation process can remove 100% of cell material.7 Residual cellular materials within the decellularised ECM may contribute to either in vitro cytocompatibility or in vivo immunogenicity. However, it is possible to quantitatively assess cell components such as double-stranded DNA (dsDNA), mitochondria or membrane-associated molecules, for example, phospholipids.
Apart from residual cellular material, antigens are also capable of inducing an immunogenic response and therefore must also be reduced to avoid immunorejection.5,8 Analysing the presence of triggering antigens before implantation in vivo is paramount; the specific components that may be measured include the α-Gal epitope and major histocompatibility complexes (MHC) present on the cell membrane.8 The α-Gal epitope is ubiquitously found in non-primate mammals, marsupials and New World Monkeys.9,10 Although absent in humans, the anti-Gal antibody (~1% of circulating immunoglobulins) is naturally generated in the human species.9,10 Thus, we have a distinct anti-α-Gal reactivity responsible for hyperacute rejection of organs transplanted from α-Gal donors like pigs.9,10 MHC can lead to T-cell and natural killer cell responses.8 While the α-Gal epitope and MHC are immunogenic molecules, some ECM structural components like collagen VI could also have potential immunogenicity.11 Recellularising the ECM with autologous cells and particularly stem cells could help to prevent host rejection: after the recellularisation and maturation of the ECM, the personalised graft would be implanted without needing long-term immunosuppression.12 However, a recellularised ECM would still retain elements of the native composition, and thus a certain degree of an immunogenic response upon implantation can be anticipated.12
Thrombogenicity
Thrombogenicity is defined as the tendency of a material in contact with blood to produce a clot, which can be either a thrombus (fixed clot) or an embolus (travelling through the bloodstream). Thrombogenicity is particularly relevant for in vivo performance of decellularised vascular grafts and whole organs.13 In vitro assessment of thrombogenicity can be carried out with platelet adhesion and activation assays.
It has been established that the exposure of collagen following vascular injury prompts immediate platelet activation that leads to thrombus formation and sealing of the wound.14 Therefore, the exposure of collagen fibres of a cardiovascular decellularised matrix would trigger a strong prothrombotic stimulus.15 It has been shown that thrombogenicity can be reduced by optimising the decellularisation protocol.16,17 Another possible solution could be to cell seed and populate the cardiovascular decellularised matrix prior to implantation.17 In the case of decellularised whole organs, the formation of fibrin clots is a critical issue once implanted/transplanted into a recipient. In normal capillary spaces, endothelial cells are scarce and directly connect the ECM to the bloodstream for oxygen and nutrient exchange with antithrombogenic status. If damaged, the ECM is directly exposed to blood flow, thereby activating the coagulation pathway, leading to fibrin clots and ultimately thrombus formation in vascular networks.
ECM alteration
A key part of the assessment of decellularisation is to evaluate the alteration produced in the decellularised ECM. This evaluation should include compositional, structural and mechanical assays to determine the functionality of the decellularised ECM.
It is well known that collagen is the main structural component of the ECM of body tissues. Depending on the tissue and organ, different collagen types are present in varying proportions.3 Other important structural proteins include elastin, which is a key molecule for tissue elasticity, laminins, a major component of the basal lamina, or fibronectin, a glycoprotein with several functions such as cell adhesion, migration, growth and differentiation. Other important components of the ECM are the GAGs, the long linear polysaccharides consisting of repeating disaccharide units, and proteoglycans (such as fibronectin), which are heavily glycosylated proteins. Ensuring that all these components are present after the decellularisation process is of key importance to maintain the functionality of the ECM. However, many studies mainly focus on collagen and a second component like GAGs. In addition, although content of the structural proteins might be equal or similar to the original tissue, the preservation of functional groups such as attachment sequences should also be investigated. We believe that thorough evaluation of the ECM composition is necessary to conclude that the structural properties of the decellularised ECM are intact. Moreover, other types of analytical techniques that allow for characterisation of the ECM’s architecture should also be included, such as scanning electron microscopy (SEM), transmission electron microscopy (TEM) or atomic force microscopy (AFM) that can allow quantification of architectural parameters such as percentage of porosity, pore size range, fibre diameter or surface roughness.
After the decellularisation process, maintaining the mechanical properties of the native tissue is of key importance to ensure adequate functionality. Mechanical properties of interest include tensile strength, elastic modulus, viscous modulus, stiffness or yield strength. Another parameter to be considered is the anisotropic or isotropic characteristics of the tissue, since they can somewhat control the orientation of the reseeded cells, as it occurs with cardiomyocytes in myocardium regeneration.18 The majority of decellularisation studies tend to look into one or two mechanical parameters; however, we believe that this area should be more thoroughly investigated. The mentioned mechanical properties are in turn regulated by the main ECM structural proteins, namely, collagen, laminin, fibronectin and elastin. Therefore, the assessment of structure and mechanics is intimately related.
Criteria
Crapo et al.6 proposed minimal criteria for elimination of residual DNA and nuclear material that, according to the authors, would be sufficient to validate decellularisation. The proposed criteria suggested that the decellularised ECM should contain less than 50 ng of dsDNA per mg of ECM (dry weight), DNA fragment length should be less than 200 bp, and the absence of nuclear material should be shown in tissue sections stained with 4′,6-diamidino-2-phenylindole (DAPI) or haematoxylin and eosin (H&E).6 Literature also states that elimination of at least 90% of host DNA should be achieved in order to consider the decellularisation process successful.5 While these criteria might be useful to evaluate the extent of cellular removal, further investigation might be needed to determine the threshold for eliciting an immune response after implantation in the host. For example, many commercially available decellularised scaffolds contain DNA fragments.7 A study by Gilbert and co-workers determined the DNA content and fragment length in commercially available decellularised ECM material, showing that most of the analysed materials contained measurable amounts of DNA, which was determined by histological staining, gel electrophoresis or fluorescent probes that bind to dsDNA. Nevertheless, the material with the highest amount of DNA (Restore, DePuy Orthopaedics, porcine small intestine) had 1.13 ± 0.03 ng of DNA/mg dry weight, which is considerably lower than the criteria proposed by Crapo et al. Also, gel electrophoresis showed that the majority of the DNA was fragments in the size range of 100–200 bp,7 consistent with the abovementioned criteria. Interestingly, some reports have shown that even when up to 88% of DNA was left in the ECM after the decellularisation process, a significant in vitro immune response (secretion of tumour necrosis factor-alpha (TNFα) and interleukin (IL)-10 after culturing human acute monocytic leukaemia THP1 cells on the decellularised scaffolds) was not measured.19 Therefore, more clarity may be needed regarding the antigenic threshold for decellularised scaffolds.
Caution should also be exerted regarding the method to evaluate cell removal. A study by Partington et al.20 detected DNA within the mucosal glands of cadaveric tracheas, even though H&E staining suggested complete cellular removal. DNA is quite adherent and can bind to ECM fibres. In the study by Partington et al.,20 the detected DNA was surrounded by intact fibronectin and laminin membranes. Further studies are also needed regarding the effect of freezing–thawing cycles prior to decellularisation, considering that both DNA and ECM proteins might be undergoing chemical/structural modifications, which makes the removal of genomic material more difficult with time.21,22
As seen in the previous paragraphs, efforts have been made towards establishment of criteria for determining successful cellular removal after decellularisation. However, no criteria have been proposed in terms of functionality. We believe that a decellularisation process cannot be deemed successful unless the criteria for both cellular removal and ECM functionality are met. Quantifiable parameters to be considered for functionality criteria should include preservation of the main structural proteins as well as some minor ECM components (e.g., binding sites for growth factors), mechanical properties relevant to the tissue of interest and key architectural parameters for recellularisation.
Methods to produce decellularised matrices
Decellularisation of tissues and organs can be achieved using different chemical, biological and/or physical methods. Different agents and techniques exist within each method category, and the most common and ubiquitous practice is to use a combination of them to achieve decellularisation (Tables 1–3).
Table 1.
Summary of chemical agents used for decellularisation discussed in this review.
| Agent and mode of action | Examples | Description |
|---|---|---|
| Surfactants: Disarrangement of the phospholipid cell membrane, thereby lysing cells |
Sodium dodecyl sulphate (SDS) | – Ionic surfactant – Effective at removing cells and genetic material – Cytotoxic: must be thoroughly washed after the decellularisation process – Damaging to structural and signalling proteins and components: will alter the mechanical properties and prevent the cells from repopulating the tissue |
| Sodium deoxycholate (SD) | – Ionic surfactant – Less damaging and cytotoxic than SDS – Causes DNA agglutination: commonly used in combination with nucleases |
|
| Triton X-100 | – Non-ionic surfactant – Less damaging to tissue structure than ionic surfactants – Ineffective on its own, therefore used in combination with other methods and agents |
|
| CHAPS | – Zwitterionic surfactant – Maintains ECM structural elements – Ineffective on its own, therefore used in combination with other methods and agents |
|
| Acids and bases: Solubilisation of the cell membrane and nuclear material due to their intrinsic charge properties |
Peracetic acid | – Highly corrosive and strongly oxidising nature – Used in combination with other methods and agents – Increases ECM stiffness |
| Alkaline treatment | – Used in combination with other methods and agents |
ECM: extracellular matrix; CHAPS: 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate.
Table 2.
Summary of biological agents used for decellularisation discussed in this review.
| Agent and mode of action | Examples | Description |
|---|---|---|
| Enzymes: Breaking down of nucleic acids and proteins |
DNase | – Nuclease – Breaks down DNA fragments – Used in combination with other methods and agents |
| RNase | – Nuclease – Breaks down RNA fragments – Used in combination with other methods and agents |
|
| Benzoase | – Genetically engineered endonuclease – Degrades DNA and RNA without proteolytic activity – Easily removed by repeated washing – Used in combination with other methods and agents |
|
| Trypsin | – Breaks down cell-matrix adhesions – Used with EDTA – Used in combination with other methods and agents |
EDTA: ethylenediaminetetraacetic acid.
Table 3.
Summary of physical methods used for decellularisation discussed in this review.
| Method | Description |
|---|---|
| Freeze–thaw | – Alternating freezing temperatures with biological temperatures
for an optimised number of cycles, thereby lysing cells – Ineffective at removing cells and genetic material, therefore used in combination with other methods and agents |
| High hydrostatic pressure | – Applying pressures above 600 MPa to dismantle the cellular
membrane – Can be used on its own |
| Supercritical CO2 | – With a critical temperature of 31.1°C and a critical pressure
of 7.40 MPa, supercritical CO2 is compatible with
biological systems – CO2 does not remain within the tissue: extensive washing is not required – CO2 is non-polar; therefore, the addition of a polar entrainer is necessary to remove the polar phospholipid membrane – Can be used on its own |
Chemical methods
These are the most widely used decellularisation methods, and the types of chemicals used as decellularising agents include surfactants, acids and bases. All of these agents offer advantages and disadvantages which are discussed below.
Surfactants
Surfactants act by disarranging the phospholipid cell membrane, thereby lysing cells. They can be ionic, presenting either a positive or a negative electrical charge; non-ionic, when no electrical charge is present; or zwitterionic, when the net charge of the surfactant molecule is zero.
Perhaps the most widely used surfactant is the ionic sodium dodecyl sulphate (SDS) because of its effectiveness in removing cells and genetic material, meaning that it meets the standard requirements of complete cell removal and elimination of at least 90% of host DNA. Numerous examples of types of tissues and organs from both human and animal origin where SDS was successfully used in their decellularisation can be found in the literature, including cornea, liver, heart valve, small intestine submucosa, kidney, vein, lungs and heart.21–28 Interestingly, SDS has also been used on whole organs, that is, lungs in a perfusion mode through the organ’s vasculature, where the blood vessels’ structure was maintained, and the deformation of alveoli was prevented.28 Subsequent seeding of the decellularised lungs with stem cells procured the recovery of the lungs’ original function. Nevertheless, it has been shown that SDS can be damaging to structural and signalling proteins and components, which will alter the tissue’s original mechanical properties and prevent the cells from repopulating the tissue.16,21 In the specific case of thin tissues and cell sheets, this damaging effect caused by SDS can be particularly evident. For example, an 80% and 62% decrease in elastic and viscous modulus, respectively, was observed for fibroblast cell sheets that were treated with a high concentration (0.5 wt%) of SDS solution.19 Another disadvantage of SDS is that it is cytotoxic, and therefore, it must be thoroughly washed after the decellularisation process. Since SDS is an ionic surfactant, it is more difficult to remove using typical solutions like phosphate-buffered saline (PBS), thus requiring a rigorous washing protocol.
Another ionic surfactant commonly used is sodium deoxycholate (SD), which, compared with SDS, is non-damaging. A study by Syed et al.25 on decellularisation of small intestine submucosa showed that a protocol using SDS/Triton X-100 surfactants yielded reduced metabolic activity due to the cytotoxic effect of residual agents compared to using SD. A disadvantage of SD is that it can cause DNA agglutination on the tissue’s surface, which could be prevented by combining the SD surfactant with deoxyribonuclease I (DNase I) enzyme that breaks down DNA fragments. However, a significant amount of DNA can still remain after this treatment, so a solution is to use additional SD/DNase I treatment cycles. For instance, in a study by Piccoli et al.29 on decellularisation of a diaphragmatic muscle from wild-type mice, a 95% decrease in native genetic material after three cycles of SD/DNase I was reported. Moreover, the authors reported a well-preserved skeletal muscle matrix with intact myofibres and protein composition and distribution similar to fresh tissue.29 The preservation of muscle fibres was key to maintain the matrix’s structure, biomechanical properties and elasticity.29 In an earlier study by Partington et al.20 using whole cadaveric tracheas harvested from male pigs, the authors used 25 cycles of SD/DNase I, after which the decellularised trachea retained the gross anatomical structure of native trachea while appearing completely acellular in the submucosa region. Nevertheless, the presence of chondrocytes was still observed and DNA was still detected, bound to ECM fibres in the lamina propia and within mucosal glands. Laminin and fibronectin, which are important for cell attachment, migration and revascularisation, were present after the decellularisation process: laminin was localised to basement membrane structures, while fibronectin was seen throughout the whole lamina propria.20 On the contrary, collagen type II, soluble collagen and GAG underwent a decline throughout the decellularisation process.20
Triton X-100 (t-octyl phenoxy polyethoxy ethanol) is an example of a non-ionic surfactant that is usually combined with SDS to aid in the wash process. It has also been combined with other chemical agents, such as in the study by Mendoza-Novelo et al.30 where decellularisation of bovine pericardium was achieved by treatment with Triton X-100, tridecyl polyethoxy ethanol (ATE), alkaline treatment and subsequent addition of nucleases (DNase/RNase). The amount of residual DNA content and the absence of nuclear structures suggested effective cell removal. However, the native tissue GAG content decreased and an alteration in the tissue stress relaxation properties was measured after alkaline treatment. The authors concluded that the decellularisation process preserved the collagen network, anisotropy, tensile modulus and strength, and maximum strain at failure of native tissue.30 In another study combining Triton X-100 with SD and DNase/RNase, Greco et al. created an acellular porcine vaginal matrix that could be used for vaginal augmentation and cloacal repair.31 Importantly, the authors reported retention of the ECM’s collagen and elastin, 50% of GAGs and an intact base membrane (positive for laminin and collagen IV).31 The decellularised scaffolds were seeded with adipose-derived stem cells (ADSCs) and vaginal epithelial cells showing attachment and growth.31 This study highlights the importance of combining different chemical agents to obtain efficient decellularisation.
Finally, an example of zwitterionic surfactants is 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate or CHAPS, which has been used in the perfusion decellularisation of rat, porcine and human lungs along with SDS and SD.28 The decellularised lungs displayed comparable loss of DNA, while the greatest preservation of ECM components was observed with the use of SDS. The authors measured the highest level of soluble collagen in SDS decellularised lungs, while a decreased amount was found using SD and CHAPS when compared with control tissue. A higher number of collagen and laminin peptide counts were measured with SDS. Moreover, the highest level of myosin components remained in SD decellularised lungs, while CHAPS-decellularised lungs had a higher level of cytoplasmic proteins, which suggested incomplete decellularisation.28
Acids and bases
The mode of action of acids and bases is solubilisation of the cell membrane and nuclear material due to their intrinsic charge properties. In many instances, acid and bases are used in combination with other decellularisation agents such as surfactants. For example, in the previously cited study by Mendoza-Novelo et al.30 regarding the decellularisation of bovine pericardium, surfactants (Triton X-100 and ATE) and calcium oxide alkaline solution were used.
Peracetic acid, which is used for sterilisation because of its highly corrosive and strongly oxidising nature, has been used as a decellularisation agent in a number of studies.25,32,33 Gilbert et al.32 evaluated the collagen fibre alignment and biaxial mechanical behaviour of decellularised porcine urinary bladder tunica mucosa and basement membrane, and urinary bladder submucosa. Specimens were decellularised by immersion in 0.1% (v/v) peracetic acid, 4% (v/v) ethanol and 96% (v/v) deionised water. As a result of differences in collagen fibre alignment, a stiffer ECM was produced in the longitudinal direction, suggesting an alteration in ECM function after treatment with peracetic acid.32 Therefore, the use of this acid may not be ideal for tissues and organs that require expandability and compliance.
Other acids that have been used as decellularisation agents are formic acid, acetic acid and citric acid. For example, Lin et al.34 used these three acids in the decellularisation of inguinal and popliteal lymph nodes harvested from Lewis rats.
Biological methods
Decellularisation approaches can be optimised by adding enzymatic treatments to aid in the removal of unwanted cellular and genetic components. Ultimately, their efficacy depends on maintaining the key ECM features needed in regenerating the desired functions of the target tissue or organ.
Enzymes
We already mentioned the use of nucleases, that is, DNase I and RNase I, in combination with the surfactant SD to avoid DNA agglutination.32 A recent and novel example by Dong et al.35 reported the use of supernuclease, a homologous nuclease of benzoase which is a genetically engineered endonuclease that can efficiently degrade DNA and RNA without proteolytic activity and is easily removed by repeated washing. In this study, the authors used supernuclease in combination with the surfactant N-lauroyl glutamate in the decellularisation of porcine corneal stroma. Reported results showed efficient removal of xenoantigen DNA within 3 h, retention of the ultrastructure, transparency and mechanical properties. The decellularised porcine corneas were then implanted in a rabbit model, and after 1 month, they showed no immune rejection and favourable transparency.35 Therefore, the decellularisation method described by Dong et al.35 offers an alternative to the shortcomings of the traditional methods used in the decellularisation of corneas, that is, long elution times for the removal of xenogenic components leading to over-swelling and reduced transparency. Another example of the use of benzoase in decellularisation protocols is the study by Khan and Bayat on the microarchitectural analysis of decellularised unscarred and scarred dermis, which they combined with a variety of chemical agents.36
Another enzyme that is often included in decellularisation protocols is trypsin, commonly used with the chelating aminopolycarboxylic acid ethylenediaminetetraacetic acid (EDTA). Trypsin/EDTA breaks cell–matrix adhesions and is routinely used in cell culture techniques. Zhou and colleagues used four different decellularisation protocols for porcine heart valves: (1) SD, (2) SDS, (3) trypsin/EDTA and (4) trypsin/EDTA–Triton X-100–phenylmethylsulfonyl fluoride (PMSF)–DNase/RNase. Results showed that SD enabled cell removal with an almost complete preservation of the ECM structures, as measured by two-photon laser scanning microscopy. However, the authors found that the four protocols affected immunogenicity and increased thrombogenicity.24 In contrast, a more recent study by Giraldo-Gomez et al.37 used trypsin/EDTA in cyclical tracheal decellularisation with no significant alteration or degradation in the components of the ECM. An interesting study by Purpura et al.38 described the use of trypsin for the decellularisation of human foreskin. The authors successfully combined trypsin with a cryofreezing method, thereby offering a regenerative approach for the reconstruction of foreskin in circumcised males.38
Physical methods
As discussed in the previous sections, the use of chemical and biological methods raises the concern of toxicity of the chemical agents used in the decellularisation process and destruction of major and minor ECM components. Therefore, other methods are being developed that do not involve the use of harsh chemicals. These methods use physical principles to lyse cells and destroy cell–matrix adhesive proteins, and a washing step is needed to remove cellular debris. The most common physical methods are freeze–thaw, high hydrostatic pressure and supercritical carbon dioxide.4
Freeze–thaw
Freeze–thaw involves alternating freezing temperatures (~ −80°C) with biological temperatures (~37°C) for an optimised number of cycles, although protocols can be tailored by modifying the temperature difference and/or number of freeze–thaw cycles.39,40 Elder et al.40 used one freeze–thaw cycle followed by exposure to 2% SDS with RNase/DNase-EDTA to achieve decellularisation of cartilage explants isolated from the distal femur of 1-week-old male claves. The authors found that exposure to 2% SDS for 8 h yielded complete decellularisation but mechanical properties were decreased, while exposure for 2 hours kept the mechanical properties but had a minimal effect on removal of DNA content.40 Therefore, it was concluded that the protocol used should be optimised to achieve complete cell removal, while maintaining mechanical properties. In the previously cited study by Xing et al. on decellularisation of fibroblast cell sheets, the authors investigated the use of a freeze–thaw cycling method, which maintained the ECM structure and mechanical strength but preserved about 88% of DNA. Interestingly, in vitro inflammatory tests suggested that the amount of DNA left would not cause a significantly higher immune response, which opens questions regarding the threshold for eliciting an immune response, as mentioned before in this review.19 Nevertheless, freeze–thaw on its own is ineffective at removing cells and genetic material; therefore, it is generally used in combination with other methods and agents.
High hydrostatic pressure
This physical method has become increasingly popular and works by applying pressures above 600 MPa to disrupt the cellular membrane.5 Unlike the methods discussed so far,41 high hydrostatic pressure can be used on its own. An example is the study by Hashimoto et al.,42 who used high hydrostatic pressure to decellularise porcine corneas. The corneas were treated at 980 MPa, and H&E staining confirmed complete removal of corneal cells. The decellularised corneas were implanted into rabbits, and no immune reaction was reported. Importantly, the turbid corneas became clear after implantation.42 The same group used high hydrostatic pressure in the decellularisation of porcine aortic blood vessels, which did not alter the mechanical properties of the decellularised vessels. Xenogenic transplant experimentation showed reduced inflammation following implantation of the decellularised vessels, while allogenic transplantation showed that they tolerated arterial blood pressure with no clot formation on the luminal surface.43
Supercritical carbon dioxide
Supercritical fluids have liquid-like density and gas-like diffusivity, and with a critical temperature of 31.1°C and a critical pressure of 7.40 MPa, supercritical CO2 is compatible with biological systems.5,44 In terms of decellularisation, the main advantage of this physical method is that the CO2 does not remain within the tissue, and therefore, extensive washing is not required.5 However, CO2 is non-polar, so the addition of a polar entrainer such as ethanol is necessary to remove the polar phospholipid membrane of cells.44 Supercritical CO2 has been used in the decellularisation of rat heart tissues, porcine corneas, porcine and bovine pericardium, and human adipose tissue.45–48 All the cited articles reported successful decellularisation with preservation of structural and mechanical properties, thus making supercritical CO2 a promising method.
Modification of decellularised matrices
As we and others have reviewed, the current decellularisation methods are not perfect, and some degree of damage to the ECM is always incurred.5 Therefore, many researchers have explored the possibility of modifying or priming the decellularised scaffolds with growth factors or ECM components to restore the functions damaged during the decellularisation process.
Wu et al.49 prepared a xenogeneic decellularised scaffold from pig peritoneum. The scaffold was combined with hyaluronic acid (HA), a GAG present in various tissues of our body, and two different concentrations of basic fibroblast growth factor (bFGF). The primed scaffold was investigated for its use for the repair of skin wounds. Results in vitro showed that HA enhanced bFGF adsorption to the scaffolds and slowed its release. Using a rabbit model, wounds covered with scaffolds containing 1 μg/mL bFGF had higher wound healing rates on days 6, 11, and 14 after surgery than non-primed scaffolds. Wounds covered with scaffolds containing 1 μg/mL bFGF showed more dermis regeneration than the other wounds. No significant differences in wound healing rates and dermal thickness between wounds covered with scaffolds containing 1 and 3 μg/mL bFGF on days 3, 6, 11, and 14 after surgery were observed. Thus, the authors showed the potential of the primed decellularised scaffolds for skin tissue engineering.49 Similarly, both Lee et al.50 and Xu et al.51 have shown the improved outcome of decellularised scaffolds coated with conjugated growth factors: platelet-derived growth factor (PDGF) conjugated with heparin and bFGF encapsulated by thermo-sensitive gel and conjugated with heparin, respectively (Figure 2). In a last example by Assmann et al.,52 the surface coating of decellularised rat aortic conduits with fibronectin accelerated autologous in vivo endothelialisation and showed a significantly increased medial recellularisation.
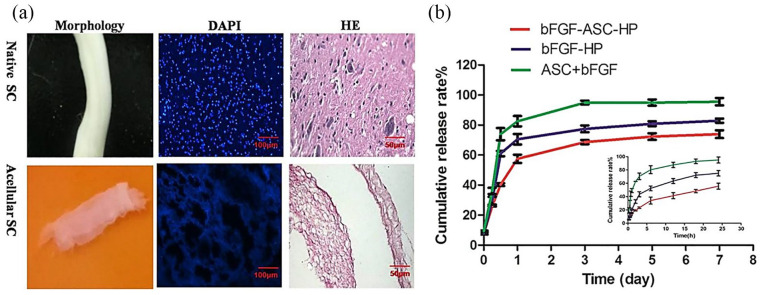
Figure 2.
(a) Decellularisation of spinal cord (SC) harvested from adult female Sprague Dawley rats using 1% Triton X-100% and 4% sodium deoxycholate (SD). Acellular SC (ASC) was transparent and with a bulk shape similar to that of native SC. 4′,6-diamidino-2-phenylindole (DAPI) and haematoxylin and eosin (H&E) staining did not show any cellular components in the acellular SC. (b) In vitro release of basic fibroblast growth factor (bFGF) from three different complexes created using ASC and heparin-modified poloxamer (HP). The bFGF–ASC complex released bFGF rapidly because of a lack of three-dimensional (3D) network in the ASC scaffold. A slower bFGF release from the HP hydrogel matrix was observed whether bFGF was combined with ASC or not (©Xu et al.51 Article distributed under a Creative Common Attribution License CC BY 4.0).
The above studies are examples of how modifying or priming decellularised scaffolds can overcome the damage resulting from the decellularisation process and create functional scaffolds for tissue and organ reconstruction. A disadvantage of this strategy might be a potentially more difficult regulatory pathway for licensing of the product for clinical use by the competent agencies, such as the Food and Drug Administration (FDA).
Bioinks with decellularised matrices for three-dimensional printing
Additive manufacturing techniques, including three-dimensional (3D) printing, offer an exciting prospect in the tissue engineering field, as scaffolds with pre-determined shapes and implants that perfectly fit a defect can be fabricated using these techniques.53,54 Furthermore, bioprinting aims to print and pattern cells and ECM material in three dimensions to generate structures similar to tissues and organs.55 An important consideration in bioprinting is that the printing process must be cytocompatible, thus restricting the choice of materials that can operate in an aqueous or aqueous gel environment. Current materials of choice are gelatin, chitosan, alginate, collagen, HA or fibrin among others.54,56 However, there are some concerns over these materials, such as the use of harsh cross-linking agents.57,58 Another important limitation is that the majority of the matrix materials used as bioinks so far for bioprinting cannot represent the complexity of the natural ECM. Therefore, researchers have been investigating decellularised ECM as bioinks.57–62 Some of the reported bioinks are derived from oesophageal, adipose, cartilage, heart, cornea or liver tissue.
For example, Pati et al.57 developed a method for bioprinting of cell-laden constructs with novel decellularised ECM bioinks, including adipose, cartilage and heart tissues. Their proposed method consisted of different steps, starting with the decellularisation of the ECM material using a combination of enzymatic, chemical and physical methods. The decellularised ECM was solubilised to a final concentration of 3%, adjusting the pH to physiological values before encapsulating cells maintaining the temperature below 10°C. The pH-adjusted solution remained as a liquid solution at temperatures below 15°C and gelled when incubated at 37°C for 30 min. After gelation, the decellularised matrix gel retained its shape and form, a prerequisite for biofabrication of cell-printed constructs. Subsequently, 3D open porous structures of decellularised ECM with polycaprolactone (PCL) as a framework were printed using a multi-head tissue/organ building system, which could position one or more polymer or hydrogel or both at specific locations (Figure 3). A cell viability greater than 90% was observed over 14 days with active cell proliferation. Furthermore, the authors evaluated tissue formation with immunohistology. Chondrogenic differentiation of human inferior turbinate-tissue-derived mesenchymal stromal cells in constructs printed with decellularised cartilage ECM was observed, where the cells synthesised collagen type II within the construct. The maturation of myoblasts in constructs printed with decellularised heart ECM was investigated by cardiac myosin heavy chain (β-MHC) staining and compared with that of collagen, which is abundantly available in the myocardium. Results showed that cells expressed a higher level of β-MHC than collagen in the construct. Adipogenic differentiation of human ADSCs in the constructs printed with decellularised adipose ECM was confirmed by substantial expression of peroxisome proliferator–activated receptor gamma (PPARγ) and collagen type IV. The authors concluded that their bioprinted constructs could be used in tissue engineering applications such as in vitro disease models and drug screening.57
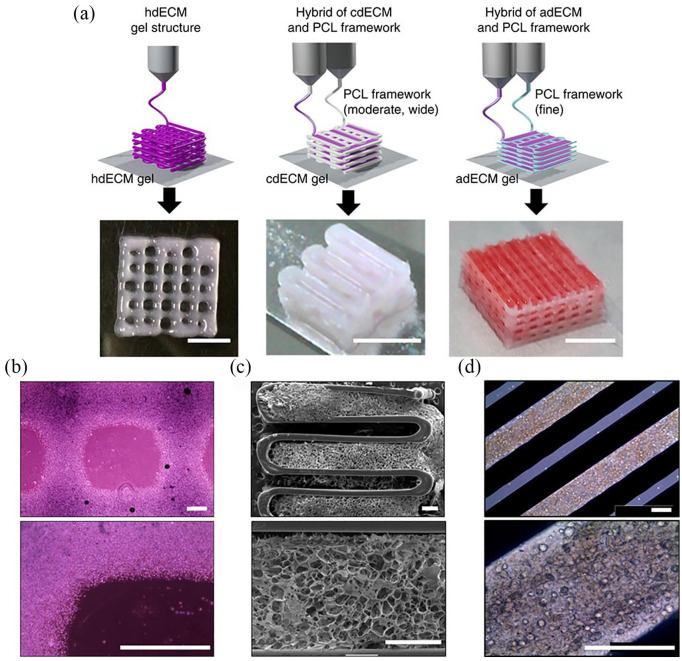
Figure 3.
(a) Construct printed with decellularised heart (hdECM), cartilage (cdECM) and adipose (adECM) tissues in combination with a polycaprolactone (PCL) framework (scale bar = 5 mm). (b) Representative microscopic images of hdECM construct (scale bar = 400 μm). (c) Scanning electron microscopy (SEM) images of cdECM construct (scale bar = 400 μm). (d) Microscopic images of adECM construct (scale bar = 400 μm). (©Pati et al.57 Article distributed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License CC BY-NC-ND 3.0).
Due to an ageing population and increased cases of laser-based surgeries, the waiting time for a donor cornea has increased. While artificial corneas were developed to meet the increase in demand, they have shown limited tissue integration resulting in material deterioration. Therefore, Kim et al.58 introduced a cornea-derived decellularised ECM as a bioink for corneal regeneration. The authors used bovine eyeballs as the source material, and they were decellularised using chemical methods. The developed bioink had similar quantitative measurement results for collagen and GAGs compared with native corneas and had the required transparency for allowing vision, as it provided over 75% in the visible spectrum of light. The differentiation potential of human turbinate–derived mesenchymal stem cells to a keratocyte lineage was observed in the developed bioink, which did not have a cytotoxic effect on encapsulated cells for 3D culture. Biocompatibility was further studied by xeno-implantation into mice and rabbits for 2 and 1 month, respectively. In vivo safety similar to clinical-grade collagen was seen with the developed bioink, which helped to maintain the keratocyte-specific characteristics in vivo, compared with collagen. To assess the design flexibility of the bioink, lattice pattern structures were 3D printed, which maintained their printed pattern after cross-linking, with no dead cells observed in the printed structure. The authors concluded that the developed bioink could potentially be used in various types of corneal diseases.58
The approach of using decellularised ECM tissues in bioinks is a growing research trend that has great potential. However, as with any biological-derived material, batch-to-batch variations in decellularised ECM are expected, and therefore in the bioinks made from them.59 On the contrary, ECM is very resistant to degradation, which allows cadavers to be used as potentially unlimited sources of tissue not only for bioinks but also for decellularised scaffolds in general.59
Hydrogels with decellularised matrices
Hydrogels are 3D networks of hydrophilic polymers that can swell and hold a large amount of water while maintaining their structure as a result of chemical or physical cross-linking of individual polymer chains.63,64 These materials also present a degree of flexibility that is very similar to natural tissues, thus the extensive research into hydrogels for tissue engineering applications.65 Hydrogels made of natural materials like fibrin, alginate or silk fibroin have been used as scaffolds for cells promoting cell growth and maturation.65 Hydrogels containing or made of decellularised ECM retain tissue-specific biochemical cues that are of key importance for organ and tissue function.66,67 As some decellularised tissues present a limited potential for recellularisation,66 a potential solution is to transform decellularised tissues into hydrogels, where cells can be encapsulated throughout their structure. These cell-containing hydrogels can then be injected for minimally invasive delivery into irregular spaces,68–70 applied topically or used for 3D printing or electrospinning.57,71
Some of the medical conditions for which decellularised ECM-based hydrogels have been investigated include type 1 diabetes,70 myocardial infarction by replacing damaged cardiac tissue,68,69 peripheral artery disease,72 skin wound healing,73 keratoconus by bioprinting a corneal stromal substitute,71 acute liver failure,74 chronic fibrotic diseases75 and inflammatory bowel disease.76 The impact of the decellularisation method on the ECM-derived hydrogel has been shown by several studies.66,77 For example, Fernández-Pérez and Ahearne66 examined the impact of different decellularisation protocols on hydrogels prepared from porcine corneas that were isolated and decellularised with SDS, Triton X-100 or freeze–thaw cycles. While all the three methods showed significant reduction of DNA, the SDS method produced cytotoxic hydrogels. On the contrary, the other two methods produced cytocompatible hydrogels.66
A disadvantage of ECM-derived hydrogels is the poor self-supporting ability due to low viscosity and mechanical properties, thereby hindering the possibility of making large and complex 3D structures with hydrogels.67,73 An interesting and novel example by Yi et al.67 described a new process for rhinoplasty, integrating tissue engineering and 3D printing approaches. The authors generated an engineered nasal cartilage implant by injecting cartilage-derived hydrogel containing human ADSCs into the 3D-printed implant containing an octahedral interior architecture. The cartilage-derived hydrogel was prepared from hyaline cartilages collected from porcine knee parts that were decellularised by using freeze-drying, enzymatic and detergent methods, followed by lyophilisation and grinding. The resulting powder was solubilised and neutralised to obtain the cartilage-derived hydrogel, which was mixed with alginate and cell suspension and injected into the assembled implant (Figure 4). The authors observed significantly higher expression levels of early chondrogenic differentiation markers (SOX9, ACAN, COL21A) in the human ADSCs grown in the engineered nasal cartilage with the cartilage-derived hydrogel compared to an alginate-only hydrogel. The engineered nasal cartilage was implanted into a mouse subcutaneous defect and exhibited maintenance of shape and structure, and formation of cartilaginous tissues for 12 weeks. The authors concluded that the developed process combining computer-aided design, 3D printing, and tissue-derived hydrogel could be also used in generating implants of other tissue types.67
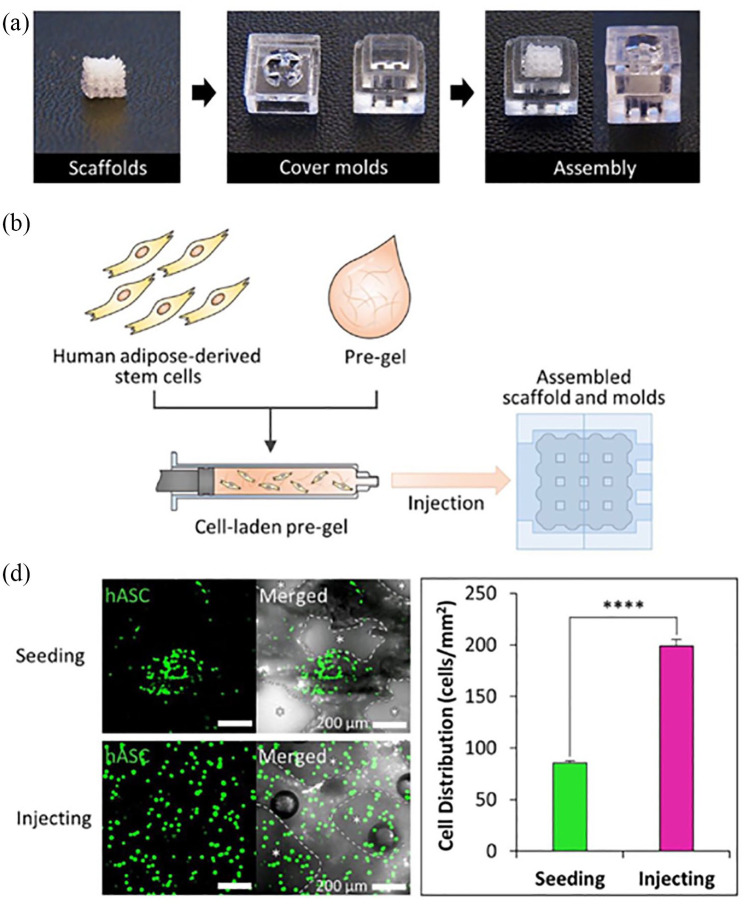
Figure 4.
(a) Assembly of cover moulds and scaffolds for cell-laden hydrogel injection. (b) Scheme of the cell-laden hydrogel pre-gel injection procedure. (c) Calcein AM staining of each scaffold after cell seeding and injecting (scale bar = 200 μm) and quantification of cell distribution in each scaffold (n = 3 per experimental group, ****p < 0.0001). (©Yi et al.67 Article distributed under a Creative Common Attribution License CC BY 4.0).
Bare versus recellularised matrices
The product obtained after the decellularisation process is a bare ECM that should retain the composition, structure and mechanical characteristics of the native ECM. Furthermore, bare decellularised matrices offer a scaffold for recellularisation, which can happen prior or post-implantation.4 Implanted bare scaffolds will be infiltrated by the patient’s own cells that in time will replace the decellularised matrix with the newly deposited ECM.52,78,79 Nevertheless, this could be a slow process that could lead to scaffold collapse and loss of structure and function, particularly for large scaffolds or whole organs. Therefore, many researchers aim at recellularising the bare matrices prior to implantation.80,81 The seeded cells will re-populate the scaffold and deposit new ECM molecules throughout the matrix, thereby preserving its structure and function.12 Furthermore, seeding the scaffolds with the patient’s own cells will create an autologous construct, offering a potentially immunologically inert therapy.12 However, seeding and culturing decellularised matrices has challenges such as choice of cell source(s) or technical matters like the seeding and culturing methods themselves.12,80 Especially for large scaffolds and whole organs, the technical difficulties can be quite challenging and the use of culturing systems such as bioreactors inevitable.12,80 Another issue is that the time for expanding the cells in order to reach an acceptable number per cm3 of tissue might imply higher costs and delay in the implantation, which may cause distress to the patient and high costs to the health system. Some studies have shown that decellularised grafts alone can lead to satisfactory clinical results,82 and therefore, careful consideration as to whether the decellularised matrix needs to be repopulated with relevant cells should be exercised.
Cell sources
In the field of tissue engineering, the question of which cell source is to be used and seeded onto decellularised scaffolds is still debatable.80 The cell sources used in tissue engineering are embryonic stem cells, foetal stem cells, somatic differentiated cells, adult stem cells, and differentiated cells; however, stem cells are preferable because of their potential to differentiate into a variety of cell types and tissues.
Table 4 shows a brief overview of the stem cell types used in tissue engineering and regenerative medicine.83 Embryonic cells are totipotent, that is, they can develop into all types of cells present in the organism; however, their setback is that their harvest is limited because of ethical and legal issues. In contrast, adult stem cells are present in every tissue of the body, can be pluripotent or multipotent and differentiate into many cell types, given that they encounter specific target tissue environment. One of the subtypes of adult stem cells that is gaining interest in tissue engineering is ADSC, given that they have the ability to differentiate into various types of cells, their abundant resources and relatively easier harvest compared to other cell types.83 A study by Ross et al.84 in decellularised rat kidney scaffolds showed that seeding the scaffolds with embryonic stem cells had the potential for the cells to develop into any adult renal cell type and form an organ ex vivo, when compared to other sources of stem cells, as they may not be able to differentiate to all types of adult renal somatic cells.
Table 4.
Summary of sources and potency of stem cells used in tissue engineering and regenerative medicine.83
| Stem cell type | Source | Potency | Definition |
|---|---|---|---|
| Embryonic | Morula | Totipotent | – Have the ability to develop into all types of cells present in the organism |
| Blastocyst | Pluripotent | – Can create any tissue in the body except the placenta | |
| Foetal | Foetus | Multipotent, pluripotent | – Able to differentiate only to a limited number of
specialised cell types – Pluripotent as above |
| Extrafetal tissues: amniotic fluid, umbilical cord | Multipotent, pluripotent | – As above | |
| Adult | Bone marrow, adipose tissue, skin, blood, skeletal muscle, heart, liver, and so on | Multipotent, pluripotent, oligopotent, bipotent or unipotent | – Pluripotent and multipotent as above – Oligopotent: able to form two or more mature cell types – Bipotent: ability to develop into two types of cells – Unipotent: can differentiate along only one lineage |
| Induced | Somatic differentiated cells | Pluripotent | – As above |
Arguably, determining the optimum cell type for each scaffold and clinical application is still debatable as there are multiple factors that play a role in integrating stem cells into biological scaffolds and differentiating them into the desired cells including the bioavailability, mechanical and architectural characteristics of the scaffold used.
Bioreactors
Bioreactors are devices that are used in tissue engineering to provide a controlled and prespecified set of environmental and operating conditions, for which biological and biochemical processes can be developed.85–87 The use of bioreactors can influence certain processes during the recellularisation of decellularised scaffolds, including cell seeding, improved mixing of nutrients throughout the medium and applying mechanical forces to accelerate regeneration.88 Cell seeding using bioreactors has shown the ability to distribute cells within a scaffold in high density and uniformly.85–87 Bioreactors can also improve the mixture of nutrients and oxygen throughout the medium compared to static culture, thus aiding in limiting tissue hypoxia seen under static conditions. The application of mechanical forces on scaffolds by the bioreactors can mimic the forces applied on native tissue, which has demonstrated accelerated tissue regeneration in vitro.85–87 Different types of bioreactor systems exist, such as perfusion-based, stirred flasks, rotating wall vessels or multi-pass filtration, among others. Numerous examples can be found in the literature regarding the use of various bioreactor systems for the recellularisation of decellularised scaffolds.81, 88–90
A recent example is the work by Talò et al.90 who used an oscillating stretch-perfusion bioreactor that combined bidirectional perfusion with programmable, uniaxial strain to functionalise cell-seeded decellularised tendons. Decellularised tendon matrices from adult horses were seeded on their surfaces and within the tendon fibres with rabbit bone marrow mesenchymal stem cells and cultured in the bioreactor system for 7 days. Results showed viable cells that were homogenously distributed on the surface of the constructs and a superior production and organisation of newly formed collagen fibres compared to constructs cultured under static conditions.90 Another example is the study by Yazdani and colleagues who found that cyclic bioreactor preconditioning (flow and pressure) accelerated the formation of a significant muscular layer on decellularised porcine carotid artery scaffolds, particularly on adventitia-denuded scaffolds. Moreover, the vascular smooth muscle cells layer of bioreactor preconditioned vessels could mobilise calcium in response to cellular depolarisation.91
Finally, recent advances in the development of bioreactor systems focus not only on building automated systems but also on the development of systems that incorporate biosensors. Therefore, the system detects any changes (e.g., temperature, pH and metabolites) in the biochemical culture of the engineered scaffold and relays it back to the bioreactor to adjust accordingly, resulting in consistent optimal tissue growth environment.92,93
Use of decellularised scaffolds as 3D ex vivo platforms
Since decellularised tissues possess the native ECM components and structure, they could be of great value in applications that require 3D ex vivo platforms, such as disease modelling to study their progression and new targets, as well as screening and investigation of drugs/therapeutics.75,76,94
Such application can be very relevant in cancer, where the biological activity of cancerous cells is not only affected by physicochemical changes in the ECM, but also they can alter the ECM by, for example, applying mechanical forces for expansion or by secreting enzymes that promote cancer spread. A tumour comprises a microenvironment that undergoes remodelling following extracellular, intercellular and intracellular signals. With the increasing understanding of the tumour microenvironment, numerous studies that investigate cell signalling, gene and small-molecule expression, and drug screening use 3D tissue culture models. Research so far shows that cancer cells grown in 3D cultures exhibit different morphologies, migration and proliferation capacities, and higher resistance to anticancer drugs compared to those grown on flat surfaces. At the moment, the most popular 3D models are spheroids and scaffolds.95–97 The drawbacks of spheroids are that they have inconsistent formation, handling difficulties and absence of ECM features. As mentioned before, there is a variety of materials used in scaffolds; however, not all of them may resemble or recapitulate the ECM features as accurately as the decellularised scaffolds. Therefore, decellularised scaffolds are being investigated in cancer research, and they can be prepared from normal or diseased tissues.98 An example is a study by Rijal and Li where they introduced a versatile 3D tissue matrix scaffold system for tumour modelling and drug screening.99 The authors used decellularised mammary or muscle tissues isolated from nonobese diabetic (NOD)/severe combined immunodeficient (SCID) female mice (8–12 weeks old) that supported cancer cell line survival, proliferation, migration, and invasion in culture and vascularised tumour formation after implantation into the mammary fat pads of the 8-week-old female NOD/SCID mice99 (Figure 5). Another example is the work by Liu et al.100 where decellularised human breast cancer biopsies were seeded with MCF-7 breast cancer cell line showing increased cell migration, proliferation and epithelial-to-mesenchymal transition. When treated with 5-fluorouracil, an antineoplastic agent to treat multiple solid tumours including breast, expression of stem cell markers was maintained in the recellularised scaffold with decreased apoptosis rates compared to monolayer cells. The authors concluded that the decellularised breast scaffold model would help to simulate the pathogenesis of breast cancer in vitro.100 A last example reported by Mollica et al.101 described a novel mammary-specific culture combining a self-gelling hydrogel solely composed of decellularised rat or human breast tissue ECM with a 3D bioprinting platform. The authors were able to show that large organoids/tumoroids could be established in the mammary-derived hydrogel.101 These studies show the great potential of decellularised scaffolds for the study of cancer and disease progression as well as the screening of new therapies.
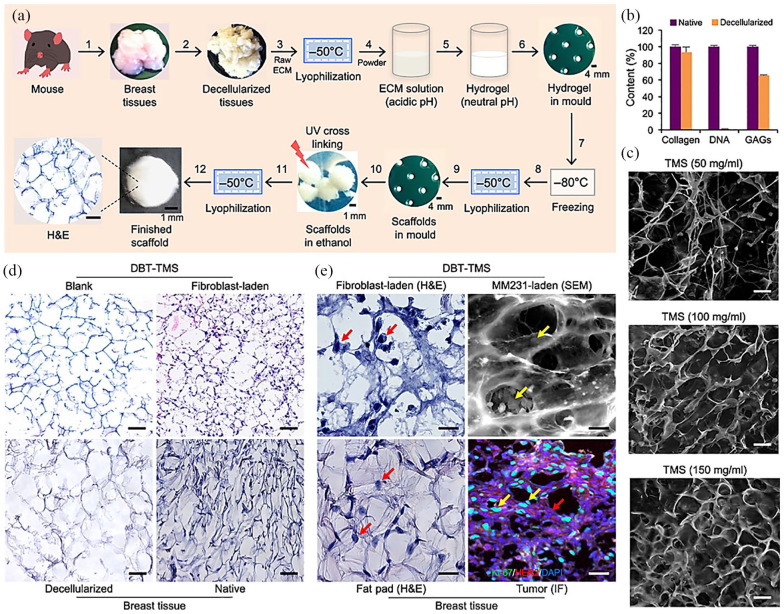
Figure 5.
Overview of the study by Rijal and Li where they introduced a versatile three-dimensional (3D) tissue matrix scaffold system for tumour modelling and drug screening. (a) Workflow of scaffold fabrication. (b) DNA, collagen and glycosaminoglycan (GAGs) composition of the decellularised tissues compared with native ones. (c) Scaffold porosity under scanning electron microscopy (SEM; TMS = tissue matrix scaffold). (d) Blank versus fibroblast-laden scaffolds and breast tissue (DBT = decellularised breast tissue). (e) Occupancies of cells grown inside the scaffolds compared with native cells that lived in mouse breast tissues. Scale bars, 100 μm (c to e). (©Rijal and Li.99 Article distributed under a Creative Common Attribution License CC BY 4.0).
Idiopathic diseases such as inflammatory bowel diseases (e.g., Crohn’s disease and ulcerative colitis) could also be modelled using decellularised matrices. The specific causes of inflammatory bowel diseases are not yet known, although genetic predisposition and immunological factors are contributing elements.102 These diseases are characterised by defects in intestinal epithelial cell barrier function and an aberrant immune response.102 The ECM is a critical component of inflammation and progression of these diseases.103 The ECM’s role, which is often overlooked, could be investigated using tissue-engineered models. Indeed, some studies have attempted to build such models using an ECM-like scaffold and relevant cells.76,103 However, much effort is still needed towards the development of models that recapitulate the complexity of inflammatory bowel diseases.
Conclusion
The use of decellularised matrices as scaffolds offers the advantage of great similarity with the tissue to be replaced. Moreover, decellularised tissues and organs can be repopulated with the patient’s own cells to produce bespoke therapies. Great progress has been made in the research and development of decellularised scaffolds. The use of decellularised ECM in hydrogels, bioinks for bioprinting and disease modelling to study new therapies are exciting areas that most likely will continue to be extensively researched in the coming decade. However, as seen in this review, much effort is still needed towards preserving the original ECM composition, especially its minor components, assessing its functionality and scaling up for large tissues and organs. Emphasis should be placed on developing new decellularisation methods that do not involve the use of harsh chemicals and enzymes and establishing minimal criteria for assessing the success of the decellularisation process. Such criteria should include both removal of genetic material and ECM functionality assessment to produce a bioactive matrix that supports full tissue remodelling after implantation.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by The Griffin Institute (Registered Charity No 1129348, UK) charitable funds.
ORCID iD: Elena García-Gareta  https://orcid.org/0000-0001-7062-9099
https://orcid.org/0000-0001-7062-9099
References
- 1. Langer R, Vacanti JP. Tissue engineering. Science 1993; 260(5110): 920-926. [DOI] [PubMed] [Google Scholar]
- 2. Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. J Cell Sci 2010; 123(24): 4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. García-Gareta E. Collagen, from tissue culture to biomaterials, tissue engineering, and beyond. 1st ed Newcastle upon Tyne, UK: Cambridge Scholars Publishing, 2019. [Google Scholar]
- 4. Hussey GS, Dziki JL, Badylak SF. Extracellular matrix-based materials for regenerative medicine. Nat Rev Mater 2018; 3(7): 159–173. [Google Scholar]
- 5. Gilpin A, Yang Y. Decellularization strategies for regenerative medicine: from processing techniques to applications (ed. C Del Gaudio). Biomed Res Int 2017; 2017: 9831534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials 2011; 32(12): 3233–3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gilbert TW, Freund JM, Badylak SF. Quantification of DNA in biologic scaffold materials. J Surg Res 2009; 152(1): 135–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wong ML, Griffiths LG. Immunogenicity in xenogeneic scaffold generation: antigen removal vs. decellularization. Acta Biomater 2014; 10(5): 1806–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Galili U. The α-gal epitope and the anti-Gal antibody in xenotransplantation and in cancer immunotherapy. Immunol Cell Biol 2005; 83(6): 674–686. [DOI] [PubMed] [Google Scholar]
- 10. Huai G, Qi P, Yang H, et al. Characteristics of α-Gal epitope, anti-Gal antibody, α1,3 galactosyltransferase and its clinical exploitation (review). Int J Mol Med 2016; 37(1): 11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Boeer U, Buettner FFR, Klingenberg M, et al. Immunogenicity of intensively decellularized equine carotid arteries is conferred by the extracellular matrix protein collagen type VI. PLoS ONE 2014; 9(8): e105964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hillebrandt KH, Everwien H, Haep N, et al. Strategies based on organ decellularization and recellularization. Transpl Int 2019; 32(6): 571–585. [DOI] [PubMed] [Google Scholar]
- 13. Kitahara H, Yagi H, Tajima K, et al. Heterotopic transplantation of a decellularized and recellularized whole porcine heart. Interact Cardiovasc Thorac Surg 2016; 22(5): 571–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gorbet MB, Sefton MV. Biomaterial-associated thrombosis: roles of coagulation factors, complement, platelets and leukocytes. Biomaterials 2004; 25(26): 5681–5703. [DOI] [PubMed] [Google Scholar]
- 15. Kasimir MT, Rieder E, Seebacher G, et al. Decellu-larization does not eliminate thrombogenicity and inflammatory stimulation in tissue-engineered porcine heart valves. J Hear Valve Dis 2006; 15(2): 278–286. [PubMed] [Google Scholar]
- 16. Kawasaki T, Kirita Y, Kami D, et al. Novel detergent for whole organ tissue engineering. J Biomed Mater Res Part A 2015; 103(10): 3364–3373. [DOI] [PubMed] [Google Scholar]
- 17. Simsa R, Padma AM, Heher P, et al. Systematic in vitro comparison of decellularization protocols for blood vessels. PLoS ONE 2018; 13(12): e0209269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang F, Guan J. Cellular cardiomyoplasty and cardiac tissue engineering for myocardial therapy. Adv Drug Deliv Rev 2010; 62(7–8): 784–797. [DOI] [PubMed] [Google Scholar]
- 19. Xing Q, Yates K, Tahtinen M, et al. Decellularization of fibroblast cell sheets for natural extracellular matrix scaffold preparation. Tissue Eng Part C Methods 2014; 21(1): 77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Partington L, Mordan NJ, Mason C, et al. Biochemical changes caused by decellularization may compromise mechanical integrity of tracheal scaffolds. Acta Biomater 2013; 9(2): 5251–5261. [DOI] [PubMed] [Google Scholar]
- 21. Pulver Shevtov A, Leybovich B, Artyuhov I, et al. Production of organ extracellular matrix using a freeze-thaw cycle employing extracellular cryoprotectants. Cryo Letters 2014; 35(5): 400–406. [PubMed] [Google Scholar]
- 22. Xu X, Li Z, Cai L, et al. Mapping the nonreciprocal micromechanics of individual cells and the surrounding matrix within living tissues. Sci Rep 2016; 6: 24272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pang K, Du L, Wu X. A rabbit anterior cornea replacement derived from acellular porcine cornea matrix, epithelial cells and keratocytes. Biomaterials 2010; 31(28): 7257–7265. [DOI] [PubMed] [Google Scholar]
- 24. Zhou J, Fritze O, Schleicher M, et al. Impact of heart valve decellularization on 3-D ultrastructure, immunogenicity and thrombogenicity. Biomaterials 2010; 31(9): 2549–2554. [DOI] [PubMed] [Google Scholar]
- 25. Syed O, Walters NJ, Day RM, et al. Evaluation of decellularization protocols for production of tubular small intestine submucosa scaffolds for use in oesophageal tissue engineering. Acta Biomater 2014; 10(12): 5043–5054. [DOI] [PubMed] [Google Scholar]
- 26. Sullivan DC, Mirmalek-Sani S-H, Deegan DB, et al. Decellularization methods of porcine kidneys for whole organ engineering using a high-throughput system. Biomaterials 2012; 33(31): 7756–7764. [DOI] [PubMed] [Google Scholar]
- 27. Schaner PJ, Martin ND, Tulenko TN, et al. Decellularized vein as a potential scaffold for vascular tissue engineering. J Vasc Surg 2004; 40(1): 146–153. [DOI] [PubMed] [Google Scholar]
- 28. Gilpin SE, Guyette JP, Gonzalez G, et al. Perfusion decellularization of human and porcine lungs: bringing the matrix to clinical scale. J Hear Lung Transplant 2014; 33(3): 298–308. [DOI] [PubMed] [Google Scholar]
- 29. Piccoli M, Urbani L, Alvarez-Fallas ME, et al. Improvement of diaphragmatic performance through orthotopic application of decellularized extracellular matrix patch. Biomaterials 2016; 74: 245–255. [DOI] [PubMed] [Google Scholar]
- 30. Mendoza-Novelo B, Avila EE, Cauich-Rodríguez JV, et al. Decellularization of pericardial tissue and its impact on tensile viscoelasticity and glycosaminoglycan content. Acta Biomater 2011; 7(3): 1241–1248. [DOI] [PubMed] [Google Scholar]
- 31. Greco KV, Jones LG, Obiri-Yeboa I, et al. Creation of an acellular vaginal matrix for potential vaginal augmentation and cloacal repair. J Pediatr Adolesc Gynecol 2018; 31(5): 473–479. [DOI] [PubMed] [Google Scholar]
- 32. Gilbert TW, Wognum S, Joyce EM, et al. Collagen fiber alignment and biaxial mechanical behavior of porcine urinary bladder derived extracellular matrix. Biomaterials 2008; 29(36): 4775–4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yamanaka H, Morimoto N, Yamaoka T. Decellularization of submillimeter-diameter vascular scaffolds using peracetic acid. J Artif Organs 2020; 23: 156–162. [DOI] [PubMed] [Google Scholar]
- 34. Lin H-J, Wang W, Huang Y-Y, et al. Decellularized lymph node scaffolding as a carrier for dendritic cells to induce anti-tumor immunity. Pharmaceutics 2019; 11(11): 553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dong M, Zhao L, Wang F, et al. Rapid porcine corneal decellularization through the use of sodium N-lauroyl glutamate and supernuclease. J Tissue Eng 2019; 10: 2041731419875876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Khan U, Bayat A. Microarchitectural analysis of decellularised unscarred and scarred dermis provides insight into the organisation and ultrastructure of the human skin with implications for future dermal substitute scaffold design. J Tissue Eng 2019; 10: 2041731419843710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Giraldo-Gomez DM, Leon-Mancilla B, Del Prado-Audelo ML, et al. Trypsin as enhancement in cyclical tracheal decellularization: morphological and biophysical characterization. Mater Sci Eng C 2016; 59: 930–937. [DOI] [PubMed] [Google Scholar]
- 38. Purpura V, Bondioli E, Cunningham EJ, et al. The development of a decellularized extracellular matrix-based biomaterial scaffold derived from human foreskin for the purpose of foreskin reconstruction in circumcised males. J Tissue Eng 2018; 9: 2041731418812613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lin M, Ge J, Wang X, et al. Biochemical and biomechanical comparisions of decellularized scaffolds derived from porcine subcutaneous and visceral adipose tissue. J Tissue Eng 2019; 10: 2041731419888168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Elder BD, Kim DH, Athanasiou KA. Developing an articular cartilage decellularization process toward facet joint cartilage replacement. Neurosurgery 2010; 66(4): 722–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Greco KV, Francis L, Somasundaram M, et al. Characterisation of porcine dermis scaffolds decellularised using a novel non-enzymatic method for biomedical applications. J Biomater Appl 2015; 30(2): 239–253. [DOI] [PubMed] [Google Scholar]
- 42. Hashimoto Y, Funamoto S, Sasaki S, et al. Preparation and characterization of decellularized cornea using high-hydrostatic pressurization for corneal tissue engineering. Biomaterials 2010; 31(14): 3941–3948. [DOI] [PubMed] [Google Scholar]
- 43. Funamoto S, Nam K, Kimura T, et al. The use of high-hydrostatic pressure treatment to decellularize blood vessels. Biomaterials 2010; 31(13): 3590–3595. [DOI] [PubMed] [Google Scholar]
- 44. Sawada K, Terada D, Yamaoka T, et al. Cell removal with supercritical carbon dioxide for acellular artificial tissue. J Chem Technol Biotechnol 2008; 83(6): 943–949. [Google Scholar]
- 45. Halfwerk FR, Rouwkema J, Gossen JA, et al. Supercritical carbon dioxide decellularised pericardium: mechanical and structural characterisation for applications in cardio-thoracic surgery. J Mech Behav Biomed Mater 2018; 77: 400–407. [DOI] [PubMed] [Google Scholar]
- 46. Huang Y-H, Tseng F-W, Chang W-H, et al. Preparation of acellular scaffold for corneal tissue engineering by supercritical carbon dioxide extraction technology. Acta Biomater 2017; 58: 238–243. [DOI] [PubMed] [Google Scholar]
- 47. Seo Y, Jung Y, Kim SH. Decellularized heart ECM hydrogel using supercritical carbon dioxide for improved angiogenesis. Acta Biomater 2018; 67: 270–281. [DOI] [PubMed] [Google Scholar]
- 48. Wang JK, Luo B, Guneta V, et al. Supercritical carbon dioxide extracted extracellular matrix material from adipose tissue. Mater Sci Eng C 2017; 75: 349–358. [DOI] [PubMed] [Google Scholar]
- 49. Wu Z, Fan L, Xu B, et al. Use of decellularized scaffolds combined with hyaluronic acid and basic fibroblast growth factor for skin tissue engineering. Tissue Eng Part A 2014; 21(1–2): 390–402. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 50. Lee K, Il Olmer M, Baek J, et al. Platelet-derived growth factor-coated decellularized meniscus scaffold for integrative healing of meniscus tears. Acta Biomater 2018; 76: 126–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Xu H-L, Tian F-R, Lu C-T, et al. Thermo-sensitive hydrogels combined with decellularised matrix deliver bFGF for the functional recovery of rats after a spinal cord injury. Sci Rep 2016; 6(1): 38332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Assmann A, Delfs C, Munakata H, et al. Acceleration of autologous in vivo recellularization of decellularized aortic conduits by fibronectin surface coating. Biomaterials 2013; 34(25): 6015–6026. [DOI] [PubMed] [Google Scholar]
- 53. Owji N, Aldaadaa A, Cha JR, et al. Synthesis, characterization, and 3D printing of an isosorbide-based, light-curable, degradable polymer for potential application in maxillofacial reconstruction. ACS Biomater Sci Eng 2020; 6: 2578-2587. [DOI] [PubMed] [Google Scholar]
- 54. Hinton TJ, Jallerat Q, Palchesko RN, et al. Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Sci Adv 2015; 1: e1500758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bedell ML, Navara AM, Du Y, et al. Polymeric systems for bioprinting. Chem Rev 2020. DOI: 10.1021/acs.chemrev.9b00834. [DOI] [PubMed] [Google Scholar]
- 56. Xu J, Zheng S, Hu X, et al. Advances in the research of bioinks based on natural collagen, polysaccharide and their derivatives for skin 3D bioprinting. Polymers 2020; 12(6): E1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pati F, Jang J, Ha D-H, et al. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat Commun 2014; 5(1): 3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kim H, Park M-N, Kim J, et al. Characterization of cornea-specific bioink: high transparency, improved in vivo safety. J Tissue Eng 2019; 10: 2041731418823382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Dzobo K, Motaung KSCM, Adesida A. Recent trends in decellularized extracellular matrix bioinks for 3D printing: an updated review. Int J Mol Sci 2019; 20(18): 4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kim J, Shim IK, Hwang DG, et al. 3D cell printing of islet-laden pancreatic tissue-derived extracellular matrix bioink constructs for enhancing pancreatic functions. J Mater Chem B 2019; 7(10): 1773–1781. [DOI] [PubMed] [Google Scholar]
- 61. Mao Q, Wang Y, Li Y, et al. Fabrication of liver microtissue with liver decellularized extracellular matrix (dECM) bioink by digital light processing (DLP) bioprinting. Mater Sci Eng C 2020; 109: 110625. [DOI] [PubMed] [Google Scholar]
- 62. Nam H, Jeong H-J, Jo Y, et al. Multi-layered free-form 3D cell-printed tubular construct with decellularized inner and outer esophageal tissue-derived bioinks. Sci Rep 2020; 10(1): 7255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ahmed EM. Hydrogel: preparation, characterization, and applications: a review. J Adv Res 2015; 6(2): 105–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bahram M, Mohseni N, Moghtader M. An introduction to hydrogels and some recent applications. In: Emerging concepts in analysis and applications of hydrogels. IntechOpen, 2016, https://www.intechopen.com/books/emerging-concepts-in-analysis-and-applications-of-hydrogels/an-introduction-to-hydrogels-and-some-recent-applications [Google Scholar]
- 65. He W, Reaume M, Hennenfent M, et al. Biomimetic hydrogels with spatial- and temporal-controlled chemical cues for tissue engineering. Biomater Sci 2020; 8: 3248-3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Fernández-Pérez J, Ahearne M. The impact of decellularization methods on extracellular matrix derived hydrogels. Sci Rep 2019; 9: 14933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yi H-G, Choi Y-J, Jung JW, et al. Three-dimensional printing of a patient-specific engineered nasal cartilage for augmentative rhinoplasty. J Tissue Eng 2019; 10: 2041731418824797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Singelyn JM, DeQuach JA, Seif-Naraghi SB, et al. Naturally derived myocardial matrix as an injectable scaffold for cardiac tissue engineering. Biomaterials 2009; 30(29): 5409–5416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ungerleider JL, Johnson TD, Rao N, et al. Fabrication and characterization of injectable hydrogels derived from decellularized skeletal and cardiac muscle. Methods 2015; 84: 53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Chaimov D, Baruch L, Krishtul S, et al. Innovative encapsulation platform based on pancreatic extracellular matrix achieve substantial insulin delivery. J Control Release 2017; 257: 91–101. [DOI] [PubMed] [Google Scholar]
- 71. Kim H, Jang J, Park J, et al. Shear-induced alignment of collagen fibrils using 3D cell printing for corneal stroma tissue engineering. Biofabrication 2019; 11(3): 035017. [DOI] [PubMed] [Google Scholar]
- 72. Hernandez MJ, Zelus EI, Spang MT, et al. Dose optimization of decellularized skeletal muscle extracellular matrix hydrogels for improving perfusion and subsequent validation in an aged hindlimb ischemia model. Biomater Sci 2020; 8: 3511-3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Engel H, Kao S-W, Larson J, et al. Investigation of dermis-derived hydrogels for wound healing applications. Biomed J 2015; 38(1): 58–64. [DOI] [PubMed] [Google Scholar]
- 74. Ahmed E, Saleh T, Yu L, et al. Decellularized ECM-rich hydrogel–silver nanoparticles mixture as a potential treatment for acute liver failure model. J Biomed Mater Res Part A. Epub ahead of print 16 May 2020. DOI: 10.1002/jbm.a.36988. [DOI] [PubMed] [Google Scholar]
- 75. Petrou CL, D’Ovidio TJ, Bölükbas DA, et al. Clickable decellularized extracellular matrix as a new tool for building hybrid-hydrogels to model chronic fibrotic diseases in vitro. J Mater Chem B. Epub ahead of print 17 April 2020. DOI: 10.1039/D0TB00613K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Dosh RH, Jordan-Mahy N, Sammon C, et al. Long-term in vitro 3D hydrogel co-culture model of inflammatory bowel disease. Sci Rep 2019; 9(1): 1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Pouliot RA, Young BM, Link PA, et al. Porcine lung-derived extracellular matrix hydrogel properties are dependent on pepsin digestion time. Tissue Eng Part C Methods 2020; 26: 332-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Quinn RW, Hilbert SL, Bert AA, et al. Performance and morphology of decellularized pulmonary valves implanted in juvenile sheep. Ann Thorac Surg 2011; 92(1): 131–137. [DOI] [PubMed] [Google Scholar]
- 79. Tudorache I, Theodoridis K, Baraki H, et al. Decellularized aortic allografts versus pulmonary autografts for aortic valve replacement in the growing sheep model: haemodynamic and morphological results at 20 months after implantation. Eur J Cardio-Thoracic Surg 2015; 49(4): 1228–1238. [DOI] [PubMed] [Google Scholar]
- 80. Scarritt ME, Pashos NC, Bunnell BA. A review of cellularization strategies for tissue engineering of whole organs. Front Bioeng Biotechnol 2015; 3: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Doryab A, Heydarian M, Amoabediny G, et al. Recellularization on acellular lung tissue scaffold using perfusion-based bioreactor: an online monitoring strategy. J Med Biol Eng 2017; 37(1): 53–62. [Google Scholar]
- 82. Etnel JRG, Suss PH, Schnorr GM, et al. Fresh decellularized versus standard cryopreserved pulmonary allografts for right ventricular outflow tract reconstruction during the Ross procedure: a propensity-matched study. Eur J Cardio-Thoracic Surg 2018; 54(3): 434–440. [DOI] [PubMed] [Google Scholar]
- 83. Bacakova L, Zarubova J, Travnickova M, et al. Stem cells: their source, potency and use in regenerative therapies with focus on adipose-derived stem cells – a review. Biotechnol Adv 2018; 36(4): 1111–1126. [DOI] [PubMed] [Google Scholar]
- 84. Ross EA, Williams MJ, Hamazaki T, et al. Embryonic stem cells proliferate and differentiate when seeded into kidney scaffolds. J Am Soc Nephrol 2009; 20(11): 2338–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. García-Gareta E, Hua J, Rayan F, et al. Stem cell engineered bone with calcium-phosphate coated porous titanium scaffold or silicon hydroxyapatite granules for revision total joint arthroplasty. J Mater Sci Mater Med 2014; 25(6): 1553–1562. [DOI] [PubMed] [Google Scholar]
- 86. Vunjak-Novakovic G, Obradovic B, Martin I, et al. Dynamic cell seeding of polymer scaffolds for cartilage tissue engineering. Biotechnol Prog 1998; 14(2): 193–202. [DOI] [PubMed] [Google Scholar]
- 87. Stephenson M, Grayson W. Recent advances in bioreactors for cell-based therapies. F1000Rese 2018; 7: F1000 Faculty Rev-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Stiehler M, Bünger C, Baatrup A, et al. Effect of dynamic 3-D culture on proliferation, distribution, and osteogenic differentiation of human mesenchymal stem cells. J Biomed Mater Res Part A 2009; 89(1): 96–107. [DOI] [PubMed] [Google Scholar]
- 89. Price AP, England KA, Matson AM, et al. Development of a decellularized lung bioreactor system for bioengineering the lung: the matrix reloaded. Tissue Eng Part A 2010; 16(8): 2581–2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Talò G, D’Arrigo D, Lorenzi S, et al. Independent, controllable stretch-perfusion bioreactor chambers to functionalize cell-seeded decellularized tendons. Ann Biomed Eng 2020; 48(3): 1112–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Yazdani SK, Watts B, Machingal M, et al. Smooth muscle cell seeding of decellularized scaffolds: the importance of bioreactor preconditioning to development of a more native architecture for tissue-engineered blood vessels. Tissue Eng Part A 2009; 15(4): 827–840. [DOI] [PubMed] [Google Scholar]
- 92. Simmons AD, Williams C, Degoix A, et al. Sensing metabolites for the monitoring of tissue engineered construct cellularity in perfusion bioreactors. Biosens Bioelectron 2017; 90: 443–449. [DOI] [PubMed] [Google Scholar]
- 93. Karami D, Richbourg N, Sikavitsas V. Dynamic in vitro models for tumor tissue engineering. Cancer Lett 2019; 449: 178–185. [DOI] [PubMed] [Google Scholar]
- 94. da Palma RK, Fratini P, Schiavo Matias GS, et al. Equine lung decellularization: a potential approach for in vitro modeling the role of the extracellular matrix in asthma. J Tissue Eng 2018; 9: 2041731418810164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Han K, Pierce SE, Li A, et al. CRISPR screens in cancer spheroids identify 3D growth-specific vulnerabilities. Nature 2020; 580: 136-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Atapattu L, Utama R, O’Mahony A, et al. Abstract 5022: precision medicine: high-throughput 3D bioprinting of embedded multicellular cancer spheroids. Cancer Res 2018; 78(13 Suppl.): 5022. [Google Scholar]
- 97. Angeloni V, Contessi N, De Marco C, et al. Polyurethane foam scaffold as in vitro model for breast cancer bone metastasis. Acta Biomater 2017; 63: 306–316. [DOI] [PubMed] [Google Scholar]
- 98. Hoshiba T. Decellularized extracellular matrix for cancer research. Mater 2019; 12(8): 1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Rijal G, Li W. A versatile 3D tissue matrix scaffold system for tumor modeling and drug screening. Sci Adv 2017; 3(9): e1700764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Liu G, Wang B, Li S, et al. Human breast cancer decellularized scaffolds promote epithelial-to-mesenchymal transitions and stemness of breast cancer cells in vitro. J Cell Physiol 2019; 234(6): 9447–9456. [DOI] [PubMed] [Google Scholar]
- 101. Mollica PA, Booth-Creech EN, Reid JA, et al. 3D bioprinted mammary organoids and tumoroids in human mammary derived ECM hydrogels. Acta Biomater 2019; 95: 201–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Cornaggia M, Leutner M, Mescoli C, et al. Chronic idiopathic inflammatory bowel diseases: the histology report. Dig Liver Dis 2011; 43: S293–S303. [DOI] [PubMed] [Google Scholar]
- 103. Hussey GS, Cramer MC, Badylak SF. Extracellular matrix bioscaffolds for building gastrointestinal tissue. Cell Mol Gastroenterol Hepatol 2018; 5(1): 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]