Key Points
Question
Can the circulating metabolome identify individuals with high cardiometabolic stress across multiple physiological systems who are at risk for long-term complications of cardiovascular disease?
Findings
In 2 distinct cohorts spanning more than 3000 individuals, a metabolite-based signature of systemic cardiometabolic stress (defined by clinical phenotypes) was defined, and its association with long-term all-cause mortality and coronary heart disease risk over nearly 30 years, independent of traditional risk factors, was demonstrated.
Meaning
These results underscore the shared molecular pathophysiology of metabolic dysfunction, cardiovascular disease, and survival and suggest pathways for modification to improve prognosis across all linked conditions.
Abstract
Importance
Cardiometabolic disease is responsible for decreased longevity and poorer cardiovascular outcomes in the modern era. Metabolite profiling provides a specific measure of global metabolic function to examine specific metabolic mechanisms and pathways of cardiometabolic disease beyond its clinical definitions.
Objectives
To define a molecular basis for cardiometabolic stress and assess its association with cardiovascular prognosis.
Design, Setting, and Participants
A prospective observational cohort study was conducted in a population-based setting across 2 geographically distinct centers (Boston Puerto Rican Health Study [BPRHS], an ongoing study of individuals enrolled between June 1, 2004, and October 31, 2009; and Atherosclerosis Risk in Communities [ARIC] study, whose participants were originally sampled between November 24, 1986, and February 10, 1990, and followed up through December 31, 2017). Participants in the BPRHS were 668 Puerto Rican individuals with metabolite profiling living in Massachusetts, and participants in the ARIC study were 2152 individuals with metabolite profiling and long-term follow-up for mortality and cardiovascular outcomes. Statistical analysis was performed from October 1, 2018, to March 13, 2020.
Exposure
The primary exposure was metabolite profiles across both cohorts.
Main Outcomes and Measures
Outcomes included associations with multisystem cardiometabolic stress and all-cause mortality and incident coronary heart disease (in the ARIC study).
Results
Participants in the BPRHS (N = 668; 491 women; mean [SD] age, 57.0 [7.4] years; mean [SD] body mass index [calculated as weight in kilograms divided by height in meters squared], 32.0 [6.5]) had higher prevalent cardiometabolic risk relative to those in the ARIC study (N = 2152; 599 African American individuals; 1213 women; mean [SD] age, 54.3 [5.7] years; mean [SD] body mass index, 28.0 [5.5]). Multisystem cardiometabolic stress was defined for 668 Puerto Rican individuals in the BPRHS as a multidimensional composite of hypothalamic-adrenal axis activity, sympathetic activation, blood pressure, proatherogenic dyslipidemia, insulin resistance, visceral adiposity, and inflammation. A total of 260 metabolites associated with cardiometabolic stress were identified in the BPRHS, involving known and novel pathways of cardiometabolic disease (eg, amino acid metabolism, oxidative stress, and inflammation). A parsimonious metabolite-based score associated with cardiometabolic stress in the BPRHS was subsequently created; this score was applied to shared metabolites in the ARIC study, demonstrating significant associations with coronary heart disease and all-cause mortality after multivariable adjustment at a 30-year horizon (per SD increase in metabolomic score: hazard ratio, 1.14; 95% CI, 1.00-1.31; P = .045 for coronary heart disease; and hazard ratio, 1.15; 95% CI, 1.07-1.24; P < .001 for all-cause mortality).
Conclusions and Relevance
Metabolites associated with cardiometabolic stress identified known and novel pathways of cardiometabolic disease in high-risk, community-based cohorts and were associated with coronary heart disease and survival at a 30-year time horizon. These results underscore the shared molecular pathophysiology of metabolic dysfunction, cardiovascular disease, and longevity and suggest pathways for modification to improve prognosis across all linked conditions.
This cohort study defines a molecular basis for cardiometabolic stress and assesses its associations with cardiovascular prognosis.
Introduction
Despite ongoing efforts in the prevention of cardiovascular disease, recent reports suggest a relative plateau in mortality owing to major cardiometabolic diseases (CMD) and cardiovascular diseases (CVD).1 Cardiometabolic disease is recognized as a shared risk factor for both cardiovascular and noncardiovascular morbidity, predating neurocognitive, renal, cardiovascular, and functional decline. These observations have been distilled into clinical prevention guidelines and definitions (eg, metabolic syndrome). Nevertheless, for therapeutic discovery, these constructs are based on clinical phenotypes (eg, blood pressure and lipid levels), lacking resolution for the molecular components of metabolic homeostasis that may pinpoint specific, mutable pathways relevant to CVD. This limitation becomes especially important at the extremes of clinical risk, where earlier detection and more specific metabolic biomarkers may be relevant to defining the hazard of CMD in association with CVD over a longer time horizon.
Our primary hypothesis was that circulating metabolites associated with a composite marker of cardiometabolic (CM) stress would identify underlying pathways relevant to CVD and survival and would be associated with these outcomes across 2 racially diverse cohorts at high prevalent CMD risk. We first studied 668 Puerto Rican adults in the Boston Puerto Rican Health Study (BPRHS) with metabolite profiling to identify molecular correlates and pathways of a validated, multiparametric clinical index of CM stress based on known and emerging CMD traits. We derived a metabolite-based score from this clinical index and subsequently applied the metabolite-based score to a separate cohort of 2152 participants in the Atherosclerosis Risk in Communities (ARIC) study to investigate its association with the development of long-term coronary heart disease (CHD) and all-cause mortality over 30 years independent of sex, race/ethnicity, and clinical CMD traits. Our ultimate goal was to define a molecular metabolic basis for CMD in 2 high-risk populations, understand the potential pathways implicated, and identify whether the implicated metabolites would help us to identify individuals at risk for CVD and mortality over a long horizon in large, multiethnic populations.
Methods
Cohort Description
Boston Puerto Rican Health Study
The BPRHS is an ongoing longitudinal study of 1499 individuals enrolled between June 1, 2004, and October 31, 2009, that sought to examine stress, nutrition, and CVD outcomes in Puerto Rican immigrants in the Boston area. The design of the overall study has been previously published.2 In brief, eligible participants were of self-identified Puerto Rican descent, able to answer questions in English or Spanish, 45 to 75 years of age, and living in the Boston, Massachusetts, area at study enrollment. Detailed survey methods have been reported2 (eAppendix in the Supplement). Blood samples were collected for biochemical measures after a 12-hour fast (dehydroepiandrosterone sulfate, hemoglobin A1c, C-reactive protein, a lipid panel, and glucose), and a 12-hour urine collection was performed for urinary epinephrine and norepinephrine levels. The BPRHS cohort study procedures were approved by the Tufts Medical Center Institutional Review Board. The current BPRHS data analysis was approved by the University of Massachusetts Lowell and Massachusetts General Hospital Institutional Review Boards.
Our construction of an analytic cohort is shown in eFigure 1 in the Supplement. Of the 1499 individuals in the baseline study visit, 817 individuals had metabolite profiling performed. The metabolite profiling was performed on archived plasma at 2 different times (“technical runs”; run 1, n = 736; run 2, n = 81). We focused on the 736 study participants, with their samples performed on the same platform to reduce technical variability. Among these, a subset of the 736 samples for metabolite profiling in the BPRHS was selected for other purposes as part of a case-control study for diabetes (120 cases and 120 controls without diabetes), and the remainder were randomly selected. After exclusion for missing clinical covariates (age, sex, body mass index [BMI], smoking status, and CM stress index) and metabolites with high missingness (>25%), 668 participants with 719 metabolites comprised our final analytic cohort.
ARIC Study
The ARIC study is a prospective cohort study of 15 792 individuals, originally sampled between November 24, 1986, and February 10, 1990. The detailed design of the ARIC study has been previously published.3 The ARIC study participants were 45 to 64 years of age from 4 US communities (Forsyth County, North Carolina; Jackson, Mississippi; suburbs of Minneapolis, Minnesota; and Washington County, Maryland) and underwent collection of standard demographic, clinical, and biochemical measures. Clinical measures included vital signs and anthropometric indices,3 lipid profiles,4,5,6 and glomerular filtration rate7 (eAppendix in the Supplement). Our analytic cohort consisted of 2152 participants with metabolites measured in 2014 from serum stored at a study visit during the period from 1987 to 1989, as well as CHD and mortality follow-up information and necessary covariates. Our primary outcomes were CHD and all-cause mortality. In the ARIC study, prevalent CHD at baseline was defined as a self-reported history of physician-diagnosed myocardial infarction, coronary artery bypass surgery, or coronary angioplasty or electrocardiogram evidence of a previous myocardial infarction. Prevalent CVD was defined as evidence of prior CHD, heart failure, or stroke. Incident CHD was defined as definite fatal CHD and definite or probable myocardial infarction that occurred on or before December 31, 2017. The cases were identified and adjudicated using information from study visits, interviews, annual follow-up calls, hospital discharge lists, medical records, and death certificates.8 Only 1947 (of 2152) participants without prevalent CHD (and with CHD follow-up and all covariates available for adjustment) at the baseline examination were included in incident CHD models. The vital status for each individual was followed from the baseline visit until death, loss to follow-up, or administrative censoring at December 31, 2017. Of the overall 2152 individuals, 2076 were included in mortality models with all covariates available for adjustment.
Metabolite Profiling
Metabolite profiling was performed at a commercial facility (Metabolon Inc) using proprietary procedures.9 For the BPRHS, the Metabolon metabolomics platform uses liquid chromatography–mass spectrometry methods with positive ion and negative ion modes (eAppendix in the Supplement). Metabolites sampled across 4 modes were detected: (1) acidic positive ion (optimized for hydrophilic molecules), (2) acidic positive ion (optimized for hydrophobic molecules), (3) basic negative ion, and (4) negative ionization from eluent of a HILIC (hydrophilic interaction liquid chromatography) column. Raw data were extracted and peaks identified using proprietary methods, with more than 3300 commercially available purified molecules as reference. Metabolites without specific chemical annotation were also detected. The relative metabolite concentration was reported as a normalized area under the curve value for each metabolite. Missing values were imputed as 50% of the minimal value across all participants, and metabolites were subsequently log2-transformed and standardized (to mean, 0; variance, 1) for entry into analysis. Methods for the BPRHS (from Metabolon) are reported in the eAppendix in the Supplement.
For the ARIC study validation cohort (performed earlier than the BPRHS metabolomics), gas chromatography was used in addition to liquid chromatography for several platforms. The details of metabolite profiling in the ARIC study have been published elsewhere for reference.10 The median relative SD for internal standards was 2% for the ARIC study and 5% for the BPRHS, with total process variability of 10% or less. Recent consortium reports for Metabolon’s platform in other samples have reported a median coefficient of variation of 14.6% across replicates for known metabolites, with a median coefficient of variation of 29% from day to day.11
Definition of CM Stress in the BPRHS
The index of CM stress used here has been previously validated in the BPRHS as a marker of multisystem metabolic stress associated with disease (Table 1).2 The stress index comprehensively quantifies metabolic, inflammatory, and neurohormonal states across multiple dimensions: (1) hypothalamus-adrenal axis (serum dehydroepiandrosterone sulfate and urinary cortisol levels), (2) sympathetic activation (12-hour urinary norepinephrine and epinephrine levels), (3) vascular dysfunction (blood pressure), (4) proatherogenic dyslipidemia, (5) insulin resistance or type 2 diabetes, (6) visceral adiposity, and (7) inflammation (C-reactive protein levels). Use of medications for type 2 diabetes, hypertension, or dyslipidemia was incorporated into this index. Each component was parameterized into a 0-point to 1-point scale or a 0-point to 20-point scale and summed to generate the final systemic CM stress index.12 The operating characteristics for biochemical assays used in the prescription of this index (urinary and plasma or serum biomarkers) have been previously reported, and the study-specific cutoffs for these biochemical measures have been prescribed by previous work in the BPRHS.2
Table 1. Definition of Cardiometabolic Stress Index and Its 9 Components in the Boston Puerto Rican Health Study.
| Criteriaa | Definition |
|---|---|
| Blood pressure and antihypertensive medication use | |
| 0 | SBP ≤140 mm Hg, DBP ≤90 mm Hg, and no antihypertensive medication use reported |
| 1 | All others |
| 2 | SBP >140 mm Hg and DBP >90 mm Hg |
| Waist circumference | |
| 0 | Other |
| 1 | >102 cm for Male patients; >88 cm for female patients |
| Dyslipidemia or use of medications for lipids | |
| 0 | HDL-C ≥40 mg/dL, total cholesterol <240 mg/dL, and no antilipidemic medications reported |
| 1 | All others |
| 2 | HDL-C <40 mg/dL and total cholesterol ≥240 mg/dL (regardless of medication) or HDL-C <40 mg/dL and total cholesterol ≤240 mg/dL with medication use |
| HbA1c and antidiabetes medication use | |
| 0 | HbA1c ≤7% and no antidiabetes medication use reported |
| 1 | HbA1c >7% or antidiabetes medication use reported |
| Urinary cortisol | |
| 0 | <41.5 μg/g Creatinine for male patients or <49.5 μg/g creatinine for female patients |
| 1 | All others |
| Urinary epinephrine | |
| 0 | <2.8 μg/g Creatinine for male patients or <3.6 μg/g creatinine for female patients |
| 1 | All others |
| Urinary norepinephrine | |
| 0 | <30.5 μg/g Creatinine for male patients or <46.9 μg/g creatinine for female patients |
| 1 | All others |
| Serum DHEA-S | |
| 0 | All others |
| 1 | ≤589.5 ng/mL or Taking androgens for male patients or ≤368.5 ng/mL or taking androgens for female patients |
| C-reactive protein | |
| 0 | All others |
| 1 | >3 mg/L |
Abbreviations: DHEA-S, dehydroepiandrosterone sulfate; DBP, diastolic blood pressure; HbA1c, hemoglobin A1c; HDL-C, high-density lipoprotein cholesterol; SBP, systolic blood pressure.
SI conversion factors: To convert total cholesterol and HDL-C to millimoles per liter, multiply by 0.0259; HbA1c to proportion of total hemoglobin, multiply by 0.01.
Components are given a score of 0, 1, or 2, which are then summed across all 9 components to generate a score from 0 to 11 that reflects cardiometabolic stress. All medication use was self-reported. Blood pressures and waist circumference were the mean of multiple measurements.
Statistical Analysis
Identification of Metabolites Associated With the CM Stress Index
Statistical analysis was performed from October 1, 2018, to March 13, 2020. After filtering and imputation, metabolites were subsequently log-transformed and then mean-centered and standardized (to mean, 0; variance, 1). We measured the association of the CM stress index as a function of each metabolite, adjusted for age, sex, BMI, and ever smoking status in separate linear models. We adjusted for multiple hypothesis testing across all models using the Benjamini-Hochberg false discovery rate threshold of 5%, finding 260 metabolites significantly associated (false discovery rate threshold <0.05) with the CM stress index (BPRHS; eFigure 1 in the Supplement).
Pathway Analysis
To provide an analysis of molecular pathways relevant to CVD based on CM stress, we identified genes whose protein products were along the enzymatic pathways of top CM stress–associated metabolites and performed a comprehensive pathway analysis on these genes. Of the 260 metabolites, 124 were annotated with KEGG Compound identification (ID) numbers using MetaboAnalyst.13 Using these ID numbers as a query set, we next constructed a network of metabolic pathways consisting of 375 metabolites, 300 reaction nodes, 210 enzyme classes, 427 genes, and 1563 interactions using the MetScape app14 in Cytoscape.15 Using the RCy3 package for R-Cytoscape scripting, we interrogated the network to identify sets of genes linked to metabolites associated with CM stress.16 We assigned minimum, mean, and maximum β coefficient values to each pathway gene based on its connectivity to analyzed metabolites via the enzyme-metabolite interactions curated by MetScape. These values were used to define metabolically harmful and metabolically healthy gene sets (eg, genes having a positive-maximum and negative-minimum metabolite-associated β coefficient value, respectively). These genes were used to perform a pathway analysis using WikiPathways17 via the clusterProfiler R package.18 In addition to the typical caveats for pathway analysis as a strictly exploratory method, the mapping from metabolites to genes via incomplete and continually updated databases may introduce bias.
Construction of Metabolite-Based Score Reflecting CM Stress (BPRHS)
From 260 metabolites associated with the CM stress index in the BPRHS, 125 metabolites were commonly expressed with the ARIC study (matched by Metabolon chemical ID number, Human Metabolome Database ID, mass, and/or name) and expressed in more than 75% of the ARIC study participants. We used elastic net regression in the BPRHS to create a metabolite-based CM stress score based on the clinical CM stress index as a function of age, sex, and the 125 common metabolites. Selection of α and λ parameters was optimized by 10-fold cross-validation (with 5 repeats), and age and sex were not penalized in regressions (using caret in R [R Foundation for Statistical Computing]). Data from the BPRHS were used to construct the score, with the knowledge that external validation against outcomes in the ARIC study would mitigate overfitting of the metabolite-based score in the BPRHS. To achieve maximal parsimony in constructing the score, we ranked all 125 metabolites by calculating the variable importance in the elastic net and specified successive linear models for the clinical CM stress index in the BPRHS as a function of age, sex, and increasing number of metabolites (with initial models including the most important metabolites). The predictive ability of linear models was assessed in 10-fold cross-validation (5 repeats). We plotted the cross-validated model R2 and variable importance as a function of sets of metabolites to determine the minimal number of metabolites necessary to optimize model fit. We report the 30-metabolite model in the ARIC study for parsimony.
Survival Analysis for 30-Year CHD and Mortality (ARIC Study)
For the ARIC study, imputation of below-detection values was done as in the BPRHS, and metabolites were log-transformed, mean-centered, and standardized for analysis. Cox proportional hazards regression models were constructed for CHD and all-cause mortality as a function of metabolite-based CM stress score. Survival analysis included 2 models: model 1 comprised age, sex, and race/ethnicity adjusted for center, and model 2 comprised all the factors in model 1, with added adjustment for traditional risk factors (smoking status, systolic blood pressure, use of medications for hypertension, diabetes status, total cholesterol, high-density lipoprotein cholesterol, and estimated glomerular filtration rate for incident CHD analysis). All-cause mortality analysis was further adjusted for BMI and prevalent CVD. For CHD, we performed a sensitivity analysis, including the Pooled Cohort Equations risk as defined19 and examining the association of CM stress score and CHD adjusted for center, sex, and Pooled Cohort Equations score. The Cox proportional hazards regression assumption was assessed by visual inspection of residual plots and by inclusion of time by metabolomic score linear interaction terms (with interaction P > .05 indicating no violation of proportionality). Effect modification by sex was assessed. We calculated the mean value and 95% CI of the area under the curve with 10-fold cross-validation with 100 replicates.20 The net reclassification index and integrated discrimination improvement were calculated as described.21,22 All statistical analyses were performed using R, version 3.5.2 or SAS, version 9.4 (SAS Institute Inc). A 2-tailed P < .05 was considered statistically significant, with adjustments for type I error as specified.
Results
Cohort Characterization
Characteristics of the BPRHS and ARIC study subsamples included in this analysis are shown in Table 2. The BPRHS subsample (n = 668) had a mean (SD) age of 57.0 (7.4) years and included 491 women (73.5%). The mean (SD) BMI was 32.0 (6.5) (calculated as weight in kilograms divided by height in meters squared), and most of the cohort was at high prevalent CM risk: 345 participants (51.6%) had a diagnosis of diabetes, 469 (70.2%) had a diagnosis of hypertension, and 432 (64.7%) had a diagnosis of dyslipidemia. The 2152 ARIC study participants (599 African American individuals and 1553 European American individuals) had a similar age distribution to the BPRHS (mean [SD] age, 54.3 [5.7] years) but with a smaller proportion of women (1213 [56.4%]) and with lower CM risk, including a lower mean (SD) BMI (28.0 [5.5]) and more favorable lipid profile and blood pressure.
Table 2. Characteristics of the BPRHS and ARIC Study Subsamples Included in This Analysis.
| Characteristica | BPRHS (n = 668) | ARIC study (n = 2152) | ||
|---|---|---|---|---|
| No. | Value | No. | Value | |
| Age, mean (SD), y | 668 | 57.0 (7.4) | 2152 | 54.3 (5.7) |
| Female sex, No. (%) | 668 | 491 (73.5) | 2152 | 1213 (56.4) |
| African American race/ethnicity, No. (%) | NA | NA | 2152 | 599 (27.8) |
| BMI, mean (SD) | 668 | 32.0 (6.5) | 2149 | 28.0 (5.5) |
| Waist circumference, mean (SD), cm | 668 | 102 (15) | 2148 | 98 (14) |
| Type 2 diabetes, No. (%) | 668 | 345 (51.6) | 2147 | 254 (11.8) |
| Hypertension, No. (%) | 668 | 469 (70.2) | 2142 | 805 (37.6) |
| Dyslipidemia, No. (%) | 668 | 432 (64.7) | 2134 | 1010 (47.3) |
| Prevalent cardiovascular disease, No. (%) | 668 | 139 (20.8) | 2094 | 269 (12.8) |
| Current smoker, No. (%) | 668 | 151 (22.6) | 2152 | 585 (27.2) |
| Blood pressure, mean (SD), mm Hg | ||||
| Systolic | 668 | 136 (19) | 2152 | 122 (20) |
| Diastolic | 668 | 81.5 (10.8) | 2152 | 73.6 (11.7) |
| Lipid panel, mean (SD), mg/dL | ||||
| Triglycerides | 668 | 166 (129) | 2146 | 136 (110) |
| High-density lipoprotein cholesterol | 668 | 45 (13) | 2146 | 52 (17) |
| Total cholesterol | 668 | 185 (42) | 2145 | 215 (42) |
| Glucose, mean (SD), mg/dL | 668 | 119 (50) | 2152 | 110 (42) |
| Hemoglobin A1c, mean (SD), % | 667 | 7.0 (1.8) | NA | NA |
| CM stress index, mean (SD) | 668 | 4.5 (1.9) | NA | NA |
Abbreviations: ARIC, Atherosclerosis Risk in Communities; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); BPRHS, Boston Puerto Rican Health Study; CM, cardiometabolic; NA, not applicable.
SI conversion factors: To convert triglycerides to millimoles per liter, multiply by 0.0113; high-density lipoprotein cholesterol and total cholesterol to millimoles per liter, multiply by 0.0259; glucose to millimoles per liter, multiply by 0.0555; and hemoglobin A1c to proportion of total hemoglobin, multiply by 0.01.
Hypertension and dyslipidemia were defined as prescribed by cutoffs used to generate the cardiometabolic stress index. Prevalent cardiovascular disease was self-reported in the BPRHS and was defined as prior evidence of coronary heart disease, heart failure, and stroke in ARIC. Type 2 diabetes is defined in the text.
Molecular Pathways of CM Stress
After adjustment for age, sex, BMI, and smoking status, we found 260 metabolites significantly associated with CM stress (eTable 1 in the Supplement). The identified metabolites fit broadly into 3 major categories: (1) those with a previously reported association with CVD, diabetes, or longevity (eg, glutamate, taurine, isoleucine, valine, 2-aminoadipate, and aconitate); (2) those associated with diseases specified in the CM stress definition (eg, glucose, cholesterol, androgens, and catecholamine intermediates); and (3) several novel biomolecules (mainly lipids) not commonly described as functional biomarkers of inflammation or CVD. We next used experimentally defined metabolite-enzyme interactions from MetScape14 to identify enriched pathways of CM stress from WikiPathways (Figure 1). Pathways shared across both metabolically harmful and metabolically healthy gene sets included amino acid metabolism, Ras signaling, eicosanoid synthesis, and the Nrf2 pathway.
Figure 1. Pathway Analyses.
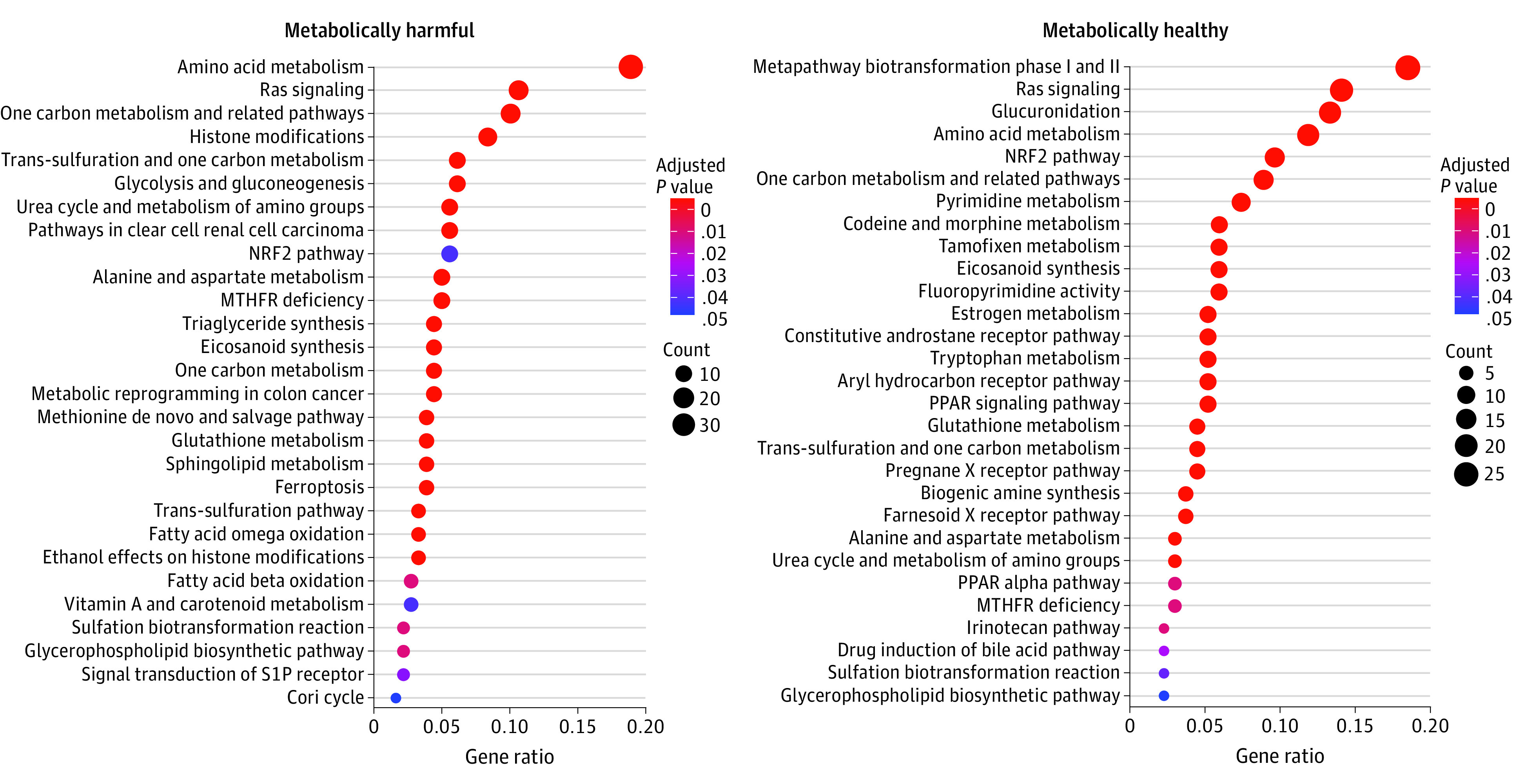
Dot plots of pathways enriched for genes linked to cardiometabolic stress (increased stress on the left; decreased on the right) based on an analysis against the WikiPathways collection. The significance (Benjamini-Hochberg–adjusted P value) is indicated by color gradient while the order, size, and x-axis are determined by the number of genes overlapping a given pathway. Gene ratio (x-axis) is the number of overlapping genes per pathway over the total possible number of overlapping genes across all pathways.
Construction of Metabolite-Based CM Stress Score
We trained an elastic net model (as specified in the Methods) for the CM stress index as a function of 125 metabolites that were matched between the ARIC study and the BPRHS, with a 10-fold cross-validation approach (5 repeats) for specification of the α and λ parameters (eTable 2 in the Supplement, ranked by variable importance). To obtain a parsimonious model that optimized the association with CM stress in the BPRHS (derivation cohort), we next conducted a series of linear regressions for the CM stress index, including successively more metabolites (ordered by variable importance) in each age-adjusted and sex-adjusted model and using a cross-validated model R2 (eFigure 2 and eTable 2 in the Supplement). We found that 30 metabolites (plus age and sex) optimized cross-validated model R2 (adjusted R2 = approximately 0.42). Both the full elastic net model (125 metabolites) and the parsimonious model (30 metabolites) demonstrated calibration against the CM stress index in the BPRHS and were in agreement with each other (Pearson r between models, 0.95; P < .001; eFigure 3 in the Supplement). Across these 30 metabolites, we observed a maximal imputation rate approximately equal to 9% in the ARIC cohort, suggesting high expression of subselected metabolites in the ARIC study. We evaluated the parsimonious model in the ARIC study (30 metabolites).
Association of Metabolite-Based CM Stress With Risk for CHD and Mortality Over 30 Years
Over 30 years of follow-up, we identified 364 cases of incident CHD (16.9%) and 1113 deaths (51.7%) in the ARIC study; a final analytic data set with full covariate availability for survival analysis included 1068 deaths (724 noncardiovascular) and 282 incident CHD events (233 myocardial infarctions and 49 fatal CHD events). The results of survival analysis are shown in Table 3 and Figure 2. We observed an adjusted 14% increased hazard of CHD and 15% increased hazard of all-cause mortality per SD of metabolomic CM stress (CHD: hazard ratio, 1.14 [95% CI, 1.00-1.31]; all-cause mortality: hazard ratio, 1.15 [95% CI, 1.07-1.24]). Although we did not observe an effect modification of the association of CM stress with CHD by sex, we did observe an effect modification of the association of CM stress with mortality; increased metabolomic CM stress was primarily associated with all-cause mortality in women (per SD of metabolomic CM stress for women: hazard ratio, 1.26 [95% CI, 1.14-1.40]; per SD of metabolomic CM stress for men: hazard ratio, 1.06 [95% CI, 0.96-1.18]; P = .01 for interaction), perhaps reflecting the female predominance of the derivation cohort. In a sensitivity analysis including the Pooled Cohort Equation score, we observed similar results (Table 3, model 3). There were no improvements in reclassification or discrimination via addition of metabolite-based CM stress score to adjustment (Table 3, model 2).
Table 3. Multivariable Survival Analysis in the Atherosclerosis Risk in Communities Studya.
| Metabolomic risk (HR per SD increase in CM stress) | No. (No. of events) | HR (95% CI) | P value | C (95% CI)b | ∆C (95% CI)b | NRI (95% CI)b | IDI (95% CI)b |
|---|---|---|---|---|---|---|---|
| CHDc | |||||||
| Model 1 | 1947 (282) | 1.40 (1.24 to 1.58) | <.001 | 0.656 (0.621 to 0.691) | 0.029 (0.005 to 0.054) | 0.142 (0.036 to 0.241) | 0.027 (0.011 to 0.048) |
| Model 2 | 1947 (282) | 1.14 (1.00 to 1.31) | .045 | 0.738 (0.704 to 0.772) | 0.001 (−0.004 to 0.006) | 0.121 (0.024 to 0.230) | 0.010 (0.002 to 0.027) |
| Model 3 | 1947 (282) | 1.13 (1.00 to 1.28) | .05 | 0.715 (0.681 to 0.750) | 0.002 (−0.004 to 0.008) | −0.007 (−0.124 to 0.104) | 0.003 (−0.001 to 0.011) |
| All-cause mortality | |||||||
| Model 1 | 2076 (1068) | 1.33 (1.24 to 1.41) | <.001 | 0.685 (0.670 to 0.701) | 0.020 (0.011 to 0.028) | 0.140 (0.071 to 0.209) | 0.029 (0.015 to 0.043) |
| Model 2 | 2076 (1068) | 1.15 (1.07 to 1.24) | <.001 | 0.734 (0.720 to 0.749) | 0.003 (0.000 to 0.006) | 0.046 (−0.043 to 0.122) | 0.005 (0.000 to 0.012) |
Abbreviations: CHD, coronary heart disease; CM, cardiometabolic; HR, hazard ratio; IDI, integrated discrimination improvement; NRI, net reclassification index.
Adjustments: model 1: age, sex, and race/ethnicity adjusted for center; model 2: model 1 plus smoking, systolic blood pressure, use of medications for hypertension, type 2 diabetes status, total cholesterol, high-density lipoprotein cholesterol, and estimated glomerular filtration rate for incident CHD analysis; all-cause mortality analysis was further adjusted for body mass index and prevalent cardiovascular disease; and model 3: for CHD only; adjusted for center, sex, and Pooled Cohort Equation.
The C statistics and NRI and IDI are computed in reference to models without the CM score added vs those with the CM score added.
Analyses for incident CHD excluded individuals with prevalent CHD at baseline (accounting for the difference in number of individuals per regression).
Figure 2. Unadjusted Kaplan-Meier Survival Curves by Quartiles of Cardiometabolic Stress Index in the Atherosclerosis Risk in Communities Study.

A, Incident coronary heart disease (CHD). B, All-cause mortality. The P values are the result of log-rank tests in Kaplan-Meier analysis. Shaded areas indicate 95% CIs.
Discussion
Our central aim was to understand the molecular basis for CM stress and its association with long-term outcomes relevant to CVD and longevity. In a cohort of Puerto Rican individuals with prevalent CMD, we identified 260 metabolites associated with multisystem CM stress, independent of age, sex, BMI, and smoking. Several metabolites had known association with common pathways of aging, insulin resistance, inflammation, and CVD (eg, glutamate,23 branched-chain amino acids,24,25 2-aminoadipate,26 aconitate,27 ceramides,28 and sphingomyelins29). Mapping these empirical associations onto known enzymatic pathways implicated known and novel genetic-metabolic networks in CMD. We observed significant and modest associations between the metabolite-based CM stress score and CHD and mortality over 30 years in ARIC, independent of CMD traits and CVD risk factors. These findings demonstrate that metabolites linked to CM stress identify important, key metabolic pathway alterations in CMD and are associated with long-term outcomes relevant to CVD for future molecular study.
A slowing in CVD improvement in the face of modern prevention efforts has prompted studies on an associated role for CMD.1 Cardiometabolic disease has classically been defined through clinical component traits of metabolic syndrome (eg, pro-atherogenic dyslipidemia, visceral adiposity, hypertension, and dysglycemia). Although this construct has been helpful in epidemiology and prevention efforts, its focus on end-phenotypes of CMD limits its ability to resolve specific mechanisms that underlie CMD. In this regard, genetic and epigenetic studies of large human populations have been conducted to understand a potential basis for CMD. In seminal studies of the circulating metabolome in humans, shared metabolic pathways have been associated with components of CMD, including physical activity, insulin resistance, and obesity (eg, fatty acids, branched-chain amino acids, and Krebs cycle). Nevertheless, most of these studies have focused on single components of CMD for discovery and do not forge a link between metabolomic signatures of CMD with CVD, a need in therapeutic discovery efforts.
Here, we studied a validated CM stress index to explore the molecular basis for CMD and its association with CVD. The index used in this study incorporates components of a traditional metabolic syndrome as well as systemic inflammation, adrenal, and sympathetic nervous system activity markers of CM stress. Several metabolites uncovered in this study have been previously linked to CMD and CVD, increasing confidence in their reproducibility (eg, glutamate). We also identified several novel molecules associated with CM stress not previously widely reported, to our knowledge (eg, phospholipids, sphingomyelins, and amino acid derivatives). More important, the integrative genetic-metabolic network methods used here suggest that these CM stress–related metabolites may link CMD to central mechanisms of CVD and health. For example, Ras signaling plays a well-established role in CMD, regulating central components of metabolism integral to nutrient processing, insulin resistance, and myocardial hypertrophy (eg, AMPK [5′ adenosine monophosphate-activated protein kinase] and mTOR [mammalian target of rapamycin]).30,31,32,33 In addition, Nrf2 has been implicated in CMD34 and aging and longevity35,36 and regulates many genes involved in oxidative stress, inflammation, drug conjugation and detoxification, and mitochondrial biogenesis.37 Eicosanoids—central mediators of initiation and resolution of inflammation—also emerged from these studies as linked to stress. Several metabolites associated with CM stress in this study have been previously modified to ameliorate mechanisms of CVD: supplementation of taurine and arginine (higher levels associated with lower CM stress) may improve endothelial function, inflammation, oxidative stress, and atherogenesis.38,39,40 Some metabolites may be associated with dietary exposures,41 suggesting the potential for nutritional interventions.
Limitations
This study has some limitations. The results of this study should be viewed in the context of its design. The effect size of the metabolite-based CM stress score for CHD and mortality was smaller relative to other protein-based markers that are closer to disease (eg, high-sensitivity troponin42), which may limit direct clinical translation. We limited the metabolites included and used a priori–defined imputation, which may introduce biased regression estimates, although restriction of metabolites expressed in most participants mitigates this risk. Second, the subset of overlapping metabolites was limited between the BPRHS and the ARIC study, which may have led to submaximal model performance. Our results are based on 2 different observational studies (the BPRHS and the ARIC study) with disparate prevalent CMD and racial composition and residual confounding (eg, cancer and HIV infection in the BPRHS), and the use of a CM stress score (as opposed to its metabolic components) may limit discovery for each individual parameter of the score. Further studies in large composite cohorts should be performed to address imputation, unannotated metabolite significance, confounding, and different end points (eg, cancer or diabetes). Third, while these studies only accessed circulating metabolites (limiting the ability to infer a direct cellular role), there are many examples of circulating metabolite–based mechanistic discoveries that begin in clinical observation.43,44,45 Pathway analysis remains a strictly exploratory method, with continuous updating of annotated metabolite-gene databases that may improve mapping and candidate pathway identification for mechanistic study.
Conclusions
Metabolite signatures linked to CM stress specify known and novel pathways and are linked to CVD and all-cause mortality at a 30-year horizon. These findings provide evidence of the shared metabolic underpinnings of CMD and CVD, indicate novel loci for mechanistic investigation, and highlight the role of metabolic characterization in human studies in the targeting and prevention of CVD.
eTable 1. Association of 260 Metabolites With CM Stress Index
eTable 2. Results of Elastic Net and Linear Regressions for Allostatic Load in BPRHS
eFigure 1. Construction of the BPRHS Study Subsample
eFigure 2. Model Optimization Across 125 Metabolites in BPRHS
eFigure 3. CM Stress and Metabolite-Based Score in BPRHS
eAppendix. Supplemental Methods
References
- 1.Shah NS, Lloyd-Jones DM, O’Flaherty M, et al. Trends in cardiometabolic mortality in the United States, 1999-2017. JAMA. 2019;322(8):780-782. doi: 10.1001/jama.2019.9161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mattei J, Demissie S, Falcon LM, Ordovas JM, Tucker K. Allostatic load is associated with chronic conditions in the Boston Puerto Rican Health Study. Soc Sci Med. 2010;70(12):1988-1996. doi: 10.1016/j.socscimed.2010.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The ARIC Investigators The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129(4):687-702. doi: 10.1093/oxfordjournals.aje.a115184 [DOI] [PubMed] [Google Scholar]
- 4.Warnick GR, Mayfield C, Benderson J, Chen JS, Albers JJ. HDL cholesterol quantitation by phosphotungstate-Mg2+ and by dextran sulfate-Mn2+-polyethylene glycol precipitation, both with enzymic cholesterol assay compared with the lipid research method. Am J Clin Pathol. 1982;78(5):718-723. doi: 10.1093/ajcp/78.5.718 [DOI] [PubMed] [Google Scholar]
- 5.Siedel J, Hägele EO, Ziegenhorn J, Wahlefeld AW. Reagent for the enzymatic determination of serum total cholesterol with improved lipolytic efficiency. Clin Chem. 1983;29(6):1075-1080. doi: 10.1093/clinchem/29.6.1075 [DOI] [PubMed] [Google Scholar]
- 6.Nägele U, Hägele EO, Sauer G, et al. Reagent for the enzymatic determination of serum total triglycerides with improved lipolytic efficiency. J Clin Chem Clin Biochem. 1984;22(2):165-174. doi: 10.1515/cclm.1984.22.2.165 [DOI] [PubMed] [Google Scholar]
- 7.Levey AS, Stevens LA, Schmid CH, et al. ; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) . A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604-612. doi: 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.White AD, Folsom AR, Chambless LE, et al. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) Study: methods and initial two years’ experience. J Clin Epidemiol. 1996;49(2):223-233. doi: 10.1016/0895-4356(95)00041-0 [DOI] [PubMed] [Google Scholar]
- 9.Evans AM, Bridgewater BR, Liu Q, et al. High resolution mass spectrometry improves data quantity and quality as compared to unit mass resolution mass spectrometry in high-throughput profiling metabolomics. Metabolomics. 2014;4:132. doi: 10.4172/2153-0769.1000132 [DOI] [Google Scholar]
- 10.Yu B, Heiss G, Alexander D, Grams ME, Boerwinkle E. Associations between the serum metabolome and all-cause mortality among African Americans in the Atherosclerosis Risk in Communities (ARIC) Study. Am J Epidemiol. 2016;183(7):650-656. doi: 10.1093/aje/kwv213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rhee EP, Waikar SS, Rebholz CM, et al. ; CKD Biomarkers Consortium . Variability of two metabolomic platforms in CKD. Clin J Am Soc Nephrol. 2019;14(1):40-48. doi: 10.2215/CJN.07070618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seeman TE, Singer BH, Rowe JW, Horwitz RI, McEwen BS. Price of adaptation—allostatic load and its health consequences: MacArthur studies of successful aging. Arch Intern Med. 1997;157(19):2259-2268. doi: 10.1001/archinte.1997.00440400111013 [DOI] [PubMed] [Google Scholar]
- 13.Chong J, Soufan O, Li C, et al. MetaboAnalyst 4.0: towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018;46(W1):W486-W494. doi: 10.1093/nar/gky310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karnovsky A, Weymouth T, Hull T, et al. Metscape 2 bioinformatics tool for the analysis and visualization of metabolomics and gene expression data. Bioinformatics. 2012;28(3):373-380. doi: 10.1093/bioinformatics/btr661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shannon P, Markiel A, Ozier O, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13(11):2498-2504. doi: 10.1101/gr.1239303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gustavsen JA, Pai S, Isserlin R, Demchak B, Pico AR. RCy3: network biology using Cytoscape from within R. F1000Res. 2019;8:1774. doi: 10.12688/f1000research.20887.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Slenter DN, Kutmon M, Hanspers K, et al. WikiPathways: a multifaceted pathway database bridging metabolomics to other omics research. Nucleic Acids Res. 2018;46(D1):D661-D667. doi: 10.1093/nar/gkx1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu G, Wang LG, Han Y, He QY. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS. 2012;16(5):284-287. doi: 10.1089/omi.2011.0118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yadlowsky S, Hayward RA, Sussman JB, McClelland RL, Min YI, Basu S. Clinical implications of revised pooled cohort equations for estimating atherosclerotic cardiovascular disease risk. Ann Intern Med. 2018;169(1):20-29. doi: 10.7326/M17-3011 [DOI] [PubMed] [Google Scholar]
- 20.Uno H, Cai T, Pencina MJ, D’Agostino RB, Wei LJ. On the C-statistics for evaluating overall adequacy of risk prediction procedures with censored survival data. Stat Med. 2011;30(10):1105-1117. doi: 10.1002/sim.4154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pencina MJ, D’Agostino RB Sr, D’Agostino RB Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27(2):157-172. doi: 10.1002/sim.2929 [DOI] [PubMed] [Google Scholar]
- 22.Pencina MJ, D’Agostino RB Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30(1):11-21. doi: 10.1002/sim.4085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng Y, Hu FB, Ruiz-Canela M, et al. Metabolites of glutamate metabolism are associated with incident cardiovascular events in the PREDIMED PREvención con DIeta MEDiterránea (PREDIMED) Trial. J Am Heart Assoc. 2016;5(9):e003755. doi: 10.1161/JAHA.116.003755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Würtz P, Tiainen M, Mäkinen VP, et al. Circulating metabolite predictors of glycemia in middle-aged men and women. Diabetes Care. 2012;35(8):1749-1756. doi: 10.2337/dc11-1838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang TJ, Larson MG, Vasan RS, et al. Metabolite profiles and the risk of developing diabetes. Nat Med. 2011;17(4):448-453. doi: 10.1038/nm.2307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang TJ, Ngo D, Psychogios N, et al. 2-Aminoadipic acid is a biomarker for diabetes risk. J Clin Invest. 2013;123(10):4309-4317. doi: 10.1172/JCI64801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng S, Larson MG, McCabe EL, et al. Distinct metabolomic signatures are associated with longevity in humans. Nat Commun. 2015;6:6791. doi: 10.1038/ncomms7791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ertunc ME, Hotamisligil GS. Lipid signaling and lipotoxicity in metaflammation: indications for metabolic disease pathogenesis and treatment. J Lipid Res. 2016;57(12):2099-2114. doi: 10.1194/jlr.R066514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Floegel A, Stefan N, Yu Z, et al. Identification of serum metabolites associated with risk of type 2 diabetes using a targeted metabolomic approach. Diabetes. 2013;62(2):639-648. doi: 10.2337/db12-0495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Slack C, Alic N, Foley A, Cabecinha M, Hoddinott MP, Partridge L. The Ras-Erk-ETS-signaling pathway is a drug target for longevity. Cell. 2015;162(1):72-83. doi: 10.1016/j.cell.2015.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Slack C. Ras signaling in aging and metabolic regulation. Nutr Healthy Aging. 2017;4(3):195-205. doi: 10.3233/NHA-160021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kennedy BK, Lamming DW. The mechanistic target of rapamycin: the grand conducTOR of metabolism and aging. Cell Metab. 2016;23(6):990-1003. doi: 10.1016/j.cmet.2016.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kodiha M, Rassi JG, Brown CM, Stochaj U. Localization of AMP kinase is regulated by stress, cell density, and signaling through the MEK→ERK1/2 pathway. Am J Physiol Cell Physiol. 2007;293(5):C1427-C1436. doi: 10.1152/ajpcell.00176.2007 [DOI] [PubMed] [Google Scholar]
- 34.Ramprasath T, Selvam GS. Potential impact of genetic variants in Nrf2 regulated antioxidant genes and risk prediction of diabetes and associated cardiac complications. Curr Med Chem. 2013;20(37):4680-4693. doi: 10.2174/09298673113209990154 [DOI] [PubMed] [Google Scholar]
- 35.Bruns DR, Drake JC, Biela LM, Peelor FF III, Miller BF, Hamilton KL. Nrf2 signaling and the slowed aging phenotype: evidence from long-lived models. Oxid Med Cell Longev. 2015;2015:732596. doi: 10.1155/2015/732596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kubben N, Zhang W, Wang L, et al. Repression of the antioxidant NRF2 pathway in premature aging. Cell. 2016;165(6):1361-1374. doi: 10.1016/j.cell.2016.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ma Q. Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol. 2013;53:401-426. doi: 10.1146/annurev-pharmtox-011112-140320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahmadian M, Roshan VD, Aslani E, Stannard SR. Taurine supplementation has anti-atherogenic and anti-inflammatory effects before and after incremental exercise in heart failure. Ther Adv Cardiovasc Dis. 2017;11(7):185-194. doi: 10.1177/1753944717711138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Katakawa M, Fukuda N, Tsunemi A, et al. Taurine and magnesium supplementation enhances the function of endothelial progenitor cells through antioxidation in healthy men and spontaneously hypertensive rats. Hypertens Res. 2016;39(12):848-856. doi: 10.1038/hr.2016.86 [DOI] [PubMed] [Google Scholar]
- 40.Ellis AC, Patterson M, Dudenbostel T, Calhoun D, Gower B. Effects of 6-month supplementation with β-hydroxy-β-methylbutyrate, glutamine and arginine on vascular endothelial function of older adults. Eur J Clin Nutr. 2016;70(2):269-273. doi: 10.1038/ejcn.2015.137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Esko T, Hirschhorn JN, Feldman HA, et al. Metabolomic profiles as reliable biomarkers of dietary composition. Am J Clin Nutr. 2017;105(3):547-554. doi: 10.3945/ajcn.116.144428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Lemos JA, Drazner MH, Omland T, et al. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA. 2010;304(22):2503-2512. doi: 10.1001/jama.2010.1768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou M, Shao J, Wu CY, et al. Targeting BCAA catabolism to treat obesity-associated insulin resistance. Diabetes. 2019;68(9):1730-1746. doi: 10.2337/db18-0927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roberts LD, Boström P, O’Sullivan JF, et al. β-Aminoisobutyric acid induces browning of white fat and hepatic β-oxidation and is inversely correlated with cardiometabolic risk factors. Cell Metab. 2014;19(1):96-108. doi: 10.1016/j.cmet.2013.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lecoutre S, Dollet L, et al. Glutamine links obesity to inflammation in human white adipose tissue. Cell Metab. 2020;31(2):375-390. doi: 10.1016/j.cmet.2019.11.019 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Association of 260 Metabolites With CM Stress Index
eTable 2. Results of Elastic Net and Linear Regressions for Allostatic Load in BPRHS
eFigure 1. Construction of the BPRHS Study Subsample
eFigure 2. Model Optimization Across 125 Metabolites in BPRHS
eFigure 3. CM Stress and Metabolite-Based Score in BPRHS
eAppendix. Supplemental Methods


