Abstract
Lipoprotein (a) [Lp(a)] is a low-density, cholesterol-containing lipoprotein that differs from other low-density lipoproteins due to the presence of apolipoprotein(a) bound to its surface apolipoprotein B100. Multiple epidemiologic studies, including Mendelian Randomization studies, have demonstrated that increasing Lp(a) levels are associated with increased risk of heart disease, including atherosclerotic cardiovascular disease and calcific aortic stenosis. The risk associated with elevations in Lp(a) appears to be independent of other lipid markers. While the current treatment options for elevated Lp(a) are limited, promising new therapies are under development, leading to renewed interest in Lp(a). This review provides an overview of the biology and epidemiology of Lp(a), available outcome studies, and insights into future therapies.
Keywords: Lipoprotein (a), Cardiovascular Disease, Residual Risk, Calcific Aortic Stenosis
Introduction:
Despite advances in treatment of traditional risk factors for cardiovascular disease (CVD), many still remain with residual CVD risk despite optimal medical therapy1.One such lipid biomarker that confers residual risk is lipoprotein (a) [Lp(a)], which has been associated with premature accelerated atherosclerosis, ischemic heart disease, and calcific aortic stenosis.2-6. Lp(a) is further recognized in the 2018 ACC/AHA cholesterol guidelines as a risk enhancer 7,8. While there is no available treatment for Lp(a) alone, multiple therapies directly targeting Lp(a) are currently being evaluated, raising the possibility for Lp(a) to become a modifiable risk factor.
The Molecular Structure of Lp(a):
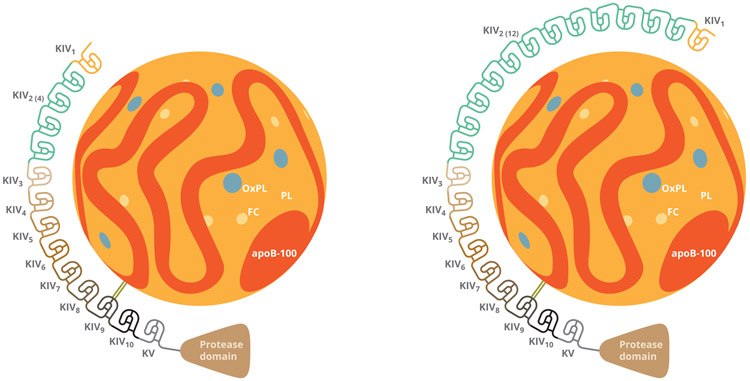
Lp(a) is a cholesterol-containing low-density lipoprotein. Similar to a low density lipoprotein cholesterol (LDL-C) particle, Lp(a) particles each contain a single apolipoprotein B100 (apoB). Where Lp(a) particles differ from LDL-C particles is the presence of an apolipoprotein (a) (apo(a)) covalently bound by a single disulfide bond to the apoB molecule (Figure 1). Apo(a) evolved from the plasminogen gene9. Normally plasminogen is composed of five kringles (KI, KII, KIII, KIV, and KV) and a protease domain. However, apo(a) does not contain KI, KII, or KIII, but instead varying numbers of KIV, one copy of KV, and an inactive protease domain. Given the differing numbers of KIV repeats, there are several different isoforms of apo(a), and thus several different sizes of Lp(a) (> 40 different isoforms)9,10. Importantly, each Lp(a) particle also contains a core of cholesterol ester and triglycerides, similar to a low density lipoprotein particle. Therefore, elevated levels of Lp(a) can falsely elevate measured plasma LDL-C levels since traditional clinically used assays cannot distinguish between Lp(a) bound LDL-C and free LDL-C10-12. Other molecules inside the Lp(a) core include oxidized phospholipids (OxPL).
Figure 1: Structure of Lp(a):
Molecular structure of two Lp(a) isoforms showing apoB bound by a disulfide bond to isomer of apo(a) with varying levels of KIV repeats. ApoB-100= apolipoprotein B100; OxPL= Oxidized Phospholipid; FC= Free Cholesterol; PL= Phospholipid; KIV= Kringle 4
Though there is not general consensus, Lp(a) is believed to cause atherosclerotic disease either through pro-atherogenic, pro-inflammatory, and/or pro-thrombotic mechanisms. Due to the concentration of OxPL, its contribution to atherogenesis may be promoted by oxidative mechanisms and the effects of these mechanisms on macrophages after entry into the vessel wall13,14. Additionally, apo(a) may have anti-fibrinolytic effects by antagonizing plasminogen-binding sites (given similar homology) and allowing more accumulation within the vascular endothelium7. The purported pro-thrombotic effect of Lp(a) needs further study, but is also felt to be mediated by inflammatory mechanisms15.
Measurement of Lp(a)
Currently there is no standardized assay used to measure serum or plasma Lp(a) levels. Immunochemical methods are the most common and can be divided into “mass dependent” or “mass independent” assays16. Mass independent assays employ antibodies that target non-repeating kringles of Lp(a) to measure the actual particle number16. These assays are reported in nanomoles per liter (nmol/L). Mass dependent assays compute the entire molecular content (proteins, lipids, carbohydrates, etc) of Lp(a) and expressed in the SI unit of milligrams per liter16. Routine clinical testing of these assays are reported in mg/dL. Mass dependent assays can be limited due to heterogeneity of the Lp(a) particle size (i.e overestimation in cases of large isoforms and underestimation in cases of small isoforms). Many laboratories use calibrators to help overcome this limitation and link the results to reference reagents7. However, the lack of standardization could result in confounding when comparing between various assays.
Genetics of Lp(a):
Approximately 90% of circulating Lp(a) levels are inherited and strongly determined by a single gene, the LPA gene17. As a result, unlike other traditional lipid measures, lifestyle changes have little impact on Lp(a) levels.18,19 Furthermore, Lp(a) levels are fairly stable over time. The size of the molecule is determined by polymorphisms of apo(a) due to a variable number of kringle IV repeats in the LPA gene allowing for numerous variants of the molecule. The LPA gene is highly expressed in the liver and the regulation of its expression is not well understood. Some variants in the gene have higher plasma concentrations of plasma Lp(a) levels. These variations include splice site mutations, non-sense mutations, single nucleotide polymorphisms (SNPs), or other non-genetic factors influencing Lp(a) levels, such as kidney, thyroid, or liver diseases17. Genetic conditions such as familial hypercholesterolemia, familial defective apoB, abetalipoproteinaemia, variants of apolipoprotein E (APOE)and others also influence Lp(a) concentrations17.
Certain Lp(a) variants have been shown to be more associated with coronary artery disease and cardiovascular events. For example, in a European genome wide association study two variants within the LPA locus (rs10455872 and rs3798220) showed an odds ratios (OR) for coronary disease of 1.70 (95% CI 1.49-1.95) and 1.92 (95% CI 1.48-2.49), respectively20. These variants were also associated with increased levels of circulating Lp(a). Moreover, there was a graded risk of coronary disease with the number of LPA gene variations20. Similarly, in a multinational study of 63,746 cases of coronary artery disease and 130,681 controls, there was a genome wide significance within the LPA locus for CVD events 21. Interestingly, this genetic association was numerically more potent than those for LDLR and PCSK921.
Furthermore, several Mendelian randomization (MR) studies have shown causal links between LPA gene variations and CVD20,22,23,24. MR methods are designed to see if a genetic exposure causes an outcome and addresses concerns about confounding that typical observational studies are often limited by. While a better methodology to help determine causality is a randomized clinical trial, clinical trials can be very expensive and time consuming if incorporating large populations. MR studies overcome some of these limitations, by determining causality based on genetic data without barriers of a clinical trial.
Epidemiology of Lp(a):
Because Lp(a) is not routinely measured as part of the traditional lipid panel, estimates of the population distribution of Lp(a) remain less clear than for traditional lipid biomarkers. The epidemiology of Lp(a) has been studied in several large cohort studies25-28. Other studies have evaluated the distribution of Lp(a) in convenience samples of those who have it measured in clinical practice, but are likely impacted by surveillance bias as healthy adults are less likely to have Lp(a) levels tested than those with a positive family history of cardiovascular disease29,30. In the United States, data from tertiary care referral centers found that approximately 35-40% of patients had Lp(a) levels > 30 mg/dL, and 24-30% have levels > 50 mg/dL25. Lp(a) levels have a similar distribution between men and women with approximately 20% in both groups having Lp(a) levels > 50 mg/dL31. Lp(a) levels can also vary by race: African Americans have been shown to have higher mean Lp(a) values when compared to Whites, Chinese, and Hispanics 32. It is currently not clear if race or gender significantly alters the clinical prognosis of elevated Lp(a), but certainly an area of future study.
Lp(a) has also been shown to be a risk modifier in patients with monogenic lipid disorders including heterozygous Familial Hypercholesterolemia (FH). Studies have found that in addition to LDL-C, FH patients have higher Lp(a) levels than non-FH patients30,33. Since the Lp(a) molecule can overestimate free LDL-C levels, it is thought that elevated Lp(a) may explain certain phenotypes of FH that are gene negative. This observation was first seen in Spanish subjects where elevated Lp(a) accounted for up to 6% of autosomal dominant hypercholesterolemia in patients without a LDLR or apoB mutation34. Furthermore, in a Danish study by Langsted et. al. the investigators found that elevated Lp(a) and LPA risk genotypes could account for a quarter of patients diagnosed with clinical FH6. However, Lp(a) also predicts CAD manifestations in patients with genetically proven FH. In a study that included two registries of patients with a genetic diagnosis of FH, patients with elevated Lp(a) were more likely to have a previous myocardial infarction (MI), stroke, or peripheral arterial disease (PAD) compared to those without elevated Lp(a)35. Thus, screening for Lp(a) in patients with FH can help with further risk stratification, and identify an already high risk population that may warrant targeted Lp(a) therapy. In the United states, serum values for Lp(a) that are considered clinically to represent negligible risk for CVD is < 30 mg/dL (<75 nmol/L)7,36. The European Atherosclerosis Society Guideline uses a threshold of 50 mg/dL to identify those at high risk. 7
Role of Lp(a) in Predicting Risk in Primary Prevention:
In one of the largest clinical studies to evaluate the impact of Lp(a) from Denmark, majority of a 40,486 patient population was free of ischemic heart disease. The investigators found that after a 16 year follow up there was a graded risk of MI across levels of Lp(a)22. Higher number of kringle IV repeats were also associated with lower Lp(a) values and less MIs, possibly suggesting that larger isoforms may be less pathogenic22. Similar results were found in a more recent, but smaller, prospective study from Greece 37.
These findings in primary prevention have also been reproduced outside of Europe, both in Pakistan and in a large United States cohort from the Atherosclerosis Risk in Community (ARIC) study38, 39. Moreover, subgroup analyses from ARIC have shown a ~41% higher risk of ischemic stroke after adjustment of lipid profiles in patients with Lp(a) > 50 mg/dL26. Lp(a) has also been seen as a marker of risk in women free of disease from the Women’s Health Study where post-menopausal participants with hypercholesterolemia had a ~88% higher risk for 10 year first CVD event if Lp(a) was > 50 mg/dL40.
Knowing that patients in a primary prevention setting with elevated Lp(a) are at risk, a question arises if this risk is potentially modulated by LDL-C levels. In a study combining two large primary prevention prospective cohorts investigators concluded that at LDL-C levels below 2.5 mmol/L (~97 mg/dL) the risk of elevated Lp(a) appeared to be attenuated, a finding not seen in secondary prevention studies41. Thus, the results suggest an importance of reducing LDL-C levels as much as possible in the primary prevention setting for a patient with elevated Lp(a), as Lp(a) can serve to re-classify risk. A summary of the noted primary prevention studies is seen in table 1.
Table 1:
Summary of Noted Primary Prevention Observational Studies of Lp(a) in CVD Risk Prediction
| Author, year |
Country | Size | Age (Years) |
Gender (Male) |
Population | Follow up | Main Outcome |
Main Results |
|---|---|---|---|---|---|---|---|---|
| Kamstrup 2009 | Denmark | 40,486 | 58 | 48% | Danish | 16 years | MI | Graded risk for MI with Elevated Lp(a) |
| Kouvari 2019 | Greece | 3,042 | 44 | 48% | Greek | 10 years | MACE | Lp(a) > 50 mg/dL with high risk of MACE |
| Saleheen, 2017 | Pakistan | 17,644 | 54 | 80% | Pakistani | MR study | MI | Lp(a) concentration associated with MI risk |
| Agarwala, 2017 | United States | 14,154 | 55 | 45% | United States | 23.4 years | MI,HF | Lp(a) levels were associated with incident MI related heart failure |
| Aronis 2017 | United States | 9,908 | 63 | 43% | United States | 15 years (median) | Stroke | High Lp(a) was associated with a 42% relative increase in stroke risk |
| Cook 2018 | United States | 8158 | 54 | 0% | US | 10 years | MACE | Direct linear relationship of Lp(a) with MACE |
| Verbeek 2018 | Denmark and UK | 26,102 | 59 | 45% | British and Danish | Minimum 5 years | MACE | Lp(a) ≥80th percentile were at increased CVD risk |
• Major Adverse Cardiovascular Event (MACE): Non-fatal MI, Non-Fatal Stroke, CV death, Coronary Revascularization
• MI: Myocardial Infarction
• HF: Heart Failure
Role of Lp(a) in Predicting Risk in Secondary Prevention:
Analyses from clinical trials have also highlighted Lp(a) as a marker for residual cardiovascular risk in those with established cardiovascular disease. In the AIM-HIGH trial (Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglyceride and Impact on Global Health Outcomes) patients with LDL-C of 62.5 mg/dL, but with Lp(a) levels > ~50 mg/dL had a 89% higher risk of major adverse cardiovascular events (MACE ) than those with similar LDL-C but lower Lp(a)42. Similarly, in the LIPID trial (Long term Intervention with Pravastatin in Ischemic Disease) patients with an LDL-C of 112 mg/dL and Lp(a) > 73.7 mg/dL had a 21% higher chance of MACE43. In the ACCELERATE trial (The assessment of the clinical effects of cholesteryl ester transfer protein inhibition with Evacetrapib in patients at high risk for vascular outcomes), increasing Lp(a) levels were associated with increasing risk of MACE even if LDL-C was < 80 mg/dL on optimal medical management44.
Investigators also evaluated the impact of the proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor evolocumab on Lp(a) in an optimally medically managed secondary prevention population from the FOURIER (Further Cardiovascular Outcomes Research with PCSK9 inhibition in Subjects with Elevated Risk) trial45. In the placebo arm, the highest quartile of Lp(a) (>165 nm/L ~>69 mg/dL) had the highest risk of MACE, independent of LDL-C45. At 48 weeks, patients in the evolocumab arm had significantly reduced Lp(a) by a median (interquartile range) of 26.9% (6.2%-46.7%), and had reduced events by 23% in those with baseline Lp(a) > median (37 nmol/L ~ 15mg/dL). Furthermore, patients with baseline Lp(a) greater than the median on evolocumab had a higher absolute risk reduction (ARR) and lower number needed to treat (NNT) compared to the those with levels less than the median (ARR 2.49%; NNT 41 vs ARR 0.95%; NNT 105)45. Similar reductions in Lp(a) levels were also seen with alirocumab over 1.5 years from a pooled analysis of 10 phase 3 studies in the ODYSSEY program, as well as a reduction in MACE in post ACS patients46,47. Thus, these findings further support the benefit of PCSK9 inhibition in patients with elevated Lp(a) and provide a rational to screen for Lp(a) in certain instances. A summary of noted secondary prevention studies is seen in table 2.
Table 2:
Summary of Noted Secondary Prevention Studies of Lp(a) in CVD Risk Prediction within Clinical Trial Populations
| Author, year | Country | Size | Age (Year) |
Gender (Male) |
Study Type | Follow up | Main Outcome |
Main Results |
|---|---|---|---|---|---|---|---|---|
| Albers 2013 | United States and Canada | 3,144 | 64 | 85% | Post Hoc Analysis | 2 years | MACE | Lp(a) was predictive of MACE |
| Nestel 2013 | Australia and New Zealand | 7,863 | 62 | 83% | Post Hoc Analysis | 6 years (median) | CV death | Baseline Lp(a) was associated with CV death |
| Lincoff 2017 | Multi-National | 12,092 | 65 | 77% | Post Hoc Analysis | 2.3 years | MACE | Lp(a) in the highest quartile had higher events |
| O’Donoghue 2019 | Multi-national | 27,564 | 63 | 75% | Post Hoc Analysis | 1 year | MACE | Lp(a) lowering with PCSK9i had reduced MACE |
| Gaudet 2017 | Multi-national | 4,915 | 62 | 60% | Post Hoc Analysis | 1.5 years | Change in Lp(a) | Alirocumab resulted in 23-29% reductions in Lp(a) |
| Willeit 2018 | Multi-national | 29,069 | 62 | 72% | Meta-analysis | 3 years (median) | MACE | Increase in MACE with high Lp(a) level on statin therapy |
• Major Adverse Cardiovascular Event (MACE): Non-fatal MI, Non-Fatal Stroke, CV death, Coronary Revascularization
The Role of Lp(a) Beyond Coronary Disease:
Lp (a) and Peripheral Arterial Disease
While there are many commonalities amongst risk factors between atherosclerotic CAD and peripheral arterial disease (PAD), the underlying epidemiologic models are different. Interestingly, Lp(a) has also been linked to the development of PAD in several observational studies. In a study from the European Prospective Investigation into Cancer and Nutrition (EPIC-Norfolk) cohort, patients within the highest Lp(a) quartiles had an increased risk of developing PAD3. This association was also not modified by LDL-C level. Similarly, in the Scottish Heart Health Extended Cohort Lp(a) levels increased the 20 year risk of PAD and mortality 27. In both registries the predictive value of Lp(a) was higher for PAD than CAD. Furthermore, in patients with established PAD, elevated Lp(a) has also been shown to be linked to limb amputations48. However, large randomized clinical trials are needed to further understand Lp(a)’s impact on PAD outcomes.
Lp(a) and Stroke:
Lp(a) is also associated with the development of strokes. For instance in a large study of 36 prospective trials the authors found an adjusted risk ratio per 3.5 fold of Lp(a) concentration above normal limits of 1.10 (95% CI, 1.02-1.18)24. Similarly, in a meta-analysis of 20 articles comprising of 90,904 subjects the pooled estimated odds ratio was 1.41 (95% CI, 1.26-1.57) for case-control studies49. Interestingly, this finding was more pronounced in younger populations which is consistent with prior literature suggesting the risk of premature atherosclerosis across various vascular beds.
Lp(a) and Diabetes:
Diabetes Mellitus is also another well-known risk factor for CVD. However, the interaction between Lp(a) and diabetes is interesting and a topic of further investigation. A small number of studies have shown an inverse relationship between Lp(a) levels and onset diabetes based on the underlying genetic composition of Lp(a)50,51. Yet, there is limited literature on whether patients with both elevated Lp(a) and diabetes have more or less CVD risk than those without diabetes. In a recent multi-centered prospective analysis of Chinese patients with stable CAD, the investigators found a higher event rate in those with elevated Lp(a) and impaired glucose regulation compared to normal glucose regulation52. Similar studies are needed in western populations since patients with diabetes and CAD may also be good candidates to consider Lp(a) screening.
Risk of Calcific Aortic Valve Disease Associated with Elevated Lp(a) Levels
Calcific aortic stenosis (AS) also shares traditional risk factors with atherosclerotic CAD, but with different underlying models of risk. In a genome wide association study to identify genetic variants associated with calcific AS, a SNP in the LPA locus reached genome wide significance (OR per allele, 2.05 (95% CI 1.63-2.57); p-9.0 x 10−10) and was significant across multiple ethnicities5. The investigators also performed a prospective analyses and found the LPA genotype was associated with a higher incidence of AS and aortic valve replacement over time5. In another MR study, based on a multivariable adjusted HR model the authors found a graded association based on the level of Lp(a) and AS similar to prior CVD studies 4. These results have also been replicated and further support causality of AS by Lp(a)53.
The link between Lp(a) and AS can been seen in clinical trial cohorts as well. In a post hoc analysis from the Effects of Rosuvastatin on Aortic Stenosis Progression (ASTRONOMER) trial, investigators found that AS progression was linear and faster in patients with Lp(a) levels greater than 58.5 mg/dL, particularly patients who were less than 57 years of age54,55. Interestingly, patients with AS progression also had elevated oxidized phospholipids on apoB-containing particles (OxPL-apoB) which could suggest a synergistic role with Lp(a) in the pathophysiology of calcific valve disease. An enzyme known as autotaxin has also been found to promote inflammation and mineralization of the aortic valve and is transported to the aortic valve by Lp(a)56. In a recent study combing two prospective cohorts the investigators used multimodality imaging to assess valvular calcification activity by 18F-sodium fluoride (18F-NaF) positron emission tomography (PET), progression of calcification by computed tomography calcium scoring (CAC), and hemodynamic progression of AS by echocardiography in patients with both elevated Lp(a) and OxPL-apoB. The authors found both molecules were associated with AS, as well as its progression 57.
Therapeutic Modification of Lp(a) Levels:
There are currently no approved medications that specifically target Lp(a). Genetic studies suggest that Lp(a) reductions of up to 100mg/dL may be needed to detect a benefit on cardiovascular events58. Lifestyle changes are thought to have minimal effects since Lp(a) is genetically based18,19. There is some evidence that plant based diets may reduce Lp(a), but further study is needed in larger populations59. Statins also do not reduce Lp(a), and there is evidence to suggest they may actually increase Lp(a) levels by 10-20%60,61. Niacin at doses ranging from 1-3g per day has been shown to reduce Lp(a) levels by about 30%, however, Niacin has not been shown to improve CVD outcomes42. Estrogen and progestin replacement therapy in postmenopausal women has also been shown to reduce Lp(a) levels by 15-20%, but these have not been tested in women with elevated Lp(a) to determine if CV events can be reduced62. In general, HRT has been shown to actually increase CVD risk in postmenopausal women in part due to rises in certain lipid parameters, prothrombotic effects, and inflammation63. Mipomersen (anti-sense oligonucleotide targeting apoB) modestly drops Lp(a) levels by approximately 25%, but also with unknown clinical effect in patients with primarily elevated Lp(a)64,65. PCSK9 inhibitors have modest reductions in Lp(a), with the most benefit seen in patients with higher absolute values45.
A therapy that has shown the most clinical benefit thus far is Lp(a) apheresis with mean percent reductions up to 73%. One study of 120 patients with established coronary artery disease and a mean Lp(a) level of 4.21 μmol/L (4, 210 nmol/L; ~1,684 mg/dL) received apheresis for a mean duration of 5 years and had an 81% reduction in annual MACE (P < 0.0001)66. Similarly another apheresis study with lower Lp(a) levels of 170 high risk patients with mean LDL-C of 99.0 mg/dL and Lp(a) of 104.9 mg/dL showed significant reductions in cardiovascular events 2 years post apheresis67. It is important to note that the data from apheresis studies is non-randomized and largely observational68. Lipoprotein apheresis is generally reserved for patients with extremely elevated lipoproteinemia despite medical therapy or homozygous FH.
Efforts are currently being made to medically target Lp(a). A particular interest has risen in anti-sense oligonucleotides (ASO) targeting apo(a) on the Lp(a) molecule. Randomized controlled trials using an ASO to apo(a) have shown much promise with 62-92% reductions in Lp(a) in a dose dependent fashion, and with effect reversal off-treatment69. A summary of Lp(a) lowering therapies can be seen in table 3.
Table 3:
Impact of lipid lowering therapy on Lp(a). CETPi= cholesterylester transfer protein inhibitor; PCSK9= proprotein convertase subsilisin/kexin type 9; apo(a)= apolipoprotein (a)
| Therapy | Percent Change in Lp(a) |
|---|---|
| Apo(a) Antisense Oligonucleotide | Reduces by 70-90% |
| Lipoprotein Apheresis | Reduces by 30-40% |
| Niacin | Reduces by 30% |
| PCSK9 inhibitor | Reduces by 20-30% |
| CETPi | Reduces by 25% |
| Mipomersen | Reduces by 25% |
| Statin | May increase by 10-20% |
Future Directions:
As the evidence on Lp(a)’s impact on cardiovascular disease and calcific valvular disease continues to rise, efforts are focused on new pharmacotherapies targeting Lp(a). Large outcome trials of therapies dedicated to Lp(a) are on the horizon with the completion of the ASO phase 2 clinical trial70. Furthermore, efforts should also be made to help providers identify patients and families who would benefit from Lp(a) screening and targeted therapy. With the current implantation of real world evidence platforms where data can be collected from multiple sources (electronic health records, claims and billing platforms, product registries, health applications, etc) more clinically relevant information will be available to help identify these at risk populations earlier and possibly broaden these guideline recommendations. Current societal screening recommendations are seen in table 471.
Table 4:
Major Societal Guidelines on Lp(a) Screening
| Class | Level of Evidence |
|
|---|---|---|
| 2018 ACC/AHA Cholesterol Guidelines | ||
| Screening Lp(a) in intermediate risk patients who are considering statin therapy | IIa | B-NR* |
| 2019 European Society of Cardiology Cholesterol Guidelines | ||
| One time screening of Lp(a) in general to identify those at higher overall risk | IIa | C |
| 2019 National Lipid Association72 | ||
| First degree relatives with premature ASCVD (men <55; women <65 years of age) | IIa | C-LD** |
| A personal history of premature ASCVD | IIa | C-LD** |
| Suspected FH or primary severe hypercholesterolemia (LDL-C ≥ 190 mg/dL) | IIa | B-NR* |
| To aid in risk discussion for intermediate risk patients ages 40-75 | IIa | B-NR* |
| Identify cause of less than expected LDL-C lowering | IIb | C-LD** |
| Use as cascade screening in family members with severe hypercholesterolemia | IIb | C-LD** |
| Identify risk of progressive aortic stenosis | IIb | C-LD** |
Nonrandomized data
Limited data
Therefore, taking in all the available information, Lp(a) should be considered in (but not limited to) patients with intermediate ASCVD risk (7.5-19.9%), those with strong family histories, children of patients with elevated levels, personal history of ASCVD without traditional risk factors, patients with FH, patients with uncontrolled LDL-C on lipid lowering therapy, patients with poor statin response, or patients with recurrent events despite optimal therapy.
Conclusions:
In conclusion, Lp(a) is a cholesterol molecule that serves as a potent mediator of cardiovascular disease. Several types of studies have highlighted Lp(a) as a major risk factor for CVD, even in patients on optimal medical management. Increased awareness of Lp(a) is needed by all providers to better understand risk and for early referrals to specialized centers. Currently, there are only a few therapies that lower Lp(a), many with uncertain clinical benefit. However, clinical trials evaluating the role of ASOs targeting Lp(a) have shown promise for the future.
References
- 1.Wong ND, Zhao Y, Quek RGW, Blumenthal RS, Budoff MJ, Cushman M, Garg P, Sandfort V, Tsai M, Lopez JAG. Residual atherosclerotic cardiovascular disease risk in statin-treated adults: The Multi-Ethnic Study of Atherosclerosis. J Clin Lipidol 2017;11(5):1223–1233. doi: 10.1016/j.jacl.2017.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stubbs P Lipoprotein(a) as a risk predictor for cardiac mortality in patients with acute coronary syndromes. Eur Heart J 1998;19(9):1355–1364. doi: 10.1053/euhj.1998.1043. [DOI] [PubMed] [Google Scholar]
- 3.Gurdasani D, Sjouke B, Tsimikas S, Hovingh GK, Luben RN, Wainwright NWJ, Pomilla C, Wareham NJ, Khaw K-T, Boekholdt SM, Sandhu MS. Lipoprotein(a) and Risk of Coronary, Cerebrovascular, and Peripheral Artery Disease. Arterioscler Thromb Vasc Biol 2012;32(12):3058–3065. doi: 10.1161/ATVBAHA.112.255521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kamstrup PR, Tybjærg-Hansen A, Nordestgaard BG. Elevated Lipoprotein(a) and Risk of Aortic Valve Stenosis in the General Population. J Am Coll Cardiol. 2014;63(5):470–477 doi: 10.1016/j.jacc.2013.09.038. [DOI] [PubMed] [Google Scholar]
- 5.Thanassoulis G, Campbell CY, Owens DS, Smith JG, Smith AV., Peloso GM, Kerr KF, Pechlivanis S, Budoff MJ, Harris TB, Malhotra R, O’Brien KD, Kamstrup PR, Nordestgaard BG, Tybjaerg-Hansen A, Allison MA, Aspelund T, Criqui MH, Heckbert SR, Hwang S-J, Liu Y, Sjogren M, van der Pals J, Kälsch H, Mühleisen TW, Nöthen MM, Cupples LA, Caslake M, Di Angelantonio E, Danesh J, Rotter JI, Sigurdsson S, Wong Q, Erbel R, Kathiresan S, Melander O, Gudnason V, O’Donnell CJ, Post WS. Genetic Associations with Valvular Calcification and Aortic Stenosis. N Engl J Med 2013;368(6):503–512. doi: 10.1056/NEJMoa1109034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Langsted A, Kamstrup PR, Benn M, Tybjærg-Hansen A, Nordestgaard BG. High lipoprotein(a) as a possible cause of clinical familial hypercholesterolaemia: a prospective cohort study. Lancet Diabetes Endocrinol 2016;4(7):577–587. doi: 10.1016/S2213-8587(16)30042-0. [DOI] [PubMed] [Google Scholar]
- 7.Tsimikas S A Test in Context: Lipoprotein(a). J Am Coll Cardiol 2017;69(6):692–711. doi: 10.1016/j.jacc.2016.11.042. [DOI] [PubMed] [Google Scholar]
- 8.Grundy Scott M., Stone Neil J., Bailey Alison L. Beam, Craig Birtcher, Kim K, Blumenthal Roger S., Braun Lynne T., Ferranti Sarah D., Faiella-Tommasino Joseph, Forman Daniel E., Goldberg Ronald, Heidenreich Paul A., Hlatky Mark A., Jones Da J. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol. J Am Coll Cardiol 2019;73 (24):e285–350. https://www.sciencedirect.com/science/article/pii/S073510971839034X?pes=vor. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt K, Noureen A, Kronenberg F, Utermann G. Structure, function, and genetics of lipoprotein (a). J Lipid Res 2016;57(8):1339–1359. doi: 10.1194/jlr.R067314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamgang P, Chazot G, Njonfang E, Ngongang NBT, Tchoua FM. Mantle sources and magma evolution beneath the Cameroon Volcanic Line: Geochemistry of mafic rocks from the Bamenda Mountains (NW Cameroon). Gondwana Res 2013;24(2):727–741. doi: 10.1016/j.gr.2012.11.009. [DOI] [Google Scholar]
- 11.Kostner GM, Ibovnik A, Holzer H, Grillhofer H. Preparation of a stable fresh frozen primary lipoprotein[a] (Lp[a]) standard. J Lipid Res 1999;40(12):2255–2263. http://www.ncbi.nlm.nih.gov/pubmed/10588951. [PubMed] [Google Scholar]
- 12.Ando Y, Qing L, Song Y, Yamada S, Kasahara K, Sawano K, Miyao M, Dery H, Hamaya K. Exchange-driven magnetoresistance in silicon facilitated by electrical spin injection. Clin Chem March 2014. http://arxiv.org/abs/1403.4509. [Google Scholar]
- 13.Steinberg D, Witztum JL. Oxidized Low-Density Lipoprotein and Atherosclerosis. Arterioscler Thromb Vasc Biol 2010;30(12):2311–2316. doi: 10.1161/ATVBAHA.108.179697. [DOI] [PubMed] [Google Scholar]
- 14.van der Valk FM, Bekkering S, Kroon J, Yeang C, Van den Bossche J, van Buul JD, Ravandi A, Nederveen AJ, Verberne HJ, Scipione C, Nieuwdorp M, Joosten LAB, Netea MG, Koschinsky ML, Witztum JL, Tsimikas S, Riksen NP, Stroes ESG. Oxidized Phospholipids on Lipoprotein(a) Elicit Arterial Wall Inflammation and an Inflammatory Monocyte Response in Humans. Circulation 2016;134(8):611–624. doi: 10.1161/CIRCULATIONAHA.116.020838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boffa MB, Koschinsky ML. Lipoprotein (a): truly a direct prothrombotic factor in cardiovascular disease? J Lipid Res 2016;57(5):745–757. doi: 10.1194/jlr.R060582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marcovina SM, Albers JJ. Lipoprotein (a) measurements for clinical application. J Lipid Res. 2016;57(4):526–537. doi: 10.1194/jlr.R061648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kronenberg F Human Genetics and the Causal Role of Lipoprotein(a) for Various Diseases. Cardiovasc Drugs Ther 2016;30(1):87–100. doi: 10.1007/s10557-016-6648-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mackinnon LT, Hubinger L, Lepre F. Effects of physical activity and diet on lipoprotein(a). Med Sci Sports Exerc 1997;29(11):1429–1436. doi: 10.1097/00005768-199711000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Hirowatari Y, Manita D, Kamachi K, Tanaka A. Effect of dietary modification by calorie restriction on cholesterol levels in lipoprotein(a) and other lipoprotein classes. Ann Clin Biochem September 2016:000456321667224. doi: 10.1177/0004563216672247. [DOI] [PubMed] [Google Scholar]
- 20.Clarke R, Peden JF, Hopewell JC, Kyriakou T, Goel A, Heath SC, Parish S, Barlera S, Franzosi MG, Rust S, Bennett D, Silveira A, Malarstig A, Green FR, Lathrop M, Gigante B, Leander K, de Faire U, Seedorf U, Hamsten A, Collins R, Watkins H, Farrall M. Genetic Variants Associated with Lp(a) Lipoprotein Level and Coronary Disease. N Engl J Med 2009;361(26):2518–2528. doi: 10.1056/NEJMoa0902604. [DOI] [PubMed] [Google Scholar]
- 21.Deloukas P, Kanoni S, Willenborg C, Farrall M, Assimes TL, Thompson JR, Ingelsson E, Saleheen D, Erdmann J, Goldstein BA, Stirrups K, König IR, Cazier J-B, Johansson Å, Hall AS, Lee J-Y, Willer CJ, Chambers JC, Esko T, Folkersen L, Goel A, Grundberg E, Havulinna AS, Ho WK, Hopewell JC, Eriksson N, Kleber ME, Kristiansson K, Lundmark P, Lyytikäinen L-P, Rafelt S, Shungin D, Strawbridge RJ, Thorleifsson G, Tikkanen E, Van Zuydam N, Voight BF, Waite LL, Zhang W, Ziegler A, Absher D, Altshuler D, Balmforth AJ, Barroso I, Braund PS, Burgdorf C, Claudi-Boehm S, Cox D, Dimitriou M, Do R, Doney ASF, Mokhtari NEl, Eriksson P, Fischer K, Fontanillas P, Franco-Cereceda A, Gigante B, Groop L, Gustafsson S, Hager J, Hallmans G, Han B-G, Hunt SE, Kang HM, Illig T, Kessler T, Knowles JW, Kolovou G, Kuusisto J, Langenberg C, Langford C, Leander K, Lokki M-L, Lundmark A, McCarthy MI, Meisinger C, Melander O, Mihailov E, Maouche S, Morris AD, Müller-Nurasyid M, Nikus K, Peden JF, Rayner NW, Rasheed A, Rosinger S, Rubin D, Rumpf MP, Schäfer A, Sivananthan M, Song C, Stewart AFR, Tan S-T, Thorgeirsson G, Schoot CE van der, Wagner PJ, Wells GA, Wild PS, et al. Large-scale association analysis identifies new risk loci for coronary artery disease. Nat Genet 2013;45(1):25–33. doi: 10.1038/ng.2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamstrup PR. Genetically Elevated Lipoprotein(a) and Increased Risk of Myocardial Infarction. JAMA 2009;301(22):2331. doi: 10.1001/jama.2009.801. [DOI] [PubMed] [Google Scholar]
- 23.Ference BA. Causal Effect of Lipids and Lipoproteins on Atherosclerosis. Cardiol Clin 2018;36(2):203–211. doi: 10.1016/j.ccl.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 24.Tipping RW, Ford CE, Simpson LM, Walldius G, Jungner I, Folsom AR, Chambless L, Panagiotakos D, Pitsavos C, Chrysohoou C, Stefanadis C, Goldbourt U, Benderly M, Tanne D, Whincup P, Wannamethee SG, Morris RW, Kiechl S, Willeit J, Santer P, Mayr A, Wald N, Ebrahim S, Lawlor D, Yarnell J, Gallacher J, Casiglia E, Tikhonoff V, Nietert PJ, Sutherland SE, Bachman DL, Cushman M, Psaty BM, Tracy R, Tybjærg-Hansen A, Nordestgaard BG, Frikke-Schmidt R, Kamstrup PR, Giampaoli S, Palmieri L, Panico S, Vanuzzo D, Pilotto L, De La Cámara AG, Gómez Gerique JA, Simons L, McCallum J, Friedlander Y, Fowkes FGR, Lee A, Smith FB, Taylor J, Guralnik JM, Phillips CL, Wallace RB, Blazer DG, Brenner H, Raum E, Müller H, Rothenbacher D, Jansson JH, Wennberg P, Nissinen A, Donfrancesco C, Salomaa V, Harald K, Jousilahti P, Vartiainen E, Woodward M, D’Agostino RB, Wolf PA, Vasan RS, Pencina MJ, Bladbjerg EM, Jørgensen T, Møller L, Jespersen J, Dankner R, Chetrit A, Lubin F, Rosengren A, Wilhelmsen L, Lappas G, Eriksson H, Björkelund C, Lissner L, Bengtsson C, Cremer P, Nagel D, Tilvis RS, Strandberg TE, Rodriguez B, Dekker J, Nijpels G, Stehouwer CDA, Rimm E, Pai JK, Sato S, et al. Lipoprotein(a) Concentration and the Risk of Coronary Heart Disease, Stroke, and Nonvascular Mortality. JAMA 2009;302(4):412. doi: 10.1001/jama.2009.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Varvel S, McConnell JP, Tsimikas S. Prevalence of Elevated Lp(a) Mass Levels and Patient Thresholds in 532 359 Patients in the United States. Arterioscler Thromb Vasc Biol 2016;36(11):2239–2245. doi: 10.1161/ATVBAHA.116.308011. [DOI] [PubMed] [Google Scholar]
- 26.Aronis KN, Zhao D, Hoogeveen RC, Alonso A, Ballantyne CM, Guallar E, Jones SR, Martin SS, Nazarian S, Steffen BT, Virani SS, Michos ED. Associations of Lipoprotein(a) Levels With Incident Atrial Fibrillation and Ischemic Stroke: The ARIC (Atherosclerosis Risk in Communities) Study. J Am Heart Assoc 2017;6(12). doi: 10.1161/JAHA.117.007372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tunstall-Pedoe H, Peters SAE, Woodward M, Struthers AD, Belch JJF. Twenty-Year Predictors of Peripheral Arterial Disease Compared With Coronary Heart Disease in the Scottish Heart Health Extended Cohort (SHHEC). J Am Heart Assoc 2017;6(9). doi: 10.1161/JAHA.117.005967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong ND, Zhao Y, Quek RGW, Blumenthal RS, Budoff MJ, Cushman M, Garg P, Sandfort V, Tsai M, Lopez JAG. Residual atherosclerotic cardiovascular disease risk in statin-treated adults: The Multi-Ethnic Study of Atherosclerosis. J Clin Lipidol 2017. doi: 10.1016/j.jacl.2017.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Willeit P, Kiechl S, Kronenberg F, Witztum JL, Santer P, Mayr M, Xu Q, Mayr A, Willeit J, Tsimikas S. Discrimination and Net Reclassification of Cardiovascular Risk With Lipoprotein(a). J Am Coll Cardiol 2014;64(9):851–860. doi: 10.1016/j.jacc.2014.03.061. [DOI] [PubMed] [Google Scholar]
- 30.Nenseter MS, Lindvig HW, Ueland T, Langslet G, Ose L, Holven KB, Retterstøl K. Lipoprotein(a) levels in coronary heart disease-susceptible and -resistant patients with familial hypercholesterolemia. Atherosclerosis 2011;216(2):426–432. doi: 10.1016/j.atherosclerosis.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 31.Nordestgaard BG, Chapman MJ, Ray K, Borén J, Andreotti F, Watts GF, Ginsberg H, Amarenco P, Catapano A, Descamps OS, Fisher E, Kovanen PT, Kuivenhoven JA, Lesnik P, Masana L, Reiner Z, Taskinen M-R, Tokgözoglu L, Tybjærg-Hansen A. Lipoprotein(a) as a cardiovascular risk factor: current status. Eur Heart J 2010;31(23):2844–2853. doi: 10.1093/eurheartj/ehq386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guan W, Cao J, Steffen BT, Post WS, Stein JH, Tattersall MC, Kaufman JD, McConnell JP, Hoefner DM, Warnick R, Tsai MY. Race Is a Key Variable in Assigning Lipoprotein(a) Cutoff Values for Coronary Heart Disease Risk Assessment. Arterioscler Thromb Vasc Biol 2015;35(4):996–1001. doi: 10.1161/ATVBAHA.114.304785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alonso R, Andres E, Mata N, Fuentes-Jiménez F, Badimón L, López-Miranda J, Padró T, Muñiz O, Díaz-Díaz JL, Mauri M, Ordovás JM, Mata P. Lipoprotein(a) levels in familial hypercholesterolemia: An important predictor of cardiovascular disease independent of the type of LDL receptor mutation. J Am Coll Cardiol 2014. doi: 10.1016/j.jacc.2014.01.063. [DOI] [PubMed] [Google Scholar]
- 34.Meriño-Ibarra E, Puzo J, Jarauta E, Cenarro A, Recalde D, Garcĩa-Otín ÁL, Ros E, Martorell E, Pintó X, Franco M, Zambón D, Brea Á, Pocoví M, Civeira F. Hyperlipoproteinaemia(a) is a common cause of autosomal dominant hypercholesterolaemia. J Inherit Metab Dis 2007. doi: 10.1007/s10545-007-0585-z. [DOI] [PubMed] [Google Scholar]
- 35.Pavanello C, Pirazzi C, Bjorkman K, Sandstedt J, Tarlarini C, Mosca L, Romeo S, Calabresi L, Mancina RM. Individuals with familial hypercholesterolemia and cardiovascular events have higher circulating Lp(a) levels. J Clin Lipidol July 2019. doi: 10.1016/j.jacl.2019.06.011. [DOI] [PubMed] [Google Scholar]
- 36.Man LC, Kelly E, Duffy D. Targeting Lipoprotein (a): an Evolving Therapeutic Landscape. Curr Atheroscler Rep 2015;17(5):25. doi: 10.1007/s11883-015-0502-0. [DOI] [PubMed] [Google Scholar]
- 37.Kouvari M, Panagiotakos DB, Chrysohoou C, Georgousopoulou EN, Yannakoulia M, Tousoulis D, Pitsavos C. Lipoprotein (a) and 10-year Cardiovascular Disease Incidence in Apparently Healthy Individuals: A Sex-based Sensitivity Analysis from ATTICA Cohort Study. Angiology 2019;70(9):819–829. doi: 10.1177/0003319719854872. [DOI] [PubMed] [Google Scholar]
- 38.Saleheen D, Haycock PC, Zhao W, Rasheed A, Taleb A, Imran A, Abbas S, Majeed F, Akhtar S, Qamar N, Zaman KS, Yaqoob Z, Saghir T, Rizvi SNH, Memon A, Mallick NH, Ishaq M, Rasheed SZ, Memon F-R, Mahmood K, Ahmed N, Frossard P, Tsimikas S, Witztum JL, Marcovina S, Sandhu M, Rader DJ, Danesh J. Apolipoprotein(a) isoform size, lipoprotein(a) concentration, and coronary artery disease: a mendelian randomisation analysis. Lancet Diabetes Endocrinol 2017;5(7):524–533. doi: 10.1016/S2213-8587(17)30088-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Agarwala A, Pokharel Y, Saeed A, Sun W, Virani SS, Nambi V, Ndumele C, Shahar E, Heiss G, Boerwinkle E, Konety S, Hoogeveen RC, Ballantyne CM. The association of lipoprotein(a) with incident heart failure hospitalization: Atherosclerosis Risk in Communities study. Atherosclerosis 2017;262:131–137. doi: 10.1016/j.atherosclerosis.2017.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cook NR, Mora S, Ridker PM. Lipoprotein(a) and Cardiovascular Risk Prediction Among Women. J Am Coll Cardiol 2018;72(3):287–296. doi: 10.1016/j.jacc.2018.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Verbeek R, Hoogeveen RM, Langsted A, Stiekema LCA, Verweij SL, Hovingh GK, Wareham NJ, Khaw K-T, Boekholdt SM, Nordestgaard BG, Stroes ESG. Cardiovascular disease risk associated with elevated lipoprotein(a) attenuates at low low-density lipoprotein cholesterol levels in a primary prevention setting. Eur Heart J 2018;39(27):2589–2596. doi: 10.1093/eurheartj/ehy334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Albers JJ, Slee A, O’Brien KD, Robinson JG, Kashyap ML, Kwiterovich PO, Xu P, Marcovina SM. Relationship of Apolipoproteins A-1 and B, and Lipoprotein(a) to Cardiovascular Outcomes. J Am Coll Cardiol 2013;62(17):1575–1579. doi: 10.1016/j.jacc.2013.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nestel PJ, Barnes EH, Tonkin AM, Simes J, Fournier M, White HD, Colquhoun DM, Blankenberg S, Sullivan DR. Plasma Lipoprotein(a) Concentration Predicts Future Coronary and Cardiovascular Events in Patients With Stable Coronary Heart Disease. Arterioscler Thromb Vasc Biol 2013;33(12):2902–2908. doi: 10.1161/ATVBAHA.113.302479. [DOI] [PubMed] [Google Scholar]
- 44.Lincoff AM, Nicholls SJ, Riesmeyer JS, Barter PJ, Brewer HB, Fox KAA, Gibson CM, Granger C, Menon V, Montalescot G, Rader D, Tall AR, McErlean E, Wolski K, Ruotolo G, Vangerow B, Weerakkody G, Goodman SG, Conde D, McGuire DK, Nicolau JC, Leiva-Pons JL, Pesant Y, Li W, Kandath D, Kouz S, Tahirkheli N, Mason D, Nissen SE. Evacetrapib and Cardiovascular Outcomes in High-Risk Vascular Disease. N Engl J Med 2017;376(20):1933–1942. doi: 10.1056/NEJMoa1609581. [DOI] [PubMed] [Google Scholar]
- 45.O’Donoghue ML, Fazio S, Giugliano RP, Stroes ESG, Kanevsky E, Gouni-Berthold I, Im K, Lira Pineda A, Wasserman SM, Češka R, Ezhov M V., Jukema JW, Jensen HK, Tokgözoğlu SL, Mach F, Huber K, Sever PS, Keech AC, Pedersen TR, Sabatine MS. Lipoprotein(a), PCSK9 Inhibition, and Cardiovascular Risk. Circulation 2019;139(12):1483–1492. doi: 10.1161/CIRCULATIONAHA.118.037184. [DOI] [PubMed] [Google Scholar]
- 46.Bittner VA, Szarek M, Aylward PE, Bhatt DL, Diaz R, Edelberg JM, Fras Z, Goodman SG, Halvorsen S, Hanotin C, Harrington RA, Jukema JW, Loizeau V, Moriarty PM, Moryusef A, Pordy R, Roe MT, Sinnaeve P, Tsimikas S, Vogel R, White HD, Zahger D, Zeiher AM, Steg PG, Schwartz GG. Effect of Alirocumab on Lipoprotein(a) and Cardiovascular Risk After Acute Coronary Syndrome. J Am Coll Cardiol 2020;75(2):133–144. doi: 10.1016/j.jacc.2019.10.057. [DOI] [PubMed] [Google Scholar]
- 47.Gaudet D, Watts GF, Robinson JG, Minini P, Sasiela WJ, Edelberg J, Louie MJ, Raal FJ. Effect of Alirocumab on Lipoprotein(a) Over ≥1.5 Years (from the Phase 3 ODYSSEY Program). Am J Cardiol 2017;119(1):40–46. doi: 10.1016/j.amjcard.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 48.Sanchez Muñoz-Torrero JF, Rico-Martín S, Álvarez LR, Aguilar E, Alcalá JN, Monreal M. Lipoprotein (a) levels and outcomes in stable outpatients with symptomatic artery disease. Atherosclerosis 2018;276:10–14. doi: 10.1016/j.atherosclerosis.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 49.Nave AH, Lange KS, Leonards CO, Siegerink B, Doehner W, Landmesser U, Steinhagen-Thiessen E, Endres M, Ebinger M. Lipoprotein (a) as a risk factor for ischemic stroke: A meta-analysis. Atherosclerosis 2015. doi: 10.1016/j.atherosclerosis.2015.08.021. [DOI] [PubMed] [Google Scholar]
- 50.Tsimikas S In search of a physiological function of lipoprotein(a): causality of elevated Lp(a) levels and reduced incidence of type 2 diabetes. J Lipid Res 2018;59(5):741–744. doi: 10.1194/jlr.C085639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mu-Han-Ha-Li D-L-D-E, Zhai T-Y, Ling Y, Gao X. LPA kringle IV type 2 is associated with type 2 diabetes in a Chinese population with very high cardiovascular risk. J Lipid Res 2018;59(5):884–891. doi: 10.1194/jlr.P082792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jin J-L, Cao Y-X, Zhang H-W, Sun D, Hua Q, Li Y-F, Guo Y-L, Wu N-Q, Zhu C-G, Gao Y, Dong Q-T, Liu H-H, Dong Q, Li J-J. Lipoprotein(a) and Cardiovascular Outcomes in Coronary Artery Disease Patients With Prediabetes and Diabetes. Diabetes Care May 2019:dc190274. doi: 10.2337/dc19-0274. [DOI] [PubMed] [Google Scholar]
- 53.Arsenault BJ, Boekholdt SM, Dubé M-P, Rhéaume É, Wareham NJ, Khaw K-T, Sandhu MS, Tardif J-C. Lipoprotein(a) Levels, Genotype, and Incident Aortic Valve Stenosis. Circ Cardiovasc Genet 2014;7(3):304–310. doi: 10.1161/CIRCGENETICS.113.000400. [DOI] [PubMed] [Google Scholar]
- 54.Capoulade R, Yeang C, Chan KL et al. Association of Mild to Moderate Aortic Valve Stenosis Progression With Higher Lipoprotein(a) and Oxidized Phospholipid Levels Secondary Analysis of a Randomized Clinical Trial. JAMA Cardiol 2018:3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Capoulade R, Chan KL, Yeang C, Mathieu P, Bossé Y, Dumesnil JG, Tam JW, Teo KK, Mahmut A, Yang X, Witztum JL, Arsenault BJ, Després J-P, Pibarot P, Tsimikas S. Oxidized Phospholipids, Lipoprotein(a), and Progression of Calcific Aortic Valve Stenosis. J Am Coll Cardiol 2015;66(11):1236–1246. doi: 10.1016/j.jacc.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 56.Bouchareb R, Mahmut A, Nsaibia MJ, Boulanger M-C, Dahou A, Lépine J-L, Laflamme M-H, Hadji F, Couture C, Trahan S, Pagé S, Bossé Y, Pibarot P, Scipione CA, Romagnuolo R, Koschinsky ML, Arsenault BJ, Marette A, Mathieu P. Autotaxin Derived From Lipoprotein(a) and Valve Interstitial Cells Promotes Inflammation and Mineralization of the Aortic Valve. Circulation 2015;132(8):677–690. doi: 10.1161/CIRCULATIONAHA.115.016757. [DOI] [PubMed] [Google Scholar]
- 57.Zheng KH, Tsimikas S, Pawade T, Kroon J, Jenkins WSA, Doris MK, White AC, Timmers NKLM, Hjortnaes J, Rogers MA, Aikawa E, Arsenault BJ, Witztum JL, Newby DE, Koschinsky ML, Fayad ZA, Stroes ESG, Boekholdt SM, Dweck MR. Lipoprotein(a) and Oxidized Phospholipids Promote Valve Calcification in Patients With Aortic Stenosis. J Am Coll Cardiol 2019;73(17):2150–2162. doi: 10.1016/j.jacc.2019.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Burgess S, Ference BA, Staley JR, Freitag DF, Mason AM, Nielsen SF, Willeit P, Young R, Surendran P, Karthikeyan S, Bolton TR, Peters JE, Kamstrup PR, Tybjærg-Hansen A, Benn M, Langsted A, Schnohr P, Vedel-Krogh S, Kobylecki CJ, Ford I, Packard C, Trompet S, Jukema JW, Sattar N, Di Angelantonio E, Saleheen D, Howson JMM, Nordestgaard BG, Butterworth AS, Danesh J. Association of LPA Variants With Risk of Coronary Disease and the Implications for Lipoprotein(a)-Lowering Therapies. JAMA Cardiol 2018;3(7):619. doi: 10.1001/jamacardio.2018.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Najjar RS, Moore CE, Montgomery BD. Consumption of a defined, plant-based diet reduces lipoprotein(a), inflammation, and other atherogenic lipoproteins and particles within 4 weeks. Clin Cardiol 2018;41(8):1062–1068. doi: 10.1002/clc.23027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yeang C, Hung M-Y, Byun Y-S, Clopton P, Yang X, Witztum JL, Tsimikas S. Effect of therapeutic interventions on oxidized phospholipids on apolipoprotein B100 and lipoprotein(a). J Clin Lipidol 2016;10(3):594–603. doi: 10.1016/j.jacl.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 61.Tsimikas S, Gordts PLSM, Nora C, Yeang C, Witztum JL. Statin therapy increases lipoprotein(a) levels. Eur Heart J 2019;1:1–10. doi: 10.1093/eurheartj/ehz310. [DOI] [PubMed] [Google Scholar]
- 62.Shlipak MG. Estrogen and Progestin, Lipoprotein(a), and the Risk of Recurrent Coronary Heart Disease Events After Menopause. JAMA 2000;283(14):1845. doi: 10.1001/jama.283.14.1845. [DOI] [PubMed] [Google Scholar]
- 63.Manson JE, Hsia J, Johnson KC, Rossouw JE, Assaf AR, Lasser NL, Trevisan M, Black HR, Heckbert SR, Detrano R, Strickland OL, Wong ND, Crouse JR, Stein E, Cushman M. Estrogen plus Progestin and the Risk of Coronary Heart Disease. N Engl J Med 2003;349(6):523–534. doi: 10.1056/NEJMoa030808. [DOI] [PubMed] [Google Scholar]
- 64.Duell PB, Santos RD, Kirwan B-A, Witztum JL, Tsimikas S, Kastelein JJP. Long-term mipomersen treatment is associated with a reduction in cardiovascular events in patients with familial hypercholesterolemia. J Clin Lipidol 2016;10(4):1011–1021. doi: 10.1016/j.jacl.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 65.Raal FJ, Giugliano RP, Sabatine MS, Koren MJ, Langslet G, Bays H, Blom D, Eriksson M, Dent R, Wasserman SM, Huang F, Xue A, Albizem M, Scott R, Stein EA. Reduction in Lipoprotein(a) With PCSK9 Monoclonal Antibody Evolocumab (AMG 145). J Am Coll Cardiol 2014;63(13):1278–1288. doi: 10.1016/j.jacc.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 66.Jaeger BR, Richter Y, Nagel D, Heigl F, Vogt A, Roeseler E, Parhofer K, Ramlow W, Koch M, Utermann G, Labarrere CA, Seidel D. Longitudinal cohort study on the effectiveness of lipid apheresis treatment to reduce high lipoprotein(a) levels and prevent major adverse coronary events. Nat Rev Cardiol 2009;6(3):229–239. doi: 10.1038/ncpcardio1456. [DOI] [PubMed] [Google Scholar]
- 67.Leebmann J, Roeseler E, Julius U, Heigl F, Spitthoever R, Heutling D, Breitenberger P, Maerz W, Lehmacher W, Heibges A, Klingel R. Lipoprotein Apheresis in Patients With Maximally Tolerated Lipid-Lowering Therapy, Lipoprotein(a)-Hyperlipoproteinemia, and Progressive Cardiovascular Disease. Circulation 2013;128(24):2567–2576. doi: 10.1161/CIRCULATIONAHA.113.002432. [DOI] [PubMed] [Google Scholar]
- 68.Waldmann E, Parhofer KG. Lipoprotein apheresis to treat elevated lipoprotein (a). J Lipid Res 2016;57(10):1751–1757. doi: 10.1194/jlr.R056549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Viney NJ, van Capelleveen JC, Geary RS, Xia S, Tami JA, Yu RZ, Marcovina SM, Hughes SG, Graham MJ, Crooke RM, Crooke ST, Witztum JL, Stroes ES, Tsimikas S. Antisense oligonucleotides targeting apolipoprotein(a) in people with raised lipoprotein(a): two randomised, double-blind, placebo-controlled, dose-ranging trials. Lancet 2016;388(10057):2239–2253. doi: 10.1016/S0140-6736(16)31009-1. [DOI] [PubMed] [Google Scholar]
- 70.Tsimikas S, Karwatowska-Prokopczuk E, Gouni-Berthold I, Tardif J-C, Baum SJ, Steinhagen-Thiessen E, Shapiro MD, Stroes ES, Moriarty PM, Nordestgaard BG, Xia S, Guerriero J, Viney NJ, O’Dea L, Witztum JL. Lipoprotein(a) Reduction in Persons with Cardiovascular Disease. N Engl J Med January 2020:NEJMoa1905239. doi: 10.1056/NEJMoa1905239. [DOI] [PubMed] [Google Scholar]
- 71.Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, Chapman MJ, De Backer GG, Delgado V, Ference BA, Graham IM, Halliday A, Landmesser U, Mihaylova B, Pedersen TR, Riccardi G, Richter DJ, Sabatine MS, Taskinen M-R, Tokgozoglu L, Wiklund O, Mueller C, Drexel H, Aboyans V, Corsini A, Doehner W, Farnier M, Gigante B, Kayikcioglu M, Krstacic G, Lambrinou E, Lewis BS, Masip J, Moulin P, Petersen S, Petronio AS, Piepoli MF, Pintó X, Räber L, Ray KK, Reiner Ž, Riesen WF, Roffi M, Schmid J-P, Shlyakhto E, Simpson IA, Stroes E, Sudano I, Tselepis AD, Viigimaa M, Vindis C, Vonbank A, Vrablik M, Vrsalovic M, Zamorano JL, Collet J-P, Koskinas KC, Casula M, Badimon L, John Chapman M, De Backer GG, Delgado V, Ference BA, Graham IM, Halliday A, Landmesser U, Mihaylova B, Pedersen TR, Riccardi G, Richter DJ, Sabatine MS, Taskinen M-R, Tokgozoglu L, Wiklund O, Windecker S, Aboyans V, Baigent C, Collet J-P, Dean V, Delgado V, Fitzsimons D, Gale CP, Grobbee D, Halvorsen S, Hindricks G, Iung B, Jüni P, Katus HA, Landmesser U, Leclercq C, Lettino M, Lewis BS, Merkely B, Mueller C, Petersen S, Petronio AS, Richter DJ, Roffi M, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 2020;41(1):111–188. doi: 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]