Phytosterols from soybean are low-cost by-products of soybean oil production and, owing to their good bioavailability in mycobacteria, are preferred as the substrates for steroid drug production via biotransformation by Mycobacterium. However, the low level of production of steroid hormone drugs due to the low aqueous solubility (below 0.1 mmol/liter) of phytosterols limits the commercial use of sterol-transformed strains. To improve the bioconversion of steroids, cyclodextrins (CDs) are generally used as an effective carrier for the delivery of hydrophobic steroids to the bacterium. CDs improve the biotransformation of steroids due to their effects on steroid solubilization and alterations in cell wall permeability for steroids. However, studies have rarely reported the effects of CDs on cell metabolic pathways related to sterols. In this study, the effects of hydroxypropyl-β-CD (HP-β-CD) on the expression of enzymes related to steroid catabolic pathways in Mycobacterium neoaurum were systematically investigated. These findings will improve our understanding of the complex functions of CDs in the regulation of sterol metabolism and guide the application of CDs to sterol production.
KEYWORDS: Mycobacterium neoaurum; androst-1,4-diene-3,17-dione; androst-4-ene-3,17-dione; biotransformation; hydroxypropyl-β-cyclodextrin; HP-β-CD; phytosterols; proteomic analysis
ABSTRACT
Androst-4-ene-3,17-dione (AD) and androst-1,4-diene-3,17-dione (ADD) are valuable steroid pharmaceutical intermediates obtained by soybean phytosterol biotransformation by Mycobacterium. Cyclodextrins (CDs) are generally believed to be carriers for phytosterol delivery and can improve the production of AD and ADD due to their effects on steroid solubilization and alteration in cell wall permeability for steroids. To better understand the mechanisms of CD promotion, we performed proteomic quantification of the effects of hydroxypropyl-β-CD (HP-β-CD) on phytosterol metabolism in Mycobacterium neoaurum TCCC 11978 C2. Perturbations are observed in steroid catabolism and glucose metabolism by adding HP-β-CD in a phytosterol bioconversion system. AD and ADD, as metabolic products of phytosterol, are toxic to cells, with inhibited cell growth and biocatalytic activity. Treatment of mycobacteria with HP-β-CD relieves the inhibitory effect of AD(D) on the electron transfer chain and cell growth. These results demonstrate the positive relationship between HP-β-CD and phytosterol metabolism and give insight into the complex functions of CDs as mediators of the regulation of sterol metabolism.
IMPORTANCE Phytosterols from soybean are low-cost by-products of soybean oil production and, owing to their good bioavailability in mycobacteria, are preferred as the substrates for steroid drug production via biotransformation by Mycobacterium. However, the low level of production of steroid hormone drugs due to the low aqueous solubility (below 0.1 mmol/liter) of phytosterols limits the commercial use of sterol-transformed strains. To improve the bioconversion of steroids, cyclodextrins (CDs) are generally used as an effective carrier for the delivery of hydrophobic steroids to the bacterium. CDs improve the biotransformation of steroids due to their effects on steroid solubilization and alterations in cell wall permeability for steroids. However, studies have rarely reported the effects of CDs on cell metabolic pathways related to sterols. In this study, the effects of hydroxypropyl-β-CD (HP-β-CD) on the expression of enzymes related to steroid catabolic pathways in Mycobacterium neoaurum were systematically investigated. These findings will improve our understanding of the complex functions of CDs in the regulation of sterol metabolism and guide the application of CDs to sterol production.
INTRODUCTION
Androst-4-ene-3,17-dione (AD) and androst-1,4-diene-3,17-dione (ADD) are valuable steroid pharmaceutical intermediates that are used to produce steroid hormone pharmaceuticals and can be obtained via the side chain cleavage of natural phytosterols by Mycobacterium (1–3). Industrialists prefer using phytosterols from soybean as the substrates for mycobacterial biotransformation because they are inexpensive and have good bioavailability in mycobacteria. However, the low level of AD(D) production from phytosterol biodegradation seriously limits the commercial use of sterol-transforming Mycobacterium. This result can be attributed to the poor water solubility of phytosterols in aqueous media (2, 4, 5). To improve the bioconversion of steroids, powdered substrate particles, surfactant-facilitated emulsification, and biotransformation in two-phase systems with oil, liquid polymers, or cyclodextrins (CDs) are generally used.
CDs are a family of cyclic oligosaccharides with a hydrophilic outer surface and a lipophilic central cavity. The ability of CDs or chemically modified CDs to form inclusion complexes with steroids makes them effective carriers for the delivery of hydrophobic steroids to cells, which enhances the biotransformation of steroid compounds (6–12) (Fig. 1). The multiple effects of CDs on the biotransformation of steroids have been widely studied. CDs are generally believed to be carriers for phytosterol delivery and can improve the biotransformation of steroids due to their effects on steroid solubilization and changes in cell wall permeability for steroids (9–11, 13, 14). The effect of CDs on gene expression during the microbial conversion of sterols has also been studied. Based on whole-transcriptome analysis, Shtratnikova et al. (12) reported that the expression levels of steroid-catabolizing genes, which are related to side chain cleavage and the initial steps of steroid core oxidation, are unaffected by the addition of methylated β-cyclodextrin (MCD) to the phytosterol fermentation system. Nevertheless, in our previous study, we found that the proportion of AD to ADD products, depending on the catalytic activity of 3-ketosteroid-Δ-1-dehydrogenase (KsdD), was increased in the presence of hydroxypropyl-β-CD (HP-β-CD) (1), thereby indicating that the enhanced catalytic activity of KsdD may prominently influence the ADD-to-AD ratio by the addition of HP-β-CD.
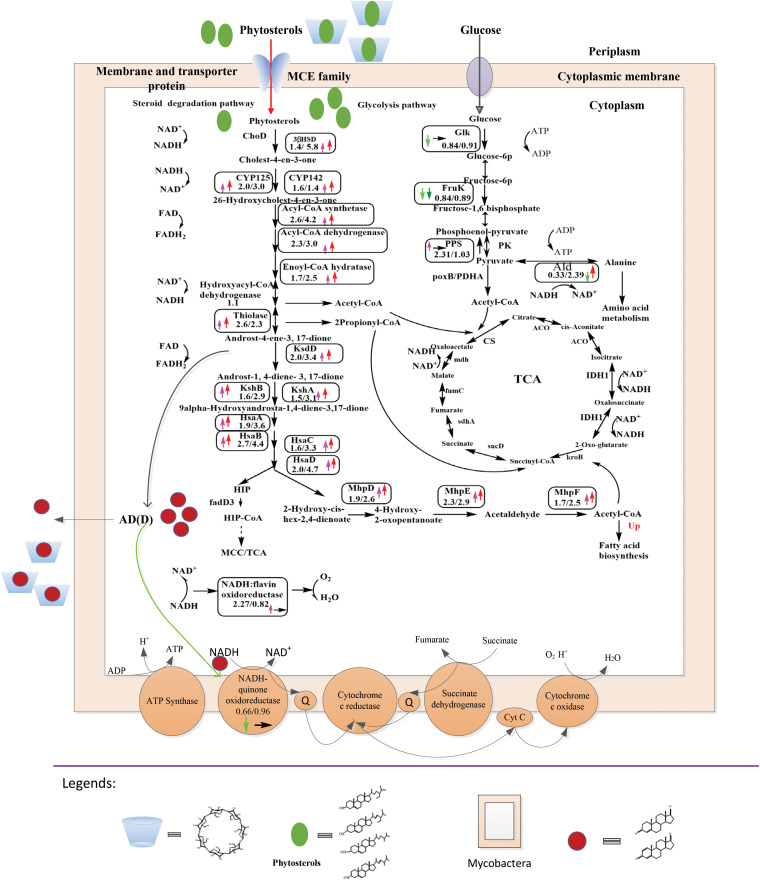
FIG 1.
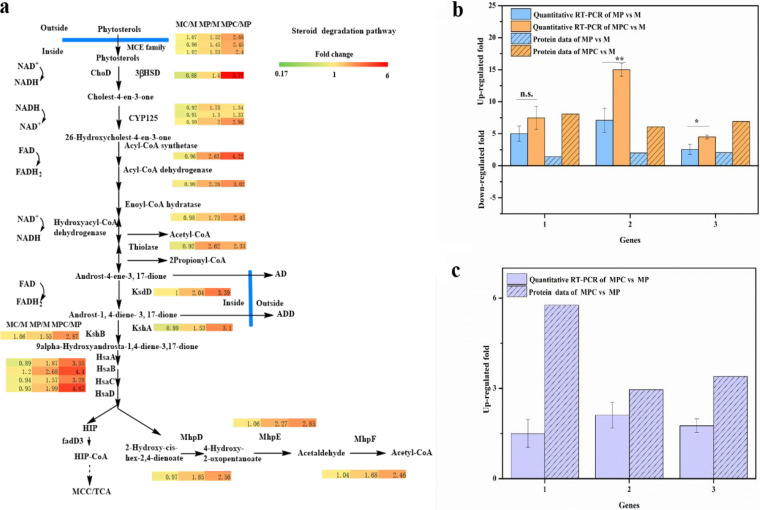
Primary differentially expressed proteins included in MP-versus-M and MPC-versus-MP tests. The numbers denote the ratios of the expression levels in MP versus M medium and MPC versus MP medium. Upward pink arrows represent upregulated proteins in MP versus M medium, upward red arrows represent upregulated proteins in MPC versus MP medium, downward light-green arrows represent downregulated proteins in MP versus M medium, downward green arrows represent downregulated proteins in MPC versus MP medium, rightward black arrows represent unchanged proteins in MPC versus MP medium, blue cups represent HP-β-CD, green ovals represent phytosterols, and red circles represent AD(D). FAD, flavin adenine dinucleotide; FADH2, reduced flavin adenine dinucleotide. PDHA, pyruvate dehydrogenase E1 component alpha subunit; PK, pyruvate kinase; CS, citrate synthase; ACO, aconitate hydratase; IDH1, isocitrate dehydrogenase; HIP, 3-[(3aS,4S,7aS)-7a-methyl-1,5-dioxo-octahydro-1H-inden-4-yl]propanoate.
Given that most cellular metabolic activities and bacterial physiology are directly mediated by proteins, proteomic technology is an effective method to monitor the quantitative protein changes that occur at the cellular level and analyze these changes during biotransformation (15–18). To clarify the role of CDs in the expression of key enzymes included in cell metabolic pathways, the effects of HP-β-CD on the expression of enzymes related to steroid catabolic pathways in Mycobacterium neoaurum TCCC 11978 C2 (M3C2) were systematically investigated using proteomics. M. neoaurum M3C2 is the ksdD gene replacement strain M. neoaurum M3 ΔksdD::ksdD-MNR (the ksdD gene of M. neoaurum M3 was replaced by the ksdD gene from M. neoaurum TCCC 11028) constructed by homologous recombination. M. neoaurum M3C2 possesses an improved and stable phenotype that is more suitable for ADD accumulation than M. neoaurum M3. These findings will elucidate the complex effects of HP-β-CD on sterol bioconversion via analysis of intracellular catabolic pathways.
RESULTS
Effect of HP-β-CD on phytosterol bioconversion and product proportions in M. neoaurum M3C2.
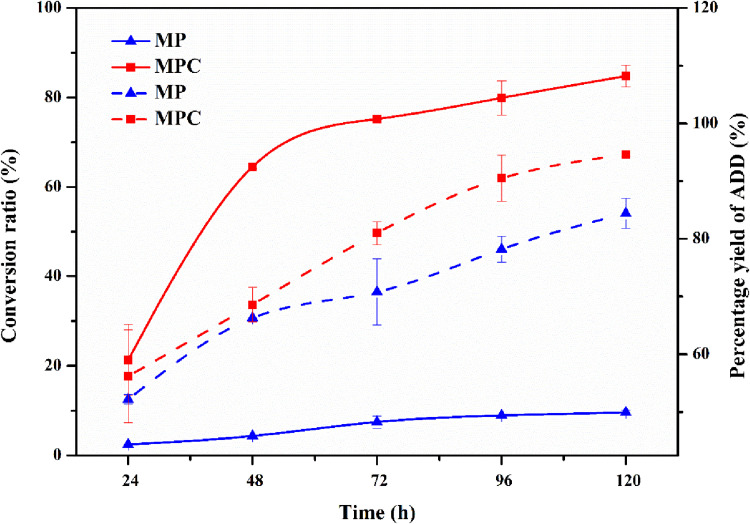
The time course of M. neoaurum M3C2 transformation was studied with phytosterols and HP-β-CD in the medium. As shown in Fig. 2, the molar conversion of phytosterols reached 84.8% in the presence of HP-β-CD. In comparison, the highest molar conversion rate in the control experiments without HP-β-CD was 9.6% at 120 h. This finding indicates an increase of 7.83 times in AD(D) production in the HP-β-CD conversion system compared with the control. This finding was in accordance with the results of previous studies indicating that CDs can enhance steroid bioconversion (10, 11, 19). The influence of HP-β-CD on product proportions is also shown in Fig. 2. At 120 h, the proportion of ADD reached 93.8% in the presence of HP-β-CD for M. neoaurum M3C2, whereas that of the control reached 84.4%. We supposed that the enhanced catalytic activity of KsdD in the presence of HP-β-CD led to an increase in the ratio of ADD to AD (1).
FIG 2.
Effect of HP-β-CD on phytosterol side chain degradation and product proportions in M. neoaurum M3C2. The percent yield of ADD in the 1-dehydrogenation reaction is defined as the mole ratio of produced ADD to AD and ADD. Dashed lines represent conversion ratios. Solid lines represent percent yields of ADD. The error bars represent the standard deviations of data from three biological replicates.
Effect of HP-β-CD on M. neoaurum M3C2 cell growth during biotransformation of phytosterols.
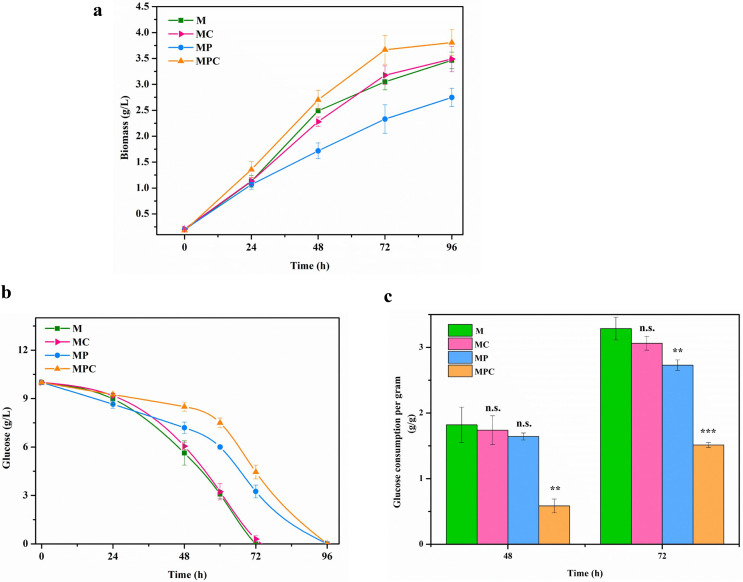
Apart from the effect of HP-β-CD on the sterol metabolic pathway, HP-β-CD can influence cell growth during the biotransformation of phytosterols. As presented in Fig. 3a, the growth curves of M. neoaurum M3C2 cultured in M, MC (M medium with HP-β-CD), MP (M medium with phytosterols), and MPC (M medium with phytosterols and HP-β-CD) media were obtained. During the first 48 h, the initial cell growth rate in MP medium (0.031 g liter−1 h−1) was lower than that in M medium (0.048 g liter−1 h−1). The biomass and initial growth rate of cells in MC medium were similar to those in M medium and higher than those in MP medium. The biomass and initial growth rates of cells in MPC medium were 9.8% and 9.4% higher than those of cells in M medium, respectively. The addition of HP-β-CD to M medium exerted minimal effects on cell growth. Phytosterols inhibited cell growth because they reduced the utilization of carbon sources (Fig. 3). Furthermore, the inhibitory effect of phytosterols on cell growth can be eliminated by the addition of HP-β-CD.
FIG 3.
Cell growth and glucose consumption of M. neoaurum M3C2 under different conditions with phytosterols or HP-β-CD. Shown are growth curves (a), glucose consumption (b), and specific glucose consumption capabilities (c) of M. neoaurum M3C2. M, minimal medium; MC, HP-β-CD (25 mM) in M medium; MP, phytosterols (3 g/liter) in M medium; MPC, HP-β-CD (25 mM) and phytosterols (3 g/liter) in M medium. Cell growth measurement in medium with phytosterols was conducted in accordance with methods described previously by Meyers et al. (53). The error bars represent the standard deviations of data from three biological replicates. **, P < 0.01; ***, P < 0.001, n.s., no significant difference (P > 0.05).
Cell growth is closely related to carbon source utilization. Glucose consumption in the four media (M, MC, MP, and MPC) was investigated. As shown in Fig. 3b, glucose consumption of M. neoaurum M3C2 in MC medium is similar to that in M medium. In contrast, the glucose consumption rate in MP medium was lower than that in M medium and higher than that in MPC medium. Given the influence of phytosterols and HP-β-CD on cell biomass, the specific glucose consumption ability (glucose consumption per gram of cells) was obtained (Fig. 3c). The specific glucose consumption levels in MP medium reached 1.64 and 2.73 g/g at 48 and 72 h, respectively, which were lower than those in M medium (1.82 and 3.29 g/g). However, the specific glucose consumption levels in MPC medium were the lowest (0.58 and 1.51 g/g at 48 and 72 h, respectively), which were significantly lower than those in M medium (P < 0.01). These results indicated that phytosterols inhibited the glucose utilization ability and that HP-β-CD strengthened the inhibition of glucose consumption from sterols.
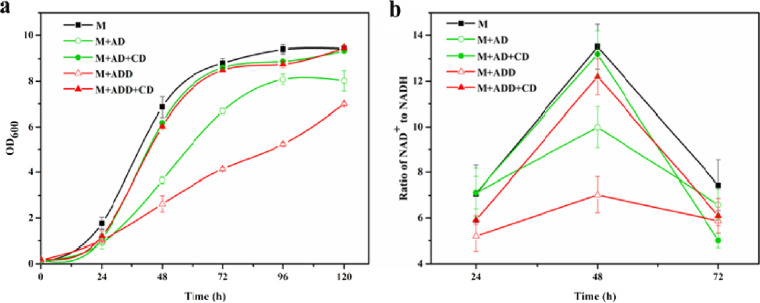
Significantly higher cytotoxicity was reportedly caused by the biotransformation products AD and ADD; the reason was possibly the fact that AD(D) can impair the electron transport chain (ETC) of cells (2, 5). To elucidate the effect of phytosterols and HP-β-CD on cell growth, the toxicities of AD and ADD on cell growth and the ETC pathway were verified. As shown in Fig. 4a, the addition of AD or ADD to M medium inhibited cell growth, and the inhibitory effect of ADD was more significant than that of AD. During the first 48 h, the initial cell growth rate in M medium (0.143/h) was significantly higher than those in the AD- and ADD-containing media (0.076 and 0.055/h, respectively; P < 0.001). Moreover, at 72 h, the biomass in M medium was significantly higher than those in media containing AD and ADD, which were 31.8% and 113%, respectively (P < 0.001). By analyzing the enzymes associated with the ETC pathway in the proteome, an ETC pathway-related enzyme, named NADH dehydrogenase (UniProt no. V5X9W2), was downregulated (0.7-fold; P < 0.01). This enzyme catalyzes the NADH electron transport chain, which catalyzes the oxidation of NADH to NAD+ (20). NADH dehydrogenase also plays a crucial role in maintaining the NAD+/NADH balance (21). Thus, to analyze the product effects on the ETC pathway, the cellular NAD+/NADH ratio was assayed. As shown in Fig. 4b, the NAD+-to-NADH ratio reached the highest value at 48 h. The ratio of NAD+ to NADH in M medium was 13.5, which was 35.1% and 92.3% higher than those in AD- and ADD-containing media, respectively (P < 0.05). Thus, AD and ADD inhibited the cellular ratio of NAD+ to NADH. Moreover, Fig. 4 presents the effects of AD and ADD on cell growth in the presence of HP-β-CD. With CD added to AD- or ADD-containing medium, the cell growth rates were similar to those in M medium (Fig. 4a). Additionally, the cellular NAD+-to-NADH ratio improved when HP-β-CD was added to sterol-containing medium (Fig. 4b). Thus, HP-β-CD can relieve the inhibitory effect of phytosterol metabolites on cell growth and the ETC pathway.
FIG 4.
Cell growth and intracellular NAD+-to-NADH ratio of M. neoaurum M3C2 under different conditions with AD(D) or HP-β-CD. Shown are cell growth (a) and intracellular NAD+-to-NADH ratios (b) of M. neoaurum M3C2 under different conditions. M, M. neoaurum M3C2 cultured in M medium; M+AD, M. neoaurum M3C2 cultured in M medium with AD (1 g/liter); M+AD+CD, M. neoaurum M3C2 cultured in M medium with AD (1 g/liter) and HP-β-CD (25 mM); M+ADD, M. neoaurum M3C2 cultured in M medium with ADD (1 g/liter); M+ADD+CD, M. neoaurum M3C2 cultured in M medium with ADD (1 g/liter) and HP-β-CD (25 mM); OD600, optical density at 600 nm. The error bars represent the standard deviations of data from three biological replicates.
Identification and quantification of differentially expressed proteins of M. neoaurum M3C2 with or without HP-β-CD in phytosterol-containing medium.
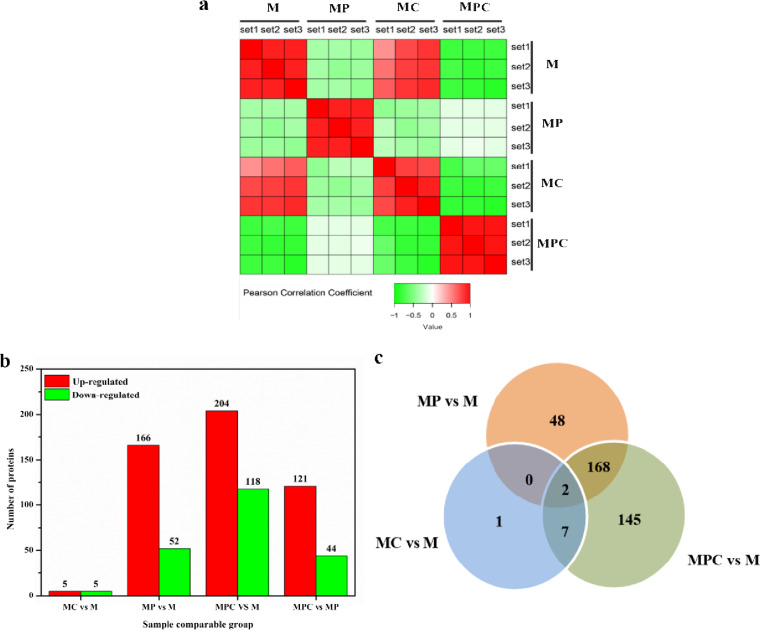
The above-mentioned phenotypic analysis showed that HP-β-CD can promote phytosterol biotransformation, perturb glucose utilization, and relieve cytotoxicity from sterol products of phytosterols in phytosterol-containing medium. Proteome analysis is a widely used approach to detect metabolic pathway disturbances (22–24). Proteome-wide profiling is necessary for the systematic and comprehensive study of protein regulation in organisms (15–18). To further elucidate the metabolism changes caused by the addition of HP-β-CD and phytosterols, the proteomes of M. neoaurum M3C2 cultured in M, MC, MP, and MPC media were investigated. Based on the criteria of a P value of <0.05, proteins with fold changes of >1.5-fold are considered significantly upregulated, and those with fold changes of <1/1.5-fold are considered significantly downregulated. Pearson correlation coefficients show the correlation of samples (Fig. 5a). The replicates of M. neoaurum M3C2 cultured in M, MC, MP, and MPC media showed excellent repeatability. The samples cultured in M medium exhibited a close correlation with those in MC medium, thereby agreeing with the results shown in Fig. 5b. Only 10 proteins were differentially expressed in comparison with MC and M media. Totals of 166 and 52 proteins were increased and decreased, respectively, in MP medium compared with those in M medium. Compared with minimal medium (MPC versus M), 204 proteins were upregulated in MPC medium, and 114 proteins were downregulated. Furthermore, 121 and 44 proteins were increased and decreased, respectively, in MPC medium compared with MP medium. Specifically, 168 differentially expressed proteins were observed in both MPC-versus-M and MP-versus-M tests (Fig. 5c). Two proteins are common among the three groups. HP-β-CD exhibited minimal effects on the protein expression of M. neoaurum M3C2 without phytosterols in the medium. However, the addition of HP-β-CD to the medium with phytosterols disturbed the protein expression of M. neoaurum M3C2 substantially.
FIG 5.
Analysis of sample correlation. (a) Pearson correlation coefficients showing correlations among the samples. (b) Changes in protein expressions in MP-versus-M, MPC-versus-MP, and MC-versus-M tests and numbers of differentially expressed proteins in the three comparisons. (c) Venn diagram showing overlaps among the three comparisons.
Phytosterols inhibited cell growth, and the addition of HP-β-CD to phytosterol-containing medium alleviated this inhibition. Pathway analysis was performed on differentially expressed proteins in MP-versus-M and MPC-versus-MP tests to preliminarily explore their potential functions in M. neoaurum M3C2 under different culture conditions (see Fig. S1 in the supplemental material). The 16S rRNA sequence of M. neoaurum M3 showed 98% query coverage and 99% similarity to the 16S rRNA sequence of M. neoaurum VKM Ac-1815D. M. neoaurum VKM Ac-1815D orthologs were subjected to bioinformatics analysis based on their known cellular location and function. Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis was conducted to determine the biological pathways of differentially expressed proteins. For the P value obtained by an enrichment test (Fisher’s exact test was used here), negative-logarithm (−log10) transformation was carried out. The higher the value obtained after conversion, the more significant the enrichment of this functional type is. The upregulated proteins in MPC versus MP medium exhibited functions similar to those included in MP versus M medium, whereas the steroid degradation pathway yielded the highest −log10 value (P value) (Fig. S1a and c). However, the downregulated proteins in MPC versus MP medium differed from those in MP versus M medium and are involved in the oxidative phosphorylation pathway (Fig. S1b and c). In our preliminary analysis, the addition of HP-β-CD to the phytosterol-containing system affected phytosterol metabolism-related pathways and energy-related metabolic pathways in M. neoaurum M3C2.
Effect of HP-β-CD on protein expression involved in phytosterol degradation pathways of M. neoaurum M3C2.
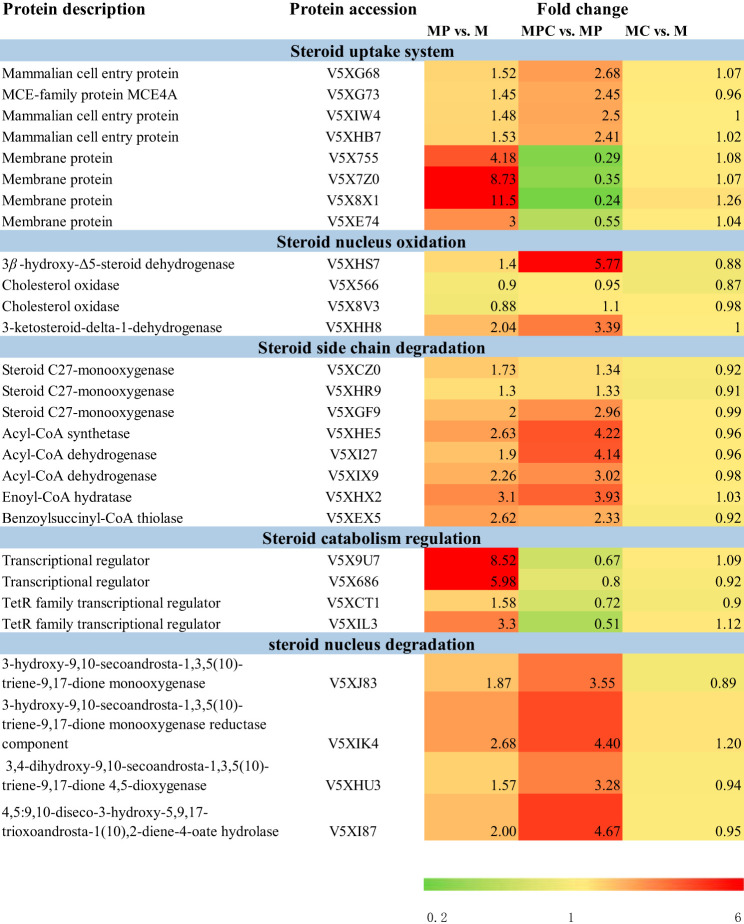
To study the mechanism of HP-β-CD in phytosterol metabolism action, we analyzed the identity and abundance of proteins involved in phytosterol degradation pathways. Table 1 and Fig. 6a present the changed proteins in MC-versus-M, MP-versus-M, and MPC-versus-MP tests. In general, mycobacterial degradation of sterol includes four-part systems, including steroid uptake systems, steroid nucleus oxidation, steroid side chain degradation, and the regulation of steroid catabolism. The enzymes involved in these pathways were identified, and most were upregulated in both phytosterol-supplemented medium and medium containing phytosterols combined with HP-β-CD.
TABLE 1.
Identified proteins involved in phytosterol degradation pathways
FIG 6.
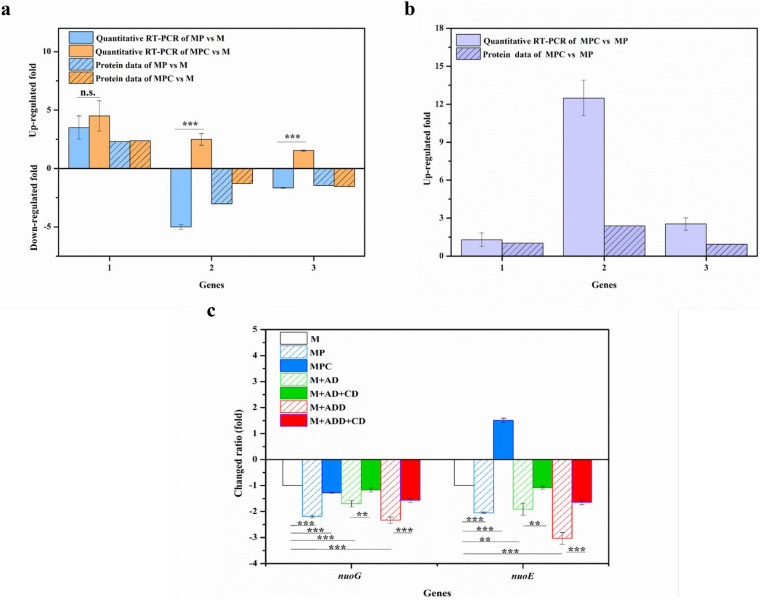
Changes in relative gene expression measured by qRT-PCR and determination of protein abundances. (a) Primary differentially expressed proteins included in the steroid degradation pathway and changes in relative expressions of selected genes determined by qRT-PCR. (b) Induction under MP and MPC conditions compared with that under M conditions. (c) Induction under MPC versus MP conditions. The values were calculated by qRT-PCR and the presented protein data. 1, 3β-hydroxy-Δ5-steroid dehydrogenase (3β-HSD); 2, steroid C27-monooxygenase (Cyp125); 3, 3-ketosteroid-Δ-1-dehydrogenase (KsdD). The error bars represent the standard deviations of data from three biological replicates. *, P < 0.05; **, P < 0.01; n.s., no significant difference (P > 0.05).
Steroid uptake system.
In previous studies, the gene clusters related to steroid degradation have been found, and the steroid transporter-mammalian cell entry (Mce) transport system was found in the gene cluster (25, 26). This system is closely related to steroid uptake. Casali and Riley (27) demonstrated that steroid uptake by mycobacterium is an active transport process. Furthermore, The Mce4 transport system in the steroid-degrading gene cluster of Mycobacterium tuberculosis affects the active uptake of steroid substrates by microorganisms (28). He et al. constructed a sterol transport system from Macrothrix elegans and improved the sterol conversion rate (29). As shown in Table 1, four Mce transport proteins were upregulated (UniProt numbers V5XG68 [1.52-fold], V5XG73 [1.45-fold], V5XIW4 [1.48-fold], and V5XHB7 [1.53-fold] [P < 0.05]) when phytosterol was added to M medium, and their expressions increased more significantly after the addition of cyclodextrin to MP medium (UniProt numbers V5XG68, 2.68-fold; V5XG73, 2.45-fold; V5XIW4, 2.5-fold; V5XHB7, 2.41-fold [P < 0.001]), indicating that sterol addition induced Mce transporter expression. Cyclodextrin addition promoted the expression of the Mce transporter. The Mce4 gene cluster reportedly encodes a steroid import system that enables mycobacteria to derive both carbon and energy from external sources (22). Apart from Mce proteins, other membrane proteins manifested significantly different expression levels; the membrane proteins in Table 1 were downregulated (UniProt numbers V5X755 [0.29-fold], V5X7Z0 [0.35-fold], V5X8X1 [0.24-fold], and V5XE74 [0.55-fold] [P < 0.001]) in the presence of HP-β-CD. Shen et al. (10) reported that with the addition of HP-β-CD, the membrane proteins accounting for solute transporters were downregulated, and the interactions between CDs and downregulated membrane proteins increased cell permeability. Thus, the downregulated membrane proteins may positively affect cell permeability.
Steroid nucleus oxidation.
3β-Hydroxy-Δ5-steroid dehydrogenase (3β-HSD) (UniProt no. V5XHS7), which initiates cholesterol catabolism by 3β-hydroxy group oxidation and Δ5→Δ4 isomerization (30), was identified in steroid nucleus oxidation (Table 1). 3β-HSD was slightly upregulated (1.4-fold; P < 0.05) when M. neoaurum M3C2 was grown in MP medium. Moreover, 3β-HSD was upregulated by 5.77-fold (P < 0.001) after the addition of HP-β-CD to phytosterol-containing medium. Cholesterol oxidase (ChoD) also initiates cholesterol catabolism (31, 32). The small change in the expression level of ChoD in phytosterol-supplemented medium could be due to the fact that ChoD is nonessential for M. neoaurum M3C2, similar to that in Mycobacterium sp. strain VKM Ac-1815D (33). KsdD is an important enzyme involved in steroid nucleus oxidation in the production of ADD from AD. KsdD (UniProt no. V5XHH8) was upregulated by 3.39-fold (P < 0.001) in the presence of HP-β-CD. The improvement of KsdD activity by HP-β-CD confirmed the hypothesis that HP-β-CD can enhance the catalytic activity of KsdD to increase the ADD-to-AD ratio (1). The other genes involved in the metabolism of the steroid nucleus, including those that encode KshA (3.10-fold; P < 0.001), KshB (2.87-fold; P < 0.001), HsaA (3.55-fold; P < 0.001), HsaB (4.40-fold; P < 0.001), HsaC (3.28-fold; P < 0.001), HsaD (4.67-fold; P < 0.001), MhpD (2.56-fold; P < 0.001), MhpE (2.85-fold; P < 0.001), and MhpF (2.46-fold; P < 0.001) (34), were upregulated with the addition of HP-β-CD to phytosterol-contained medium (Table S1).
Steroid side chain degradation.
The expression levels of most enzymes involved in the side chain degradation pathway were increased due to the addition of HP-β-CD to the biotransformation system. Steroid C27-monooxygenase (Cyp125) is the enzyme that catalyzes hydroxylation at C-26 (or C-27) (35–38). Three Cyp125-encoding genes (UniProt numbers V5XCZ0, V5XHR9, and V5XGF9) were identified from the proteome of M. neoaurum M3C2. V5XGF9 presented the highest expression level (2.96-fold; P < 0.001) among the three Cyp125 proteins, indicating its important role in hydroxylation at C-26 or C-27 of sterols. Subsequent cleavage of the side chain to 17-ketosteroid occurs stepwise in three consecutive cycles of fatty acid β-oxidation, thereby forming two molecules of propionyl-CoA and one molecule of acetyl-CoA from the side chain (39). In addition to Cyp125, other degradation-related proteins (acyl-CoA synthetase [4.22-fold], acyl-CoA dehydrogenase [4.13-fold and 3.02-fold], enoyl-CoA hydratase [3.93-fold], and benzoylsuccinyl-CoA thiolase [2.33-fold] [P < 0.001]) included in the β-oxidation metabolism pathway were also identified in the present study. Most of these proteins were highly expressed when MP was compared with M medium and when MPC was compared with MP medium.
Steroid catabolism regulation.
The transcription of genes involved in steroid metabolism is strongly regulated by transcriptional repressors of TetR-type KstR and KstR2 (34, 39, 40). As shown in Table 1, four transcriptional regulators exhibited changes in expression levels in MP-versus-M (UniProt numbers V5X9U7 [8.52-fold], V5X686 [5.98-fold], V5XCT1 [1.59-fold], and V5XIL3 [3.3-fold] [P < 0.001]) and MPC-versus-MP (UniProt numbers V5X9U7 [0.67-fold], V5X686 [0.8-fold], V5XCT1 [0.72-fold], and V5XIL3 [0.51-fold] [P < 0.001]) tests. These transcriptional regulators may be related to steroid catabolism and can be influenced by HP-β-CD.
To validate the proteomic analysis results, we analyzed the correlation between the production of several representative proteins and their gene expression levels by using reverse transcription-quantitative PCR (qRT-PCR). The following proteins with known important functions involved in phytosterol metabolism were selected: 3β-HSD, Cyp125, and KsdD. As shown in Fig. 6b and c, the patterns of gene expression fold changes determined by qRT-PCR were consistent with the proteomics data.
Effect of HP-β-CD on protein expression involved in energy-related metabolic pathways of M. neoaurum M3C2 in phytosterol biotransformation.
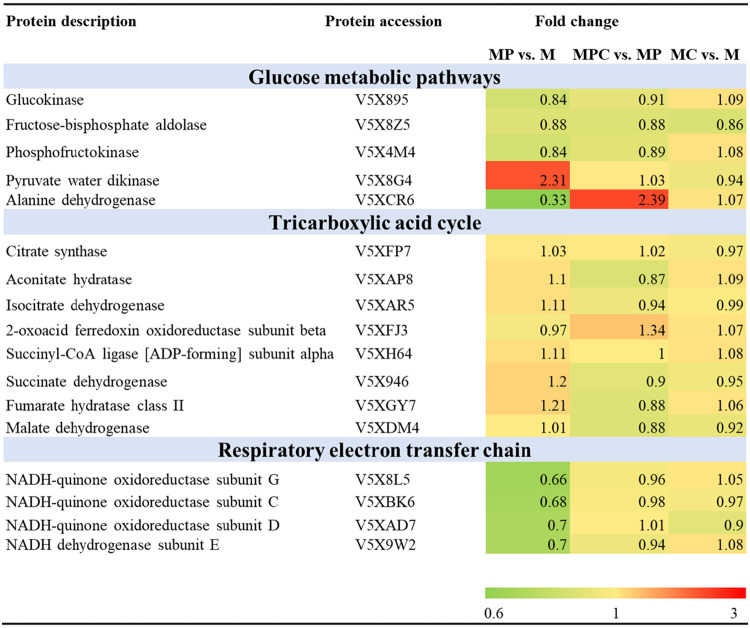
According to the results shown in Fig. 3, the glucose consumption of M. neoaurum M3C2 was inhibited and cell growth was enhanced after HP-β-CD was added during sterol biotransformation. Based on the phenotypic analyses, HP-β-CD promoted cell growth and instead suppressed glucose metabolism. We analyzed the characteristics of the proteins involved in the glucose metabolism, tricarboxylic acid (TCA), and ETC pathways, which exhibit a close relationship with the energy metabolism of M. neoaurum M3C2. Table 2 shows the proteins differentially expressed in MPC-versus-MP and MP-versus-M tests.
TABLE 2.
Identified proteins involved in glucose metabolic, tricarboxylic acid cycle, and respiratory electron transfer chain pathways
Glucokinase (Glk) (UniProt no. V5X895), phosphofructokinase (FruK) (UniProt no. V5X4M4), and fructose-bisphosphate aldolase (UniProt no. V5X8Z5) are enzymes involved in the glycolysis pathway. As shown in Table 2, these enzymes were downregulated to some extent in the presence of phytosterols in M medium (UniProt numbers V5X895 [0.84-fold], V5X4M4 [0.84-fold], and V5X8Z5 [0.88-fold] [P < 0.001]) or in the presence of HP-β-CD in MP medium (UniProt numbers V5X895 [0.91-fold], V5X4M4 [0.89-fold], and V5X8Z5 [0.88-fold] [P < 0.001]). Pyruvate water dikinase (V5X8G4), which can mediate the conversion of pyruvate to phosphoenolpyruvate as the first committed step of gluconeogenesis, was upregulated (2.31-fold; P < 0.001) in MP versus M medium. The changed expression of proteins indicated that the glycolytic pathway was inhibited and that the gluconeogenesis pathway was enhanced when phytosterols or HP-β-CD was present within the biotransformation system. Thus, glucose consumption was inhibited by phytosterols, and inhibition was enhanced by adding HP-β-CD to MP medium. Acyl-CoA produced from the glycolysis and phytosterol degradation pathways enters the TCA cycle. However, the enzymes involved in the TCA cycle were not differentially expressed when phytosterols or phytosterols and HP-β-CD were incorporated into the medium (Table 2). Alanine dehydrogenase (V5XCR6), which catalyzes the reversible reaction between pyruvate and alanine, was downregulated by 0.31-fold (P < 0.001) in the presence of phytosterols. However, this enzyme was upregulated by 2.39-fold (P < 0.001) in the presence of HP-β-CD in the medium with phytosterols. As shown in Fig. 7a and b, the patterns of gene expression fold changes of pyruvate water dikinase and alanine dehydrogenase determined by qRT-PCR were consistent with the proteome data.
FIG 7.
Changes in relative expressions of selected genes in glucose and energy metabolic pathways determined by qRT-PCR. (a) Induction under MP and MPC conditions compared with that under M conditions. (b) Induction under MPC versus MP conditions. 1, pyruvate water dikinase; 2, alanine dehydrogenase; 3, NADH dehydrogenase subunit E (nuoE). (c) Differential transcription of genes under different conditions determined by qRT-PCR compared with those under M conditions. M, M. neoaurum M3C2 cultured in M medium; MP, M. neoaurum M3C2 cultured in M medium with phytosterols (1.6 g/liter); MPC, M. neoaurum M3C2 cultured in M medium with phytosterols (1.6 g/liter) and HP-β-CD (25 mM); M+AD, M. neoaurum M3C2 cultured in M medium with AD (1 g/liter); M+AD+CD, M. neoaurum M3C2 cultured in M medium with AD (1 g/liter) and HP-β-CD (25 mM); M+ADD, M. neoaurum M3C2 cultured in M medium with ADD (1 g/liter); M+ADD+CD, M. neoaurum M3C2 cultured in M medium with ADD (1 g/liter) and HP-β-CD (25 mM). The error bars represent the standard deviations of data from three biological replicates. **, P < 0.01; ***, P < 0.001; n.s., no significant difference (P > 0.05).
Adequate energy and oxygen supply can enhance cell growth and phytosterol conversion (41, 42). Oxidative phosphorylation utilizes oxygen as the terminal electron acceptor and generates energy through the electron transfer chain; therefore, the differentially expressed proteins involved in electron transfer and oxidative phosphorylation were analyzed. The expressions of NADH-quinone oxidoreductase (UniProt numbers V5X8L5 [0.66-fold], V5XBK6 [0.68-fold], and V5XAD7 [0.70-fold] [P < 0.001]) and NADH dehydrogenase (V5X9W2 [0.70-fold] [P < 0.01]), which are involved in the ETC pathway, were partly downregulated in phytosterol medium compared to those in M medium. After HP-β-CD was added to sterol-containing medium, the expression levels of NADH-quinone oxidoreductase and NADH dehydrogenase showed no changes (Table 2). Thus, HP-β-CD can alleviate the inhibition of sterol biotransformation on the respiratory chain of cells. As shown in Fig. 4, AD and ADD inhibited cell growth and the cellular ratio of NAD+ to NADH. The reason was possibly the fact that AD(D) can impair the respiratory chain of cells (2, 5). Figure 7c shows the transcription levels of NADH-quinone oxidoreductase subunit G (nuoG) and NADH dehydrogenase subunit E (nuoE), whose expression levels were downregulated in the presence of phytosterols, in medium with AD (1 g/liter), ADD (1 g/liter), or phytosterols (1.6 g/liter, with molarity similar to those of AD and ADD). The nuoG transcription levels of M. neoaurum M3C2 in AD-, ADD-, and phytosterol-containing media were downregulated by 1.70-, 2.33-, and 2.19-fold compared with those in M medium, respectively. The nuoE transcription level was downregulated by 1.91-, 3.03-, and 2.05-fold when M. neoaurum M3C2 was cultured with AD, ADD, and phytosterols, respectively. Thus, AD and ADD can impair the respiratory chain of cells and inhibit cell growth. Compared with sterol-containing medium and after the addition of HP-β-CD, the relative transcription levels of nuoG and nuoE increased by 0.91- and 3.56-fold, respectively (Fig. 7c). Based on the above-described proteomic analysis, HP-β-CD can relieve the inhibitory effect of sterols on cell growth and the ETC pathway (Table 2 and Fig. 7).
DISCUSSION
CDs can enhance the biotransformation of steroids through the improvement of steroid solubilization and cell permeability mediated by CDs. Apart from the positive effect of HP-β-CD on steroid conversion, the enhanced catalytic activity of KsdD in the presence of HP-β-CD increases the ADD-to-AD ratio. In plant cells, CDs are used as elicitors to improve the production of artemisinin, trans-resveratrol, indole alkaloid, phytosterols, and phenolic compounds from cell suspension cultures of plants (43–46). However, studies have rarely reported the effect of CDs on steroid metabolic pathways of M. neoaurum. Based on these studies, we propose that HP-β-CD affects the expression of the genes involved in the steroid catabolic pathway of mycobacteria.
To further understand the metabolic changes caused by the addition of HP-β-CD and phytosterols, the proteome of M. neoaurum M3C2 cultured in different media was investigated. According to the proteomic analysis, HP-β-CD upregulated the expression of most proteins involved in steroid metabolism, thereby enhancing phytosterol bioconversion and changing the product proportions in M. neoaurum M3C2. In this work, a hypothesis for the mechanism by which CDs affect phytosterol catabolism is proposed. CDs have comparatively high molecular weights and low octanol-water partition coefficients. With the presence of multiple hydrogen donors and acceptors on CD molecules, lipophilic biological membranes are unlikely to be directly permeable to CDs. Therefore, the structure of CDs reduces the possibility of their entry into cells to affect the expression of intracellular enzymes. As CDs are a family of cyclic oligosaccharides with a hydrophilic outer surface and a lipophilic central cavity, they form an inclusion with sterols, thereby increasing the solubility of hydrophobic sterols in aqueous solution. However, CD also provides an isolated environment for the products to protect AD and ADD from degradation. Therefore, the reasons for the positive effect of HP-β-CD on steroid metabolism were suspected to be as follows. In the presence of HP-β-CD, more substrates can be dissolved and can enter the cell, thereby enhancing the substrate’s induction effect on the expressions of proteins related to the sterol metabolism pathway (Fig. 1). However, by RNA sequencing of the whole transcriptomes with different combinations of phytosterols and MCD, Shtratnikova et al. (12) stated that the genes related to the KstR regulon were responsible for side chain cleavage and the initial steps of steroid core oxidation. No significant difference or only a slight upregulation was observed during phytosterol supplementation with MCD.
We analyzed the correlation between the levels of several representative proteins and their gene expression levels by using qRT-PCR to validate the results of the proteomic analysis. Although the patterns of gene expression fold changes determined by qRT-PCR were consistent with the proteome data, the fold change levels differed at the transcriptional and translational levels (Fig. 6b and c). The correlation between proteome and transcriptome abundances in Saccharomyces cerevisiae or Escherichia coli has been studied extensively; weak positive correlations of fold changes are observed between the transcription and translation of genes (47, 48). Nevertheless, proteome-wide profiling of a strain is generally believed to directly reflect the protein regulation of an organism and changes in the metabolic processes.
Apart from the effect of HP-β-CD on the sterol metabolic pathway, HP-β-CD can influence the energy-related metabolic pathways of M. neoaurum M3C2 during the biotransformation of phytosterols (Fig. 1). The characteristics of proteins involved in the glucose metabolism, tricarboxylic acid (TCA), and electron transfer chain (ETC) pathways, which exhibit a close relationship with the energy metabolism of M. neoaurum M3C2, were analyzed. The changed expressions of proteins indicated that glucose consumption was inhibited by phytosterols, and inhibition was enhanced with the addition of HP-β-CD to MP medium. Cells can obtain carbon and energy from the degradation of side chain (41). The addition of CDs promotes the degradation of phytosterols to produce acyl-CoA, which will replace the acyl-CoA produced by glucose metabolism. As shown in Table 1, the expression of acyl-CoA synthetase was upregulated. To maintain the intracellular acyl-CoA content, the glycolysis pathway was inhibited. Acyl-CoA is among the main driving forces of mycobacterial carbon metabolism. Accelerating the metabolism of propionyl-CoA can increase biomass (49). Cyclodextrin increases the permeability of cell walls to nutrients and promotes cell growth. The expression of enzymes involved in the TCA cycle did not differ with phytosterols alone or with phytosterols in combination with HP-β-CD in the medium. Alanine dehydrogenase was upregulated with the addition of HP-β-CD in the medium with phytosterols. A high level of this enzyme is linked to the generation of alanine for peptidoglycan biosynthesis (50) and maintenance of the NAD+ pool when the terminal electron acceptor oxygen becomes limiting (51); these are important for cell growth and proliferation. The NADH-quinone oxidoreductase and NADH dehydrogenase involved in the ETC pathway were downregulated to some extent in the medium with phytosterols. However, the expressions of these enzymes showed no change after HP-β-CD was added to sterol-containing medium. The phytosterol addition induced the expression of enzymes in sterol metabolism and inhibited the glucose metabolic and ETC pathways (Fig. 1). After adding HP-β-CD to the sterol-containing system, the inhibitory effect of phytosterols on sterol and glucose metabolisms was further strengthened, but its influence on the ETC pathway was invariable.
These results indicate that HP-β-CD can alleviate the inhibition of sterol biotransformation on the respiratory chain of cells. The products of AD and ADD can impair the respiratory chain of cells and inhibit cell growth, and the inhibitory effect of ADD was more significant than that of AD. During phytosterol biotransformation, the phytosterol inhibitory effect on the transcription of enzymes involved in the ETC pathway was greater than that of AD and less than that of ADD. Thus, the inhibitory effect on cell growth and the ETC pathway during phytosterol conversion was mediated mainly by the toxicity of the biotransformation products AD and ADD. When HP-β-CD was added to sterol-containing (AD and ADD) medium, cell growth, the cellular NAD+-to-NADH ratio, and the transcription levels of nuoG and nuoE involved in the ETC pathway improved (Fig. 4 and 7). The inhibitory effect on cell growth and the ETC pathway during phytosterol conversion can be detoxified by HP-β-CD. In the presence of CD, intracellular AD(D) can be rapidly transported out of the cell to avoid the inhibitory effect of the products on the electron transport chain and cell growth (Fig. 1).
Adding HP-β-CD as a phytosterol carrier to phytosterol-containing medium promoted the steroid metabolic pathway and detoxified the toxic effects of metabolic products (AD and ADD) on the electron transfer system of the cell. This work analyzed the effect of phytosterol on the sterol and energy metabolism of Mycobacterium and clarified the mechanism of CD as a transporter for sterol delivery to promote the metabolic pathway in relation to phytosterol and cell growth. This study provides a theoretical basis for the study of the sterol metabolic pathway and insight into the complex functions of CDs as mediators of the regulation of sterol metabolism.
MATERIALS AND METHODS
Chemicals.
The phytosterol substrate used is a sterol mixture composed of 51.7% β-sitosterol, 27.2% stigmasterol, 17.1% campesterol, and 4.0% brassicasterol. Standard AD and ADD were purchased from Sigma-Aldrich Co. All chemical solvents and salts were of analytical grade or higher. HP-β-CD (31.7% degree of substitution; 1,523 average relative molecular mass) was procured from Xi’an Deli Biology and Chemical Industry Co., Ltd.
Microorganisms and culture conditions.
M. neoaurum M3C2 is the ksdD gene replacement strain M. neoaurum M3ΔksdD::ksdD-MNR constructed by homologous recombination (52). M. neoaurum M3C2 possesses an improved and stable phenotype that is more suitable for ADD accumulation than that of M. neoaurum M3. The AD(D) concentration refers to the total sum of the products ADD and AD. The cultivation and bioconversion of microorganisms and the preparation and analysis of transformation products were performed according to procedures described previously by Shen et al. (1) M. neoaurum M3C2 was grown in a previously described transformation culture medium, which is designated further minimal medium (M medium). M medium contained glucose (10 g/liter), MgSO4 (0.5 g/liter), K2HPO4 (0.5 g/liter), (NH4)2HPO4 (3.5 g/liter), citric acid (2 g/liter), and ammonium iron citrate (0.05 g/liter), with a pH of 7.2. Experiments were conducted under different culture conditions with phytosterols (3 g/liter) or HP-β-CD (25 mM) in M medium. MC medium contained M medium and HP-β-CD. MP medium contained M medium and phytosterols. MPC medium contained M medium, phytosterols, and HP-β-CD. Cells were grown in 250-ml shake flasks containing 50 ml of the culture medium on a rotary shaker (140 rpm) at 30°C. The seed culture was inoculated with fermentation broth at a concentration of 7%. All experiments were performed in triplicate, and data were statistically analyzed by one-way analysis of variance (ANOVA).
Quantification of bioconversion products.
AD and ADD were analyzed by high-performance liquid chromatography (HPLC) using a C18 column (250 mm by 4.6 mm) (Agilent, USA) with UV absorbance detection at 254 nm. For sample preparation, 0.8 ml fermentation liquid was absorbed into a tube, and ethyl acetate at the same volume was added for ultrasonic extraction. After centrifugation, 100 μl of the supernatant was placed into the tube and dried naturally. The solid extracts were redissolved in the HPLC mobile phase composed of methanol-water (4:1, vol/vol) at 30°C.
Growth estimations.
Cell growth measurement in medium with phytosterols was conducted according to methods described previously by Meyers et al. (53). Hot NaOH was used to release the total cellular protein, and the protein concentration was determined from the following equation: protein (micrograms per milliliter) = (183 × A230) − (75.8 × A260). The amount of protein was related to the dry cell weight (DCW) obtained using an adequate calibration curve, as follows: DCW (milligrams per milliliter) = 2.10 × protein (milligrams per milliliter) − 0.17 (R2 = 0.99). Glucose concentrations were quantitated with a biosensor (Shandong Academy of Sciences, Jinan, China).
Protein analysis and identification.
M. neoaurum M3C2 was grown in the above-described transformation culture media (M, MC, MP, and MPC media). The cells were harvested after 60 h of cultivation (late logarithmic phase). The collected sediment was sonicated on ice using a high-intensity ultrasonic processor (Scientz) in lysis buffer (8 M urea, 1% Triton X-100, 10 mM dithiothreitol, and 1% protease inhibitor cocktail) and centrifuged at 20,000 × g for 10 min at 4°C. The protein was redissolved in 8 M urea. The protein concentration was determined with a bicinchoninic acid (BCA) kit according to the manufacturer’s instructions. Proteins were prepared and analyzed using tandem mass tag (TMT)-based liquid chromatography-tandem mass spectrometry (LC-MS/MS). Each protein sample (300 μg) was reduced, alkylated, and subjected to tryptic hydrolysis. TMT labeling was performed according to the manufacturer’s protocols (Pierce). The three samples cultured in M medium were labeled with reagent 127. Samples cultured in MP medium were labeled with reagent 128. Samples cultured in MC medium were labeled with reagent 129. Samples cultured in MPC medium were labeled with reagent 130. TMT-labeled samples were diluted in 10 mM HCOONH4 buffer (NH3H2O, pH 10) before HPLC on a Waters XBridge Shield C18 reversed phase column. Twenty fractions were pooled for each sample and dried with a vacuum centrifuge. The peptides were separated by a linear gradient formed from 2% acetonitrile (ACN)–0.1% formic acid (FA) (mobile phase A) and 80% ACN–0.1% FA (mobile phase B) at a flow rate of 300 μl/min. MS analysis was performed on a Thermo Scientific Q Exactive Plus instrument. Raw data (.raw) were converted into peak lists (.mgf) by Proteome Discoverer. The 16S rRNA sequence of M. neoaurum M3 was subjected to a BLAST search at the National Center for Biotechnology Information database. Results showed 98% query coverage and 99% similarity to the 16S rRNA of M. neoaurum VKM Ac-1815D. Thus, the database used for the search was the M. neoaurum VKM Ac-1815D UniProt reference proteome (UniProt accession no. UP000018763). The median value of the ratio obtained from repeated comparisons of all organisms of different samples to be compared was taken as the fold change of the difference between the final samples. The relative quantitative value of each protein in the sample was tested by a t test, and the corresponding P value was calculated as the significance index. When the fold change was ≥1.5 and the P value was ≤0.05, protein expression was significantly different. Gene Ontology (http://www.geneontology.org/) analysis was used to investigate the potential functions of peptide precursors. The KEGG database (http://www.genome.jp/kegg/) was used to annotate the protein pathways.
Isolation of RNA and qRT-PCR analyses.
The cells were harvested at the late logarithmic phase and preserved at −80°C. Isolation of RNA and qRT‑PCR analyses were conducted according to methods described previously by Su et al. (54). Table 3 lists the primer pairs used. Each gene was measured in triplicate from three independent tests.
TABLE 3.
Primer sequences used for qRT-PCR
| Protein description (abbreviation) | Protein accession no. | Primer sequence |
|---|---|---|
| 16S rRNA | ACCAGCGTCCTGTGCATGTC/AGTACGGCCGCAAGGCTAAAAC | |
| 3β-Hydroxy-Δ5-steroid dehydrogenase (3β-HSD) | V5XHS7 | TGGAACGGCTTTACCTGAA/TGTCGAACAGCTCGGTGTAGTA |
| Steroid C27-monooxygenase (Cyp125) | V5XGF9 | TGTCCTCCTTCGAGCTCATC/CACGAAGAAACCGAACTC |
| 3-Ketosteroid-Δ-1-dehydrogenase (KsdD) | V5XHH8 | ACTATGTCCGGCTCAACC/CCATACCGACCAGGTTCTT |
| NADH dehydrogenase subunit E (NuoE) | V5X9W2 | GATCTCCAGAATCGCGTCA/GTCGCCACGTTCTATTCG |
| NADH-quinone oxidoreductase (NuoG) | V5X8L5 | GCACCTACCGGACAGATCT/ACCCGTTCATCGAGTTGTTG |
| Pyruvate water dikinase | V5X8G4 | CGGTCGAAGAAGAACACG/AACAGACCAAGGCGAAGATG |
| Alanine dehydrogenase | V5XCR6 | AGCGTTTGACCCTGTCGTAG/CATCACCGACAACGACTTCA |
Data availability.
The Protein Data Bank accession numbers for proteins identified in this study can be found in Tables 1 and 2.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Key R&D Program of China, Synthetic Biology Research (no. 2019YFA0905300), and the National Natural Science Foundation of China (no. 21978221 and 21276196).
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Shen YB, Wang M, Li HN, Wang YB, Luo JM. 2012. Influence of hydroxypropyl-beta-cyclodextrin on phytosterol biotransformation by different strains of Mycobacterium neoaurum. J Ind Microbiol Biotechnol 39:1253–1259. doi: 10.1007/s10295-012-1130-0. [DOI] [PubMed] [Google Scholar]
- 2.Malaviya A, Gomes J. 2008. Androstenedione production by biotransformation of phytosterols. Bioresour Technol 99:6725–6737. doi: 10.1016/j.biortech.2008.01.039. [DOI] [PubMed] [Google Scholar]
- 3.Xiong LB, Sun WJ, Liu YJ, Wang FQ, Wei DZ. 2017. Enhancement of 9alpha-hydroxy-4-androstene-3,17-dione production from soybean phytosterols by deficiency of a regulated intramembrane proteolysis metalloprotease in Mycobacterium neoaurum. J Agric Food Chem 65:10520–10525. doi: 10.1021/acs.jafc.7b03766. [DOI] [PubMed] [Google Scholar]
- 4.Wang FQ, Yao K, Wei DZ. 2011. From soybean phytosterols to steroid hormones, p 231–252. In El-Shemy H. (ed), Soybean and health. InTech, Rijeka, Croatia. [Google Scholar]
- 5.Donova MV. 2007. Transformation of steroids by actinobacteria: a review. Appl Biochem Microbiol 43:1–14. doi: 10.1134/S0003683807010012. [DOI] [PubMed] [Google Scholar]
- 6.Fernandes P, Cruz A, Angelova B, Pinheiro HM, Cabral JMS. 2003. Microbial conversion of steroid compounds: recent developments. Enzyme Microb Technol 32:688–705. doi: 10.1016/S0141-0229(03)00029-2. [DOI] [Google Scholar]
- 7.Hesselink PGM, van Vliet S, de Vries H, Witholt B. 1989. Optimization of steroid side chain cleavage by cyclodextrins. Enzyme Microb Technol 11:398–404. doi: 10.1016/0141-0229(89)90133-6. [DOI] [Google Scholar]
- 8.Manosroi A, Saowakhon S, Manosroi J. 2008. Enhancement of androstadienedione production from progesterone by biotransformation using the hydroxypropyl-β-cyclodextrin complexation technique. J Steroid Biochem Mol Biol 108:132–136. doi: 10.1016/j.jsbmb.2007.05.032. [DOI] [PubMed] [Google Scholar]
- 9.Zhang LT, Wang M, Shen Y, Ma Y, Luo J. 2009. Improvement of steroid biotransformation with hydroxypropyl-β-cyclodextrin induced complexation. Appl Biochem Biotechnol 159:642–654. doi: 10.1007/s12010-008-8499-2. [DOI] [PubMed] [Google Scholar]
- 10.Shen Y, Liang J, Li H, Wang M. 2015. Hydroxypropyl-β-cyclodextrin-mediated alterations in cell permeability, lipid and protein profiles of steroid-transforming Arthrobacter simplex. Appl Microbiol Biotechnol 99:387–397. doi: 10.1007/s00253-014-6089-5. [DOI] [PubMed] [Google Scholar]
- 11.Geng HZ, Kim KK, So KP, Lee YS, Chang Y, Lee YH. 2007. Effect of acid treatment on carbon nanotube-based flexible transparent conducting films. J Am Chem Soc 129:7758–7759. doi: 10.1021/ja0722224. [DOI] [PubMed] [Google Scholar]
- 12.Shtratnikova VY, Schelkunov MI, Dovbnya DV, Bragin EY, Donova MV. 2017. Effect of methyl-β-cyclodextrin on gene expression in microbial conversion of phytosterol. Appl Microbiol Biotechnol 101:4659–4667. doi: 10.1007/s00253-017-8288-3. [DOI] [PubMed] [Google Scholar]
- 13.Ma YH, Wang M, Fan Z, Shen YB, Zhang LT. 2009. The influence of host-guest inclusion complex formation on the biotransformation of cortisone acetate 1-dehydrogenation. J Steroid Biochem Mol Biol 117:146–151. doi: 10.1016/j.jsbmb.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 14.Mohamed SS, El-Refai A-MH, Hashem A-GM, Ali HA. 2014. Approaches to improve the solubility and availability of progesterone biotransformation by Mucor racemosus. Biocatal Biotransformation 32:141–150. doi: 10.3109/10242422.2014.894983. [DOI] [Google Scholar]
- 15.Andrés-Barrao C, Saad MM, Chappuis M-L, Boffa M, Perret X, Ortega Pérez R, Barja F. 2012. Proteome analysis of Acetobacter pasteurianus during acetic acid fermentation. J Proteomics 75:1701–1717. doi: 10.1016/j.jprot.2011.11.027. [DOI] [PubMed] [Google Scholar]
- 16.Shen HJ, Cheng BY, Zhang YM, Tang L, Li Z, Bu YF, Li XR, Tian GQ, Liu JZ. 2016. Dynamic control of the mevalonate pathway expression for improved zeaxanthin production in Escherichia coli and comparative proteome analysis. Metab Eng 38:180–190. doi: 10.1016/j.ymben.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 17.Zhang LL, Zhang Y, Ren JN, Liu YL, Li JJ, Tai YN, Yang SZ, Pan SY, Fan G. 2016. Proteins differentially expressed during limonene biotransformation by Penicillium digitatum DSM 62840 were examined using iTRAQ labeling coupled with 2D-LC-MS/MS. J Ind Microbiol Biotechnol 43:1481–1495. doi: 10.1007/s10295-016-1826-7. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Kang Y, Zhou Q, Zhou J, Wang H, Jin H, Liu X, Ma D, Li X. 2014. Quantitative proteomic analysis of serum from pregnant women carrying a fetus with conotruncal heart defect using isobaric tags for relative and absolute quantitation (iTRAQ) labeling. PLoS One 9:e111645. doi: 10.1371/journal.pone.0111645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Druzhinina AV, Andryushina VA, Stytsenko TS, Voishvillo NE. 2008. Conversion of 17α-methyltestosterone to methandrostenolone by the bacterium Pimelobacter simplex VKPM Ac-1632 with the presence of cyclodextrins. Appl Biochem Microbiol 44:580–584. doi: 10.1134/S0003683808060045. [DOI] [PubMed] [Google Scholar]
- 20.Awasthy D, Ambady A, Narayana A, Morayya S, Sharma U. 2014. Roles of the two type II NADH dehydrogenases in the survival of Mycobacterium tuberculosis in vitro. Gene 550:110–116. doi: 10.1016/j.gene.2014.08.024. [DOI] [PubMed] [Google Scholar]
- 21.Vilcheze C, Weisbrod TR, Chen B, Kremer L, Hazbon MH, Wang F, Alland D, Sacchettini JC, Jacobs WR Jr. 2005. Altered NADH/NAD+ ratio mediates coresistance to isoniazid and ethionamide in mycobacteria. Antimicrob Agents Chemother 49:708–720. doi: 10.1128/AAC.49.2.708-720.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van der Geize R, Yam K, Heuser T, Wilbrink MH, Hara H, Anderton MC, Sim E, Dijkhuizen L, Davies JE, Mohn WW, Eltis LD. 2007. A gene cluster encoding cholesterol catabolism in a soil actinomycete provides insight into Mycobacterium tuberculosis survival in macrophages. Proc Natl Acad Sci U S A 104:1947–1952. doi: 10.1073/pnas.0605728104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uhia I, Galan B, Kendall SL, Stoker NG, Garcia JL. 2012. Cholesterol metabolism in Mycobacterium smegmatis. Environ Microbiol Rep 4:168–182. doi: 10.1111/j.1758-2229.2011.00314.x. [DOI] [PubMed] [Google Scholar]
- 24.Mohn WW, Wilbrink MH, Casabon I, Stewart GR, Liu J, van der Geize R, Eltis LD. 2012. Gene cluster encoding cholate catabolism in Rhodococcus spp. J Bacteriol 194:6712–6719. doi: 10.1128/JB.01169-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garcia JL, Uhia I, Galan B. 2012. Catabolism and biotechnological applications of cholesterol degrading bacteria. Microb Biotechnol 5:679–699. doi: 10.1111/j.1751-7915.2012.00331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rathor N, Chandolia A, Saini NK, Sinha R, Pathak R, Garima K, Singh S, Varma-Basil M, Bose M. 2013. An insight into the regulation of mce4 operon of Mycobacterium tuberculosis. Tuberculosis (Edinb) 93:389–397. doi: 10.1016/j.tube.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 27.Casali N, Riley LW. 2007. A phylogenomic analysis of the Actinomycetales mce operons. BMC Genomics 8:60. doi: 10.1186/1471-2164-8-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arruda S, Bomfim G, Knights R, Huima-Byron T, Riley LW. 1993. Cloning of an M. tuberculosis DNA fragment associated with entry and survival inside cells. Science 261:1454–1457. doi: 10.1126/science.8367727. [DOI] [PubMed] [Google Scholar]
- 29.He K, Sun H, Song H. 2018. Engineering phytosterol transport system in Mycobacterium sp. strain MS136 enhances production of 9α-hydroxy-4-androstene-3,17-dione. Biotechnol Lett 40:673–678. doi: 10.1007/s10529-018-2520-9. [DOI] [PubMed] [Google Scholar]
- 30.Uhia I, Galan B, Morales V, Garcia JL. 2011. Initial step in the catabolism of cholesterol by Mycobacterium smegmatis mc2155. Environ Microbiol 13:943–959. doi: 10.1111/j.1462-2920.2010.02398.x. [DOI] [PubMed] [Google Scholar]
- 31.Kreit J, Sampson NS. 2009. Cholesterol oxidase: physiological functions. FEBS J 276:6844–6856. doi: 10.1111/j.1742-4658.2009.07378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fernández de Las Heras L, Mascaraque V, García Fernández E, Navarro-Llorens JM, Perera J, Drzyzga O. 2011. ChoG is the main inducible extracellular cholesterol oxidase of Rhodococcus sp. strain CECT3014. Microbiol Res 166:403–418. doi: 10.1016/j.micres.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 33.Ivashina TV, Nikolayeva VM, Dovbnya DV, Donova MV. 2012. Cholesterol oxidase ChoD is not a critical enzyme accounting for oxidation of sterols to 3-keto-4-ene steroids in fast-growing Mycobacterium sp. VKM Ac-1815D. J Steroid Biochem Mol Biol 129:47–53. doi: 10.1016/j.jsbmb.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 34.Shtratnikova VY, Schelkunov MI, Fokina VV, Pekov YA, Ivashina T, Donova MV. 2016. Genome-wide bioinformatics analysis of steroid metabolism-associated genes in Nocardioides simplex VKM Ac-2033D. Curr Genet 62:643–656. doi: 10.1007/s00294-016-0568-4. [DOI] [PubMed] [Google Scholar]
- 35.Capyk JK, Kalscheuer R, Stewart GR, Liu J, Kwon H, Zhao R, Okamoto S, Jacobs WR Jr, Eltis LD, Mohn WW. 2009. Mycobacterial cytochrome p450 125 (Cyp125) catalyzes the terminal hydroxylation of C27 steroids. J Biol Chem 284:35534–35542. doi: 10.1074/jbc.M109.072132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosloniec KZ, Wilbrink MH, Capyk JK, Mohn WW, Ostendorf M, van der Geize R, Dijkhuizen L, Eltis LD. 2009. Cytochrome P450 125 (CYP125) catalyses C26-hydroxylation to initiate sterol side-chain degradation in Rhodococcus jostii RHA1. Mol Microbiol 74:1031–1043. doi: 10.1111/j.1365-2958.2009.06915.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Driscoll MD, McLean KJ, Levy C, Mast N, Pikuleva IA, Lafite P, Rigby SEJ, Leys D, Munro AW. 2010. Structural and biochemical characterization of Mycobacterium tuberculosis CYP142: evidence for multiple cholesterol 27-hydroxylase activities in a human pathogen. J Biol Chem 285:38270–38282. doi: 10.1074/jbc.M110.164293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnston JB, Ouellet H, Ortiz de Montellano PR. 2010. Functional redundancy of steroid C26-monooxygenase activity in Mycobacterium tuberculosis revealed by biochemical and genetic analyses. J Biol Chem 285:36352–36360. doi: 10.1074/jbc.M110.161117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kendall SL, Burgess P, Balhana R, Withers M, ten Bokum A, Lott JS, Gao C, Uhia-Castro I, Stoker NG. 2010. Cholesterol utilization in mycobacteria is controlled by two TetR-type transcriptional regulators: kstR and kstR2. Microbiology 156:1362–1371. doi: 10.1099/mic.0.034538-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kendall SL, Withers M, Soffair CN, Moreland NJ, Gurcha S, Sidders B, Frita R, Ten Bokum A, Besra GS, Lott JS, Stoker NG. 2007. A highly conserved transcriptional repressor controls a large regulon involved in lipid degradation in Mycobacterium smegmatis and Mycobacterium tuberculosis. Mol Microbiol 65:684–699. doi: 10.1111/j.1365-2958.2007.05827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Szentirmai A. 1990. Microbial physiology of sidechain degradation of sterols. J Ind Microbiol Biotechnol 6:101–115. doi: 10.1007/BF01576429. [DOI] [Google Scholar]
- 42.Su L, Shen Y, Gao T, Luo J, Wang M. 2017. Improvement of AD biosynthesis response to enhanced oxygen transfer by oxygen vectors in Mycobacterium neoaurum TCCC 11979. Appl Biochem Biotechnol 182:1564–1574. doi: 10.1007/s12010-017-2418-3. [DOI] [PubMed] [Google Scholar]
- 43.Durante M, Caretto S, Quarta A, De Paolis A, Nisi R, Mita G. 2011. β-Cyclodextrins enhance artemisinin production in Artemisia annua suspension cell cultures. Appl Microbiol Biotechnol 90:1905–1913. doi: 10.1007/s00253-011-3232-4. [DOI] [PubMed] [Google Scholar]
- 44.Chu M, Pedreno MA, Alburquerque N, Faize L, Burgos L, Almagro L. 2017. A new strategy to enhance the biosynthesis of trans-resveratrol by overexpressing stilbene synthase gene in elicited Vitis vinifera cell cultures. Plant Physiol Biochem 113:141–148. doi: 10.1016/j.plaphy.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 45.Almagro L, Gutierrez J, Pedren MA, Sottomayor M. 2014. Synergistic and additive influence of cyclodextrins and methyl jasmonate on the expression of the terpenoid indole alkaloid pathway genes and metabolites in Catharanthus roseus cell cultures. Plant Cell Tissue Organ Cult 119:543–551. doi: 10.1007/s11240-014-0554-9. [DOI] [Google Scholar]
- 46.Miras-Moreno B, Almagro L, Pedreño MA, Sabater-Jara AB. 2016. Enhanced accumulation of phytosterols and phenolic compounds in cyclodextrin-elicited cell suspension culture of Daucus carota. Plant Sci 250:154–164. doi: 10.1016/j.plantsci.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 47.Griffin TJ, Gygi SP, Ideker T, Rist B, Eng J, Hood L, Aebersold R. 2002. Complementary profiling of gene expression at the transcriptome and proteome levels in Saccharomyces cerevisiae. Mol Cell Proteomics 1:323–333. doi: 10.1074/mcp.m200001-mcp200. [DOI] [PubMed] [Google Scholar]
- 48.Taniguchi Y, Choi PJ, Li GW, Chen H, Babu M, Hearn J, Emili A, Xie XS. 2010. Quantifying E. coli proteome and transcriptome with single-molecule sensitivity in single cells. Science 329:533–538. doi: 10.1126/science.1188308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou X, Zhang Y, Shen Y, Zhang X, Zhang Z, Xu S, Luo J, Xia M, Wang M. 2019. Economical production of androstenedione and 9α-hydroxyandrostenedione using untreated cane molasses by recombinant mycobacteria. Bioresour Technol 290:121750. doi: 10.1016/j.biortech.2019.121750. [DOI] [PubMed] [Google Scholar]
- 50.Starck J, Kallenius G, Marklund BI, Andersson DI, Akerlund T. 2004. Comparative proteome analysis of Mycobacterium tuberculosis grown under aerobic and anaerobic conditions. Microbiology 150:3821–3829. doi: 10.1099/mic.0.27284-0. [DOI] [PubMed] [Google Scholar]
- 51.Hutter B, Dick T. 1998. Increased alanine dehydrogenase activity during dormancy in Mycobacterium smegmatis. FEMS Microbiology Lett 167:7–11. doi: 10.1016/S0378-1097(98)00360-7. [DOI] [PubMed] [Google Scholar]
- 52.Xie RL, Shen YB, Qin N, Wang YB, Su LQ, Wang M. 2015. Genetic differences in ksdD influence on the ADD/AD ratio of Mycobacterium neoaurum. J Ind Microbiol Biotechnol 42:507–513. doi: 10.1007/s10295-014-1577-2. [DOI] [PubMed] [Google Scholar]
- 53.Meyers PR, Bourn WR, Steyn LM, van Helden PD, Beyers AD, Brown GD. 1998. Novel method for rapid measurement of growth of mycobacteria in detergent-free media. J Clin Microbiol 36:2752–2754. doi: 10.1128/JCM.36.9.2752-2754.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Su L, Shen Y, Zhang W, Gao T, Shang Z, Wang M. 2017. Cofactor engineering to regulate NAD(+)/NADH ratio with its application to phytosterols biotransformation. Microb Cell Fact 16:182. doi: 10.1186/s12934-017-0796-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The Protein Data Bank accession numbers for proteins identified in this study can be found in Tables 1 and 2.