The biodegradation pathway of pyridine, a notorious toxicant, is relatively unexplored, as no genetic data related to this process have ever been presented. In this paper, we describe the plasmid-borne pyr gene cluster, which includes the complete set of genes responsible for the degradation of pyridine. A key enzyme, the monooxygenase PyrA, which is responsible for the first step of the catabolic pathway, performs an oxidative cleavage of the pyridine ring without typical activation steps such as reduction or hydroxylation of the heterocycle. This work provides new insights into the metabolism of N-heterocyclic compounds in nature.
KEYWORDS: Arthrobacter, biodegradation, plasmid, pyridine, two-component flavin-dependent monooxygenase
ABSTRACT
Pyridine and its derivatives constitute the majority of heterocyclic aromatic compounds that occur largely as a result of human activities and contribute to environmental pollution. It is known that they can be degraded by various bacteria in the environment; however, the degradation of unsubstituted pyridine has not yet been completely resolved. In this study, we present data on the pyridine catabolic pathway in Arthrobacter sp. strain 68b at the level of genes, enzymes, and metabolites. The pyr gene cluster, responsible for the degradation of pyridine, was identified in a catabolic plasmid, p2MP. The pathway of pyridine metabolism consisted of four enzymatic steps and ended by the formation of succinic acid. The first step in the degradation of pyridine proceeds through a direct ring cleavage catalyzed by a two-component flavin-dependent monooxygenase system, encoded by pyrA (pyridine monooxygenase) and pyrE genes. The genes pyrB, pyrC, and pyrD were found to encode (Z)-N-(4-oxobut-1-enyl)formamide dehydrogenase, amidohydrolase, and succinate semialdehyde dehydrogenase, respectively. These enzymes participate in the subsequent steps of pyridine degradation. The metabolites of these enzymatic reactions were identified, and this allowed us to reconstruct the entire pyridine catabolism pathway in Arthrobacter sp. 68b.
IMPORTANCE The biodegradation pathway of pyridine, a notorious toxicant, is relatively unexplored, as no genetic data related to this process have ever been presented. In this paper, we describe the plasmid-borne pyr gene cluster, which includes the complete set of genes responsible for the degradation of pyridine. A key enzyme, the monooxygenase PyrA, which is responsible for the first step of the catabolic pathway, performs an oxidative cleavage of the pyridine ring without typical activation steps such as reduction or hydroxylation of the heterocycle. This work provides new insights into the metabolism of N-heterocyclic compounds in nature.
INTRODUCTION
Pyridine is the simplest aromatic N-heterocycle found in a myriad of natural compounds and widely used as a solvent as well as a starting material for the synthesis of herbicides, pesticides, and pharmaceuticals (1). Large amounts of pyridine are often found in effluents from coking plants, pharmaceutical factories, and other related industries. Due to its toxicity, the emission of pyridine-containing waste causes significant damage to human health and the quality of the environment (1). Despite its widespread application, the data on the pathways of pyridine degradation in nature remain obscure. “It appears that pyridine affords a magnificent diet for bacteria and provides the simple case of two or three species feeding directly on the substance itself or on its decomposition products,” wrote Buddin in 1914 (2). Many bacteria that are involved in the biodegradation of pyridine have been discovered since then, including Arthrobacter (3–5), Nocardia (6), Bacillus (6), Paracoccus (7, 8), Pseudomonas (9), Rhodococcus (10), Shewanella (11), Alcaligenes (12), Shinella (13), Gordonia (14), Pimelobacter (15), and Achromobacter (16) species. Despite more than 100 years of investigation of pyridine ring metabolism, only recently the enzymes and genes essential for biodegradation of the hydroxylated or carboxylated pyridine derivatives have been identified (1, 17–19). Nevertheless, none of the genes encoding the biodegradation of pyridine have been described. Moreover, the enzymes involved in the initial stages of ring cleavage are not known (6, 20).
Several distinct pathways of the ring fission of pyridine have been proposed during the years of investigation (Fig. 1). The first pathway involves cleavage of the ring between C-2 and C-3 to yield a semialdehyde, which is hydrolyzed to formamide and succinate semialdehyde (6, 21). Another pathway of pyridine catabolism involves C-2–N ring cleavage and is accompanied by the accumulation of glutarate semialdehyde (6). Both pathways are proposed to proceed through a ring reduction step before the ring opening (Fig. 1B and C). There are also suggestions that the initial pyridine degradation may proceed without a reduction step when pyridine is first hydroxylated by Rhodococcus opacus (5) and Arthrobacter sp. cells (5, 22) (Fig. 1A and D). However, the enzymes of the proposed pathways have not been identified.
FIG 1.

A principal scheme of the proposed pathways of aerobic bacterial degradation of pyridine. (A) Arthrobacter crystallopoietes (5). (B) Corynebacterium sp. (48), Bacillus sp. (6), and Micrococcus luteus (21). (C) Nocardia sp. (59). (D) Rhodococcus opacus (5). Compounds were detected and/or isolated except one depicted in brackets. Hyphenated arrows, hypothetical reactions (enzymes or genes were not detected); solid arrows, recorded enzymatic activities.
Recently, it has been shown that an initial pyridine ring cleavage may proceed without reduction or hydroxylation steps. Hence, it has been confirmed that oxygenase is responsible for the opening of the pyridine ring of N-methylnicotinate (19). The similar reactions are catalyzed by pyrimidine monooxygenase RutA, which cleaves pyrimidine ring between N-3 and C-4 directly by adding oxygen atoms (23), and tetramethylpyrazine monooxygenase (24) cleaving a pyrazine ring between C-2 and C-3 with the formation of (Z)-N,N´-(but-2-ene-2,3-dinyl)diacetamide. The existence of oxygenases capable of attacking the nonreduced heterocyclic rings suggests the possibility of an analogous reaction in the cleavage of the unsubstituted pyridine.
In this study, we report the entire catabolic pathway of pyridine in Arthrobacter sp. strain 68b. This strain was previously isolated from the contaminated soil based on its ability to utilize phthalic acid as a source of carbon and energy (25). It turned out that the Arthrobacter sp. strain 68b could use nicotine, pyridine, and 2-methylpyridine as a sole carbon source. We determined that the bacterium harbors two catabolic plasmids containing the degradation genes of the abovementioned aromatic and heterocyclic compounds. A gene cluster responsible for pyridine degradation, which we called pyr, was located in one of the plasmids. The genes coding the pyridine-degrading proteins were cloned and expressed in Escherichia coli cells, and the metabolites of the pyridine pathway were identified. These findings allowed us to elucidate the catabolic pathway of pyridine metabolism with the implementation of a novel flavin-dependent monooxygenase as the first step of this process.
RESULTS
The aim of this study was to investigate Arthrobacter sp. strain 68b (DSM103156), which is capable of degrading pyridine and assimilating it as the sole carbon source. In addition, this bacterium utilizes 2-methylpyridine, nicotine, and phthalic acid. As the ability to degrade xenobiotic compounds often is encoded by catabolic plasmids, we decided to search for them in the Arthrobacter sp. 68b strain. The analysis of genome composition revealed that the Arthrobacter sp. 68b strain harbors at least two high-molecular-weight plasmids (Fig. 2). To ascertain whether the genes for pyridine biodegradation are plasmid-borne, we performed plasmid curing and obtained mutant strains which were unable to metabolize pyridine (mut4 and mut5). After the analysis of genome composition and growth substrate specificity, it was concluded that the catabolic plasmid named pNIC harbors nicotine degradation genes, whereas the plasmid designated p2MP contains genes involved in pyridine, 2-methylpyridine, and phthalate degradation (Fig. 2). The plasmid p2MP is 112,864 bp in length with GC content of 61 mol% (GenBank accession number KJ410765) and contains 99 putative open reading frames.
FIG 2.

The pyridine catabolic genes are located on the p2MP plasmid. Analysis of the total DNA from Arthrobacter sp. 68b and its mutants (mut4 and mut5) by agarose gel electrophoresis and growth on mineral medium supplemented with the indicated substrate.
The analysis of pyridine-induced proteins in the Arthrobacter sp. 68b strain by SDS-PAGE revealed the overexpression of a 40-kDa protein designated PyrA. De novo sequencing of this protein allowed the identification of the PyrA-encoding gene (Fig. 3). It turned out that two identical copies of the pyrA gene were located 8 kb apart from each other on the plasmid p2MP in a disorganized gene cluster (pyr) (22.1 kb) (Table 1). The amino acid sequence of PyrA protein showed the highest identity (35 to 40%) to bacterial flavin-dependent monooxygenases from the luciferase-like monooxygenase (LLM) family. According to structural and functional properties, the members of LLM belong to the group C monooxygenases (Fig. 4). For catalytic activity, they require reduced flavin mononucleotide (FMN) that is produced by a supplementary flavin reductase. The mechanism of LLMs is thought to involve the formation of a C-4a-(hydro)peroxyflavin intermediate that is responsible for the oxygenation of the substrate (26). Generally, genes encoding flavin reductase and monooxygenase are located in the same operon (27). In the case of the Arthrobacter sp. 68b strain, the flavin reductase gene, designated pyrE, was located upstream of the second pyrA gene. This gene organization allowed us to assume that the PyrA and PyrE proteins could comprise two-component monooxygenase. Earlier, it was supposed that pyridine degradation pathways might include reductive steps before ring cleavage (28); however, the latest data show that oxygenases are involved in the initial ring cleavage of some pyridine ring-containing compounds (19, 24, 29). Moreover, the aromatic N-heterocycle ring-cleaving monooxygenases (trigonelline monooxygenase [TgnB] and tetramethylpyrazine monooxygenase [TpdA]) clustered with PyrA in the same branch of the phylogenetic tree (Fig. 4). Thus, we hypothesized that the two-component monooxygenase participated in the pyridine ring cleavage. To test this idea, we cloned the pyrA and pyrE genes and expressed them in E. coli BL21(DE3) cells. Then we used the recombinant cells or the purified proteins for the pyridine conversion experiments in vivo and in vitro, respectively.
FIG 3.
PyrA monooxygenase is induced by pyridine in Arthrobacter sp. 68b. The sequence of PyrA protein is presented in a frame with four highlighted peptides, which were determined by de novo protein sequencing; the arrow shows PyrA protein in cell extracts. Cells were grown with succinic acid (1), glucose (2), 2-methylpyridine (3), and pyridine (4); samples were analyzed by SDS-PAGE.
TABLE 1.
Functional annotation of the pyr gene clustera
| Gene/protein (GenPept accession no.) | Protein length (aa) | Nearest homolog, GenPept accession no. | Identity (%) |
|---|---|---|---|
| pyrA1 AKG47386.1 | 384 | LLM class flavin-dependent oxidoreductase (Gordonia aichiensis), WP_005179081.1 | 42 |
| orf43 AKG47381.1 | 165 | Riboflavin kinase (Arthrobacter sp. DCT5), WP_113719793.1 | 62 |
| pyrB AKG47382.1 | 465 | NAD-dependent succinate-semialdehyde dehydrogenase (Streptomyces sp.), WP_100452979.1 | 74 |
| pyrC AKG47383.1 | 570 | Amidohydrolase (Pseudonocardia spinosispora), WP_028937455.1 | 61 |
| pyrE AKG47384.1 | 178 | Flavin reductase (Micrococcus luteus ATCC 49442), WP_164207067.1 | 90 |
| pyrA2 AKG47385.1 | 384 | LLM class flavin-dependent oxidoreductase (Gordonia aichiensis), WP_005179081.1 | 42 |
| orf48 AKG47387.1 | 41 | Pirin family protein (Leptospira adleri), WP_100787178.1 | 85 |
| orf49 AKG47388.1 | 157 | 3,4-Dihydroxy-2-butanone-4-phosphate synthase (Arthrobacter cupressi), WP_074587626.1 | 77 |
| orf50 AKG47434.1 | 139 | Nuclear transport factor 2 family protein (Pseudarthrobacter sp. strain ATCC 49987), WP_160667908.1 | 85 |
| orf51 AKG47389.1 | 396 | Helix-turn-helix domain-containing protein (Micrococcus luteus ATCC 49442), WP_164207088.1 | 99 |
| orf52 AKG47435.1 | 115 | VOC family protein (Pseudarthrobacter sp. strain ATCC 49987), WP_160667916.1 | 77 |
| orf53 AKG47436.1 | 100 | DUF1330 domain-containing protein (Sphingobium herbicidovorans), WP_051908118.1 | 37 |
| pyrD AKG47390.1 | 494 | NAD-dependent succinate-semialdehyde dehydrogenase (Arthrobacter sp. ok909), WP_091254750.1 | 94 |
Enzymes involved in the catabolic pathway of pyridine are bold.
FIG 4.
Evolutionary relationship of PyrA monooxygenase in the context of class C flavin-dependent monooxygenases. PyrA, pyridine monooxygenase; TpdA, tetramethylpyrazine monooxygenase; TgnB, trigonelline monooxygenase. The assignment of the indicated accession numbers of the sequences are provided in Table S3 in the supplemental material. Scale bar, 20 substitutions per site.
The liquid chromatography-mass spectrometry (LC-MS) analysis of the in vivo conversion reaction mixture of pyridine (with recombinant cells containing pyrA and pyrE genes) showed the decrease of pyridine and generation of a new metabolite (later identified to be compound 2). The wild-type E. coli cells did not metabolize pyridine at all (data not shown). The mass-to-charge ratio (m/z) of the formed pyridine metabolite 2 was 114 [M+H]+ (Fig. S1 in the supplemental material). This new compound diminished from in vivo reaction samples during prolonged incubation (24 h) and was assumed to be slowly metabolized by E. coli. However, attempts to purify this compound for nuclear magnetic resonance (NMR) identification were unsuccessful due to its decomposition and proved it to be rather unstable. Next, we tried to capture the intermediate metabolites of the PyrA reaction during the in vitro experiments. However, the purified recombinant flavin reductase PyrE was unstable and was replaced by a more stable FMN reductase IifD (30) and the enzymatic system for NADPH regeneration (NADPH-dependent glucose-6-phosphate dehydrogenase and its substrate). The same compound 2 was identified in the in vitro reaction mixture as in in vivo. Metabolite 2 was not synthesized in the control reaction samples containing the monooxygenase PyrA or flavin reductase only. Thus, both enzymes were required for the initial step of pyridine degradation and the formation of metabolite 2. Considering all possible compounds that could be formed in this reaction, we presumed that it might be a pyridine ring cleavage product; thus, it should have an aldehyde group. To verify this hypothesis, we performed the derivatization of the reaction product with 2-nitrophenylhydrazine (2-NPH). The LC-MS analysis revealed the formation of a new compound (i.e., compound 6) (m/z 249 [M+H]+) instead of metabolite 2. Furthermore, the new compound had an absorption maximum at 445 nm, consistent with the 2-NPH derivatives of aldehydes and ketones. Compound 6 was extracted from the bioconversion reaction mixture and purified (Fig. S2). The NMR analysis revealed that the structure of compound 6 corresponded to a mixture of two rotamers of (Z)-N-4-(2-nitrophenyl)hydrazone(but-1-enyl)formamide (Fig. S9). Similar rotamers were observed for structurally related compounds described previously (31).
Hence, (Z)-N-(4-oxobut-1-enyl)formamide (metabolite 2) was identified to be the first intermediate metabolite of pyridine degradation. The mass difference between substrate (pyridine) and the product of the PyrA reaction is 34 Da. As flavin-dependent monooxygenases rarely incorporate both oxygen atoms from O2, the simplest explanation would be that a single oxygen atom and additional water molecule were incorporated during the PyrA-catalyzed cleavage of the pyridine ring. To confirm the origin of oxygen atoms in the product of pyridine ring cleavage catalyzed by the PyrA monooxygenase, we performed the pyridine conversion by the recombinant E. coli cells expressing pyrA and pyrE genes in the 18O-labeled water (H218O). LC-MS data showed (Fig. 5) that the m/z ratio of the 18O-labeled metabolite 2 was 116 [M+H]+ (compared to m/z = 114 [M+H]+ obtained in H2O), indicating that one 18O atom from the labeled water was incorporated (Fig. 5). This led to the conclusion that another oxygen atom in metabolite 2 arose from molecular oxygen; thus, pyridine-oxidizing enzyme PyrA acted as a monooxygenase. After the analogous derivatization with 2-NPH, the LC-MS analysis revealed that the m/z ratio of derivative 6 produced in the presence of H218O was 249 [M+H]+, and it did not differ from the one observed when the reaction was carried out in H2O (Fig. 5). Thus, we were able to determine end positions of oxygen atoms, which were derived from different substrates (O2 and water). The oxygen atom from the O2 molecule was incorporated into the C-2 position of pyridine, and water was added to the C-3 position (Fig. 5). For the elucidation of the potential substrates for PyrA, we tested various compounds (Table 2) as putative substrates using whole recombinant cells of E. coli. Hence, in addition to pyridine, the enzyme could convert 2-methylpyridine and 3-methylpyridine. The conversion of those compounds was confirmed by the in vitro experiments too (Fig. S3 and S4). The products of the corresponding reactions were identified as resulting from analogous ring fission of pyridine between the C-2 and C-3 positions. Furthermore, monooxygenase PyrA was determined to be active not only with FMN but also with flavin adenine dinucleotide (FAD) as a cosubstrate (Fig. S1).
FIG 5.
The action of PyrA monooxygenase. (a) Reaction catalyzed by PyrA. (b) MS spectra of the pyridine metabolite formed in the unlabeled and 18O-labeled water. The peaks of m/z 114 and m/z 116 are the metabolite 2, (Z)-N-(4-oxobut-1-enyl)formamide [M+H]+, ions and the peaks of m/z 155 and m/z 157 are metabolite 2 [M+CH3CN]+ ions formed in the unlabeled and 18O-labeled water, respectively; the peaks of m/z 249 are of the derivative 6, (Z)-N-(4-(2-nitrophenyl)hydrazono(but-1-enyl)formamide), [M+H]+ ion formed in the unlabeled and 18O-labeled water.
TABLE 2.
Substrates used for testing PyrA activity
| Substrate type | Compoundsa |
|---|---|
| Hydroxylated pyridines | 2-Hydroxypyridine, 3-hydroxypyridine, 4-hydroxypyridine, 2,3-dihydroxypyridine, 2,4-dihydroxypyridine, 2,5-dihydroxypyridine |
| Alkylated pyridines | 2-Methylpyridine, 3-methylpyridine, 4-methylpyridine, 2,3-dimethylpyridine, 2,4-dimethylpyridine, 2,5-dimethylpyridine, 2,6-dimethylpyridine, 3,4-dimethylpyridine, 3,5-dimethylpyridine, 2,3,5-trimethylpyridine, 2,4,6-trimethylpyridine, 2-ethylpyridine, 4-ethylpyridine |
| Carboxylated pyridines | 2-Pyridinecarboxylic acid, 3-pyridinecarboxylic acid, 2,3-pyridinedicarboxylic acid, 2,4-pyridinedicarboxylic acid, 2,5-pyridinedicarboxylic acid, 2,6-pyridinedicarboxylic acid, 3,4-pyridinedicarboxylic acid, 3,5-pyridinedicarboxylic acid |
| Pyridine and other pyridine derivatives | Pyridine, 2-hydroxy-3-methylpyridine, 2-hydroxy-6-methylpyridine, 5-hydroxy-2-methylpyridine, 2-hydroxy-4-methyl-3-nitropyridine, 2-hydroxymethylpyridine, 3-hydroxy-2-methylpyridine, 2-methylpyridine N-oxide, 2-amino-3-methylpyridine, 2-amino-4-methylpyridine, 6-amino-3-methylpyridine, 2,3-cyclopentenopyridine, 2,4-pyridinedicarbonitrile, 4-methyl-2-pyridinecarbonitrile, 6-methyl-2-pyridinecarbonitrile, 3,5-dimethylpyridine-2-carbonitrile |
| Pyrazines | 2,3-Dimethylpyrazine, 2,5-dimethylpyrazine, 2,6-dimethylpyrazine, 2,3,5-trimethylpyrazine, 2,3,5,6-tetramethylpyrazine |
| Quinolines | 2,2ʹ-Bipyridine, 4,4ʹ-bipyridine, 5-aminoquinoline, 8-hydroxyquinoline |
Compounds consumed by the enzyme are marked in bold.
To elucidate the subsequent part of the degradation pathway of pyridine, we heterologously expressed the genes from the pyr cluster (Table 1) and purified two putative aldehyde dehydrogenases (PyrB and PyrD) and amidohydrolase (PyrC) (Fig. 6). We determined that PyrB (homologous to NAD-dependent succinate semialdehyde dehydrogenase) could not use succinate semialdehyde as a substrate, but metabolite 2 was a substrate for PyrB in reaction with NAD as an electron acceptor. Consequently, the PyrB could be the second enzyme in the pyridine catabolic pathway. A new metabolite (i.e., metabolite 3) with an m/z ratio of 130 [M+H]+ was formed in both in vitro and in vivo pyridine conversion reactions proceeding in the presence of the PyrA, flavin reductase, and PyrB (Fig. 6). The NMR analysis of the purified metabolite 3 revealed a mixture of two rotamers of (Z)-4-formamidobut-3-enoic acid (Fig. S5 and S9). The same formation of two rotamers was observed for compound 6. Thus, hypothetical dehydrogenase PyrB was confirmed to catalyze oxidation of the aldehyde group in compound 2.
FIG 6.
In vitro reconstruction of the pyridine degradation pathway. (a) Analysis of pyridine metabolites by LC-MS and the metabolic pathway of pyridine. Samples were analyzed in the positive or negative ionization mode; the extracted ion chromatograms correspond to the quasimolecular ions of pyridine (1), (Z)-N-(4-oxobut-1-enyl)formamide (2), (Z)-4-formamidobut-3-enoic acid (3), succinic acid semialdehyde (4), and succinate (5). (b) Purified proteins participating in pyridine pathway; (c) pyr gene cluster in the p2MP plasmid. PyrA, pyridine monooxygenase, PyrE and FR, flavin reductase; PyrB, (Z)-N-(4-oxobut-1-enyl)formamide dehydrogenase; PyrC, amidohydrolase; PyrD, succinate semialdehyde dehydrogenase.
We then hypothesized that amidohydrolase PyrC could be the third enzyme in the pyridine degradation pathway. Indeed, the PyrC protein was active in vitro toward (Z)-4-formamidobut-3-enoic acid (metabolite 3), and the LC-MS analysis showed a new metabolite (i.e., metabolite 4) with an m/z ratio of 101 [M-H]−. The retention time and mass spectrum of the product 4 matched those of succinic acid semialdehyde (Fig. 6 and Fig. S6a). The formation of this metabolite during pyridine degradation was reported previously (6, 32–34). Next, PyrD, a putative succinate semialdehyde dehydrogenase, was tested toward succinic acid semialdehyde or the PyrC reaction product 4 as the substrates. Not surprisingly, the PyrD showed a succinate semialdehyde dehydrogenase activity and produced succinic acid (Fig. 6 and Fig. S6b). It should be stressed that neither PyrC nor PyrD used metabolite 2 as a substrate.
DISCUSSION
In this report, we describe the degradation of pyridine in Arthrobacter sp. 68b. It turned out that the pyridine degradation genes are located in the 112-kb metabolic plasmid p2MP. The elimination of the plasmid resulted in the loss of pyridine and 2-methylpyridine catabolism (Fig. 2). Earlier, plasmid-dependent catabolism of 2-methylpyridine has been described in Bacillus cereus (35). In the p2MP plasmid, we identified a gene cluster (pyr), which contains genes involved in pyridine catabolism. The cloning and expression of five genes in E. coli allowed a reconstruction of the pyridine degradation pathway from pyridine to a well-documented intermediate succinic acid (Fig. 6).
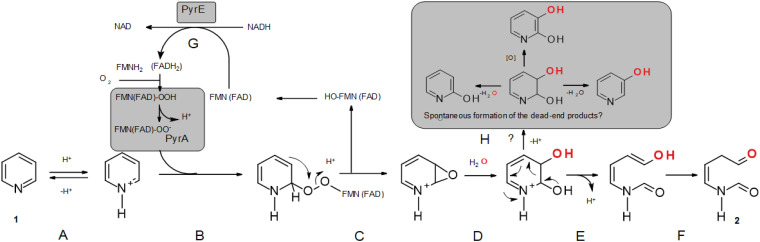
The pathway of degradation of pyridine in Arthrobacter sp. 68b starts with an attack at the C-2–C-3 bond by a two-component flavin-dependent monooxygenase PyrA. The ability of another flavin reductase (IifD) to readily substitute PyrE suggests that the flavin reductase does not form a specific complex with the monooxygenase component. Several two-component monooxygenase systems belonging to the group of LLM have already been studied. These include F420-dependent N5,N10-methylenetetrahydrome-thanopterin reductase (36), alkanesulfonate monooxygenase (37), alkane monooxygenase (38), pyrimidine monooxygenase (23), nitrilotriacetate monooxygenases (39), and EDTA monooxygenase (40). These two-component systems consist of an oxidoreductase providing FMNH2 for the oxygenation reaction catalyzed by the second component. They react with molecular oxygen to form the activated species C-4a-(hydro)peroxyflavin, which is capable of incorporating a single oxygen atom into an organic substrate and catalyzing the hydroxylation, epoxidation, Baeyer-Villiger oxidation, and heteroatom oxidation of a wide range of substrates. Recently, it has been demonstrated that it is possible to open a pyridine ring by the action of two-component flavin-dependent monooxygenase without a hydroxylation or reduction step through direct cleavage of the C-5–C-6 bond of N-methylnicotinate (19). It has been proposed that a similar reaction takes place during chemical oxidation of 1-methyl- and 1-benzyl-3-carbamoylpyridinium in the presence of hydrogen peroxide (41). In addition, the P450-mediated epoxidation/peroxidation with a subsequent ring cleavage of the C-5–C-6 bond of the 2-substituted pyridine ring in vismodegib has been also anticipated (29). Moreover, 2-methyl-3-hydroxypyridine-5-carboxylic acid oxygenase and 5-pyridoxic acid oxygenase, which cleave a C–C bond in the pyridine ring and which, initially, were assigned as dioxygenases, have been reclassified as flavin-dependent monooxygenases based on bioinformatics analysis, 18O2 experiments, and mechanistic investigations. Besides, the protonation of the N atom of the substrate has been proposed as a factor facilitating the reaction (for review, see reference 42). Based on the published data and the results of this study, we proposed a hypothetical mechanism of the first step of pyridine catabolism catalyzed by PyrA and PyrE (Fig. 7).
FIG 7.
Hypothetical scheme of the PyrA-catalyzed reaction. (A) Protonation of pyridine takes place. FMN (FAD) is first reduced to FMNH2 (FADH2) through a hydride ion transfer from NAD(P)H by PyrE (G). (B) The reduced flavin accepts a molecular oxygen at C-4a of the isoalloxazine ring and becomes 4a-hydroperoxyflavin, which is deprotonized to 4a-peroxyflavin (FMN[FAD]-OO−), which attacks a substrate to form a peroxide at the C-2 position. (C) A peroxide rearranges, and the unstable epoxy group is formed during this reaction. (D to F) The epoxy group is attacked by water molecule and, during an internal rearrangement, the C-2–C-3 bond is cleaved to form a stable compound. (H) The proposed spontaneous side reactions, which can occur if an intermediate leaves the active center.
It is well accepted that pyridine is extremely resistant to oxidative processes because the nitrogen heteroatom drains electrons from the ring, resulting in a considerable activation energy barrier. The nitrogen heteroatom with a higher electronegativity than carbon causes a depletion of π-electrons in γ- and α-positions, leads to regioselective electrophilic substitution in β-positions, and, conversely, facilitates nucleophilic attack, mainly in α-positions. It should be noted that pyridine is a base (pKa = 5.19 to 5.23) (43, 44). Hence, on protonating or quaternizing pyridine (as in the case of N-methylnicotinate) (19) to a pyridinium salt, the above chemical features of π-electron deficiency become more marked. In addition, a new reaction becomes possible, namely, ring opening, due to the higher electronegativity of the heteroatom, especially when the electron-withdrawing group is connected to the heteroatom (43, 45, 46). Moreover, pyridinium salt can be effectively oxidized by the milder oxidants such as potassium permanganate and active manganese dioxide instead of Fremy’s salt, lead(II) and mercuric acetates, chromic acid, and peracids (47).
Keeping in mind the abovementioned chemical features of pyridine, the protonated pyridine is proposed as a substrate of PyrA (Fig. 1A). The second substrate might be a 4a-peroxyflavin (FMN[FAD]-OO−) that attacks quaternized pyridine to form a peroxide (Fig. 1B), which, via an unstable epoxy group, rearranges to metabolite 2. This compound with a molecular weight of 113 Da has been detected by LC-MS analysis both in the reaction by recombinant cells harboring the PyrA and PyrE monooxygenase system and in the in vitro PyrA- and PyrE-coupled reaction. The structure of metabolite 2 is determined by NMR as its 2-nitrophenylhydrazone derivative (compound 6; Fig. S9 in the supplemental material). Interestingly, the pyridine degradation compound with a molecular weight of 113 Da has been detected previously in Paracoccus sp. NJUST30 and Arthrobacter sp. KM-4 (8, 22); however, the structures of these compounds have not been determined.
The further reaction of pyridine degradation by Arthrobacter sp. 68b is catalyzed by the aldehyde dehydrogenase PyrB. The product of this reaction, identified by the NMR (metabolite 3; Fig. S9), also supports our hypothesis that the pyridine ring is cleaved at the C-2–C-3 position during the PyrA-catalyzed reaction. The last step involves succinate semialdehyde dehydrogenase PyrD, which has been widely detected in various pyridine-degrading microorganisms (5, 6, 21, 48). Interesting to note, degradation of pyridine by several bacteria is accompanied by overproduction of riboflavin (49). The accumulation of riboflavin was also observed during the 2-methylpyridine metabolism by Arthrobacter sp. (50, 51). In the case of the Arthrobacter sp. 68b strain, a yellow unidentified pigment was accumulated during growth on pyridine and 2-methylpyridine (data not shown). The fact that riboflavin serves as the precursor for FMN and FAD in almost all organisms that utilize the redox-active isoalloxazine ring system as a coenzyme in enzymatic reactions (52) may show that flavin-dependent enzymes are involved in those metabolic pathways. The occurrence of the riboflavin synthesis genes in the pyr gene cluster (Table 1) does not contradict this statement.
The biodegradation of pyridine has been reported to occur via two different mechanisms: ring reduction or ring hydroxylation followed by ring cleavage. Resistance of the pyridine ring to oxidation, as well as the detection of the reduced metabolites, inclines it toward an existence of a reductive mechanism. Recently, several reduced cyclic compounds, including the derivatives of hydroxypyridines produced during pyridine metabolism by Paracoccus sp. NJUST30, have been hypothesized based on MS analyses (8); however, none of the structures of these compounds are confirmed by NMR, and no enzymes utilizing these substrates have been identified. On the other hand, the identification of numerous hydroxylated pyridine derivatives (5, 8) has raised the question of the enzymatic oxidation of the unsubstituted pyridine ring, and this has been under debate for many years. Besides, formation of various hydroxypyridines due to a biological N-oxidation of N-heterocycles by the promiscuous bacterial enzymes does not have to be ruled out, especially when the whole-cell experiments are carried out. For example, methane, 2-hydroxypyridine, methylpyrazine, phenol, and cyclohexanone monooxygenases (53–58) have been shown to oxidize various N-heterocycles, including pyridine, with a formation of the corresponding N-oxides, which can be further abiotically or enzymatically transformed to hydroxylated pyridines under appropriate conditions as, for example, in Nocardia sp. cells when the degradation of pyridine-N-oxide begins with an enzymatic transformation to 2-hydroxypyridine before undergoing further catabolism (59). To sum up, a characterization of the catabolic enzymes is required to ascertain the origin of the previously identified metabolites of pyridine confidently. The data obtained in this study let us reconstruct the entire catabolic pathway (Fig. 6) involving an enzymatic cleavage of the C-2–C-3 bond of pyridine as the first step. In addition, 2-methylpyridine (2-MP) was converted to succinic acid in the same way as pyridine, sharing all abovementioned enzymes (Fig. S4, S7, S8, and S9); however, 3-MP was metabolized only by PyrA (Fig. S3). These data coincide with plentiful observations that many pyridine-degrading microorganisms are capable of utilizing the methylated derivatives and vice versa (22, 51, 60).
In conclusion, we revealed that the pyr gene cluster from Arthrobacter sp. 68b encodes the complete set of genes responsible for the degradation of pyridine. PyrA monooxygenase, which is responsible for the first step of the catabolic pathway, performs an oxidative cleavage of the pyridine ring without typical activation steps, such as reduction or hydroxylation of the heterocycle. Certainly, further studies are needed to elucidate the mechanism of this reaction in detail, and definitely, we cannot exclude that other pathways of pyridine catabolism, including reductive ones, exist in nature. In summary, we can say that a 100-year-long journey of investigating the degradation of pyridine in microorganisms reaches a milestone; however, there is a long way to understanding the entire diversity and complexity of this process.
MATERIALS AND METHODS
Pyridine, 2-methylpyridine, succinate semialdehyde, NAD(P), NAD(P)H, 2-nitrophenylhydrazine, and other chemicals and solvents of the highest purity available were purchased from Sigma-Aldrich and Fluka. Restriction endonucleases and DNA ligase were from Thermo Scientific, Lithuania. All bacterial strains and plasmids used in this study are listed in Table 3.
TABLE 3.
Bacterial strains and plasmids
| Strain or plasmid | Description | Source or reference no. |
|---|---|---|
| Strain | ||
| E. coli BL21(DE3) | F– ompT gal dcm lon hsdSB(rB− mB−) λ(DE3 [lacI lacUV5-T7 gene 1 ind1 sam7 nin5]) | Invitrogen |
| E. coli Rosetta(DE3) pLysS | F– ompT hsdSB(rB− mB−) gal dcm λ(DE3 [lacI lacUV5-T7 gene 1 ind1 sam7 nin5]) pLysSRARE (Camr) | Novagen |
| Arthrobacter sp. 68b DSM 103156 | Soil isolate capable of degrading 2-methylpyridine, nicotine, phthalic acid, and pyridine | 25 |
| Plasmid | ||
| pET21b | T7 tag (N terminus), His tag (C terminus), Apr | Novagen |
| pET28b | T7 tag (N terminus), His tag (C and N termini), Kmr | Novagen |
| pETM11 | N-terminal His tag (MetLysHisHisHisHisHisHisProMetSerAspTyrAspIleProThrThrGluAsnLeuTyrPheGluGlyAla) was fused to the PyrB | 70 |
| pCDFduet | T7 tag (two), Smr | Novagen |
| pRSFduet | T7 tag (two), Kmr | Novagen |
| pACYCduet1 | T7 tag (two), Cmr | Novagen |
Bacterial growth medium and conditions.
E. coli BL21(DE3) and Rosetta(DE3) pLysS (Novagen) strains were grown aerobically on nutrient agar (NA) (Oxoid) or cultivated in nutrient broth (NB) (Oxoid) at 30°C. Arthrobacter sp. 68b strain was grown on EFA [10 g/liter K2HPO4, 4.0 g/liter KH2PO4, 1.0 g/liter (NH4)2SO4, 0.5 g/liter yeast extract, 0.4 g/liter MgSO4 · 7H2O, 10 ml/liter salt solution (2.0 g/liter CaCl2 · 2H2O, 1.0 g/liter MnSO4 · 4H2O, 0.5 g/liter FeSO4 · 7H2O dissolved in 0.1 N HCl)] (25) medium with 0.1% 2-methylpyridine, 0.1% pyridine, 0.1% nicotine, 0.1% phthalic acid, or 0.3% succinic acid at 30°C.
Ampicillin (50 μg/ml), chloramphenicol (20 μg/ml), streptomycin (20 μg/ml), and kanamycin (50 μg/ml) were used as required.
DNA manipulations.
DNA isolation, digestion, ligation, and electrophoresis were carried out according to standard procedures (61). The genome of Arthrobacter sp. 68b was sequenced using the 454 platform and Illumina technology, and the sequence of p2MP plasmid was assembled using VectorNTI v. 10.
Gene cloning.
DNA fragments were amplified using Phusion high-fidelity PCR master mix with GC buffer with added dimethyl sulfoxide (DMSO) (Thermo Scientific, Lithuania) according to the recommendations of the manufacturer. Specific primers (Metabion) were used to amplify the genes of the pyr gene cluster; the obtained fragments were digested with the appropriate restriction endonucleases and cloned to the suitable vector. All details are summarized in Table 4.
TABLE 4.
Details for gene cloning of the pyr gene cluster
| Gene | Primer and sequencea | Restriction endonucleaseb | Vector/plasmid for: |
|
|---|---|---|---|---|
| In vitro expts | In vivo expts | |||
| pyrA | M1, AACCCATATGAAGTTCGGAGCTATTAACG | NdeI | pET21b (Ap) with N-His of pET28b/pMNX28-NHis | pCDFDuet (Sm)/pMNXCDF7 |
| M2, TCGGGATCCTCAGATGAGGAACGACTTCG | BamHI | |||
| pyrE | FMNRF, GAGCAAAGCCATGGTCAGCTCAAACACC | NcoI | pACYCDuet1 (Cm)/pFrd (C-His tag) | pACYCDuet1 (Cm)/pFrd (C-His tag) |
| FMNRHisR, GGTAGACAAGCTTGGTACAGACGCCGA | HindIII | |||
| pyrB | Spadh1NF, CAGAGAGGGAACCATGGGCACTGCAC | NcoI | pET21b (Ap) with N-His tag of pETM11/pSdh1M11 | pRSFDuet (Km)/pSdh1Rsf |
| Spadh1R, CTCCGGGTTAAGCTTCAGCGCTTG | HindIII | |||
| pyrC | AmhdNheF, CTGCCAGCTAGCGTGAGGCGCATG | NheI | pET21b (Ap)/pAmh (C-His tag) | pET21b (Ap)/pAmh (C-His tag) |
| AmhdHNR, GCATCTTTGCTCCTCTCGAGCCAGTTGC | XhoI | |||
| pyrD | Sdh2NdeF, TTTCATATGACTAAATTCTCTACC | NdeI | pET21b (Ap)/pSDH2 | |
| Sdh2HindR, AATGAAGCTTAACCGGCGTGGG | HindIII | |||
The restriction endonuclease site is underlined.
Used for cloning.
Preparation of electrocompetent cells and electroporation.
E. coli BL21(DE3), Rosetta(DE3) pLysS, and Arthrobacter sp. 68b competent cells were prepared by the method described by Sharma and Schimke (62). Electroporation was performed as reported previously (63).
Plasmid curing.
Electrocompetent Arthrobacter sp. 68b cells were used for electroporation without the addition of any plasmid. After recovery, dilutions of the culture were spread plated on mineral agar medium (64) supplemented with pyridine (0.1%), and after 3 to 6 days of incubation, the smallest colonies were picked. The selected clones were grown again on the same medium to confirm the lack of growth on pyridine. Total DNAs of wild type of Arthrobacter sp. 68b and mutants from the cured strains were analyzed by agarose gel electrophoresis.
Expression and purification of recombinant proteins.
E. coli BL21(DE3) bacteria were transformed with appropriate plasmid to produce PyrA, PyrB, PyrC, or PyrE recombinant proteins. In the case of PyrD, the E. coli Rosetta(DE3) pLysS strain was used. Cells harboring plasmids were grown overnight in NB medium with a suitable antibiotic aerobically at 30°C, and then 2 ml of night culture were transferred to 200 ml of the same medium, and cells were grown aerobically at 30°C to an optical density (OD600) of 0.8. The expression was induced by isopropyl-β-d-thiogalactopyranoside (IPTG) (final concentration of 0.5 mM for PyrA, PyrB, PyrC, and PyrE and 0.25 mM for PyrD); later, bacteria were grown aerobically for 18 h at 20°C. Cells were collected by centrifugation (20 min at 3,220 × g at 4°C), then suspended in 50 mM Tris-HCl (pH 8) buffer, and disrupted by sonication (5 min at 22 kHz in ice-water bath, 40% of amplitude). Cell debris was removed by centrifugation at 10,000 × g for 10 min at 4°C. For purification of PyrA, PyrB, PyrC, and PyrE, cell extracts were loaded onto a Ni-NTA SF cartridge 5-ml column (Qiagen) equilibrated with the same buffer, and 6-His tagged proteins were eluted with 0.5 M imidazole in 50 mM Tris-HCl buffer, pH 8. Fractions with purified proteins were confirmed by SDS-PAGE; later, they were combined and loaded on the HiTrap desalting column (GE Healthcare) in 50 mM Tris-HCl buffer, pH 8. For desalting of PyrB protein, the same buffer supplemented with 10% glycerol and 1 mM CaCl2 was used.
For purification of PyrD protein, the collected cells were suspended in SP-10 buffer (10 mM potassium phosphate, pH 7.5, 1 mM EDTA, 1 mM dithiothreitol [DTT], and 1% sucrose) and disrupted by sonication. Cell debris and unbroken cells were removed by centrifugation. The sample was applied to an ANX Sepharose Fast Flow (high sub) column (10 ml) (GE Healthcare) preequilibrated with SP-10 buffer. Elution of enzyme was carried out with a linear gradient of 0 to 1 M NaCl. Pooled and concentrated fractions were loaded onto a phenyl-Sepharose 6 (low sub) column (10 ml) (GE Healthcare) equilibrated with SP-10 buffer containing 1.5 M (NH4)2SO4. PyrD protein was eluted with (NH4)2SO4 by a decreasing gradient from 1.5 to 0 M in SP-10 buffer. Fractions showing succinate semialdehyde dehydrogenase activity were pooled.
Protein concentration was determined by the Lowry method (65) using crystalline serum albumin as a standard. The purified proteins were stored at –20°C for further use.
Bioconversion of pyridine.
In vivo. Recombinant E. coli cells were grown as for protein purification, harvested by centrifugation, washed with 50 mM potassium phosphate buffer, pH 7.2, resuspended in the 10 ml of the same buffer, and used for in vivo experiments. We added 5 mM pyridine or 1 mM 2-methylpyridine to the reaction mixture. Bioconversion reactions were carried out for 3 h at 30°C with shaking at 180 rpm. The conversion was followed by changes in the UV absorption spectrum of 200 to 400 nm or by LC-MS analysis.
In vitro experiments.
Purified recombinant proteins were used for the in vitro experiments. The standard reaction mixture for PyrA was composed of 50 mM Tris-HCl (pH 8) buffer, 1 mM pyridine, 40 μM FMN, 1 mM NADPH, 5.75 nmol PyrA, and 3.12 nmol flavin reductase (PyrE or IifD) (total volume, 500 μl). We added 20 μg of glucose-6-phosphate dehydrogenase and 10 mM glucose-6-phosphate to the reaction mixture for the regeneration of NADPH. The reaction was started by adding the PyrA enzyme, and the formation of the product was observed by LC-MS analysis after 4 and 24 h of incubation at 30°C.
The standard reaction mixture for PyrB consisted of 50 mM Tris-HCl (pH 8) buffer supplemented with 5% of glycerol, 1 mM pyridine, 40 μM FMN, 1 mM NADPH, 5.75 nmol PyrA, 3.12 nmol flavin reductase (PyrE or IifD), and 2.0 nmol PyrB (total volume 500 μl). The formation of the product was elucidated as in the case of PyrA.
The standard reaction mixture for PyrC was composed of 50 mM potassium phosphate (pH 7.5) buffer, 100 μl of PyrA-PyrB in vivo reaction product ([Z]-4-formamidobut-3-enoic acid), and 810 pmol PyrC (total volume, 500 μl). The formation of the product was observed by LC-MS analysis after 16 h incubation at 30°C.
The standard reaction mixture of PyrD was composed of 190 pmol PyrD, 1 mM NADP, and the mixture of the PyrC reaction. To detect the produced compound, the LC-MS analysis was performed after 1 h of incubation at 30°C.
Activity assay.
The standard reaction mixture for PyrB or PyrD was 50 mM potassium phosphate buffer, pH 8, 10 μM succinate semialdehyde, 150 μM NAD or NADP, and enzyme (total volume, 1 ml). The formation of NADH or NADPH was followed spectrophotometrically at 340 nm.
Flavin reductase assay was carried out in 1 ml 50 mM Tris-HCl buffer, pH 7.5, containing 0.3 mM NADH or NADPH and 30 μM FMN, FAD, or riboflavin and enzyme at room temperature. The oxidation of NAD(P)H was monitored spectrophotometrically by absorbance decrease at 340 nm.
LC-MS analysis.
The same volume of chloroform was added to the samples of in vivo and in vitro reactions. The insoluble particles and chloroform phase were separated by centrifugation (10,000 × g at 5 min) from the water-soluble fraction that was analyzed by liquid chromatography-mass spectrometry (LC-MS) using high-performance liquid chromatography (HPLC) system (CBM-20A controller, two LC-2020AD pumps, SIL-30AC autosampler, and CTO-20AC column oven; Shimadzu, Japan) equipped with a photodiode array (PDA) detector (SPD-M20A prominence diode array detector; Shimadzu, Japan) and mass spectrometer (LCMS-2020, Shimadzu, Japan) equipped with an electrospray ionization (ESI) source. The chromatographic separation was conducted using a Hydrosphere C18 column, 4 by 150 mm (YMC, Japan), at 40°C and a mobile phase that consisted of 0.1% formic acid (solvent A) and acetonitrile (solvent B) delivered in gradient elution mode at a flow rate of 0.6 ml min−1. The elution program was used as follows: isocratic 5% B for 1 min, 5% to 95% B over 5 min, isocratic 95% B for 2 min, 95% to 5% B over 1 min, and isocratic 5% B for 4 min. Mass scans were measured from m/z 10 up to 500, at 350°C interface temperature, 250°C desolvation line (DL) temperature, ±4,500 V interface voltage, and neutral DL/Qarray, using N2 as nebulizing and drying gas. Mass spectrometry data were acquired in both positive and negative ionization modes. The data were analyzed using LabSolutions LCMS software.
Derivatization with 2-nitrophenylhydrazine.
After the PyrA in vivo reaction, bacteria were removed by centrifugation at 4,000 × g for 20 min, then 10:1 (vol/vol) 2-nitrophenylhydrazine (2-NPH) reagent was added, and the mixture was incubated at 4°C overnight. The 2-NPH reagent consisted of 62 mg 2-NPH (97%; Sigma-Aldrich) in 5 ml ethanol, 2 ml 1 M HCl, and 3 ml Milli-Q water (0.04 M 2-NPH in 50% aqueous ethanol) (66).
Purification of bioconversion products.
The accumulation of bioconversion products was monitored by thin-layer chromatography (TLC) using an aluminum plate coated with silica gel 60 F254 (Merck), and chloroform:methanol (9:1, vol/vol) was used as a mobile phase. The reaction mixtures were evaporated under reduced pressure. For the purification of formed (Z)-4-formamidobut-3-enoic acid and (Z)-4-acetamidobut-3-enoic acid, they were dissolved in water, and reverse-phase chromatography was carried out on C18 Grace flash cartridges in 80% methanol. For the purification of (Z)-N-4-(2-nitrophenyl)hydrazone(but-1-enyl)formamide, the reaction mixture was dissolved in chloroform and loaded onto silica gel 60 columns (particle size, 0.04 to 0.063 mm; Fluka) equilibrated with chloroform. The unbound 2-NPH was eluted with chloroform, then (Z)-N-4-(2-nitrophenyl)hydrazone(but-1-enyl)formamide was removed from the column with a 50:1 chloroform:methanol mixture. The isolated compounds were used for structural analysis.
1H NMR spectra were recorded in DMSO-D6 on Bruker Ascend 400, 400 MHz, and 13C NMR were recorded on Bruker Ascend 400, 100 MHz. Chemical shifts were reported in ppm relative to solvent resonance signal as an internal standard.
MS-MS analysis.
After protein separation in SDS-PAGE gel, the band corresponding to the induced 40-kDa protein was excised and subjected to de novo sequencing based on matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) mass spectrometry (MS) and subsequent computational analysis at the Proteomics Centre of the Institute of Biochemistry, Vilnius University (Vilnius, Lithuania). The sample was purified as described by Hellman et al. (67). The analysis of peptides was performed as described previously (24).
Other techniques.
The chromatograms of sequencing were analyzed using FinchTV version 1.4.0 and VectorNTI AdvanceTM 9.0 programs. The homology search was performed using BLAST algorithm at NCBI. The alignments were accomplished with ClustalW. The phylogenetic analysis was performed using MEGA 7.0. The evolutionary history was inferred using the neighbor-joining method (68); the evolutionary distances were computed using the number of differences method (69) and are in the units of the number of amino acid differences per sequence. The NCBI reference sequence identifiers, in addition to the species names, are presented in Table S1.
Data availability.
The data that support the findings of this study are available within the paper and its supplementary information file. The 112,851-bp sequence of the p2MP plasmid is available in the NCBI reference sequence database with accession number KJ410765.
Supplementary Material
ACKNOWLEDGMENTS
We thank M. Valius and A. Kaupinis for MS-MS analyses and G. Stier for the expression plasmid pETM11.
R.M. and V.Č. designed the experiments; R.M., R.S., J.V., D.T., and V.Č. analyzed the data; and R.S., R.R., J.V., D.T., R.G., and V.Č. performed experiments. J.V., R.M., and V.Č. prepared the manuscript, which was revised and approved by all authors.
We declare no competing financial interests.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Gupta N, O’Loughlin EJ, Sims GK. 2019. Microbial degradation of pyridine and pyridine derivatives, p 1–31. In Arora P. (ed), Microbial metabolism of xenobiotic compounds. Microorganisms for sustainability, vol 10 Springer, Singapore. [Google Scholar]
- 2.Buddin W. 1914. Partial sterilisation of soil by volatile and non-volatile antiseptics. J Agric Sci 6:417–451. doi: 10.1017/S0021859600002264. [DOI] [Google Scholar]
- 3.Kolenbrander PE, Lotong N, Ensign JC. 1976. Growth and pigment production by Arthrobacter pyridinolis n. sp. Arch Microbiol 110:239–245. doi: 10.1007/BF00690233. [DOI] [PubMed] [Google Scholar]
- 4.Kolenbrander PE, Weinberger M. 1977. 2-Hydroxypyridine metabolism and pigment formation in three Arthrobacter species. J Bacteriol 132:51–59. doi: 10.1128/JB.132.1.51-59.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zefirov NS, Agapova SR, Terentiev PB, Bulakhova IM, Vasyukova NI, Modyanova LV. 1994. Degradation of pyridine by Arthrobacter crystallopoietes and Rhodococcus opacus strains. FEMS Microbiol Lett 118:71–74. doi: 10.1111/j.1574-6968.1994.tb06805.x. [DOI] [Google Scholar]
- 6.Watson GK, Cain RB. 1975. Microbial metabolism of the pyridine ring. Metabolic pathways of pyridine biodegradation by soil bacteria. Biochem J 146:157–172. doi: 10.1042/bj1460157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bai Y, Sun Q, Zhao C, Wen D, Tang X. 2008. Microbial degradation and metabolic pathway of pyridine by a Paracoccus sp. strain BW001. Biodegradation 19:915–926. doi: 10.1007/s10532-008-9193-3. [DOI] [PubMed] [Google Scholar]
- 8.Wang J, Jiang X, Liu X, Sun X, Han W, Li J, Wang L, Shen J. 2018. Microbial degradation mechanism of pyridine by Paracoccus sp. NJUST30 newly isolated from aerobic granules. Chem Eng J 344:86–94. doi: 10.1016/j.cej.2018.03.059. [DOI] [Google Scholar]
- 9.Mohan SV, Sistla S, Guru RK, Prasad KK, Kumar CS, Ramakrishna SV, Sarma PN. 2003. Microbial degradation of pyridine using Pseudomonas sp. and isolation of plasmid responsible for degradation. Waste Manag 23:167–171. doi: 10.1016/S0956-053X(02)00150-2. [DOI] [PubMed] [Google Scholar]
- 10.Do JH, Lee WG, Theodore K, Chang HN. 1999. Biological removal of pyridine in heavy oil by Rhodococcus sp. KCTC 3218. Biotechnol Bioprocess Eng 4:205–209. doi: 10.1007/BF02931930. [DOI] [Google Scholar]
- 11.Mathur AK, Majumder CB. 2008. Biofiltration of pyridine by Shewanella putrefaciens in a corn-cob packed biotrickling filter. CLEAN Soil Air Water 36:180–186. doi: 10.1002/clen.200700090. [DOI] [Google Scholar]
- 12.Ronen Z, Horvath-Gordon M, Bollag JM. 1994. Biological and chemical mineralization of pyridine. Environ Toxicol Chem 13:21–26. doi: 10.1002/etc.5620130105. [DOI] [Google Scholar]
- 13.Bai Y, Sun Q, Zhao C, Wen D, Tang X. 2009. Aerobic degradation of pyridine by a new bacterial strain, Shinella zoogloeoides BC026. J Ind Microbiol Biotechnol 36:1391–1400. doi: 10.1007/s10295-009-0625-9. [DOI] [PubMed] [Google Scholar]
- 14.Yoon JH, Lee JJ, Kang SS, Takeuchi M, Shin YK, Lee ST, Kang KH, Park YH. 2000. Gordonia nitida sp. nov., a bacterium that degrades 3-ethylpyridine and 3-methylpyridine. Int J Syst Evol Microbiol 50:1203–1210. doi: 10.1099/00207713-50-3-1203. [DOI] [PubMed] [Google Scholar]
- 15.Lee ST, Rhee SK, Lee GM. 1994. Biodegradation of pyridine by freely suspended and immobilized Pimelobacter sp. Appl Microbiol Biotechnol 41:652–657. doi: 10.1007/BF00167280. [DOI] [PubMed] [Google Scholar]
- 16.Deng X, Wei C, Ren Y, Chai X. 2011. Isolation and identification of Achromobacter sp. DN-06 and evaluation of its pyridine degradation kinetics. Water Air Soil Pollut 221:365–375. doi: 10.1007/s11270-011-0796-7. [DOI] [Google Scholar]
- 17.Petkevičius V, Vaitekūnas J, Stankevičiūtė J, Gasparavičiūtė R, Meškys R. 2018. Catabolism of 2-hydroxypyridine by Burkholderia sp. strain MAK1: a 2-hydroxypyridine 5-monooxygenase encoded by hpdABCDE catalyzes the first step of biodegradation. Appl Environ Microbiol 84:e00387-18. doi: 10.1128/AEM.00387-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vaitekūnas J, Gasparavičiūtė R, Rutkienė R, Tauraitė D, Meškys R. 2016. A 2-hydroxypyridine catabolism pathway in Rhodococcus rhodochrous strain PY11. Appl Environ Microbiol 82:1264–1273. doi: 10.1128/AEM.02975-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perchat N, Saaidi P, Darii E, Pellé C, Petit J, Besnard-Gonnet M, de Berardinis V, Dupont M, Gimbernat A, Salanoubat M, Fischer C, Perret A. 2018. Elucidation of the trigonelline degradation pathway reveals previously undescribed enzymes and metabolites. Proc Natl Acad Sci U S A 115:E4358–E4367. doi: 10.1073/pnas.1722368115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaiser J, Feng Y, Bollag J. 1996. Microbial metabolism of pyridine, quinoline, acridine, and their derivatives under aerobic and anaerobic conditions. Microbiol Rev 60:483–498. doi: 10.1128/MMBR.60.3.483-498.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sims GK, Sommers LE, Konopka A. 1986. Degradation of pyridine by Micrococcus luteus isolated from soil. Appl Environ Microbiol 51:963–968. doi: 10.1128/AEM.51.5.963-968.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khasaeva F, Vasilyuk N, Terentyev P, Troshina M, Lebedev AT. 2011. A novel soil bacterial strain degrading pyridines. Environ Chem Lett 9:439–445. doi: 10.1007/s10311-010-0299-6. [DOI] [Google Scholar]
- 23.Mukherjee T, Zhang Y, Abdelwahed S, Ealick SE, Begley TP. 2010. Catalysis of a flavoenzyme-mediated amide hydrolysis. J Am Chem Soc 132:5550–5551. doi: 10.1021/ja9107676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kutanovas S, Stankeviciute J, Urbelis G, Tauraite D, Rutkiene R, Meskys R. 2013. Identification and characterization of a tetramethylpyrazine catabolic pathway in Rhodococcus jostii TMP1. Appl Environ Microbiol 79:3649–3657. doi: 10.1128/AEM.00011-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stanislauskienė R, Rudenkov M, Karvelis L, Gasparavičiūtė R, Meškienė R, Časaitė V, Meškys R. 2011. Analysis of phthalate degradation operon from Arthrobacter sp. 68b. Biologija 57:45–54. doi: 10.6001/biologija.v57i2.1828. [DOI] [Google Scholar]
- 26.Huijbers MME, Montersino S, Westphal AH, Tischler D, van Berkel W. 2014. Flavin dependent monooxygenases. Arch Biochem Biophys 544:2–17. doi: 10.1016/j.abb.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 27.Ellis HR. 2010. The FMN-dependent two-component monooxygenase systems. Arch Biochem Biophys 497:1–12. doi: 10.1016/j.abb.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 28.Fetzner S. 1998. Bacterial degradation of pyridine, indole, quinoline, and their derivatives under different redox conditions. Appl Microbiol Biotechnol 49:237–250. doi: 10.1007/s002530051164. [DOI] [Google Scholar]
- 29.Khojasteh SC, Yue Q, Ma S, Castanedo G, Chen JZ, Lyssikatos J, Mulder T, Takahashi R, Ly J, Messick K, Jia W, Liu L, Hop C, Wong H. 2014. Investigations into the mechanisms of pyridine ring cleavage in vismodegibs. Drug Metab Dispos 42:343–351. doi: 10.1124/dmd.113.055715. [DOI] [PubMed] [Google Scholar]
- 30.Sadauskas M, Vaitekūnas J, Gasparavičiūtė R, Meškys R. 2017. Indole biodegradation in Acinetobacter sp. strain O153: genetic and biochemical characterization. Appl Environ Microbiol 83:e01453-17. doi: 10.1128/AEM.01453-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Friestad GK, Wu Y. 2009. Intermolecular nonreductive alkylation of enamides via radical−polar crossover. Org Lett 11:819–822. doi: 10.1021/ol8028077. [DOI] [PubMed] [Google Scholar]
- 32.Sims GK, O'Loughlin EJ, Crawford RL. 1989. Degradation of pyridines in the environment. Crit Rev Environ Control 19:309–340. doi: 10.1080/10643388909388372. [DOI] [Google Scholar]
- 33.Shukla O, Kaul S. 1975. Succinate semialdehyde, an intermediate in degradation of pyridine by Brevibacterium sp. Indian J Biochem Biophys 12:326–330. [Google Scholar]
- 34.Korosteleva LA, Kost AN, Vorob’eva LI, Modyanova LV, Terent’ev PB, Kulikov NS. 1981. Microbiological degradation of pyridine and 3-methylpyridine. Appl Biochem Microbiol 17:276–283. [Google Scholar]
- 35.Madhusudan Reddy D, Paul D, Jogeswar M, Reddy G. 2009. Biodegradation of alpha picoline – a plasmid borne activity. Int J Environ Stud 66:737–745. doi: 10.1080/00207230903178030. [DOI] [Google Scholar]
- 36.Vaupel M, Thauer RK. 1995. Coenzyme F420-dependent N5,N10-methylenetetrahydromethanopterin reductase (Mer) from Methanobacterium thermoautotrophicum strain Marburg. Cloning, sequencing, transcriptional analysis, and functional expression in Escherichia coli of the mer gene. Eur J Biochem 231:773–778. doi: 10.1111/j.1432-1033.1995.0773d.x. [DOI] [PubMed] [Google Scholar]
- 37.Gao B, Ellis HR. 2005. Altered mechanism of the alkanesulfonate FMN reductase with the monooxygenase enzyme. Biochem Biophys Res Commun 331:1137–1145. doi: 10.1016/j.bbrc.2005.04.033. [DOI] [PubMed] [Google Scholar]
- 38.Li L, Liu X, Yang W, Xu F, Wang W, Feng L, Bartlam M, Wang L, Rao Z. 2008. Crystal structure of long-chain alkane monooxygenase (LadA) in complex with coenzyme FMN: unveiling the long-chain alkane hydroxylase. J Mol Biol 376:453–465. doi: 10.1016/j.jmb.2007.11.069. [DOI] [PubMed] [Google Scholar]
- 39.Uetz T, Schneider R, Snozzi M, Egli T. 1992. Purification and characterization of a two-component monooxygenase that hydroxylates nitrilotriacetate from “Chelatobacter” strain ATCC 29600. J Bacteriol 174:1179–1188. doi: 10.1128/jb.174.4.1179-1188.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jun S-Y, Lewis KM, Youn B, Xun L, Kang C. 2016. Structural and biochemical characterization of EDTA monooxygenase and its physical interaction with a partner flavin reductase. Mol Microbiol 100:989–1003. doi: 10.1111/mmi.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bristol DW, Dittmer DC. 1970. Reactions of pyridinium salts with alkaline hydrogen peroxide. Formation of pyrrolidinone hydroperoxides from 1-methyl- and 1-benzyl-3-carbamoylpyridinium chloride. J Org Chem 35:2487–2495. doi: 10.1021/jo00833a005. [DOI] [Google Scholar]
- 42.Chaiyen P. 2010. Flavoenzymes catalyzing oxidative aromatic ring-cleavage reactions. Arch Biochem Biophys 493:62–70. doi: 10.1016/j.abb.2009.08.021. [DOI] [PubMed] [Google Scholar]
- 43.Balaban AT. 2009. Aromaticity of six-membered rings with one heteroatom, p 204–246. In Krygowski TM, Cyrański MK (ed), Aromaticity in heterocyclic compounds, vol. 19 Springer, Berlin, Germany. [Google Scholar]
- 44.Binetin S, Devillers J. 1994. QSAR for organic chemical sorption in soils and sediments. Chemosphere 28:1171–1188. doi: 10.1016/0045-6535(94)90335-2. [DOI] [Google Scholar]
- 45.Bertuzzi G, Bernardi L, Fochi M. 2018. Nucleophilic dearomatization of activated pyridines. Catalysts 8:632–636. doi: 10.3390/catal8120632. [DOI] [Google Scholar]
- 46.Sowmiah S, Esperança J, Rebelo LPN, Afonso C. 2018. Pyridinium salts: from synthesis to reactivity and applications. Org Chem Front 5:453–493. doi: 10.1039/C7QO00836H. [DOI] [Google Scholar]
- 47.Comins DL, O’Connor S, Al-Awar RS. 2008. Pyridines and their benzo derivatives: reactivity at the ring, p 41–99. In Katritzky AR, Ramsden CA, Scriven EFV, Taylor RJK (ed), Comprehensive heterocyclic chemistry III. A review of the literature 1995-2007, 1st ed Elsevier Ltd, Oxford, UK. [Google Scholar]
- 48.Shukla OP, Kaul SM. 1974. A constitutive pyridine degrading system in Corynebacterium sp. Indian J Biochem Biophys 11:201–207. [PubMed] [Google Scholar]
- 49.Sims GK, O'loughlin EJ. 1992. Riboflavin production during growth of Micrococcus luteus on pyridine. Appl Environ Microbiol 58:3423–3425. doi: 10.1128/AEM.58.10.3423-3425.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shukla OP. 1974. Microbial decomposition of alpha-picoline. Indian J Biochem Biophys 11:192–200. [PubMed] [Google Scholar]
- 51.O'Loughlin EJ, Sims GK, Traina SJ. 1999. Biodegradation of 2-methyl, 2-ethyl, and 2-hydroxypyridine by an Arthrobacter sp. isolated from subsurface sediment. Biodegradation 10:93–104. doi: 10.1023/A:1008309026751. [DOI] [PubMed] [Google Scholar]
- 52.Macheroux P, Kappes B, Ealick SE. 2011. Flavogenomics – a genomic and structural view of flavin‐dependent proteins. FEBS J 278:2625–2634. doi: 10.1111/j.1742-4658.2011.08202.x. [DOI] [PubMed] [Google Scholar]
- 53.Colby J, Stirling DI, Dalton H. 1977. The soluble methane mono oxygenase of Methylococcus capsulatus (Bath). Its ability to oxygenate n alkanes, n alkenes, ethers, and alicyclic, aromatic and heterocyclic compounds. Biochem J 165:395–402. doi: 10.1042/bj1650395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stankevičiūtė J, Vaitekūnas J, Petkevičius V, Gasparavičiūtė R, Tauraitė D, Meškys R. 2016. Oxyfunctionalization of pyridine derivatives using whole cells of Burkholderia sp. MAK1. Sci Rep 6:39129. doi: 10.1038/srep39129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sun JQ, Xu L, Tang YQ, Chen FM, Zhao JJ, Wu XL. 2014. Bacterial pyridine hydroxylation is ubiquitous in environment. Appl Microbiol Biotechnol 98:455–464. doi: 10.1007/s00253-013-4818-9. [DOI] [PubMed] [Google Scholar]
- 56.Kutanovas S, Rutkienė R, Urbelis G, Tauraitė D, Stankevičiūtė J, Meškys R. 2013. Bioconversion of methylpyrazines and pyridines using novel pyrazines-degrading microorganisms. Chemija 24:67–73. [Google Scholar]
- 57.Petkevičius V, Vaitekūnas J, Tauraitė D, Stankevičiūtė J, Šarlauskas J, Čėnas N, Meškys R. 2018. A biocatalytic synthesis of heteroaromatic N‐oxides by whole cells of Escherichia coli; expressing the multicomponent, soluble di‐iron monooxygenase (SDIMO) PmlABCDEF. Adv Synth Catal 361:2456–2465. doi: 10.1002/adsc.201801491. [DOI] [Google Scholar]
- 58.Zhao Y, Qian G, Ye Y, Wright S, Chen H, Shen Y, Liu F, Du L. 2016. Heterocyclic aromatic N-oxidation in the biosynthesis of phenazine antibiotics from Lysobacter antibioticus. Org Lett 18:2495–2498. doi: 10.1021/acs.orglett.6b01089. [DOI] [PubMed] [Google Scholar]
- 59.Shukla OP, Kaul SM. 1986. Microbiological transformation of pyridine N-oxide and pyridine by Nocardia sp. Can J Microbiol 32:330–341. doi: 10.1139/m86-065. [DOI] [Google Scholar]
- 60.Houghton C, Cain RB. 1972. Microbial metabolism of the pyridine ring. Formation of pyridinediols (dihydroxypyridines) as intermediates in the degradation of pyridine compounds by micro-organisms. Biochem J 130:879–893. doi: 10.1042/bj1300879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sambrook J, Fritsch E, Maniatis T. 1989. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 62.Sharma RC, Schimke RT. 1996. Preparation of electro-competent E. coli using salt-free growth medium. Biotechniques 20:42–44. doi: 10.2144/96201bm08. [DOI] [PubMed] [Google Scholar]
- 63.Stanislauskienė R, Kutanovas S, Kalinienė L, Bratchikov M, Meškys R. 2018. Tetramethylpyrazine-inducible promoter region from Rhodococcus jostii TMP1. Molecules 23:1530. doi: 10.3390/molecules23071530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Casaitė V, Povilonienė S, Meškienė R, Rutkienė R, Meškys R. 2011. Studies of dimethylglycine oxidase isoenzymes in Arthrobacter globiformis cells. Curr Microbiol 62:1267–1273. doi: 10.1007/s00284-010-9852-6. [DOI] [PubMed] [Google Scholar]
- 65.Lowry OH, Rosebrough NJ, Farr LA, Randall RJ. 1951. Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275. [PubMed] [Google Scholar]
- 66.Peters R, Hellenbrand J, Mengerink Y, Van der Wal S. 2004. On-line determination of carboxylic acids, aldehydes and ketones by high-performance liquid chromatography-diode array detection-atmospheric pressure chemical ionisation mass spectrometry after derivatization with 2-nitrophenylhydrazine. J Chromatogr A 1031:35–50. doi: 10.1016/j.chroma.2003.10.100. [DOI] [PubMed] [Google Scholar]
- 67.Hellman U, Wernstedt C, Góñez J, Heldin CH. 1995. Improvement of an “in-gel” digestion procedure for the micropreparation of internal protein fragments for amino acid sequencing. Anal Biochem 224:451–455. doi: 10.1006/abio.1995.1070. [DOI] [PubMed] [Google Scholar]
- 68.Saitou N, Nei M. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 69.Nei M, Kumar S. 2000. Molecular evolution and phylogenetics, p 333 Oxford University Press, Oxford, UK. [Google Scholar]
- 70.Dümmler A, Lawrence AM, de Marco A. 2005. Simplified screening for the detection of soluble fusion constructs expressed in E. coli using a modular set of vectors. Microb Cell Fact 4:34. doi: 10.1186/1475-2859-4-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available within the paper and its supplementary information file. The 112,851-bp sequence of the p2MP plasmid is available in the NCBI reference sequence database with accession number KJ410765.