This study describes the generation of a nisin variant with superior characteristics for use in the NICE protein expression system. The variant, termed nisin M, retains an induction capacity comparable to that of wild-type nisin A but exhibits significantly reduced antimicrobial activity and can therefore be used at concentrations that are normally toxic to the expression host.
KEYWORDS: Lactococcus lactis, antimicrobial activity, autoinduction, nisin, protein expression, signal transduction
ABSTRACT
Nisin A is a potent antimicrobial with potential as an alternative to traditional antibiotics, and a number of genetically modified variants have been created that target clinically relevant pathogens. In addition to antimicrobial activity, nisin autoregulates its own production via a signal transduction pathway, a property that has been exploited in a protein expression system termed the nisin-controlled gene expression (NICE) system. Although NICE has become one of the most popular protein expression systems, one drawback is that the inducer peptide, nisin A, also has inhibitory activity. It has already been demonstrated that the N-terminal region of nisin A contributes to antimicrobial activity and signal transduction properties; therefore, we conducted bioengineering of nisin at positions Pro9 and Gly10 within ring B to produce a bank of variants that could potentially be used as alternative induction peptides. One variant, designated nisin M, has threonines at positions 9 and 10 and retains induction capacity comparable to that of wild-type nisin A, while most of the antimicrobial activity is abolished. Further analysis confirmed that nisin M produces a mix of peptides as a result of different degrees of dehydration of the two threonines. We show that nisin M exhibits potential as a more suitable alternative to nisin A for the expression of proteins that may be difficult to express or for production of proteins in strains that are sensitive to wild-type nisin. Moreover, it may address the increasing demand by industry for optimization of peptide fermentations to increase yields or production rates.
IMPORTANCE This study describes the generation of a nisin variant with superior characteristics for use in the NICE protein expression system. The variant, termed nisin M, retains an induction capacity comparable to that of wild-type nisin A but exhibits significantly reduced antimicrobial activity and can therefore be used at concentrations that are normally toxic to the expression host.
INTRODUCTION
Producing high quantities of proteins of biotechnological and pharmaceutical value from their natural sources can have economic challenges. Although Escherichia coli has been the dominant player in the production of recombinant proteins for decades, several issues, including the presence of endotoxin or lipopolysaccharide, requires expensive and often problematic downstream purification processes (1). The lactic acid bacterium (LAB) Lactococcus lactis has gained importance as a host for heterologous protein expression due to its well-understood genetics and metabolism, its GRAS (generally regarded as safe) status, and the availability of a wide range of genetic tools. Indeed, a major advance with regard to protein expression in L. lactis was the discovery and use of gene expression systems based on a number of inducible promoters. These include promoters that respond to the environment, such as P170, which is upregulated at low pH (2), and zinc-based systems that respond to zinc availability (3).
One of the best-known and most widely employed expression systems is the nisin-inducible controlled gene expression (NICE) system (4, 5), which stems from the nisin biosynthetic operon (nisABTCIPRKFEG) found in some L. lactis strains (6). Nisin is a 34-amino-acid peptide and is the most extensively studied bacteriocin (bacteriocins are ribosomally synthesized antimicrobial peptides produced by bacteria) (7, 8). It targets a wide range of Gram-positive bacteria, including food pathogens such as Listeria monocytogenes, Staphylococcus aureus, Bacillus cereus, and clostridia (9, 10). Nisin induces its own biosynthesis via a two-component signal transduction pathway, NisRK (6), and has led to the development and application of a food-grade expression system using L. lactis as the host (11). The NICE system encompasses both regulatory elements of the nisin operon: PnisA, the nisin-inducible promoter (cloned into several expression vectors), and nisRK, encoding the two-component histidine kinase response regulator system (harbored by compatible plasmids or inserted on the chromosome of a suitable host strain). The system is switched on by the addition of nisin in the nanomolar range, which activates the receptor NisK. NisK activates NisR by phosphorylation, and the activated NisR induces expression at the nisin A promoter (4).
The NICE system has been extensively used to produce proteins in L. lactis, such as bacteriophage lysins and metalloendopeptidases, to demonstrate their potential in dairy fermentations (12, 13). Moreover, NICE can, under certain conditions and with some modifications to the system components, also be used in other species of LAB and in other Gram-positive bacteria (11, 14). Its numerous advantages include ease of use, exquisitely controlled and efficiently induced expression, and amenability to large-scale production processes. As an example, nisin-induced fermentations of the antimicrobial lysostaphin have been carried out and even identified areas of the NICE system that need improvement (5). However, for industrial applications, nisin addition remains costly (15). Another drawback of the system is that the inducing peptide is also toxic due to its potent antimicrobial activity. Therefore, a nisin peptide that retains its induction capacity while having little or no antimicrobial activity would be highly desirable.
The gene-encoded nature of the nisin peptide makes genetic engineering to develop certain characteristics of the molecule an attractive and feasible option. Although the bioengineering of nisin commenced over 3 decades ago, the majority of studies have largely focused on identifying nisin variants with enhanced antimicrobial activity or an extended antimicrobial spectrum (10, 16, 17). The importance of the N-terminal rings A and B with respect to induction has been highlighted on a number of occasions (6, 16, 18). Those studies involved either combinatorial saturation mutagenesis of rings A and B (18) or the application of alanine scanning approaches to assess the antimicrobial activity and induction properties of various nisin derivatives (16).
In this study, we carried out a more comprehensive bioengineering approach and created banks of nisin derivatives that have been randomized at positions 9 (P9X) and 10 (G10X) individually and in combination (P9X/G10X) and assessed them for antimicrobial activity in conjunction with their ability to induce the nisin promoter using GFP and β-galactosidase reporter systems.
RESULTS
Creation and screening nisin derivatives for antimicrobial activity and induction capacity.
Previous studies utilizing site‐directed and alanine scanning mutagenesis of nisin revealed that the N-terminal ring structures are an important region required to activate NisRK (16, 18). In particular, mutagenesis of ring B has been shown to modulate antimicrobial and induction activity. We selected this location as a suitable target for the generation of variants to screen for our desired activities. In order to fully exploit the potential of the nisin ring B, we undertook a complete randomization of the amino acids Pro9 and Gly10, alone (P9X and G10X) and in combination (P9X/G10X), using NNK scanning of both codons in the nisin A structural gene (nisA) as previously described (7). A bank consisting of 1,452 individual variants created in L. lactis NZ9800 pCI372nisA (pDF05) was screened for antimicrobial activity using deferred-antagonism agar diffusion assays and their ability to induce the nisin promoter, PnisA, fused to a gfp reporter gene. The impact of mutations targeting position 9 (proline) on antimicrobial activity was assessed using an overlay assay and resulted in zones ranging from activity comparable to that of the wild-type control to a lack of any observable activity. Analysis of colonies using mass spectrometry and/or DNA sequencing identified 12 different amino acid substitutions: P9H, P9E, P9S, P9T, P9N, P9A, P9M, P9I, P9V, P9L, P9W, and P9F (Fig. 1A). Substituting P9 with an alanine (P9A) had no impact on either antimicrobial activity or induction capacity. A number of variants (P9H, P9E, P9W, and P9F) displayed a loss of both properties. Several others (P9M, P9L, P9N, P9V, and P9I) displayed a significant reduction in antimicrobial activity (between 50% and 65% of wild type) as well as a reduced ability to induce the gfp reporter. Notably, P9T and P9S exhibited a slight reduction in antimicrobial activity (70% to 75%) but retained 100% and 75% induction capacity, respectively, compared to the wild-type control (Fig. 1A), which was in agreement with previous studies (16, 18). It is significant that replacement of P9 with either threonine or serine introduces hydroxylated residues which could act as substrates for the lanthionine modification machinery. Indeed, colony mass spectrometry (CMS) of the P9T and P9S producers revealed the presence of masses corresponding to the presence of both unmodified residues (threonine or serine) and modified residues (dehydrobutyrine [Dhb] or dehydroalanine [Dha]) (Table 1).
FIG 1.
Induction and antimicrobial activity analyses of nisin mutants with substitutions at position 9 (A) and position 10 (B). Induction capacity (red) and antimicrobial activity (green) are shown as percentages and ordered from highest to lowest based on biological activity of the variants.
TABLE 1.
Mass spectrometry analysis of selected derivatives
| Ring B derivative | Mass (Da) |
No. of dehydrations |
Reference(s) | ||
|---|---|---|---|---|---|
| Predicted | Actual | Observed | Lacking | ||
| P9A | 3,328 | 3,327.87 | 8 | 0 | This study, 16 |
| P9T | 3,357 | 3,356.67 | 8 | 1 | This study |
| 3,339.62 | 9 | 0 | |||
| P9S | 3,343 | 3,342.73 | 8 | 1 | This study |
| 3,324.69 | |||||
| G10A | 3,366 | 3,367.14 | 8 | 0 | This study, 18 |
| G10T | 3,398 | 3,397.76 | 8 | 1 | This study, 18 |
| G10S | 3,384 | 3,384.57 | 8 | 1 | This study, 18 |
| 3,367.23 | 9 | 0 | |||
| P9T/G10T (nisin M) | 3,402 | 3,399.86 | 8 | 2 | This study |
| 3,382.91 | 9 | 1 | |||
| 3,365.33 | 10 | 0 | |||
| Nisin A | 3,354 | 3,353.44 | 8 | 0 | 7 |
Analysis of 144 variants in which position 10 (glycine) was targeted revealed that the majority of clones either exhibited wild-type activity or displayed a complete loss of both antimicrobial activity and induction capacity. Mass spectrometry (MS) and DNA sequencing determined that almost all of the active variants had retained the original glycine at position 10 (wild type), but we also detected variants corresponding to G10T and G10S (Fig. 1B). Here, too, CMS identified a mixture of both modified and nonmodified residues in the case of G10S (i.e., G10S and G10Dha), but this was not observed when threonine was present at position 10. A variant that displayed little reduction in activity (>50%) had an alanine (G10A) substitution (Fig. 1B). A selection of variants that lacked both antimicrobial activity and induction capacity were subjected to DNA sequencing analysis, which identified the substitutions G10F, G10W, and G10L. The inability to detect as wide a range of active variants with substitutions at this position may arise from the fact that several variations in this position (including G10D, G10N, G10H, G10R, G10L, and G10P) have been linked with the loss of threonine dehydration at position 8, meaning that ring B does not undergo cyclization (18).
We then set out to vary both residues 9 and 10 simultaneously. As expected, screening of the doubly randomized P9X/G10X bank revealed far fewer bioactive variants (approximately 5.6% of the total), of which the majority were wild type (38/64). The remainder exhibited various degrees of antimicrobial activity ranging from 10% to 50% with a concomitant loss in induction capacity (data not shown). However, one clone was conspicuous in that despite its apparent lack of antimicrobial activity, it retained an induction capability comparable to that of wild-type nisin A (Fig. 2A). DNA sequencing analysis revealed a P9T/G10T variant (both residues replaced with a threonine) (Fig. 2B). Furthermore, CMS revealed the presence of masses corresponding to the doubly modified TT peptide (Dhb9/Dhb10) but also species with one residue modified to Dhb and a mass close to that of a peptide with no modified threonine residues (Fig. 2A; Table 1). Purification of the P9T/G10T derivative, which we termed nisin M, was carried out with our standard nisin purification protocol, and subsequent high-performance liquid chromatography (HPLC) evaluation revealed the presence of two major peaks (data not shown). Mass spectrometry analysis of these fractions revealed the presence of one peptide of 3,365 Da (consistent with the presence of two Dhb residues) and a second peptide of 3,383 Da (consistent with a peptide with one threonine and one Dhb). Additionally, a mass in close agreement with a nonmodified peptide with threonines in both positions was also observed (data not shown).
FIG 2.
(A) (Top) Biological activity of nisin A and nisin M, as determined by deferred-antagonism assays and assessment of induction capacity following induction of a L. lactis strain containing gfp+ under the control of the nisin promoter. (Bottom) Colony mass spectrometry of wild-type nisin A (3,353.44 Da) and nisin M comprising a combination of no modification, single dehydration, or two dehydrations at P9T/G10T (3,399.86 Da, 3,382.91 Da, and 3,365.33 Da, respectively). (B) Structure of nisin A. Amino acids are represented by their single-letter codes, and modified residues are indicated as follows: Dha, dehydroalanine; Dhb, dehydrobutyrine; Abu, 2-aminoabutyric acid; Ala-S-Ala, lanthionine; Abu-S-Ala, methyllanthionine. Residues in orange and pink show amino acid substitutions for nisin M, producing 4 possible forms of the peptide.
MIC of nisin M.
Following HPLC and freeze-drying of combined fractions to obtain pure peptides, MIC assays were carried out using equimolar concentrations of nisin A and nisin M against a range of Gram-positive targets, including genera into which the NICE system has been previously introduced (Table 2). The MIC was determined to be the lowest concentration of peptide that resulted in the absence of visible growth of the target strain after 16 h under the appropriate growth conditions. We established that the MIC of nisin M against the standard laboratory indicator L. lactis HP was 2.5 μg ml−1, reflecting a 16-fold increase in MIC compared to that of wild-type nisin A (0.156 μg ml−1). Nisin M displayed a similar decrease in potency against the L. lactis NZ9000 gfp reporter strain and its isogenic equivalent L. lactis NZ9000 (Table 2). Several lactobacilli have been used as hosts of the NICE system, including Lactobacillus plantarum, Lactobacillus helveticus, and Lactobacillus brevis (14, 19). When Lb. plantarum UCC16 and Lb. brevis SA-C12 were assessed, MICs of >2.5 μg ml−1 and 1.25 μg ml−1 were observed, demonstrating >4-fold and 16-fold decreases in antimicrobial activity for nisin M, respectively, in comparison to wild-type peptide (Table 2).
TABLE 2.
MICs of nisin A and nisin M against standard indicator strains (including those reported to have had the NICE system introduced)
| Indicator organism | Concn [μg ml−1 (μM)] of: |
Fold decrease in activity | |
|---|---|---|---|
| Nisin A | Nisin M | ||
| Lb. plantarum UCC16 | 0.625 (0.1875) | >2.5 (>0.750) | >4 |
| L. lactis NZ9000 pNZ8150gfp+ | 0.156 (0.046) | >2.5 (>0.750) | >16 |
| L. lactis NZ9000 pNZ8150 | 0.156 (0.046) | 2.5 (0.750) | 16 |
| Lb. brevis SA-C12 | 0.078 (0.0234) | 1.25 (0.375) | 16 |
| L. lactis subsp. cremoris HP | 0.156 (0.0468) | 2.5 (0.750) | 16 |
Induction capacity of nisin A and nisin M at 10 ng ml−1.
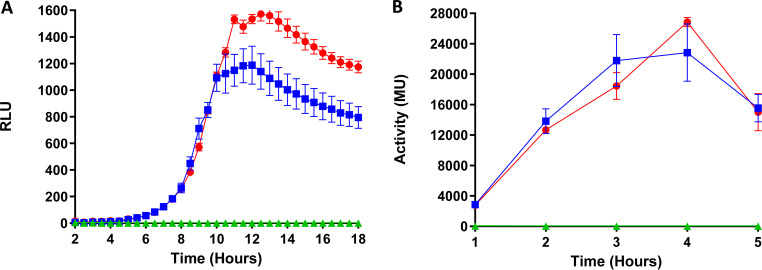
The induction capacity of nisin A and nisin M at a concentration of 10 ng ml−1 was determined using two reporter systems, by way of measurement of green fluorescent protein (GFP) and β-galactosidase production. There was no statistical difference in the dynamics of RLU detection when the GFP reporter strain was induced with nisin A and nisin M (P > 0.05) (Fig. 3A). Similarly, induction of the LacZ+ reporter strain also revealed no significant difference between nisin A and nisin M at equivalent concentrations (10 ng ml−1) (P > 0.05) (Fig. 3B). Moreover, the rate of expression, and therefore the rate of induction, was identical for both nisin A and nisin M under the conditions tested in both GFP and β-galactosidase assays.
FIG 3.
Induction capacity of nisin A (red circles) and nisin M (blue squares) determined by expression of GFP (A) and β-galactosidase (B) reporter genes under the control of the PnisA promoter in L. lactis NZ9000 pNZ8150gfp+ and L. lactis NZ9000 pPTPLLacZ+, respectively, when induced at a final concentration of 10 ng ml−1. Negative controls (green triangles) were uninduced test strains. Statistical analysis shows no significant difference between the induction capacities of nisin M and nisin A in both methods tested (P > 0.05).
Induction capacity and effect on growth of the nisin reporter strain at higher induction concentrations.
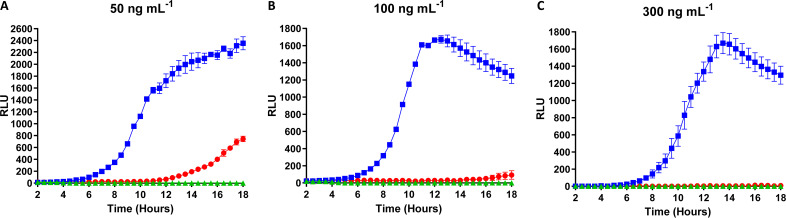
Next, we employed 50 ng ml−1, 100 ng ml−1, and 300 ng ml−1 of peptide to determine the effect of higher concentrations of nisin A and M on protein expression and on growth of the expression host. Fluorescence (in relative light units [RLU]) was measured to determine GFP expression, and absorbance readings, measured as optical density at 595 nm (OD595), were taken to observe growth of induced strains. For each of these higher concentrations, a significant difference between the levels of induction by nisin A and nisin M (P < 0.0005) was noted (Fig. 4A to C). Interestingly, the highest RLU reading attained was from cells induced with nisin M at a final concentration of 50 ng ml−1. At this concentration, induction continued for the course of the experiment (18 h), whereas at 100 ng ml−1 and 300 ng ml−1, induction reached a maximum and decreased after 10 to 12 h. Notably, there was no delay in the rate of GFP expression following induction with nisin M at 50 ng ml−1 and 100 ng ml−1 (Fig. 4A and B) compared to 10 ng ml−1 (Fig. 3A), where fluorescence intensified at approximately 6 h postinduction for all experiments; meanwhile, there was a minor delay of 30 min in expression with induction at 300 ng ml−1 (Fig. 4C).
FIG 4.
Comparison of induction capacities of nisin A (red circles) and nisin M (blue squares) determined by expression of GFP under the control of the PnisA promoter in L. lactis NZ9000 pNZ8150gfp+ induced at final concentrations of 50 ng ml−1 (A), 100 ng ml−1 (B), and 300 ng ml−1 (C). Statistical analysis demonstrates a significant difference between induction capacity of the two peptides at all concentrations tested (P < 0.0005). The negative control in this assay was uninduced L. lactis NZ9000 pNZ8150gfp+ (green triangles).
When the effects on growth were analyzed, no significant impact on the growth of the GFP reporter strain was observed following induction with nisin M at 50 ng ml−1 (P > 0.05), 100 ng ml−1 (P > 0.05), and 300 ng ml−1 (P > 0.05). The OD595 of both noninduced cells and cells induced with nisin M increased approximately 5 h postinduction. However, induction with nisin A at the same concentrations resulted in a significant lag time in growth. An increase in OD595 was not observed until 7.5, 8, and 10 h after induction at 50 ng ml−1 (P < 0.05 for comparison of nisin M to wild type [WT]; P < 0.0005 for comparison of WT to uninduced samples), 100 ng ml−1 (P < 0.0005), and 300 ng ml−1 (P < 0.0005), respectively. It is worth noting that although growth was observed in samples at these times following induction with WT nisin, no fluorescence was detected from the same samples until 12, 16, and 18 h, respectively (Fig. 4 and 5).
FIG 5.
Effects of nisin A (red circles) and nisin M (blue squares) on growth of L. lactis NZ9000 pNZ8150gfp+ induced at concentrations of 50 ng ml−1 (A), 100 ng ml−1 (B), and 300 ng ml−1 (C) compared to an uninduced control (green triangles) determined by absorbance at OD595. Results show no significant difference between growth of the uninduced control and cells induced with nisin M at all concentrations tested (P > 0.05), while there is a significant difference between the growth of cells induced with WT nisin and those of both the uninduced control (50 ng ml−1, P < 0.0005; 100 ng ml−1, P < 0.0005; 300 ng ml−1, P < 0.0005) and samples induced with nisin M (50 ng ml−1, P < 0.05; 100 ng ml−1, P < 0.0005; 300 ng ml−1, P < 0.0005).
DISCUSSION
Technological advancements to improve the production of protein biopharmaceuticals and industrial enzymes by microorganisms are highly desirable. Potential methods to optimize the efficiency of an inducible gene expression system may involve adjustment of inducer dosage and/or the timing of inducer addition. The Gram-positive NICE system is somewhat unusual in that the inducer peptide also has the capacity to kill the expression host, and thus, induction and killing capacity must be balanced. The generation of a nisin derivative that retains its induction properties but has reduced antimicrobial properties would represent a significant improvement to the NICE system that could be applied to more sensitive strains.
Previous work, where the focus has been on the nisin peptide itself, involved randomized mutagenesis of rings A and B (18) and described mutants that retained considerable autoinduction abilities but with lower antimicrobial properties (and vice versa). Similarly, Ge and coworkers applied a complete alanine scanning mutagenesis approach and reported that the N-terminal ring structures (ring A and ring B) in nisin were involved in activating NisK to act as an inducing molecule (16). In this study, we focused our attention on ring B with a more systematic mutagenesis approach to identify novel derivatives with altered activity and/or induction properties. This proved to be successful in that we identified a nisin variant that retains induction capacity comparable to that of the wild type peptide but exhibits significantly less antimicrobial activity.
Notably, another lantibiotic, subtilin, is structurally closely related to nisin and contains the same lanthionine ring structure but does not induce PnisA. Indeed, in the study by Spieß et al., the failure of subtilin to induce the histidine kinase NisK was shown to mostly depend on the presence of an N-terminal tryptophan, as its replacement with the aliphatic amino acid residues isoleucine, leucine, and valine led to activation of NisK (20). This suggests that further bioengineering at position 1 and indeed other amino acid locations in the nisin M background could potentially enhance induction and reduce antimicrobial activity even further.
Although this study highlighted ring B of nisin as a critical region in our quest to separate antimicrobial activity from induction and pheromone activity, more residue positions could and should be targeted. Studies with the natural variant nisin Z have revealed that derivatives with the mutations T2S and M17W exhibited 11-fold and 2-fold increases in induction capacity relative to the parent peptide, respectively, while S5T and S3T derivatives had significantly reduced induction capacity (6).
A computational approach evaluating the antimicrobial activity, induction capacity, production levels, and immunity/sensor kinase components of natural and bioengineered nisin derivatives could provide a blueprint for the design of more efficient peptide inducers. For example, the abilities of nisin P, A, and H to activate PnisA fused to a gfp reporter were assessed and found to differ (21). The promoter was more sensitive to nisin A (1 ng ml−1 to 1 μg ml−1) than nisin H (10 ng ml−1 to 1 μg ml−1) and nisin P (100 ng ml−1 to 10 μg ml−1). Higher concentrations of nisin P were required to activate the promoter, but it continued to induce promoter activity at higher concentrations (10 μg ml−1), whereas nisin A and H were capable of inducing the promoter only up to 1-μg ml−1 concentrations of peptides. The ability to use higher concentrations of nisin P is most likely due to its decreased antimicrobial activity compared with nisin A and nisin H. While this might advocate the use of nisin P as an alternative inducer to nisin A, the peptide does not induce at the lower and commonly used inducing concentration (10 ng ml−1). Notably, the nisin M mutant generated in this study induces at both low and high concentrations. While no significant difference in growth of the induced strain compared to the uninduced control was observed, even at the maximum concentration applied (300 ng ml−1), further evaluation with even higher concentrations of nisin M and with a variety of expression host strains are necessary. However, the practicality of using such high concentrations in terms of industrial applications would need to be considered, given that cell-free supernatant from a nisin M producer would be the most likely option for induction (rather than expensive purified nisin peptides), though a fermentate analogous to nisaplin (2.5% nisin A) would enable a range of concentrations to be applied irrespective of the sensitivity of host strains (e.g., induction levels above 10 ng ml−1 nisin A result in inhibitory effects on the expression strain L. lactis NZ9000) (22).
Additionally, the natural variant nisin Q also displays antimicrobial capabilities similar to that of nisin A but differs in its ability to induce the nisA promoter (23). Directed mutagenesis and analysis of the four amino acids which differ between nisin A and nisin Q (A15V, M21L, H27N, and I30V) may help us to more completely understand the pheromone activity of nisin. Remarkably, in the study by Ge and coworkers, the derivatives with the substitutions L16D, L16A, L16H, L16V, M21A, M21D, and M21N all exhibited enhanced induction properties when assessed by β-galactosidase assays, with the L16D variant being particularly notable, given that it also displays a significant reduction in antimicrobial activity (16). Other regions of nisin subjected to bioengineering approaches and shown to affect induction activity include the C terminus and in particular serine and isoleucine at positions 29 and 30, respectively (24). Although the specifics of the interaction between the nisin peptide and NisK have yet to be fully elucidated, a recent study has provided some insight through mutational analysis of NisK. Mutagenesis of conserved residues in the extracellular region of NisK revealed that several hydrophobic residues, including two aromatic residues (Tyr113 and Phe133) are crucial for NisK in sensing nisin and regulating nisin biosynthesis (25).
Elimination of the antimicrobial activity of nisin is a priority in the effort to improve the nisin peptide in terms of its suitability as a peptide inducer, such as in the NICE system. For example, Reunanen and Saris (26) developed a method for the quantification of nisin in food samples, through the construction of a non-nisin-producing L. lactis strain (LAC240), with a plasmid containing a gfp gene under the control of the nisF promoter and the constituent genes of the nisin two-component regulatory system, nisRK. It was reported that upon the addition of nisin peptide concentrations greater than 20 ng ml−1, the LAC240 cells became stressed, resulting in a reduction in the quantity of GFP produced, and the signal reached the background level when the concentration of nisin was approximately 60 ng ml−1 (26). Moreover, in a study that aimed to improve the response of L. lactis to freezing damage through expression of an antifreeze peptide (SF-P), the recombinant strain L. lactis NZ3900 SF-P was incubated with different concentrations of nisin (25, 50, or 100 ng ml−1) and at various pHs and growth temperatures (27). Notably, maximal expression was observed at 25 ng ml−1, with a much lower level of expression at 50 ng ml−1 and virtually no expression at 100 ng ml−1, most likely due to the inhibitory effects of nisin A, though pH and temperature values were also a factor (27). In another study that sought to optimize the NICE system for the expression of lysostaphin for both laboratory (1 liter) and industrial-scale (3,000 liters) applications and at high cell densities, the authors noted that the addition of too much nisin was detrimental to product formation. Notably, when the culture was induced at higher cell densities, 160 mg liter−1 lysostaphin was formed with 20 ng ml−1 nisin and 220 mg liter−1 lysostaphin was produced when 40 ng ml−1 nisin was used for induction, indicative of a clear correlation between the cell density at induction and the amount of nisin that is needed for maximal induction (5). While this group reported that maximum protein yield in the NICE system is achieved by induction carried out at a cell density (OD600) of 5 with a final concentration of 40 ng ml−1 of nisin, we suggest that nisin M provides greater flexibility with respect to inducer concentration by virtue of the attenuated antimicrobial activity of the peptides, so that the application of high concentrations of inducer peptide is not now a limiting factor.
To date, a multitude of peptides, enzymes, and vaccines of clinical and biotechnological interest have been overexpressed using nisin, including the antibacterial protein lysostaphin (5), a hemagglutinin of the H5N1 influenza virus (28), and rotavirus VP6 protein (29), to name but a few. Though several improvements have been made to the NICE system, further improvements are possible. For example, streamlined-genome mutants of L. lactis NZ9000 were generated by deletion of four large nonessential DNA regions accounting for 2.83% of the genome and evaluated as microbial cell factories for recombinant protein production. Indeed, following nisin induction, not only was the transcriptional efficiency improved, but also the production levels of the expressed reporters were approximately 3- to 4-fold enhanced compared with the wild strain (30). Additionally, expression from the ΔlacF host strain L. lactis NZ3900 (a strain unable to utilize lactose) enabled food-grade, lactose-based plasmid selection and induction (31), while deletion of a specific proteinase gene (NZ9000 ΔhtrA) led to increased stability of heterologously secreted proteins (32).
While the aforementioned studies focused on improving the host strain for expression of proteins, this study focused on potential improvements that can be made to the inducing peptide via mutagenesis of ring B, which has already been reported to play an important role in induction capacity (16, 18). This study has demonstrated that a nisin A variant with modifications to ring B retained induction capacity comparable to that of the wild-type nisin A peptide yet exhibited reduced inhibitory effects on the growth of the strain L. lactis NZ9000 when applied at concentrations as high as 300 ng ml−1 (0.09 μM). It was also determined this combination has between >4- and >16-fold less activity against various genera and species of bacteria into which the NICE system has been introduced, therefore supporting the claim that nisin M exhibits potential as a suitable alternative to nisin A for use in the NICE system.
This study confirms that random mutagenesis experiments continue to be beneficial with a view to enhancing the functional properties of the nisin peptide for specific applications and provide novel nisin variants that exhibit potential for future applications in the pharmaceutical, biotechnological, and industrial fields.
MATERIALS AND METHODS
Bacterial strains and plasmids.
Bacterial strains and plasmids used in this study are listed in Table 3.
TABLE 3.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Characteristic(s) | Reference(s) and/or source |
|---|---|---|
| L. lactis NZ9000 | MG1363 derivative, nisRK integrated into the pepN gene | 22 |
| Integration results in a pepN-negative phenotype | ||
| Most commonly used host of the NICE system | ||
| L. lactis NZ9000 pNZ8150 | NZ9000 strain harboring pNZ8150; pNZ8150: ScaI site for translational fusions, standard vector for NICE system, Cmr | 4 |
| L. lactis NZ9000 pNZ8150gfp+ | NZ9000 strain harboring pNZ8150 gfp+ under the control of the PnisA promoter; Cmr | 24 |
| L. lactis NZ9000 pPTPLLacZ+ | NZ9000 strain harboring low-copy-number plasmid pPTPL with a β-galactosidase-expressing gene under the control of the PnisA promoter; Tetr | 7 |
| L. lactis NZ9800 | Derivative of NZ9700 with 4-bp deletion rendering an inactive nisin operon, except nisRK genes; host of the NICE system | 22, 36 |
| L. lactis NZ9800 pDF05 | NZ9800 harboring pDF05 (pCI372 with nisA under the control of its own promoter); wild-type nisin A producer, Cmr | 7, 22, 36 |
| L. lactis NZ9800 pDF05nisM | pDF05 where codons 9 and 10 of nisA have been randomized; nisin M producer, Cmr | UCC Culture Collection (APC3920) (this work) |
| Lb. plantarum UCC16 | Nisin-sensitive indicator species in which the NICE system has been utilized | UCC Culture Collection (4, 19) |
| Lb. brevis SA-C12 | Nisin-sensitive indicator species in which the NICE system has been utilized | UCC Culture Collection (4, 37) |
| L. lactis subsp. cremoris HP | Nisin-sensitive indicator strain | UCC Culture Collection |
Creation and analysis of a bank of nisin A ring B derivatives.
Mutagenesis of the nisA gene was carried out as described previously (7). Briefly, saturation mutagenesis was carried out using pDF05 (pCI372-nisA) as the template and using oligonucleotides as listed in Table 4 containing an NNK codon in place of each native codon. PCR amplification was performed in a total volume of 50 μl with 0.5 ng of target DNA (pCI372-nisA), 1 U Phusion high-fidelity DNA polymerase (Finnzymes, Finland), 1 mM deoxynucleoside triphosphates (dNTPs), and 500 ng each of the appropriate forward and reverse oligonucleotides. The reaction mixture was preheated at 98°C for 2 min, incubated for 29 cycles of 98°C for 30 s, 55°C for 15 s, and 72°C for 3 min 30 s, and finally incubated at 72°C for 3 min 30 s. Amplified products were treated with DpnI (Stratagene) for 60 min at 37°C to digest template DNA and purified using the QIAquick PCR purification kit. Following transformation of E. coli Top10 cells, plasmid DNA was isolated and sequenced using primers pCI372FOR and pCI372REV (Table 4) to verify that mutagenesis had taken place. The purified products were subsequently introduced by electroporation into the strain L. lactis NZ9800, which has all the genes necessary for nisin production. Approximately 150 transformants were chosen at random for each single position (P9X and G10X) and 1,152 transformants for the randomized P9X/G10X bank. Isolated colonies were inoculated into 96-well plates containing GM17 with chloramphenicol at 10 μg ml−1, incubated overnight, and stored at −20°C after addition of 80% glycerol. Deferred-antagonism assays were performed by replicating strains on GM17 agar plates and allowing them to grow overnight before overlaying them with GM17 agar (0.75% [wt/vol] agar) seeded with the L. lactis HP indicator strain. Induction assays were carried out by replicating strains from each 96-well plate in a fresh 96-well plate containing GM17 broth preinoculated with L. lactis NZ9000 pNZ8150gfp+, in which GFP acts as a reporter of expression from a nisin-inducible promoter (24). Induction of GFP was monitored over 20 h in terms of relative fluorescence units (RFU) using a TECAN Genios fluorescence, absorbance, and luminescence reader using excitation and emission spectra of 485 nm and 535 nm, respectively.
TABLE 4.
Oligonucleotides used in this study
| Primer name | Sequence |
|---|---|
| NisP9degFOR | 5′ CTA TGT ACA NNK GGT TGT AAA ACA GGA GCT CTG ATG GGT 3′ |
| NisP9degREV | 5′ TTT ACA ACC MNN TGT ACA TAG CGA AAT ACT TGT AAT GCG 3′ |
| NisG10degFOR | 5′ TGT ACA CCC NNK TGT AAA ACA GGA GCT CTG ATG GGT TGT 3′ |
| NisG10degREV | 5′ TGT TTT ACA MNN GGG TGT ACA TAG CGA AAT ACT TGT AAT 3′ |
| NisP9G10degFOR | 5′ CTA TGT ACA NNK NNK TGT AAA ACA GGA GCT CTG ATG GGT 3′ |
| NisP9G10degREV | 5′ TTT ACA MNN MNN TGT ACA TAG CGA AAT ACT TGT AAT GCG 3′ |
| pCI372For | 5′-CGGGAAGCTAGAGTAAGTAG-3′ |
| pCI372Rev | 5′-ACCTCTCGGTTATGAGTTAG-3′ |
MALDI-TOF mass spectrometry.
For colony mass spectrometry (CMS), bacterial colonies of P9X and G10X mutants were collected with sterile plastic loops and mixed with 50 μl of 70% isopropyl alcohol (IPA) containing 0.1% trifluoroacetic acid (TFA). The suspension was vortexed, the cells were centrifuged in a benchtop centrifuge at 8,260 × g for 2 min, and the supernatant was removed for analysis. For matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometry of nisin M cell-free supernatant (CFS) was purified as follows; a 1% inoculum of nisin mutant-producing strains was grown overnight in 50 ml clarified tryptone-yeast extract (TY) broth and incubated overnight at 30°C. Following incubation, cells were centrifuged at 5,000 rpm for 20 min at 4°C. CFS was removed and passed through a 1-g (6-ml) Strata C18 E column (Phenomenex) pre-equilibrated with 6 ml methanol (Fisher Scientific, UK) and 6 ml HPLC-grade H2O. The column was washed with 12 ml of 30% ethanol, and nisin was eluted using 5 ml of 70% isopropanol–0.1% TFA. Mass spectrometry in all cases was performed with an Axima TOF2 MALDI-TOF mass spectrometer (Shimadzu Biotech, Manchester, UK). A 0.5-μl aliquot of matrix solution (α-cyano-4-hydroxycinnamic acid [CHCA], 10 mg ml−1 in 50% acetonitrile–0.1% [vol/vol] TFA) was placed on the target and left for 1 to 2 min before being removed. The residual solution was then air dried, and the sample solution (resuspended lyophilized powder or CMS supernatant) was positioned on the precoated sample spot. Matrix solution (0.5 μl) was added to the sample and allowed to air dry. The sample was subsequently analyzed in a positive-ion linear mode.
Purification of nisin A and nisin M.
Purifications of nisin A and variant nisin M were carried out as per a previously employed method (33), with modifications. Briefly, overnight cultures of L. lactis NZ9800 pDF05nisM (APC3920) and L. lactis NZ9700 were inoculated at 0.5% into separate portions of purified tryptone-yeast extract (TY) broth (900 ml each) supplemented with 20% glucose and 20% β-glycerophosphate and incubated at 30°C overnight. Following incubation, the cultures were centrifuged at 6,500 × g at 4°C for 15 min. The supernatant was passed through a column containing ∼70 g Amberlite XAD-16 beads and subsequently washed with 500 ml of 30% ethanol. The nisin was eluted from the column using 70% isopropanol containing 0.1% TFA. Simultaneously, bacterial cell pellets were resuspended in 300 ml 70% isopropanol–0.1% TFA and stirred at room temperature for 3 h. This cell suspension was then centrifuged at 5,000 × g at 4°C for 10 min, and the supernatant was retained. The column eluant was pooled with the postcentrifugation supernatant and isopropanol evaporated using a rotary evaporator (Rotavapor R-205; Büchi, Switzerland). The sample was adjusted to pH 4.0 and was subsequently passed through a 10-g (60-ml) Strata C18 E column (Phenomenex) pre-equilibrated with 60 ml methanol (Fisher Scientific, UK) and 60 ml HPLC-grade H2O. After 120 ml of 30% ethanol was applied, nisin was eluted from the column using 60 ml of 70% isopropanol–0.1% TFA. For HPLC purification, 12-ml volumes were concentrated to a volume of 2 ml by rotary evaporation and applied to a Phenomenex C12 reverse-phase HPLC column (Jupiter 4-μm Proteo, 90 Å, 250 mm by 10.0 mm, 4 μm) previously equilibrated with 25% acetonitrile–0.1% TFA. Nisin was eluted via a gradient of 25 to 50% acetonitrile–0.1% TFA that was developed from 10 to 40 min at a flow rate of 3.2 ml min−1. Nisin-containing fractions were pooled, and acetonitrile was removed by rotary evaporation. The purified peptides were lyophilized and stored at –20°C.
MIC assays.
MICs were also determined for strains into which the NICE system was reported to have been introduced, including Lactobacillus plantarum and Lactobacillus brevis, in order to determine the potential of nisin M as an alternative to nisin A in the NICE system.
MIC determinations for strains were carried out in triplicate in 96-well microtiter plates (Sarstedt) as described previously (34). Plates were pretreated with bovine serum albumin (BSA) prior to addition of the peptides. Briefly, to each well of the microtiter plate, 200 μl of phosphate-buffered saline (PBS) containing 1% (wt/vol) BSA was added, and plates were incubated at 37°C for 30 min. The wells were washed with 200 μl PBS and allowed to dry. The target strains L. lactis spp. cremoris HP and L. lactis NZ9000 pNZ8150gfp+ were grown overnight in M17 broth (Sigma) supplemented with glucose (0.5%) at 30°C. Lb. plantarum and Lb. brevis were grown overnight in MRS broth (Oxoid) at 30°C. Strains were subcultured into fresh broth, allowed to grow to an OD600 of ∼0.5, and diluted to a final concentration of 105 CFU ml−1 in a volume of 0.2 ml. Nisin A and nisin M peptides were adjusted to a 750 nM starting concentration, and 2-fold serial dilutions of each peptide were added to the target strain. After incubation for 16 h at 30°C, the MIC was read as the lowest peptide concentration causing inhibition of visible growth.
Comparison of nisin A and nisin M induction capacity using β-galactosidase activity.
The β-galactosidase activity assay was performed as previously described (35), with modifications. Cultures of L. lactis NZ9000 pPTPLLacZ+ were inoculated in M17 broth (Sigma), supplemented with glucose at 0.5% (GM17) and tetracycline (10 μg ml−1), and incubated at 30°C overnight. Following incubation, a 1% inoculum of each replicate was subcultured into fresh GM17 medium and incubated at 30°C until an OD600 of 0.2 to 0.3 was reached. Cells were then treated separately with nisin A and nisin M purified peptides to a final concentration of 10 ng ml−1. Every hour, 1-ml samples of each test were transferred to an Eppendorf tube and centrifuged at 13,000 rpm for 2 min (Sorvall Legend Micro 17 centrifuge; Thermo Scientific) to harvest cells. Cells were resuspended in 1 ml LacZ buffer, and 0.5 ml of this was treated with 12.5 μl of 0.1% sodium dodecyl sulfate (SDS) and 25 μl of chloroform and incubated at 30°C for 5 min to dissolve cell membranes. Following incubation, 100 μl of 2-nitrophenyl-β-d-galactopyranoside (ONPG) (4 mg ml−1) (Sigma-Aldrich) was added to each sample and incubated at 37°C until a yellow color developed. To stop the reaction, samples were treated with 250 μl of a 1 M sodium carbonate solution and centrifuged at 8,000 rpm for 5 min (Thermo Scientific). Absorbance readings of supernatant were taken at 420 and 550 nm (SpectraMax M3 spectrophotometer; Molecular Devices, Sunnyvale, CA, USA). Measurement of β-galactosidase activity of samples was calculated as {1,000 × [OD420 – (1.75 × OD550)]}/(t × v × OD600), where t is reaction time (time taken for yellow color to develop) and v is volume of culture tested, as previously described (16).
Assessment of purified nisin A and nisin M induction capacity using a green fluorescent protein reporter system.
Induction assays were performed as previously described (24) with modifications. Briefly, cultures of L. lactis NZ9000 pNZ8150gfp+ were inoculated in M17 broth (Sigma), supplemented with glucose at 0.5% (GM17) with chloramphenicol (10 μg ml−1) and incubated at 30°C overnight. Following incubation, a 1% inoculum of each replicate was subcultured into fresh GM17 medium and incubated at 30°C until an OD600 of ∼0.5 was reached. Cells were then diluted to a final concentration of 105 CFU ml−1 and treated with nisin A and nisin M at final concentrations of 10 ng ml−1, 50 ng ml−1, 100 ng ml−1, and 300 ng ml−1. Subsequently, 2 ml was transferred to black 24-well microtiter plates (PerkinElmer) for induction and 200 μl into a 96-well plate (Sarstedt) for absorbance readings. Fluorescence was detected using a SpectraMax M3 spectrophotometer (Molecular Devices, Sunnyvale, CA, USA) where excitation and emission parameters were set to 485 nm and 528 nm, respectively, for fluorescence, while absorbance readings were taken at OD595 using a Multiskan FC microplate photometer, v1.01.14 (Thermo Scientific, Waltham, MA, USA). Baseline absorbance of uncultured GM17 was subtracted from the fluorescence and absorbance readings of all test samples using SoftMax Pro v6.3 and SkanIt RE v4.1 software, respectively. Fluorescence was reported as relative light units (RLU) and absorbance as OD595. Tests were carried out in triplicate.
Statistical analysis.
Statistical analysis was carried out with SPSS Statistics v2. A test of normality was performed to determine whether data for each test were normally distributed. For normally distributed data, a repeated-measures analysis of variance (ANOVA) was performed. For data not normally distributed, Levene’s test of homogeneity was performed, where if equal variances were assumed, the repeated-measures ANOVA was carried out, and if equal variances were not assumed, the nonparametric Friedman test was performed to determine if differences between the two nisin variants’ induction capacities and between the growth of the strains when induced with the peptides at higher concentrations and those of an uninduced control were significant. For ANOVA and Friedman’s results with a significant difference between groups (P < 0.05), a post hoc test was performed. The post hoc test for normally distributed and equal-variances-assumed samples was the Bonferroni test, and for nonnormally distributed and equal-variances-not-assumed samples, Dunnett’s T3 test was performed. The significance threshold for all ANOVA and nonparametric tests performed was set at 0.05.
ACKNOWLEDGMENTS
This work was supported by the Irish Government under the National Development Plan, through Science Foundation Ireland Investigator awards 10/IN.1/B3027, SFI/12/RC/2273, and SFI/12/RC/2273 P2. D.F. acknowledges receipt of a Society for Applied Microbiology Students into Work Grant for A.G.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
REFERENCES
- 1.Cano-Garrido O, Rueda FL, Sànchez-García L, Ruiz-Ávila L, Bosser R, Villaverde A, García-Fruitós E. 2014. Expanding the recombinant protein quality in Lactococcus lactis. Microb Cell Fact 13:167. doi: 10.1186/s12934-014-0167-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Madsen SM, Arnau J, Vrang A, Givskov M, Israelsen H. 1999. Molecular characterization of the pH‐inducible and growth phase‐dependent promoter P170 of Lactococcus lactis. Mol Microbiol 32:75–87. doi: 10.1046/j.1365-2958.1999.01326.x. [DOI] [PubMed] [Google Scholar]
- 3.Llull D, Poquet I. 2004. New expression system tightly controlled by zinc availability in Lactococcus lactis. Appl Environ Microbiol 70:5398–5406. doi: 10.1128/AEM.70.9.5398-5406.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mierau I, Kleerebezem M. 2005. 10 years of the nisin-controlled gene expression system (NICE) in Lactococcus lactis. Appl Microbiol Biotechnol 68:705–717. doi: 10.1007/s00253-005-0107-6. [DOI] [PubMed] [Google Scholar]
- 5.Mierau I, Olieman K, Mond J, Smid EJ. 2005. Optimization of the Lactococcus lactis nisin-controlled gene expression system NICE for industrial applications. Microb Cell Fact 4:16. doi: 10.1186/1475-2859-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuipers OP, Beerthuyzen MM, de Ruyter PG, Luesink EJ, de Vos WM. 1995. Autoregulation of nisin biosynthesis in Lactococcus lactis by signal transduction. J Biol Chem 270:27299–27304. doi: 10.1074/jbc.270.45.27299. [DOI] [PubMed] [Google Scholar]
- 7.Field D, Connor PM, Cotter PD, Hill C, Ross RP. 2008. The generation of nisin variants with enhanced activity against specific Gram-positive pathogens. Mol Microbiol 69:218–230. doi: 10.1111/j.1365-2958.2008.06279.x. [DOI] [PubMed] [Google Scholar]
- 8.Wirawan RE, Klesse NA, Jack RW, Tagg JR. 2006. Molecular and genetic characterization of a novel nisin variant produced by Streptococcus uberis. Appl Environ Microbiol 72:1148–1156. doi: 10.1128/AEM.72.2.1148-1156.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naghmouchi K, Drider D, Baah J, Teather R. 2010. Nisin A and polymyxin B as synergistic inhibitors of Gram-positive and Gram-negative bacteria. Probiotics Antimicrob Proteins 2:98–103. doi: 10.1007/s12602-009-9033-8. [DOI] [PubMed] [Google Scholar]
- 10.Field D, Cotter PD, Ross RP, Hill C. 2015. Bioengineering of the model lantibiotic nisin. Bioengineered 6:187–192. doi: 10.1080/21655979.2015.1049781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Ruyter P, Kuipers OP, de Vos WM. 1996. Controlled gene expression systems for Lactococcus lactis with the food-grade inducer nisin. Appl Environ Microbiol 62:3662–3667. doi: 10.1128/AEM.62.10.3662-3667.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Ruyter PG, Kuipers OP, Meijer WC, de Vos WM. 1997. Food-grade controlled lysis of Lactococcus lactis for accelerated cheese ripening. Nat Biotechnol 15:976–979. doi: 10.1038/nbt1097-976. [DOI] [PubMed] [Google Scholar]
- 13.Hickey RM, Ross RP, Hill C. 2004. Controlled autolysis and enzyme release in a recombinant lactococcal strain expressing the metalloendopeptidase enterolysin A. Appl Environ Microbiol 70:1744–1748. doi: 10.1128/aem.70.3.1744-1748.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kleerebezem M, Beerthuyzen MM, Vaughan EE, de Vos WM, Kuipers OP. 1997. Controlled gene expression systems for lactic acid bacteria: transferable nisin-inducible expression cassettes for Lactococcus, Leuconostoc, and Lactobacillus spp. Appl Environ Microbiol 63:4581–4584. doi: 10.1128/AEM.63.11.4581-4584.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Özel B, Şimşek Ö, Akçelik M, Saris PE. 2018. Innovative approaches to nisin production. Appl Microbiol Biotechnol 102:6299–6307. doi: 10.1007/s00253-018-9098-y. [DOI] [PubMed] [Google Scholar]
- 16.Ge X, Teng K, Wang J, Zhao F, Wang F, Zhang J, Zhong J. 2016. Ligand determinants of nisin for its induction activity. J Dairy Sci 99:5022–5031. doi: 10.3168/jds.2015-10809. [DOI] [PubMed] [Google Scholar]
- 17.Zhou L, van Heel AJ, Montalban-Lopez M, Kuipers OP. 2016. Potentiating the activity of nisin against Escherichia coli. Front Cell Dev Biol 4:7. doi: 10.3389/fcell.2016.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rink R, Wierenga J, Kuipers A, Kluskens LD, Driessen AJ, Kuipers OP, Moll GN. 2007. Dissection and modulation of the four distinct activities of nisin by mutagenesis of rings A and B and by C-terminal truncation. Appl Environ Microbiol 73:5809–5816. doi: 10.1128/AEM.01104-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pavan S, Hols P, Delcour J, Geoffroy M-C, Grangette C, Kleerebezem M, Mercenier A. 2000. Adaptation of the nisin-controlled expression system in Lactobacillus plantarum: a tool to study in vivo biological effects. Appl Environ Microbiol 66:4427–4432. doi: 10.1128/aem.66.10.4427-4432.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spieß T, Korn SM, Kötter P, Entian K-D. 2015. Activation of histidine kinase SpaK is mediated by the N-terminal portion of subtilin-like lantibiotics and is independent of lipid II. Appl Environ Microbiol 81:5335–5343. doi: 10.1128/AEM.01368-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia-Gutierrez E, O’Connor PM, Saalbach G, Walsh CJ, Hegarty JW, Guinane CM, Mayer MJ, Narbad A, Cotter PD. 2020. First evidence of production of the lantibiotic nisin P. Sci Rep 10:3738. doi: 10.1038/s41598-020-60623-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuipers OP, de Ruyter PG, Kleerebezem M, de Vos WM. 1998. Quorum sensing-controlled gene expression in lactic acid bacteria. J Biotechnol 64:15–21. doi: 10.1016/S0168-1656(98)00100-X. [DOI] [Google Scholar]
- 23.Yoneyama F, Fukao M, Zendo T, Nakayama J, Sonomoto K. 2008. Biosynthetic characterization and biochemical features of the third natural nisin variant, nisin Q, produced by Lactococcus lactis 61-14. J Appl Microbiol 105:1982–1990. doi: 10.1111/j.1365-2672.2008.03958.x. [DOI] [PubMed] [Google Scholar]
- 24.Field D, Blake T, Mathur H, O' Connor PM, Cotter PD, Ross RP, Hill C. 2019. Bioengineering nisin to overcome the nisin resistance protein. Mol Microbiol 111:717–731. doi: 10.1111/mmi.14183. [DOI] [PubMed] [Google Scholar]
- 25.Ge X, Teng K, Wang J, Zhao F, Zhang J, Zhong J. 2017. Identification of key residues in the NisK sensor region for nisin biosynthesis regulation. Front Microbiol 8:106. doi: 10.3389/fmicb.2017.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reunanen J, Saris P. 2003. Microplate bioassay for nisin in foods, based on nisin-induced green fluorescent protein fluorescence. Appl Environ Microbiol 69:4214–4218. doi: 10.1128/aem.69.7.4214-4218.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang L, Jin Q, Luo J, Wu J, Wang S, Wang Z, Gong S, Zhang W, Lan X. 2018. Intracellular expression of antifreeze peptides in food grade Lactococcus lactis and evaluation of their cryoprotective activity. J Food Sci 83:1311–1320. doi: 10.1111/1750-3841.14117. [DOI] [PubMed] [Google Scholar]
- 28.Szczepankowska AK, Szatraj K, Sałański P, Rózga A, Górecki RK, Bardowski JK. 2017. Recombinant Lactococcus lactis expressing haemagglutinin from a Polish avian H5N1 isolate and its immunological effect in preliminary animal trials. Biomed Res Int 2017:6747482. doi: 10.1155/2017/6747482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Esteban LE, Temprana CF, Argüelles M, Glikmann G, Castello AA. 2013. Antigenicity and immunogenicity of rotavirus VP6 protein expressed on the surface of Lactococcus lactis. Biomed Res Int 2013:298598. doi: 10.1155/2013/298598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu D, Fu Y, Liu F, Xu H, Saris PEJ, Qiao M. 2017. Enhanced heterologous protein productivity by genome reduction in Lactococcus lactis NZ9000. Microb Cell Fact 16:1. doi: 10.1186/s12934-016-0616-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Platteeuw C, van Alen-Boerrigter I, van Schalkwijk S, De Vos W. 1996. Food-grade cloning and expression system for Lactococcus lactis. Appl Environ Microbiol 62:1008–1013. doi: 10.1128/AEM.62.3.1008-1013.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lindholm A, Smeds A, Palva A. 2004. Receptor binding domain of Escherichia coli F18 fimbrial adhesin FedF can be both efficiently secreted and surface displayed in a functional form in Lactococcus lactis. Appl Environ Microbiol 70:2061–2071. doi: 10.1128/aem.70.4.2061-2071.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith MK, Draper LA, Hazelhoff P-J, Cotter PD, Ross RP, Hill C. 2016. A bioengineered nisin derivative, M21A, in combination with food grade additives eradicates biofilms of Listeria monocytogenes. Front Microbiol 7:1939. doi: 10.3389/fmicb.2016.01939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Field D, Begley M, O'Connor PM, Daly KM, Hugenholtz F, Cotter PD, Hill C, Ross RP. 2012. Bioengineered nisin A derivatives with enhanced activity against both Gram positive and Gram negative pathogens. PLoS One 7:e46884. doi: 10.1371/journal.pone.0046884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Israelsen H, Madsen SM, Vrang A, Hansen EB, Johansen E. 1995. Cloning and partial characterization of regulated promoters from Lactococcus lactis Tn917-lacZ integrants with the new promoter probe vector, pAK80. Appl Environ Microbiol 61:2540–2547. doi: 10.1128/AEM.61.7.2540-2547.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuipers OP, Beerthuyzen MM, Siezen RJ, De Vos WM. 1993. Characterization of the nisin gene cluster nisABTCIPR of Lactococcus lactis: requirement of expression of the nisA and nisI genes for development of immunity. Eur J Biochem 216:281–291. doi: 10.1111/j.1432-1033.1993.tb18143.x. [DOI] [PubMed] [Google Scholar]
- 37.Åvall-Jääskeläinen S, Kylä-Nikkilä K, Kahala M, Miikkulainen-Lahti T, Palva A. 2002. Surface display of foreign epitopes on the Lactobacillus brevis S-layer. Appl Environ Microbiol 68:5943–5951. doi: 10.1128/aem.68.12.5943-5951.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]