Significance
Recent advances in bioelectronics have opened avenues toward hybrid engineered tissues with embedded devices that could monitor, modulate, or otherwise augment cellular function. In order to achieve these “cyborgs,” however, both devices and electronic interconnects must stably integrate with the surrounding tissue. We investigated a photo–cross-linkable silk fibroin (PSF) derivative that could be selectively cross-linked using conventional photolithography. We found that PSF was an ideal candidate for bioelectronic scaffolds: it was electrically insulating and so could passivate electronic interconnects, presented amenable surface chemistry to promote cellular adhesion, and could be realized as freestanding, three-dimensional constructs. PSF-based scaffolds are compatible with a wide variety of devices and tissues, and so could enable new classes of hybrid systems.
Keywords: silk fibroin, photo–cross-linkable, bioelectronics, cell affinity, scaffold
Abstract
Bioelectronic scaffolds that support devices while promoting tissue integration could enable tissue hybrids with augmented electronic capabilities. Here, we demonstrate a photo–cross-linkable silk fibroin (PSF) derivative and investigate its structural, electrical, and chemical properties. Lithographically defined PSF films offered tunable thickness and <1-µm spatial resolution and could be released from a relief layer yielding freestanding scaffolds with centimeter-scale uniformity. These constructs were electrically insulating; multielectrode arrays with PSF-passivated interconnects provided stable electrophysiological readouts from HL-1 cardiac model cells, brain slices, and hearts. Compared to SU8, a ubiquitous biomaterial, PSF exhibited superior affinity toward neurons which we attribute to its favorable surface charge and enhanced attachment of poly-d-lysine adhesion factors. This finding is of significant importance in bioelectronics, where tight junctions between devices and cell membranes are necessary for electronic communication. Collectively, our findings are generalizable to a variety of geometries, devices, and tissues, establishing PSF as a promising bioelectronic platform.
Advances in bioelectronics promise to blur the line between living and artificial systems, enabling two-way communication between those disparate but complementary components (1–3). Examples of bioelectronic devices include multielectrode arrays (MEAs) and field-effect transistors (FETs) which have been interfaced with neuron or cardiac cells for multiplexed, real-time readouts of electrophysiological activity (4, 5), while other classes of devices have achieved on-demand drug delivery, localized stimulation, power generation, and chemical sensing capabilities (6, 7). Advances in nanoscience have played a key role in each of these areas by enabling cellular-scale, noninvasive devices such as transparent graphene MEAs (8) and freestanding nanowire or nanopillar arrays (9–11) that could access the cytosol for intracellular measurements.
One important challenge in bioelectronics—particularly with regard to three-dimensional (3D) device configurations—relates to the device substrate, which must support devices and interconnects, provide adequate electrical passivation to prevent short circuits, and be biocompatible. Organ-level studies have been demonstrated using flexible, stretchable, and bioresorbable materials (12–14). More recently, highly flexible, macroporous mesh electronics were demonstrated (15). Composed of devices and metallic interconnects sandwiched between layers of SU8, a photo–cross-linkable epoxy, mesh electronics functioned as device supports, electronic passivation, and tissue scaffold and have been fabricated with tunable geometries using conventional photolithography processes. Significantly, they solved the problem of mechanical mismatches presented by conventional, rigid devices such as microwires and silicon-based microchips, thereby enabling excellent 3D device integration within engineered or primary tissues with minimal immune response. They have been implemented to realize synthetic bioelectronics-innervated cardiac tissues (16, 17) and organoids (18) and have also been injected into the brain (19) or retina (20) of live animal models to achieve chronically stable electrophysiological readouts. Collectively, these studies demonstrate the extraordinary potential for highly flexible electronics for achieving 3D bioelectronic interfaces with a wide variety of tissues.
Recent efforts have focused on materials that are not only biocompatible but also bioactive. These materials, which bond surrounding tissues through chemical or mechanical interactions, are commonly used with implants to achieve biointegration and mitigate potential fibrotic responses. One common example is hydroxyapatite, which is similar to bone and is frequently used as a coating for metallic implants to promote osseointegration (21). Bioactive dielectrics could be especially relevant in bioelectronics, as they would promote seamless integration between interconnects and the surrounding tissue. Bioactive materials with tunable conductivity include glasses (22) and ceramics (23), but these materials are often brittle and so are not ideal candidates for flexible and stretchable electronics. Natural polymers represent an alternative approach, playing a central role in tissue engineering (24). One especially promising bioactive polymer is silk fibroin, derived from the cocoon of Bombyx mori. Silk exhibits tunable mechanical properties (25, 26), excellent biocompatibility (27), and programmable biodegradability (28), and moreover has potential for chemical customization through reactive amino acid side-chain groups (29). It has been demonstrated in a wide range of tissue models (30), including those that incorporate cocultures and functional innervation (31). Bioelectronic devices containing silk as a major dielectric component would be complementary to existing tissue scaffolds, potentially enhancing tissue/device integration.
Here, we describe a photo–cross-linkable silk fibroin (PSF) derivative and report how it may be used as a passivating dielectric for bioelectronic devices. While photo–cross-linkable forms of silk fibroin have been demonstrated previously (32, 33), their dielectric properties in bioelectronic systems have not been explored, nor has their ability to adhere delicate cell lines such as human induced neural stem cells (hiNSCs) (34) which have been integrated into 3D tissue cocultures and brain tissue models. We achieved PSF via an isocyanate-hydroxyl reaction and demonstrated a photoresist that was compatible with conventional photolithography. We found that the thickness of the cross-linked PSF film could be tuned over an order of magnitude and after exposure and developing exhibited features with ∼1-µm resolution, rivaling the performance of SU8. We also found that PSF stably passivated MEA interconnects, enabling bioelectronic readouts for up to 8 d and stable passivation for at least 21 d. Significantly, hiNSCs demonstrated remarkably improved adhesion to PSF compared to SU8, which we attribute to PSF’s favorable surface charge and enhanced attachment of poly-d-lysine (PDL) adhesion factors. This finding is of significant importance in bioelectronics, where tight junctions between devices and cell membranes are necessary for efficient electronic communication. These findings establish PSF as a promising bioelectronic platform which could be extended to freestanding 3D configurations that could be stably integrated into a wide variety of tissue models.
Results
Synthesis of PSF.
Fig. 1 outlines our synthetic route toward PSF. First, native silk fibroin was extracted from cocoons and freeze dried as described previously (SI Appendix, Text) (35). Photo–cross-linkable methacrylate groups were then grafted onto pendant hydroxyl groups on the fibroin backbone by reaction with 2-isocyanatoethyl methacrylate (IEM) in 60 °C dimethyl sulfoxide (DMSO) with 1 M lithium chloride and dibutyltin dilaurate catalyst under nitrogen atmosphere. The reaction product was thoroughly rinsed with a mixture of acetone and ethanol, dried and lyophilized to form a PSF powder, then dissolved in hexafluoroisopropanol (HFIP), to achieve a clear amber-colored solution. We confirmed successful engraftment of the methacrylate groups by 1H NMR. While the PSF reserved most of the functional groups in native fibroin, additional chemical shifts in PSF spectra were ascribed to the IEM modification: 1.88 ppm (methyl group), 5.7 and 6.09 ppm (vinyl group), 4.97 ppm (methylene bridge adjacent to O), and 3.27 and 3.35 ppm (methylene bridge adjacent to N) (SI Appendix, Fig. S1). We also investigated the influence of the methacrylate-esterification reaction on the silk fibroin structure using attenuated total reflectance Fourier-transform infrared spectroscopy (SI Appendix, Fig. S2). PSF preserved characteristic crystalline peaks (1,621 and 1,701 cm−1) and random coil peak (1,641 cm−1) of native silk fibroin (36), with the intensity of crystalline peaks higher and random coil peak lower compared to native silk fibroin, indicating that PSF contained a higher fraction of crystalline domains.
Fig. 1.
Synthetic route toward PSF. Native fibroin derived from cocoons was methacrylated through the hydroxyl groups on the chain to achieve PSF. After purification and freeze drying, PSF was readily dissolved in HFIP, yielding a clear amber-colored solution.
Characterization of Lithographically Defined Structures.
After synthesizing PSF we assessed its feasibility as a photoresist (Fig. 2A). We chose Irgacure 2959 as the photoinitiator (PI) to trigger the cross-linking of PSF under ultraviolet (UV) since Irgacure 2959 is a high-efficacy PI and exhibits minimal toxicity to cells (PI refers to Irgacure 2959 in the following text other than special note) (37, 38). We prepared solutions of PSF in HFIP at concentrations of 2, 4, 6, and 8% (wt/vol) with fixed PI concentration at 0.4% (wt/vol), then spin-coated them on silicon substrates. After prebaking the substrates at 80 °C for 10–15 min and selectively cross-linking the PSF by UV photolithography, we postbaked the substrates for another 10–15 min at 80 °C and then immersed them into a LiCl/DMSO solution (1 M) for 0.5–2 min to remove the unreacted PSF. Thermogravimetric analysis and differential scanning calorimetry both revealed that the glass transition of PSF was in a broad range, from about 110 °C to over 170 °C, substantially higher than our pre- and postbake temperatures (SI Appendix, Fig. S3).
Fig. 2.
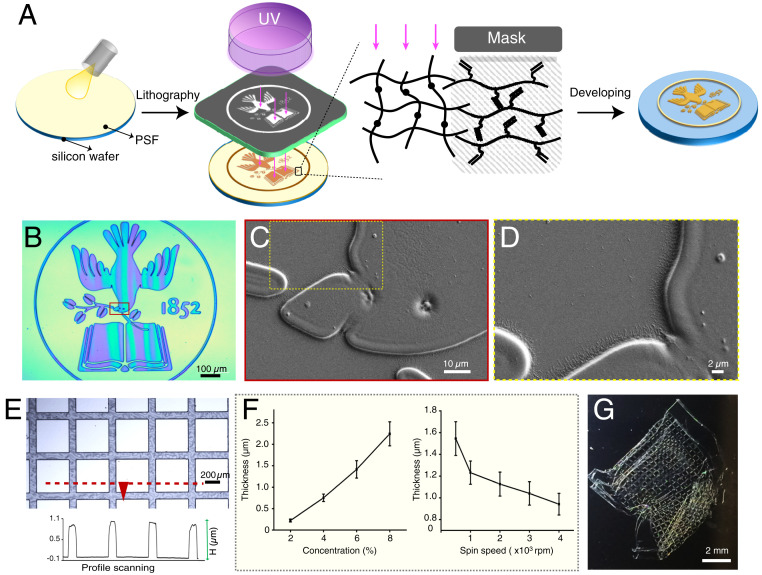
Microfabrication with PSF. (A) Fabrication scheme using PSF as a negative photoresist, whereby an arbitrary pattern (e.g., Tufts logo) may be achieved by selective cross-linking of the PSF by conventional lithography followed by a developing step to wash away the unreacted material. (B) Optical image of a PSF Tufts logo pattern and (C and D) SEM expansions of regions in dotted boxes. (E) Optical image of PSF cross-bar pattern on glass substrate. (Inset) The curve represents the height profile of structures shown in red dashed line. (F) PSF film thickness as a function of PSF photoresist solution concentration (n = 15) or spin speed during spin coating (n = 4). (G) Photo of a highly flexible, freestanding PSF construct achieved through etching underlying zinc relief layer.
We next assessed the suitability of PSF for achieving microscale structures by photolithography. First, we created a Tufts University logo pattern in which minimum feature sizes of ∼1 µm were clearly resolved (Fig. 2 B–D). Next, we demonstrated cross-bar motifs similar to those achieved with mesh electronics (15). Fig. 2E depicts the cross-sectional profile of the cross-bars along the dashed red line, highlighting the sharp edges of the pattern. Significantly, height of the pattern (thickness of PSF film) could be controlled by varying the concentration of PSF in HFIP solution as well as the spin-coating speed (Fig. 2F), allowing us to tune the thickness between ∼200 and 2,500 nm. We also found that PSF could be resolved over a very broad exposure window, which makes the processing time more flexible. For example, films formed from 6% PSF solution and 2,000-rpm spin rate exposed at doses between 1,000 and 12,000 mJ/cm2 yielded similar high-quality patterns after development (SI Appendix, Fig. S4). In addition, the as-prepared PSF patterns were stable against common solvents frequently used in microfabrication and cell culture including isopropyl alcohol, ethanol, methanol, DMSO, cell culture medium, and phosphate-buffered saline for at least 1 mo (SI Appendix, Fig. S5), showing no evidence of swelling or degradation. Finally, we demonstrated that our silk films could be released from an underling relief layer to achieve highly flexible, freestanding constructs that are amenable to macroporous, conformable electronics with device arrangements in 3D configurations (Fig. 2G). Collectively, these features demonstrated the suitability of PSF for bioelectronic applications in which stable, high-resolution structures and flexible conformations, including those in 3D, would be desirable.
Bioelectronic Interfaces with Silk-Based MEAs.
We next explored the feasibility of our silk-based structures to achieve stable bioelectronic interfaces with cell culture medium and living cells. Since bioelectronic devices are typically addressed by passivated interconnects, we first assessed the dielectric properties of PSF by electrical impedance spectroscopy. Gold working electrodes coated with dielectric films were submerged in PBS to mimic the conditions of a typical bioelectronic measurement. We then measured the impedance spectra across those films using a typical three-electrode setup consisting of a Pt wire counterelectrode and Ag/AgCl reference electrode (Fig. 3 A and B and SI Appendix, Text) (39). Films cast from 2% PSF demonstrated poor passivation, potentially because of pinholes in the films, which were evident under scanning electron microscopy (SEM) (SI Appendix, Fig. S6). In contrast, films cast from 4, 6, and 8% PSF exhibited excellent dielectric properties over the range 10–106 Hz, with corresponding impedance amplitude ratio (|ZPSF,c|/|Zbare_Au|) of 14.6, 17.2, and 16.6 at 103 Hz, for c = 4, 6, and 8% PSF, respectively. Significantly, these PSF films exhibited better insulating properties than the typically used dielectric layer of sputtered 200-nm SiO2 films, which exhibited a ratio of only 2.79 times at 103 Hz. Moreover, we found that the insulating property of PSF was stable against PBS solution, with impedance at 1 kHz dropping by less than 20% over 21 d (SI Appendix, Fig. S7).
Fig. 3.
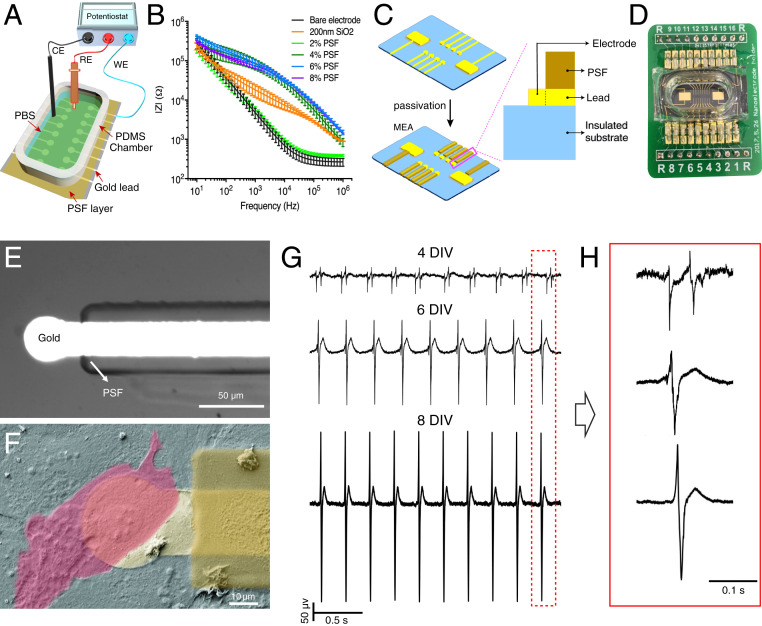
Bioelectronic measurements with PSF-based devices. (A) Electrical impedance measurement setup. (B) Impedance spectrum of PSF films spin-coated from 2 to 8% (wt/vol) HFIP solutions, compared to bare gold electrodes and electrodes coated with a silicon dioxide layer with thickness of 200 nm (n = 16). (C) Schematics of the MEA with interconnects passivated by PSF. The expansion illustrates a side view of a single device element and passivated interconnect. (D) Photograph of bioelectronic chip assembly including MEA, PDMS well, and PCB interface. (E) Optical image of a single device with interconnect passivated by PSF. (F) False-colored SEM image of HL-1 cultured on the PSF passivated device highlighting (bright yellow) gold layer, (deep yellow) PSF passivation layer, and (pink) single HL-1 cell. (G) Extracellular signal from a spontaneously beating HL-1 cell monolayer recorded on days 4, 6, and 8. (H) Magnified signals in the red dashed box in G.
We next assessed the ability of silk-passivated devices to form bioelectronic interfaces with living cells or tissue. Our devices consisted of MEAs with circular 30-µm-diameter Au recording elements whose interconnects were passivated by 6% PSF films (Fig. 3 C–F). Fig. 3 C and D are a model and photograph of our MEA built on a printed circuit board (PCB) with a polydimethylsiloxane (PDMS) chamber adhered to enable cell culturing (SI Appendix, Text). We initially assessed our devices with HL-1 cardiac muscle models (40), which remained viable on our MEA substrates for at least 8 d in vitro (DIV). Fig. 3F shows a false-colored SEM image of the HL-1 monolayer at 4 DIV, highlighting the cell (pink), recording element (light yellow), and silk passivation layer (yellow). Throughout the culture period HL-1 monolayers beat spontaneously with stable firing intervals of 0.27 ± 0.01, 0.29 ± 0.02, and 0.26 ± 0.01 s at 4, 6, and 8 DIV, respectively (n = 3). Fig. 3 G and H show extracellular recordings from a representative recording element at each of those time points, showing an increase in magnitude over time—48.2 ± 0.9, 218.3 ± 0.5, and 441.8 ± 0.6 µV at 4, 6, and 8 DIV, respectively—consistent with an expected increase in cell–cell junctions and electrical synchrony throughout the culture over time (SI Appendix, Fig. S8). Significantly, the filtered baseline noise was consistently less than 4 µV across all time points, and the strongest bioelectronic interfaces demonstrated signal-to-noise >78.
Our route toward silk-passivated bioelectronics represents a general platform that is readily extendable to a wide variety of applications. We anticipate that our devices will enable bioelectronic interfaces with a wide variety of cell or tissue types; to demonstrate this principle we achieved bioelectronic interfaces with ex vivo heart and mouse brain. Female BALB/c mice were killed at age of 6 wk, and the isolated hearts and brains were immediately interfaced to silk-passivated MEA devices to record signals with signal-to-noise of 48 and 9.2, respectively (SI Appendix, Fig. S9). In addition, we note that our silk films were highly transparent, with >90% transmission at visible wavelengths. This transmissivity was similar to SU8 films (41, 42) as well as silk films that were not photo–cross-linked (13), opening avenues for simultaneous imaging and recording (SI Appendix, Fig. S10).
Characterization of Cell Adhesion.
A crucial feature of bioelectronic scaffolds is that they must exhibit appropriate chemical properties to form tight, seamless interfaces with the surrounding cells. We studied this criterion using hiNSCs, which spontaneously differentiated into neurons and have shown great potential in engineered brain and other innervated tissue models (34, 43). For the purposes of these studies we examined two-dimensional systems, which enabled quantitative studies of cellular adhesion, migration, and surface chemistry.
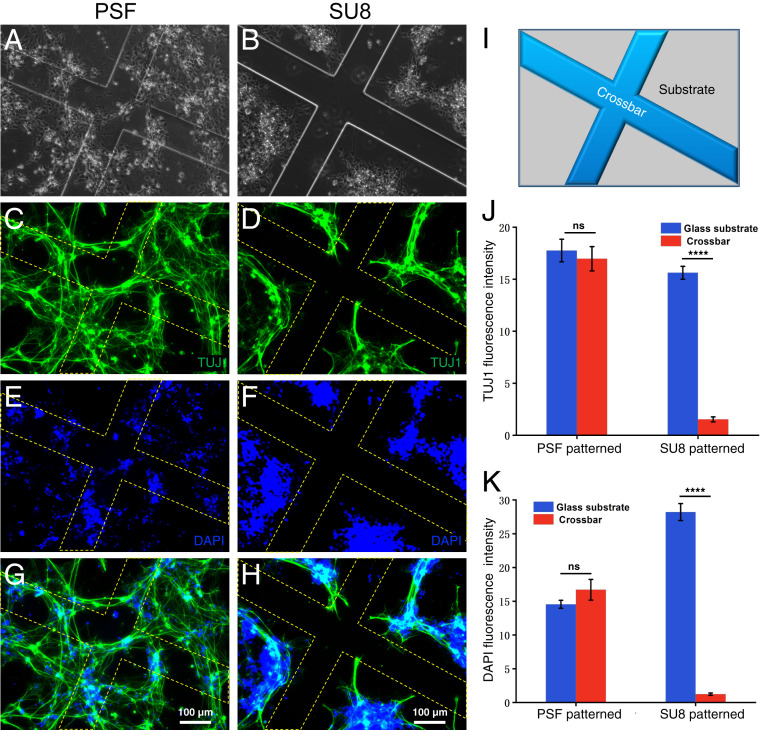
We plated hiNSCs on PSF-patterned glass wafer surfaces and maintained their growth in culture medium (Materials and Methods). SU8-patterned glass wafers and pristine glass wafers were also seeded with hiNSCs at the same density as contrast and control groups. Immediately prior to plating the cells, all of the surfaces were coated with PDL to promote hiNSC adhesion. After 5 d of culturing, the cells were fixed and stained with the neuron-specific marker β-III tubulin (TUJ-1) antibody and DAPI. Fig. 4 A and B are, respectively, the bright-field images of hiNSCs on the PSF- and SU8-patterned glass substrate. Fig. 4 C–F are corresponding fluorescence images stained with TUJ1 antibody (DAPI). Fig. 4 G and H are the merged fluorescent images. The yellow dashed lines outline edges of the PSF and SU8 cross-bars (Fig. 4I) to aid visualization. All of these images show that hiNSCs distributed differently on the two types of surfaces: While hiNSCs appear both on the PSF cross-bars and the interspaced glass substrates on the PSF patterned surfaces, they mostly avoided contact with the SU8 cross-bars on the patterned surfaces. Fig. 4 J and K are statistical analysis of the fluorescence intensity of the different regions on the PSF- and SU8-patterned surfaces. This quantitative comparison demonstrates that the fluorescence intensity on the PSF cross-bars was similar to that on the glass substrate irrespective of statistics based on TUJ-1 staining (Fig. 4J) or DAPI staining (Fig. 4K). However, the fluorescence intensity on the SU8 cross-bars was much lower than that on the interspaced glass substrates, indicating fewer hiNSCs adhered to the SU8 cross-bars. This distribution difference was even more apparent in the wide-field imaging, as shown from the bright-field and fluorescent images in SI Appendix, Fig. S11, where the pristine glass wafer substrate was used as the control surface.
Fig. 4.
hiNSC adhesion study on microfabricated PSF and SU8 structures. (A and B) Bright-field microscope images of cells plated on (Left) PSF- and (Right) SU8-patterned surfaces. The cells were stained with neuron-specific marker antibody TUJ1 (C and D) and DAPI (E and F), with G and H merged images for both surfaces. (I) Schematic of the patterned structure. (J and K) Statistical analysis of fluorescence intensity for TUJ1 and DAPI on PSF- and SU8-patterned surfaces, showing that cells distribute evenly on the PSF-patterned surfaces, while avoiding SU8 framework (****P < 0.0001; n = 7).
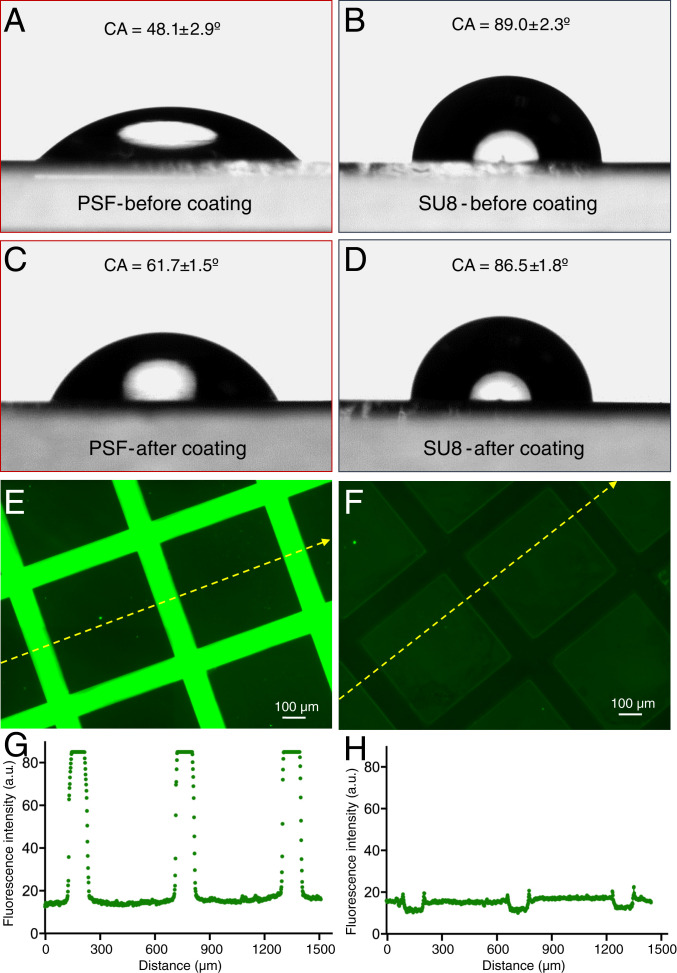
Since hiNSCs were plated on all surfaces at the same density and cultured under identical conditions, we hypothesized that the final distribution behavior arose from differential cell affinity to SU8 vs. PSF surfaces. To address this point, we assessed the wettability of uniform PSF and SU8 films both before and after the PDL coating. These films were prepared in a fashion identical to the cross-bar arrays presented in Fig. 4 and SI Appendix, Fig. S12, except that they were uniformly cross-linked by excluding the photomask during exposure. Before coating, the PSF and SU8 film showed water contact angles (WCA) of 48.1 ± 2.9° and 89.0 ± 2.3°, respectively (Fig. 5 A and B). After PDL coating at room temperature overnight, the WCA on the PSF film has increased to 61.7 ± 1.5°, a change of approximate 13.6° although still in the hydrophilic domain, indicating successful coating (Fig. 5C) (44), while the WCA on the SU8 decreased a little, to 86.5 ± 1.8°, kept more hydrophobic than the postcoated PSF film (Fig. 5D). (n = 5 for all WCA measurements.) Since hydrophobicity is adverse to cell adhesion and moderate hydrophilicity promotes cell adhesion, this difference in the wettability on both surfaces in turn affects cell distribution (45).
Fig. 5.
(A–D) Contact angle measurements on PSF (A and C) or SU8 (B and D) films either with or without PDL coating. (E and F) Fluorescence microscopy of FITC-PLL coated glass surfaces patterned with PSF or SU8 cross-bars. (G and H) Profile of the fluorescence intensity along the dashed yellow line in PSF- and SU8-patterned glass substrate, respectively.
To visualize the differential coating effect on PSF and SU8, we used fluorescein isothiocyanate (FITC)-labeled poly-l-lysine (PLL) to coat the PSF- and SU8-patterned surfaces and imaged them under a fluorescent microscope. FITC-labeled PLL had the same coating mechanism as PDL on the substrate (physical absorption) but was also fluorescent (46). Fig. 5 E and F show the PSF-and SU8-patterned surfaces captured under the same exposing time. The PSF cross-bars are brighter than the interspaced glass substrate, which is further brighter than the SU8 cross-bars. Fig. 5 G and H are the fluorescence intensity profile along the yellow dashed line in Fig. 5 E and F. For both surfaces, the interspaced glass substrates keep the same value of around 16, but the PSF cross-bars have a much higher value of nearly 90 and SU8 cross-bars have a lower value of around 10. The different coating effect of PLL on the PSF, glass wafer, and SU8 surfaces can also be found in SI Appendix, Fig. S12 in wide-field imaging. All of these results confirmed that PLL was physically absorbed more efficiently on the PSF surface compared to SU8, leading to differing cell behaviors. The probable reason for the different physical absorption is the varied surface electrical properties: PSF shows large negative charges in neutral solution, glass surface is less negative charged, and SU8 is more prone to be neutral (47). When those surfaces are immersed in the positively charged PLL or PDL solution, the electrostatic interaction is strongest on the PSF surfaces, leading to being coated most and attracting the most cells, vice versa for the situation of SU8 surfaces.
Discussion
In this work we have investigated PSF, a photo–cross-linkable derivative of silk fibroin which we achieved as a photoresist and used to develop silk-based bioelectronic devices. While similar materials have been investigated previously, we specifically assessed the unique properties of photo–cross-linkable silk as they related to bioelectronics. We demonstrated that PSF-based photoresists, compatible with standard photolithography, could achieve patterned features with centimeter-scale uniformity and feature sizes with critical length < 1µm. This feature is important since it opens avenues for a wide variety of bioelectronic devices, ranging from short channel-length FETs to organ-level structures, including bioelectronics-embedded brain or cardiac tissues. Moreover, we showed that our PSF exhibited not only promising structural properties but also excellent electrical insulating properties, which demonstrated that the material could be used both to support devices and also passivate electrical interconnects. This is a crucial feature for a wide range of bioelectronic devices, ranging from active FETs to passive MEAs (4). As proof-of-principle and to demonstrate the robustness of our technique, we demonstrated that MEAs with silk-passivated interconnects could record signals both from cultured cells (HL-1) as well as primary tissues (brain, heart). Significantly, in the case of HL-1 cells we recorded signals with signal-to-noise >78, which compared favorably to other devices recently used to study cardiac monolayers, including graphene MEAs with SU8 passivation (8).
A significant contribution of this study—and perhaps the one that most distinguishes it from recent works involving SU8—is our observation that PSF exhibited substantially improved properties for cell culture: It was more hydrophilic in its as-prepared state, more effectively bound the poly-d-lysine adhesion factor, and at least in the case of hiNSC-derived neurons far more effectively promoted soma adhesion as well as neurite extension. While highly flexible SU8 scaffolds have demonstrated remarkable promise in bioelectronics, providing stable recordings from brain or retina with minimal immune response, these studies have been largely confined to mature cells in vivo. The few demonstrations of SU8 in engineered tissue systems required a chemically distinct matrix [e.g., coated poly (lactic-coglycolic acid) fibers] to promote 3D adhesion and spreading. The ability of PSF to promote adhesion is likely to improve coupling between cell membranes and devices, thereby improving signal-to-noise (48). This property is especially apropos within the context of nerve tissue engineering where neurons are relatively sparse compared to cardiac tissue. Since 3D models for brain and other innervated tissues have been achieved in silk scaffolds (30, 31), freestanding PSF-based bioelectronic scaffolds similar to the proof-of-concept shown in Fig. 2G represent a logical route toward stable bioelectronics-embedded hybrids.
As with all bioactive materials, the mechanical properties of PSF are an important consideration. A similar formulation of PSF had a reported elastic modulus of 15.6 ± 1.1 GPa (32). This was comparable to SU8, which had elastic modulus between 3.0 ± 0.1 and 4.4 ± 0.2 GPa (49). We note that the mechanical properties of PSF could be further tuned through 1) molecular weight at the point of fibroin protein extraction from silk fibers, 2) methacrylate or other ligand content during chemical modification, or 3) photoinitiator concentration (50). Additionally, silk fibroin thin films had a reported tensile strength of ∼100 MPa, which is about three times greater than other polymer systems including SU8 (50, 51). This property is especially desirable for flexible electronics and in load-bearing applications, e.g., bioelectronics-embedded cardiac tissues. Finally, the adhesion strength between PSF and gold is an important consideration. Because of its amino and carboxylate groups, silk fibroin adhered tightly to gold and moreover has been used as an adhesion layer between gold and silicon oxide for optically active devices (52). Since the amino groups in PSF are unreacted, we expect good adhesion with our systems as well; we have verified this point experimentally through our long-term silicon oxide adhesion (SI Appendix, Fig. S5) and impedance (SI Appendix, Fig. S7) studies.
Taken together, our results demonstrate that PSF is a promising route toward bioelectronic scaffolds, representing a general platform that could be applied to a wide variety of cell and tissue types. The functionality of PSF described here could be further extended through covalently attached ligands, e.g., to achieve integrin-specific binding, direct neurite outgrowths, or achieve sustained drug release capabilities (53). Moreover, we note that silk fibroin bioinks are compatible with 3D printing processes (54), which in conjunction with techniques to print flexible and stretchable circuits (55) could enable complex bioelectronic scaffolds with geometry tailored to the specific application. Taken together, the advantages of PSF could enable new classes of hybrid tissues with seamlessly integrated bioelectronics that monitor, modulate, or otherwise augment tissue function.
Materials and Methods
Synthesis of PSF.
Silk fibroin refinement procedures are described in SI Appendix. We dried 1.6 g lyophilized silk fibroin in a 60 °C oven overnight prior to dissolving in 160 mL 1 M LiCl/DMSO solution to achieve a final concentration of 1% (wt/vol). The mixture was maintained at 60 °C with continuous stirring and degassing via nitrogen purge. After the solution became clear (∼20 min), 3.18 mL IEM and 60 µl dibutyltin dilaurate (DBTDL) were added into the solution quickly. The reaction was allowed to proceed for 5 h before pouring into a mixture of cold acetone and ethanol (volume ratio of 1:1) to precipitate the white flocculent postmodified fibroin. The product was rinsed 3× with acetone/ethanol mixture, and washed 2× with deionized water, then lyophilized for 1–2 d, yielding dried and purified PSF (32, 35).
HL-1 Culturing and Fixation for SEM.
Culture.
HL-1 cells were plated at a density of 2 × 105 cells per cm2 on the fibronectin precoated flask and MEA surfaces and maintained in the Claycomb medium supplemented with 10% fetal bovine serum, 2 mM l-glutamine, 0.1 mM norepinephrine, and 100 U ml−1 penicillin and 100 mg/mL streptomycin in a cell-culturing incubator. Medium was changed daily.
SEM studies.
HL-1 cells were fixed with 2% glutaraldehyde at 4 °C for 1 h, then dehydrated with a gradient series of ethanol at 50, 60, 70, 80, 90, and 100% concentrations (vol/vol), 10 min for each concentration. Afterward, the sample was dried using a liquid CO2 critical point dryer. Prior to SEM, samples were sputter coated with a 10-nm gold layer to impart conductivity (11, 56).
hiNSC Culturing and Immunohistochemistry.
Culture.
hiNSC lines derived from adult human adipose stem cells were generated as previously described. Surfaces were treated with 0.05mg/mL PDL solution at room temperature overnight, then washed with PBS for 2–3 times. Cells (passage 16) were plated at 1.6 × 105 cells per cm2 and the hiNSC maintaining medium (knockout Dulbecco’s Modified Eagle’s Medium supplemented with 20% knockout xeno-free SR, 1% Glutamax, 1% antibiotic-antimycotic and 0.1 mM β-mercapthanol) was changed once every 3 d.
Immunohistochemistry.
Cells were fixed with 4% paraformaldehyde at 4 °C for 15 min and then incubated with a blocking solution consisting of 6% goat serum and 0.1% TritonX-100 in PBS. The primary antibody (Rabbit TUJ-1) was added to the blocking buffer and incubated for 2 h at RT and then the sample was washed with PBS thoroughly. Cells were next incubated with the fluorescent secondary antibody (Abcam anti-rabbit IgG conjugated to Alexa 488) in blocking buffer for 1 h and washed again with PBS. Nuclei were counterstained by incubating cells with DAPI solution for 10 min (34).
Electrophysiology.
Chip fabrication and assembly details are found in SI Appendix. Extracellular signals were measured using a 16-channel data acquisition system (USB-ME16-FAI-System, Multichannel Systems) at a sampling frequency of 25 KHz. All signals were preamplified 1,000×. They were filtered with a digital bandpass filter: 1 Hz to 2.25 kHz for HL-1 cell measurements and 1 Hz to 1.25 kHz for brain slice measurements.
Mouse Heart and Brain Signal Recording.
BALB/c mice (6 wk, female) were killed by carbon dioxide asphyxiation. Their hearts and brains were carefully isolated from the surrounding tissue, then thoroughly washed with PBS (RT) to remove the blood. They were immediately interfaced with MEA devices to record signals. All experiments were performed in accordance with US Animal Welfare Act and institutional guidelines and were approved by the Institutional Animal Care and Use Committee at Tufts University.
Surface Wettability Characterization.
Contact angles were evaluated using a custom-built measurement apparatus and calculated with OCA 20 software (DataPhysics). Each surface was measured at five different positions.
Statistics.
Calculations were performed in GraphPad Prism or OriginLab. All of the values are represented as means ± SEM. Statistical analyses were performed using one-way ANOVA, with P ≤ 0.05 considered statistically significant.
Data Availability Statement.
Raw data associated with Figs. 1–5 are available in the Harvard/Tufts University Dataverse at https://dataverse.harvard.edu/dataset.xhtml?persistentId=doi:10.7910/DVN/SXSI8O (57).
Additional methods relating to silk fibroin refinement and characterization, chip assembly, and PSF stability, as well as SI Appendix, Figs. S1–S12, can be found in SI Appendix.
Supplementary Material
Acknowledgments
We thank Prof. David Kaplan for invaluable advice. We thank Dr. Will Collins, Dr. Tao Yang, and Dr. Chengchen Guo for experimental assistance. We acknowledge a Tufts Collaborates Grant to B.P.T., a Tufts Research Advancement Fund award to B.P.T., a National Science Foundation of China Grant (2190050415) to J.J., and a China Research Council Award ([2016]3100) to H.L. All authors thank the NIH for grant support through the Tissue Engineering Resource Center (P41 EB002520). This work was performed in part at the Center for Nanoscale Systems (CNS), a member of the National Nanotechnology Coordinated Infrastructure Network, which is supported by the NSF under Award 1541959. CNS is part of Harvard University. This work was also performed in part at the Tufts Micro and Nano Fabrication Facility with the assistance of Dr. James Vlahakis.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
Data deposition: Raw data associated with Figs. 1–5 are available in the Harvard/Tufts University Dataverse at https://dataverse.harvard.edu/dataset.xhtml?persistentId=doi:10.7910/DVN/SXSI8O.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2003696117/-/DCSupplemental.
References
- 1.Zhang A., Lieber C. M., Nano-bioelectronics. Chem. Rev. 116, 215–257 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu H.et al., Nanobiotechnology: 1D nanomaterial building blocks for cellular interfaces and hybrid tissues. Nano Res. 11, 5372–5399 (2018). [Google Scholar]
- 3.Luo Z., Weiss D. E., Liu Q., Tian B., Biomimetic approaches toward smart bio-hybrid systems. Nano Res. 11, 3009–3030 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spira M. E., Hai A., Multi-electrode array technologies for neuroscience and cardiology. Nat. Nanotechnol. 8, 83–94 (2013). [DOI] [PubMed] [Google Scholar]
- 5.Bolonduro O. A., Duffy B. M., Rao A. A., Black L. D., Timko B. P., From biomimicry to bioelectronics: Smart materials for cardiac tissue engineering. Nano Res. 13, 1253–1267 (2020). [Google Scholar]
- 6.Feiner R.et al., Engineered hybrid cardiac patches with multifunctional electronics for online monitoring and regulation of tissue function. Nat. Mater. 15, 679–685 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ding W., Wang A. C., Wu C., Gao H., Wang Z., Human-machine interfacing enabled by triboelectric nanogenerators and tribotronics. Adv. Mater. Technol. 4, 1800487 (2019). [Google Scholar]
- 8.Rastogi S. K.et al., Graphene microelectrode arrays for electrical and optical measurements of human stem cell-derived cardiomyocytes. Cell. Mol. Bioeng. 11, 407–418 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tian B.et al., Three-dimensional, flexible nanoscale field-effect transistors as localized bioprobes. Science 329, 830–834 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGuire A. F., Santoro F., Cui B., Interfacing cells with vertical nanoscale devices: Applications and characterization. Annu. Rev. Anal. Chem. (Palo Alto, Calif.) 11, 101–126 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu H.et al., Heart-on-a-chip model with integrated extra- and intra-cellular bioelectronics for monitoring cardiac electrophysiology under acute hypoxia. Nano Lett. 20, 2585–2593 (2020). [DOI] [PubMed] [Google Scholar]
- 12.Timko B. P.et al., Electrical recording from hearts with flexible nanowire device arrays. Nano Lett. 9, 914–918 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim D.-H.et al., Dissolvable films of silk fibroin for ultrathin conformal bio-integrated electronics. Nat. Mater. 9, 511–517 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Y., Pharr M., Salvatore G. A., Lab-on-skin: A review of flexible and stretchable electronics for wearable health monitoring. ACS Nano 11, 9614–9635 (2017). [DOI] [PubMed] [Google Scholar]
- 15.Dai X., Hong G., Gao T., Lieber C. M., Mesh nanoelectronics: Seamless integration of electronics with tissues. Acc. Chem. Res. 51, 309–318 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dai X., Zhou W., Gao T., Liu J., Lieber C. M., Three-dimensional mapping and regulation of action potential propagation in nanoelectronics-innervated tissues. Nat. Nanotechnol. 11, 776–782 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalmykov A.et al., Organ-on-e-chip: Three-dimensional self-rolled biosensor array for electrical interrogations of humans electrogenic spheroids. Sci. Adv. 5, eaax0729 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Q.et al., Cyborg organoids: Implantation of nanoelectronics via organogenesis for tissue-wide electrophysiology. Nano Lett. 19, 5781–5789 (2019). [DOI] [PubMed] [Google Scholar]
- 19.Fu T.-M.et al., Stable long-term chronic brain mapping at the single-neuron level. Nat. Methods 13, 875–882 (2016). [DOI] [PubMed] [Google Scholar]
- 20.Hong G.et al., A method for single-neuron chronic recording from the retina in awake mice. Science 360, 1447–1451 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harun W. S. W.et al., A comprehensive review of hydroxyapatite-based coatings adhesion on metallic biomaterials. Ceram. Int. 44, 1250–1268 (2018). [Google Scholar]
- 22.Miguez-Pacheco V., Hench L. L., Boccaccini A. R., Bioactive glasses beyond bone and teeth: Emerging applications in contact with soft tissues. Acta Biomater. 13, 1–15 (2015). [DOI] [PubMed] [Google Scholar]
- 23.Salinas A. J., Vallet-Regí M., Bioactive ceramics: From bone grafts to tissue engineering. RSC Adv. 3, 11116–11131 (2013). [Google Scholar]
- 24.Khademhosseini A., Langer R., A decade of progress in tissue engineering. Nat. Protoc. 11, 1775–1781 (2016). [DOI] [PubMed] [Google Scholar]
- 25.Mitropoulos A. N.et al., Transparent, nanostructured silk fibroin hydrogels with tunable mechanical properties. ACS Biomater. Sci. Eng. 1, 964–970 (2015). [DOI] [PubMed] [Google Scholar]
- 26.Zhu Z.et al., High-strength, durable all-silk fibroin hydrogels with versatile processability toward multifunctional applications. Adv. Funct. Mater. 28, 1704757 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kapoor S., Kundu S. C., Silk protein-based hydrogels: Promising advanced materials for biomedical applications. Acta Biomater. 31, 17–32 (2016). [DOI] [PubMed] [Google Scholar]
- 28.Brenckle M. A.et al., Modulated degradation of transient electronic devices through multilayer silk fibroin pockets. ACS Appl. Mater. Interfaces 7, 19870–19875 (2015). [DOI] [PubMed] [Google Scholar]
- 29.Murphy A. R., Kaplan D. L., Biomedical applications of chemically-modified silk fibroin. J. Mater. Chem. 19, 6443–6450 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abbott R. D., Kimmerling E. P., Cairns D. M., Kaplan D. L., Silk as a biomaterial to support long-term three-dimensional tissue cultures. ACS Appl. Mater. Interfaces 8, 21861–21868 (2016). [DOI] [PubMed] [Google Scholar]
- 31.Wang S.et al., In vitro 3D corneal tissue model with epithelium, stroma, and innervation. Biomaterials 112, 1–9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kurland N. E., Dey T., Kundu S. C., Yadavalli V. K., Precise patterning of silk microstructures using photolithography. Adv. Mater. 25, 6207–6212 (2013). [DOI] [PubMed] [Google Scholar]
- 33.Pal R. K.et al., Fabrication of precise shape-defined particles of silk proteins using photolithography. Eur. Polym. J. 85, 421–430 (2016). [Google Scholar]
- 34.Cairns D. M.et al., Expandable and rapidly differentiating human induced neural stem cell lines for multiple tissue engineering applications. Stem Cell Reports 7, 557–570 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rockwood D. N.et al., Materials fabrication from Bombyx mori silk fibroin. Nat. Protoc. 6, 1612–1631 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim S.et al., All-water-based electron-beam lithography using silk as a resist. Nat. Nanotechnol. 9, 306–310 (2014). [DOI] [PubMed] [Google Scholar]
- 37.Mironi-Harpaz I., Wang D. Y., Venkatraman S., Seliktar D., Photopolymerization of cell-encapsulating hydrogels: Crosslinking efficiency versus cytotoxicity. Acta Biomater. 8, 1838–1848 (2012). [DOI] [PubMed] [Google Scholar]
- 38.Williams C. G., Malik A. N., Kim T. K., Manson P. N., Elisseeff J. H., Variable cytocompatibility of six cell lines with photoinitiators used for polymerizing hydrogels and cell encapsulation. Biomaterials 26, 1211–1218 (2005). [DOI] [PubMed] [Google Scholar]
- 39.Xie C.et al., Three-dimensional macroporous nanoelectronic networks as minimally invasive brain probes. Nat. Mater. 14, 1286–1292 (2015). [DOI] [PubMed] [Google Scholar]
- 40.Claycomb W. C.et al., HL-1 cells: A cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc. Natl. Acad. Sci. U.S.A. 95, 2979–2984 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vernekar V. N.et al., SU-8 2000 rendered cytocompatible for neuronal bioMEMS applications. J. Biomed. Mater. Res. A 89, 138–151 (2009). [DOI] [PubMed] [Google Scholar]
- 42.Zhang Y., et al. , “Application of SU-8 as the insulator toward a novel planar microelectrode array for extracellular neural recording,” in 2010 IEEE 5th International Conference on Nano/Micro Engineered and Molecular Systems (Institute of Electrical and Electronics Engineers, Piscataway, NJ, 2010), pp. 395-398.
- 43.Gage F. H., Temple S., Neural stem cells: Generating and regenerating the brain. Neuron 80, 588–601 (2013). [DOI] [PubMed] [Google Scholar]
- 44.Moy E., Lin F. Y. L. H., Vogtle J. W., Policova Z., Neumann A. W., Contact angle studies of the surface properties of covalently bonded poly-L-lysine to surfaces treated by glow-discharge. Colloid Polym. Sci. 272, 1245–1251 (1994). [Google Scholar]
- 45.Valamehr B.et al., Hydrophobic surfaces for enhanced differentiation of embryonic stem cell-derived embryoid bodies. Proc. Natl. Acad. Sci. U.S.A. 105, 14459–14464 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim Y. H., Baek N. S., Han Y. H., Chung M.-A., Jung S.-D., Enhancement of neuronal cell adhesion by covalent binding of poly-D-lysine. J. Neurosci. Methods 202, 38–44 (2011). [DOI] [PubMed] [Google Scholar]
- 47.Lammel A. S., Hu X., Park S.-H., Kaplan D. L., Scheibel T. R., Controlling silk fibroin particle features for drug delivery. Biomaterials 31, 4583–4591 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blau A., Cell adhesion promotion strategies for signal transduction enhancement in microelectrode array in vitro electrophysiology: An introductory overview and critical discussion. Curr. Opin. Colloid Interface Sci. 18, 481–492 (2013). [Google Scholar]
- 49.Robin C. J., Vishnoi A., Jonnalagadda K. N., Mechanical behavior and anisotropy of spin-coated SU-8 thin films for MEMS. J. Microelectromech. Syst. 23, 168–180 (2014). [Google Scholar]
- 50.Jiang C.et al., Mechanical properties of robust ultrathin silk fibroin films. Adv. Funct. Mater. 17, 2229–2237 (2007). [Google Scholar]
- 51.Feng R., Farris R. J., Influence of processing conditions on the thermal and mechanical properties of SU8 negative photoresist coatings. J. Micromech. Microeng. 13, 80–88 (2003). [Google Scholar]
- 52.Min K., Umar M., Ryu S., Lee S., Kim S., Silk protein as a new optically transparent adhesion layer for an ultra-smooth sub-10 nm gold layer. Nanotechnology 28, 115201 (2017). [DOI] [PubMed] [Google Scholar]
- 53.Chen J., Venkatesan H., Hu J., Chemically modified silk proteins. Adv. Eng. Mater. 20, 1700961 (2018). [Google Scholar]
- 54.Kim S. H.et al., Precisely printable and biocompatible silk fibroin bioink for digital light processing 3D printing. Nat. Commun. 9, 1620 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mohammed M. G., Kramer R., All-printed flexible and stretchable electronics. Adv. Mater. 29, 1604965 (2017). [DOI] [PubMed] [Google Scholar]
- 56.Grob L.et al., Printed 3D electrode arrays with micrometer‐scale lateral resolution for extracellular recording of action potentials. Adv. Mater. Technol. 5, 1900517 (2019). [Google Scholar]
- 57.Ju J., Hu N., Cairns D. M., Liu H., Timko B. P., “Photo-crosslinkable, insulating silk fibroin for bioelectronics with enhanced cell affinity.” Harvard/Tufts University Dataverse. 10.7910/DVN/SXSI8O. Deposited 11 May 2020. [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw data associated with Figs. 1–5 are available in the Harvard/Tufts University Dataverse at https://dataverse.harvard.edu/dataset.xhtml?persistentId=doi:10.7910/DVN/SXSI8O (57).
Additional methods relating to silk fibroin refinement and characterization, chip assembly, and PSF stability, as well as SI Appendix, Figs. S1–S12, can be found in SI Appendix.