Abstract
Background :
Raltegravir (RTG) and dolutegravir (DTG) have different pharmacokinetic patterns in the gastrointestinal tract. To determine if this results in pharmacodynamic differences, we compared HIV RNA, HIV DNA, and immunological markers in gut-associated lymphoid tissue (GALT) of HIV-infected participants receiving RTG or DTG with tenofovir+emtricitabine (TDF/FTC).
Methods:
GALT specimens from the terminal ileum, splenic flexure, and rectum were obtained by colonoscopy at a single time point in 20 adults treated with RTG (n=10) or DTG (n=10) with HIV RNA <50 copies/mL. Flow cytometry, drug concentrations, and HIV RNA and DNA were analyzed in tissue. CD4/8+ T cells were tested for γδ TCR, and markers of T cell activation and exhaustion. Data are reported as median (Q1,Q3).
Results:
15 men and 5 women were enrolled. There was no difference in time since HIV diagnosis for those on RTG [9.5 (4–22) yr] and DTG [17 (1–24) yr] (p = 0.6), although time on RTG [5.4 (2.3–6.7) yr] was greater than DTG [1.0 (0.1–1.5) yr] (P < 0.001). Concentrations of RTG and DTG in rectal tissue (RT) were similar to previous reports: median tissue:plasma ratio was 11.25 for RTG and 0.44 for DTG. RNA:DNA ratios were [1.14 (0.18–5.10)] for the RTG group and [0.90 (0.30–18.87)] for the DTG group (p = 0.95). No differences (p ≥ 0.1) between CD4+ and CD8+ T cell markers were found.
Conclusions:
RTG produced higher tissue exposures than DTG, but no significant differences in GALT HIV RNA, DNA, or most immunologic markers were observed.
Introduction
Since the first approval of AZT in 1987, significant advances have been made in the management of chronic HIV infection.[1] The current standard of care is for patients to be treated with combination antiretroviral therapy (cART) that includes at least 3 drugs.[2] Integrase strand transfer inhibitors (INSTIs), which include raltegravir (RAL) and dolutegravir (DTG), are first line options.[2]
Despite adequate suppression of HIV replication in the blood, HIV replication persists in tissue reservoirs, such as gut-associated lymphoid tissue (GALT).[3] This persistent replication results in persistent inflammation which may significantly contribute to morbidity and mortality in HIV infected persons, even after CD4 T cell reconstitution.[4,5] We previously described differing GALT pharmacokinetic distribution of two INSTIs: RTG exposure is >100-fold higher in tissue compared to blood plasma (BP) while DTG exposure is >5-fold lower in tissue compared to BP.[6,7] It’s unknown if this difference affects local virologic replication or immune activation. The primary objectives of this study were to compare HIV RNA, HIV DNA, and immunological markers in the GALT of HIV-infected participants receiving RTG or DTG, with a back bone of tenofovir disoproxyl fumarate (TDF) + emtricitabine (FTC).
Methods
Study design and participant selection
A Phase IV, open label study was conducted in 20 HIV-infected volunteers who were on cART containing TDF plus FTC, with either RTG or DTG. The study was conducted at the University of North Carolina at Chapel Hill (UNC), was approved by the UNC Biomedical Institutional Review Board, and registered with ClinicalTrials.gov (NCT02218320). All visits were conducted at the UNC Clinical Translational Research Center (CTRC). Participants were enrolled from December 2014 to October 2015 and provided written informed consent prior to study procedures. Participants were eligible to participate if they were aged 18–65 years (inclusive on the date of screening) and had documentation of at least one positive HIV test. Participants must have been receiving an antiretroviral regimen containing TDF (300mg once daily) and FTC (200mg once daily) with RAL (400mg twice daily) or DTG (50mg once daily) for at least 3 months prior to enrollment. Participants must have self-reported at least 80% adherence to cART, with no missed doses in the 3 days prior to the inpatient visit. Participants were required to have a blood plasma HIV RNA of <50 copies/mL for at least 4 weeks prior to enrollment, assessed at screening and on the day of biopsy/sample collection.
Women of childbearing potential were required to use at least one acceptable form of birth control. All participants agreed to refrain from insertion of any device or product into the rectum for 72 hours prior to the inpatient visit and through 7 days after the colonoscopy. Participants were excluded for any history of inflammatory bowel disease, significant procedures altering the GI tract, or clinically significant abnormal laboratory test, physical finding, or clinical condition that would interfere with study procedures.
Screening procedures consisted of a complete medical history and physical examination, 12-lead electrocardiogram (ECG), and comprehensive laboratory studies (complete blood count with differential, serum HIV RNA viral load, immunologic markers (i.e. CD4), liver function tests, serum chemistries, urinalysis, and urine toxicology). Participants were screened for sexually transmitted infections including gonorrhea, chlamydia, and syphilis. Study participation consisted of the screening period of 0–42 days, a 2-day inpatient visit including a colonoscopy with tissue sampling, and a follow-up period of 1–14 days.
Study visits
Participants were admitted to the CTRC inpatient unit 18–24 hours prior to the colonoscopy. Participants ate a low-fiber diet for the 7 days preceding the procedure. On the morning of the first inpatient day, participants began a diet consisting of only clear liquids. Once admitted, participants underwent an observed full bowel preparation consisting of bisacodyl 10mg and 4 L polyethylene glycol and electrolytes oral solution (GoLYTELY) consumed in two 2 hour periods 18 and 6 hours prior to the colonoscopy, respectively. On the morning of the colonoscopy, participants took an observed antiretroviral dose. Two hours after the dose, a blood sample was collected for drug concentration analysis and HIV RNA. Participants then underwent the colonoscopy procedure with tissue sampling performed by a board-certified gastroenterologist 2–6 hours after their dose of RAL or DTG. All participants were observed for at least 6 hours post-colonoscopy. Participants returned for a follow-up safety visit 1–14 days after the colonoscopy.
Sample Collection and Analysis
Blood plasma collection and processing.
Whole blood was obtained using EDTA collection tubes (Becton, Dickinson and Company, Franklin Lakes, NJ) and centrifuged at 2680 rpm at 4°C for 10 minutes. Aliquots of the resulting blood plasma were distributed into labeled cryovials and stored at −80°C until analysis.
Gastrointestinal tissue collection and processing.
Tissue samples were obtained during colonoscopy performed at the UNC Hospitals or the UNC Hospitals Endoscopy Center according to standard Gastroenterology procedures. Monitored conscious sedation was provided for all participants. A total of 18 tissue biopsies were taken: 6 from each of the 3 sampling sites (the terminal ileum/ascending colon (TI), the splenic flexure (SF), and the rectum/sigmoid colon (RT)) using a Radial Jaw 4 Large Capacity Forceps with needle (Boston Scientific Corporation, Natick, MA). Samples were placed into separate tubes for flow cytometry (TI and SF tissues), drug concentration analysis (RT), and HIV RNA/DNA quantification (TI+SF+RT combined). Biopsy specimens were distributed either into labeled cryovials and immediately snap frozen in liquid nitrogen (HIV RNA/DNA and drug concentrations) and stored at −80°C or screw-capped polypropylene tubes and placed on ice until analysis (flow cytometry).
Blood Plasma Concentration Analysis.
RTG concentrations were determined in plasma samples by a protein precipitation extraction followed by LC-MS/MS analysis. Briefly, 50μL of plasma was mixed with 50μL of methanol:water containing the isotopically-labeled internal standard RTG-d3 (Toronto Research Chemicals, Toronto, Ontario, Canada) and 250μL of methanol. Following vortex and centrifugation steps, 50μL of the supernatant was mixed with 450μL of water prior to LC-MS/MS analysis. Using a Shimadzu high performance liquid chromatography system (Shimadzu, Columbia, MD) LC separation was achieved using a Phenomenex Synergi Polar-RP (50×2mm, 2.5μm particle size) analytical column (Phenomenex, Torrance, CA) under gradient conditions with water with 0.1% formic acid and acetonitrile with 0.1% formic acid as mobile phases. RTG and RTG-d3 were detected on an AB Sciex API-5000 triple quadrupole mass spectrometer (AB Sciex, Foster City, CA) operated in positive ion electrospray mode. Data were collected using AB Sciex Analyst Chromatography Software. The dynamic range of this assay was 5–5000 ng/mL.
DTG concentrations were determined in plasma samples by a protein precipitation extraction followed by LC-MS/MS analysis. Briefly, 30μL of plasma was mixed with 120μL of methanol containing the isotopically-labeled internal standard DTG-13C, d5 (Alsachim, Illkirch Graffenstaden, France). Following vortex and centrifugation steps, 40μL of the supernatant was mixed with 40μL of water prior to LC-MS/MS analysis. LC separation was achieved using a Waters Atlantis T3 (50×2.1mm, 3μm particle size) analytical column (Waters, Milford, MA) under gradient conditions with water with 0.1% formic acid and acetonitrile with 0.1% formic acid as mobile phases. DTG and DTG-13C, d5 were detected on an AB Sciex API-5000 triple quadrupole mass spectrometer operated in positive ion electrospray mode. The dynamic range of this assay was 1–5000 ng/mL. Calibrators and quality control samples were within 15% of the nominal value during both analyses.
Tissue Drug Concentration Analysis.
RT biopsies for RTG and DTG were homogenized in a solution of 70:30 acetonitrile:1mM ammonium phosphate (pH 7.4). The resulting homogenates were then extracted with methanol containing the isotopically-labeled internal standards RTG-d3 and DTG-13C, d5. Following vortex and centrifugation steps the extracts were analyzed by LC-MS/MS. LC separation was achieved similarly to plasma with 0.1% formic acid and methanol as mobile phases. All analytes and internal standards were detected on an AB Sciex API-5000 triple quadrupole mass spectrometer operated in positive ion electrospray mode. The dynamic range of this assay was 5–1000 ng/mL for RTG and 0.4–1000ng/mL for DTG. Calibrators and quality control samples were within 15% of the nominal value during this analysis.
Tissue Drug Distribution Analysis.
RTG and DTG visualization within RT biopsies was also characterized using infrared matrix-assisted laser desorption electrospray ionization (IR-MALDESI) mass spectrometry imaging (MSI) as previously described.[8–10] Briefly, biopsied tissues were grouped by dosing cohort and embedded within a 2:1 gelatin/carboxymethylcellulose gel block to provide support during sectioning.[11] Blocks were snap frozen and stored at −80°C. Embedded tissues were sectioned for IR-MALDESI analysis at 10μm thickness using a cryostat (Leica Biosystems, Wetzlar, Germany) and thaw mounted onto a glass microscope slide, with adjacent sections of each sample collected for parallel analysis by immunohistochemistry (IHC) and LC-MS.
Mounted tissues were placed inside the humidity-controlled source chamber and completely ablated with an infrared laser at 100um spot-to-spot distance. Desorbed sample material then intersected an orthogonal electrospray, and the resulting ions (associated with both endogenous compounds and ARVs) were sampled into a Q Exactive Plus mass spectrometer (Thermo Fisher Scientific, Bremen, Germany) operated in positive ion mode. Raw mass spectrometry data was converted to the mzXML format with MSConvert (ProteoWizard), then to the imzML format for interrogation using MSiReader, which allows for generation of images of ion distributions across the tissue slice. The spatial distribution of Heme B, a blood biomarker, was used to identify areas of the tissue samples where residual blood contamination may be present. Regions of intense Heme response were evaluated based on image thresholding using Otsu’s method.[12] All response to ARVs measured in these regions of high Heme b intensity was removed in order to isolate the presence of ARVs in tissue.
Absolute quantitation of ARV concentration was achieved by spotting a series of calibration standards of known ARV concentration onto non-dosed “blank” non-human primate RT slices from identical tissue matrices (rectum; Bioreclamation IVT, Baltimore, MD) as described previously.
Immunofluorescence (IF) for CD3 was performed on frozen sections in the Leica Bond fully-automated slide staining system (Leica Microsystems) using Bond Polymer Refine Detection kit (DS9800). Slides were allowed to sit at room temperature for 15 minutes, then fixed in 10% NBF for 15 minutes. They were then placed in Bond wash solution (AR9590). Antigen retrieval was performed at 100°C in Bond-epitope retrieval solution 2 pH9.0 (AR9640) for 10 minutes. Staining was performed first using CD4 1F6 antibody (Abcam clone BC/ 1F6) at 1:50 dilution for 1h with Bond Polymer and Post-Primary reagents and Cy5 fluorochrome (Perkin Elmer) for 15 minutes. Antigen retrieval was done at again 100°C in Bond-epitope retrieval solution 2 pH9.0 (AR9640) between protocols. Slides were then stained with CD3 (Leica clone LN10) Ready-to-Use antibody for 15 minutes and Dako Envision mouse secondary for 30 minutes. Cy3 fluorochrome (Perkin Elmer) was applied for 15 minutes. IF slides were counterstained with Hoechst 33258 (Invitrogen, Carlsbad, CA) and mounted with ProLong Gold antifade reagent (P36934, Life Technologies).
Image analysis was performed using Matlab. MSI and IF imaging data from adjacent tissue sections were co-registered by manual selection of feature control points, and similarity in spatial distributions was evaluated based on Pearson’s correlation coefficients.
RNA and DNA Extraction of Intestinal Tissue.
Previously frozen biopsy punch pairs from the three sites (TI, SF, RT) were combined for extraction using the ToTALLY RNA Total RNA Isolation Kit (Ambion®, Thermo Fisher Scientific, Waltham, MA). Tissues were homogenized in 200μL of Denaturation Solution plus 200μL Disruption Beads for Tissues (Research Products International, Mt. Prospect, IL). A Mini BeadBeater-8 (BioSpec Products, Bartlesville, OK) was used 1 minute at a time for 3 total vortexes. The homogenates were spun for 3 min at 13,000g in a microcentrifuge, and the liquid was transferred to a new tube. The rest of the extraction was done according to the manufacturer’s instructions except that the organic phases of each extraction were saved for DNA recovery. The organic phases were combined and DNA was extracted using an Ambion® protocol.[13] The RNA was resuspended in 15 μL RNase-free water; the DNA was resuspended in 18 μL 1X TE.
Droplet Digital PCR Analysis.
Primers and probes for detection of HIV were from the single copy HIV assay of Palmer et al.[14] RNA was reverse transcribed using the iScript cDNA Synthesis Kit (Bio-Rad Laboratories, Hercules, CA) in a reaction volume of 20 μL, using all 15 μL of RNA. Droplet Digital PCR was done using the ddPCR Supermix for Probes (Bio-Rad, Hercules, CA). For cDNA, 9 μL of cDNA was added to the Supermix in duplicate reactions containing HIV primers and probes. For DNA, 15 μL of DNA was added to the Supermix in single reactions with the HIV primers and probe. For cell number quantitation, the PrimePCR ddPCR Expression Probe Assay for Human CCR5 (Bio-Rad, Hercules, CA) was used with 15μL of 1:100 dilution of DNA. If applicable, CCR5 DNA was repeated at either 1:10 or 1:1000 dilution. CCR5 DNA was used to normalize the RNA and DNA to copies/1,000 cells. Droplets were generated and detected using the QX100 Droplet Digital System (Bio-Rad, Hercules, CA). Lower limit of quantification (LLQ) for RNA and DNA was 1 copy/biopsy of tissue. Positive droplet counts were adjusted for dilution for total number of copies per set of biopsies processed together.
GALT Tissue Processing.
Two fresh biopsies (2×2mm) each from the SF and TI were placed into sterile petri dishes with 5 mL digestion media consisting of RPMI 1640 with collagenase II (0.5mg/mL) (Sigma, St. Louis, MO) and 40 U/mL DNAse (Roche Diagnostics Incorporation, Indianapolis, IN). Biopsies were dissected into smaller fragments with scalpels, transferred to a sterile tube, and incubated in a shaking water bath for 30 minutes at 37°C. After the incubation, the digested samples were filtered through a 100 μm sieve into a 50 mL centrifuge tube that was filled with PBS to stop the enzymatic activity. The samples were centrifuged at 1800 rpm (800g) for 10 min. The cell pellet was resuspended in RPMI1640 and centrifuged a second time. Cells were resuspended in PBS and counted. In case <0.7×106 cells were obtained/ biopsy site, cells from the TI and SF were combined (n=11); otherwise biopsy samples from different sites were processed separately.
Flow Cytometric Analysis.
Cells were stained by standard protocols as described previously.[15,16] The following antibodies were used to define T cell subsets (CD3, CD4, CD8, γδTCR), T cell activation (CD38, HLA-DR, CCR5, CD69, PD-1), and T cell differentiation into naïve and memory T cells, including tissue resident memory cells, (CCR7, CD45RA, CD69). The analysis of γδ T cells was included because γδ T cells are frequently found at mucosal surfaces, are rapidly depleted in HIV infection, and have also been demonstrated to serve as a viral reservoir for HIV.[17,18] All antibodies were obtained from BD Biosciences (San Jose, CA), except for PD-1 that was obtained from eBioscience (San Diego, CA). A live/dead cell discriminator dye (Qdot 585; Invitrogen, ThermoFisher Scientific; Waltham, MA) was included in all samples. Samples were acquired on a LSRII instrument (Beckton Dickinson, San Jose, CA) recording a minimum of 300,000 events. Samples were analyzed with FlowJo Software (Treestar, Ashland, OR) applying Boolean gating strategies.
Statistical analysis
Sample size was determined based on a difference in the mean tissue HIV RNA. Using a Wilcoxon rank-sum test, a sample size of n=10 per group was calculated to provide 80% power to detect a 1.0 log10 difference (two-sided; tested at α = 0.05). All other analyses were considered exploratory. Comparisons of RTG and DTG data were made using Wilcoxon rank-sum tests with exact p-values, except for sex and race, where a Fisher’s exact test was utilized. For the flow cytometry analysis, only 9 of the 20 patients had enough tissue from both TI and SF sites to compare these individually. Therefore, a Wilcoxon signed-rank test with an exact p-value was used on the data from these 9 to determine whether a statistical difference was seen between the two tissue sites. Since no consistent difference was noted (data not shown), the average of the two sites was used for a pooled analysis. Data are presented as [median (25th-75th percentile)] unless otherwise noted. To calculate tissue penetration ratios, tissue density was assumed to be 1.04 g/mL.[19] No adjustment was made for multiple hypothesis testing; all testing was two-sided with α = 0.05. Analyses were performed using SAS software, version 9.4 (SAS, Inc., Cary, NC) and the R statistical programming language version 3.3.0.
Results
Participant demographics
Summary participant demographics can be found in Table 1. All summary statistics presented in this section are median (minimum-maximum). Twenty-three participants were screened with 20 enrolling and completing the protocol. The median age of enrolled participants completing the study was 53 years. The participants taking RTG were statistically significantly older, 55 (48–64) years compared to those taking DTG 50 (32–56) years (p = 0.03). Time since HIV diagnosis did not differ significantly (p = 0.62) between RTG (9.5 (4.0–22.0) years) and DTG (17.0 (1.0–24.0) years) groups. Patients in the RTG group were on RTG-based for a significantly longer time (5.4 (2.3–6.7) years)) compared to time on DTG-based therapy (1.0 (0.1–1.5) years) prior to enrollment (p < 0.001). However, total time on ART was similar between the two groups at 9.2 (5–18) years in the RTG group versus 10.8 (1–22) years in the DTG group.
Table 1:
Participant Demographics
| Raltegravir (n=10) | Dolutegravir (n=10) | P-value | ||
|---|---|---|---|---|
| Age (years) | 55 (48–64) | 50 (32–56) | 0.03 | |
| Male | 6 (60%) | 9 (90%) | 0.30 | |
| Race | ||||
| Caucasian | 3 | 3 | ||
| BMI | 30.7 (18.7–41.4) | 28.9 (21.0–35.6) | 0.67 | |
| Time Since HIV Diagnosis (years) | 9.5 (4.0–22.0) | 17.0 (1.0–24.0) | 0.62 | |
| Time on study drug (years) | 5.4 (2.3–6.7) | 1.0 (0.1–1.5) | < 0.001 | |
| Current CD4+ | 812 (594–956) | 620 (223–1300) | 0.32 | |
| CD4+ nadir (cells/mm3) | 356 (9.–476) | 74 (2–458) | 0.18 | |
| Peak HIV RNA (copies/mL) | 30580 (137–298700) | 47427 (463–650200) | 0.91 | |
Data are reported as median (min-max). BMI = body mass index.
During the study, there were no serious adverse events and no individuals discontinued the study as the result of an adverse event. No ARV-related adverse events occurred. Adverse events potentially related to the bowel preparation and/or colonoscopy included mild diarrhea (n=1), abdominal cramping and constipation (n=2), external anal irritation (n=1), and moderately elevated blood pressure (n=1). All were classified as Grade 1 or 2 and resolved within the week following the study sampling.
Pharmacokinetics
Participants were scheduled to have one BP sample taken 2-hours after their morning dose of study medication. Two participants in each group had an out of window BP sample taken earlier at 0.5–1 hours post-dose. RTG BP concentrations were 434 (271–2,720) ng/mL, and DTG BP concentrations were 1,845 (1,198–3,170) ng/mL, consistent with previous literature values. Tissue biopsy samples were taken 3.8 (2.18–5.41) h post-dose. Using LC-MS/MS analysis, RT concentrations of RTG were 5,308 (3,938–19,600) ng/g, and of DTG were 810 (510–1,408) ng/g. Tissue:plasma concentration ratios were 11.25 (7.71–25.54) for RTG and 0.44 (0.29–0.65) for DTG. These concentrations and ratios were also consistent with previous published data.
Drug distribution within the tissues was visualized by infrared matrix-assisted laser desorption electrospray ionization (IR-MALDESI) mass spectrometry imaging (MSI). Figure 1 contains representative images of the distribution of both RTG and DTG in RT. MSI also noted greater penetration of RTG into tissue. The fraction of total tissue indicating measurable drug exposure was 57% (42–62%) for RTG and 51% (35–63%) for DTG. Intra-tissue concentration gradients were 5–57 fold for RTG and 1.4–24 fold for DTG, indicating greater variability in tissue distribution for RTG than DTG, although relative standard deviation was similar (RTG, 57% (45–62%)). To evaluate the relationship between drug exposure in the locations of CD3+ cells, Pearson correlation coefficients were 0.09 (.05–0.16) for the RTG samples and 0.23 (0.17–0.31) for the DTG samples where 13% (11–23%) and 11% (5–24%), respectively, of all CD3+ cells colocalized with measurable exposure to drug.
Figure 1.
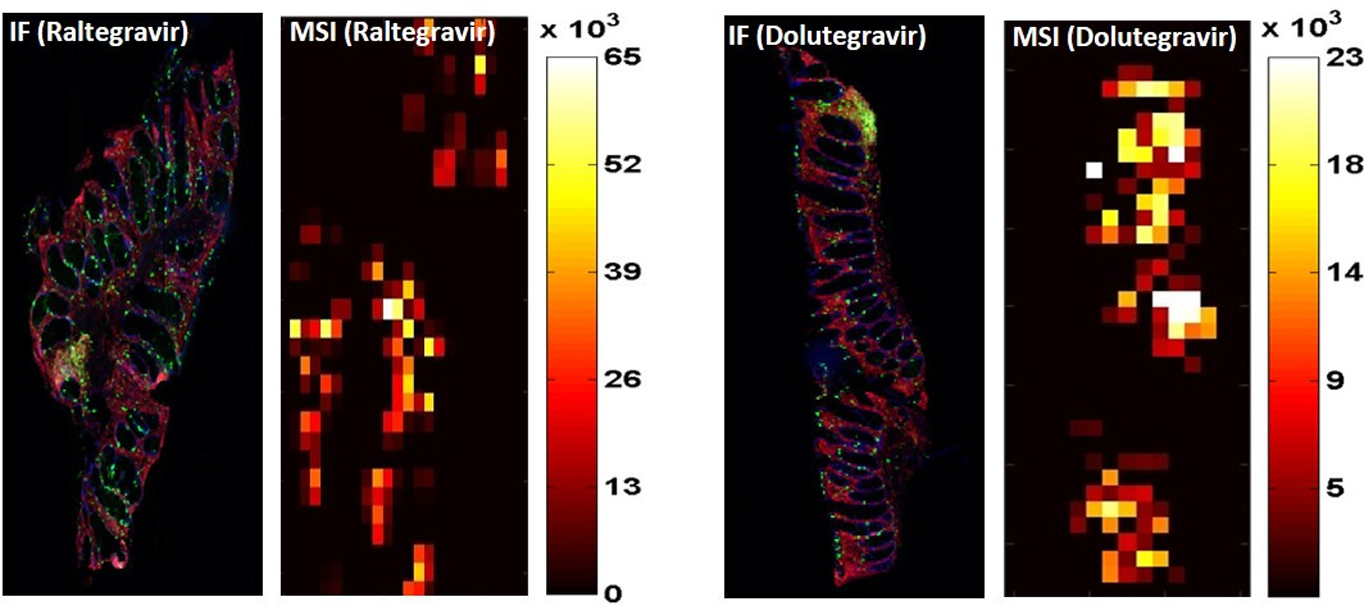
Immunofluorescence and IR-MALDESI MSI images for RTG and DTG tissue distribution. Serially sliced samples were used to obtain samples for immunofluorescence (IF) staining for nuclei (blue) and CD3+ cells (green) and for drug concentration intensity (scaled from dark red to white). Paired images for a representative raltegravir (RTG) tissue biopsy is on the left, and for a dolutegravir (DTG) tissue biopsy is on the right. A scale of mass spectrometry imaging (MSI) signal intensity, representing localized tissue concentration of RTG or DTG, is adjacent to each MSI image. IR-MALDESI, infrared matrix-assisted laser desorption electrospray ionization.
Pharmacodynamics
RNA and DNA.
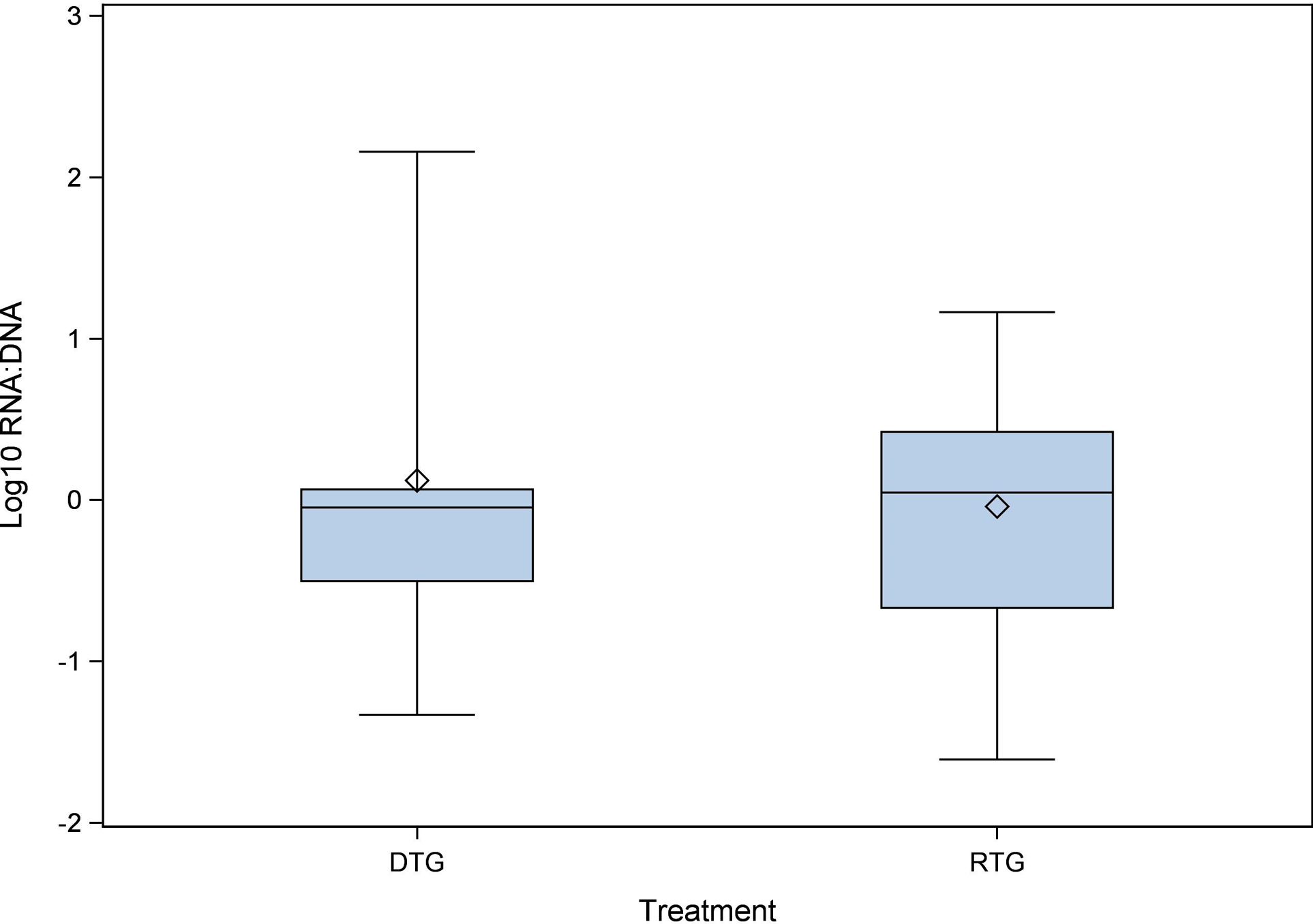
Since the yield of CD4+ T cells varied between sites and study participants, concentrations of HIV RNA copies/106 cells were normalized to CD4+ T cell content using the results from flow cytometry. For the RTG group, ≤ 20% had undetectable HIV RNA and DNA, compared to 40% in the DTG group. No statistical difference was detected between RTG and DTG groups in the amount of RNA or DNA found in the tissues. RNA concentrations for the RTG group were 0.16 (0.01–36.19) copies/1,000 cells, and for the DTG group were 0.05 (0.003–0.34) copies/1,000 cells (p = 0.48). DNA concentrations for the RTG group were 0.13 (0.04–11.39) copies/1,000 cells, and for the DTG group were 0.05 (0.01–0.17) copies/1,000 cells (p = 0.14). There was no difference detected in the RNA:DNA ratio between the RTG group [1.14 (0.18–5.10)] and the DTG group [0.90 (0.30–18.87)] (p = 0.95; Figure 3). Notably, the self-reported adherence to ART within 3 and 7 days prior to biopsy/sample collection was 100% in both groups. In the 30 days prior to the biopsy/sample collection one participant in the RTG group reported missing 2 doses and three participants in the DTG group reported missing 1 or 2 doses.
Figure 3.
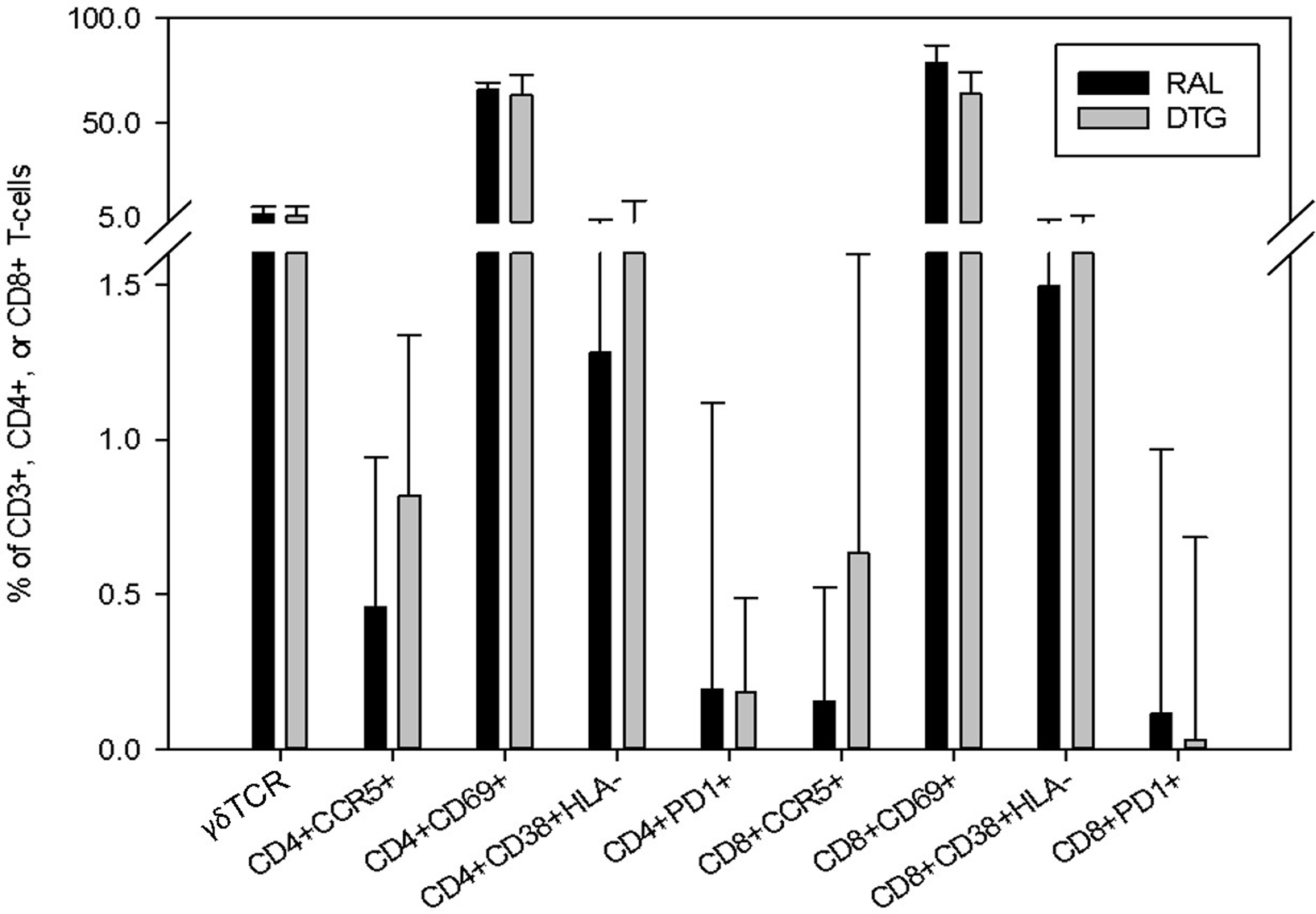
CD3+ , CD4+ and CD8+ T-cell markers. Cells from terminal ileum/ascending colon (TI) and splenic flexure (SF) tissue biopsies were combined and analysed for T-cell marker activation. To quantify T-cell activation and exhaustion, the following proteins and receptors were measured on CD4+ and CD8+ T-cells: CCR5+, CD69+, PD1+ and CD38+HLA-DR+. All CD3+ T-cells were also analysed for the presence of the [3] TCR. Data are presented as a proportion of activation marker expression by cell type. DTG, dolutegravir; RTG, raltegravir.
T cell activation.
To quantify T cell activation and exhaustion, the following proteins and receptors were measured on CD4+ and CD8+ T cells: CCR5+, CD69+, PD1+, and CD38+HLA-DR+ (Figure 2). All CD3+ T cells were also analyzed for the presence of the γδ TCR cell surface receptor. Patients had tissue samples taken from both TI and SF for T-cell marker quantification. No differences between CD4+ and CD8+ T cells were found in the percentage of CD3+ cells [43% vs 31% and 46% vs 52% (P ≥ 0.1)] or in the absolute quantity of CD4+ or CD8+ cells [1.51 ×105 vs 1.31 ×105 and 1.57×105 vs 2.19×105 (p ≥ 0.3)]. There was also no difference found between RTG (6.51%) and DTG (5.66%) (p = 1.0) in the expression of the γδ TCR cell surface receptor. CCR5 expression on CD8+ T cells was significantly lower in participants treated with RTG [0.15 (0.07–0.52) %] vs DTG [0.64 (0.24–1.60) %] (p = 0.02). All other immunological markers showed no significant difference between groups (See Table 2 & Figure 2).
Figure 2.
Tissue RNA:DNA ratio. Terminal ileum/ascending colon, splenic flexure and rectum/sigmoid colon tissue biopsies were combined and analysed for HIV RNA and DNA quantification. Once quantified, the RNA:DNA ratio was calculated and is portrayed on a log10 scale. DTG, dolutegravir; RTG, raltegravir.
Table 2.
Immunology Marker Results as a Percentage of CD3+, CD4+, or CD8+ T-cells
| Cell Population | Raltegravir | Dolutegravir | P-value | |
|---|---|---|---|---|
| CD3 | 3.2 (2.4–5.8) x105 | 4.1 (1.7–5.2) x105 | 0.80 | |
| γδ TCR | 6.51% (3.44–10.32) (n=5) | 5.66% (3.70–10.20) (n=7) | 1.0 | |
| CD4 | 1.5 (0.9–3.3) x105 | 1.3 (0.3–1.7) x105 | 0.35 | |
| CCR5 | 0.46% (0.35–0.94) | 0.82% (0.10–1.34) | 0.54 | |
| CD69 | 65.88% (55.53–69.49) | 63.45% (45.30–73.13) | 0.87 | |
| CD38+HLA-DR+ | 1.28% (0.49–4.15) | 1.86% (0.94–13.22) | 0.24 | |
| PD1 | 0.20% (0.08–1.11) | 0.19% (0.05–0.49) | 0.35 | |
| CD8 | 1.6 (1.0–2.0) x105 | 2.2 (1.0–3.3) x105 | 0.39 | |
| CCR5 | 0.15% (0.07–0.52) | 0.64% (0.24–1.60) | 0.02* | |
| CD69 | 78.80% (64.21–87.28) | 64.20% (55.71–74.40) | 0.17 | |
| CD38+HLA-DR+ | 1.50% (0.65–4.21) | 2.73% (0.83–5.87) | 0.43 | |
| PD1 | 0.11% (0.03–0.97) | 0.03% (0.01–0.69) | 0.43 | |
Data are reported as median (25th, 75th percentile).
Discussion
RTG and DTG have potent virologic and immunologic treatment effects which are well maintained with prolonged therapy.[20,21] DTG has the additional utility of once daily dosing, although a new once-daily RTG formulation has recently been approved for use. It has been noted that these INSTIs have differing pharmacokinetic distribution patterns in GALT. At the end of the dosing interval, RTG has been shown to achieve tissue concentrations >1,000-fold above the protein-adjusted (PA) 90% inhibitory concentration (IC90) and DTG achieves tissue concentrations >2-fold above the PA-IC90.[6,7,22,23] Although the reasons for these contrasting tissue concentrations are unclear,[24] they may have implications for complete suppression of viral replication: it has been shown that sites of poor antiviral drug diffusion can favor residual replication, viral persistence, and a chronic state of inflammation, which potentiates the risk on non-AIDS morbidity and mortality.[25–29] GALT is a logical evaluation site of drug diffusion effect on immunological and virological markers since CD4+ T cells in the GALT of HIV-infected individuals may support the persistence of HIV replication and chronic inflammation.[3,26]
This study is the first to compare virologic and immunologic responses in GALT between RTG and DTG-treated participants using a backbone of TDF+FTC, and to visualize drug distribution in tissue biopsies. Consistent with previous studies, higher tissue concentrations were noted with RTG compared to DTG.[6,7] New information identified using IR-MALDESI MSI demonstrated that the heterogeneous distributions of RTG and DTG within biopsies was similar, and that the co-localization of these distributions with CD3+ cells was greater for DTG than RTG. Despite these pharmacologic differences, no significant differences in tissue HIV RNA, DNA, or most immunologic markers were observed from tissue homogenates, although it is important to note that these approaches preclude comparison of spatial distribution with that of RTG and DTG by IR-MALDESI MSI. These findings are consistent with previous GALT studies that showed no globally consistent significant reduction in local HIV RNA, DNA, or immunological markers when virologically suppressed infected individuals intensified their cART regimen with the addition of RTG.[30,31] Some antiretroviral drugs have been reported to alter the composition of the intestinal microbiota, and this could potentially result in altered local immune activation.[32] However, there are no conclusive data indicating that distinct drugs differently affect immune activation.[33,34] The only potential difference between the two groups was lower single positive CCR5+ expression on CD8+ cells in the RTG-treated participants, but the biological significance of this finding is questionable. CCR5+ expression on CD8+ T cells is typically seen in regions of low level viral replication due to the localized migration of both effector and memory T cells.[35] This migration is due to the β-chemokines RANTES and MIP- 1β which are produced by local inflammation in the presence of ongoing viral replication.[26,35] This inter-group difference could be an indirect marker of lower level HIV replication, although there were no statistically significant differences in HIV RNA between the groups.
There were some limitations to this investigation. We powered this feasibility investigation on a difference of 1 log10 in tissue HIV RNA between the groups and limited the other measures to exploratory endpoints. This precluded identifying more subtle differences in HIV RNA but was necessary due to the limitations in finding a large cohort on these regimens willing to undergo intestinal biopsies. No adjustment was made for multiple comparisons Controlling for time on study drug by stratification was not possible because time on study drug did not overlap between the groups (minimum time on study drug was 2.3 years for the RTG group, and maximum time on study drug was 1.5 years for the DTG group). However, a stratified Wilcoxon rank-sum test was used to compare CCR5+ expression by study drug group, stratified by dichotomized (median split) current CD4+ count. Since the stratified Wilcoxon test was statistically significant (p = 0.04), this suggests that the observed association between CCR5+ expression and study drug group may not be attributable to differences in time on study drug. Tissue samples from multiple GALT locations were combined for virologic and immunologic analyses, which limited site-specific findings, while pharmacologic assessments were based on colorectal drug concentrations only. This was based on our previous investigations of raltegravir concentrations throughout the gastrointestinal tract.[6] However, with no data on dolutegravir concentrations in the ileum or at the splenic flexure, a bias in this approach cannot be ruled out.
In conclusion, although the RTG participants exhibited greater tissue exposures than DTG, these increased concentrations did not have a consistent impact on GALT HIV RNA or other immunologic and virologic markers of interest.
Acknowledgements
This study was supported by an investigator-initiated study grant to UNC from Merck. Participants were seen in the Clinical Trial Research Center within the NC Translational and Clinical Sciences Institute (NC TraCS), which is supported through Grant Award Number UL1TR001111. This publication resulted in part from Core activities of the University of North Carolina at Chapel Hill Center for AIDS Research (CFAR), an NIH funded program (P30 AI50410). The authors would also like to acknowledge and thank the study volunteers and the UNC Gastrointestinal Procedure nurses for their efforts.
ADM Kashuba has previously served as a consultant for Merck and Viiv.
Footnotes
References
- 1.Cihlar T, Fordyce M. Current status and prospects of HIV treatment. Curr Opin Virol 2016; 18:50–56. doi: 10.1016/j.coviro.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. (Updated 28 January 2016 Accessed 12 May 2016) Available from http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf. [Google Scholar]
- 3.Chun T-W, Nickle DC, Justement JS, et al. Persistence of HIV in Gut-Associated Lymphoid Tissue despite Long-Term Antiretroviral Therapy. J Infect Dis 2008; 197:714–720. doi: 10.1086/527324. [DOI] [PubMed] [Google Scholar]
- 4.George MD, Asmuth DM. Mucosal immunity in HIV infection: what can be done to restore gastrointestinal-associated lymphoid tissue function? Curr Opin Infect Dis 2014; 27:275–281. doi: 10.1097/QCO.0000000000000059. [DOI] [PubMed] [Google Scholar]
- 5.Deeks SG, Kitchen CMR, Liu L, et al. Immune activation set point during early HIV infection predicts subsequent CD4+ T-cell changes independent of viral load. Blood 2004; 104:942–947. doi: 10.1182/blood-2003-09-3333. [DOI] [PubMed] [Google Scholar]
- 6.Patterson KB, Prince HA, Stevens T, et al. Differential Penetration of Raltegravir throughout Gastrointestinal Tissue: Implications for Eradication and Cure. AIDS 2013; 27:1413–1419. doi: 10.1097/QAD.0b013e32835f2b49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greener BN, Patterson KB, Prince HMA, et al. Dolutegravir pharmacokinetics in the genital tract and colorectum of HIV-negative men after single and multiple dosing. J Acquir Immune Defic Syndr 2013; 64:39–44. doi: 10.1097/QAI.0b013e31829ed7a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robichaud G, Barry JA, Garrard KP, Muddiman DC. Infrared matrix-assisted laser desorption electrospray ionization (IR-MALDESI) imaginf source coupled to a FT-ICR mass spectrometer. J Am Soc Mass Spectrom 2013; 24:92–100. doi: 10.1007/s13361-012-0505-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barry JA, Robichaud G, Bokhart MT, et al. Mapping antiretroviral drugs in tissue by IR-MALDESI MSI coupled to the Q Exactive and comparison with LC-MS/MS SRM Assay. J Am Soc Mass Spectrom 2014; 25:2038–2047. doi: 10.1007/s13361-014-0884-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bokhart MT, Rosen E, Thompson C, Sykes C, Kashuba ADM, Muddiman DC. Quantitative mass spectrometry imaging of emtricitabine in cervical tissue model using infrared matrix-assisted laser desorption electrospray ionization. Anal Bioanal Chem 2015; 407:2073–2084. doi: 10.1007/s00216-014-8220-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nelson KA, Daniels GJ, Fournie JW, Hemmer MJ. Optimization of Whole-Body Zebrafish Sectioning Methods for Mass Spectrometry Imaging. J Biomol Tech 2013; 24:119–127. doi: 10.7171/jbt.13-2403-002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Otsu N IEEE Trans Syst Man Cybern 1979; 9:62. doi: 10.1109/TSMC.1979.4310076. [DOI] [PubMed] [Google Scholar]
- 13.ThermoFisher Scientific. Simultaneous Extraction of RNA and DNA. (Accessed 12 May 2016) Available from http://www.thermofisher.com/us/en/home/references/ambion-tech-support/rna-isolation/tech-notes/simultaneous-extraction-of-rna-and-dna.html. [Google Scholar]
- 14.Palmer S, Wiegand AP, Maldarelli F, et al. New Real-Time Reverse Transcriptase-Initiated PCR Assay with Single-Copy Sensitivity for Human Immunodeficiency Virus Type 1 RNA in Plasma. J Clin Microbiol 2003; 41:4531–4536. doi: 10.1128/JCM.41.10.4531-4536.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jensen K, Nabi R, Van Rompay KKA, et al. Vaccine-Elicited Mucosal and Systemic Antibody Responses Are Associated with Reduced Simian Immunodeficiency Viremia in Infant Macaques. J Virol 2016; 90:7285–7302. doi: 10.1128/JVI.00481-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rahangdale L, De Paris K, Kashuba ADM, et al. Immunologic, Virologic, and Pharmacologic Characterization of the Female Upper Genital Tract in HIV-Infected Women. J Acquir Immune Defic Syndr 2015; 68:420–424. doi: 10.1097/QAI.0000000000000480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pauza CD, Poonia B, Li H, et al. γδ T Cells in HIV Disease: Past, Present, and Future. Front Immunol. 2015; 5:687. doi: 10.3389/fimmu.2014.00687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soriano-Sarabia N, Archin NM, Bateson R, et al. Peripheral Vγ9Vδ2 T Cells Are a Novel Reservoir of Latent HIV Infection. PLoS Pathog. 2015. October 16;11(10):e1005201. doi: 10.1371/journal.ppat.1005201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cristy M, Eckerman KF. Specific Absorbed Fractions of Energy at Various Ages from Internal Photon Sources. I Methods Oak Ridge National Laboratory Report ORNL/NUREG/TM-367. Oak Ridge, TN: Oak Ridge National Laboratory; 1987. [Google Scholar]
- 20.Rockstroh JK, DeJesus E, Lennox JL, et al. Durable Efficacy and Safety of Raltegravir Versus Efavirenz When Combined With Tenofovir/Emtricitabine in Treatment-Naive HIV-1–Infected Patients: Final 5-Year Results From STARTMRK. J Acquir Immune Defic Syndr 2013; 63:77–85. doi: 10.1097/QAI.0b013e31828ace69. [DOI] [PubMed] [Google Scholar]
- 21.Raffi F, Rachlis A, Stellbrink H-J, et al. Once-daily dolutegravir versus raltegravir in antiretroviral-naive adults with HIV-1 infection: 48 week results from the randomised, double-blind, non-inferiority SPRING-2 study. Lancet 2013; 381:735–743. doi: 10.1016/S0140-6736(12)61853-4. [DOI] [PubMed] [Google Scholar]
- 22.Song I, Chen S, Piscitelli S. Meta-analysis of Pharmacokinetic-Pharmacodynamic Relationship of Integrase Inhibitors 11th International Workshop on Clinical Pharmacology of HIV Therapy. 7–9 April 2010, Sorrento, Italy: Available from http://regist2.virology-education.com/11th/docs/30_Song.pdf. [Google Scholar]
- 23.Cottrell ML, Hadzic T, Kashuba ADM. Clinical Pharmacokinetic, Pharmacodynamic and Drug-Interaction Profile of the Integrase Inhibitor Dolutegravir. Clin Pharmacokinet 2013; 52:981–994. doi: 10.1007/s40262-013-0093-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thompson CG, Gay CL, Kashuba ADM. HIV Persistence in Gut-Associated Lymphoid Tissues: Pharmacological Challenges and Opportunities. AIDS Res Hum Retroviruses 2017; 33:513–523. doi: 10.1089/AID.2016.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bourry O, Mannioui A, Sellier P, et al. Effect of a short-term HAART on SIV load in macaque tissues is dependent on time of initiation and antiviral diffusion. Retrovirology 2010; 7:78. doi: 10.1186/1742-4690-7-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klatt NR, Chomont N, Douek DC, Deeks SG. Immune activation and HIV persistence: Implications for curative approaches to HIV infection. Immunol Rev 2013; 254:326–342. doi: 10.1111/imr.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boulware DR, Hullsiek KH, Puronen CE, et al. Higher Levels of CRP, D-dimer, IL-6, and Hyaluronic Acid Before Initiation of Antiretroviral Therapy (ART) Are Associated With Increased Risk of AIDS or Death. J Infect Dis 2011; 203:1637–1646. doi: 10.1093/infdis/jir134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hunt PW, Cao HL, Muzoora C, et al. Impact of CD8+ T Cell Activation on CD4+ T Cell Recovery and Mortality in HIV-infected Ugandans Initiating Antiretroviral Therapy. AIDS 2011; 25:2123–2131. doi: 10.1097/QAD.0b013e32834c4ac1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ledwaba L, Tavel JA, Khabo P, et al. Pre-ART Levels of Inflammation and Coagulation Markers Are Strong Predictors of Death in a South African Cohort with Advanced HIV Disease. PLOS ONE 2012; 7:e24243. doi: 10.1371/journal.pone.0024243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yukl SA, Shergill A, McQuaid K, et al. Effect of Raltegravir-containing Intensification on HIV Burden and T Cell Activation in Multiple Gut Sites of HIV+ Adults on Suppressive Antiretroviral Therapy. AIDS 2010; 24:2451–2460. doi: 10.1097/QAD.0b013e32833ef7bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hatano H, Hayes TL, Dahl V, et al. A Randomized, Controlled Trial of Raltegravir Intensification in Antiretroviral-treated, HIV-infected Patients with a Suboptimal CD4+ T Cell Response. J Infect Dis 2011; 203:960–968. doi: 10.1093/infdis/jiq138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Villanueva-Millán MJ, Pérez-Matute P, Recio-Fernández E, et al. Differential effects of antiretrovirals on microbial translocation and gut microbiota composition of HIV-infected patients. J Int AIDS Soc 2017; 20:21526. doi: 10.7448/IAS.20.1.21526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kelesidis T, Moser C, Stein JH, et al. Changes in Markers of T-Cell Senescence and Exhaustion With Atazanavir-, Raltegravir-, and Darunavir-Based Initial Antiviral Therapy: ACTG 5260s. J Infect Dis 2016; 214:748–52. doi: 10.1093/infdis/jiw253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ananworanich J, Chomont N, Fletcher JL, et al. Markers of HIV reservoir size and immune activation after treatment in acute HIV infection with and without raltegravir and maraviroc intensification. J Virus Erad 2015; 1:116–122. [PMC free article] [PubMed] [Google Scholar]
- 35.Fukada K, Sobao Y, Tomiyama H, Oka S, Takiguchi M. Functional Expression of the Chemokine Receptor CCR5 on Virus Epitope-Specific Memory and Effector CD8+ T Cells. J Immunol 2002; 168:2225–2232. doi: 10.4049/jimmunol.168.5.2225. [DOI] [PubMed] [Google Scholar]


