Abstract
Factors that impact first year morbidity and mortality in adults undergoing myeloablative allogeneic hematopoietic cell transplantation with ex-vivo CD34+ selection have not been previously reported. We assessed all toxicities ≥ grade 3 from the start of conditioning to date of death, relapse, or last contact in 200 patients during the first year post-transplantation, identifying 1885 individual toxicities among 17 organ-based toxicity groups. The most prevalent toxicities in the 1st year were of infectious, metabolic, hematologic, oral/GI, hepatic, cardiac and pulmonary etiologies. Renal complications were minimal. Grades II–IV and III–IV acute GVHD at day 100 were 11.5% and 3% respectively. In separate multivariate models, cardiovascular, hematologic, hepatic, neurologic, pulmonary and renal toxicities negatively impacted NRM and OS during the first year. A higher than targeted busulfan level, patient CMV seropositivity, and an HCT-CI of ≥ 3 were associated with increased risk of NRM and all cause death. Ex-vivo CD34+ selection had a favorable 1-year OS of 75%, NRM of 17%, and a low incidence of SOS. These data establish a benchmark to focus efforts in reducing toxicity burden while improving patient outcomes.
Keywords: toxicities, ex vivo CD34 selection, T-cell depletion, allogeneic hematopoietic cell transplantation
Introduction:
While potentially curative for many hematologic malignancies, allogeneic hematopoietic stem cell transplantation (allo-HCT) is a procedure with known inherent risks. Harnessing the efficacy of intensive conditioning and the graft vs tumor (GVT) effect while reducing the impacts of graft vs host disease (GVHD) and non-relapse mortality (NRM) has proven a difficult balancing act. Historically, several strategies pursuant to this goal have demonstrated certain degrees of success. One approach using ex vivo CD34+ selected stem cells as a method of T-Cell depletion (TCD) has demonstrated substantive reductions in both acute and chronic GVHD while maintaining good disease control in patients with acute leukemia or MDS in complete remission1–9. This method obviates the need for post-transplant administration of calcineurin inhibitors (CNIs) and their resultant toxicities, but currently requires myeloablative conditioning (MAC), which influences the spectrum of toxicities incurred by patients within the first year post-transplantation.
Studies have demonstrated that toxicities that occur early after transplant have an impact on longer term survival10–12. To date, a detailed analysis of acute events associated with ex-vivo CD34+ selected HCT in adults has not been described. Given the ongoing Blood and Marrow Transplantation Clinical Trials Network (BMT CTN) phase III randomized trial (BMT CTN 1301, NCT02345850) comparing the GVHD prophylactic strategies of CD34+ selection with the CliniMACS® Reagent System, post-transplantation cyclophosphamide (PTCy), and standard methotrexate and tacrolimus, we aimed to comprehensively examine and report the toxicities, associated risk factors, and their impact on outcomes in the 1st year post-transplantation for adult patients undergoing ex-vivo CD34+ selected allo-HCT for hematologic malignancies.
Methods:
Patients, Graft Sources and Conditioning Regimens
Eligible patients (N=200) were ≥ 18 years of age (no upper age limit pre-specified) with adequate pre-transplant organ function who underwent matched-related donor (MRD), mismatched-related donor (MMRD), matched-unrelated donor (MUD) or mismatched unrelated donor (MMUD) granulocyte colony-stimulating factor (G-CSF) mobilized peripheral blood stem cell (PBSC) allo-HCT for any hematologic malignancy with myeloablative conditioning (MAC) using ex-vivo CD34+ selection (CliniMACS® CD34 Reagent System, Miltenyi Biotech, Gladbach, Germany) as GVHD prophylaxis. No further pharmacologic GVHD prophylaxis was given post-transplant. All consecutive patients who met the above criteria and were transplanted between 2006 and 2012 were included. Human leukocyte antigen (HLA) typing was performed using high-resolution DNA sequence-specific oligonucleotide typing for the HLA-A, -B, -C, -DRB1 and -DQB1 loci. Patients who had been previously typed using intermediate resolution methods were re-typed with high-resolution methods that were used to define the level of mismatch.
All patients were treated with one of the following conditioning regimens at the discretion of the treating physician based on age, comorbidities, and disease type: busulfan, melphalan, and fludarabine (Bu/Mel/Flu); clofarabine, melphalan, and thiotepa (Clo/Mel/Thio); total body irradiation, thiotepa, and cyclophosphamide (TBI/Thio/Cy); or TBI, thiotepa, and fludarabine (TBI/Thio/Flu) as previously described3,4,13. All patients received rabbit anti-thymocyte globulin (ATG) 2.5 to 5 mg/kg/day on days −3, −2. Patients who received a TBI-based preparative regimen received palifermin (keratinocyte growth factor, KGF) 60 mcg/kg IV on days −13, −12, −11 and 0, +1, +214. G-CSF was initiated on day +7 until absolute neutrophil count (ANC) recovery. First dose Bu pharmacokinetic monitoring was done to target an area-under-the-curve (AUC) range of 4100 – 5300 micromol*min/L. All patients received standard supportive care for prevention of sinusoidal obstruction syndrome (SOS) and anti-microbial prophylaxis according to MSKCC institutional guidelines.
Toxicity Collection and Bio-statistical Methods
Data was extracted from the electronic medical record with a data-cutoff of December 31, 2015. The 1st year was defined from the start of conditioning chemotherapy to 12 months after the infusion of stem cells. We then retrospectively collected all grade ≥ 3 toxicities using the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 from the start of the conditioning regimen to the date of last contact, relapse or progression of disease, or death if the patient had relapsed. To enhance accuracy among reviewers, we performed audits with discrepancies settled by discussion with a 3rd investigator. Individual toxicities were organized into 17 organ-based groups (Supplement 1). To avoid multiple counting, infectious toxicities were separated into febrile neutropenia without a source, febrile neutropenia with an identified source, neutropenic sepsis, non-neutropenic sepsis, and non-neutropenic bacteremia. Viral reactivations without organ disease were excluded and recurrence intervals for all infections were based on the CIBMTR classification15. One individually graded toxicity per specified post-HCT time period (start of conditioning to day 100, day 100–180, day 181–270, day 271–365) was recorded and used for the purposes of statistical analyses.
Patients were grouped by disease into acute leukemia/MDS, multiple myeloma, or other. The cumulative incidences (CI) of all individual toxicity groups were assessed treating death and relapse as competing events. The association between baseline characteristics and the risk of individual toxicities was assessed using cause-specific Cox proportional hazards regression.
Progression-free survival (PFS) and overall survival (OS) were estimated using the Kaplan-Meier method. Univariate and multivariate Cox proportional hazards regression was used to compare the risk of death and NRM across patient and treatment characteristics. The association between the respective toxicities and each outcome was assessed using time-dependent covariates within the Cox model. All analyses were conducted using the R v3.3.2.
Results
Baseline patient and HCT characteristics are shown in Table 1. The median age of the 200 included patients was 56.7 (range 19–73), with 52% being male. The majority of patients had acute myeloid leukemia (AML), acute lymphocytic leukemia (ALL), or myelodysplastic syndrome (MDS) and received chemotherapy only based conditioning (72%). Within the 1st year, there were 1885 individual ≥ grade 3 toxicities among the 200 patients. Per patient, the median number of individual toxicities incurred within the first 100 days was 6. Of the 1885 toxicities, 474 (25 %) were infectious, 442 (23 %) were metabolic, 317 (17 %) were hematologic, 186 (10 %) were oral/gastrointestinal (GI), 121 (6.5 %) were hepatic, 104 (5.5%) were cardiovascular, and 57 (3.0 %) were pulmonary and 10% were “other.” The cumulative incidences of the most common toxicities within the 1st year are shown in Figure 1.
Table 1.
Baseline Patient and HCT Characteristics
| Characteristic | N=200, (%) |
|---|---|
| Age | 56.7 (19–73) |
| ≥ 60 | 80 (40) |
| Sex (male) | 104 (52) |
| Disease | |
| AML | 76 (38) |
| ALL | 17 (9) |
| Acute Leukemia | 3 (2) |
| MDS | 47 (24) |
| MM | 31 (16) |
| CLL/T-PLL | 2 (1) |
| CML | 10 (5) |
| MPD | 9 (5) |
| NHL | 4 (2) |
| FHLH | 1 (1) |
| Regimen | |
| Bu/Mel/Flu | 131 (66) |
| Clo/Mel/Thio | 13 (7) |
| TBI/Thio/CY | 55 (28) |
| TBI/Thio/Flu | 1 (1) |
| HLA | |
| MRD | 76 (38) |
| MMRD | 1 (1) |
| MURD | 78 (39) |
| MMURD | 45 (23) |
AML, acute myeloid leuekemia, ALL, acute lymphocytic leukemia; MDS, myelodysplastic syndrome; MM, multiple myeloma; CLL, chronic lymphocytic leukemia; T-PLL, T-prolymphocytic leukemia; CML, chronic myeloid leukemia; MPD, myeloproliferative disorder; NHL, non-Hodgkin lymphoma; FHLH, familial hemophagocytic lymphohistiocytosis; Bu, busulfan; Mel, melphalan; Flu, fludarabine; Clo, clofarabine; Thio, thiotepa; TBI, total-body irradiation; Cy, cyclophosphamide; HLA, human-leukocyte antigen; MRD, matched related donor; MMRD, mismatched-related donor; MURD, matched-unrelated donor; MMURD, mismatched unrelated donor.
Figure 1:
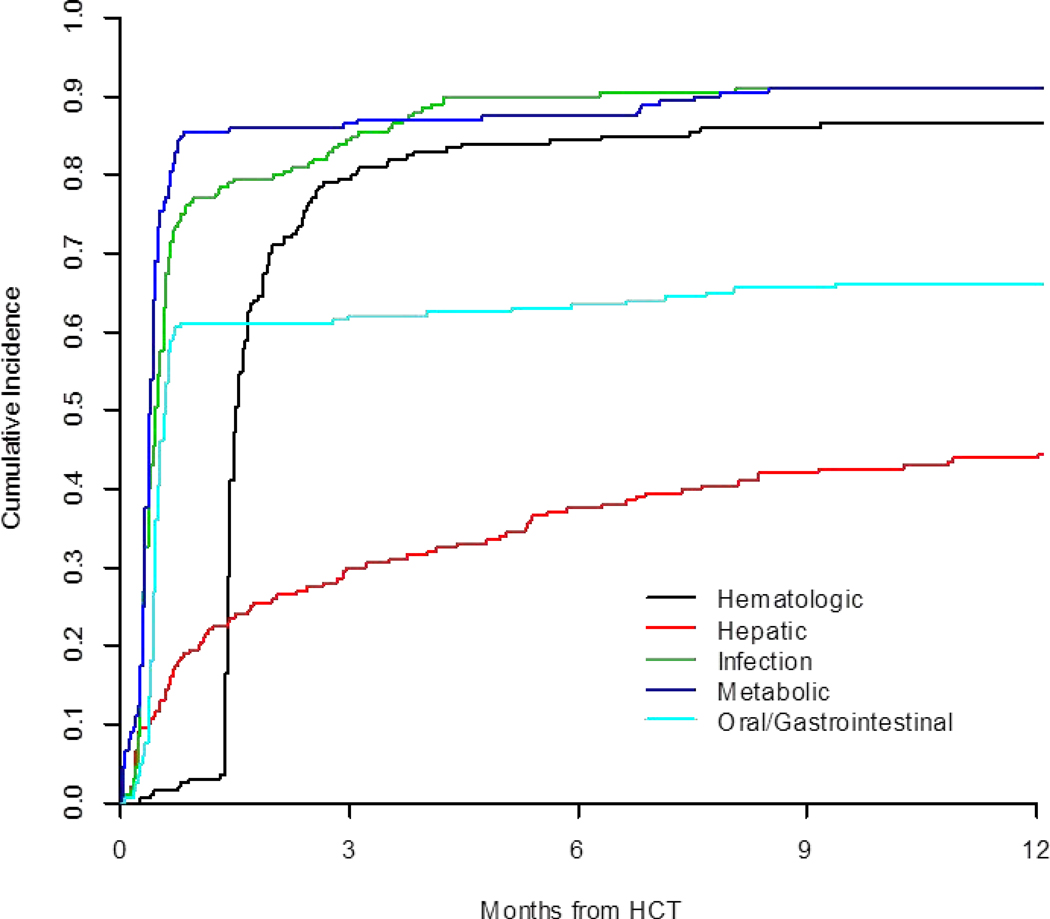
Cumulative Incidence of 1st Year Toxicities after Transplant
Specific Toxicities:
Infectious:
Among the 474 toxicities related to infection in the first year: 83 (18%) of which were febrile neutropenia without a clear source, 61 (13%) febrile neutropenia with a source. There were 61 (13%) lung infections, 50 (11%) non-neutropenic bacteremias, 24 (5%) episodes of Clostridium difficile, and 24 (5%) Epstein-Barr virus post-transplant lymphoproliferative disorders (EBV-PTLD). There were also 13 ≥ grade 3 urinary tract infections, 9 upper respiratory infections, and 11 episodes each of neutropenic and non-neutropenic sepsis. Rarer viral complications included BK cystitis (16/474), cytomegalovirus organ disease (13/474), adenovirus organ disease (9/474) and human herpes virus 6 organ disease (4/474).
Metabolic:
There were 442 metabolic toxicities with the most common being electrolyte abnormalities (197, 45%), anorexia (111, 25%), and hyperglycemia warranting therapy (103, 23%). Most cases of electrolyte abnormalities (137/197, 70%) occurred from the time of conditioning to day +30.
Hematologic:
There were 317 individual hematologic toxicities. Cytopenias after day +30 accounted for 285 (90%) and were mainly within the first 6 months (272/315, 86%). Rarer toxicities included coagulopathies (n=13), venous thromboembolism (n=10), and hemolytic uremic syndrome/thrombotic thrombocytopenic purpura (n=3), disseminated intravascular coagulation (n=2) and hemolysis (n=1).
Oral/GI:
There were 186 oral/GI toxicities, with the most common being mucositis (103, 55%). Other adverse events included diarrhea (37/186, 20%), colitis (9%), nausea/vomiting (9%), GI hemorrhage (4%), and ascites (<1%). Mucositis occurred almost exclusively (101/103, 98%) within the first 30 days, and was likely conditioning regimen related.
Hepatic:
There were 121 hepatic toxicities. Liver function test abnormalities accounted for 86%, while cholecystitis occurred in 8%. Liver function test abnormalities occurred throughout the 1st year however were concentrated (87/104, 84%) mainly within the first 180 days. Rarer complications included hepatic failure (n=2) and portal hypertension (n=1). There were 4 cases of SOS.
Cardiac:
There were 104 cardiac toxicities. The most prevalent were hypertension (23%), hypotension (19%), atrial fibrillation (14%), and heart failure (13%). All cases of hypertension occurred within the first 6 months and mostly prior to day +30 (19/24, 79%). Heart failure occurred throughout the 1st year; however, 62% (8/13) developed in the first 6 months, and 5 of those cases (63%) developed by day +30.
Pulmonary:
There were 57 pulmonary toxicities. Volume overload was the most common toxicity as evidenced by pulmonary edema (7/57, 12%) and pleural effusions (10/57, 18%). Grade ≥3 dyspnea (2/57), hypoxia including needing intubation (4/57), and acute respiratory distress syndrome (ARDS, 11/57) were less frequent. Rarer complications included asymptomatic reductions in diffusion capacity (4/57), pneumothoraces (3/57), organizing pneumonia (2/57), diffuse alveolar hemorrhage (1/57), and idiopathic pulmonary syndrome (1/57). ARDS occurred within the first 6 months for the majority of cases (8/11, 73%), and pulmonary edema was mostly by day +30 (5/7, 71%).
Renal:
There were only 27 renal toxicities in the first year after transplant. Of these, 17 (63%) were grade ≥3 creatinine increases, with 13/17 (76%) in the first 6 months. Chronic kidney disease accounted for 3 toxicities, as did hematuria.
Risk Factors for Toxicities
Using univariate Cox regression analysis, we assessed the associations between patient and transplantation characteristics and the development of common toxicities.
The risk of developing infectious complications was decreased by being male [HR 0.63 (95% CI 0.47 – 0.84), p= 0.002]; no factors increased the risk. Patients with a CMV positive donor had a higher chance of developing hematologic complications [HR 1.4 (95% CI 1.04 – 1.9), p=0.028], while this risk was ameliorated for patients entering transplant with an absolute lymphocyte count (ALC) > 0.5 K/mcl [HR 0.41 (95% CI 0.26 – 0.63), p <0.001]. Male gender [HR 0.58 (95% CI 0.41 – 0.82), p=0.002], having an unrelated donor (URD) [HR 0.67 (95% CI 0.47 – 0.95), p=0.023] and high ALC [HR 0.57 (95% CI 0.35 – 0.93), p=0.024] conferred a decreased risk of oral/GI toxicity, while patients who received TBI [HR 1.76 (95% CI 1.22 – 2.53), p=0.002] were at higher risk. The chance of developing metabolic complications was lessened in patients having an URD [HR 0.74 (95% CI 0.55 – 1), p=0.049]. Patients with ferritin level >1000 were at a significantly greater risk to develop hepatic complications in the 1st year (HR 3.46 (95% CI 1.9 – 6.32), p<0.001). Hematopoietic cell transplantation co-morbidity index (HCT-CI)16 was not associated with an increased risk of specific toxicities.
Outcomes:
At day 100, 23 patients (11.5%) experienced grade II-IV acute GVHD (aGVHD), while 6 patients (3%) developed grade III–IV aGVHD. At one year after transplant, 149 of 200 patients were alive. Of the 51 who died, 17 (9%) died of disease and 34 (17%) died of NRM. Of these 34 patients, 18 died of infection, 7 of GVHD, 3 from conditioning related toxicities, 2 from hepatic failure, 1 of interstitial pneumonia, 1 dementia, and 1 cause of death was unknown. Sixteen patients were alive with relapsed disease. At 1 year after allo-HCT, PFS and OS for the entire cohort were 67% and 75% respectively, with a NRM of 17% (Figure 2). Using a landmark, there was no OS difference between patients above or below the median number of toxicities by 100 days.
Figure 2:
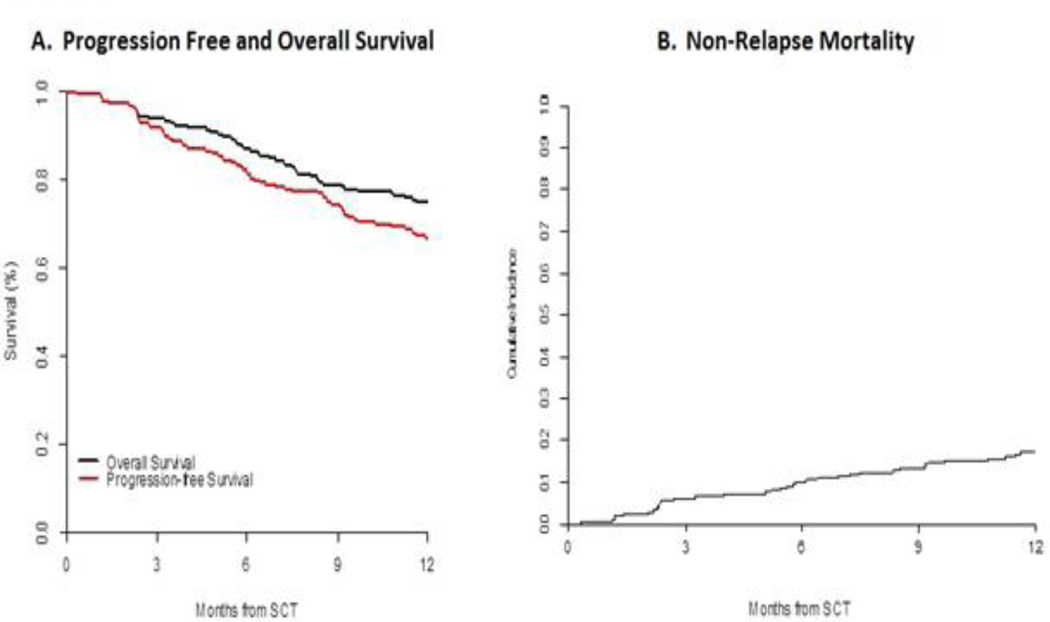
OS and NRM at 1 Year.
Upon univariate analysis of risk factors, hematologic, hepatic, neurologic, pulmonary, and renal toxicities were associated with an increased risk of all cause mortality and NRM in the 1st year (Supplement 2). A higher than target level of busulfan [HR 2.79 (1.32 to 5.91), p <0.001], an HCT-CI of ≥ 3 [HR 2.45 (1.08 to 5.57), p-0.01] and CMV seropositivity of the patient [HR 1.82 (1 to 3.29), p-0.05] were risk factors for poorer OS. Increased NRM risk was also noted in patients with higher than target Busulfan levels [HR 3.09 (1.33 to 7.18), p-0.001] and an HCT-CI of ≥ 3 [HR 3 (1.03 to 8.7), p-0.02].
Due to univariate associations and overall sample size, we investigated two multivariate models. The first analyzed the association between individual toxicities and the risk of all cause mortality and NRM while adjusting for change in busulfan dosing due to off-target levels based on 1st dose pharmacokinetics and HCT-CI, in the 131 patients who received busulfan in conditioning. The second analyzed similar associations but adjusted for CMV serostatus of the patient and HCT-CI, and therefore could include all 200 patients. In the multivariate model adjusting for busulfan levels and HCT-CI, hematologic, hepatic, neurologic, pulmonary, and renal toxicities all separately remained significantly associated with increased risk of death within the first year. Cardiac and hepatic toxicities were associated with increased NRM risk in the adjusted models (Table 2). While adjusting for patient CMV seropositivity and HCT-CI, hematologic, hepatic and neurologic toxicities separately increased mortality risk, while pulmonary and renal toxicities increased both all cause mortality and NRM risk (Table 3).
Table 2: Multivariate Model.
Association of risk of death or non-relapsed mortality when controlling for change in busulfan dose based on 1st dose pharmacokinetics, and toxicities that were significant based on a univariate analysis. Separate multivariate models were fit to each toxicity.
| Risk of death* | Risk of NRM* | |||||
|---|---|---|---|---|---|---|
| HR (95% CI ) | P-value | HR (95% CI ) | P-value | |||
| Cardiovascular | 1.47 (0.81 to 2.67) | 0.201 | 2.66 (1.23 to 5.77) | 0.013 | ||
| Age | 1.03 (0.99 to 1.07) | 0.093 | 1.01 (0.96 to 1.05) | 0.73 | ||
| Busulfan Change | No change | (reference) | (reference) | |||
| Decrease | 2.59 (1.25 to 5.36) | 0.01 | 2.99 (1.27 to 7.06) | 0.012 | ||
| Increase | 0.89 (0.45 to 1.75) | 0.736 | 0.5 (0.19 to 1.33) | 0.164 | ||
| HCT-CI | 0 | (reference) | (reference) | |||
| 1–2 | 1.53 (0.53 to 4.39) | 0.43 | 1.01 (0.24 to 4.2) | 0.988 | ||
| >=3 | 2.34 (0.88 to 6.2) | 0.087 | 1.83 (0.52 to 6.48) | 0.348 | ||
| Hepatic | 4.97 (1.52 to 16.21) | 0.008 | ||||
| Age | 1.04 (1 to 1.08) | 0.036 | ||||
| Busulfan Change | No change | (reference) | ||||
| Decrease | 2.71 (1.31 to 5.6) | 0.007 | ||||
| Increase | 1.02 (0.52 to 2.01) | 0.952 | ||||
| HCT-CI | 0 | (reference) | ||||
| 1–2 | 1.61 (0.56 to 4.64) | 0.378 | ||||
| >=3 | 2.57 (0.98 to 6.73) | 0.054 | ||||
| Hematologic | 2.96 (1.63 to 5.35) | <0.001 | 7.58 (3.14 to 18.27) | <0.001 | ||
| Age | 1.05 (1.01 to 1.08) | 0.014 | 1.03 (0.99 to 1.08) | 0.146 | ||
| Busulfan Change | No change | (reference) | (reference) | |||
| Decrease | 2.36 (1.14 to 4.88) | 0.021 | 2.5 (1.06 to 5.91) | 0.037 | ||
| Increase | 0.94 (0.48 to 1.86) | 0.867 | 0.55 (0.2 to 1.46) | 0.229 | ||
| HCT-CI | 0 | (reference) | (reference) | |||
| 1–2 | 1.91 (0.66 to 5.48) | 0.231 | 1.61 (0.39 to 6.68) | 0.514 | ||
| >=3 | 2.87 (1.09 to 7.57) | 0.033 | 2.67 (0.76 to 9.39) | 0.126 | ||
| Neurologic | 2.38 (1.22 to 4.64) | 0.011 | 5.75 (2.52 to 13.13) | <0.001 | ||
| Age | 1.03 (0.99 to 1.06) | 0.141 | 0.99 (0.95 to 1.04) | 0.795 | ||
| Busulfan Change | No change | (reference) | (reference) | |||
| Decrease | 2.25 (1.07 to 4.72) | 0.032 | 2.1 (0.86 to 5.12) | 0.103 | ||
| Increase | 0.86 (0.44 to 1.69) | 0.66 | 0.45 (0.16 to 1.22) | 0.114 | ||
| HCT-CI | 0 | (reference) | (reference) | |||
| 1–2 | 1.46 (0.51 to 4.2) | 0.486 | 1.04 (0.25 to 4.33) | 0.96 | ||
| >=3 | 2.29 (0.87 to 6.04) | 0.093 | 1.94 (0.55 to 6.79) | 0.301 | ||
| Pulmonary | 2.99 (1.65 to 5.41) | <0.001 | 6.4 (2.96 to 13.82) | <0.001 | ||
| Age | 1.03 (0.99 to 1.07) | 0.117 | 1.01 (0.96 to 1.05) | 0.729 | ||
| Busulfan Change | No change | (reference) | (reference) | |||
| Decrease | 2.2 (1.06 to 4.57) | 0.034 | 2.18 (0.92 to 5.17) | 0.076 | ||
| Increase | 0.88 (0.45 to 1.74) | 0.717 | 0.52 (0.19 to 1.38) | 0.189 | ||
| HCT-CI | 0 | (reference) | (reference) | |||
| 1–2 | 1.56 (0.54 to 4.45) | 0.409 | 1.07 (0.26 to 4.39) | 0.93 | ||
| >=3 | 2.51 (0.95 to 6.6) | 0.062 | 2.05 (0.59 to 7.17) | 0.26 | ||
| Renal | 2.49 (1.14 to 5.48) | 0.023 | 3.73 (1.48 to 9.4) | 0.005 | ||
| Age | 1.03 (1 to 1.07) | 0.08 | 1.01 (0.97 to 1.06) | 0.641 | ||
| Busulfan Change | No change | (reference) | (reference) | |||
| Decrease | 2.18 (1.04 to 4.58) | 0.039 | 2.19 (0.91 to 5.29) | 0.082 | ||
| Increase | 1.03 (0.52 to 2.06) | 0.924 | 0.65 (0.24 to 1.77) | 0.398 | ||
| HCT-CI | 0 | (reference) | (reference) | |||
| 1–2 | 1.64 (0.57 to 4.69) | 0.355 | 1.26 (0.31 to 5.19) | 0.748 | ||
| >=3 | 2.2 (0.82 to 5.85) | 0.116 | 1.8 (0.5 to 6.43) | 0.366 | ||
within the first year. HCT-CI, HCT-CI, hematopoietic cell transplantation-comorbidity index
Table 3: Multivariate Model.
Association of risk of death or non-relapsed mortality when controlling for change in CMV serostatus, and toxicities that were significant based on a univariate analysis. Separate multivariate models were fit to each toxicity.
| Risk of death* | Risk of NRM* | |||||
|---|---|---|---|---|---|---|
| HR (95% CI ) | P-value | HR (95% CI ) | P-value | |||
| Cardiovascular | 1.2 (0.72 to 2.02) | 0.48 | 2.29 (1.15 to 4.57) | 0.018 | ||
| Age | 1.03 (1 to 1.05) | 0.032 | 1.02 (0.98 to 1.05) | 0.339 | ||
| ptCMV | 1.66 (0.99 to 2.76) | 0.053 | 1.86 (0.91 to 3.82) | 0.089 | ||
| HCT-CI | 0 | (reference) | (reference) | |||
| 1–2 | 1.41 (0.64 to 3.11) | 0.391 | 1.21 (0.36 to 4.04) | 0.754 | ||
| >=3 | 2.21 (1.06 to 4.61) | 0.035 | 2.81 (0.97 to 8.18) | 0.058 | ||
| Hepatic | 2.72 (1.07 to 6.89) | 0.035 | ||||
| Age | 1.03 (1.01 to 1.06) | 0.015 | ||||
| ptCMV | 1.54 (0.92 to 2.57) | 0.098 | ||||
| HCT-CI | 0 | (reference) | ||||
| 1–2 | 1.43 (0.65 to 3.15) | 0.369 | ||||
| >=3 | 2.23 (1.07 to 4.63) | 0.032 | ||||
| Hematologic | 2.65 (1.6 to 4.39) | <0.001 | 7.49 (3.34 to 16.79) | <0.001 | ||
| Age | 1.03 (1.01 to 1.06) | 0.011 | 1.03 (1 to 1.06) | 0.075 | ||
| ptCMV | 1.45 (0.87 to 2.42) | 0.158 | 1.42 (0.69 to 2.94) | 0.343 | ||
| HCT-CI | 0 | (reference) | (reference) | |||
| 1–2 | 1.68 (0.76 to 3.7) | 0.201 | 1.67 (0.5 to 5.56) | 0.406 | ||
| >=3 | 2.43 (1.17 to 5.06) | 0.017 | 3.53 (1.22 to 10.25) | 0.02 | ||
| Neurologic | 2.21 (1.24 to 3.94) | 0.007 | 5.79 (2.86 to 11.74) | <0.001 | ||
| Age | 1.03 (1 to 1.05) | 0.045 | 1.01 (0.98 to 1.05) | 0.486 | ||
| ptCMV | 1.63 (0.98 to 2.72) | 0.06 | 1.74 (0.85 to 3.58) | 0.131 | ||
| HCT-CI | 0 | (reference) | (reference) | |||
| 1–2 | 1.29 (0.58 to 2.86) | 0.526 | 0.93 (0.27 to 3.14) | 0.902 | ||
| >=3 | 2.04 (0.97 to 4.26) | 0.059 | 2.19 (0.74 to 6.5) | 0.158 | ||
| Pulmonary | 3.11 (1.87 to 5.2) | <0.001 | 9.01 (4.53 to 17.91) | <0.001 | ||
| Age | 1.02 (1 to 1.05) | 0.048 | 1.02 (0.99 to 1.05) | 0.281 | ||
| ptCMV | 1.78 (1.07 to 2.98) | 0.028 | 2.22 (1.07 to 4.61) | 0.032 | ||
| HCT-CI | 0 | (reference) | (reference) | |||
| 1–2 | 1.32 (0.6 to 2.91) | 0.492 | 1.01 (0.3 to 3.4) | 0.982 | ||
| >=3 | 2.1 (1.01 to 4.38) | 0.047 | 2.53 (0.87 to 7.4) | 0.09 | ||
| Renal | 4.07 (2.15 to 7.72) | <0.001 | 9.88 (4.54 to 21.52) | <0.001 | ||
| Age | 1.02 (1 to 1.05) | 0.07 | 1.01 (0.97 to 1.04) | 0.65 | ||
| ptCMV | 1.88 (1.12 to 3.15) | 0.017 | 2.59 (1.24 to 5.41) | 0.011 | ||
| HCT-CI | 0 | (reference) | (reference) | |||
| 1–2 | 1.36 (0.62 to 2.99) | 0.447 | 1.17 (0.35 to 3.89) | 0.803 | ||
| >=3 | 1.74 (0.82 to 3.72) | 0.149 | 1.94 (0.64 to 5.89) | 0.241 | ||
within the first year. HCT-CI, HCT-CI, hematopoietic cell transplantation-comorbidity index
Discussion:
Although previous studies have reported upon the important relationship between toxicities that develop early after transplant and their effect on patient outcomes11,12,17–20, few have focused on the frequency of specific organ toxicities. This current analysis is the first and most comprehensive investigation of risk factors for developing serious toxicities and their subsequent impact on 1-year outcomes in patients undergoing ex-vivo CD34+ selected allo-HCT. Although utilizing MAC might be expected to yield increased NRM and decreased OS, we found excellent survival with favorable rates of aGVHD and NRM. Furthermore, when compared to BMT-CTN data evaluating toxicities with MAC conventional transplants21, the toxicity burden seems similar; although, this would need to be substantiated in a prospective study.
In our study, patients experiencing pulmonary, renal, hepatic and neurologic toxicities had an increased risk of death and NRM while adjusting for either first dose busulfan pharmacokinetics or patient CMV serostatus. This highlights that certain individual toxicities can harbor significant clinical impact in the first year after transplant. Surprisingly, infectious complications as a whole, which were the most prevalent toxicity, did not have a significant impact upon 1 year NRM or OS. This may be due to the fact that the majority of these infections are easily detectable and treated with early intervention. Also, despite a higher incidence than that reported in unmodified transplants, patients with viral disease resistant to standard therapies have access to viral specific cytotoxic immune therapies at our center22,23, which may abrogate the risk of poor outcomes. Since almost all patients had a grade ≥ 3 infection in the first 1–2 months following transplant, and for the majority, these infections were easily treated, infectious toxicity – as a predictor – was unable to discriminate those who will and will not die of NRM. Particularly of interest in the TCD setting is the incidence of CMV organ disease, which was 7.5% (15/200) in the 1st year with 85% (13/15) occurring within the first 6 months. These findings are consistent with previously published data from our center by Huang et al., which revealed a day +180 incidence of CMV organ disease of 5%24. In our study, CMV seropositive patients who developed pulmonary and renal toxicities experienced a higher risk of death and NRM in the 1st year. These findings parallel our previous report in which patients who were CMV seropositive undergoing CD34+ selected allo-HCT were more likely to develop CMV viremia and had higher mortality within the first 3 months. We believe CMV status must be emphasized in this setting perhaps even more than with conventional approaches. With the possible approval of letermovir for prevention of CMV infection in high-risk allo-HCT recipients, it will be interesting to assess the impact of CMV serostatus in patients undergoing CD34 selected allo-HCT who receive prophylaxis. Our study also highlights the risk of additional viral complications beyond CMV that also appear more prevalent in this patient population. In another recent study by Huang et al from our center, we specifically assessed the risk of infection by double stranded DNA viruses (CMV, adenovirus, human herpesvirus 6 and EBV), including viremia, end-organ disease, and infection-related mortality, in a cohort of 156 patients25. We showed that viremia by double stranded DNA viruses occurred in 85% of CD34+ HCT recipients by day +100 and 33% of patients experienced ≥2 viremias by day +180. Specifically, the cumulative incidences for CMV, HHV6, ADV, and EBV viremia were 44%, 61%, 7% and 16%, respectively, with 28 patients (18%) developing end organ disease at 1 year post HCT.
Certain patient and transplant specific characteristics were either associated with the development of specific toxicities or had an effect on outcomes. Similar to the conventional setting26, a high pre-transplant ferritin was an important predictor for the development of serious hepatic complications. High ALC at time points after transplant has been associated with improvements in post-transplant survival27. We noted that pre-transplant ALC was also related to a lower risk of developing hematologic and oral/GI toxicities. These assays, as well as others, may be important adjunct studies in this patient population28,29. Although HCT-CI was not associated with an increased risk of developing toxicities, in the multivariate model adjusting for CMV serostatus of the patient, an HCT-CI ≥3 was independently predictive of death in those incurring cardiac, hematologic, and pulmonary toxicities while increasing the risk of both outcome measures in patients with hepatic complications. Similar findings were noted by Barba et al30.
Regarding the conditioning regimen, we found TBI increased the risk of developing oral/GI complications consistent with data showing increased intestinal barrier disruption and GI toxicity after conventional transplant31. In recipients of busulfan, higher than target first dose busulfan AUC prompting reduction in subsequent doses was independently predictive of poorer NRM and OS in models incorporating cardiac and hepatic toxicities; and was associated with the risk of death in models including hematologic, neurologic, pulmonary and renal toxicities. These findings build upon data by our own group which revealed higher total busulfan exposure resulted in adverse outcomes in patients undergoing ex-vivo CD34 selected allo-HCT32,33. This finding runs parallel with other studies which have found a relationship between busulfan exposure and outcomes in patients undergoing conventional transplantation34–38. Although investigation of differences between various busulfan level cohorts are beyond the scope of this study, synergisms between higher drug exposures, underlying inflammatory processes, and other factors which contribute to NRM in this population may contribute to the association with outcomes.
Two important transplant measures that compared favorably to MAC conventional platforms were aGVHD and SOS. At day 100, grade II–IV aGVHD was 11.5% and grade III–IV was 6%. This reaffirmed findings by Barba et al., which specifically evaluated GVHD and outcomes in 248 patients who underwent CD34+ selected allo-HCT39. By day 180, patients developed grade II–IV and grade III–IV aGVHD at a rate of 16% and 5%, respectively. Our incidence of aGVHD compares favorably with that of myeloablative conventional transplants40,41; however, this should be substantiated by head to head comparison as ongoing in the BMT CTN 1301 study. The long-term outcomes of patients who developed GVHD within the 1st year have been analyzed separately by our group42. TBI and busulfan have historically resulted in a higher risk of SOS, but it developed in only 4 of our patients (2%). This is favorable when compared to the incidence seen after MAC conventional transplants which usually range anywhere from 10–15%43–45. Utilizing CD34+ selection as a method of T-cell depletion may result in downregulation or modulation of the immune response integral to the development of SOS and, therefore, decrease the risk. This is supported by other analyses of TCD transplants46,47. Furthermore, using 1st dose adjusted IV busulfan instead of the oral formulation can result in more stable drug bioavailability and exposure. Finally, the total amount of renal toxicities was low (27/1885, <2%) with only 8% of patients having acute kidney injury (AKI) in the first year after transplant. This compares very favorably to regimens requiring calcineurin inhibitors for GVHD prophylaxis, which have been reported to have up to 70% of patients with AKI after transplant48.
Consideration must be given to the retrospective nature of our findings and the resulting inherent limitations of the study. For example, one finding that could be seen as somewhat surprising was the low incidence of some GI toxicities. While mucositis and diarrhea are recorded daily in the medical record, nausea and vomiting are harder to capture unless done in a prospective manner. Most patients in this series who required TPN did so because of mucositis since they underwent ablative conditioning. This could in part account for some underscoring of nausea and vomiting. Furthermore, patients did not receive any post transplant GVHD prophylaxis with CNI or methotrexate, which may contribute to additional GI toxicity post transplant. In addition, the very low incidence of GVHD is also a likely contributor to low incidence of both upper and lower GI complaints of nausea/vomiting and diarrhea, that would be expected in recipients of unmodified grafts.
Transplant toxicity assessments done in a retrospective manner may either over- or under-estimate the incidence or severity of adverse events. This is understandable given the distinct complexity of transplant hospitalizations in which multiple parallel events occur with sometimes shared etiologies perhaps blurring correlations. While grading some events can be subjective, we made extra efforts to improve consistency of data collection by conducting multiple cross audits. We also incorporated all objective data such as laboratory values and culture results to confirm the inclusion of relevant toxicities.
In conclusion, patients who underwent an ex-vivo CD34+ selected allo-HCT using MAC experienced low rates of aGVHD and SOS, with good rates of NRM, PFS and OS at 1 year after transplantation. Overall, we show that patient and transplant factors can predict toxicities after CD34+ selected transplants, and that these toxicities are independently predictive of outcomes. The toxicities expected with an intensive regimen may be potentially balanced by the absence of complications related to methotrexate and calcineurin inhibitors, and this work provides a benchmark for strategies to improve the tolerability of and outcomes after transplant. These findings emphasize the importance of the BMT CTN 1301 prospective study, which may provide further progress in optimizing patient care.
Supplementary Material
Highlights:
This is a comprehensive assessment of early toxicities after CD34+ selected allo HCT.
Organ toxicities, sub-optimal busulfan targeting, and CMV serostatus increased the risk of death and NRM.
Renal toxicities were minimal in this calcineurin inhibitor free GVHD prophylaxis strategy.
Acknowledgments:
This research was supported in part by National Institutes of Health award number P01 CA23766 and NIH/NCI Cancer Center Support Grant P30 CA008748. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Additional support was provided by the Bergstein Family Foundation Fund and the Dave and Merle Brown Fund (SK, MSKCC).
Footnotes
Conflicts of Interest: There are no other relevant conflicts of interests in relation to the work described.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Reisner Y, Kapoor N, O’Reilly RJ, Good RA. Allogeneic bone marrow transplantation using stem cells fractionated by lectins: VI, in vitro analysis of human and monkey bone marrow cells fractionated by sheep red blood cells and soybean agglutinin. Lancet. 1980;2(8208):1320–1324. http://www.ncbi.nlm.nih.gov/pubmed/6109148. Accessed April 24, 2017. [DOI] [PubMed] [Google Scholar]
- 2.Young JW, Papadopoulos EB, Cunningham I, et al. T-Cell-Depleted Allogeneic Bone Marrow Transplantation in Adults With Acute Nonlymphocytic Leukemia in First Remission. Blood. 1992. [PubMed] [Google Scholar]
- 3.Papadopoulos EB, Carabasi MH, Castro-Malaspina H, et al. T-Cell–Depleted Allogeneic Bone Marrow Transplantation as Postremission Therapy for Acute Myelogenous Leukemia: Freedom From Relapse in the Absence of Graft-Versus-Host Disease. Blood. 1998;91(3):1083–1090. [PubMed] [Google Scholar]
- 4.Castro-Malaspina H, Jabubowski AA, Papadopoulos EB, et al. Transplantation in remission improves the disease-free survival of patients with advanced myelodysplastic syndromes treated with myeloablative T cell-depleted stem cell transplants from HLA-identical siblings. BBMT. 2008;14(4):458–468. doi: 10.1016/j.bbmt.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pasquini MC, Devine S, Mendizabal A, et al. Comparative outcomes of donor graft CD34+ selection and immune suppressive therapy as graft-versus-host disease prophylaxis for patients with acute myeloid leukemia in complete remission undergoing HLA-matched sibling allogeneic hematopoietic cell transpl. JCO. 2012;30(26):3194–3201. doi: 10.1200/JCO.2012.41.7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bayraktar UD, de Lima M, Saliba RM, et al. Ex vivo T cell-depleted versus unmodified allografts in patients with acute myeloid leukemia in first complete remission. BBMT. 2013;19(6):898–903. doi: 10.1016/j.bbmt.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldberg JD, Linker A, Kuk D, et al. T cell-depleted stem cell transplantation for adults with high-risk acute lymphoblastic leukemia: long-term survival for patients in first complete remission with a decreased risk of graft-versus-host disease. BBMT. 2013;19(2):208–213. doi: 10.1016/j.bbmt.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tamari R, Chung SS, Papadopoulos EB, et al. CD34-Selected Hematopoietic Stem Cell Transplants Conditioned with Myeloablative Regimens and Antithymocyte Globulin for Advanced Myelodysplastic Syndrome: Limited Graft-versus-Host Disease without Increased Relapse. BBMT. 2015;21(12):2106–2114. doi: 10.1016/j.bbmt.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hobbs GS, Hamdi A, Hilden PD, et al. Comparison of outcomes at two institutions of patients with ALL receiving ex vivo T-cell-depleted or unmodified allografts. BMT. 2015;50(4):493–498. doi: 10.1038/bmt.2014.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bearman SI, Appelbaum FR, Buckner CD, et al. Regimen-related toxicity in patients undergoing bone marrow transplantation. JCO. 1988;6(10):1562–1568. doi: 10.1200/JCO.1988.6.10.1562. [DOI] [PubMed] [Google Scholar]
- 11.Patriarca F, Skert C, Bonifazi F, et al. Effect on survival of the development of late-onset non-infectious pulmonary complications after stem cell transplantation. Haematologica. 2006;91(9):1268–1272. http://www.ncbi.nlm.nih.gov/pubmed/16956831. Accessed May 2, 2017. [PubMed] [Google Scholar]
- 12.Tichelli A, Bhatia S, Socié G. Cardiac and cardiovascular consequences after haematopoietic stem cell transplantation. BJH. 2008;142(1):11–26. doi: 10.1111/j.1365-2141.2008.07165.x. [DOI] [PubMed] [Google Scholar]
- 13.Jakubowski AA, Small T, Kernan N, et al. T-Cell Depleted Unrelated Donor Stem Cell Transplantation Provides Favorable Disease Free Survival for Adults With Hematologica Malignancies. BBMT. 2011;17(9):1335–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldberg JD, Zheng J, Castro-Malaspina H, et al. Palifermin is efficacious in recipients of TBI-based but not chemotherapy-based allogeneic hematopoietic stem cell transplants. BMT. 2012;48(May):1–6. doi: 10.1038/bmt.2012.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tomblyn M CIBMTR Infection Data CIBMTR Infection Data and the New Infection and the New Infection Inserts Inserts. https://www.cibmtr.org/Meetings/Materials/CRPDMC/Documents/2007/february/TomblynM_Infection.pdf. Published 2007. Accessed November 14, 2016.
- 16.Sorror ML, Storb RF, Sandmaier BM, et al. Comorbidity-age index: A clinical measure of biologic age before allogeneic hematopoietic cell transplantation. JCO. 2014;32(29):3249–3256. doi: 10.1200/JCO.2013.53.8157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Druley TE, Hayashi R, Mansur DB, et al. Early outcomes after allogeneic hematopoietic SCT in pediatric patients with hematologic malignancies following single fraction TBI. BMT. 2009;43(4):307–314. doi: 10.1038/bmt.2008.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohty B, Mohty M. Long-term complications and side effects after allogeneic hematopoietic stem cell transplantation: an update. Blood Cancer J. 2011;1(4):e16. doi: 10.1038/bcj.2011.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wingard JR, Majhail NS, Brazauskas R, et al. Long-term survival and late deaths after allogeneic hematopoietic cell transplantation. JCO. 2011;29(16):2230–2239. doi: 10.1200/JCO.2010.33.7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohty M, Apperley JF. Long-term physiological side effects after allogeneic bone marrow transplantation. Am Soc Hematol Educ B. 2010:229–236. doi: 10.1182/asheducation-2010.1.229; 10.1182/asheducation-2010.1.229. [DOI] [PubMed] [Google Scholar]
- 21.Maziarz RT, Lazarus HM, Riches ML, Mudrick C, Mendizabal A. BMT CTN trials: A rich source for regimen related toxicity assessments in the modern era. BBMT. 2016;22(3 SUPPL. 1):S291–S292. doi: 10.1016/j.bbmt.2015.11.746. [DOI] [Google Scholar]
- 22.Trivedi D, Williams RY, O’Reilly RJ, Koehne G. Generation of CMV-specific T lymphocytes using protein-spanning pools of pp65-derived overlapping pentadecapeptides for adoptive immunotherapy. Blood. 2005;105(7):2793–2801. doi: 10.1182/blood-2003-05-1433. [DOI] [PubMed] [Google Scholar]
- 23.Koehne G, Hasan A, Doubrovina E, et al. Immunotherapy with Donor T Cells Sensitized with Overlapping Pentadecapeptides for Treatment of Persistent Cytomegalovirus Infection or Viremia. BMT. 2015;21(9):1663–1678. doi: 10.1016/j.bbmt.2015.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang Y-T, Neofytos D, Foldi J, et al. Cytomegalovirus Infection after CD34+-Selected Hematopoietic Cell Transplantation. BBMT. 2016;22(8):1480–1486. doi: 10.1016/j.bbmt.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang Y-T, Kim SJ, Lee YJ, et al. Co-Infections by Double-Stranded DNA (dsDNA) Viruses after Ex Vivo T-Cell Depleted, CD34+ Selected Hematopoietic Cell Transplantation (HCT). BBMT. 2017;In Press. doi: 10.1016/j.bbmt.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pullarkat V, Blanchard S, Tegtmeier B, et al. Iron overload adversely affects outcome of allogeneic hematopoietic cell transplantation. BMT. 2008;42(12):799–805. doi: 10.1038/bmt.2008.262. [DOI] [PubMed] [Google Scholar]
- 27.Goldberg JD, Zheng J, Ratan R, et al. Early recovery of T-cell function predicts improved survival after T-cell depleted allogeneic transplant. Leuk Lymphoma. 2017;58(8):1859–1871. doi: 10.1080/10428194.2016.1265113. Epub 2017 Jan 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Artz AS, Logan B, Zhu X, et al. The prognostic value of serum C-reactive protein, ferritin, and albumin prior to allogeneic transplantation for acute myeloid leukemia and myelodysplastic syndromes. Haematologica. 2016;101(11):1426–1433. doi: 10.3324/haematol.2016.145847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rashidi A, DiPersio JF, Westervelt P, et al. Peritransplant Serum Albumin Decline Predicts Subsequent Severe Acute Graft-versus-Host Disease after Mucotoxic Myeloablative Conditioning. BBMT. 2016;22(6):1137–1141. doi: 10.1016/j.bbmt.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 30.Barba P, Ratan R, Cho C, et al. The Hematopoietic Cell Transplantation Comorbidity Index (HCT-CI) Predicts Outcomes in Patients with Acute Myeloid Leukemia and Myelodysplastic Syndromes Receiving CD34+ Selected Grafts for Allogeneic Hematopoietic Cell Transplantation. BBMT. 2016;23(1):67–74. doi: 10.1016/j.bbmt.2016.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johansson J-E, Ekman T. Gut Toxicity During Hemopoietic Stem Cell Transplantation May Predict Acute Graft-Versus-Host Disease Severity in Patients. Dig Dis Sci. 2007;52(9):2340–2345. doi: 10.1007/s10620-006-9404-x. [DOI] [PubMed] [Google Scholar]
- 32.Proli AJ, Tamari R, Zheng J, et al. Impact of Busulfan Exposure on Transplant Outcomes for Patients with Advanced Myelodysplastic Syndrome Undergoing CD34 Selected Allogeneic Hematopoietic Stem Cell Transplantation. BBMT. 2016;22(3):S348. doi: 10.1016/j.bbmt.2015.11.841. [DOI] [Google Scholar]
- 33.Beyer K, Proli A, Zheng J, et al. Outcomes of Pharmacokinetically Targeted Busulfan-Based Conditioning Regimen for Patients with Myelodysplastic Syndrome and Acute Myelogenous Leukemia Undergoing CD34 Selected Allogeneic Hematopoietic Stem Cell Transplantation. Blood. 2016;128(22). http://www.bloodjournal.org/content/128/22/3392. Accessed May 2, 2017. [Google Scholar]
- 34.Geddes M, Kangarloo SB, Naveed F, et al. High Busulfan Exposure Is Associated with Worse Outcomes in a Daily i.v. Busulfan and Fludarabine Allogeneic Transplant Regimen. BBMT. 2008;14(2):220–228. doi: 10.1016/j.bbmt.2007.10.028. [DOI] [PubMed] [Google Scholar]
- 35.Bartelink IH, Bredius RGM, Belitser S V., et al. Association between Busulfan Exposure and Outcome in Children Receiving Intravenous Busulfan before Hematologic Stem Cell Transplantation. BBMT. 2009;15(2):231–241. doi: 10.1016/j.bbmt.2008.11.022. [DOI] [PubMed] [Google Scholar]
- 36.Perkins J, Field T, Kim J, et al. Pharmacokinetic targeting of i.v. BU with fludarabine as conditioning before hematopoietic cell transplant: the effect of first-dose area under the concentration time curve on transplant-related outcomes. BMT. 2011;46(11):1418–1425. doi: 10.1038/bmt.2010.315. [DOI] [PubMed] [Google Scholar]
- 37.Perkins JB, Kim J, Anasetti C, et al. Maximally Tolerated Busulfan Systemic Exposure in Combination with Fludarabine as Conditioning before Allogeneic Hematopoietic Cell Transplantation. BBMT. 2012;18(7):1099–1107. doi: 10.1016/j.bbmt.2011.12.584. [DOI] [PubMed] [Google Scholar]
- 38.Palmer J, McCune JS, Perales M-A, et al. Personalizing Busulfan-Based Conditioning: Considerations from the American Society for Blood and Marrow Transplantation Practice Guidelines Committee. BBMT. 2016;22(11):1915–1925. doi: 10.1016/j.bbmt.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 39.Barba P, Hilden P, Devlin SM, Maloy M, Ciolino C. CD34 + Selected Ex-Vivo T-Cell Depleted ( TCD ) Grafts for Allogeneic Hematopoietic Cell Transplantation ( Allo-HSCT ) Is Associated with Low Incidence of Acute and Chronic Graft-Versus-Host Disease ( GVHD ) and High Chronic-Gvhd / Relapse-Free Survival. BBMT. 2017;23(3):452–458. doi:doi: 10.1016/j.bbmt.2016.12.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cutler C, Logan B, Nakamura R, et al. Tacrolimus/sirolimus vs tacrolimus/methotrexate as GVHD prophylaxis after matched, related donor allogeneic HCT. Blood. 2014;124(8):1372–1377. doi: 10.1182/blood-2014-04-567164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anasetti C, Logan BR, Lee SJ, et al. Peripheral-blood stem cells versus bone marrow from unrelated donors. NEJM. 2012;367(16):1487–1496. doi: 10.1056/NEJMoa1203517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barba P, Hilden P, Devlin SM, et al. Ex Vivo CD34+–Selected T Cell–Depleted Peripheral Blood Stem Cell Grafts for Allogeneic Hematopoietic Stem Cell Transplantation in Acute Leukemia and Myelodysplastic Syndrome Is Associated with Low Incidence of Acute and Chronic Graft-versus- Host Disease. BBMT. 2017;23(3):452–458. doi: 10.1016/j.bbmt.2016.12.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ho VT, Revta C, Richardson PG. Hepatic veno-occlusive disease after hematopoietic stem cell transplantation: update on defibrotide and other current investigational therapies. BMT. 2008;41(3):229–237. doi: 10.1038/sj.bmt.1705899. [DOI] [PubMed] [Google Scholar]
- 44.Mohty M, Malard F, Abecassis M, et al. Sinusoidal obstruction syndrome/veno-occlusive disease: current situation and perspectives-a position statement from the European Society for Blood and Marrow Transplantation (EBMT). BMT. 2015;50(6):781–789. doi: 10.1038/bmt.2015.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dalle J-H, Giralt SA. Hepatic Veno-Occlusive Disease after Hematopoietic Stem Cell Transplantation: Risk Factors and Stratification, Prophylaxis, and Treatment. BBMT. 2016;22(3):400–409. doi: 10.1016/j.bbmt.2015.09.024. [DOI] [PubMed] [Google Scholar]
- 46.Moscardó F, Sanz G, De La Rubia J, et al. Post-transplant complications Marked reduction in the incidence of hepatic veno-occlusive disease after allogeneic hematopoietic stem cell transplantation with CD34 + positive selection. BMT. 2001;27:983–988. [DOI] [PubMed] [Google Scholar]
- 47.Moscardó F, Urbano-Ispizua A, Sanz GF, et al. Positive selection for CD34+ reduces the incidence and severity of veno-occlusive disease of the liver after HLA-identical sibling allogeneic peripheral blood stem cell transplantation. Exp Hematol. 2003;31(6):545–550. http://www.ncbi.nlm.nih.gov/pubmed/12829031. Accessed May 2, 2017. [DOI] [PubMed] [Google Scholar]
- 48.Kogon A, Hingorani S. Acute Kidney Injury in Hematopoietic Cell Transplantation. Semin Nephrol. 2010;30(6):615–626. doi: 10.1016/j.semnephrol.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


