Abstract
A case-control study was performed to determine whether an association exists between exposure to synthetic oxytocin and a subsequent autism spectrum disorder diagnosis; 171 children under age 18 meeting Diagnostic and Statistical Manual of Mental Disorders (5th ed.) autism spectrum disorder criteria were compared to 171 children without autism spectrum disorder diagnosis matched by gender, birth year, gestational age, and maternal age at birth. A conditional logistic regression model was used to examine the association of clinical variables and autism spectrum disorder. Significantly elevated odds ratios for autism spectrum disorder were associated with first-time Cesarean section (odds ratio = 2.56), but not a repeat Cesarean section. Odds ratios were also significantly elevated for subjects whose mother’s body mass index was 35 or higher at birth (odds ratio = 2.34) and subjects in which the reason for delivery was categorized as “fetal indication” (odds ratio = 2.00). When controlling for these and other variables, the odds of developing autism spectrum disorder were significantly elevated in males with long duration of exposure (odds ratio = 3.48) and high cumulative dose of synthetic oxytocin (odds ratio = 2.79). No significant associations of synthetic oxytocin dosing and autism spectrum disorder were noted in female subjects. The association of elevated autism spectrum disorder odds found with high duration and high cumulative dose synthetic oxytocin in male subjects suggests the need for further investigation to fully elucidate any cause and effect relationship.
Lay abstract
Oxytocin is a hormone naturally produced in the human body that can make the womb (uterus) contract during labor. Manufactured oxytocin is frequently given to mothers in labor to strengthen the contractions or in some cases to start labor. This study compared children with a diagnosis of autism and children without autism to see whether children with autism received more oxytocin during labor. The odds of a child having an autism diagnosis were significantly higher if the delivery was a first-time Cesarean section, if the mother had a body mass index of 35 or higher, or if the reason for delivery were a range of fetal problems that made delivery necessary. It was found that boys who were exposed to oxytocin for longer periods of time during labor and received higher total doses of oxytocin had significantly higher odds of developing autism. There were no significant associations of oxytocin dosing and autism noted in female children. As this is the first study to look at any relationship between the dose of oxytocin received during labor and the odds of developing autism, further study needs to be done to determine whether there is any cause and effect relationship. Thus, at this time, there is no recommended change in clinical practice.
Keywords: autism spectrum disorders, Cesarean, labor, oxytocin
Oxytocin (OT) is a central nervous system (CNS) neuropeptide that plays a critical role in social bonding; abnormalities in OT activity have been associated with autism spectrum disorder (ASD; Carter et al., 2009; Champagne & Meaney, 2006; Hashemi et al., 2013; Israel et al., 2008; Modahl et al., 1998; Takayanagi et al., 2005; Wahl, 2004). Synthetic oxytocin (sOT) is utilized to induce or augment uterine contractions during labor (Simpson, 2011). Several groups have investigated whether sOT utilization during labor is associated with future diagnosis of ASD; however, the results are conflicting. While some studies report no association (Brimacombe et al., 2007; Burstyn et al., 2010; Fein et al., 1997; Gale et al., 2003; Laxer et al., 1988; Maimburg & Vaeth, 2006; Mason-Brothers et al., 1990; Oberg et al., 2016; Stein et al., 2006), others found a modestly elevated odds of developing ASD based on exposure to sOT during labor induction/augmentation (Dodds et al., 2011; Gardener et al., 2011; Glasson et al., 2004; Gregory et al., 2013; Hollander et al., 1998; Juul-Dam et al., 2001; Weisman et al., 2015).
These contradictory conclusions may be predicated on analyses that did not specify the method(s) utilized for augmentation and/or labor induction, sOT dosing, and any association of induction/augmentation methods with the eventual mode of delivery. For example, Gregory et al. (2013) reported increased odds of ASD after labor induction/augmentation, an effect that was most pronounced in male infants. This study was criticized (Vintzileos & Ananth, 2013) due to the lack of information regarding the methods used for induction/augmentation. The study utilizing the North Carolina Birth Record and Education Research Databases included individuals who had been diagnosed with Pervasive Developmental Disorder Not Otherwise Specified (including atypical autism; PDD-NOS) in the ASD group. This inclusion was based on the Diagnostic and Statistical Manual of Mental Disorders (4th ed.; DSM-IV; American Psychiatric Association, 1994) criteria, which were criticized (Vintzileos & Ananth, 2013) as resulting in overdiagnosis of ASD. In DSM-IV, the criteria for PDD-NOS required severe impairment in reciprocal social interaction, communication, or presence of stereotyped behavior, while the diagnosis of an autistic disorder required the presence of all three. In the Diagnostic and Statistical Manual of Mental Disorders (4th ed., text rev.; DSM-IV-TR; American Psychiatric Association, 2000), the criterion for PDD-NOS was changed to require severe impairment in reciprocal social interaction along with either impaired communication or the presence of stereotyped behavior. When subsequent analysis of the data collected by Gregory et al. (2013) was performed using DSM-IV-TR criteria, no association was found between induction of labor and subsequent ASD (Vintzileos & Ananth, 2013). Consequently, the American College of Obstetricians and Gynecologists (ACOG) Committee on Obstetric Practice (2014) recommended against a change in practice.
The current study focuses on these discrepancies and examines whether intrapartum induction or augmentation with sOT (as a function of dosage, rate, and time of exposure) and other modes of delivery are associated with a subsequent ASD diagnosis based on current Diagnostic and Statistical Manual of Mental Disorders (5th ed.; DSM-5; American Psychiatric Association, 2013) criteria. As ASD is more prevalent in males (Baio et al., 2018), this study examines whether gender was a factor related to the odds of an ASD diagnosis after intrapartum exposure to sOT.
Methods
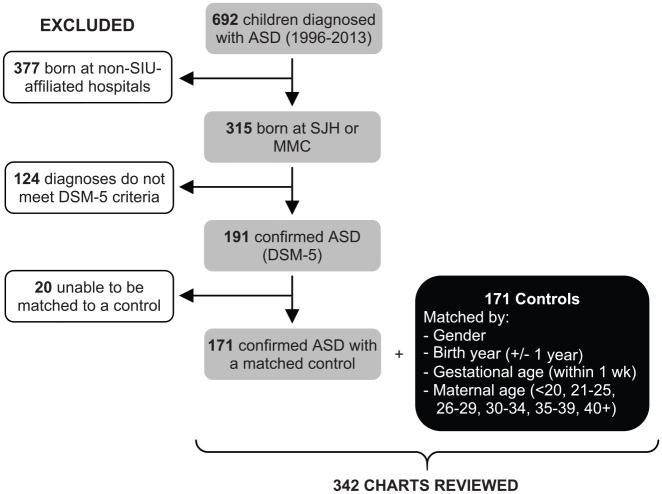
This study was approved by the Institutional Review Board (IRB) of Southern Illinois University (SIU) School of Medicine (#15-324). A retrospective case-control chart review was performed including all children (⩽18 years old at the time of IRB approval) who were seen by SIU School of Medicine providers between 1996 and 2013. Potential subjects were diagnosed with ASD utilizing the following ninth revision of the International Classification of Diseases (ICD-9) codes (299.00, 299.80, 299.10), which distinguish the DSM-IV and DSM-5 (American Psychiatric Association, 2013) ASD criteria (n = 692; Figure 1). Of these, only records belonging to children born at either of the two SIU-affiliated hospitals in Springfield, Illinois (n = 315), were included in the analysis. In order to ascertain the accurate ASD diagnosis by DSM-5 criteria, the review of ASD diagnosis was performed by a board-certified child psychiatrist who was blinded to intrapartum sOT exposure. The electronic medical records (EMRs) were carefully reviewed for documentation to assure the subjects met DSM-5 criteria for persistent deficits in social communication and social interaction as well as for restricted, repetitive patterns of behavior, interests, or activities that had been present in the early developmental period and were causing clinically significant impairment in important areas of functioning. The EMR needed sufficient information to clarify that any behavioral disturbances were not better explained by intellectual disability or global developmental delay. Exclusion criteria were incomplete/unavailable birth records and those diagnosed with any genetic abnormalities or congenital diseases associated with ASD (e.g. Fragile X, Angelman syndrome, or tuberous sclerosis). This review found 191 subjects with confirmed ASD.
Figure 1.
Subject selection and study population.
Control subjects were selected from a population of children seen by SIU School of Medicine providers and born at either of the two SIU-affiliated hospitals between 1997 and 2011. To eliminate individuals with known psychiatric disorders, children diagnosed with ICD-9 codes 290–319 were excluded. This resulted in a potential matching pool of approximately 165 control subjects per birth year, per gender. Each potential control subject was then matched to one ASD subject based on gender, birth year (±1 year), gestational age (GA) at birth (within the same week of gestation), and maternal age range (⩽24, 25–29, 30–34, 35–39, 40+) by research team members who were blinded to intrapartum sOT exposure. The first potential control subject confirmed to match on all four criteria would then have their EMR reviewed to ensure the absence of any psychiatric diagnosis, if this was confirmed they were selected as a control subject. Twenty ASD subjects were unable to be matched to a control and were excluded, resulting in a final population of 171 ASD subjects (137 males and 34 females) and their matched controls (Supplemental Table S1).
Maternal demographics which included body mass index (BMI), parental age, intrapartum duration, mode of delivery, maternal health information, and family history of ASD were extracted from the electronic health record (Supplemental Table S2).
Three factors related to sOT dosing were chosen to be examined. In obstetric practice, sOT is administered via a continuous infusion at a rate specified in milliunits (mU) per minute. The rate is then increased until the desired frequency and strength of contractions are achieved while monitoring for signs of fetal distress and side effects from sOT administration such as hyponatremia. Since this was the first known study looking at dosing, there were no data available regarding which dosing parameters should be examined with regard to sOT and ASD. It was decided to look at the maximum rate of sOT administration, the total time sOT was administered at any rate (exposure), and the total cumulative dose of sOT administered (mU/minute × duration of administration for each rate utilized and then totaled).
In order to further evaluate the relationship between sOT dosing and ASD, cumulative sOT dosing, maximum rate of sOT administration, and duration of sOT exposure were categorized by levels of exposure. Subjects who had no exposure to sOT (n = 136) were assigned as the reference group (sOT = 0 mU). The remaining subjects were divided into three groups (designated as levels of exposure) of approximately equal numbers for each variable. This division was arbitrarily determined because there is no established definition of low, moderate, and high sOT dosing levels. The three cumulative sOT dosing groups were labeled as 1st level (low dosing, 104–1760 mU, n = 69), 2nd level (moderate dosing, 1761–5492 mU, n = 69), and 3rd level (high dosing, 5493–40,338 mU, n = 68) for cumulative sOT exposure.
A similar approach was used to create levels of exposure for maximum rate of sOT administration in mU per minute (mU/min): 1st level (low rate: 0.5–7.0 mU/min, n = 65), 2nd level (moderate rate: 8.0–13.0 mU/min, n = 71), and 3rd level (high rate: 14.0–32.0 mU/min, n = 70). The levels for duration of sOT exposure were defined as 1st level (low duration: 79–364 min, n = 69), 2nd level (moderate duration: 366–672 min, n = 69), and 3rd level (high duration: 678–3343 min, n = 68).
The following example illustrates how the dosing and exposure time for sOT were calculated: a woman who begins receiving sOT at 7:00 a.m. with an infusion rate of 2 mU/min which is subsequently increased to 4 mU/min at 9:00 a.m., 6 mU/min at 10:00 a.m., 8 mU/min at 12:00 p.m., 10 mU/min at 1:30 p.m., 11 mU/min at 2:30 with a final increase to a maximum rate of 12 mU/min at 3:50 p.m. would have received a cumulative sOT dose of 3580 mU over 545 min of exposure (9.08 h) by the time of delivery (4:05 p.m.). The sOT data from this delivery would be categorized in Level 2 for cumulative dose, maximum rate of administration, and time of exposure.
Data analysis
To account for the matched study design, a conditional logistic regression model was used to assess the association between each variable and ASD status. The outcome was a binary (yes or no) measure of the presence or absence of ASD. The variables were labor induction, augmentation, exposure to sOT (for either induction or augmentation), exposure to prostaglandins, sOT cumulative dosage, sOT maximum rate, and sOT duration of exposure. The threshold for statistical significance was set at p < 0.05 (noted in bold in tables). The sOT-ASD association was estimated based on odds ratios (ORs) with 95% confidence intervals (CIs) around the point estimate. All statistical analyses were conducted using Stata 14.0 (StataCorp, College Station, TX, USA).
Results
Univariate conditional logistic regression analyses investigated differences of demographic and clinical features between the ASD and non-ASD groups (Table 1). The odds of having ASD were significantly lower in non-Caucasian subjects (OR = 0.27, 95% CI = 0.13–0.55, p = 0.001).
Table 1.
Univariate conditional logistic regression analyses of demographic and clinical features of sample, n = 342 or 171 pairs.
| Variable | Category | Non-ASD n = 171 n (%) |
ASD n = 171 n (%) |
Test statistic OR (95% CI) |
p value |
|---|---|---|---|---|---|
| Child race | Caucasian | 131 (45.5) | 157 (54.5) | Reference | |
| Not Caucasian | 40 (74.1) | 14 (25.9) | 0.27 (0.13–0.55) | 0.001 | |
| Marital status | Married | 102 (50.5) | 100 (49.5) | Reference | 0.782 |
| Not married | 69 (49.3) | 71 (50.7) | 1.08 (0.62–1.86) | ||
| Maternal hypertension | None | 154 (50.5) | 151 (49.5) | Reference | 0.602 |
| Chronic/gestational hypertension/preeclampsia | 17 (45.9) | 20 (54.0) | 1.2 (0.60–2.38) | ||
| Mode of delivery | Vaginal | 125 (56.3) | 97 (43.7) | 1 | |
| Vacuum/forceps | 10 (45.4) | 12 (54.5) | 1.38 (0.59–3.26) | 0.451 | |
| Cesarean | 36 (36.7) | 62 (63.3) | 2.22 (1.33–3.70) | 0.002 | |
| Indication for delivery | Spontaneous | 90 (55.7) | 78 (46.4) | Reference | |
| Elective | 14 (63.6) | 8 (36.4) | 0.61 (0.23–1.57) | 0.306 | |
| Hypertensive disease and diabetes | 12 (36.4) | 21 (63.6) | 2.04 (0.92–4.50) | 0.077 | |
| Repeat-Cesarean sectiona | 10 (66.7) | 5 (33.3) | 0.61 (0.19–1.91) | 0.403 | |
| Dates | 23 (46.9) | 26 (53.1) | 1.33 (0.59–2.98) | 0.483 | |
| Fetal indication | 22 (40.0) | 33 (60.0) | 2.00 (1.01–3.94) | 0.044 | |
| BMI | BMI below 30 | 79 (56.0) | 62 (44.0) | 1 | |
| BMI 30–34.99 | 48 (57.8) | 35 (42.2) | 1.03 (0.57–.87) | 0.902 | |
| BMI 35+ | 44 (37.3) | 74 (62.7) | 2.34 (1.33–4.10) | 0.003 | |
| Smoking during pregnancy | No | 134 (51.2) | 128 (48.8) | 1 | |
| Yes | 37 (46.3) | 43 (53.7) | 1.21 (0.74–1.97) | 0.454 | |
| Alcohol use during pregnancy | No | 169 (49.8) | 170 (50.2) | 1 | |
| Yes | 2 (66.7) | 1 (33.3) | 0.50 (0.04–5.51) | 0.571 | |
| Paternal age (years) | <24 | 158 (50.8) | 153 (49.2) | Reference | |
| 25–29 | 8 (36.4) | 14 (63.6) | 2.36 (0.80–6.96) | 0.119 | |
| 30–34 | 4 (66.7) | 2 (33.3) | 0.50 (0.09–2.73) | 0.423 | |
| 35–39 | 1 (33.3) | 2 (66.7) | 2.73 (0.23–32.70) | 0.428 |
Fetal indication: placental anomalies, placental abruption, premature rupture of membranes, chorioamnionitis, breech, intrauterine growth restriction, oligohydramnios, multiple fetuses, polyhydramnios, macrosomia, abnormal fetal heart tracings, and congenital anomalies. ASD: autism spectrum disorder; OR: odds ratio; CI: confidence interval; BMI: body mass index.
Compared with spontaneous vaginal delivery, the odds of ASD were significantly higher for children who were delivered via a Cesarean section (C/S; OR = 2.22, 95% CI = 1.33–3.70, p = 0.002; Table 1). Among patients undergoing C/S, the percentages of repeat C/S were 22.5% in the ASD group and 33.3% in the non-ASD group. Repeat C/S is usually a scheduled procedure without sOT administration except in uncommon eases where parents want to attempt a vaginal birth during a subsequent pregnancy. In our sample, only 8% of repeat C/S subjects received sOT as opposed to 62.5% of the first-time C/S subjects (Supplemental Table S3). Thus, first-time C/S and repeat C/S groups were examined separately. Results indicated that a first-time C/S was associated with significantly elevated odds of having ASD (OR = 2.56, 95% CI = 1.44–4.58, p = 0.001; Table 2).
Table 2.
Univariate conditional logistic regression analysis of delivery procedures, n = 342 or 171 pairs.
| Category | Non-ASD n (%) |
ASD n (%) |
OR (95% CI) | p value |
|---|---|---|---|---|
| Vaginal | 125 (56.3) | 97 (43.7) | 1 | Reference |
| Vacuum/forceps | 10 (45.4) | 12 (54.5) | 1.33 (0.57–3.12) | 0.512 |
| Cesarean, first-time | 24 (33.3) | 48 (66.6) | 2.56 (1.44–4.58) | 0.001 |
| Cesarean, first-time no fetal indications | 14 (29.8) | 33 (70.2) | 3.94 (1.54–10.09) | 0.004 |
| Cesarean, first-time fetal indications | 10 (40.0) | 15 (60.0) | 1.18 (0.39–3.51) | 0.769 |
| Cesarean, repeata | 12 (46.1) | 14 (53.9) | 1.55 (0.69–3.49) | 0.284 |
ASD: autism spectrum disorder; OR: odds ratio, CI: confidence interval.
Some first-time C/S were done due to the presence of fetal indications (defined in Table 1) and were more likely to go directly to surgery without receiving sOT (40% received sOT; Supplemental Table S3). More subjects in the ASD group had a fetal indication for a first-time C/S (Table 2) than in the non-ASD group, but this did not reach statistical significance, possibly due to the small number of subjects in these groups.
In the absence of fetal indications, patients who eventually underwent first-time C/S were much more likely to have received sOT to augment or induce delivery (74% received sOT; Supplemental Table S3). Compared to the spontaneous vaginal delivery group, first-time C/S with no fetal indication had significantly elevated odds of ASD (OR = 3.94, p = 0.004; Table 2). Given these results, mode of delivery was included as a covariate in the subsequent data analysis.
Subjects whose mother’s BMI was ⩾35 had significantly elevated odds of an ASD diagnosis (Table 1; OR = 2.34, 95% CI = 1.33–4.10, p = 0.003). Because elevated BMI is associated with the potential need for higher cumulative sOT dose for successful labor induction (Roloff et al., 2015) and a higher maternal BMI is associated with an increased risk for ASD (Getz et al., 2016; Li et al., 2016), this variable was included as a covariate in subsequent analysis of the data.
Using spontaneous labor as a reference (no exposure to sOT), cases categorized as “fetal indication” for delivery (Table 1) as a group had significantly higher odds of ASD (OR = 2.00, 95% CI = 1.01–3.94, p = 0.044). To control for the possibility that fetal indications could confound the results in Tables 3 to 6, this variable was included as a covariate in subsequent analysis as was the presence of maternal hypertension (Curran et al., 2018), maternal diabetes (Li et al., 2016; OR = 2.04, p = 0.077), maternal education (as a substitute for the lack of socioeconomic status data), birth weight (Burstyn et al., 2010), maternal smoking (Caramaschi et al., 2018) or alcohol use during pregnancy (Bölte et al., 2019), the child’s race (Baio et al., 2018), and paternal age (Taylor et al., 2019).
Table 3.
Conditional logistic regression results on the association between clinical variables and the odds of ASD among all subjects (n = 342 or 171 pairs).
| Variable | Levels | Non-ASD | ASD | OR | 95% CI | p value |
|---|---|---|---|---|---|---|
| Cumulative dose of sOT | 0 mU | 70 | 66 | 1 | ||
| 1st level | 44 | 25 | 0.64 | 0.30–1.37 | 0.252 | |
| 2nd level | 34 | 35 | 1.41 | 0.65–3.06 | 0.381 | |
| 3rd level | 23 | 45 | 2.31 | 1.03–5.19 | 0.043 | |
| Maximum dose rate | 0 mU/min | 70 | 66 | 1 | ||
| 1st level | 37 | 28 | 0.82 | 0.40–1.72 | 0.614 | |
| 2nd level | 37 | 34 | 1.29 | 0.61–2.74 | 0.505 | |
| 3rd level | 27 | 43 | 1.82 | 0.85–3.86 | 0.121 | |
| Time of exposure | 0 min | 70 | 66 | 1 | ||
| 1st level | 45 | 24 | 0.63 | 0.29–1.35 | 0.237 | |
| 2nd level | 31 | 38 | 1.48 | 0.71–3.01 | 0.296 | |
| 3rd level | 25 | 43 | 2.25 | 0.96–5.24 | 0.060 |
All variables are categorical and coded so that OR > 1 indicates higher odds of ASD. Variables controlled for were maternal hypertension, maternal diabetes, maternal education, indication for delivery (including fetal indications), birth weight, maternal body mass index, mode of delivery, child race, smoking during pregnancy, alcohol use during pregnancy, and paternal age. The exposure variables (cumulative dose of sOT, maximum dose rate, and time of exposure) were run in separate models. Each model consisted of the exposure variable, covariates, and the outcome. ASD: autism spectrum disorder; OR: odds ratio; CI: confidence interval; sOT: synthetic oxytocin.
Table 6.
Follow-up tests for effect modification: conditional logistic regression results on cumulative dose of sOT and mode of delivery as variables associated with ASD (n = 294).
| Variable | OR | 95% CI | p value |
|---|---|---|---|
| Cumulative dose of sOT per 500 units | 1.040 | 0.985–1.099 | 0.157 |
| C/S first-time no fetal indication versus normal delivery (reference) | 4.623 | 1.122–19.042 | 0.034 |
| Cumulative dose of sOT per 500 units × mode of delivery | 0.974 | 0.899–1.056 | 0.531 |
OR > 1 indicates higher odds of ASD. Variables controlled for were maternal hypertension, maternal diabetes, maternal education, birth weight, maternal body mass index, child race, smoking during pregnancy, alcohol use during pregnancy, and paternal age. sOT: synthetic oxytocin; ASD: autism spectrum disorder; OR: odds ratio, CI: confidence interval; C/S: Cesarean section.
The ASD and non-ASD groups were compared for any differences in induction and augmentation procedures utilized during the intrapartum period without taking into consideration sOT dosing (exposure vs no exposure). Induction and augmentation by any method and exposure to prostaglandins were not associated with significantly greater odds of ASD (Supplemental Table S4).
However, when sOT dosing was categorized by levels of exposure, a conditional logistic regression analysis of the data (which controlled for the potential confounding factors previously described) found that exposure to the highest level of cumulative sOT dosing (5493 mU or higher) was associated with significantly elevated odds of ASD (OR = 2.31, 95% CI = 1.03–5.19, p = 0.043; Table 3).
Furthermore, the odds of an ASD diagnosis were elevated for subjects exposed to the longest duration of sOT (678 min or longer; OR = 2.25), although not quite significantly (p = 0.060). There was no significant association between the maximum rate of sOT administration and the odds of developing ASD (Table 3).
Due to the greater prevalence of ASD in males (Baio et al., 2018), a conditional logistic regression analysis was performed examining the sOT utilization in subjects stratified by gender. Male infants who had been exposed to the highest level of cumulative sOT dosing (5493 mU or higher) had significantly greater odds of a subsequent ASD diagnosis (Table 4; OR = 2.79, 95% CI = 1.10–7.46, p = 0.031). Time of exposure to sOT in males at the highest level was also significantly associated with elevated odds of ASD (OR = 3.48, 95% CI = 1.29–9.41, p = 0.014).
Table 4.
Conditional logistic regression results on the association between clinical variables and the odds of ASDs among boys (n = 274 or 137 pairs).
| Variable | Levels | Non-ASD | ASD | OR | 95% CI | p value |
|---|---|---|---|---|---|---|
| Cumulative dose of sOT | 0 mU | 57 | 51 | 1 | ||
| 1st level | 37 | 20 | 0.61 | 0.26–1.41 | 0.250 | |
| 2nd level | 25 | 25 | 1.62 | 0.63–4.16 | 0.310 | |
| 3rd level | 18 | 41 | 2.79 | 1.10–7.06 | 0.031 | |
| Maximum dose rate | 0 mU/min | 57 | 51 | 1 | ||
| 1st level | 29 | 23 | 0.85 | 0.37–1.97 | 0.709 | |
| 2nd level | 31 | 26 | 1.27 | 0.55–2.95 | 0.575 | |
| 3rd level | 20 | 37 | 1.97 | 0.83–4.69 | 0.127 | |
| Time of exposure | 0 min | 57 | 51 | 1 | ||
| 1st level | 39 | 17 | 0.52 | 0.22–1.22 | 0.134 | |
| 2nd level | 23 | 28 | 1.79 | 0.69–4.61 | 0.229 | |
| 3rd level | 18 | 41 | 3.48 | 1.29–9.41 | 0.014 |
All variables are categorical and coded so that OR > 1 indicates higher odds of ASD. Variables controlled for were maternal hypertension, maternal diabetes, maternal education, indication for delivery (including fetal indications), birth weight, maternal body mass index, mode of delivery, child race, smoking during pregnancy, alcohol use during pregnancy, and paternal age. The exposure variables (cumulative dose of sOT, maximum dose rate, and time of exposure) were run in separate models. Each model consisted of the exposure variable, covariates, and the outcome. ASD: autism spectrum disorder; OR: odds ratio, CI: confidence interval; sOT: synthetic oxytocin.
There was no significant association between clinical variables—sOT cumulative dosing, rate of administration, or duration of exposure—and the odds of ASD in female subjects (Supplemental Table S5).
For both male and female subjects (Table 4 and Supplemental Table S5), the odds of ASD were reduced (but not to the point of statistical significance) for those subjects in the lowest ranges of cumulative dose, maximum rate, and duration of exposure to sOT.
To recheck the association between the various dosing variables and the odds of ASD without the arbitrary classification of low, moderate, and high levels of sOT exposure, a conditional logistic regression analysis was performed that treated the three dosing variables as continuous. Among boys, the OR significantly increased an average of 1.059 with every 500 mU increase in cumulative dosing (p = 0.008), 1.628 with every 10 mU/min increase in maximum dosage rate (p = 0.037), and 2.065 with every 500 min increase in time of exposure (p = 0.004) (Table 5). No significant association between the dosing and the odds of ASD was noted in females (Supplemental Table S6).
Table 5.
Conditional logistic regression results on the association between clinical variables and the odds of ASDs among boys (n = 274 or 137 pairs).
| Variablea | OR | 95% CI | p value |
|---|---|---|---|
| Cumulative dose of sOT per 500 mU | 1.059 | 1.015–1.105 | 0.008 |
| Maximum dose rate per 10 mU/min | 1.628 | 1.029–2.575 | 0.037 |
| Time of exposure per 500 min | 2.065 | 1.255–3.398 | 0.004 |
OR > 1 indicates higher odds of ASD. Variables controlled for were maternal hypertension, maternal diabetes, maternal education, indication for delivery (including fetal indications), birth weight, maternal body mass index, mode of delivery, child race, smoking during pregnancy, alcohol use during pregnancy, and paternal age. ASDs: autism spectrum disorders; OR: odds ratio, CI: confidence interval; sOT: synthetic oxytocin.
The exposure variables (cumulative dose of sOT, maximum dose rate, and time of exposure) were run in separate models. Each model consisted of the exposure variable, covariates, and the outcome.
The finding that first-time C/S in deliveries where there were no fetal indications on admission was associated with significantly increased odds of ASD (Table 2) raises the question of whether this finding is related to the mothers receiving higher cumulative doses of sOT prior to the procedure. The multivariate conditional logistic regression for interaction between these variables (Table 6) suggests that the cumulative dose of sOT and first-time C/S with no fetal indications are independent variables and that the association of first-time C/S with an increased OR for ASD is not a function of cumulative sOT dosing in these patients.
Discussion
An association between an ASD diagnosis and intrapartum sOT exposure remains open and controversial. A review of the literature found no study that considered dose or duration of exposure to sOT and the odds of developing ASD. Consequently, this study is the first to examine whether cumulative dose, rate of administration, and duration of exposure to sOT, along with other methods used for induction and/or augmentation, may be associated with a subsequent diagnosis of ASD. When these three variables were broken down into exposure levels, two significant impacts were noted on the odds of developing ASD. The odds of male subjects having an ASD diagnosis were significantly elevated with high cumulative dose and long duration of exposure to sOT. These results cannot be used to imply causality but suggest the need for further investigation via prospective studies.
Another intriguing result was that the odds of ASD were reduced (but not to the point of statistical significance) in both males and females in the lowest ranges of cumulative dose, maximum rate, and duration of exposure to sOT. If this study were to be repeated in a larger population, it would be interesting to see whether this finding is replicated and found to be statistically significant.
The possibility that there is a varied association of sOT exposure with the odds of developing ASD in humans (lower dose sOT possibly reducing the odds of ASD and elevated sOT dosing increasing the odds) is highlighted by the literature on animal behavior. Male voles who received a 3 µg dose of OT on postnatal day 1 exhibited enhanced pair bonding upon maturity, while those receiving a 6 µg dose showed no pair bonding. A 12 µg dose resulted in bonding capability being reinstated (Carter et al., 2008, 2009). In contrast, female voles receiving 3 µg of OT neonatally had minimal behavioral impacts, a 6 µg dose enhanced pair bonding, and 12 µg demonstrated no bonding facilitation. Neonatal doses of 24 µg in females resulted in an atypical preference for unfamiliar males (Bales & Carter, 2003; Carter et al., 2009).
Prior human studies examining the possible associations between sOT use and ASD considered only sOT exposure but not dosage. These prior studies have produced a range of results suggesting both no association and a positive association with ASD. If varying doses of sOT in humans have differing impacts on the odds of developing ASD, the potential associations found in this study need to be confirmed with a larger sample size that allows for categorization of much smaller dosage ranges and exposure duration periods of sOT with the odds of ASD.
Studies examining the possible association of C/S and ASD have found both increased odds of an ASD diagnosis with C/S (Brimacombe et al., 2007; Curran et al., 2014) and no association (Curran et al., 2015, 2016). In this study, first-time C/S, but not repeat C/S, was associated with significantly higher odds of developing ASD.
Mothers admitted with no fetal indications who were induced or augmented and subsequently required a first-time C/S had significantly elevated odds of ASD that did not appear to be a function of receiving increased doses of sOT. Intrapartum sOT administration has many recognized potential side effects, the most common being uterine hyperstimulation that can have a progressive negative effect on fetal oxygenation, acid–base status, and heart rate (Simpson, 2011). Failure of labor to progress with sOT, coupled with evidence of fetal distress, often leads to a first-time C/S. Future studies will need to discern whether the association of sOT and ASD may involve direct impact of sOT on CNS OT receptors and CNS OT production or other impacts due to sOT-induced side effects on the fetal brain.
The current study found that male infants with the highest levels of sOT duration of exposure and cumulative dosing had significantly higher odds of an ASD diagnosis than male infants who received no sOT, while no such association was found in female subjects. Weisman et al. (2015) reported that augmented labor with OT in males had a moderately increased risk of ASD, while sOT augmentation in females did not. Our current results support the findings of Weisman et al. (2015), and build upon them, by suggesting that this effect in males may be a function of the duration of sOT exposure and cumulative sOT dose.
The apparent specific susceptibility of males to high sOT exposures is intriguing, given the male preponderance of ASD diagnoses (presently, 1:38 males will be diagnosed with ASD vs 1:152 females; Baio et al., 2018). The intrapartum phase is a critical period of sexual differentiation of the mammalian brain (Grumbach et al., 2002). Specifically, manipulations of typical steroid exposures during this period of rodent development can reverse brain sex differences and interfere with the development of juvenile social behaviors (McCarthy & Ball, 2008). Whether sOT alters sex differences in the brain with subsequent alterations of behavior remains an open question. However, recent reports indicate that development of the central OT system is sexually dimorphic in rodents (Carter et al., 2009; Scott et al., 2015; Tamborski et al., 2016; Yamasue et al., 2009), suggesting intrapartum manipulations of this system could impair or alter the development of related social behaviors.
Since exogenous administration of sOT to the mother can result in reduced fetal production of OT (Dawood et al., 1978), the association between the odds of having ASD and high cumulative doses/time of exposure of sOT suggests that downregulation of fetal OT activity in the intrapartum period may play a role in increasing the odds of ASD development. Reduced endogenous OT functioning has previously been correlated with ASD (Modahl et al., 1998). To supplement these findings, future studies utilizing animal models are needed to determine the impact of induction/augmentation with various intrapartum sOT doses on subsequent behavior and OT levels in the brain of the offspring.
It is important to note the limitations of this study. First, the finding that being from a non-Caucasian background was associated with lower odds of having an ASD diagnosis is consistent with other studies reporting racial and ethnic disparities in recognizing ASD in minority populations (Mandell et al., 2009). It has been reported that non-Hispanic Black children were less likely than non-Hispanic White children to have been diagnosed with less severe forms of ASD, such as Asperger’s disorder and PDD-NOS (Durkin et al., 2017; Jarquin et al., 2011). The Centers for Disease Control and Prevention (CDC) reported that the prevalence of ASD was higher among Caucasian children (17.2/1000) as opposed to African American children (16/1000) and Hispanic children (14/1000) at sites in the Autism and Developmental Disabilities Monitoring Network (Baio et al., 2018). This same report noted that there has been a trend since 2002 for the ASD prevalence ratios for Caucasian:minority children to be decreasing. Speculation on the reason for this has included socioeconomic, language, and cultural barriers to accessing care and having the symptoms of ASD recognized. In the case of the current study, the OR for ASD was markedly lower for minority children. This result is likely due to children from lower socioeconomic backgrounds in this community receiving child psychiatric care from university faculty at organizations which maintain EMRs separate from the university record. Such children would not have had a university EMR, and thus were excluded from the study.
Second, only four key variables were used for matching controls and ASD subjects because of the relatively small patient population that controls could be drawn from (approximately 165 potential control subjects per gender, per birth year). This resulted in 10.5% of the potential ASD study subjects being dropped from the study because of a lack of a matched control to utilize. In many cases, only one suitable control case could be found matching all four control variables. Thus, there was not a randomization process where a matched control could be chosen from a pool of potential matches. Since the drop-out rate and lack of randomized controls could have affected our data analysis, the results of this study should be regarded as tentative until performed with a larger patient population.
Third, the analysis in Tables 3 to 6 were controlled for an additional 11 variables that were potential confounders for which there were complete data sets. However, since the EMR from which the data were obtained was not part of a prospectively developed research database, the data regarding some variables that would have been collected and controlled for were not present to a degree that would allow them to be considered in this study. For example, current everyday clinical methods to report a family history of ASD preclude precise observations of associations between family history, any intrapartum sOT exposure, and eventual ASD diagnosis. It was noted that many records stated “No pertinent history” without specifying about which mental disorder(s) the clinician inquired about. The ACOG Antepartum Record for Genetic Screening and Counseling, which is routinely given to prenatal patients, combines the subject regarding history of “mental retardation/autism” into one question which could result in confusion and bias regarding what a “yes” would indicate. As only about one third of the individuals with ASD have mental retardation (Leyfer et al., 2006), these disorders should be reviewed separately during prenatal visits when documenting family history to avoid potential false-negative answers.
Fourth, it should be noted that case-control studies looking at associations between variables and the odds of ASD have reported stronger associations than those found in cohort studies looking at the same association (Curran et al., 2014).
Finally, this study was conducted on a population of patients from a smaller midwestern city with a large rural population and may not be generalizable to a broader population.
Even with these limitations in mind, this study highlights important issues that merit further investigation. This study provides the first evidence to suggest that sOT utilization may either raise or lower the odds of ASD depending on the dosage or duration of exposure. If this finding is replicated in studies on larger populations, then it would suggest that further study on the reasons for the variation of ASD odds with dosage may be fruitful.
The data from this study should not be used to make immediate changes in sOT utilization for established indications for use. However, if future studies confirm the current findings, then a balanced risk/benefit approach toward continued utilization of sOT for labor induction or augmentation in the context of subsequent risk of ASD diagnosis could be developed.
Supplemental Material
Supplemental material, AUT902903_Supplemental_material for An association of intrapartum synthetic oxytocin dosing and the odds of developing autism by Stephen M Soltys, Jill Rose Scherbel, Joseph R Kurian, Todd Diebold, Teresa Wilson, Lindsay Hedden, Kathleen Groesch, Paula L Diaz-Sylvester, Albert Botchway, Pamela Campbell and Julio Ricardo Loret de Mola in Autism
Acknowledgments
The authors would like to thank Jerry Kruse, MD, and Anna Austin, MPH, for their helpful comments in reviewing the paper.
Footnotes
Authors’ Note: The data of this paper were presented at the Annual Meeting of the American Psychiatric Association, New York, 5–9 May 2018.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Stephen M Soltys  https://orcid.org/0000-0001-8783-7900
https://orcid.org/0000-0001-8783-7900
Supplemental material: Supplemental material for this article is available online.
References
- American Psychiatric Association. (1994). Diagnostic and statistical manual of mental disorders (4th ed.). [Google Scholar]
- American Psychiatric Association. (2000). Diagnostic and statistical manual of mental disorders (4th ed., text rev.). [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). [Google Scholar]
- Baio J., Wiggins L., Christensen D. L., Maenner M. J., Daniels J., Warren Z., . . . Dowling N. F. (2018). Prevalence of autism spectrum disorder among children aged 8 years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2014. Morbidity & Mortality Weekly Report. Surveillance Summaries, 67(6), 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bales K. L., Carter C. S. (2003). Sex differences and developmental effects of oxytocin on aggression and social behavior in prairie voles (Microtus ochrogaster). Hormones and Behavior, 44, 178–184. [DOI] [PubMed] [Google Scholar]
- Bölte S., Girdler S., Marschik P. B. (2019). The contribution of environmental exposure to the etiology of autism spectrum disorder. Cellular and Molecular Life Sciences, 76(7), 1275–1297. 10.1007/s00018-018-2988-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brimacombe M., Ming X., Lamendola M. (2007). Prenatal and birth complications in autism. Maternal and Child Health Journal, 11, 73–79. [DOI] [PubMed] [Google Scholar]
- Burstyn I., Sithole F., Zwaigenbaum L. (2010). Autism spectrum disorders, maternal characteristics and obstetric complications among singletons born in Alberta, Canada. Chronic Diseases in Canada, 30(4), 125–134. [PubMed] [Google Scholar]
- Caramaschi D., Taylor A. E., Richmond R. C., Havdahl K. A., Golding J., Relton C. L., . . . Rai D. (2018). Maternal smoking during pregnancy and autism: Using causal inference methods in a birth cohort study. Translational Psychiatry, 8(1), 262 10.1038/s41398-018-0313-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter C. S., Boone E. M., Bales K. L. (2008). Early experience and the developmental programming of oxytocin and vasopressin. In Bridges R. S. (Ed.), Neurobiology of the parental brain (pp. 417–433). Elsevier. [Google Scholar]
- Carter C. S., Boone E. M., Pournajafi-Nazarloo H., Bales K. L. (2009). Consequences of early experiences and exposure to oxytocin and vasopressin are sexually dimorphic. Developmental Neuroscience, 31(4), 332–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne F. A., Meaney M. J. (2006). Stress during gestation alters postpartum maternal care and the development of the offspring in a rodent model. Biological Psychiatry, 59, 1227–1235. [DOI] [PubMed] [Google Scholar]
- Committee on Obstetric Practice. (2014). ACOG Committee Opinion No. 597: Labor induction or augmentation and autism. Obstetrics & Gynecology, 123(5), 1140–1142. [DOI] [PubMed] [Google Scholar]
- Curran E. A., Cryan J. F., Kenny L. C., Dinan T. G., Kearney P. M., Khashan A. S. (2016). Obstetrical mode of delivery and childhood behavior and psychological development in a British cohort. Journal of Autism and Developmental Disorders, 46, 603–614. [DOI] [PubMed] [Google Scholar]
- Curran E. A., Dalman C., Kearney P. M., Kenny L. C., Cryan J. F., Dinan T. G., Khashan A. S. (2015). Association between obstetric mode of delivery and autism spectrum disorder: A population-based sibling design study. Journal of the American Medical Association Psychiatry, 72(9), 935–942. [DOI] [PubMed] [Google Scholar]
- Curran E. A., O’Keeffe G. W., Looney A. M., Moloney G., Hegarty S. V., Murray D. M., . . . Kenny L. C. (2018). Exposure to hypertensive disorders of pregnancy increases the risk of autism spectrum disorder in affected offspring. Molecular Neurobiology, 55, 5557–55564. [DOI] [PubMed] [Google Scholar]
- Curran E. A., O’Neill S. M., Cryan J. F., Kenny L. C., Dinan T. G., Khashan A. S., Kearney P. M. (2014). Research review: Birth by caesarean section and development of autism spectrum disorder and attention-deficit/hyperactivity disorder: A systematic review and meta-analysis. Journal of Child Psychology and Psychiatry, 56(5), 500–508. 10.1111/jcpp.12351 [DOI] [PubMed] [Google Scholar]
- Dawood M. Y., Wang C. F., Gupta R., Fuchs F. (1978). Fetal contribution to oxytocin in human labor. Obstetrics & Gynecology, 52(2), 205–209. [PubMed] [Google Scholar]
- Dodds L., Fell D. B., Shea S., Armson B. A., Allen A. C., Bryson S. (2011). The role of prenatal, obstetric and neonatal factors in the development of autism. Journal of Autism and Developmental Disorders, 41(7), 891–902. [DOI] [PubMed] [Google Scholar]
- Durkin M. S., Maenner M. J., Baio J., Christensen D. L., Daniels J., Fitzgerald R., . . . Yeargin-Allsopp M. (2017). Autism spectrum disorder among US children (2002–2010): Socioeconomic, racial, and ethnic disparities. American Journal of Public Health, 107(11), 1818–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein D., Allen D., Dunn M., Feinstein C., Green L., Morris R., . . . Waterhouse L. (1997). Pitocin induction and autism. American Journal of Psychiatry, 154(3), 438–439. [DOI] [PubMed] [Google Scholar]
- Gale S., Ozonoff S., Lainhart J. (2003). Brief report: Pitocin induction in autistic and nonautistic individuals. Journal of Autism and Developmental Disorders, 33(2), 205–208. [DOI] [PubMed] [Google Scholar]
- Gardener H., Spiegelman D., Buka S. L. (2011). Perinatal and neonatal risk factors for autism: A comprehensive meta-analysis. Pediatrics, 128(2), 344–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getz K. D., Anderka M. T., Werler M. M., Jick S. S. (2016). Maternal pre-pregnancy body mass index and autism spectrum disorder among offspring: A population-based case-control study. Paediatric and Perinatal Epidemiology, 30(5), 479–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasson E. J., Bower C., Petterson B., de Klerk N., Chaney G., Hallmayer J. F. (2004). Perinatal factors and the development of autism: A population study. Archives of General Psychiatry, 61(6), 618–627. [DOI] [PubMed] [Google Scholar]
- Gregory S. G., Anthopolos R., Osgood C. E., Grotegut C. A., Miranda M. L. (2013). Association of autism with induced or augmented childbirth in North Carolina Birth Record (1990–1998) and Education Research (1997–2007) databases. Journal of the American Medical Association Pediatrics, 167(10), 959–966. [DOI] [PubMed] [Google Scholar]
- Grumbach M. M., Hughes I. A., Conte F. A. (2002). Disorders of sex differentiation. In Larsen P., Kronenberg H., Melmed S., Polonsky K. (Eds.), Williams textbook of endocrinology (10th ed, pp. 842–1002). Saunders. [Google Scholar]
- Hashemi F., Tekes K., Laufer R., Szegi P., Tóthfalusi L., Csaba G. (2013). Effect of a single neonatal oxytocin treatment (hormonal imprinting) on the biogenic amine level of the adult rat brain: Could oxytocin-induced labor cause pervasive developmental diseases? Reproductive Science, 20(10), 1255–1263. [DOI] [PubMed] [Google Scholar]
- Hollander E., Cartwright C., Wong C. M., DeCaria C. M., DelGiuduce-Asch G., Buchsbaum M. S., Aronowitz B. R. (1998). A dimensional approach to the autism spectrum. Central Nervous System Spectrums, 3(3), 22–39. [Google Scholar]
- Israel S., Lerer E., Shalev I., Uzefovsky F., Reibold M., Bachner-Melman R., . . . Ebstein R. P. (2008). Molecular genetic studies of the arginine vasopressin 1a receptor (AVPR1a) and the oxytocin receptor (OXTR) in human behaviour: From autism to altruism with some notes in between. Progress in Brain Research, 170, 435–449. [DOI] [PubMed] [Google Scholar]
- Jarquin V. G., Wiggins L. D., Schieve L. A., Van Naarden-Braun K. (2011). Racial disparities in community identification of autism spectrum disorders over time; Metropolitan Atlanta, Georgia, 2000–2006. Journal of Development and Behavioral Pediatrics, 32(3), 179–187. [DOI] [PubMed] [Google Scholar]
- Juul-Dam N., Townsend J., Courchesne E. (2001). Prenatal, perinatal, and neonatal factors in autism, Pervasive Developmental Disorder-Not Otherwise Specified, and the general population. Pediatrics, 107(4), Article e63. [DOI] [PubMed] [Google Scholar]
- Laxer G., Rey M., Ritvo E. R. (1988). A comparison of potentially pathologic factors in European children with autism, Down’s syndrome, and multiple physical handicaps. Journal of Autism and Developmental Disorders, 18(2), 309–313. [DOI] [PubMed] [Google Scholar]
- Leyfer O. T., Folstein S. E., Bacalman S., Davis N. O., Dinh E., Morgan J., . . . Lainhart J. E. (2006). Comorbid psychiatric disorders in children with autism: Interview development and rates of disorders. Journal of Autism and Developmental Disorders, 36(7), 849–861. [DOI] [PubMed] [Google Scholar]
- Li M., Fallin M. D., Riley A., Landa R., Walker S. O., Silverstein M., . . . Wang X. (2016). The association of maternal obesity and diabetes with autism and other developmental disabilities. Pediatrics, 137(2), Article e20152206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maimburg R. D., Vaeth M. (2006). Perinatal risk factors and infantile autism. Acta Psychiatrica Scandinavica, 114, 557–264. [DOI] [PubMed] [Google Scholar]
- Mandell D. S., Wiggins L. D., Carpenter L. A., Daniels J., DiGuiseppi C., Durkin M. S., . . . Kirby R. S. (2009). Racial/ethnic disparities in the identification of children with autism spectrum disorders. American Journal of Public Health, 99(3), 493–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason-Brothers A., Ritvo E. R., Pingree C., Petersen P. B., Jenson W. R., McCahon W. M., . . . Mo A. (1990). The UCLA-University of Utah epidemiologic survey of autism: Prenatal, perinatal, and postnatal factors. Pediatrics, 86(4), 514–519. [PubMed] [Google Scholar]
- McCarthy M. M., Ball G. F. (2008). The neuroendocrine control of sex specific behavior in vertebrates: Lessons from mammals and birds. Current Topics in Developmental Biology, 83, 213–248. [DOI] [PubMed] [Google Scholar]
- Modahl C., Green L., Fein D., Morris M., Waterhouse L., Feinstein C., Levin H. (1998). Plasma oxytocin levels in autistic children. Biological Psychiatry, 43(4), 270–277. [DOI] [PubMed] [Google Scholar]
- Oberg A. S., D’Onofrio B. M., Rickert M. E., Hernandez-Diaz S., Ecker J. L., Almqvist C., . . . Bateman B. T. (2016). Association of labor induction with offspring risk of autism spectrum disorders. Journal of the American Medical Association Pediatrics, 170(9), Article e160965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roloff K., Peng S., Sanchez-Ramos L., Valenzuela G. J. (2015). Cumulative oxytocin dose during induction of labor according to maternal body mass index. International Journal of Gynaecology & Obstetrics, 131(1), 54–58. [DOI] [PubMed] [Google Scholar]
- Scott N., Prigge M., Yizhar O., Kimchi T. (2015). A sexually dimorphic hypothalamic circuit controls maternal care and oxytocin secretion. Nature, 525(7570), 519–522. [DOI] [PubMed] [Google Scholar]
- Simpson K. R. (2011). Clinician’s guide to the use of oxytocin for labor: Induction and augmentation. Journal of Midwifery & Women’s Health, 56, 214–221. [DOI] [PubMed] [Google Scholar]
- Stein D., Weizman A., Ring A., Barak Y. (2006). Obstetric complications in individuals diagnosed with autism and in healthy controls. Comprehensive Psychiatry, 47(1), 69–75. [DOI] [PubMed] [Google Scholar]
- Takayanagi Y., Yoshida M., Bielsky I. F., Ross H. E., Kawamata M., Onaka T., . . . Nishimori K. (2005). Pervasive social deficits, but normal parturition, in oxytocin receptor-deficient mice. Proceedings of the National Academy of Sciences of the United States of America, 102(44), 16096–16101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamborski S., Mintz E. M., Caldwell H. K. (2016). Sex differences in the embryonic development of the central oxytocin system in mice. Journal of Neuroendocrinology, 28(4). 10.1111/jne.12364 [DOI] [PubMed] [Google Scholar]
- Taylor J. L., Debost J. C. P.G., Morton S. U., Wigdor E. M., Heyne H. O., Lai D., . . . Robinson E. B. (2019). Paternal-age-related de novo mutations and risk for five disorders. Nature Communications, 10(1), Article 3043. 10.1038/s41467-019-11039-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vintzileos A. M., Ananth C. V. (2013). Does augmentation or induction of labor with oxytocin increase the risk for autism? American Journal of Obstetrics & Gynecology, 209(6), 502–504. [DOI] [PubMed] [Google Scholar]
- Wahl R. U. R. (2004). Could oxytocin administration during labor contribute to autism and related behavioral disorders?—A look at the literature. Medical Hypotheses, 63(3), 456–460. [DOI] [PubMed] [Google Scholar]
- Weisman O., Agerbo E., Carter C. S., Harris J. C., Uldbjerg N., Henriksen T. B., . . . Dalsgaard S. (2015). Oxytocin-augmented labor and risk for autism in males. Behavioral Brain Research, 284, 207–212. [DOI] [PubMed] [Google Scholar]
- Yamasue H., Kuwabara H., Kawakubo Y., Kasai K. (2009). Oxytocin, sexually dimorphic features of the social brain, and autism. Psychiatry and Clinical Neurosciences, 63, 129–140. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, AUT902903_Supplemental_material for An association of intrapartum synthetic oxytocin dosing and the odds of developing autism by Stephen M Soltys, Jill Rose Scherbel, Joseph R Kurian, Todd Diebold, Teresa Wilson, Lindsay Hedden, Kathleen Groesch, Paula L Diaz-Sylvester, Albert Botchway, Pamela Campbell and Julio Ricardo Loret de Mola in Autism