Abstract
Purpose
The objective of this study was to analyse the expression and cellular localization of FOXO3, pFOXO3 and PTEN throughout human ovary development both before and after birth.
Methods
Foetal, pubertal and adult paraffin-embedded ovarian samples were analysed by immunohistochemistry for cellular localization of FOXO3, pFOXO3 and PTEN proteins. Protein and mRNA expression were analysed by western blot and real time PCR, respectively, from fresh biopsies.
Results
PTEN was not detected by immunohistochemistry in germ cells and follicles of foetal, pubertal and adult ovaries. Occasional PTEN immunoreactive granulosa cells were found in atretic antral follicles in the adult ovary. Western blot analysis showed low levels of PTEN protein. Nuclear FOXO3-expressing primordial follicles represented a variable proportion of the ovarian reserve. The presence of FOXO3-expressing primordial follicles was very low in foetal ovary; although always represented in a low proportion, prevalence increased during pubertal and adult life.
Conclusion
Our results seem to indicate that two subpopulations of primordial follicles, i.e. nuclear FOXO3-expressing and no FOXO3-expressing primordial follicles are found in the postnatal human ovary. This scenario suggests that FOXO3 could be acting as in the mouse model, preventing primordial follicle activation. However, the strategy would not be an “all or nothing” system as in mouse ovary but rather a selected subpopulation of primordial follicles preserved to ensure long-term fertility.
Electronic supplementary material
The online version of this article (10.1007/s10815-020-01790-x) contains supplementary material, which is available to authorized users.
Keywords: PTEN , FOXO3 , pFOXO3 , Primordial follicle activation , Foetal human ovary , Pubertal and adult human ovary
Introduction
Primordial germ cells, originating in the extra-embryonic compartment of the newly implanted conceptus [1], reach and colonise the genital ridges at approximately 6–7 weeks post-conception (wpc) [2]. After colonisation, as the embryo has been committed to the female fate, definitive ovarian histogenesis takes place. Germ cells start to be surrounded by somatic cells about 12–13 wpc in a process referred to as follicular assembly. Follicular assembly yields by birth an endowment of resting, non-growing primordial follicles, comprising an oocyte surrounded by a single layer of flattened somatic cells, which forms the ovarian reserve (OR). Primordial follicles in the OR remain quiescent in women for years or decades until they are lost by atresia or activated to enter a process of maturation in the so-called growing follicular pool. At puberty, quiescent primordial follicles cyclically selected for maturation undergo folliculogenesis yielding just one preovulatory follicle-enclosed dominant oocyte at each menstrual cycle [3].
Genetic factors involved in maintaining the quiescent state or activating the dormant primordial follicle to enter the growing follicular pool are diverse, and they are expressed either in the germ cell proper or in the granulosa cells [4]. Among the different genes involved in dormancy/activation of the OR, the forkhead transcription factors, FOXOS, are key effectors of the PI3K/AKT signalling pathway [5, 6]. In the mouse ovary, Foxo3 is expressed in the nuclei of primordial follicles and translocates to the cytoplasm of primary follicles coming from activated primordial follicles leaving the OR. The potent effect of Foxo3 in maintaining the dormant state becomes evident in Foxo3−/− mice whose primordial follicles start growing without control voiding the OR by the age of 15 weeks [7]. Similarly, Foxo3 seems to regulate dormancy/activation of the primordial follicle in the OR of other rodents such as the rat and the deer mouse [8]. However, in non-rodent mammals as diverse as cow, cat, dog, pig and some non-human primates, no specific localization of nuclear or cytoplasmic FOXO3 was detectable in primordial or primary oocytes [8]. In humans, the only sample analysed, the adult ovary of a 38-year-old woman, showed no FOXO3 expression as in the other non-rodent mammals [8].
Other factors involved in the PI3K/AKT signalling pathway participate in the control of dormancy/activation of primordial follicles in the OR. In mice, for example, the ablation of Pten, the phosphatase and tensin homologue detected on chromosome 10 gene, a negative regulator of PI3K, was found to promote massive recruitment of primordial follicles from the OR [9] as shown for Foxo3 knockout mice. At present, attempts to relate PTEN [10, 11] or FOXO3 [12] expression with human premature ovarian failure or primary amenorrhea were unsuccessful showing that haploinsufficiency, i.e. mutation of a single allele, at least in FOXO3, does not seem to contribute to ovarian failure. However, the inhibition of PTEN in human ovary in vitro promotes an increased activation of primordial follicles [13].
Although the Foxo3 and Pten functioning in mouse ovary seems to be specific for maintaining dormancy in the OR, our knowledge in humans is up to day very scarce and not conclusive. We report here the analysis of expression and immunolocalisation of FOXO3, pFOXO3 and PTEN throughout human ovary development both before and after birth including infant, pubertal and adult ovary, especially focusing in primordial and primordial-to-primary follicle transition.
Materials and methods
Collection of ovarian samples
A total of 59 samples of human ovarian tissue were collected and analysed. Foetal ovaries (n = 23) were obtained as preserved paraffin-embedded tissues (8 to 30 wpc) from the Maternidad y Hospital Infantil Ramón Sardá, Buenos Aires City, and as fresh tissue (30 to 41 wpc) from the Hospital Municipal de Merlo, Merlo, Buenos Aires Province (Table S1). Fresh foetal ovaries were obtained from spontaneous abortion; the age of the foetuses was estimated from the date of the last menstruation of the mother and the cephalocaudal length at the time of the autopsy. Foetuses with normal morphological appearance without signs of necrosis, in which death was considered to have occurred just before the autopsy, were included. Infant and pubertal tissue samples (n = 13) were obtained by laparoscopic surgery at the Hospital de Niños “Dr. Ricardo Gutiérrez”, Buenos Aires City, including pre-menarcheal patients of 6 (n = 1), 9 (n = 2) and 11 (n = 1) years old and post-menarcheal patients of 12 (n = 2), 13 (n = 2), 14 (n = 3), 15 (n = 1) and 16 (n = 1) years old (Table S1). Adult ovary samples (n = 23) ranging from 29 to 52 years old, obtained by laparoscopic surgery, were recovered at the Hospital General de Agudos J. A. Penna and the Hospital Israelita, Buenos Aires City. According to the age of donors, samples were divided into three groups: group A (n = 7), 29–35 years old, group B (n = 9), 37–45 years old and group C (n = 6), 46–52 years old. All adult samples were examined in haematoxylin-eosin stained sections; only those classified as histological normal tissue were included in this study. The exclusion criteria included: ovarian cancer, endometriosis in ovary or pelvic cavity and polycystic ovary. Samples collected at the time of surgery under sterile conditions were preserved in 10% formaldehyde until embedded in paraffin, serially sectioned at 5 μm thickness, mounted onto cleaned slides and kept at room temperature until used. When possible (cf. Table S1), two small fresh-tissue fragments were preserved either in an RNAse/DNAse-free sterile cryotube or in 1 ml RNA later (QiaGen, Ambion Inc., Austin, TX, USA) and stored at − 80 °C until used. The present study was reviewed and approved by the Institutional Research Ethics Committee, Universidad Maimónides, Buenos Aires, Argentina, and Research Ethics Committees from collaborating hospitals. Samples were used after obtaining the patients’, and/or parents or legal representatives’, informed consent.
Immunohistochemistry
Mounted paraffin sections were dewaxed in xylene, rehydrated in graded alcohols and washed in distilled water. Endogenous peroxidase activity was inhibited with 0.5% H2O2/methanol (v/v) for 20 min at room temperature. Sections were then blocked for 1 h with 15% normal horse serum or normal rabbit serum in phosphate buffered saline (PBS) and incubated overnight at room temperature with the 1:100 diluted primary antibodies: mouse monoclonal anti-FKHRL1 (FOXO3) (sc-48348), goat polyclonal anti-p-FKHRL1 (pFOXO3) (sc-12357) and mouse polyclonal anti-PTEN (sc-7974), all from Santa Cruz Biotechnology, Dallas, TX, USA. After overnight incubation, slides were rinsed thrice in PBS and incubated for 1 h at room temperature with the appropriate 1:200-diluted biotinylated secondary antibody (Vector Labs, Peterborough, UK). After further washing in PBS, sections were incubated for 30 min with 1:100 diluted streptavidin-peroxidase complexes (ABC kit, Vector Labs, UK). Sections were then washed twice with PBS, and development of peroxidase activity was revealed with 0.05% 3,3′-diaminobenzidine (w/v) and 0.1% H2O2 (v/v) in Tris-HCl. Finally, sections were washed with distilled water and mounted in Canada balsam (Biopack, Buenos Aires, Argentina). In preparative assays, we performed comparative immunohistochemistry without and with citrate buffer-antigen retrieval. As no differences were found in protein detection, antigen retrieval was omitted since in some cases citrate buffer incubation damaged the fixed tissue. Negative controls were processed simultaneously by omitting the primary antibody and/or preincubating the primary antibody with the specific commercial synthetic peptide when available. Sections were examined in an Olympus BX40 microscope. Semiquantification of positive follicles was carried out counting the number of positive follicles with respect to the total of each follicular stage present in all the tissue section analysed.
Western blot analysis of PTEN
Ovarian fragments preserved at − 80 °C were homogenized in ice-cold lysis buffer containing a protease inhibitor cocktail [0.5 mM phenylmethylsulfonyl fluoride (PMSF);10 mM leupeptin; 10 mM pepstatin; 10 mM aprotinin], and centrifuged at 1.200g at 4 °C for 10 min. The supernatant was collected and proteins were quantified using the Bradford Protein Assay (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Total proteins (20 μg) from tissue extracts were separated by one-dimensional SDS-PAGE 15% and then transferred onto polyvinylidene fluoride (PVDF) membrane (Amersham Hybond-P, GE Healthcare). Membrane was then blocked for 1 h in PBS + 0.1% Tween20 with 5% non fat dry milk. After that, it was incubated 1 h at room temperature with the mouse polyclonal anti-PTEN diluted 1/100 (A2B1: sc-7974 Santa Cruz Biotechnology. Dallas, TX, USA). After washing, membrane was incubated with a goat anti-mouse IgG horseradish peroxidase conjugated secondary antibody (Bio- Rad, 1:5000). The immunoreactive product was visualized using the enhanced chemiluminescence system ECL plus GE (Amersham, Fairfield, Connecticut, USA) and Image Quant 350. To confirm equal loading, each membrane was analysed for β-actin protein expression demonstrating that the band intensities did not show significant changes between the samples analysed. Briefly, membrane was incubated with mouse monoclonal anti-β-actin (Sigma, Saint Louis, MO, USA) diluted 1/1000. After washing, membrane was incubated with a goat anti-mouse IgG horseradish peroxidase conjugated secondary antibody (Bio-Rad, 1:5000). Stained protein molecular weight markers were used as standards (Fermentas, Vilnius, Lithuania). Densitometry was performed on Scion Image for Windows software (Scion Corporation, Frederick, MD, USA) and PTEN expression was normalized to β-actin. Human uterine fibroblast (HUF) cell line was utilized as a positive control for PTEN expression [14].
RNA isolation and real time-PCR
Samples recovered in RNA later at the surgery room were maintained in that solution for 48 h and then stored a − 80 °C until used. Total ovary RNA was extracted with TRIzol (Invitrogen, Waltham, MA, USA) according to the manufacturer’s instructions. Total RNA (3 μg) was treated with DNAse I (Invitrogen, Waltham, MA, USA) and used for reverse transcription in a 20-μl reaction containing M-MLV reverse transcriptase (200 U/μl, Promega, Madison, WI, USA) and random hexamers primers (Biodynamics, Buenos Aires, Argentina). Reverse-transcribed cDNA was employed for quantitative polymerase chain reaction (PCR) using SYBR Green PCR Master Mix and specific forward (F) and reverse (R) primers (PTEN: F 5′-CCAATGTTCAGTGGCGGAACT-3′, R 5′-GAACTTGTCTTCCCGTCGTGT-3′. FOXO3: F 5´-TCTACGAGTGGATGGTGCGTT-3′, R 5′-CGACTATGCAGTGACAGGTTGT-3′, ACTIN: F 5′-CTTCCCCTCCATCGTGGG-3′, R 5′-GTGGTACGGCCAGAGGCG-3′), in a Stratagene MPX500 cycler (Stratagene, La Jolla, CA, USA). Primers were used at a concentration of 0.3 μM in each reaction. The cycling conditions were as follows: step 1, 10 min at 95 °C; step 2, 15 s at 95 °C; step 3, 30 s at 60 °C; step 4, 30 s at 72 °C, repeating steps 2 to 4 forty-five times. Data from the reaction were collected and analysed by the complementary computer software (MxPro3005P v4.10 Build 389, Schema 85, Stratagene, La Jolla, CA, USA). Melting curves were run to confirm specificity of the signal. Relative quantization of gene expression was performed using standard curves and normalized to β-actin in each sample. For assessment of quantitative differences in the cDNA target between samples, the mathematical model of Pfaffl was applied. The expression ratio was determined for each sample by calculating (Etarget)ΔCt(target)/(EGβACTIN)ΔCt(βactin), where E is the efficiency of the primer set and CT is threshold cycle with ΔCt = Ct (normalization cDNA) − Ct (experimental cDNA). The amplification efficiency of each primer set was calculated from the slope of a standard amplification curve of log (ng cDNA) per reaction vs. Ct value (E = 10-(1/slope)). Efficiencies of 2 ± 0.1 were considered optimal.
Statistical analysis
Mean and standard error (SEM) were calculated and the InfoStat Software (Version 2012, Grupo InfoStat, Universidad Nacional de Córdoba, Córdoba, Argentina) was used for one-way analysis of variance. A log10 transformation of data was done. Tukey’s test was used when differences between more than two groups were compared. A p value of less than 0.05 was considered statistically significant.
Results
PTEN, FOXO3 and p-FOXO3 protein expression in foetal human ovaries
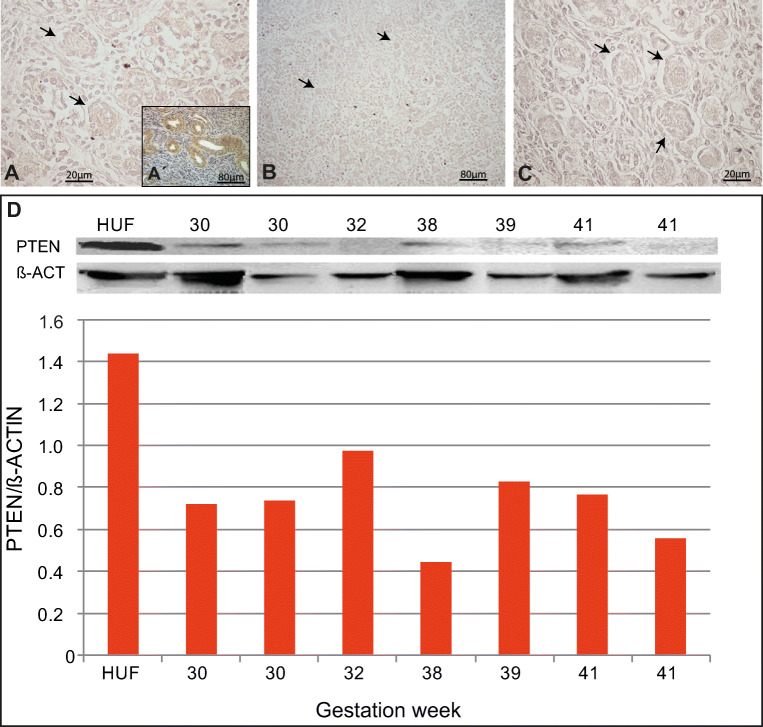
PTEN protein was not specifically detected by immunohistochemistry in oogonia, primordial follicles or other follicle types in foetal ovaries from 8 to 30 wpc (Fig. 1a–c). During the last trimester of pregnancy (30 to 41 wpc), very low levels of PTEN were detected by western blot (Fig. 1d), most likely indicating its expression from other somatic compartments, e.g. smooth muscle from blood vessels (cf. Fig. 1a’) which served as an internal positive control.
Fig. 1.
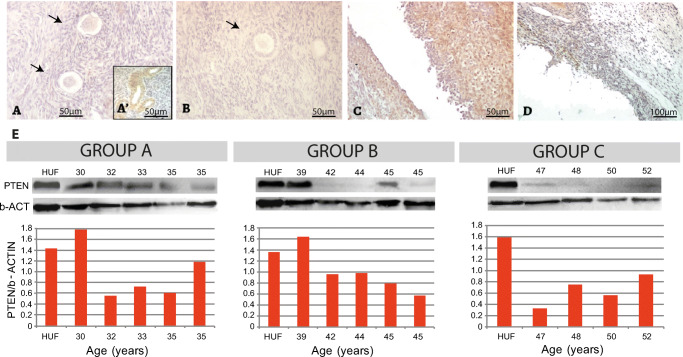
Expression of PTEN in human foetal ovary. (a–c) General view of ovarian section at 13, 18 and 30 wpc, respectively, negative for PTEN immunolocalisation both in oogonia and primordial follicles; arrows point to primordial follicles. An example of negative control staining performed by avoiding primary antibody is shown in Fig. 1S. (a’) Internal positive control showing FOXO3 expression in blood vessels from the same histological section. (d) Western blots and quantification showing low PTEN detection in all samples from 30 wpc to birth. HUF, Human uterine fibroblast cell line used as positive control (see “Materials and methods”); wpc, weeks post-conception
FOXO3 was negative in oogonia and the great majority of follicles apart from very rare primordial follicles showing oocyte nuclear expression from 18 wpc onwards (Fig. 2a, b). pFOXO3 protein was negative in all foetal samples (Fig. 2c, d).
Fig. 2.
Immunohistochemical detection of FOXO3 and pFOXO3 in human foetal ovary. (a) General view of a 16wpc histological section showing no FOXO3 localisation in oogonia. (a’) internal positive control, foetal lung epithelium. (b) Occasional primary follicle (18wpc) showing nuclear FOXO3 localisation. (c, d) Negative immunolocalisation of pFOXO3 at 13 and 28 wpc, respectively. (c′) Internal positive control, foetal lung epithelium. Wpc, weeks post-conception. Arrows point to primordial follicles
PTEN, FOXO3 and p-FOXO3 protein expression in infant and pubertal human ovaries
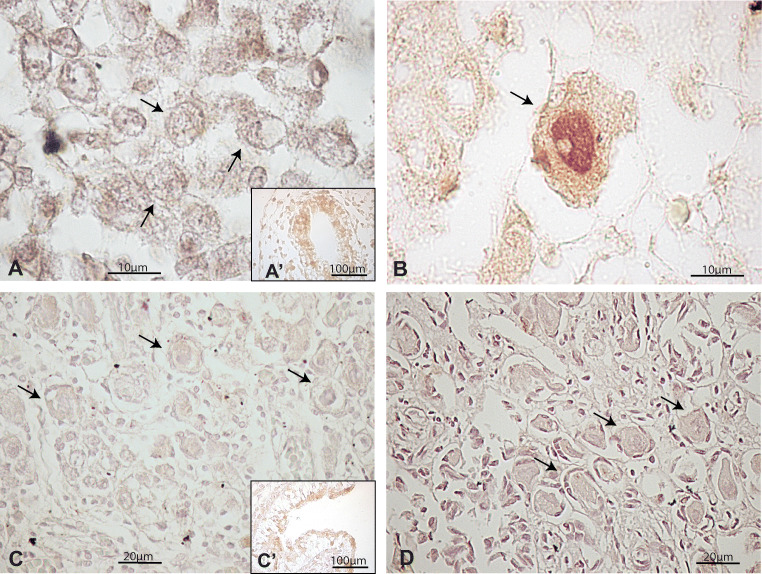
The ovarian sections of infant and pubertal ovaries analysed by immunohistochemistry only showed primordial and primary follicles. In all cases, both types of follicles were negative for PTEN (Fig. 3a), with the exception of a single primary follicle found in the 16-year-old patient (Fig. 3b). Although PTEN expression in other types of follicles cannot be discarded, it seems unlikely since detection by western blot was very low in all patients (Fig. 3c).
Fig. 3.
Expression of PTEN in pubertal human ovary. (a) Illustrative primordial follicle with no signal for PTEN from a 14-year-old sample. An example of negative control staining performed by avoiding primary antibody is shown in Fig. 1S. (a’) Internal positive control. (b) Unique primary-to-secondary transition follicle showing cytoplasmic localisation of PTEN protein found in the 16-year-old sample. (c) Western blot quantification showing low detection in almost all samples analysed
FOXO3 protein showed a variable pattern of expression in the pool of primordial follicles; some primordial follicles were negative while others showed nuclear expression of FOXO3, with the exception of one follicle displaying cytoplasmic signal (Fig. 4a, b). Table S2 shows the counting of FOXO-positive and FOXO-negative primordial follicles in the only four available paraffin-fixed samples from infant ovaries. The prevalence of FOXO-expressing follicles was variable and low, with nuclear localization, except for one case of cytoplasmic localization. Primary, secondary and antral follicles showed nuclear FOXO3 signal in some granulosa cells (Fig. 4c, d). In all cases, p-FOXO3 immunostaining was negative (Fig. 4e, f).
Fig. 4.
Immunohistochemical detection of FOXO3 and pFOXO3 in pubertal human ovary. (a) Pubertal ovary showing negative FOXO3 primordial (green arrowhead) and primary (red arrowhead) follicle. (b) Illustrative primordial follicle (arrow) showing nuclear FOXO3 expression. (c) FOXO3-expressing granulosa cells in pre-antral follicle. (d) Detail of FOXO-expressing granulosa cells in antral follicle; (d’) inset, arrow indicates the approximate region magnified in D. (e) Primordial follicles negative for pFOXO immunolocalosation. (f) Illustrative primary follicle negative for pFOXO immunolocalisation. Insets a´ and c´, internal positive control
PTEN, FOXO3 and p-FOXO3 protein expression in adult human ovaries
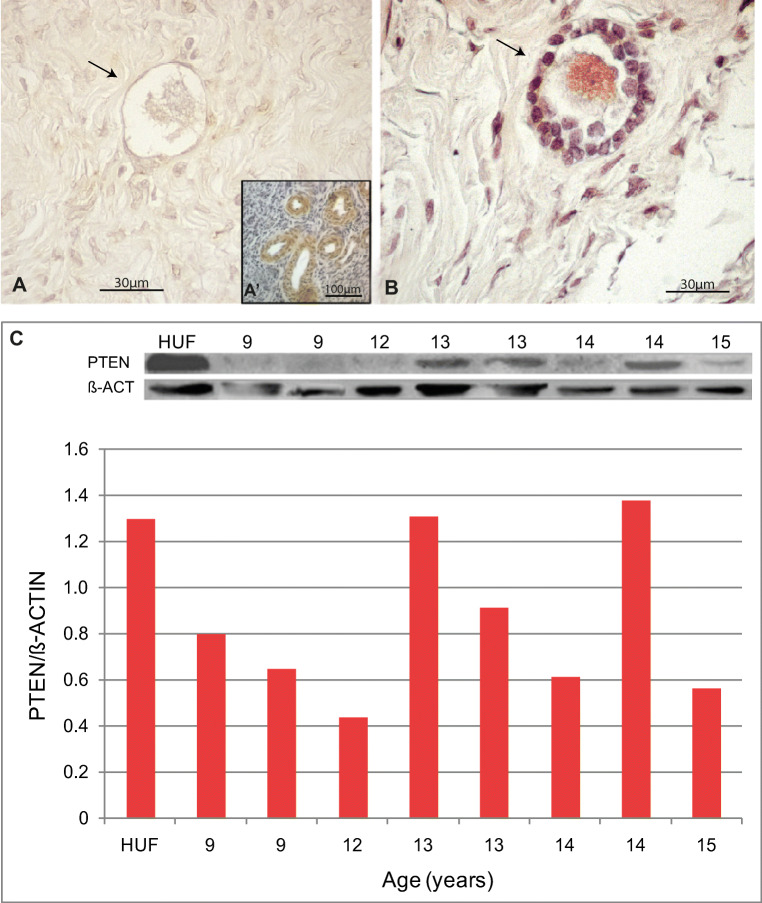
PTEN was not detectable in primordial, primary and secondary follicles in adult ovaries from groups A, B and C (Fig. 5a, b); some positive granulosa cells were observed in atretic antral follicles (Fig. 5c). AIbicans bodies were negative (Fig. 5d). Detection by western blot was low in all samples from each group (Fig. 5e). FOXO3 protein immunodetection was variable in primordial follicles.
Fig. 5.
PTEN expression in the adult human ovary. (a) Illustrative example of negative PTEN expression in primordial follicles. (a’) Internal positive control, blood vessels. (b) PTEN negative staining in primary follicle. (c) Granulosa cells from an atretic follicle showing positive PTEN expression. (d) PTEN negative albicans body. (e) Western blot analysis showing low PTEN detection in almost all cases. HUF, Human uterine fibroblast cell line used as positive control (see “Materials and methods”)
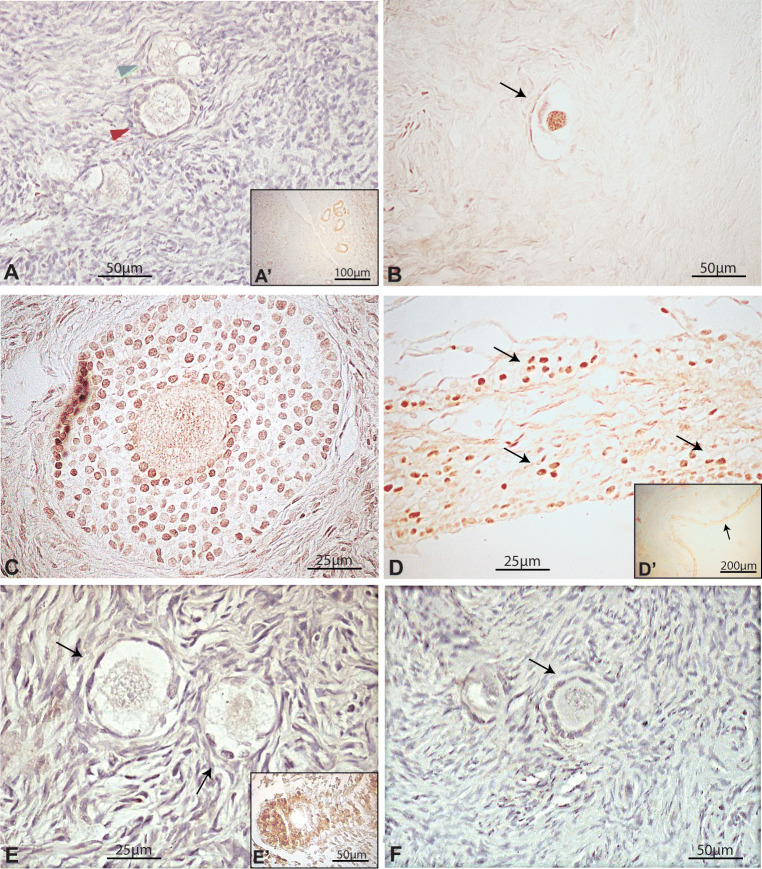
As seen in pubertal ovaries, adult ovaries displayed a variable expression of FOXO3 in primordial follicles. FOXO3-expressing primordial follicles mostly expressed nuclear FOXO3; however, cytoplasmic expression of FOXO3 was also found (Fig. 6a). Six out of 8 available paraffin-fixed samples were used for counting FOXO expression (Table S3). As shown for infant ovarian samples, a low proportion of primordial follicles displayed nuclear FOXO3 signal, with great variability among samples. FOXO3 was also immunodetected in the nucleus of granulosa cells of secondary follicles, atretic antral follicles and luteal bodies (Fig. 6b). Contrary to what was observed in the infant ovary, cytoplasmic FOXO3- and pFOXO3-expressing primordial follicles were more evident in these samples (Fig. 6c, d; Tables S3 and S4). Primary, secondary and antral follicles were found negative for pFOXO3 (Fig. 6e, f).
Fig. 6.
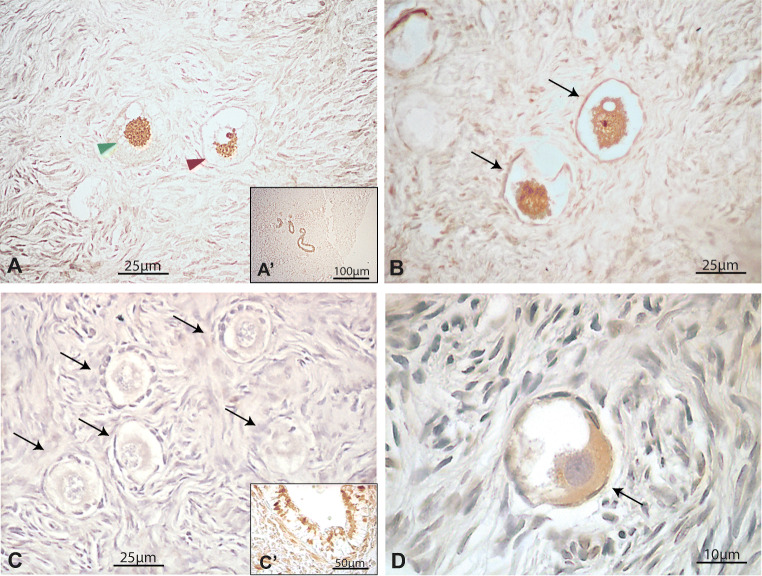
Immunohistochemical detection of FOXO3 and pFOXO3 in the human adult ovary. (a) Illustrative primordial follicles showing nuclear (green arrowhead) and cytoplasmic (red arrowhead) expression of FOXO3 and pFOXO3. (b) Nuclear FOXO3 expression in primordial follicles from a 39-year-old patient. (c) Negative primordial follicles for pFOXO3 staining. (d) Illustrative primordial follicle showing cytoplasmic localisation of pFOXO3. Insets a´ and c´, internal positive controls. An example of negative control staining performed by avoiding primary antibody is shown in Fig. 1S
FOXO3 and PTEN transcription throughout ovary development
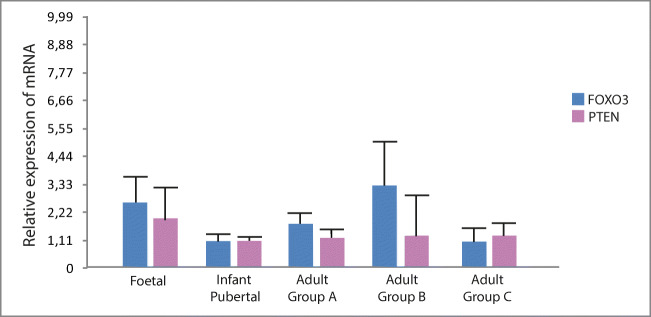
FOXO3- and PTEN-mRNA detected levels did not show any significant difference throughout the different developmental stages analysed herein (Fig. 7). Both genes showed comparable levels of transcription at foetal, infant and adult periods, with a considerable variability among samples for any given age interval.
Fig. 7.
PTEN- and FOXO3-mRNA expression at different developmental stages of the human ovary. mRNA was analysed in all samples from which fresh tissue was available (cf. Suppl. Table 1B). Adult groups: a, 29–35 years; b, 37–45 years old; c, 46–52 years old
Discussion
The current study is the first report analysing PTEN, FOXO3 and pFOXO3 expression throughout development of the human ovary. In general, we found that PTEN does not immunolocalise in primordial follicles during the different developmental stages including foetal, pubertal and adult ovary and shows low levels of mRNA detection. On the contrary, nuclear FOXO3 was expressed in a proportion of primordial follicles that varied with ovarian age.
FOXO3 expression pattern found in the human ovary differed from that reported for the mouse model in which FOXO3 is globally expressed in the nucleus of all primordial follicle-enclosed oocytes, and its translocation to the cytoplasm is coincident with follicle activation and growth [5, 6]. In contrast to what is seen in mice and other muroid rodents such as the deer mouse and the rat, primordial follicles in adult ovary of non-rodent mammals, including non-human primates, do not express FOXO3 [8, 15]. However, our results show that this is also not the case for the human adult ovary in which a variable number of primordial follicles express nuclear FOXO3. Moreover, FOXO3 expression varied with human ovarian age from a very low prevalence in foetal stage which seems to increase in pubertal age and in the adult ovary. In any case, this apparent increase in the number of FOXO3-expressing oocytes in primordial follicles has to be seen cautiously and needs further in-depth analysis. The number of samples analysed is low, preventing quantification and statistical analysis. Moreover, available sections were highly variable between them, as expected, in terms of germ cell abundance. Although no differences were observed in mRNA levels between both genes, the detection of FOXO3 protein in primordial follicles might reflect a different regulation of translation or, simply, a preferential elimination through apoptosis of no FOXO3-expressing oocytes from primordial follicles (see below). Strikingly, the rhesus macaque ovary displays an opposite behaviour. Highest prevalence of FOXO3 is found in germ cells before follicle assembly, progressively decreasing in primordial follicles during foetal and prepubertal stages to finally disappear in adult monkey ovary [15].
PTEN and FOXO3 expression in germ cells, primordial and primary follicles in foetal and neonatal ovaries of some mammals, like pig and sheep, suggest that these proteins could participate in follicular assembly and growth during foetal life [16, 17]. As stated above, our results in the foetal human ovary showed an almost absent expression of PTEN and a very low FOXO3 expression in primordial follicles. In the rhesus macaque foetal ovary, FOXO3 is sparingly expressed in mitotically active germ cells in the cortical region and at a lower prevalence in assembled primordial follicles [15]. According to these authors, the possibility that FOXO3 is just expressed when primordial follicles are activated cannot be ruled out. Nevertheless, it is difficult to reconcile the very low expression of FOXO3 in the foetal human ovary with classical studies showing that primordial follicles enter the growth phase from the third trimester of gestation leading to approximately 69% of primary follicles at birth [2]. Therefore, PTEN and FOXO3 as part of the PI3K/AKT pathway do not seem to be a relevant player in follicular assembly, activation and growth during the human foetal period.
In the infant and pubertal ovary, the number of primordial follicles that express FOXO3 becomes more evident. The majority of the follicles that begin to grow during this developmental stage will undergo a process of atresia through apoptosis [18] that can be favoured by FOXO3 expression as well as PTEN expression in granulosa cells in antral follicles [19–24]. It has been shown that FOXO3 activates apoptosis through upregulation of BH3-only proteins or through extrinsic apoptotic factors such as FASL and TRIAL [25]. Thus, nuclear FOXO3 expression in primordial follicle might indicate the entrance to the apoptosis pathway or, alternatively, the prevention to activate as seen in mice. Our results, based on an observational description and a limited number of samples, do not allow discerning between those possibilities; however, the low prevalence of cytoplasmic FOXO3 we found might indicate a role in preventing activation rather than entrance in apoptosis. Moreover, considering that the human ovary displays the highest rate of intraovarian germ cell elimination through apoptosis seen in mammals, reaching by puberty approximately 95% of the original OR established during foetal life [26, 27], it is tempting to suppose that nuclear FOXO3 expression is preserving the activation of a proportion of primordial follicles and, consequently, fertility during the adult reproductive life. Thus, the correlation between nuclear FOXO3 expression and preservation of primordial follicle activation deserves a closer experimental examination in the human ovary. Moreover, other FOXO genes, e.g. FOXO1 and FOXO4, also require a thorough examination.
The adult ovary also showed a variable number of primordial follicles expressing nuclear FOXO3. Although the number of samples available for counting FOXO3 expression was limited, the prevalence of nuclear FOXO3-expressing primordial follicles was comparable to that seen in the pubertal ovary. Further, in the adult ovary, cytoplasmic pFOXO3 was first detected (cf. Table S3) indicating a transition to the activated state or a possible entrance in the apoptotic pathway (see above).
Taking together the results of FOXO3 expression from foetal to adult ovary, with due caution of the limited number of analysed samples, our results seem to indicate that two subpopulations of primordial follicles, i.e. nuclear FOXO3-expressing and no FOXO3-expressing primordial follicles, seem to coexist in the postnatal human ovary. Until 30wpc we found a very low prevalence of FOXO3-expressing follicles. At puberty, the prevalence of FOXO3-expressing primordial follicles becomes more evident and still persists in the adult ovary. In this scenario, FOXO3 could be acting as in the mouse model, preventing primordial follicle activation. In this case, however, the strategy would not be an “all or nothing” system as in mouse ovary but rather a selected subpopulation of primordial follicles preserved to ensure long-term fertility. It is worth to mention that such a difference complies with the disparate reproductive strategies in mice and humans. A global preservation and regulation of the OR dormancy/activation seems adaptive to female mice entering quickly in reproductive life, with consecutive pregnancies, large litters and a short lifespan. On the other hand, preserving a subpopulation of primordial follicle-enclosed oocytes that must wait for years and even decades to enter the growing follicular pool, ovulation and fertilisation fits in the long lifespan of women.
Finally, the existence of a FOXO3-expressing subpopulation in the ovarian reserve of pubertal and adult human ovary that could regulate primordial follicle activation has a significant interest of clinical implication. For instance, variability in the size of this subpopulation can have an impact on primary ovarian insufficiency that has at present elusive causal genetic basis. Moreover, FOXO3-expressing primordial follicle could be a suitable target for in vitro follicle maturation or pharmacological manipulation of the PI3K/AKT pathway.
Electronic supplementary material
(DOC 5325 kb)
Acknowledgements
The authors would like to thank Ms. María Sol Clausi-Schettini for histology technical assistance, and the participating hospitals and patients who contributed samples to this study.
Funding source
This work was supported by intramural grant from Fundación Científica Felipe Fiorellino-Universidad Maimónides, Buenos Aires, Argentina.
Compliance with ethical standards
All procedures in the study carried out with human samples were approved by the Institutional Research Ethics Committee from Universidad Maimónides and collaborating Hospitals Research Ethics Board.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
María Itatí Albamonte, Email: albamonte.itati@maimonides.edu.
Lara Y. Calabró, Email: calabrolara@gmail.com
Mirta S. Albamonte, Email: albamonte.mirta@maimonides.edu
Luis Zuccardi, Email: luiszuccardi@hotmail.com.
Inés Stella, Email: iystella@yahoo.com.ar.
Julia Halperin, Email: halperin.julia@maimonides.edu.
Alfredo Daniel Vitullo, Email: vitullo.alfredo@maimonides.edu.
References
- 1.Kobayashi T, Surani MA. On the origin of human germline. Development. 2018;145:dev150433. doi: 10.1242/dev.150433. [DOI] [PubMed] [Google Scholar]
- 2.Kurilo LF. Oogenesis in antenatal development in man. Hum Genet. 1981;57:86–92. doi: 10.1007/BF00271175. [DOI] [PubMed] [Google Scholar]
- 3.McGee EA, Hsueh AJ. Initial and cyclic recruitment of ovarian follicles. Endocr Rev. 2000;21:200–214. doi: 10.1210/edrv.21.2.0394. [DOI] [PubMed] [Google Scholar]
- 4.Pelosi E, Forabosco A, Schlesssinger D. Genetics of the ovarian reserve. Front Genet. 2015;6:308. doi: 10.3389/fgene.2015.00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.John GB, Shirley LJ, Gallardo TD, Castrillon DH. Specificity of the requirement for Foxo3 in primordial follicle activation. Reproduction. 2007;133:855–863. doi: 10.1530/REP-06-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.John GB, Gallardo TD, Shirley LJ, Castrillon DH. Foxo3 is a PI3K-dependent molecular switch controlling the initiation of oocyte growth. Dev Biol. 2008;321:197–204. doi: 10.1016/j.ydbio.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castrillon DH, Miao L, Kollipara R, Horner JW, DePinho RA. Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science. 2003;301:215–218. doi: 10.1126/science.1086336. [DOI] [PubMed] [Google Scholar]
- 8.Tarnawa ED, Baker MD, Aloisio GM, Carr BR, Castrillon DH. Gonadal expression of Foxo1, but not Foxo3, is conserved in diverse mammalian species. Biol Reprod. 2013;88:103. doi: 10.1095/biolreprod.112.105791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reddy P, Shen L, Ren C, Boman K, Lundin E, Ottander U, Lindgren P, Liu YX, Sun QY, Liu K. Activation of Akt (PKB) and suppression of FKHRL1 in mouse and rat oocytes by stem cell factor during follicular activation and development. Dev Biol. 2008;281:160–170. doi: 10.1016/j.ydbio.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 10.Shimuzu Y, Kimura F, Takebayashi K, Fujiwara M, Takakura K, Takahashi K. Mutational analysis of the PTEN gene in women with premature ovarian failure. Acta Obstet Gynecol. 2009;88:824–825. doi: 10.1080/00016340902971458. [DOI] [PubMed] [Google Scholar]
- 11.Zhao Z, Qin Y, Ma J, Zhao H, Li J, Wang L, Ren C, Che L, Chen ZJ. PTEN gene analysis in premature ovarian failure patients. Acta Obstet Gynecol Scand. 2011;90:678–679. doi: 10.1111/j.1600-0412.2011.01118.x. [DOI] [PubMed] [Google Scholar]
- 12.Gallardo TD, John GB, Bradshaw K, Welt C, Reijo-Pera R, Vogt PH, Touraine P, Bione S, Toniolo D, Nelson LM, Zinn AR, Castrillon DH. Sequence variation at the human FOXO3 locus: a study of premature ovarian failure and primary amenorrhea. Hum Reprod. 2008;23:216–221. doi: 10.1093/humrep/dem255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McLaughlin M, Kinnell HL, Anderson RA, Telfer EE. Inhibition of phosphatase and tensin homologue (PTEN) in human ovary in vitro results in increased activation of primordial follicles but compromises development of growing follicles. Mol Hum Reprod. 2014;20:736–744. doi: 10.1093/molehr/gau037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fouzia S, Summayya S, Shahnazv IK. Differential expression of phosphatase and tensin homologue in normal, hyperplastic and neoplastic endometrium. J Pak Med Assoc. 2014;64:1103–1108. [PubMed] [Google Scholar]
- 15.Ting AY, Zelinski MB. Characterization of FOXO1, 3 and 4 transcription factors in ovaries of fetal, prepubertal and adult rhesus macaques. Biol Reprod. 2017;96:1052–1059. doi: 10.1093/biolre/iox034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Froment P, Bontoux M, Pisselet C, Monget P, Dupont J. PTEN expression in ovine granulosa cells increases during terminal follicular growth. FEBS Lett. 2005;579:2376–2382. doi: 10.1016/j.febslet.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 17.Ding W, Wang W, Zhou B, Zhang W, Huang P, Shi F, Taya K. Formation of primordial follicles and immunolocalization of PTEN, PKB and FOXO3A proteins in the ovaries of fetal and neonatal pigs. J Reprod Dev. 2010;56:162–168. doi: 10.1262/jrd.09-094H. [DOI] [PubMed] [Google Scholar]
- 18.Albamonte MI, Albamonte MS, Stella I, Zuccardi L, Vitullo AD. The infant and pubertal human ovary: Balbiani’s body-associated VASA expression, immunohistochemical detection of apoptosis-related BCL2 and BAX proteins, and DNA fragmentation. Hum Reprod. 2013;28:698–706. doi: 10.1093/humrep/des453. [DOI] [PubMed] [Google Scholar]
- 19.Goto M, Iwase A, Ando H, Kurotsuchi S, Harata T, Kikkawa F. PTEN and Akt expression during growth of human ovarian follicles. J Assist Reprod Genet. 2007;24:541–546. doi: 10.1007/s10815-007-9156-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goto M, Iwase A, Harata T, Takigawa S, Suzuki K, Manabe S, Kikkawa F. IGF1-induced AKT phosphorylation and cell proliferation are suppressed with the increase in PTEN during luteinization in human granulosa cells. Reproduction. 2009;137:835–842. doi: 10.1530/REP-08-0315. [DOI] [PubMed] [Google Scholar]
- 21.Fan HY, Liu Z, Cahill N, Richards JS. Targeted disruption of Pten in ovarian granulosa cells enhances ovulation and extends the life span of luteal cells. Mol Endocrinol. 2008;22:2128–2140. doi: 10.1210/me.2008-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pisarska MD, Kuo FT, Tang D, Zarrini P, Khan S, Ketefian A. Expression of forkhead transcription factors in human granulosa cells. Fertil Steril. 2009;91:1392–1394. doi: 10.1016/j.fertnstert.2008.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsuda F, Inoue N, Maeda A, Cheng YA, Sai T, Gonda H, Goto Y, Sakamaki K, Manabe N. Expression and function of apoptosis initiator FOXO3 in granulosa cells during follicular atresia in pig ovaries. J Reprod Dev. 2011;57:151–158. doi: 10.1262/jrd.10-124H. [DOI] [PubMed] [Google Scholar]
- 24.Liu Z, Ren YA, Pangas SA, Adams J, Zhou W, Castrillon DH, Richards JS. FOXO1/3and PTEN depletion in granulosa cells promotes ovarian granulosa cell tumor development. Mol Endocrinol. 2015;29:1006–1024. doi: 10.1210/me.2015-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang X, Tang N, Hadden TJ, Rishi AK. Akt, FoxO and regulation of apoptosis. Biochim Biophys Acta. 1813;2011:1978–1986. doi: 10.1016/j.bbamcr.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 26.Baker TG. A quantitative and cytological study of germ cells in human ovaries. Proc R Soc Lond B. 1963;158:417–433. doi: 10.1098/rspb.1963.0055. [DOI] [PubMed] [Google Scholar]
- 27.Forabosco A, Sforza C, De Pol A, Vizzotto L, Marzona L, Ferrario VL. Morphometric study of the human neonatal ovary. Anat Rec. 1991;231:201–208. doi: 10.1002/ar.1092310208. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 5325 kb)