Abstract
Introduction
This post hoc exploratory analysis examined the effects of ertugliflozin on liver enzymes in patients with type 2 diabetes mellitus (T2DM).
Methods
Data were pooled from seven randomized, double-blind VERTIS phase 3 trials that evaluated ertugliflozin (5 mg and 15 mg) versus non-ertugliflozin (placebo, glimepiride, or sitagliptin) treatment in patients with T2DM. Change from baseline at week 52 of treatment in alanine and aspartate aminotransferase (ALT and AST, respectively) serum levels (overall and categorized into tertiles by baseline ALT and AST), Fibrosis-4 Index (FIB-4), glycated hemoglobin (HbA1c), and body weight were evaluated, along with the association between changes in ALT and AST and changes in HbA1c and body weight by treatment.
Results
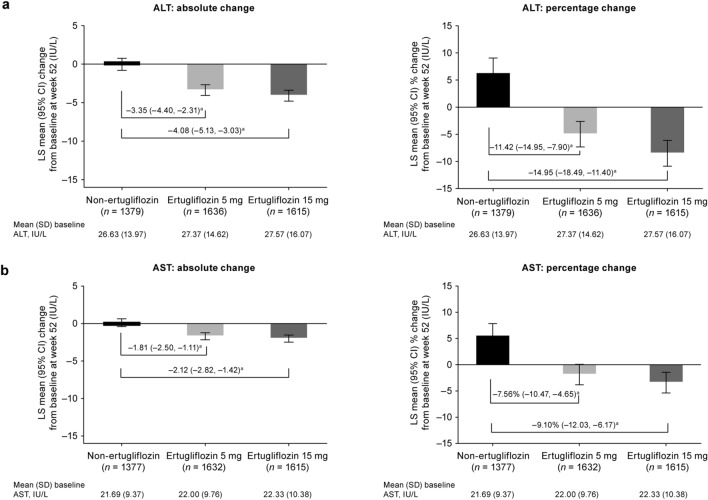
Baseline characteristics were balanced across treatment groups (ertugliflozin 5 mg, n = 1716; ertugliflozin 15 mg, n = 1693; non-ertugliflozin, n = 1450). At week 52 of treatment, serum levels of ALT and AST were reduced in patients in the ertugliflozin treatment groups (5 and 15 mg, respectively) compared with those in the non-ertugliflozin group. The comparator-adjusted mean (95% confidence interval [CI]) difference in change from baseline at week 52 for ALT was − 3.35 (− 4.40, − 2.31) IU/L for ertugliflozin 5 mg and − 4.08 (− 5.13, − 3.03) IU/L for ertugliflozin 15 mg; for AST, the respective values were − 1.81 (− 2.50, − 1.11) IU/L and − 2.12 (− 2.82, − 1.42) IU/L. The effects of ertugliflozin were detected across all baseline ALT and AST tertiles, with the highest tertile showing the greatest treatment differences. No meaningful differences were observed between treatment groups for FIB-4. Changes in ALT and AST showed a weak but statistically significant association with changes in HbA1c and body weight in all treatment groups.
Conclusions
Treatment with ertugliflozin resulted in a reduction in the levels of hepatic transaminases compared with the non-ertugliflozin group after 52 weeks of treatment. Changes in body weight and HbA1c contributed at least in part to the effects of ertugliflozin on liver enzymes.
Trial Registration
Clinicaltrials.gov registry numbers: NCT02033889, NCT01958671, NCT02036515, NCT01986855, NCT02099110, NCT02226003, NCT01999218.
Keywords: Alanine aminotransferase, Aspartate aminotransferase, Body weight, Ertugliflozin, Fibrosis-4 index, HbA1c, Liver enzymes, SGLT2 inhibitor, Type 2 diabetes mellitus
Key Summary Points
| Why carry out this study? |
| A frequently underappreciated comorbidity of type 2 diabetes mellitus (T2DM) is non-alcoholic fatty liver disease (NAFLD). Sodium-glucose cotransporter 2 (SGLT2) inhibitors have been shown to reduce serum levels of alanine and aspartate aminotransferase (ALT and AST, respectively) in patients with T2DM with and without NAFLD. |
| The aim of this pooled, post hoc analysis of seven randomized, double-blind phase 3 VERTIS trials was to assess the impact of ertugliflozin on liver enzymes in patients with T2DM. |
| What was learned from the study? |
| Ertugliflozin reduced serum ALT and AST levels after 52 weeks of treatment compared with the non-ertugliflozin treatment group (placebo, glimepiride, sitagliptin), similar to results obtained with other SGLT2 inhibitors. The reduction was greatest in patients with higher baseline ALT and AST levels. No significant differences were observed between treatment groups in the Fibrosis-4 Index. |
| Changes in ALT and AST showed a weak but statistically significant association with changes in glycated hemoglobin (HbA1c) and body weight in all treatment groups at week 52, suggesting that these changes in hepatic transaminases are partially accounted for by reductions in body weight and HbA1c. |
Introduction
A frequently underappreciated comorbidity of type 2 diabetes mellitus (T2DM) is non-alcoholic fatty liver disease (NAFLD), with a global prevalence of 55.5% among patients with T2DM [1]. Patients with NAFLD are usually asymptomatic and are often presumptively identified through increased serum levels of alanine transaminase (ALT) and aspartate transaminase (AST) [2, 3]. In a subset of patients, NAFLD can progress to non-alcoholic steatohepatitis (NASH) and fibrosis, leading to adverse liver outcomes, including mortality, and a greater need for liver transplantations. Underscoring the importance of early diagnosis, the risk of progression to NASH appears to be greater in patients with T2DM [4, 5].
Sodium-glucose cotransporter 2 (SGLT2) inhibitors are an important addition to approved therapies for T2DM [6, 7]. They have an insulin-independent mode of action and reduce renal glucose reabsorption, thereby enhancing urinary glucose excretion and reducing plasma glucose and glycated hemoglobin (HbA1c) levels [8]. The SGLT2 inhibitor drug class is associated with a reduction in blood pressure and body weight [9, 10]. In addition, studies have shown that treatment with SGLT2 inhibitors reduces the serum levels of ALT and AST, as found with canagliflozin and dapagliflozin in patients with T2DM [11, 12], with empagliflozin in patients with T2DM with or without NAFLD [13, 14], and with dapagliflozin in patients with T2DM and NAFLD or NASH [15–17]. Furthermore, there is some evidence that SGLT2 inhibitors (empagliflozin and dapagliflozin) may improve markers of hepatic injury, hepatic steatosis, and liver fibrosis in patients with T2DM and NAFLD or NASH [13, 15–17].
Ertugliflozin, a selective inhibitor of SGLT2, is approved in the USA, European Union, and other countries for the treatment of adults with T2DM. The VERTIS (eValuation of ERTugliflozin effIcacy and Safety) phase 3 clinical program has demonstrated the efficacy and safety of oral, once-daily 5 and 15 mg ertugliflozin, respectively, either as monotherapy or in combination with other antihyperglycemic agents [18–25]. In the post hoc analysis reported here, we explored the effects of ertugliflozin on ALT, AST, and the Fibrosis-4 Index (FIB-4; a non-invasive marker of hepatic fibrosis) and examined their association with changes in glycemic control (HbA1c) and body weight.
Methods
Study Design and Patient Population
This post hoc, exploratory analysis used a pooled data set comprising seven randomized, double-blind VERTIS phase 3 trials that evaluated the efficacy and safety of ertugliflozin compared with non-ertugliflozin (placebo, glimepiride, or sitagliptin) treatment (Table 1) [18–24]. In these studies, patients aged ≥ 18 years with inadequately controlled T2DM per the American Diabetes Association criteria and baseline HbA1c of 7.0–11% were randomized to treatment with ertugliflozin 5 mg, ertugliflozin 15 mg, or non-ertugliflozin treatment for 26–52 weeks (Table 1). Glycemic rescue therapy was prescribed for patients who exceeded protocol-specified glycemic thresholds. Patients were excluded from these studies if the ALT or AST level was > 2× the upper limit of normal at screening. Laboratory assessments were performed by one central laboratory with the following limits of normal: ALT female: ≤ 33 IU/L; ALT male: ≤ 41 IU/L, AST female: ≤ 31 IU/L, AST male: ≤ 37 IU/L.
Table 1.
VERTIS phase 3 studies included in the analysis
| VERTIS study name | MONO [24, 26] | MET [23, 27] | SITA2 [18] | SU [20, 28] | RENAL [19] | SITA [21] | FACTORIAL [22] |
|---|---|---|---|---|---|---|---|
| Clinicaltrials.gov identifier | NCT01958671 | NCT02033889 | NCT02036515 | NCT01999218 | NCT01986855 | NCT02226003 | NCT02099110 |
| Number of patients randomized | N = 461 | N = 621 | N = 464 | N = 1326 | N = 468 | N = 291 | N = 1233 |
| HbA1c inclusion criterion | 7.0–10.5% | 7.0–10.5% | 7.0–10.5% | 7.0–9.0% | 7.0–10.5% | 8.0–10.5% | 7.5–11.0% |
| Background therapy | Diet and exercise | Metformin | Metformin and sitagliptin | Metformin | Diet/exercise with or without AHA | Diet and exercise | Metformin |
| Ertugliflozin treatment groups |
Ertugliflozin 5 mg Ertugliflozin 15 mg |
Ertugliflozin 5 mg Ertugliflozin 15 mg |
Ertugliflozin 5 mg Ertugliflozin 15 mg |
Ertugliflozin 5 mg Ertugliflozin 15 mg |
Ertugliflozin 5 mg Ertugliflozin 15 mg |
Ertugliflozin 5 mg + sitagliptin 100 mg Ertugliflozin 15 mg + sitagliptin 100 mg |
Ertugliflozin 5 mg, Ertugliflozin 15 mg, each with and without sitagliptin 100 mg |
| Comparator | Placebo | Placebo | Placebo | Glimepiride | Placebo | Placebo | Sitagliptin |
| Duration | 26 weeks + 26-week extension | 26 weeks + 78-week extension | 26 weeks + 26-week extension | 52 weeks + 52-week extension | 26 weeks + 26-week extension | 26 weeks | 26 weeks + 26-week extension |
Table was adapted from Fig. 1 in Liu et al. [29]
AHA Antihyperglycemic agent, HbA1c glycated hemoglobin
Endpoint and Assessments
Endpoints were change from baseline to week 52 of treatment in serum levels of ALT and AST, and FIB-4. The association between changes in ALT and AST and changes in HbA1c and body weight were also assessed.
FIB-4 was calculated using the following equation [30, 31]:
No additional safety assessments were evaluated in this post hoc analysis. Safety analyses relating to each study have been published previously [18–24].
Statistical Analyses
This report presents data following 52 weeks of treatment. The post hoc analyses (SAS v9.4; SAS Institute, Cary, NC, USA) examined the data at both 26 and 52 weeks, and the results were generally similar. One of the seven VERTIS studies [21] had a duration of only 26 weeks, but because the results of the analyses at 26 and 52 weeks in the other studies were similar, these data were included in the statistical analyses (as 26-week data), and new analyses without these data and based only on week 52 data were not deemed necessary.
Analyses on ALT, AST, FIB-4, HbA1c, and body weight were conducted on all randomized patients who received ≥ 1 dose of study medication and had a baseline measurement and at least one post-baseline measurement. All analyses included data after the initiation of rescue therapy. There was no correction for type 1 error with multiple testing; the p values provided from the analysis model should be considered nominal.
Change from baseline in ALT, AST, FIB-4, HbA1c, and body weight at week 52 were compared between treatment groups using a repeated-measures analysis of covariance (ANCOVA) model with fixed effects for treatment, time, trial, baseline value of the response variable, and the interaction of time by treatment. Time was fitted as a categorical term. This analysis was also conducted on pooled sets of patients grouped into tertiles according to baseline levels of ALT and AST.
The association between changes in ALT and AST and changes in HbA1c and body weight by treatment was assessed by Pearson correlation by treatment. In addition, a repeated-measures ANCOVA model including body weight and HbA1c change from baseline, with the baseline value of the response variables as covariates and with fixed effects for treatment, time, trial, baseline value of the response variable, and the interaction of time by treatment, was performed to assess the contributions of weight loss and improvements in glycemic control to the changes in ALT and AST at week 52.
Statement of Ethics Compliance
Each of the seven studies included in the analysis was conducted in compliance with the ethical principles of the Declaration of Helsinki and in compliance with all International Conference on Harmonization Good Clinical Practice Guidelines. The final protocols and informed consent documentation were reviewed and approved by the Institutional Review Boards or Independent Ethics Committees at each investigational center. Written informed consent was obtained from all participants.
Results
Patient Disposition and Baseline Characteristics
A total of 4859 patients were included in the final analysis (ertugliflozin 5 mg, n = 1716; ertugliflozin 15 mg, n = 1693; non-ertugliflozin, n = 1450). Patients had a mean (standard deviation [SD]) duration of T2DM of 7.9 (6.4) years and a mean age of 57.8 (10.2) years; baseline characteristics were similar across the three treatment groups (Table 2).
Table 2.
Baseline demographics and disease characteristics of all patients included in the analysis
| Baseline demographics and disease characteristics | Treatment group | ||
|---|---|---|---|
| Non-ertugliflozin (n = 1450) | Ertugliflozin 5 mg (n = 1716) | Ertugliflozin 15 mg (n = 1693) | |
| Age (years) | 57.8 (10.3) | 57.9 (10.2) | 57.8 (10.2) |
| Male, n (%) | 787 (54.3%) | 885 (51.6%) | 844 (49.9%) |
| Body weight (kg)a | 89.2 (21.3) | 89.1 (20.8) | 87.5 (19.3) |
| BMI (kg/m2) | 31.6 (6.3) | 31.9 (6.1) | 31.5 (5.8) |
| Duration of T2DM (years) | 7.8 (6.3) | 7.9 (6.5) | 8.0 (6.3) |
| HbA1c (%)b | 8.1 (0.9) | 8.2 (0.9) | 8.2 (0.9) |
| ALT (IU/L)c | 26.6 (14.0) | 27.4 (14.6) | 27.6 (16.1) |
| AST (IU/L)d | 21.7 (9.4) | 22.0 (9.8) | 22.3 (10.4) |
| FIB-4e | 1.12 (0.56) | 1.11 (0.57) | 1.10 (0.53) |
| Platelet count (109/L)f | 239.2 (61.2) | 241.6 (60.4) | 245.1 (62.0) |
Data are presented as the mean with the standard deviation (SD) in parenthesis, unless otherwise specified
ALT Alanine aminotransferase, AST aspartate aminotransferase, BMI body mass index, FIB-4 Fibrosis-4 Index, HbA1c glycated hemoglobin, T2DM type 2 diabetes mellitus
a–fn is the number of patients in the non-ertugliflozin, ertugliflozin 5 mg, and ertugliflozin 15 mg treatment arms, respectively. an = 1442, n = 1706, and n = 1686; bn = 1439, n = 1695, and n = 1679; cn = 1417, n = 1677, and n = 1664; dn = 1415, n = 1672, and n = 1664; en = 1377, n = 1626, and n = 1621; fn = 1407, n = 1657, and n = 1651
Serum Levels of ALT and AST
Treatment with ertugliflozin 5 mg and 15 mg was associated with a decrease in serum levels of ALT and AST from baseline at week 52 compared with the non-ertugliflozin treatment, which showed increases in ALT and AST. Both the absolute and percentage decrease from baseline were greater with ertugliflozin 5 mg and 15 mg than with non-ertugliflozin treatment (Fig. 1).
Fig. 1.
Least squares (LS) mean absolute and percentage change from baseline at week 52 in alanine aminotransferase (ALT) (a) and aspartate aminotransferase (AST) (b). aDifference in LS means with the 95% confidence interval (CI) in parenthesis. SD Standard deviation
Patients were categorized into tertiles according to baseline serum levels of ALT and AST as follows:
ALT: ≤ 19, > 19 to ≤ 29, and > 29 IU/L;
AST: ≤ 17, > 17 to ≤ 23, and > 23 IU/L.
Across all tertiles, ertugliflozin 5 mg and 15 mg provided a greater benefit (reduction) on ALT and AST levels than did the non-ertugliflozin treatment at week 52 (Fig. 2). For both ertugliflozin doses, the largest reduction from baseline was observed in the highest tertile. For the highest baseline ALT tertile (> 29 IU/L), ertugliflozin 5 mg and 15 mg provided a least squares (LS) mean (95% confidence interval [CI]) reduction from baseline of − 10.69 (− 12.51, − 8.88) IU/L and − 11.02 (− 12.82, − 9.23) IU/L, respectively, which is more than twice the reduction shown in the non-ertugliflozin treatment arm (LS mean − 4.19 IU/L; 95% CI − 6.21, − 2.17). For the highest baseline AST tertile (> 23 IU/L), ertugliflozin 5 mg and 15 mg provided a LS mean (95% CI) reduction from baseline of − 6.79 (− 7.98, − 5.60) IU/L and − 7.17 (− 8.33, − 6.01) IU/L, respectively; LS mean change from baseline in the non-ertugliflozin arm was − 4.32 (− 5.64, − 3.01) IU/L.
Fig. 2.
Least squares (LS) mean absolute change from baseline at week 52 in ALT (a) and AST (b) by tertiles of baseline ALT and AST. aDifference in LS means with the 95% CI in parenthesis. ALT Alanine aminotransferase,AST aspartate aminotransferase,CI confidence interval,SD standard deviation
FIB-4
At week 52, the mean (SD) FIB-4 value was 1.11 (0.51) for the ertugliflozin 5 mg group, 1.09 (0.55) for the ertugliflozin 15 mg group, and 1.16 (0.52) for the non-ertugliflozin group. There were no meaningful differences in absolute LS mean change from baseline at week 52 in FIB-4 between the treatment groups (Table 3).
Table 3.
Least squares mean absolute change from baseline at week 52 in Fibrosis-4 Index
| Treatment arms | Baseline FIB-4 | Week 52 FIB-4 | Change in FIB-4 from baseline at week 52 | |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | LS mean (95% CI) | Difference in LS mean relative to non-ertugliflozin (95% CI) | |
| Non-ertugliflozin | 1.12 (0.56) | 1.16 (0.52) | 0.01 (− 0.02, 0.03) | – |
| Ertugliflozin 5 mg | 1.11 (0.57) | 1.11 (0.51) | 0.02 (0.00, 0.04) | 0.01 (− 0.02, 0.04) |
| Ertugliflozin 15 mg | 1.10 (0.53) | 1.09 (0.55) | 0.01 (− 0.01, 0.03) | 0.00 (− 0.03, 0.03) |
CI Confidence interval, LS least squares, FIB-4 Fibrosis-4 Index, SD standard deviation
Association of ALT and AST With Reductions in HbA1c and Body Weight
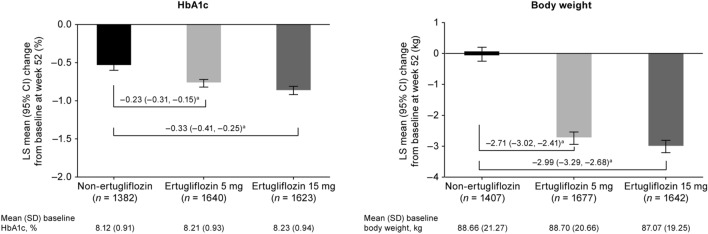
At week 52, both doses of ertugliflozin led to greater reductions from baseline in HbA1c compared with the non-ertugliflozin group (Fig. 3). Ertugliflozin also led to reductions from baseline in body weight, in contrast to the non-ertugliflozin group, which showed no overall change from baseline (Fig. 3).
Fig. 3.
Least squares (LS) mean change from baseline at week 52 in glycated hemoglobin (HbA1c) and body weight. aDifference in LS means (95% CI). CI confidence interval, SD standard deviation
The changes in ALT and AST from baseline to week 52 were associated with changes in HbA1c and body weight in all treatment groups, with r values ranging from 0.12 to 0.16 for HbA1c and from 0.13 to 0.23 for body weight (all p ≤ 0.0002).
With the addition of body weight and HbA1c as covariates in the statistical model, both doses of ertugliflozin remained more effective than non-ertugliflozin therapies in reducing serum levels of ALT and AST at week 52 (Table 4). However, the LS mean differences between treatment groups (ertugliflozin vs. non-ertugliflozin) when body weight and HbA1c were included as covariates (Table 4) were smaller than those obtained when body weight and HbA1c were not included as covariates (Fig. 1).
Table 4.
Treatment difference in least squares mean absolute and percentage change from baseline to week 52 in alanine and aspartate transaminases (repeated-measures analysis with body weight and glycated hemoglobin as covariates)
| Treatment | Difference in LS mean change from baseline relative to non-ertugliflozin (95% CI) | |
|---|---|---|
| Absolute change, IU/L | Percentage change | |
| ALT | ||
| Ertugliflozin 5 mg | – 2.35 (– 3.38, – 1.33) | – 8.08 (– 11.71, – 4.44) |
| Ertugliflozin 15 mg | – 2.93 (– 3.96, – 1.90) | – 10.77 (– 14.42, – 7.11) |
| AST | ||
| Ertugliflozin 5 mg | – 1.37 (– 2.06, – 0.68) | – 5.75 (– 8.84, – 2.66) |
| Ertugliflozin 15 mg | – 1.61 (– 2.30, – 0.92) | – 7.07 (– 10.17, – 3.96) |
ALT Alanine aminotransferase, AST aspartate aminotransferase, CI confidence interval, HbA1c glycated hemoglobin, LS least squares
Discussion
In this post hoc pooled analysis of patients with T2DM, treatment with ertugliflozin 5 mg and 15 mg reduced serum levels of ALT and AST after 52 weeks of treatment compared with non-ertugliflozin treatment. When patients were grouped by baseline ALT and AST tertiles, the greatest reduction from baseline was seen in the highest tertile for ALT (> 29 IU/L) and AST (> 23 IU/L), and was more prominent for ALT. Given that these clinical trials excluded patients with baseline ALT or AST > 2× upper limit of normal, the potential effect of ertugliflozin in reducing hepatic transaminases may be underestimated—especially for patient groups with higher transaminase levels, as found in some patients with NAFLD. Overall, these findings are consistent with those reported for other SGLT2 inhibitors in patients with T2DM [11, 12, 14].
The present analysis also assessed the impact of ertugliflozin on liver fibrosis using the FIB-4, a calculated score that has been shown to correlate with the stage of fibrosis (by histologic status) in patients with NAFLD [32]. The FIB-4 scores observed in this study at baseline and at week 52 likely correspond to fibrosis stage 0–2 [32], and are consistent with previously published FIB-4 scores in patients with T2DM and no diagnosis of liver disease [12]. No difference between treatment groups in the change from baseline to week 52 in FIB-4 score was observed. This lack of effect in patients receiving ertugliflozin may be due to the patient population enrolled; there was no requirement for liver disease, thereby resulting in low levels of baseline fibrosis. In studies of patients with T2DM and NAFLD [33, 34] or biopsy-confirmed NASH [35] treated with SGLT2 inhibitors, on average, baseline FIB-4 scores (mean values were 1.42 and 2.08 in two studies; median value was 1.75 in another study) were higher than those in the present analysis, suggesting that at least some patients in those studies may fall within fibrosis histology stage 3–4 [32]. In those studies, improvements in the FIB-4 scores were observed following SGLT2 inhibitor treatment.
Changes in ALT and AST showed a weak but statistically significant association with changes in HbA1c and body weight in all treatment groups at week 52. When body weight and HbA1c were added as covariates in the repeated-measures ANCOVA model, the effect of ertugliflozin versus non-ertugliflozin on ALT and AST remained, but the effect sizes were smaller than those from the analysis without these factors as covariates. This suggests that changes in HbA1c and body weight may partly contribute to the effects of ertugliflozin on aminotransferases, but do not account entirely for the observed reductions in this post hoc analysis. Relationships between changes in liver enzymes and changes in body weight or HbA1c may not be linear, and this could account for the weak correlation between the parameters.
Similar analyses with other SGLT2 inhibitors have produced divergent findings. In a pooled analysis of patients with T2DM treated with canagliflozin, the significant reductions in ALT and AST at week 26 were fully attributed to lowered body weight and HbA1c level [11]. However, in a pooled analysis of patients treated with empagliflozin, the significant reductions in ALT after 28 weeks of treatment were found to be largely independent of changes in body weight and glycemic control [14]. Similarly, in a clinical study of ipragliflozin in patients with T2DM-associated liver dysfunction, changes in serum ALT levels did not correlate with changes in body weight [36]. The reason for these discrepancies is unclear but may be related to methodologic and patient population differences among studies and their associated statistical models for pooled analyses.
There are limitations associated with this analysis. The post hoc nature of the analysis, the exclusion of patients with elevated levels of ALT and AST from the studies in this analysis, and the heterogeneous non-ertugliflozin control group could be considered potential limitations. Further, the analysis was not conducted in patients specifically with any chronic liver disease, such as NAFLD or NASH. Lastly, there was no correction for type 1 error with multiple testing.
Conclusion
In this post hoc, exploratory analysis, ertugliflozin was shown to be associated with reductions in hepatic transaminases in patients treated for 52 weeks compared with the group receiving non-ertugliflozin treatment (placebo, glimepiride, or sitagliptin). Weak correlations were observed with body weight and HbA1c. No differences in the FIB-4 score were observed. Prospective and mechanistic studies are needed to assess the effects of ertugliflozin and other SGLT2 inhibitors on liver enzymes in patients with T2DM with and without NAFLD.
Acknowledgements
The authors would like to thank all study participants, physicians, investigators, and staff at the participating study centers for their contributions to the individual studies.
Funding
This study was sponsored by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA (MSD), in collaboration with Pfizer Inc., New York, NY, USA. The Rapid Service Fee was funded by MSD in collaboration with Pfizer Inc., New York, NY, USA.
Medical Writing Assistance
Medical writing support was provided by Diane Hoffman, PhD, of Engage Scientific Solutions and was funded by MSD and Pfizer Inc.
Authorship
All authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Prior Presentation
Some of these data were presented at the 55th Annual Meeting of the European Association for the Study of Diabetes (EASD), September 16–20, 2019, Barcelona, Spain, and the American Association for the Study of Liver Diseases (AASLD)/The Liver Meeting 2019, November 8–12, 2019, Boston, MA, USA.
Disclosures
Annpey Pong and Annaswamy Raji are employees of MSD and may own stock in Merck & Co., Inc., Kenilworth, NJ, USA. Silvina Gallo, Roberto A. Calle, Steven G. Terra, and Lisa Tarasenko are employees of Pfizer Inc. and may hold stock and/or stock options in Pfizer Inc.
Compliance with Ethics Guidelines
Each of the seven studies included in the analysis was conducted in compliance with the ethical principles of the Declaration of Helsinki and in compliance with all International Conference on Harmonisation Good Clinical Practice Guidelines. The final protocols and informed consent documentation were reviewed and approved by the Institutional Review Boards or Independent Ethics Committees at each investigational center. Written informed consent was obtained from all participants.
Data Availability
Upon request, and subject to certain criteria, conditions, and exceptions (see https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information), Pfizer will provide access to individual de-identified participant data from Pfizer-sponsored global interventional clinical studies conducted for medicines, vaccines, and medical devices (1) for indications that have been approved in the USA and/or EU or (2) in programs that have been terminated (i.e., development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The de-identified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.
Footnotes
Digital Features
To view digital features for this article go to: 10.6084/m9.figshare.12481244.
References
- 1.Younossi ZM, Golabi P, de Avila L, Paik JM, Srishord M, Fukui N, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J Hepatol. 2019;71:793–801. doi: 10.1016/j.jhep.2019.06.021. [DOI] [PubMed] [Google Scholar]
- 2.Tomic D, Kemp WW, Roberts SK. Nonalcoholic fatty liver disease: current concepts, epidemiology and management strategies. Eur J Gastroenterol Hepatol. 2018;30:1103–1115. doi: 10.1097/MEG.0000000000001235. [DOI] [PubMed] [Google Scholar]
- 3.Spengler EK, Loomba R. Recommendations for diagnosis, referral for liver biopsy, and treatment of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Mayo Clin Proc. 2015;90:1233–1246. doi: 10.1016/j.mayocp.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol. 2013;10:330–344. doi: 10.1038/nrgastro.2013.41. [DOI] [PubMed] [Google Scholar]
- 5.Cotter TG, Rinella M. Nonalcoholic fatty liver disease 2020: The state of the disease. Gastroenterology. 2020;158:1851–1864. doi: 10.1053/j.gastro.2020.01.052. [DOI] [PubMed] [Google Scholar]
- 6.Cosentino F, Grant PJ, Aboyans V, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41:255–323. doi: 10.1093/eurheartj/ehz486. [DOI] [PubMed] [Google Scholar]
- 7.American Diabetes Association 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes—2019. Diabetes Care. 2019;42:S90–S102. doi: 10.2337/dc19-S009. [DOI] [PubMed] [Google Scholar]
- 8.Chao EC. SGLT-2 inhibitors: a new mechanism for glycemic control. Clin Diabetes. 2014;32:4–11. doi: 10.2337/diaclin.32.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang H, Yang J, Chen X, Qiu F, Li J. Effects of sodium-glucose cotransporter 2 inhibitor monotherapy on weight changes in patients with type 2 diabetes mellitus: a bayesian network meta-analysis. Clin Ther. 2019;41(322–34):e11. doi: 10.1016/j.clinthera.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Mazidi M, Rezaie P, Gao HK, Kengne AP. Effect of sodium-glucose cotransport-2 inhibitors on blood pressure in people with type 2 diabetes mellitus: a systematic review and meta-analysis of 43 randomized control trials with 22 528 patients. J Am Heart Assoc. 2017;6(6):e004007. [DOI] [PMC free article] [PubMed]
- 11.Leiter LA, Forst T, Polidori D, Balis DA, Xie J, Sha S. Effect of canagliflozin on liver function tests in patients with type 2 diabetes. Diabetes Metab. 2016;42:25–32. doi: 10.1016/j.diabet.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 12.Gastaldelli A, Repetto E, Guja C, et al. Exenatide and dapagliflozin combination improves markers of liver steatosis and fibrosis in patients with type 2 diabetes. Diabetes Obes Metab. 2020;22:393–403. doi: 10.1111/dom.13907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuchay MS, Krishan S, Mishra SK, et al. Effect of empagliflozin on liver fat in patients with type 2 diabetes and nonalcoholic fatty liver disease: a randomized controlled trial (e-lift trial) Diabetes Care. 2018;41:1801–1808. doi: 10.2337/dc18-0165. [DOI] [PubMed] [Google Scholar]
- 14.Sattar N, Fitchett D, Hantel S, George JT, Zinman B. Empagliflozin is associated with improvements in liver enzymes potentially consistent with reductions in liver fat: results from randomised trials including the EMPA-REG OUTCOME(R) trial. Diabetologia. 2018;61:2155–2163. doi: 10.1007/s00125-018-4702-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shimizu M, Suzuki K, Kato K, et al. Evaluation of the effects of dapagliflozin, a sodium-glucose co-transporter-2 inhibitor, on hepatic steatosis and fibrosis using transient elastography in patients with type 2 diabetes and non-alcoholic fatty liver disease. Diabetes Obes Metab. 2019;21:285–292. doi: 10.1111/dom.13520. [DOI] [PubMed] [Google Scholar]
- 16.Tobita H, Sato S, Miyake T, Ishihara S, Kinoshita Y. Effects of dapagliflozin on body composition and liver tests in patients with nonalcoholic steatohepatitis associated with type 2 diabetes mellitus: a prospective, open-label, uncontrolled study. Curr Ther Res Clin Exp. 2017;87:13–19. doi: 10.1016/j.curtheres.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eriksson JW, Lundkvist P, Jansson PA, et al. Effects of dapagliflozin and n-3 carboxylic acids on non-alcoholic fatty liver disease in people with type 2 diabetes: a double-blind randomised placebo-controlled study. Diabetologia. 2018;61:1923–1934. doi: 10.1007/s00125-018-4675-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dagogo-Jack S, Liu J, Eldor R, et al. Efficacy and safety of the addition of ertugliflozin in patients with type 2 diabetes mellitus inadequately controlled with metformin and sitagliptin: the VERTIS SITA2 placebo-controlled randomized study. Diabetes Obes Metab. 2018;20:530–540. doi: 10.1111/dom.13116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grunberger G, Camp S, Johnson J, et al. Ertugliflozin in patients with stage 3 chronic kidney disease and type 2 diabetes mellitus: the VERTIS RENAL randomized study. Diabetes Ther. 2018;9:49–66. doi: 10.1007/s13300-017-0337-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hollander P, Liu J, Hill J, et al. Ertugliflozin compared with glimepiride in patients with type 2 diabetes mellitus inadequately controlled on metformin: the VERTIS SU randomized study. Diabetes Ther. 2018;9:193–207. doi: 10.1007/s13300-017-0354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller S, Krumins T, Zhou H, et al. Ertugliflozin and sitagliptin co-initiation in patients with type 2 diabetes: the VERTIS SITA randomized study. Diabetes Ther. 2018;9:253–268. doi: 10.1007/s13300-017-0358-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pratley RE, Eldor R, Raji A, et al. Ertugliflozin plus sitagliptin versus either individual agent over 52 weeks in patients with type 2 diabetes mellitus inadequately controlled with metformin: the VERTIS FACTORIAL randomized trial. Diabetes Obes Metab. 2018;20:1111–1120. doi: 10.1111/dom.13194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosenstock J, Frias J, Pall D, et al. Effect of ertugliflozin on glucose control, body weight, blood pressure and bone density in type 2 diabetes mellitus inadequately controlled on metformin monotherapy (VERTIS MET) Diabetes Obes Metab. 2018;20:520–529. doi: 10.1111/dom.13103. [DOI] [PubMed] [Google Scholar]
- 24.Terra SG, Focht K, Davies M, et al. Phase III, efficacy and safety study of ertugliflozin monotherapy in people with type 2 diabetes mellitus inadequately controlled with diet and exercise alone. Diabetes Obes Metab. 2017;19:721–728. doi: 10.1111/dom.12888. [DOI] [PubMed] [Google Scholar]
- 25.Ji L, Liu Y, Miao H, et al. Safety and efficacy of ertugliflozin in Asian patients with type 2 diabetes mellitus inadequately controlled with metformin monotherapy: VERTIS Asia. Diabetes Obes Metab. 2019;21:1474–1482. doi: 10.1111/dom.13681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aronson R, Frias J, Goldman A, Darekar A, Lauring B, Terra SG. Long-term efficacy and safety of ertugliflozin monotherapy in patients with inadequately controlled T2DM despite diet and exercise: VERTIS MONO extension study. Diabetes Obes Metab. 2018;20:1453–1460. doi: 10.1111/dom.13251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gallo S, Charbonnel B, Goldman A, et al. Long-term efficacy and safety of ertugliflozin in patients with type 2 diabetes mellitus inadequately controlled with metformin monotherapy: 104-week VERTIS MET trial. Diabetes Obes Metab. 2019;21:1027–1036. doi: 10.1111/dom.13631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hollander P, Hill J, Johnson J, et al. Results of VERTIS SU extension study: safety and efficacy of ertugliflozin treatment over 104 weeks compared to glimepiride in patients with type 2 diabetes mellitus inadequately controlled on metformin. Curr Med Res Opin. 2019;35:1335–1343. doi: 10.1080/03007995.2019.1583450. [DOI] [PubMed] [Google Scholar]
- 29.Liu J, Patel S, Cater NB, et al. Efficacy and safety of ertugliflozin in East/Southeast Asian patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2020;22:574–582. doi: 10.1111/dom.13931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–1325. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- 31.Vallet-Pichard A, Mallet V, Nalpas B, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and fibrotest. Hepatology. 2007;46:32–36. doi: 10.1002/hep.21669. [DOI] [PubMed] [Google Scholar]
- 32.Shah AG, Lydecker A, Murray K, Tetri BN, Contos MJ, Sanyal AJ. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2009;7:1104–1112. doi: 10.1016/j.cgh.2009.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Itani T, Ishihara T. Efficacy of canagliflozin against nonalcoholic fatty liver disease: a prospective cohort study. Obes Sci Pract. 2018;4:477–482. doi: 10.1002/osp4.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ohki T, Isogawa A, Toda N, Tagawa K. Effectiveness of ipragliflozin, a sodium-glucose co-transporter 2 inhibitor, as a second-line treatment for non-alcoholic fatty liver disease patients with type 2 diabetes mellitus who do not respond to incretin-based therapies including glucagon-like peptide-1 analogs and dipeptidyl peptidase-4 inhibitors. Clin Drug Investig. 2016;36:313–319. doi: 10.1007/s40261-016-0383-1. [DOI] [PubMed] [Google Scholar]
- 35.Seko Y, Nishikawa T, Umemura A, et al. Efficacy and safety of canagliflozin in type 2 diabetes mellitus patients with biopsy-proven nonalcoholic steatohepatitis classified as stage 1-3 fibrosis. Diabetes Metab Syndr Obes. 2018;11:835–843. doi: 10.2147/DMSO.S184767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Komiya C, Tsuchiya K, Shiba K, et al. Ipragliflozin improves hepatic steatosis in obese mice and liver dysfunction in type 2 diabetic patients irrespective of body weight reduction. PLoS One. 2016;11:e0151511. doi: 10.1371/journal.pone.0151511. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Upon request, and subject to certain criteria, conditions, and exceptions (see https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information), Pfizer will provide access to individual de-identified participant data from Pfizer-sponsored global interventional clinical studies conducted for medicines, vaccines, and medical devices (1) for indications that have been approved in the USA and/or EU or (2) in programs that have been terminated (i.e., development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The de-identified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.