Abstract
Purpose
The objective of this systematic review and metaanalysis was to examine if the probability of pregnancy after ovarian stimulation for in vitro fertilization (IVF), using GnRH analogues and gonadotrophins is associated with serum estradiol level (Ε2) on the day of triggering final oocyte maturation with human chorionic gonadotrophin (hCG).
Methods
Twenty-one studies were eligible for this systematic review, including 19,598 IVF cycles, whereas three studies were eligible for metaanalysis, including 641 IVF cycles. The main outcome measure was achievement of ongoing pregnancy/live birth and, if not available, clinical pregnancy or biochemical pregnancy.
Results
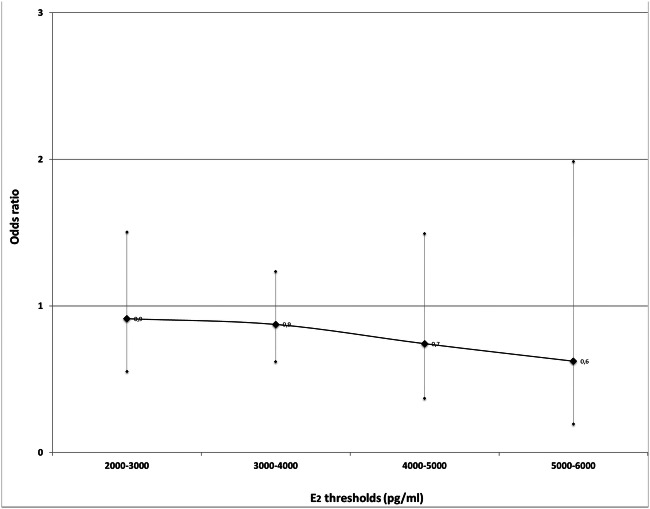
Pooling of data showed no differences in the probability of clinical pregnancy between patients with high and low Ε2 levels on the day of triggering final oocyte maturation. The pooled effect sizes for the Ε2 thresholds groups constructed, regarding clinical pregnancy were 2000–3000 pg/mL—OR 0.91, 95% CI 0.55 to 1.50, (fair quality/moderate risk of bias, n = 1 study), 3000–4000 pg/mL—OR 0.89, 95% CI 0.46 to 1.70, (fair quality/moderate risk of bias, n = 1 study, good quality/no information on which to base a judgement about risk of bias n = 2 studies), 4000–5000 pg/mL—OR 0.74, 95% CI 0.37 to 1.49 fair quality/moderate risk of bias, n = 1 study), 5000–6000 pg/mL—OR 0.62, 95% CI 0.19 to 1.98, (fair quality/moderate risk of bias, n = 1 study). In addition, no difference was observed in the probability of ongoing pregnancy for the Ε2 threshold group of 3000–4000 pg/mL OR 0.85, 95% CI 0.40 to 1.81(good quality/no information on which to base a judgement about risk of bias, n = 1 study).
Conclusion
Currently, there is insufficient evidence to support or deny the presence of an association between the probability of pregnancy and serum Ε2 levels on the day of triggering final oocyte maturation with hCG in women undergoing ovarian stimulation for IVF.
Electronic supplementary material
The online version of this article (10.1007/s10815-020-01829-z) contains supplementary material, which is available to authorized users.
Keywords: Estradiol, Clinical pregnancy, IVF, Ovarian stimulation
Introduction
Based on the latest European in vitro fertilization (IVF) monitoring (EIM) data, clinical pregnancy rates per aspiration after IVF or intracytoplasmic sperm injection (ICSI) were 29.9% and 28.4%, respectively [1]. Thus the majority of these couples are not going to achieve pregnancy in the fresh cycle. In order to gain insights into this problem, throughout the years, research has been carried out regarding the association between the achievement of pregnancy on the one hand and patients’ characteristics, embryo quality or ovarian stimulation variables on the other.
In this respect, it is well known that multifollicular development, aiming to ensure the presence of good quality embryos [2], leads to higher than normal hormone values of sex steroids [3]. High serum estradiol (E2) levels at the end of stimulation are associated with an increased number of oocytes retrieved, and in theory with more embryos of good quality available for transfer or cryopreservation, potentially increasing in this way the probability of pregnancy [4]. However, high Ε2 levels might be detrimental for implantation, adversely affecting endometrial receptivity [5] as well as oocyte maturation and quality [6], while they are associated with the occurrence of severe ovarian hyperstimulation syndrome (OHSS) [7–9].
Not unexpectedly, the association between serum Ε2 levels on the day of triggering final oocyte maturation with human chorionic gonadotrophin (hCG) and the probability of pregnancy after IVF has been in the focus of research for many years with conflicting, however, results [5, 10, 11]. Both a positive as well as a negative association between serum E2 levels on the day of triggering final oocyte maturation and the probability of pregnancy have been reported, while some studies deny the presence of such an association overall.
The mechanism by which E2 exerts its potential detrimental effect on the endometrium is not clear, although interference of high E2 levels with the synthesis and secretion of glycogen in the endometrial epithelial cells [12] and/ or induction of increased uterine contractility [13] have been implicated. In addition, high serum E2 concentrations have been shown to induce apoptosis of endometrial glandular cells (EGCs) that result in impaired endometrial receptivity [14].
On the other hand, sustained supraphysiological serum E2 levels do not seem to adversely affect the quality of developing oocytes and embryos. This view is supported by studies that evaluate pregnancy outcome in frozen-thawed embryo transfer cycles [15, 16] and oocyte donation cycles [17–19]. Embryo quality does not appear to be associated with high E2 levels, since similar pregnancy rates were achieved by transferring excess embryos from different E2 groups in subsequent frozen-thawed embryo transfer cycles. Similarly, in oocyte donation cycles, high concentrations of estradiol were not associated with the probability of pregnancy rate in the recipients.
No conclusive data on the association between E2 levels on the day of triggering final oocyte maturation and pregnancy achievement were provided by a systematic review published in 2004 [20], which included nine studies, the data of which were not synthesized in a metaanalysis. Since the publication of that systematic review [20], several additional relevant studies have been published.
The research question asked in the current systematic review and metaanalysis was whether the probability of pregnancy, expressed as ongoing pregnancy (≥ 12 weeks of gestation)/live birth, clinical pregnancy (up to 6–8 weeks of gestation) or biochemical pregnancy, after ovarian stimulation for IVF, using gonadotropin-releasing hormone (GnRH) analogues and gonadotrophins is associated with serum Ε2 levels on the day of triggering final oocyte maturation with hCG.
Methods
A systematic review and metaanalysis was performed, and the study was registered in the PROSPERO international prospective register of systematic reviews (42017059445).
Identification of studies
A computerized literature search in MEDLINE, SCOPUS, Science Direct and EMBASE covering the period until May 2019 was performed independently by two reviewers. For this purpose, a search strategy with keywords targeting the term estradiol combined with the term ‘IVF’ was constructed. Various synonyms describing each term were entered as free-text terms in the electronic databases in an attempt to maximize the sensitivity of the search strategy. The search terms used in all databases were hyperstimulation, overstimulation, superovulation, high responders, supraphysiological/high o?stradiol/estradiol/estrogen levels, pregnancy, reproduction, implantation, live birth rate, in vitro fertili?ation, in-vitro fertili?ation, IVF (Supplementary Table 1). Additionally, the citation lists of all relevant publications and review articles were hand-searched.
Inclusion/exclusion criteria
Both prospective and retrospective studies were eligible for inclusion, if they evaluated the association between serum Ε2 levels on the day of triggering final oocyte maturation with hCG and pregnancy achievement in fresh IVF/ICSI cycles. The predefined criteria for inclusion of a study in this systematic review were (a) the study had to evaluate women undergoing fresh IVF/ICSI cycles, (b) the study had to provide extractable data on pregnancy rates among women classified as those with high or low serum Ε2 levels on the day of triggering final oocyte maturation and (c) ovarian stimulation should have been performed with the use of gonadotrophins and GnRH analogues (agonists or antagonists).
Studies were excluded if (a) ovarian stimulation was carried out with the concomitant use of antioestrogens or without the use of GnRH analogues, (b) pregnancy was achieved after oocyte donation or after transfer of frozen-thawed embryos and (c) criteria used for triggering final oocyte maturation included serum Ε2 levels.
Evaluation of eligibility and data extraction were performed independently by two of the reviewers (G.K., E.M.K). Any disagreement was resolved by discussion.
Data extraction
The following data were recorded from the eligible studies, where feasible, demographic (citation data, country, study period, type of study, type of population, inclusion and exclusion criteria, number of cycles and number of patients included), methodological (power analysis, E2 threshold and reason for choosing this threshold, details regarding the assay used to determine E2 concentration) and procedural, (type of gonadotrophin used for ovarian stimulation, type of GnRH analogue and protocol used for LH surge inhibition, the criteria for timing of administration of hCG, the type of in vitro fertilization (IVF/ICSI), day of embryo transfer (ET), the number of embryos transferred, ET cancellation due to OHSS and type of luteal support administered).
Main outcome measure
The main outcome measure chosen for the current metaanalysis was achievement of pregnancy. To calculate this outcome, data from each study were primarily extracted on ongoing pregnancy (≥ 12 weeks of gestation)/live birth, and if not available, this was performed for data on clinical pregnancy (up to 6–8 weeks of gestation) or biochemical pregnancy.
Quality assessment
Quality assessment for the included studies was performed by using both the Newcastle-Ottawa form for cohort studies and the Risk Of Bias In Non-randomized Studies of Interventions (ROBINS-I) assessment tool (version for cohort-type studies) as all the studies included in this systematic review were cohort studies.
Data synthesis
From the eligible studies of the current systematic review, data synthesis was performed only in studies where (a) no interventions aiming to prevent OHSS in the presence of high serum E2 levels were performed, (b) similar numbers of embryos were transferred between the E2 groups compared and (c) women offered only one cycle for analysis.
The dichotomous data for each of the eligible studies were extracted in a 2 × 2 table and expressed as the odds ratio (OR) with 95% confidence interval (CI). These data were extracted based on the Ε2 threshold values provided by each study or dataset. When a study provided data on more than one Ε2 threshold, then all available information was extracted (e.g. one dataset for 1000 pg/ml and one dataset for 2000 pg/ml). However, no dataset was included in the same analysis more than once. Given the known variability in the thresholds employed in order to classify patients as those with high or low Ε2 levels [20], pooling of data was performed, where feasible, for groups of studies/datasets constructed based on these published Ε2 thresholds, using ranges of 1000 pg/ml. Thus the following threshold groups were constructed (a) 0–1000 pg/ml, (b) 1000–2000 pg/ml, (c) 2000–3000 pg/ml, (d) 3000–4000 pg/ml and (e) 4000–5000 pg/ml.
Pooling of data was performed using the inverse variance method [21], in the case of fixed effects model and the DerSimonian and Laird method [22], in the case of the random effects model. When the outcome of interest was of a continuous nature, the differences were pooled across the studies which provided information on this outcome, resulting in a weighted mean difference (WMD) with 95% CI. All results were combined for metaanalysis with STATA (Version 13.0, College Station, TX: StataCorp LP).
Study-to-study variation was assessed by using the chi-square statistic (the hypothesis tested was that the studies are all drawn from the same population, i.e. from a population with the same effect size). In addition, the use of the I2 index was employed in order to indicate the proportion of inconsistency between studies that could not be attributed to chance, with I2 ≥ 40% indicating significant heterogeneity [23]. A fixed effects model was used where no statistically significant heterogeneity was present, whereas in the presence of significant heterogeneity, as indicated either by the chi-square statistic or the I2 index, a random effects model was applied. Statistical significance was set at P < 0.05. The presence of publication bias was assessed, where feasible, by constructing and visually examining funnel plots, as well as by performing Egger’s test [24].
Results
Literature search
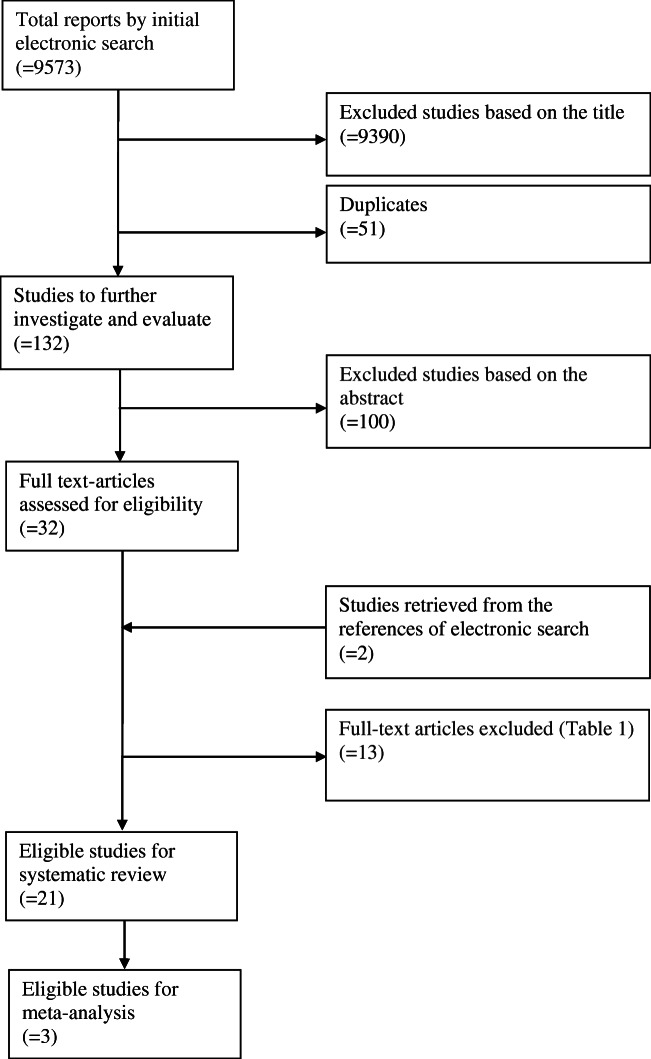
The initial electronic search yielded 9573 studies, and after the removal of duplicates, 9393 studies comprised the initial dataset. Based on the titles of these studies, 129 publications were considered relevant for answering the research question of this systematic review and were further evaluated by reading their abstracts.
Thirty studies were selected for full text evaluation. Following the screening of full texts (Table 1) and after the addition of two records retrieved by searching the references of these studies, 21 publications were eligible for inclusion in the present systematic review (Fig. 1).
Table 1.
Excluded studies from systematic review based on the full text
| Reasons | Studies excluded |
|---|---|
| Analysis did not take into account the complete range of E2 levels available | Sharara and McClamrock 1999, Friedler et al. 2005, Bahçeci et al. 2006, Chen et al. 2007, Yoldemir and Fraser 2009, Moraloglu et al. 2012 |
| No extractable data | Blazar et al. 2004, Papageorgiou et al. 2002 |
| Inclusion of serum E2 levels in the hCG administration criteria | Mettler et al. 1989, Chenette et al. 1990, Dor et al. 1992, Gelety and Buyalos 1995, Simon et al. 1995 |
Fig. 1.
Study selection process
Systematic review
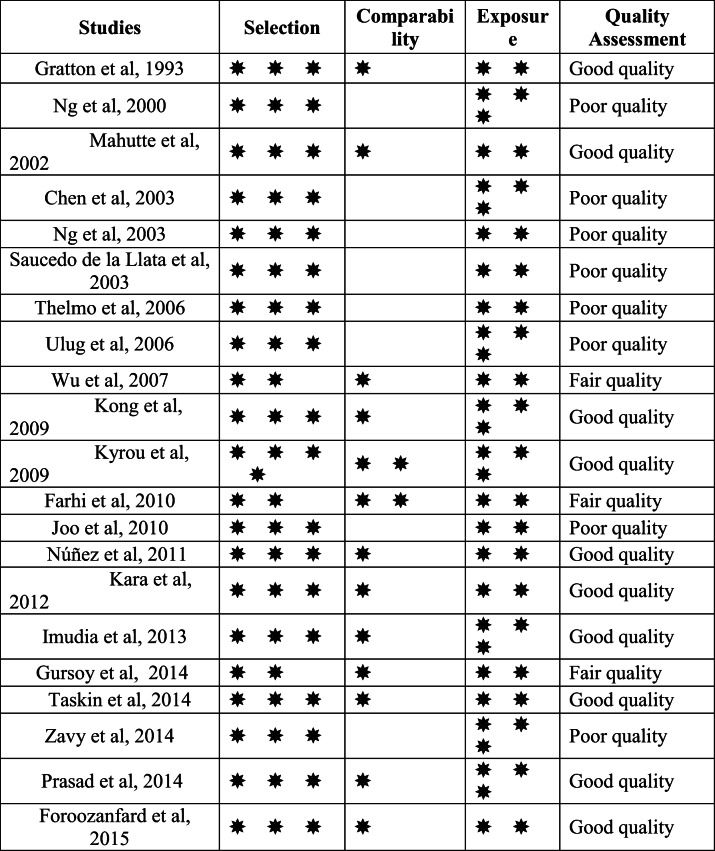
Quality assessment for the included studies that based on the Newcastle-Ottawa form for cohort studies, evaluated eight studies for having poor quality, three studies for fair quality and ten studies for good quality (Table 2).
Table 2.
Newcastle-Ottawa Quality Assessment Form for Cohort Studies
According to the quality assessment performed by using ROBINS-I assessment tool, nine studies judged to be at serious risk of bias, six at moderate risk of bias, and there was no clear indication of serious or critical risk of bias, and there was a lack of information in one or more key domains of bias at six studies (Supplementary Table 2).
Characteristics of the eligible studies
Sample size
The twenty-one eligible studies (Supplementary Table 3A) were published between 1993 and May 2019 and included 19,598 cycles, with a sample size ranging from 113 to 5778 cycles (median size 280). Power analysis was not performed in any of these studies.
Type of studies
With the exception of one prospective study [25], all the remaining eligible studies were retrospective.
Inclusion and exclusion criteria
The patient inclusion criteria varied considerably among studies (Supplementary Table 3A). In nine studies, an unselected population was analysed [8, 26–33], whereas specific inclusion/exclusion criteria where used in the remaining studies (Supplementary Table 3A).
Ovarian stimulation
Regarding the type of GnRH analogue used for the inhibition of a premature endogenous luteinizing hormone (LH) surge, which could lead to premature luteinisation, GnRH agonists were used in 14 studies, while GnRH antagonists were used in a single study. In five studies, LH suppression was achieved by using either GnRH agonists or GnRH antagonists, while in a single study this information was not reported (Supplementary Table 3A).
In nine studies, ovarian stimulation was performed with the use of follicle stimulation hormone (FSH) or human menopausal gonadotropin (hMG) or a combination of both. In the remaining studies, patients were stimulated exclusively with gonadotrophins (either urinary or recombinant) without LH activity (FSH; n = 6) or exclusively with gonadotrophins with LH activity (hMG; n = 4). In two studies, the type of gonadotrophin used for ovarian stimulation was not reported (Supplementary Table 3A).
E2 assessment/threshold
The Ε2 assays utilised varied among the eligible studies (Supplementary Table 3A). This was also true regarding the Ε2 thresholds used to classify the specific number of patients into those with high and low Ε2 levels on the day of triggering of final oocyte maturation (Supplementary Table 4). Classification of patients into those with high and low Ε2 on the day of hCG administration was accomplished by a receiver operating characteristic (ROC) analysis in two studies, by percentile analysis in five studies and by logistic regression in a single study. In 13 studies, Ε2 thresholds used to classify patients into those with high and low serum Ε2 levels on the day of triggering of final oocyte maturation were chosen arbitrarily.
Criteria for triggering final oocyte maturation type of fertilization
The criteria for hCG administration varied among studies. The most commonly used criteria were the presence of ≥ 2–3 follicles with a mean diameter of 17 or 18 mm (Supplementary Table 3A).
The method of fertilization varied among studies. IVF was used in four studies, ICSI in six studies, while both IVF/ICSI were used in the remaining 11 studies (Supplementary Table 3A).
Luteal phase support
Luteal phase support involved vaginal or intramuscular progesterone administration at various dosages in 18 studies. In the remaining three studies, either hCG or progesterone was administered (Supplementary Table 3A).
Embryo transfer
ET was performed at the cleavage stage (day 2 or day 3) in 14 studies, on day 3 or on day 5 in five studies, on either day 2 or 3 or 5 in a single study, while this information was not reported in a single study (Supplementary Table 3A). In the six studies in which ET was performed either on day 3 or day 5, the probability of blastocyst transfer significantly increased in parallel with the increase in serum Ε2 levels in three studies, while this data was not provided in three studies (Supplementary Table 3B).
A significantly higher number of embryos were transferred in the high Ε2 group compared with the low Ε2 group in seven studies while the reverse was true in two studies (Supplementary Table 3B). Moreover, no difference between Ε2 groups regarding the number of embryos transferred was present in six studies, while this information was not available in six studies (Supplementary Table 3B).
ET was cancelled in the presence of high risk of OHSS in two studies. OHSS patients were also excluded from the analysis of seven studies (Supplementary Table 3A). ET policy in the presence of high risk for OHSS was not reported in the remaining 12 studies.
Primary outcome measure
Regarding the primary outcome measure, achievement of pregnancy, it was feasible to extract data on live birth/delivery from five studies, on ongoing pregnancy from five studies, on clinical pregnancy from nine studies and on biochemical pregnancy from a single study. The outcome “pregnancy” was not defined in a single study (Supplementary Table 3B).
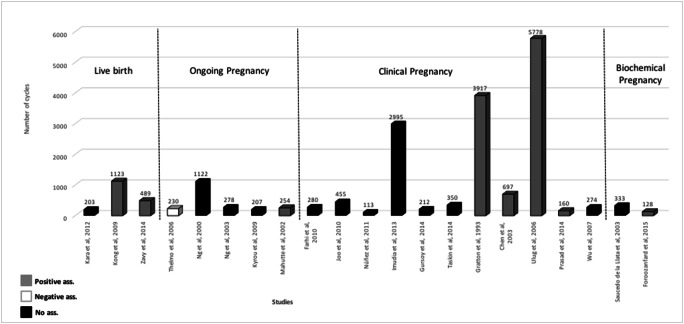
A positive association between serum Ε2 levels on the day of triggering of final oocyte maturation and delivery or live birth achievement after ET was reported in two studies [8, 34], between serum Ε2 levels on the day of triggering of final oocyte maturation and ongoing pregnancy in a single study [27], between serum Ε2 levels on the day of triggering of final oocyte maturation and clinical pregnancy in four studies [26, 28, 35, 36] and between serum Ε2 levels on the day of triggering of final oocyte maturation and biochemical pregnancy in a single study [32]. A negative association was reported between serum Ε2 levels on the day of triggering of final oocyte maturation and ongoing pregnancy in a single study [37]. No significant association was present between serum Ε2 levels on the day of triggering of final oocyte maturation and delivery in three studies [29, 30, 38], between serum Ε2 levels on the day of triggering of final oocyte maturation and ongoing pregnancy in three studies [7, 15, 25], between serum Ε2 levels on the day of triggering of final oocyte maturation and clinical pregnancy in four studies [31, 33, 39, 40] and between serum Ε2 levels on the day of triggering of final oocyte maturation and undefined pregnancy in two studies [19, 41] (Fig. 2).
Fig. 2.
Studies included in the systematic review evaluating the association between serum E2 levels on the day of triggering final oocyte maturation with hCG with the achievement of pregnancy
Secondary outcome measures
A statistically significant positive association between serum Ε2 levels on the day of triggering of final oocyte maturation and the occurrence of OHSS was reported in three studies, while positive but no statistically significant association was present in a single study (Supplementary Table 3B).
Regarding FSH dosage required for ovarian stimulation, cumulus-oocyte complexes (COCs) retrieved and number of embryo transferred, extractable data between Ε2 groups where only available in a single study, [31]. Comparison of these characteristics among cycles with high and low serum estradiol levels on the day of hCG administration showed significantly lower FSH requirement in the group with high serum Ε2 levels (2284 ± 1043 vs 2916 ± 1824, respectively, p = 0.001) and significantly higher COCs in the group with high serum estradiol levels (15 ± 5 vs 10 ± 5, respectively, p = 0.001). Similar number of embryos were transferred between patients with high and low serum Ε2 levels (2.3 ± 0.9 vs 2.2 ± 0.8, respectively, p = 0.60).
Metaanalysis
Excluding studies in which women participated more than once, those in which significantly different number of embryos was transferred between the Ε2 groups compared, as well as studies in which cycles were cancelled due to high risk of OHSS; three studies were eligible for metaanalysis, including in total 641 cycles.
Ongoing pregnancy per ET was the primary outcome in a single study, which also offered data for clinical pregnancy, while clinical pregnancy per ET was the primary outcome in three studies (Supplementary Table 3B). Therefore, it was possible to synthesize data regarding primary outcome measure only for clinical pregnancy.
Association between serum E2 levels and clinical pregnancy achievement in different E2 threshold groups constructed:
-
0–1000 pg/ml E2 threshold and 1000–2000 pg/ml E2 threshold
No studies offered data for the Ε2 thresholds of 0–1000 pg/ml and 1000–2000 pg/ml.
2000–3000 pg/ml E2 threshold
Primary outcome measure
Clinical pregnancy per ET
No important benefit or harm was detected in the probability of clinical pregnancy per ET between women with high and low serum Ε2 levels on the day of triggering of final oocyte maturation (OR 0.91, 95% CI 0.55–1.50; fixed effects model) in a single study (n = 274 patients) available for analysis which evaluated to have fair quality/moderate risk of bias [41].
3000–4000 pg/ml E2 threshold
Primary outcome measure
Clinical pregnancy per ET
No important benefit or harm was detected in the probability of clinical pregnancy per ET between women with high and low serum Ε2 levels on the day of triggering of final oocyte maturation (OR 0.89, 95% CI 0.46–1.70; heterogeneity: P = 0.07, I2 = 62.1%; random effects model) in three studies (n = 641 patients) available for analysis which evaluated to have good quality/ no information on which to base a judgement about risk of bias [27], good quality/no information on which to base a judgement about risk of bias [40] and fair quality/moderate risk of bias [41]. Publication bias was not detected by Egger’s test (p = 0.998).
Ongoing pregnancy per ET
No important benefit or harm was detected in the probability of clinical pregnancy per ET between women with high and low serum Ε2 levels on the day of triggering of final oocyte maturation (OR 0.85, 95% CI 0.40 to 1.81; fixed effects model) in a single study (n = 254 patients) which evaluated to have good quality/no information on which to base a judgement about risk of bias [27].
4000–5000 pg/ml E2 threshold
Primary outcome measure
Clinical pregnancy per ET
No important benefit or harm was detected in the probability of clinical pregnancy per ET between women with high and low serum Ε2 levels on the day of triggering of final oocyte maturation (OR 0.74, 95% CI 0.37–1.49; fixed effects model) in a single study (n = 274 patients) available for analysis which evaluated to have fair quality/moderate risk of bias [41].
5000–6000 pg/ml E2 threshold
Primary outcome measure
Clinical pregnancy per ET
No important benefit or harm was detected in the probability of clinical pregnancy per ET between women with high and low serum Ε2 levels on the day of triggering of final oocyte maturation (OR 0.62, 95% CI 0.195–1.985; fixed effects model) in a single study (n = 274 patients) available for analysis which evaluated to have fair quality/moderate risk of bias [41].
Overall, no important benefit or harm was detected in the probability of clinical pregnancy per ET between women with high and low serum Ε2 levels on the day of triggering of final oocyte maturation for all the Ε2 thresholds examined (Fig. 3).
Fig. 3.
Pooled ORs for clinical pregnancy achievement per ET in women with high serum E2 levels when compared with women with low serum E2 levels in different E2 threshold groups
Discussion
The current systematic review and metaanalysis cannot support the presence of an association between probability of pregnancy after ovarian stimulation for IVF using GnRH analogues and gonadotrophins and serum Ε2 levels on the day of triggering final oocyte maturation with hCG. This finding was present in all Ε2 threshold groups evaluated.
There is only one previous relevant systematic review [20], which evaluated the association between serum Ε2 levels on the day of triggering of final oocyte maturation and the probability of pregnancy in cycles in which a fresh embryo transfer was performed using patient’s oocytes. In that systematic review, nine retrospective studies, performed with GnRH agonists, were included (n = 3508 cycles). The primary outcome was clinical pregnancy in six studies and pregnancy (undefined) in three studies; however, no meta-analysis was possible to be performed. Five of these studies (n = 2018 cycles) did not support the presence of an association between serum Ε2 levels on the day of triggering of final oocyte maturation and pregnancy achievement. Two studies (n = 191 cycles) suggested that the higher the serum Ε2 levels on the day of triggering of final oocyte maturation, the higher the pregnancy rates achieved, while two studies (n = 1299 cycles) suggested a detrimental role of high serum Ε2 levels on the day of triggering of final oocyte maturation for pregnancy achievement.
The studies analysed in the present metaanalysis are retrospective, and thus, the presence of bias cannot be excluded. However, in order to minimize bias, studies with imbalances in the characteristics of the Ε2 groups compared, such as differences in the number of embryos transferred in women with high and low serum Ε2 levels on the day of triggering of final oocyte maturation, were excluded. Moreover, studies which included serum Ε2 levels in the criteria used for triggering of final oocyte maturation were also excluded, since they may confound the true association between Ε2 levels and the probability of pregnancy. Similarly, to avoid bias, studies in which women participated more than once, as well as studies in which cycles were cancelled due to high risk of OHSS, were not considered eligible for meta-analysis.
Interestingly, power calculation was not performed in any of the studies included in the present systematic review and metaanalysis. This could have provided information on the ability of the individual studies to detect the presence or not of an association between serum Ε2 levels on the day of triggering of final oocyte maturation and pregnancy achievement, if such an association exists, and could have avoided a type II error.
Regarding study design, arbitrary thresholds of serum Ε2 levels on the day of triggering of final oocyte maturation have been used to create groups of patients with high or low Ε2 levels. Not unexpectedly, studies supporting the presence of a negative association between serum Ε2 levels and pregnancy achievement failed to converge to a similar threshold level of Ε2 at which the probability of pregnancy was significantly decreased. It is not known whether this would have been feasible if an objective method to define these thresholds, such as the use of receiver operating curve characteristics (ROC) had been used. Moreover, the heterogeneity present in the inclusion exclusion criteria (Supplementary Table 3A) limits the external validity of the conclusions drawn.
It has to be noted that the association of serum Ε2 levels and pregnancy outcome, might depend on the ET policy used. Theoretically, in cases where a single embryo is transferred, the potential advantage of the high Ε2 regarding the selection of a high quality embryo might be less prominent compared with cases with two or three embryos transferred. This is because the selection of a high quality embryo might still be possible in women with low serum Ε2 levels on the day of triggering final oocyte maturation and thus with a lower number of oocytes/embryos. However, the potential advantage of high Ε2 levels might be balanced by the negative association of Ε2 levels and endometrial receptivity [13, 14, 42, 43].
Alternatively if a negative association exists between serum Ε2 levels the day of triggering final oocyte maturation and pregnancy achievement, high serum Ε2 levels, beyond a certain currently undefined threshold should be avoided and instead these women would benefit from embryo transfer cancellation and a freeze all strategy. Moreover besides efficacy, it should always be kept in mind that high serum Ε2 levels compromise safety since they have been associated with the occurrence of severe OHSS [7, 8, 15].
The true association between the Ε2 levels the day of hCG administration and achievement of pregnancy might depend on several other factors besides ET policy. However, this could only be revealed by performing an individual patient data (IPD) metaanalysis which was not feasible. Such an IPD metaanalysis of sufficient size would allow the identification of variables (specific patient characteristics, type of stimulation protocol, type of ovarian response, quantity and quality of available embryos, type of luteal support, criteria for triggering final oocyte maturation, method of Ε2 assessment, etc.) that might confound the true association of serum Ε2 levels and pregnancy achievement and allow the calculation of the independent effect of serum Ε2 levels on the probability of pregnancy, assisting in the development of a multivariate, predictive model that could be useful to clinicians after validation.
By understanding the drawbacks present in the studies included in the current systematic review, one could describe the characteristics of a high quality relevant study. The same gonadotrophin type with an initiating dose defined according to specific criteria should be used. The FSH dose should be preferably fixed or adjusted according to certain follicular development criteria. Criteria for hCG administration should not include Ε2 levels and should be strict (administration of hCG as soon as a predefined criterion for follicular development is reached). Due to the important role of progesterone on the day of triggering final oocyte maturation [3], progesterone should be assessed in the Ε2 groups created, and its effect should also be taken into consideration in the analysis of the probability of pregnancy [44], which was not the case with the eligible studies in the current review.
All patients should have the same number of embryos transferred (ideally a single embryo) at the same stage of embryonic development (e.g. blastocyst) to ensure that all Ε2 groups are comparable for pregnancy rates. Luteal phase should be supported in a similar way for all patients. Moreover, inclusion criteria should be broad enough to allow generalizability of the results obtained.
In conclusion, acknowledging the lack of high quality studies, the current literature does not support an association between serum Ε2 levels value on the day of triggering of final oocyte maturation and the achievement of pregnancy in fresh IVF/ICSI cycles using gonadotrophins and GnRH analogues. However, the trend for a decrease in pregnancy rates in parallel with an increase of serum Ε2 levels (Fig. 3) needs to be further evaluated.
Electronic supplementary material
(DOCX 60 kb).
(DOCX 135 kb).
(DOCX 27 kb).
(DOCX 16 kb).
(DOCX 108 kb).
Author contributions
Karatasiou G. performed the literature search and data analysis, manuscript writing and editing. Bosdou J., Venetis C. Zepiridis L., Chatzimeletiou K., Tarlatzi T., Lainas G., Tarlatzis B. and Grimbizis G. critically revised the work, and Kolibianakis E. had the idea for the article, performed data analysis and manuscript editing.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.De Geyter C, Calhaz-Jorge C, Kupka MS, Wyns C, Mocanu E, Motrenko T, et al. ART in Europe, 2014: results generated from European registries by ESHRE: The European IVF-monitoring Consortium (EIM) for the European Society of Human Reproduction and Embryology (ESHRE) Human reproduction (Oxford, England) 2018;33(9):1586–1601. doi: 10.1093/humrep/dey242. [DOI] [PubMed] [Google Scholar]
- 2.Venetis CA, Tilia L, Panlilio E, Kan A. Is more better? A higher oocyte yield is independently associated with more day-3 euploid embryos after ICSI. Human reproduction (Oxford, England) 2019;34(1):79–83. doi: 10.1093/humrep/dey342. [DOI] [PubMed] [Google Scholar]
- 3.Venetis CA, Kolibianakis EM, Bosdou JK, Tarlatzis BC. Progesterone elevation and probability of pregnancy after IVF: a systematic review and meta-analysis of over 60 000 cycles. Hum Reprod Update. 2013;19(5):433–457. doi: 10.1093/humupd/dmt014. [DOI] [PubMed] [Google Scholar]
- 4.Sunkara SK, Rittenberg V, Raine-Fenning N, Bhattacharya S, Zamora J, Coomarasamy A. Association between the number of eggs and live birth in IVF treatment: an analysis of 400 135 treatment cycles. Human reproduction (Oxford, England) 2011;26(7):1768–1774. doi: 10.1093/humrep/der106. [DOI] [PubMed] [Google Scholar]
- 5.Simon C, Cano F, Valbuena D, Remohi J, Pellicer A. Clinical evidence for a detrimental effect on uterine receptivity of high serum oestradiol concentrations in high and normal responder patients. Hum Reprod. 1995;10(9):2432–2437. doi: 10.1093/oxfordjournals.humrep.a136313. [DOI] [PubMed] [Google Scholar]
- 6.Clement P. Consequences of stimulation on oocyte quality. Gynecol Obstet Fertil. 2007;35(9):890–897. doi: 10.1016/j.gyobfe.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 7.Ng EH, Lau EY, Yeung WS, Ho PC. Oocyte and embryo quality in patients with excessive ovarian response during in vitro fertilization treatment. J Assist Reprod Genet. 2003;20(5):186–191. doi: 10.1023/A:1023670010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kong GWS, Cheung LP, Haines CJ, Lam PM. Comprehensive assessment of serum estradiol impact on selected physiologic markers observed during in-vitro fertilization and embryo transfer cycles. J Exp Clin Assist Reprod. 2009;6:5. [PMC free article] [PubMed]
- 9.Moraloglu O, Tonguc EA, Ozel M, Ozaksit G, Var T, Sarikaya E. The effects of peak and mid-luteal estradiol levels on in vitro fertilization outcome. Arch Gynecol Obstet. 2012;285(3):857–862. doi: 10.1007/s00404-011-2090-8. [DOI] [PubMed] [Google Scholar]
- 10.Mettler L, Tavmergen EN. Significance of oestradiol values in IVF–ET under a combined GnRH analogue—desensitization and simultaneous gonadotrophin stimulation for the outcome of pregnancies. Hum Reprod. 1989;4(suppl 1):59–64. doi: 10.1093/humrep/4.suppl_1.59. [DOI] [PubMed] [Google Scholar]
- 11.Dor J, Seidman DS, Ben-Shlomo I, Levran D, Karasik A, Mashiach S. The prognostic importance of the number of oocytes retrieved and estradiol levels in poor and normal responders in in vitro fertilization (IVF) treatment. J Assist Reprod Genet. 1992;9(3):228–232. doi: 10.1007/BF01203818. [DOI] [PubMed] [Google Scholar]
- 12.Van Santen MR, Haspels AA, Heijnen HFG, Rademakers LHPM. Interfering with implantation by postcoital estrogen administration II. Endometrium epithelial cell ultrastructure. Contraception. 1988;38(6):711–724. doi: 10.1016/0010-7824(88)90052-2. [DOI] [PubMed] [Google Scholar]
- 13.de Ziegler D, Fanchin R, de Moustier B, Bulletti C. The hormonal control of endometrial receptivity: estrogen (E2) and progesterone. J Reprod Immunol. 1998;39(1–2):149–166. doi: 10.1016/s0165-0378(98)00019-9. [DOI] [PubMed] [Google Scholar]
- 14.Chen SU, Chou CH, Chen MJ, Chen TH, Yang YS, Yang JH. Apoptotic effects of high estradiol concentrations on endometrial glandular cells. J Clin Endocrinol Metab. 2014;99(6):E971–E980. doi: 10.1210/jc.2013-3794. [DOI] [PubMed] [Google Scholar]
- 15.Ng EHY, Yeung WSB, Lau EYL, So WWK, Ho PC. High serum oestradiol concentrations in fresh IVF cycles do not impair implantation and pregnancy rates in subsequent frozen-thawed embryo transfer cycles. Hum Reprod. 2000;15(2):250–255. doi: 10.1093/humrep/15.2.250. [DOI] [PubMed] [Google Scholar]
- 16.Chen QJ, Sun XX, Li L, Gao XH, Wu Y, Gemzell-Danielsson K, et al. Effects of ovarian high response on implantation and pregnancy outcome during controlled ovarian hyperstimulation (with GnRH agonist and rFSH) Acta Obstet Gynecol Scand. 2007;86(7):849–854. doi: 10.1080/00016340701415152. [DOI] [PubMed] [Google Scholar]
- 17.Bianco K, Mahutte NG, Arici A, Sakkas D, Taylor HS. Effect of estradiol on oocyte development. Int J Gynaecol Obstet. 2009;104(3):230–232. doi: 10.1016/j.ijgo.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pena JE, Chang PL, Chan LK, Zeitoun K, Thornton MH, 2nd, Sauer MV. Supraphysiological estradiol levels do not affect oocyte and embryo quality in oocyte donation cycles. Human reproduction (Oxford, England) 2002;17(1):83–87. doi: 10.1093/humrep/17.1.83. [DOI] [PubMed] [Google Scholar]
- 19.de la Llata ES, Moraga Sanchez MR, Pezino Rodriguez J, Trevino A, Leal Almeida M, Sepulveda J, et al. High concentrations of serum estradiol in assisted reproduction. Ginecol Obstet Mex. 2003;71:585–589. [PubMed] [Google Scholar]
- 20.Kosmas IP, Kolibianakis EM, Devroey P. Association of estradiol levels on the day of hCG administration and pregnancy achievement in IVF: a systematic review. Human reproduction (Oxford, England) 2004;19(11):2446–2453. doi: 10.1093/humrep/deh473. [DOI] [PubMed] [Google Scholar]
- 21.Hedges L, Olkin I. P. 369 in Statistical methods for meta-analysis. New York: New York Academic Press; 1985. [Google Scholar]
- 22.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 23.Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical research ed) 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kyrou D, Popovic-Todorovic B, Fatemi HM, Bourgain C, Haentjens P, Van Landuyt L, et al. Does the estradiol level on the day of human chorionic gonadotrophin administration have an impact on pregnancy rates in patients treated with rec-FSH/GnRH antagonist? Hum Reprod. 2009;24(11):2902–2909. doi: 10.1093/humrep/dep290. [DOI] [PubMed] [Google Scholar]
- 26.Gratton RJ, Nisker JA, Daniel S, Toth S, Gunter J, Kaplan BR, Tummon IS, Yuzpe AA. An aggressive philosophy in controlled ovarian stimulation cycles increases pregnancy rates. Hum Reprod. 1993;8(4):528–531. doi: 10.1093/oxfordjournals.humrep.a138089. [DOI] [PubMed] [Google Scholar]
- 27.Mahutte NG, Duleba AJ, Taylor HS, Arici A, Jones E, Sakkas D. Elevated estradiol levels are associated with increased miscarriage rates in women undergoing IVF/ICSI. Fertil Steril. 2002;78(Supplement 1):S253. [Google Scholar]
- 28.Chen CH, Zhang X, Barnes R, Confino E, Milad M, Puscheck E, Kazer RR. Relationship between peak serum estradiol levels and treatment outcome in in vitro fertilization cycles after embryo transfer on day 3 or day 5. Fertil Steril. 2003;80(1):75–79. doi: 10.1016/s0015-0282(03)00504-1. [DOI] [PubMed] [Google Scholar]
- 29.Joo BS, Park SH, An BM, Kim KS, Moon SE, Moon HS. Serum estradiol levels during controlled ovarian hyperstimulation influence the pregnancy outcome of in vitro fertilization in a concentration-dependent manner. Fertil Steril. 2010;93(2):442–446. doi: 10.1016/j.fertnstert.2009.02.066. [DOI] [PubMed] [Google Scholar]
- 30.Kara M, Kutlu T, Sofuoglu K, Devranoglu B, Cetinkaya T. Association between serum estradiol level on the hCG administration day and IVF-ICSI outcome. Iran J Reprod Med. 2012;10(1):53–58. [PMC free article] [PubMed] [Google Scholar]
- 31.Imudia AN, Goldman RH, Awonuga AO, Wright DL, Styer AK, Toth TL. The impact of supraphysiologic serum estradiol levels on peri-implantation embryo development and early pregnancy outcome following in vitro fertilization cycles. J Assist Reprod Genet. 2013;31(1):65–71. doi: 10.1007/s10815-013-0117-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Foroozanfard F, Moraveji SA, Taghavi SA, Karimi F. Association between serum estradiol level on the day of hCG administration and IVF–ICSI outcome. J Obstet Gynecol India. 2015. [DOI] [PMC free article] [PubMed]
- 33.Taskin EA, Atabekoglu CS, Musali N, Oztuna D, Sonmezer M. Association of serum estradiol levels on the day of hCG administration with pregnancy rates and embryo scores in fresh ICSI/ET cycles down regulated with either GnRH agonists or GnRH antagonists. Arch Gynecol Obstet. 2014;289(2):399–405. doi: 10.1007/s00404-013-2984-8. [DOI] [PubMed] [Google Scholar]
- 34.Zavy MT, Craig LB, Wild RA, Kahn SN, O'Leary D, Hansen KR. In high responding patients undergoing an initial IVF cycle, elevated estradiol on the day of hCG has no effect on live birth rate. Reprod Biol Endocrinol. 2014;12:119. doi: 10.1186/1477-7827-12-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ulug U. Subclinical pregnancy losses among women undergoing in-vitro fertilization with ICSI. J Assist Reprod Genet. 2006;23(6):261–267. doi: 10.1007/s10815-006-9038-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prasad S, Kumar Y, Singhal M, Sharma S. Estradiol level on day 2 and day of trigger: a potential predictor of the IVF-ET success. J Obstet Gynecol India. 2014;64(3):202–207. doi: 10.1007/s13224-014-0515-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thelmo MC, Acacio BD, Nouriani M. P-149: Peak serum estradiol (E2) is a predictor of pregnancy outcome in in vitro fertilization (IVF) Fertil Steril. 2006;86(3, Supplement):S187. [Google Scholar]
- 38.Farhi J, Ben-Haroush A, Andrawus N, Pinkas H, Sapir O, Fisch B, et al. High serum oestradiol concentrations in IVF cycles increase the risk of pregnancy complications related to abnormal placentation. Reprod BioMed Online. 2010;21(3):331–337. doi: 10.1016/j.rbmo.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 39.Gursoy Erzincan S, Corbacioglu Esmer A, Baysal B. Does the estradiol level on the day of human chorionic gonadotropin administration predict the clinical outcome of controlled ovarian hyperstimulation? Clin Exp Obstet Gynecol. 2014;41(6):709–712. [PubMed] [Google Scholar]
- 40.Núñez JV, Pascual DG, Martín ER, Vera ER, de Oliveira NM. Estradiol levels higher than 3000pg/ml on the day of hCG decreases pregnancy and implantation rate only in women over 35 years old. Rev Iberoam Fertil Reprod Humana. 2011;28(2):6. [Google Scholar]
- 41.Wu CH, Kuo TC, Wu HH, Yeh GP, Tsai HD. High serum estradiol levels are not detrimental to in vitro fertilization outcome. Taiwan J Obstet Gynecol. 2007;46(1):54–59. doi: 10.1016/S1028-4559(08)60108-4. [DOI] [PubMed] [Google Scholar]
- 42.Roque M, Lattes K, Serra S, Sola I, Geber S, Carreras R, et al. Fresh embryo transfer versus frozen embryo transfer in in vitro fertilization cycles: a systematic review and meta-analysis. Fertil Steril. 2013;99(1):156–162. doi: 10.1016/j.fertnstert.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 43.Margalioth EJ, Taney FH, Cooper GW, Scholl GM, Rosenfeld DL. Correlations between serum oestradiol level on day of HCG and speed of embryonic development in in-vitro fertilization. Hum Reprod. 1993;8(5):752–754. doi: 10.1093/oxfordjournals.humrep.a138134. [DOI] [PubMed] [Google Scholar]
- 44.Venetis CA, Kolibianakis EM, Bosdou JK, Lainas GT, Sfontouris IA, Tarlatzis BC, et al. Estimating the net effect of progesterone elevation on the day of hCG on live birth rates after IVF: a cohort analysis of 3296 IVF cycles. Human reproduction (Oxford, England) 2015;30(3):684–691. doi: 10.1093/humrep/deu362. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 60 kb).
(DOCX 135 kb).
(DOCX 27 kb).
(DOCX 16 kb).
(DOCX 108 kb).