Abstract
Objective
This study was performed to investigate the effect of hypothermic machine perfusion (HMP) after cold storage (CS) on ovarian transplantation.
Methods
Rats aged 8–10 weeks were used as the donors and recipients for allotransplantation. Eighteen donor rats were divided into three groups: the fresh control (n = 6), cold storage (CS; n = 6), and hypothermic machine perfusion (HMP; n = 6) groups. The preservation solution contained Dulbecco’s modified Eagle’s medium/Ham’s F-12 (1:1, v/v), 10% fetal bovine serum, 10 μg/ml insulin, 10 μg/ml transferrin, and 50 mIU/ml follicle-stimulating hormone (FSH). The donor ovaries in the CS and HMP groups were excised and then respectively subjected to 4 h of CS and 2 h of CS combined with 2 h of HMP at 4 °C, and then transplanted beneath the recipient’s left renal capsule. At 7 days after transplantation, the ovaries were removed and blood samples were obtained for histological analysis, immunohistochemistry for CD31 and Ki67, and serum anti-Mullerian hormone (AMH) level estimation.
Results
The HMP group showed significant increases in serum AMH and CD31-positive areas when compared to these values in the CS group (P < 0.05). However, no differences were noted in the total number of follicles or the Ki67-positive areas among the three groups.
Conclusion
Hypothermic machine perfusion after static cold storage is more effective than static CS alone for the short-term preservation of whole ovaries during transport. Whole ovary transplantation with vascular pedicle is our future research direction.
Graphical Abstract.

The black rectangle in the figure shows the place where ligation and disconnection are required, the black dotted line shows the place where vascular forceps are used to clamp, and the black circle shows the place where the cannula is inserted
This diagram was made for reviewers to understand more intuitively how my hypothermia mechanical perfusion model was built. Organs obtained in this way can be used for subsequent perfusion and whole ovarian transplantation
Keywords: Fertility preservation, Ovarian transplantation, Ovary, Rat, Hypothermic machine perfusion
Introduction
After menopause, women are at greater risk for bone loss, cardiovascular disease, weakened immune systems, and more. According to the latest reports, approximately 300,000 teenagers (under the age of 19) worldwide have had cancer [1]. Progress in cancer treatment has improved the prognosis of cancer patients, and the 5-year survival rate of female cancer patients has increased to 68% [2]. However, the toxic effects of chemotherapy drugs and radiotherapy can cause premature ovarian insufficiency, leading to infertility. With the development of organ transplantation technology, ovarian transplantation is expected to be an effective method to preserve the fertility of women with premature ovarian failure and improve the health of postmenopausal women. Ovarian tissue cryopreservation has made the long-term storage of reproductive cells and tissues possible, and their transplantation can restore fertility to cancer patients. To date, more than 130 babies have been born to patients who underwent transplantation of cryopreserved reproductive tissues [3]. Whole fresh human ovarian transplantation has been confirmed to successfully restore ovulatory function reported by Sherman Silber [4]. And we are also working on the whole ovary transplantation with vascular pedicle and trying to improve its effects.
In addition, a large number of studies have shown that postmenopausal animal ovary transplantation can reduce the incidence of cardiomyopathy [5], prevent postmenopausal bone loss [6], prevent the decline of immune and kidney function [7], and even increase life span [8]. Cryopreservation ovarian tissue can be performed in many medical institutions, but transplantation is not done in a few centers, and the storage conditions during transportation before cryopreservation may affect the quality of the ovarian tissue.
Over the last decade, machine perfusion (MP) has attracted increasing attention as an organ preservation method for its superiority to traditional static cold storage (SCS) [9]. Hypothermic machine perfusion (HMP perfusion at 4–12 °C) has been widely used for kidney, liver, and pancreas transplants and has been shown to be respectively associated with a reduced incidence of delayed graft function in donation after circulatory death kidneys [10], reduced vascular complications in the liver [11], mitigation of oxidative stress [12], and reduced inflammation and cell damage [13]. However, the effects of HMP on ovarian transplantation and whether it improves the quality of donor ovaries is unknown.
Therefore, the present study was performed to investigate the differences between two methods of organ preservation, cold storage (CS) and HMP, using rat ovaries. The objective of this study was to evaluate the viability of preserved transplanted ovaries using hormonal assessments and histological and immunohistochemical methods.
Materials and methods
Experimental protocol
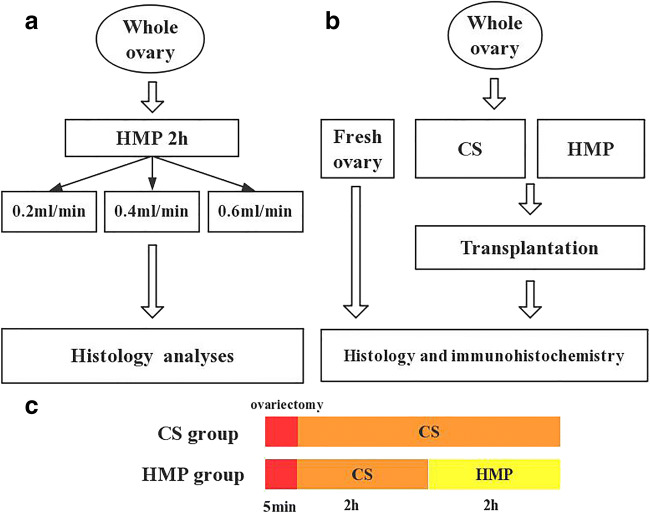
Figure 1 shows a schematic of the experimental design. Donor ovaries were collected from 14 rats and were randomly divided into three groups (fresh, CS, and HMP). The ovary preservation solution contained Dulbecco’s modified Eagle’s medium/Ham’s F-12 (1:1, v/v), 10% fetal bovine serum, 10 μg/ml insulin, 10 μg/ml transferrin, and 50 mIU/ml follicle-stimulating hormone (FSH). The conditions were referred to the Kamoshita K reported in 2016, including in the composition of preservation medium, the storage temperature, and duration. [14]. Six ovaries were perfused at three different rates (0.2, 0.4, and 0.6 ml/min) with preservation solution containing 0.5% methylene blue for 2 h to determine the optimal flow parameters. Next, the ovaries were either stored at 4 °C or perfused at a rate of 0.2 ml/min (the optimum perfusion flow rate) and then transplanted. For the histological examinations, hematoxylin and eosin (H&E) staining and Ki-67 and CD-31 immunostaining were performed, serum AMH was measured, and the numbers of follicles were counted. The expression of Ki67, a marker of cell proliferation, was assessed as an indicator of ovary health and the expression of CD31 was an indicator of re-vascularization of blood vessels in grafts. AMH is an indicator of reproductive reserve and does not affected by the reproductive cycle.
Fig. 1.
Schematic for the study design. a The experimental scheme of the determination the optimum perfusion flow. b The rats were divided into three groups: fresh control group, CS group, HMP group, respectively. The preservation solution contains Dulbecco’s modified Eagle’s medium/Ham’s F-12(1:1, v/v), 10% fetal bovine serum, 10 μg/ml insulin, 10 μg/ml transferrin, and 50 mlU/ml follicle-stimulating hormone (FSH). c Duration of operation in two groups
Experimental animals
Female SD rats were obtained from the Center for Animal Experiments of Wuhan University (Wuhan, China). The rats were housed in a pathogen-free facility at 22 °C and 55% humidity under a 12-h light/dark cycle. All rats (8–9 weeks old, weighing 200–220 g) were fed rat chow and water ad libitum and kept in transparent cages with three rats per cage. The experimental protocols and animal surgical procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of the Institution of Model Animals of Wuhan University.
Ovariectomy
Ovaries were collected from 8- to 9-week-old female SD rats. In the fresh and CS groups, anesthesia was performed by an intraperitoneal injection of 1% pentobarbital sodium (40–60 mg/kg body weight). The dorsal fur was shaved, and the skin was sterilized with 75% (v/v) alcohol. A longitudinal skin incision (about 2 cm) was made along the midline of the lower back, and then small bilateral incisions were made in the muscles and fascia above the ovary, and the donor ovary was removed.
Ovarian cold storage and hypothermia mechanical perfusion
In the CS group, ovaries were stored in preservation solution at 4 °C for 4 h. After 4 h of CS, each ovary was allografted into a recipient rat. In the HMP1 and HMP2 groups, the donor ovaries were excised and then subjected to 2 h of CS combined with 2 h of HMP. Rats in the HMP group were anesthetized with sodium pentobarbital (40 mg/kg), and then a midline incision from the xiphoid process to the pubic bone was made. The lateral vessels of the aorta/cava and left adrenal gland, the uterine branch of the ovarian artery, and the left uterine horn were ligatured and amputated by using 3–0 silk, preserving the main ovary artery and vein. The vessels were ligated between the left and right renal arteries. Isolation and severance of the right common renal vascular trunk and ovarian vessels were performed systematically. Then, an aortic cannula was performed above the branching of the iliolumbar vessels in a retrograde direction using an epidural guiding tube tied with 3–0 mersilk. The left renal pedicle was clipped with a vessel clamp to ensure that the solution only flowed into the ovary through the ovarian artery. An incision was made on the vena cava to allow drainage of blood. At this step, with the cannula connected to the pump, the ovaries were perfused with the preservation solution at a rate of 0.2 ml/min. All solutions were refrigerated at 4 °C before use, and all perfusions were carried out on ice. The average ovary weight in each group was ~ 0.05 g.
Ovarian transplantation
After anesthesia, a midabdominal incision was made, and the ovary was transplanted beneath the left renal capsule. The recipient’s ovaries were removed at the same time, and the incision and skin were closed and sutured [15].
Histology and follicle counting
Rats were sacrificed at 7 days posttransplantation, and the transplanted ovaries were dissected, fixed in 4% paraformaldehyde, and embedded in paraffin wax. Sections (5 μm thick) were stained with H&E, and the numbers of follicles were determined by examination under a light microscope. Ovarian follicles were classified into three groups as follows: immature follicles (including primordial, primary, and preantral), antral follicles, and corpus luteum [14, 16]. Evaluation of the normality of follicles was based on the cell density and the integrity of the oocyte and basement membrane. The total number of follicles in each graft, excluding primordial follicles, was counted.
Immunohistochemical analyses of transplanted ovaries
Ovarian sections were immunostained for CD31 to assess the density of blood vessels and for Ki67 to assess cell proliferation. The ovarian tissue sections were put in citric acid buffer (pH 6.5) for antigen retrieval using microwave for 23 min after deparaffinization. Then, the sections were incubated in 0.3% hydrogen peroxide while shielded from light for 25 min, and then blocked with 3% BSA for 30 min at RT. The paraffin-embedded slides were then incubated overnight at 4 °C with anti-CD31 (Servicebio, China) and Ki-67 (Servicebio, China) antibodies and treated with liquid diaminobenzidine according to the manufacturer’s instructions. Then, the sections were counterstained with hematoxylin (Servicebio, China) and dehydrated in ethanol and xylene. Finally, the density of the immunopositive cells in the ovaries was analyzed under high magnification (× 200).
Viability assessment of transplanted ovaries
Seven days after transplantation, blood samples were collected from the rats for serum preparation. After resting at 25 °C, the blood samples were then centrifuged at 3000×g for 15 min to obtain serum, which was stored at − 20 °C until use. Anti-Müllerian hormone (AMH) was measured by using an enzyme-linked immunosorbent assay kit (Servicebio, China). Six bilateral ovariectomized rats served as the negative control group (OX group) and were sacrificed 7 days later to collect blood.
Statistical analyses
Data (CD31, Ki-67, serum AMH, and the total number of follicles) were analyzed by one-way analysis of variance. GraphPad Prism 5.0 (GraphPad Software, USA) and Statistical Package for the Social Sciences version 12.0 software (SPSS, USA) were used for the statistical analyses. P values less than 0.05 were considered significant.
Results
Determination of the optimal perfusion flow rate
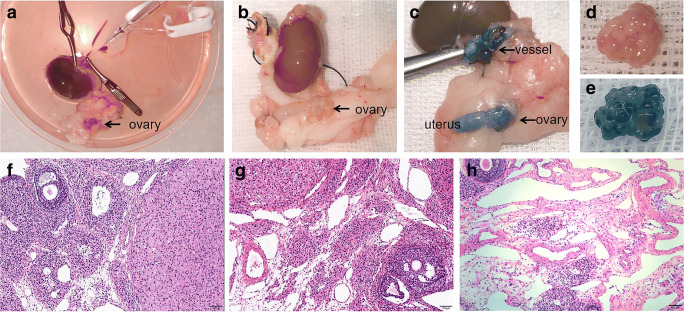
Generally, almost all of the ovarian tissue and its vascular pedicle and uterus were stained blue after successful perfusion (Fig. 2 c and e). After 30 min of perfusion, the surrounding adipose tissues were also stained blue. The blood in the ovaries and uterus was flushed out of the blood vessels (Fig. 2 b and d). Histological examination showed different levels of tissue edema and vascular dilatation in the 0.4 and 0.6 ml/min groups, but not in the 0.2 ml/min group (Fig. 2f–h).
Fig. 2.
The result of the experiment to explore the appropriate perfusion flow. a The images of ovary before perfusion. b, d The images of ovary after perfusion. c, e The image of ovary perfused by preservation solution added with 0.5% methylene blue. The ovary and uterus were dyed blue. f, g, h Representative histologic images of ovary tissue at three different perfusion rates (0.2 ml/min, 0.4 ml/min, and 0.6 ml/min), respectively. The black arrow indicate ovary (original magnification × 100, bar = 100 μm)
Macroscopic examination of the grafts
Ovarian grafts were removed 7 days after transplantation. There was a small amount of adhesion and congestion between the graft and the kidney, but the transplanted ovaries could be easily detached from the kidneys. New blood vessel formation was observed under the renal capsule and on the graft surface in both HMP and CS group (Fig. 3).
Fig. 3.
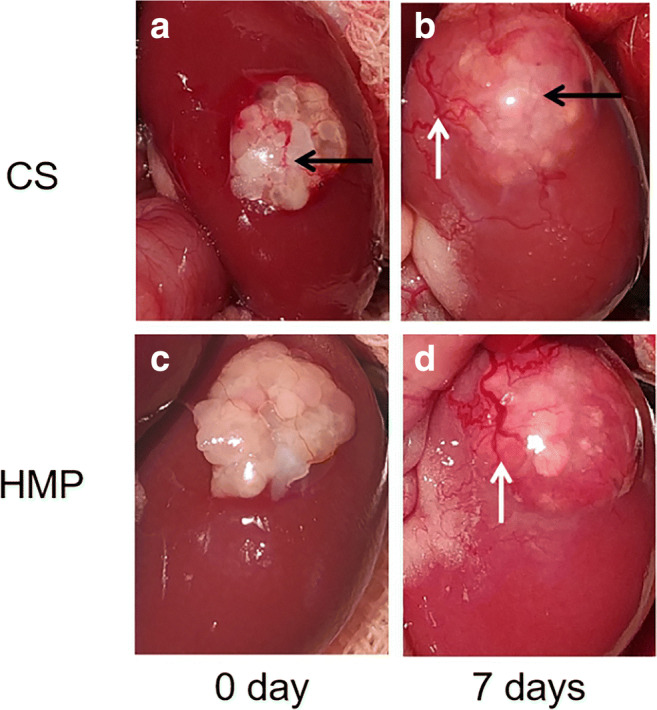
Representative images of rats ovarian tissue grafts. Ovarian transplantation beneath the left renal capsule, without vascular anastomosis. a, b Cold storage group. c, d Hypothermia mechanical perfusion group; a, c 0 day; b, d 7 days after transplantation. The black arrow and white arrow indicate ovary and blood vessel, respectively
Histological examination of the ovaries and follicle counts
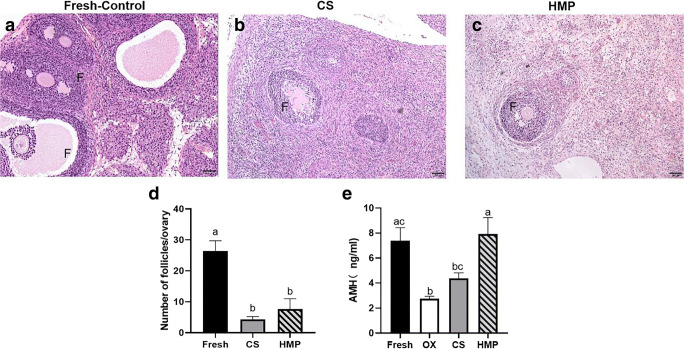
On day 7 after transplantation, intact follicles at different stages of development, blood vessels, and corpora lutea were detected in all groups. The total numbers of follicles in each graft (except primordial follicles) were counted (Fig. 4). A significant reduction in the total number of follicles was observed in the transplantation groups when compared with the Fresh-control group (fresh: 26.3 ± 3.4, CS: 4.3 ± 0.9, HMP: 7.7 ± 3.3). However, there was no significant difference in the total number of follicles between the transplantation groups (P > 0.05; Fig. 4d).
Fig. 4.
Morphology of follicles in grafts 7 days after transplantation in fresh-control group (a), CS group (b), and HMP group (c). F indicates follicles. d Mean follicle number per section. e Serum anti-Mullerian hormone (AMH) levels in rats. The serum AMH levels were detected by enzyme-linked immunosorbent assay. Data were analyzed by one-way analysis of variance. Graphs are presented as mean + SEM, and different letters indicate significant differences (P < 0.05) (original magnification × 100, bar = 100 μm)
Changes in serum AMH levels
Serum AMH levels were measured by ELISA 7 days after transplantation to assess ovarian activity (Fig. 4e). Serum AMH levels were significantly higher in the HMP group and fresh group than in the OX group and CS group. A significant increase in serum AMH levels in the HMP group was observed when compared with the CS group (P < 0.05) (Table 1).
Table 1.
The serum AMH levels of rats in all experimental groups
| Groups | ||||
|---|---|---|---|---|
| Fresh | OX | CS | HMP | |
| AMH (ng/ml) | 7.39 ± 1.04ac | 2.75 ± 0.19b | 4.39 ± 0.43bc | 7.93 ± 1.31a |
Values are presented as mean ± SEM. Groups with different letters indicate statistically significant differences (P < 0.05) (n = 6 in each group)
CD31 and Ki67 immunohistochemistry
Figure 5 shows representative images of immunohistochemistry for CD31 and in the ovaries at 7 days after transplantation. A significantly greater CD31-positive area was observed in the HMP group than in the CS group (P < 0.05; Fig. 5g). From the image we can see, Ki67 is mainly expressed in granulosa cells in growing follicles(Fig. 5 d and f). However, there was no significant difference in the Ki-67 positive areas between the three groups (Fig. 5h).
Fig. 5.
Photomicrographs of immunohistochemistry analysis for CD 31 and Ki-67 in ovarian tissue. A brown coloring of the cytoplasm/nucleus of the cells was specified as positive staining. The area was measured and analyzed by ImageJ, and data were analyzed by one-way analysis of variance. Graphs are presented as mean + SEM, and different letters indicate statistically significant differences (P < 0.05). Fo follicles (original magnification × 200, scale bar = 50 μm).
Discussion
Hypothermic machine perfusion (HMP) has been proved as an efficient way to improve graft function, such as liver and kidney, when compared with cold storage (CS) [17]. Our previous researches have already found that HMP may decrease kidney inflammation by upregulating the expression of KLF2 after transplantation in rabbit model [18] and alleviate liver injury through activating autophagy in mouse model [19]. For the first time, we have explored the effects of HMP on ovarian preservation before transplantation. By comparing with CS, HMP improved follicular function, especially the increase of AMH. Although the number of follicles did not increase, significant angiogenesis was observed in HMP group.
After transplantation, most ovarian follicles are lost (approximately 60–95%) due to ischemia during revascularization [20]. To reduce ischemic injury and follicle loss, rapid reconstruction of blood vessels in ovarian grafts is essential [21]. Therefore, decreasing ischemic damage during ovarian transplantation is crucial to ensure the survival of the graft. Although several researchers have reported that the main cause of ovarian injury is ischemic damage after transplantation rather than cryoinjury, slow freezing and vitrification has recently been used for the cryopreservation of human ovarian tissue, and several live births have been reported [22]. In 2016, Jaewang Lee et al. [23] demonstrated that posttransplantation ischemia is more deleterious than cryoinjury but that hypothermia injury during cryopreservation hindered posttransplant recovery by reducing revascularization. Several studies have concluded that freezing has little impact on follicular quality, and most injuries after cryopreservation and transplantation occur not after freezing but after grafting [24]. In 2016, Kamoshita et al. investigated the relationship between fertility and storage time, and the results suggested that although there were no obvious histological changes in mouse ovaries after storage at 4 °C for 24 h, fertility declined as storage time increased. Before transplantation, there was no morphological difference between ovaries preserved at 4 °C for 4 h and control fresh ovaries [14]. However, the fertility of cryopreserved, thawed, and transplanted ovaries after storage was not investigated. In our study, 65–85% of cryopreserved follicles were lost compared with the number in fresh control ovaries because of ischemic damage after transplantation (Fig. 4d). The larger follicles had a significantly higher recovery rate than smaller follicles after grafting [25]. So, we just evaluated the larger follicles (primary, secondary, and antral follicles) to assess ovarian function after transplantation. From the results, the follicles are mainly secondary follicles after grafts. According to references, the mean follicle number per section of ovary in our study is consistent with actual situation.
In our study, we measured serum AMH levels in transplanted rats because AMH is expressed and secreted by granulosa cells in growing follicles [26]. Serum AMH has been widely used in the clinic, mainly because it reflects the number of antral and pre-antral follicles in the ovaries. AMH is a member of the transforming growth factor beta (TGF-β) family, and it can affect the transition from non-growing follicles to growing follicles. It has been considered to be a reliable indicator of ovarian reserve, because its levels are not affected by the estrous cycle [27]. Some research shows that the serum level of AMH would decrease 36.4% (3.032 ng/mL) after 7 days in ovariectomized mouse [28]. This would be similar to the result of serum AMH measured in rats. In the present study, serum AMH was significantly higher in the HMP group than in the CS group at 7 days after transplantation (Fig. 4e), indicating a higher ovarian reserve. In light of these results, we propose that the number of AMH-secreting growing follicles was significantly higher in the HMP group than in the CS group, indicating that HMP has beneficial effects on the restoration of ovarian function at 7 days after transplantation.
In rodent models, revascularization of blood vessels occurs within 2 days, indicating that any tissue damage caused by ischemia develops during this 2-day period [29]. Since ovarian tissue is transplanted without vascular anastomosis, the graft goes through a period of hypoxia and ischemia that lasts 3–5 days before progressive revascularization. It typically shows functional vessels from day 7 onwards. CD31-positive areas are indicator of re-vascularization of blood vessels in grafts, which is crucial in terms of reducing the period of ischemia. Our study shows that the CD31-positive areas were significantly larger in the HMP group than in the CS group (Fig. 5g), indicating that HMP contributes to revascularization of the ovaries after transplantation. However, the exact mechanism underlying this phenomenon is unclear. Onions et al. reported that whole ovarian cryopreservation and transplantation leads to deleterious changes in the number of ovarian follicles that can be attributed to an acute loss of vascular patency, and both ovarian perfusion alone and the combination of perfusion and cryopreservation can induce the expression of genes related to vascular tone, wound repair, and/or hypoxia in endothelial cells [30]. Perfusion can down-regulate thrombospondin 1 (THBS1), which is commonly associated with angiogenic stimulation and wound repair. THBS1 has been shown to antagonize the effects of nitric oxide to limit the vasodilation of contracted vascular smooth muscle cells and inhibit angiogesis [31]. There is an apparent inverse relationship between the expression levels of THBS1 and VEGF in bovine ovarian granulosa cells during normal follicular development. It is well known in the field of cancer treatment research that THBS can inhibit angiogenesis by inhibiting proangiogenic genes, such as VEGF [32]. This may be one reason why HMP promotes angiogenesis. Many researchers have studied various methods for enhancing vascularization once the ovarian tissue is grafted. Studies have shown that the addition of a certain amount of FSH during in vitro culture of ovaries can promote the expression of VEGF and bFGF after transplantation and stimulate follicular development in ovarian tissue [33]. Manavella et al. showed that adipose tissue-derived stem cells can enhance neovascularization in a peritoneal ovarian grafting site [34].
The expression of Ki67, a marker of cell proliferation, has been shown to be increased following both short- and long-term exposure to hypoxia [35]. Ki67 expression was observed in both stromal cells and follicles in ovary tissue, suggesting that these follicles can grow and develop and stromal cells can provide structure and support for follicular development after transplantation [36]. However, in our study, there was no significant difference between the fresh control and experimental groups, which may be due to the short observation time. However, most follicles and granulosa cells were stained for Ki67, indicating that the follicles in the HMP and CS groups were viable and could survive.
Cryopreservation of ovarian tissue is a valid way to preserve the fertility of women with cancer [37]. Cryopreservation of whole ovaries with vascular pedicles and transplantation should reduce post-transplantation ischemic injury, and research has shown that optimized perfusion of cryoprotectants is more effective than immersion in cryoprotectants for cryopreserving whole ovaries [38, 39]. Therefore, we hypothesized that combined static CS and HMP is more effective than static CS alone for short-term preservation of whole ovaries, and this was supported by our experimental results. HMP can wash away microthrombi and metabolites, improving the microenvironment of endothelial cells and parenchymal cells, and prevent the exhaustion of adenosine triphosphate (ATP) and mitochondrial edema [19]. However, the diameters of the blood vessels in the ovary differ, and blood vessels with small diameters, such as those around the corpus luteum may be particularly vulnerable to obstruction, resulting in inadequate perfusion [40]. Therefore, it is of great importance to investigate the optimal perfusion parameters for improving ovarian function after preservation and transplantation.
The present study has several limitations. For example, the effects of different flow rates were only preliminarily evaluated in this study, and there were no detailed groupings based on perfusion flow rates. Because of the low measure methods, our result should be taken with caution. According to Soner Celik et al. [41], AMH immunohistochemistry results in the frozen/thawed and transplanted group; the expression of AMH in granulosa cells was similar to those in fresh-transplanted group. We think the result of serum AMH would be more accurate and convincing than AMH immunohistochemistry results. In the present study, our destination is to establish a new perfusion model that can be applied to the field of ovarian transplantation. Therefore, we paid more attention to the functional indicators. The serum of AMH and the re-vascularization have demonstrated the effectiveness of hypothermia mechanical perfusion to some extent. Whole ovary transplantation with vascular pedicle is our ultimate research direction. Therefore, the whole ovary transplantation with vascular pedicle after hypothermia mechanical perfusion could be more significant than ovarian tissue transplantation. In addition, the mechanism underlying the angiogenesis-promoting effects of HMP were not further explored. In the future, we will also carry out the research of whole ovary transplantation with vascular pedicle.
Conclusions
With the decline of ovarian function, women have a significantly increased risk of disease. Ovarian transplantation has a positive effect on restoration of health in postreproductive females and thus is attracting increasing attention. The results of the present study indicated that HMP combined with static CS is more effective than static CS alone for short term, whole ovary preservation during transport. After perfusion, whole ovary transplantation with vascular pedicles may be a useful technique in fertility preservation in the future, but additional surgical techniques may be required for microvascular anastomosis. Although our experimental conditions and parameters need to be further optimized, they provide meaningful insights for improving whole ovary transplantation.
Funding information
This study was supported by the Natural Science Foundation of China (81570079).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.International Agency for Research on Cancer. 2019. https://www.iarc.fr. Accessed 15 Feb 2019.
- 2.Siegel R, Ma J, Zou Z, Jemal A. Each Year TACS. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 3.Donnez J, Dolmans MM. Fertility preservation in women. N Engl J Med. 2017;377(17):1657–1665. doi: 10.1056/NEJMra1614676. [DOI] [PubMed] [Google Scholar]
- 4.Silber SJ. Fresh ovarian tissue and whole ovary transplantation. Semin Reprod Med. 2009;27(6):479–485. doi: 10.1055/s-0029-1241058. [DOI] [PubMed] [Google Scholar]
- 5.Mason JB, Cargill SL, Griffey SM, Reader JR, Anderson GB, Carey JR. Transplantation of young ovaries restored cardioprotective influence in postreproductive-aged mice. Aging Cell. 2011;10(3):448–456. doi: 10.1111/j.1474-9726.2011.00691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mason JB, Terry BC, Merchant SS, Mason HM, Nazokkarmaher M. Manipulation of ovarian function significantly influenced trabecular and cortical bone volume, architecture and density in mice at death. PLoS One. 2015;10(12):e0145821. doi: 10.1371/journal.pone.0145821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peterson RL, Parkinson KC, Mason JB. Restoration of immune and renal function in aged females by re-establishment of active ovarian function. Reprod Fertil Dev. 2017;29(10):2052–2059. doi: 10.1071/RD16333. [DOI] [PubMed] [Google Scholar]
- 8.Mason JB, Cargill SL, Anderson GB, Carey JR. Transplantation of young ovaries to old mice increased life span in transplant recipients. J Gerontol Ser A Biol Med Sci. 2009;64A(12):1207–1211. doi: 10.1093/gerona/glp134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Westerkamp AC, Karimian N, Matton APM, Mahboub P, van Rijn R, Wiersema-Buist J, de Boer MT, Leuvenink HGD, Gouw ASH, Lisman T, Porte RJ. Oxygenated hypothermic machine perfusion after static cold storage improves hepatobiliary function of extended criteria donor livers. Transplantation. 2016;100(4):825–835. doi: 10.1097/TP.0000000000001081. [DOI] [PubMed] [Google Scholar]
- 10.Bathini V, McGregor T, McAlister VC, et al. Renal perfusion pump vs cold storage for donation after cardiac death kidneys: a systematic review. J Urol. 2013;189(6):2214–2220. doi: 10.1016/j.juro.2012.11.173. [DOI] [PubMed] [Google Scholar]
- 11.Henry SD, Guarrera JV. Protective effects of hypothermic ex vivo perfusion on ischemia/reperfusion injury and transplant outcomes. Transplant Rev. 2012;26(2):163–175. doi: 10.1016/j.trre.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Dutkowski P, Guarrera JV, de Jonge J, Martins PN, Porte RJ, Clavien PA. Evolving trends in machine perfusion for liver transplantation. Gastroenterology. 2019;156(6):1542–1547. doi: 10.1053/j.gastro.2018.12.037. [DOI] [PubMed] [Google Scholar]
- 13.Karcz M, Cook HT, Sibbons P, Gray C, Dorling A, Papalois V. An ex-vivo model for hypothermic pulsatile perfusion of porcine pancreata: hemodynamic and morphologic characteristics. Exp Clin Transplant. 2010;8(1):55–60. [PubMed] [Google Scholar]
- 14.Kamoshita K, Okamoto N, Nakajima M, Haino T, Sugimoto K, Okamoto A, Sugishita Y, Suzuki N. Investigation of in vitro parameters and fertility of mouse ovary after storage at an optimal temperature and duration for transportation. Hum Reprod. 2016;31(4):774–781. doi: 10.1093/humrep/dew023. [DOI] [PubMed] [Google Scholar]
- 15.Lee J, Kim EJ, Kong HS, Youm HW, Lee JR, Suh CS, Kim SH. A combination of simvastatin and methylprednisolone improves the quality of vitrified-warmed ovarian tissue after auto-transplantation. Hum Reprod. 2015;30(11):2627–2638. doi: 10.1093/humrep/dev222. [DOI] [PubMed] [Google Scholar]
- 16.Damous LL, Silva SMD, Carbonel AAF, et al. Progressive evaluation of apoptosis, proliferation, and angiogenesis in fresh rat ovarian autografts under remote ischemic preconditioning. Reprod Sci. 2016;23(6):803–811. doi: 10.1177/1933719115620493. [DOI] [PubMed] [Google Scholar]
- 17.Fu Z, Ye Q, Zhang Y, Zhong Z, Xiong Y, Wang Y, Hu L, Wang W, Huang W, Ko DSC. Hypothermic machine perfusion reduced inflammatory reaction by downregulating the expression of matrix metalloproteinase 9 in a reperfusion model of donation after cardiac death. Artif Organs. 2016;40(6):E102–E111. doi: 10.1111/aor.12658. [DOI] [PubMed] [Google Scholar]
- 18.Liu Z, Zhong Z, Lan J, Li M, Wang W, Yang J, Tang C, Wang J, Ye S, Xiong Y, Wang Y, Ye Q. Mechanisms of hypothermic machine perfusion to decrease donation after cardiac death graft inflammation: through the pathway of upregulating expression of KLF2 and inhibiting TGF-β signaling. Artif Organs. 2017;41(1):82–88. doi: 10.1111/aor.12701. [DOI] [PubMed] [Google Scholar]
- 19.Zeng X, Wang S, Li S, Yang Y, Fang Z, Huang H, Wang Y, Fan X, Ye Q. Hypothermic oxygenated machine perfusion alleviates liver injury in donation after circulatory death through activating autophagy in mice. Artif Organs. 2019;43(12):E320–E332. doi: 10.1111/aor.13525. [DOI] [PubMed] [Google Scholar]
- 20.Henry L, Labied S, Fransolet M, et al. Isoform 165 of vascular endothelial growth factor in collagen matrix improves ovine cryopreserved ovarian tissue revascularisation after xenotransplantation in mice. Reprod Biol Endocrinol. 2015;13:12. doi: 10.1186/s12958-015-0015-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tavana S, Valojerdi MR, Azarnia M, Shahverdi A. Restoration of ovarian tissue function and estrous cycle in rat after autotransplantation using hyaluronic acid hydrogel scaffold containing VEGF and bFGF. Growth Factors. 2016;34(3–4):97–106. doi: 10.1080/08977194.2016.1194835. [DOI] [PubMed] [Google Scholar]
- 22.Silber S, Pineda J, Lenahan K, DeRosa M, Melnick J. Fresh and cryopreserved ovary transplantation and resting follicle recruitment. Reprod BioMed Online. 2015;30(6):643–650. doi: 10.1016/j.rbmo.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 23.Lee J, Kong HS, Kim EJ, Youm HW, Lee JR, Suh CS, Kim SH. Ovarian injury during cryopreservation and transplantation in mice: a comparative study between cryoinjury and ischemic injury. Hum Reprod. 2016;31(8):1827–1837. doi: 10.1093/humrep/dew144. [DOI] [PubMed] [Google Scholar]
- 24.Amorim CA, David A, Dolmans MM, Camboni A, Donnez J, van Langendonckt A. Impact of freezing and thawing of human ovarian tissue on follicular growth after long-term xenotransplantation. J Assist Reprod Genet. 2011;28(12):1157–1165. doi: 10.1007/s10815-011-9672-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chiti MC, Dolmans MM, Orellana R, et al. Influence of follicle stage on artificial ovary outcome using fibrin as a matrix. Hum Reprod. 2015:dev299. [DOI] [PubMed]
- 26.Dewailly D, Andersen CY, Balen A, Broekmans F, Dilaver N, Fanchin R, Griesinger G, Kelsey TW, la Marca A, Lambalk C, Mason H, Nelson SM, Visser JA, Wallace WH, Anderson RA. The physiology and clinical utility of anti-Mullerian hormone in women. Hum Reprod Update. 2014;20(3):370–385. doi: 10.1093/humupd/dmt062. [DOI] [PubMed] [Google Scholar]
- 27.Li J, Mao Q, He J, et al. Human umbilical cord mesenchymal stem cells improve the reserve function of perimenopausal ovary via a paracrine mechanism. Stem Cell Res Ther. 2017;8(1). [DOI] [PMC free article] [PubMed]
- 28.Detti L, Fletcher NM, Saed GM, Sweatman TW, Uhlmann RA, Pappo A, Peregrin-Alvarez I. Xenotransplantation of pre-pubertal ovarian cortex and prevention of follicle depletion with anti-Müllerian hormone (AMH) J Assist Reprod Genet. 2018;35(10):1831–1841. doi: 10.1007/s10815-018-1260-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee J, Lee JR, Youm HW, Suh CS, Kim SH. Effect of preoperative simvastatin treatment on transplantation of cryopreserved-warmed mouse ovarian tissue quality. Theriogenology. 2015;83(2):285–293. doi: 10.1016/j.theriogenology.2014.09.027. [DOI] [PubMed] [Google Scholar]
- 30.Onions VJ, Webb R, Pincott-Allen C, Picton HM, Campbell BK. The effects of whole ovarian perfusion and cryopreservation on endothelial cell-related gene expression in the ovarian medulla and pedicle. Mol Hum Reprod. 2013;19(4):205–215. doi: 10.1093/molehr/gas053. [DOI] [PubMed] [Google Scholar]
- 31.Isenberg JS, Frazier WA, Roberts DD. Thrombospondin- is a secreted protein that modulates vascular cell behavior via several cell surface receptors. In vitro NCOT. Thrombospondin-1: a physiological regulator of nitric oxide signaling. Cell Mol Life Sci. 2008;65(5):728–742. doi: 10.1007/s00018-007-7488-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greenaway J, Gentry PA, Feige JJ, LaMarre J, Petrik JJ. Thrombospondin and vascular endothelial growth factor are cyclically expressed in an inverse pattern during bovine ovarian follicle development. Biol Reprod. 2005;72(5):1071–1078. doi: 10.1095/biolreprod.104.031120. [DOI] [PubMed] [Google Scholar]
- 33.Ma W, Zheng X, Hei C, et al. Optimal FSH usage in revascularization of allotransplanted ovarian tissue in mice. J Ovarian Res. 2017;10(1). [DOI] [PMC free article] [PubMed]
- 34.Manavella DD, Cacciottola L, Desmet CM, et al. Adipose tissue-derived stem cells in a fibrin implant enhance neovascularization in a peritoneal grafting site: a potential way to improve ovarian tissue transplantation. Human Reprod. 2018;33(2):270–279. doi: 10.1093/humrep/dex374. [DOI] [PubMed] [Google Scholar]
- 35.Xu J, Long YS, Gozal D, Epstein PN, Intermittent Hypoxia IH. SAOI. Beta-cell death and proliferation after intermittent hypoxia: role of oxidative stress. Free Radic Biol Med. 2009;46(6):783–790. doi: 10.1016/j.freeradbiomed.2008.11.026. [DOI] [PubMed] [Google Scholar]
- 36.Onions VJ, Mitchell MRP, Campbell BK, Webb R. Ovarian tissue viability following whole ovine ovary cryopreservation: assessing the effects of sphingosine-1-phosphate inclusion. Hum Reprod. 2008;23(3):606–618. doi: 10.1093/humrep/dem414. [DOI] [PubMed] [Google Scholar]
- 37.Dittrich R, Lotz L, Keck G, Hoffmann I, Mueller A, Beckmann MW, van der Ven H, Montag M. Live birth after ovarian tissue autotransplantation following overnight transportation before cryopreservation. Fertil Steril. 2012;97(2):387–390. doi: 10.1016/j.fertnstert.2011.11.047. [DOI] [PubMed] [Google Scholar]
- 38.Gerritse R, Beerendonk CC, Westphal JR, et al. Glucose/lactate metabolism of cryopreserved intact bovine ovaries as a novel quantitative marker to assess tissue cryodamage. Reprod BioMed Online. 2011;23(6):755–764. doi: 10.1016/j.rbmo.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 39.Ding Y, Shao J, Li J, et al. Successful fertility following optimized perfusion and cryopreservation of whole ovary and allotransplantation in a premature ovarian insufficiency rat model. J Ovarian Res. 2018;11(1). [DOI] [PMC free article] [PubMed]
- 40.Torre A, Ben Brahim F, Popowski T, Boudjenah R, Salle B, Lornage J. Factors related to unstained areas in whole ewe ovaries perfused with a metabolic marker. Hum Reprod. 2013;28(2):423–429. doi: 10.1093/humrep/des390. [DOI] [PubMed] [Google Scholar]
- 41.Celik S, Celikkan FT, Ozkavukcu S, Can A, Celik-Ozenci C. Expression of inhibitor proteins that control primordial follicle reserve decreases in cryopreserved ovaries after autotransplantation. J Assist Reprod Genet. 2018;35(4):615–626. doi: 10.1007/s10815-018-1140-6. [DOI] [PMC free article] [PubMed] [Google Scholar]