Abstract
Purpose
To characterize the likelihood of cryopreserving enough oocytes for 50%, 60%, or 70% estimated live birth rate (eLBR) with 1–2 planned oocyte cryopreservation (Pl-OC) cycles.
Methods
We performed a retrospective cohort study utilizing all patients completing ≥ 1 Pl-OC cycle from 2016 to 2018 at a large single-center OC program. Subjects were categorized by age at retrieval and number of cycles. We extrapolated age-based oocyte thresholds for 50%, 60%, or 70% eLBR from previously published data. We calculated the proportion of subjects overall, and for each age group, whose number of frozen oocytes was greater than or equal to their age-based threshold for a 50%, 60%, or 70% eLBR after 1 and 2 cycles. OR for 60% eLBR with one cycle was calculated for age and AMH cutoff values and corroborated with logistic regression.
Results
A total of 1241 subjects, completing 1799 Pl-OC cycles, were included. With one cycle, 66% (819/1241) achieved ≥ 50% eLBR and 51% (634/1241) achieved 70% eLBR. With two cycles, 79.6% (988/1241) attained ≥ 50% eLBR and 65.5% (813/1241) achieved 70% eLBR. Achieving 50%, 60%, or 70% eLBR with 1–2 cycles was significantly associated with both age (p < 0.001) and AMH (p < 0.001). Age < 37.5 and AMH > 1.995 were independently associated with attaining 60% eLBR with one cycle (age: OR 13.73; 95%CI 9.16–20.57, p < 0.001; AMH: OR 7.32; 95% CI 5.50–9.76, p < 0.001).
Conclusions
Younger age and higher AMH were associated with achieving 50%, 60%, or 70% eLBR thresholds with Pl-OC. Nevertheless, almost all subjects were successfully able to preserve enough oocytes for ≥ 50% eLBR in 1–2 cycles.
Electronic supplementary material
The online version of this article (10.1007/s10815-020-01791-w) contains supplementary material, which is available to authorized users.
Keywords: Oocyte cryopreservation, Fertility preservation, Live birth rate
Introduction
Demand for planned oocyte cryopreservation (Pl-OC) has increased dramatically over the past few years. According to the Society for Assisted Reproductive Technologies (SART), fertility preservation cycles saw a 25% increase in utilization from 2015 to 2016 [1], with utilization projected to steadily increase in the coming years. However, with increased demand comes greater responsibility. Providers have the responsibility to counsel prospective patients regarding realistic expectations and estimated outcomes with the process. In 2018, the American Society for Reproductive Medicine (ASRM) Ethics Committee called for increased research regarding the number of oocytes “needed to have a particular chance of pregnancy when those oocytes are used,” recognizing that these findings may “often require that a woman undergo multiple cycles if she wishes to attain a reasonable chance of having a child in the future.” [2].
Patients undergoing in vitro fertilization (IVF) for conception have a concrete outcome, namely livebirth, by which to judge the success of their cycle and the decision to proceed with additional treatment. Ample data exist to gauge the infertility patients’ prognosis and counsel them appropriately. Conversely, women considering Pl-OC must weigh the potential for a future livebirth with the present-day investment of personal and financial resources. Therefore, it is of critical importance that they be presented with both appropriate estimates for live birth rates based on age and number of oocytes, along with realistic evidence-based expectations for how many cycles it may take for them to achieve those thresholds.
Unfortunately, data are severely limited with respect to expected number of cycles based on oocyte yield and/or estimated live birth rates (eLBRs), particularly in women undergoing Pl-OC. The primary objective of our study was to characterize the likelihood of cryopreserving sufficient oocytes to achieve a 50%, 60%, or 70% eLBR with one or two Pl-OC cycles. We hypothesized that at least half of subjects would achieve at least a 70% eLBR with their first cycle and that those who were younger at retrieval and/or with a higher ovarian reserve would be more likely to achieve a high eLBR threshold than subjects who were older at retrieval and/or had a lower ovarian reserve.
Material and methods
We conducted a retrospective cohort study of all patients undergoing Pl-OC cycles at Extend Fertility Medical Practice, a large single-center oocyte cryopreservation program, from April 2016 to December 2018. Clinical decisions regarding stimulation protocols, dosing, trigger day, and trigger type were determined at the discretion of the provider prior to abstraction for the study. Generally, controlled ovarian stimulation was initiated on cycle day 2–3, with a recombinant FSH and hMG. Based on estradiol levels and follicular growth, the clinicians initiated GnRH-antagonist on stimulation day 4–6. Those with poor response to stimulation were counseled individually by the physicians and given the option to proceed as planned or defer stimulation to a future cycle. Cycles with no response were canceled at the recommendation of the clinician, and stimulation resumed in a future cycle. Trigger was recommended on stimulation day 8–12, based on clinical judgment of the clinician. No specific criteria for trigger or cancelation were utilized. The oocyte retrieval was performed 36 h after trigger. Determination of maturity and subsequent vitrification of mature (MII) oocytes were performed at the discretion of the embryology team. Subjects were eligible for inclusion in the study following their first retrieval, regardless of the number of oocytes retrieved. Only cycles culminating in a retrieval were included in the cycle analyses.
Anti-Müllerian hormone (AMH) studies were performed on all subjects no more than 6 months prior to their stimulation cycle. Monitoring assays for all participants were performed on the in-house Architect i1000SR platform [3]. The study was granted exempt status by IntegReview Institutional Review Board (Austin, TX).
The lead author abstracted and categorized demographic, clinical, and embryologic data from the electronic medical record. Demographic and cycle data were collected for each subject. Parametric data were categorized by mean ± SD and non-parametric by median ± IQR. Subjects were then categorized by number of cycles and by the Society for Assisted Reproductive Technology reporting age groups (< 34, 35–37, 38–40, 41–42, > 43) based on their age at retrieval. Differences between age groups and cycle number groups were calculated using ANOVA for parametric variables or Kruskall-Wallis for non-parametric variables, where appropriate.
We extrapolated age-based oocyte number thresholds for 50%, 60%, or 70% eLBR from Doyle et al. [4] and Goldman et al. [5], as shown in Table 2. We then calculated the proportion of subjects overall and for each age group whose number of frozen oocytes from their first cycle was greater than or equal to their age-based threshold for a 50%, 60%, or 70% eLBR. Where the two studies differed, thresholds from Doyle et al. were utilized. Associations between subjects who did and did not reach each eLBR threshold were made using Student t test and Mann-Whitney U test, where appropriate.
Table 2.
Number of MII frozen oocytes needed to attain each estimated live birth rate thresholds by age group, extrapolated from Doyle et al. [4] and Goldman et al. [5]
| Age | 50% | 60% | 70% | |
|---|---|---|---|---|
| < 35 | Doyle et al. (8.2%)1 | 6 | 8 | 9 |
| Goldman et al. | 6 | 8 | 9 | |
| 35–37 | Doyle et al. (7.3%)1 | 7 | 8 | 10 |
| Goldman et al. | 8–10 | 10–14 | 14–18 | |
| 38–40 | Doyle et al. (4.5%)1 | 11 | 13 | 16 |
| Goldman et al. | 12–20 | 16–26 | 21–34 | |
| 41–42 | Doyle et al. (2.5%)1 | 20 | 24 | 28 |
| Goldman et al. | 24–31 | 33–40 | 40–50 | |
| > 42 | Doyle et al. | n/a | n/a | n/a |
| Goldman et al. | 50 | 70 | 80 |
1Estimated live birth rate per oocyte
Utilizing a ROC curve and Youden’s J-index, we then calculated the best cutoff value for both age and AMH to predict whether a subject would cryopreserve enough oocytes in her first cycle for a 60% eLBR. Odds ratio for achieving a 60% eLBR was calculated for the age and AMH cutoff value with X2 analysis. The findings were corroborated using a logistic regression model with age and AMH as covariates.
Comparisons were also made between subjects completing a single cycle versus those who completed two or more cycles, and those who achieved a 50%, 60%, or 70% threshold in two or fewer cycles compared with those who did not. Associations were made using Student t test and Mann-Whitney U test, where appropriate. Analyses of demographic and cycle data for subjects who were able to achieve 50%, 60%, or 70% threshold with one or two cycles by age group were conducted using ANOVA or Kruskal-Wallis, where appropriate.
For all analyses, we considered a two-tailed p value of < 0.05 significant. Statistical analyses were conducted using IBM SPSS Version 25 (Armonk, NY).
Results
A total of 1241 subjects underwent Pl-OC treatment and were included in the study. During the study period, 1923 Pl-OC cycles were initiated. Of these,1799 Pl-OC cycles were completed and included in the cycle analyses. 56/1923 (2.9%) cycles were canceled for poor response and 68/1923 (3.5%) cycles were canceled for non-medical reasons. There were no included subjects who did not complete at least one retrieval during the study period.
Mean age at retrieval and median AMH at first cycle were 35.6 ± 3.26 years and 2.15 ng/dL (IQR 2.43). Mean number of cycles per subject was 1.45 ± 0.79 overall and 1.28 ± 0.61 for subjects < 34, 1.46 ± 0.77 for 35–37, 1.60 ± 0.93 for 38–40, 1.76 ± 0.97 for 41–42, and 2.29 ± 1.49 for > 42 years old. Mean number of cycles completed significantly increased with age (p < 0.001).
Table 1 summarizes first and second cycle demographic and cycle data for each age group and the entire cohort. Among first cycles, AMH, peak estradiol (E2), total gonadotropin (GND), oocytes retrieved, and frozen MII oocytes were all significantly associated with age (p < 0.001, for all comparisons).
Table 1.
Demographic and cycle data for entire cohort, categorized by age group. Parametric data are presented as mean ± SD and non-parametric data are presented as median ± IQR. Comparisons performed with ANOVA or Kruskal-Wallis, where appropriate
| Age (years) | < 35 | 35–37 | 38–40 | 41–42 | > 42 | Total | p value |
|---|---|---|---|---|---|---|---|
| First cycle | |||||||
| n | 415 | 524 | 234 | 51 | 17 | 1241 | |
| AMH (median ± IQR) | 2.57 ± 2.89 | 2.05 ± 2.15 | 1.86 ± 2.31 | 1.31 ± 1.97 | 1.07 ± 1.40 | 2.15 ± 2.43 | < 0.01 |
| Peak E2 (mean ± SD) | 2733 ± 1639 | 2433 ± 1475 | 2361 ± 1493 | 1812 ± 1319 | 1898 ± 1175 | 2489 ± 1539 | < 0.01 |
| Total GND (mean ± SD) | 3118 ± 1302 | 3591 ± 1383 | 3815 ± 1489 | 4373 ± 1689 | 4328 ± 1572 | 3517 ± 1431 | < 0.01 |
| Oocytes retrieved (mean ± SD) | 21.37 ± 12.40 | 17.12 ± 11.02 | 14.24 ± 9.83 | 10.49 ± 9.34 | 9.47 ± 7.00 | 17.62 ± 11.61 | < 0.01 |
| Frozen MII oocytes (mean ± SD) | 15.41 ± 9.53 | 12.12 ± 8.26 | 9.75 ± 7.72 | 7.25 ± 6.76 | 6.12 ± 4.73 | 12.49 ± 8.86 | < 0.01 |
| Second cycle | |||||||
| n | 90 | 181 | 94 | 26 | 10 | 401 | |
| AMH (median ± IQR) | 1.59 ± 1.25 | 1.50 ± 1.27 | 1.51 ± 1.36 | 1.30 ± 1.89 | 0.94 ± 0.74 | 1.50 ± 1.31 | 0.20 |
| Total GND (mean ± SD) | 3964 ± 1564 | 4323 ± 1495 | 4202 ± 1389 | 4345 ± 1448 | 4613 ± 1963 | 4223 ± 1497 | 0.36 |
| Peak E2 (mean ± SD) | 2302 ± 1235 | 2158 ± 1387 | 2032 ± 1016 | 2131 ± 1109 | 1623 ± 794 | 2145 ± 1247 | 0.43 |
| Oocytes retrieved (mean ± SD) | 15.00 ± 9.60 | 13.29 ± 7.74 | 11.28 ± 6.74 | 11.85 ± 10.17 | 6.5 ± 4.38 | 12.94 ± 8.14 | < 0.01 |
| Frozen MII oocytes (mean ± SD) | 10.87 ± 7.74 | 9.60 ± 6.44 | 7.84 ± 4.84 | 8.12 ± 6.90 | 4.30 ± 2.95 | 9.24 ± 6.51 | < 0.01 |
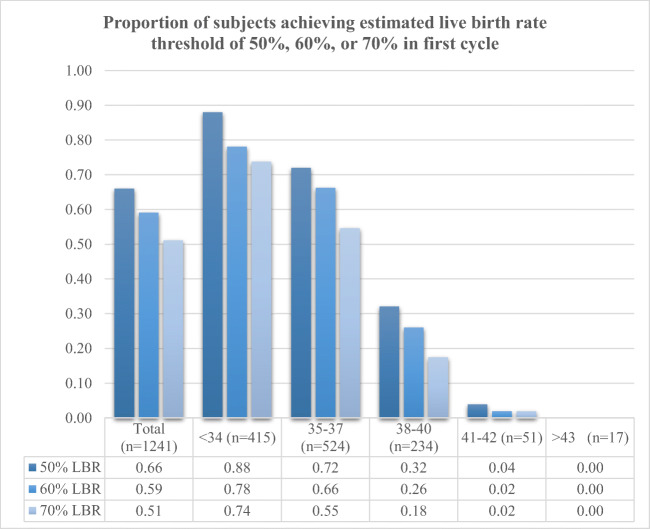
Age-based oocyte thresholds for 50%, 60%, or 70% eLBR were extrapolated from Doyle et al. and Goldman et al., as summarized in Table 2. Overall, two-thirds of subjects (819/1241, 66%) were able to achieve at least a 50% eLBR and just over half (634/1241, 51%) were able to achieve a 70% eLBR with their first cycle. The likelihood of cryopreserving enough oocytes for a 50%, 60%, or 70% eLBR in the first cycle was significantly associated with age (Fig. 1). Association between age group and eLBR threshold was statistically significant for an eLBR of 50% (p < 0.001), 60% (p < 0.001), and 70% (p < 0.001). Comparisons between subjects who did or did not achieve 50%, 60%, or 70% eLBR with their first cycle demonstrate that subjects achieving any eLBR threshold were significantly younger and had significantly higher AMH levels, as well as significantly higher peak E2 levels and significantly lower total GND usage, than those who did not achieve a threshold (Table 3).
Fig. 1.
Proportion of subjects cryopreserving enough oocytes to achieve a 50%, 60%, or 70% estimated live birth rate with their first cycles. Younger age was significantly associated with higher probability of attaining threshold at each level (p < 0.001 for 50%, 60%, and 70%). Associations made with X2 analyses
Table 3.
Characteristics of subjects who achieved 50%, 60%, and 70% eLBR in first cycle compared with those who did not. Comparisons performed with Student t test and Mann-Whitney U, where appropriate
| AMH | Age | Peak E2 | Total GND | ||
|---|---|---|---|---|---|
| 50% eLBR | Yes (n = 819) | 3.60 ± 3.08 | 34.56 ± 2.95 | 2935 ± 1596 | 3172 ± 1245 |
| No (n = 422) | 1.62 ± 1.50 | 37.67 ± 2.83 | 1619 ± 938 | 4187 ± 1530 | |
| p value | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |
| 60% eLBR | Yes (n = 733) | 3.80 ± 3.17 | 34.53 ± 2.95 | 3058 ± 1620 | 3108 ± 1205 |
| No (n = 508) | 1.68 ± 1.49 | 37.18 ± 3.04 | 1665 ± 923 | 4108 ± 1524 | |
| p value | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |
| 70% eLBR | Yes (n = 634) | 4.01 ± 3.13 | 34.34 ± 3.02 | 3177 ± 1668 | 3025 ± 1170 |
| No (n = 607) | 1.81 ± 1.87 | 36.95 ± 2.95 | 1763 ± 953 | 4031 ± 1498 | |
| p value | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
Utilizing a ROC curve and Youden’s J-index (Supplemental Fig. 1), we identified a cutoff AMH value of 1.995 ng/dL for predicting attaining a 60% eLBR in the first cycle. Overall, subjects with AMH > 1.995 ng/dL were more than twice as likely to achieve this threshold in their first cycle compared with those who had an AMH < 1.995 ng/dL (503/638 vs. 189/547; OR 2.65 95% CI 2.28–3.09, p < 0.001). When controlling for age in the logistic regression model, subjects with AMH > 1.995 ng/dL were seven times more likely to attain a 60% eLBR in their first cycle compared with those with AMH < 1.995 ng/dL (OR 7.32; 95% CI 5.50–9.76, p < 0.001) regardless of age group. The ROC curve and Youden’s J-index for age cutoff (Supplemental Fig. 2) yielded an age of 37.5 years for attaining a 60% eLBR in the first cycle. Subjects younger 37.5 were significantly more likely to attain 60% eLBR in their first cycle than those older than 37.5 (671/939 vs. 62/302, OR 1.74, 95%CI 1.59–1.89, p < 0.001), regardless of AMH value. This analysis remained significant in the logistic regression model even when controlling for AMH level (OR 13.73, 95%CI 9.16–20.57, p < 0.001).
A total of 401 (32.3%) subjects completed a second cycle. Table 1 summarizes the clinical data for the second cycles. Subjects with only a single cycle were significantly younger (35.18 ± 3.3 vs. 36.54 ± 2.9, p < 0.001) and had higher AMH level (3.45 ± 2.98 vs. 1.85 ± 2.05) compared with subjects completing two cycles. While there was no statistically significant difference between age groups in AMH, peak E2, and total GND among subjects undergoing a second cycle, the number of oocytes retrieved and frozen remained significantly associated with age (p = 0.002, for both comparisons).
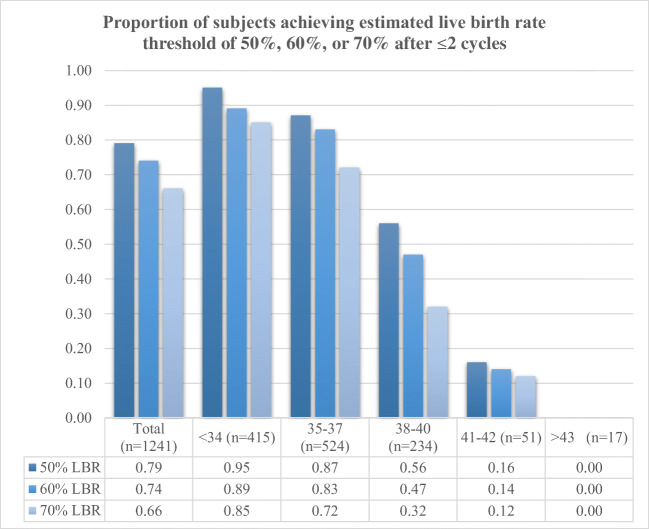
With two cycles, 79.6% (988/1241) of the entire cohort attained at least 50% eLBR, 74.0% (918/1241) reached a 60% eLBR, and 65.5% (813/1241) achieved a 70% eLBR. Of the 422 subjects who did not attain at least a 50% eLBR in first cycle, 245 (58.1%) completed a second cycle, of which 169 (69.3%) were able to reach at least a 50% eLBR after two cycles. As with first cycles, age was significantly associated with subjects who were able to achieve 50%, 60%, or 70% eLBR thresholds with two cycles (p < 0.001). As shown in Fig. 2, nearly all subjects in the youngest age groups (95.4% in < 34 and 86.5% in 35–37) were able to cryopreserve enough oocytes for at least a 50% eLBR and the vast majority were able to attain a 70% eLBR (85.1% in < 34 and 72.3% in 35–37) with 1 or 2 cycles. In addition, a significantly higher proportion of subjects in the older age groups were unable to achieve any of the thresholds, even with two cycles, than in the youngest group (0/17 in > 42, 8/51 in 41–42 vs. 19/415 in < 34, p < 0.001 for both).
Fig. 2.
Proportion of subjects cryopreserving enough oocytes to achieve a 50%, 60%, or 70% estimated live birth rate within two cycles. Younger age was significantly associated with higher probability of attaining threshold at each level (p < 0.001 for 50%, 60%, and 70%). Associations made with X2 analyses
Discussion
The present study represents a novel evidence-based model integrating both age at retrieval and AMH levels, for individualized counseling to women seeking planned oocyte cryopreservation. The results of our study enable potential patients and their provider to create realistic expectations for the likelihood of achieving a high estimated live birth threshold in her first cycle, so that she may consider potential outcomes in light of her reproductive goals and personal/financial resources.
More than half of the subjects in our study were able to achieve a 70% eLBR, and two-thirds were able to achieve at least a 50% eLBR with one cycle. Younger women had a significantly higher chance of achieving a 50%, 60%, or 70% eLBR with their first cycle, with > 85% of subjects in the ≤ 34 group achieving a 70% eLBR in 1–2 cycles. These findings underscore the compounded impact of advancing age on Pl-OC. As age increases, the number of oocytes needed to achieve an equivalent eLBR increases. Concurrently, as age increases, response to GND stimulation and oocyte yield decrease. Therefore, older women are more likely to need multiple cycles, with higher doses of stimulation, to achieve similar results as younger women. Conversely, younger women, even those with lower ovarian reserve, can achieve a high eLBR threshold with 1–2 cycles, because per-oocyte live birth rates are higher for younger women than for older women, likely as a result of lower aneuploidy rates.
Prior studies have attempted to evaluate the optimal timing and cost effectiveness of Pl-OC with conflicting results [6–10]. These theoretical analyses rely on forecasts of future age-related outcomes, as well as changing financial landscapes. Given that Pl-OC is relatively new, coupled with inherently delayed pregnancy outcomes, studies of actual livebirth rates with OC rather than IVF are scarce [4, 5, 11]. The two publications utilized to generate eLBRs in our study, Goldman et al. [5] and Doyle et al. [4], are the best available tools for predicting live birth based on age and number of oocytes retrieved. Nevertheless, both studies still have limitations. While Goldman et al. is the more comprehensive tool, it uses advanced analytics to extrapolate Pl-OC outcomes from a cohort of IVF patients without a diagnosis of female infertility, using non-frozen oocytes, rather than a true non-infertile Pl-OC patients. Doyle et al. represents one of the most helpful studies to date of pregnancy outcomes from oocyte cryopreservation. However, the cohort is small, combines medical and Pl-OC cycles, and includes outcomes from both slow-freeze and vitrification techniques. The authors themselves admit that their findings likely underestimate true live birth rates, particularly in the younger cohort. A third study, Cobo et al. [11], analyzed 137 warmed “elective” oocyte cycles. Based on the results, they recommended 8–10 oocytes to “achieve reasonable success” in women under 35 at retrieval, with steep decline in live birth rates after the age of 35. While the results are valuable, it fails to address the question women considering Pl-OC face, namely how many cycles might it take to achieve a certain potential for success. The present study addresses this question, so that counseling can be individualized by age, AMH, and personal reproductive goals.
The present study represents a large-scale analysis of true Pl-OC retrievals in non-infertile women. Our study’s primary strength lies in the size of our Pl-OC cohort, 1241 participants, completing 1799 Pl-OC cycles. All participants were treated at the same practice; while this limited clinical variations in their treatment, it may also limit generalizability to other practices and patient populations.
Most importantly, our novel approach of analyzing number of cycles needed to achieve a threshold of estimated live birth directly addresses a pressing question facing women who consider Pl-OC. The findings of this study allow women considering Pl-OC to evaluate both appropriate estimates for live birth rates based on age at retrieval and number of oocytes, as well as realistic evidence-based expectations for how many cycles it may take for them to achieve those thresholds.
The study’s primary weakness is the extrapolation of eLBR from prior datasets, one of which is not based on OC cycles. Our analyses, while critical to the counseling of women considering Pl-OC, are therefore inherently limited by the fact that it is not based on actual live birth outcomes from our participants. For several age groups, the two studies did not concur. In these cases, we chose to utilize Doyle et al., since this study’s data were collected from OC cycles, rather than extrapolated from IVF cycles. This resulted in using the lower of the two estimates for several age groups. However, Doyle relied on age categories, while Goldman utilized discreet years of age. This may account for some of the discrepancies between the two and would be a potential limitation of both Doyle and our analyses.
Canceled cycles were not included in the analysis. While cycles canceled for poor response represent a very small percentage of the overall cycles (< 3%), they could have potentially skew the results. Given that the objective of the study was to focus on an individual’s overall probability of reaching a particular oocyte threshold, rather than a specific analysis, we felt the results were best represented by first and second “retrieval cycles,” rather than initiated cycles overall. Furthermore, none of the patients attempting Pl-OC terminated the process without undergoing at least one retrieval, so there were no unaccounted subjects.
The risk for cycle cancelation due to poor response is a critical element of proper counseling for Pl-OC. Those who are anticipated to have poor or no response should be extensively counseled in advance. Our practice does not have minimal criteria for retrieval, in favor of individualized counseling and shared decision making with each patient. Furthermore, patients who chose to defer or terminate their treatment due to poor response were free to do so without incurring financial loss. Cycles with no response were canceled at the recommendation of the clinician. However, as noted above, no patient was unable to complete a retrieval.
The retrospective nature of the study also limits its generalizability. While future prospective studies are necessary and ongoing, no prospective data are currently available with which to counsel those interested in Pl-OC. Studies such as this one set the foundation for future exploration of the natural decline of female fertility and the role of Pl-OC. Until adequate pregnancy outcome data from Pl-OC cycles are available for analysis, women should be provided with the best available evidence and counseling regarding its potential limitations. While complexities and nuances exist, the ASRM Ethics Committee maintains “Patients should be trusted to comprehend information when full and appropriate medical counseling is presented and should not have options removed due to potentially biased underestimation of their capabilities” [2].
Recent studies have identified a growing desire for Pl-OC both in the USA and abroad [12–14], while also demonstrating a significant “knowledge gap” [2] and deficit in appropriate counseling [15, 16]. Additionally, several studies have raised concerns of “false security” [2] or “regret” [17] in women undergoing Pl-OC. These concerns have been underscored by sensationalism in the lay media, highlighting failed attempts of conception with cryopreserved oocytes. By and large, these episodes represent individual women in their mid-40s who cryopreserved a small number of oocytes in their late 30s, often prior to the advent of vitrification. While the likelihood of achieving 50%, 60%, or 70% eLBR thresholds decreased with increasing age at retrieval and declining AMH, almost all women in our study were successfully able to preserve enough oocytes for at least a 50% eLBR in one or two cycles. Considering that national live birth rates in IVF cycles range from 54.5% in women under 35 to 25.9% at 38–40 and 4.1% for women over 42 [1], our data demonstrate that a single cycle of fertility preservation can afford most women an opportunity to increase their chances of genetically related child should they experience infertility later in life.
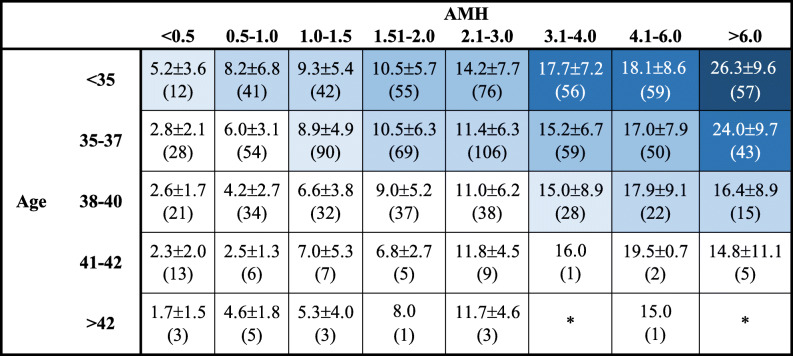
Ultimately, appropriate counseling is paramount in ensuring that women recognize the potential benefits, without developing unrealistic expectations, of what Pl-OC may offer them. Table 4 represents a summary of the results of the present study, highlighting the mean number of oocytes frozen by age/AMH and overlaid with the eLBR by age. This could serve as a critical tool for counseling women regarding Pl-OC. A counseling methodology such as the one proposed here empowers women to make educated choices about their reproductive goals, while setting realistic expectations for the number of cryopreserved oocytes needed to achieve live birth, as well as the number of Pl-OC cycles needed to achieve those goals, particularly in older women.
Table 4.
Number of frozen MII oocytes in first retrieval by age and AMH, presented as mean ± SD (n). Gradations in shading represent 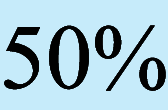 ,
,  ,
,  ,
,  ,
,  eLBR
eLBR
Electronic supplementary material
ROC curve for AMH levels achieving a 60% eLBR with first cycle. AUC 0.80 (95% CI 0.77–0.82, p < 0.001). Youden’s J-index analysis yielded AMH 1.995 as best cutoff for prediction of achieving 60% eLBR in first cycle. (DOCX 33 kb).
ROC curve for age as a predictor of achieving a 60% eLBR with first cycle. AUC 0.74 (95% CI 0.71–0.77, p < 0.001). Youden’s J-index analysis yielded 37.5 year as best age cutoff for prediction of achieving 60% eLBR in first cycle (DOCX 49 kb) (DOCX 29 kb).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.National Summary Report [Internet]. [cited 2019 Feb 13];Available from: https://www.sartcorsonline.com/rptCSR_PublicMultYear.aspx?reportingYear=2016
- 2.Daar J, Benward J, Collins L, Davis J, Davis O, Francis L, Gates E, Ginsburg E, Gitlin S, Klipstein S, McCullough L, Paulson R, Reindollar R, Ryan G, Sauer M, Tipton S, Westphal L, Zweifel J. Planned oocyte cryopreservation for women seeking to preserve future reproductive potential: an ethics committee opinion. Fertil Steril. 2018;110(6):1022–1028. doi: 10.1016/j.fertnstert.2018.08.027. [DOI] [PubMed] [Google Scholar]
- 3.Rukhsana J, Perrotta PL, Okorodudu AO, Petersen JR, Mohammad AA. Fit-for-purpose evaluation of architect i1000SR immunoassay analyzer. Clin Chim Acta Int J Clin Chem. 2010;411(11–12):798–801. doi: 10.1016/j.cca.2010.02.061. [DOI] [PubMed] [Google Scholar]
- 4.Doyle JO, Richter KS, Lim J, Stillman RJ, Graham JR, Tucker MJ. Successful elective and medically indicated oocyte vitrification and warming for autologous in vitro fertilization, with predicted birth probabilities for fertility preservation according to number of cryopreserved oocytes and age at retrieval. Fertil Steril. 2016;105(2):459–466.e2. doi: 10.1016/j.fertnstert.2015.10.026. [DOI] [PubMed] [Google Scholar]
- 5.Goldman RH, Racowsky C, Farland LV, Munné S, Ribustello L, Fox JH. Predicting the likelihood of live birth for elective oocyte cryopreservation: a counseling tool for physicians and patients. Hum Reprod. 2017;32(4):853–859. doi: 10.1093/humrep/dex008. [DOI] [PubMed] [Google Scholar]
- 6.van Loendersloot LL, Moolenaar LM, Mol BWJ, Repping S, van der Veen F, Goddijn M. Expanding reproductive lifespan: a cost-effectiveness study on oocyte freezing. Hum Reprod. 2011;26(11):3054–3060. doi: 10.1093/humrep/der284. [DOI] [PubMed] [Google Scholar]
- 7.Hirshfeld-Cytron J, Grobman WA, Milad MP. Fertility preservation for social indications: a cost-based decision analysis. Fertil Steril. 2012;97(3):665–670. doi: 10.1016/j.fertnstert.2011.12.029. [DOI] [PubMed] [Google Scholar]
- 8.Devine K, Mumford SL, Goldman KN, Hodes-Wertz B, Druckenmiller S, Propst AM, et al. Baby budgeting: oocyte cryopreservation in women delaying reproduction can reduce cost per live birth. Fertil Steril. 2015;103(6):1446–1453.e2. doi: 10.1016/j.fertnstert.2015.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mesen TB, Mersereau JE, Kane JB, Steiner AZ. Optimal timing for elective egg freezing. Fertil Steril. 2015;103(6):1551–1556.e4. doi: 10.1016/j.fertnstert.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Devesa M, Tur R, Rodríguez I, Coroleu B, Martínez F, Polyzos NP. Cumulative live birth rates and number of oocytes retrieved in women of advanced age. A single centre analysis including 4500 women ≥38 years old. Hum Reprod. 2018;33(11):2010–2017. doi: 10.1093/humrep/dey295. [DOI] [PubMed] [Google Scholar]
- 11.Cobo A, García-Velasco JA, Coello A, Domingo J, Pellicer A, Remohí J. Oocyte vitrification as an efficient option for elective fertility preservation. Fertil Steril. 2016;105(3):755–764.e8. doi: 10.1016/j.fertnstert.2015.11.027. [DOI] [PubMed] [Google Scholar]
- 12.Inhorn MC, Birenbaum-Carmeli D, Birger J, Westphal LM, Doyle J, Gleicher N, et al. Elective egg freezing and its underlying socio-demography: a binational analysis with global implications. Reprod Biol Endocrinol. 2018;16(1) [Internet]. [cited 2019 Jan 29]. Available from: https://rbej.biomedcentral.com/articles/10.1186/s12958-018-0389-z. [DOI] [PMC free article] [PubMed]
- 13.Baldwin K, Culley L. Women’s experience of social egg freezing: perceptions of success, risks, and “going it alone.”. Hum Fertil (Camb). 2018:1–7. 10.1080/14647273.2018.1522456. [DOI] [PubMed]
- 14.Baldwin K, Culley L, Hudson N, Mitchell H, Lavery S. Oocyte cryopreservation for social reasons: demographic profile and disposal intentions of UK users. Reprod BioMed Online. [Internet] [cited 2015 Jul 8];Available from: 10.1016/j.rbmo.2015.04.010. [DOI] [PubMed]
- 15.Fritz R, Klugman S, Lieman H, Schulkin J, Taouk L, Castleberry N, Buyuk E. Counseling patients on reproductive aging and elective fertility preservation-a survey of obstetricians and gynecologists’ experience, approach, and knowledge. J Assist Reprod Genet. 2018;35(9):1613–1621. doi: 10.1007/s10815-018-1273-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stevenson EL, Gispanski L, Fields K, Cappadora M, Hurt M. Knowledge and decision making about future fertility and oocyte cryopreservation among young women. Hum Fertil. 2019;0(0):1–10. [DOI] [PubMed]
- 17.Greenwood EA, Pasch LA, Hastie J, Cedars MI, Huddleston HG. To freeze or not to freeze: decision regret and satisfaction following elective oocyte cryopreservation. Fertil Steril. 2018;109(6):1097–1104.e1. doi: 10.1016/j.fertnstert.2018.02.127. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ROC curve for AMH levels achieving a 60% eLBR with first cycle. AUC 0.80 (95% CI 0.77–0.82, p < 0.001). Youden’s J-index analysis yielded AMH 1.995 as best cutoff for prediction of achieving 60% eLBR in first cycle. (DOCX 33 kb).
ROC curve for age as a predictor of achieving a 60% eLBR with first cycle. AUC 0.74 (95% CI 0.71–0.77, p < 0.001). Youden’s J-index analysis yielded 37.5 year as best age cutoff for prediction of achieving 60% eLBR in first cycle (DOCX 49 kb) (DOCX 29 kb).