Abstract
The current treatment for Asherman syndrome is limited and not very effective. The aim of this review is to summarize the most recent evidence for stem cells in the treatment of Asherman syndrome. The advent of stem cell therapy has propagated experimentation on mice and humans as a novel treatment. The consensus is that the regenerative capacity of stem cells has demonstrated improved outcomes in terms of fertility and fibrosis in both mice and humans with Asherman syndrome. Stem cells have effects on tissue repair by homing to the injured site, recruiting other cells by secreting chemokines, modulating the immune system, differentiating into other types of cells, proliferating into daughter cells, and potentially having antimicrobial activity. The studies reviewed examine different origins and administration modalities of stem cells. In preclinical models, therapeutic systemic injection of stem cells is more effective than direct intrauterine injection in regenerating the endometrium. In conjunction, bone marrow-derived stem cells have a stronger effect on uterine regeneration than uterine-derived stem cells, likely due to their broader differentiation potency. Clinical trials have demonstrated the initial safety and effectiveness profiles of menstrual, bone marrow, umbilical cord, and adipose tissue-derived stem cells in resumption of menstruation, fertility outcomes, and endometrial regeneration.
Keywords: Intrauterine adhesions, Stem cell therapy, Asherman syndrome, Cell homing, Endometrial regeneration
This is a review of the most recent literature regarding the role of stem cells in natural regeneration of the uterine endometrium as well as in experimental treatments for Asherman syndrome in humans and animals. A PubMed search was done for journal articles published through February 2020 using combinations of the terms “Asherman syndrome,” “stem cell therapy,” “endometrial stem cells,” “uterine regeneration,” and “intrauterine adhesions.” This article reviews epidemiology, pathologic features, classification systems, treatments, and areas of research regarding stem cells. It focuses on and analyzes the different types and characteristics of stem cells, their effects on tissue repair, and the different administration modalities used for the experimental treatment of Asherman syndrome.
Asherman syndrome is a gynecologic disorder first described by Israeli gynecologist Joseph Asherman in 1948 that is marked by intrauterine adhesions (IUA, formerly known as gynatresia or synechiae), causing a partial or complete obliteration of the cavity combined with symptomatology. The acquired syndrome arises from the destruction of the endometrium and often leads to infertility or recurrent pregnancy loss, chronic pelvic pain, menstrual irregularities (hypo- or amenorrhea), or dysmenorrhea. The most common risk factors include post-abortion or postpartum curettage, infection, myomectomy, and hysteroscopic surgery, i.e., procedures that disrupt the endometrial basalis layer. The syndrome is characterized by a loss of functional endometrium, a uterine cavity obliterated by scar tissue, recurrent pregnancy loss or infertility, as well as obstetric issues, such as abnormal placentation, i.e., previa or accreta. Hysterosalpingography (HSG) and hysterosonography/saline infusion sonography (SIS) are useful and sensitive diagnostic modalities but are not as specific as hysteroscopy (HSC); therefore, they should only be used if HSC is not available. MRI should not be used for diagnosis. HSG has a 75% sensitivity, 95% specificity, 50% positive predictive value (PPV), and 98% negative predictive value (NPV), while SIS has a sensitivity of 75%, specificity of 93%, PPV of 43%, and NPV of 98% for diagnosing IUA [1]. There is no standard classification system for intrauterine adhesions; they are all based on the severity of adhesions visible on hysteroscopy, with the prognosis correlating to severity (Table 1) [2]. The various classification systems make comparative studies difficult to analyze. In the status quo, there are no promising modalities for full recovery, but the three standard modes of treatment center on surgery, adhesion prevention, and restoration of the normal endometrium with hormones.
Table 1.
Classifications of intrauterine adhesions
| March, 1978 | Minimal (< ¼ of cavity; thin, filmy), moderate (¼–¾ of cavity; no agglutination of walls, partial occlusion of ostia) or severe (> ¾ of cavity, agglutination of walls, thick bands or involving tubal ostia) based on hysteroscopic (HSC) assessment of cavity involvement |
| Hamou, 1983 | Isthmic, marginal, central or severe based on HSC |
| Valle, 1988 | Mild (filmy adhesions composed of basal endometrium producing partial or complete uterine cavity occlusion), moderate (fibromuscular adhesions with thick bands covered with endometrium that may bleed when divided) or severe (bands composed of connective tissue lacking endometrium) based on HSC and extent of occlusion based on HSG |
| American Fertility Society, 1988 | Mild, moderate or severe based on endometrial cavity obliteration, appearance of adhesions on HSC/HSG and patient menstrual characteristics |
| European Society of Hysteroscopy, 1989 | Grade I-IV based on HSC, HSG and clinical symptoms |
| Donnez, 1994 | Six grades based on location; HSC, HSG used for assessment |
| Nasr, 2000 | Prognostic score by incorporating menstrual and obstetric history with IUA findings on HSC |
Adopted from: AAGL practice report: practice guidelines on intrauterine adhesions developed in collaboration with the European Society of Gynecological Endoscopy (ESGE) [2]
Intrauterine adhesion formation
The etiology of intrauterine adhesions is believed to be due to fibrosis of the opposing uterine walls after destruction of the endometrial basalis layer. It is a catastrophic process whereby uncontrolled deposition of extracellular matrix (ECM) and fibrillar collagens occurs. The stromal compartment is replaced by fibrous tissue, and the glands are replaced by inactive cubo-columnar epithelium. Myofibroblasts, which are activated by CTGF (connective tissue growth factor) via TGF-β, are highly metabolically active and express α-smooth muscle actin (SMA), which is involved in the production of ECM, e.g., fibrillar collagen types I, III, V, and VI [3]. TGF-β is a central mediator of fibrogenesis and is found in significantly higher concentration in the endometrium scarred with IUA compared to normal; it modulates fibroblast phenotype and function as well as myofibroblast trans-differentiation [3]. Fibrotic factors, such as TGF-β1, α-SMA, CTGF, collagen I and III, are key to development of IUA, and their increased expression leads to an altered environmental niche, thereby inhibiting normal regeneration by endometrial mesenchymal stem cells (eMSC); the subsequent scarring inhibits normal myometrial contractility and reduces the perfusion by steroids, resulting in atrophy [3, 4]. Moreover, increased expression of a disintegrin and metalloproteinase (ADAMs) has been found in uterine adhesions, thereby inhibiting the activities of ECM-degradation enzymes [5]. ADAM-17 activates TNF-α as well as membrane-associated ligands of the epidermal growth factor receptor (EGFR), triggering an immune and inflammatory cascade; similarly, ADAM-15 interacts with beta-integrin-3 and Src protein-tyrosine kinases, suggesting its involvement in cell adhesion and signaling [6]. Therefore, a catastrophic pathologic cascade follows, disrupting the normal endometrial regeneration [3, 4]. Although some studies have not demonstrated significant benefit, one study concluded that postoperative hormonal therapy after surgical abortion improves endometrial thickness, indicating an enhanced regeneration of the uterus while preventing adhesion formation [7]. Similarly, injection of hyaluronic acid (HA) in the uterine cavity has been shown to decrease these fibrotic factors by acting as a mechanical barrier in the uterine cavity to prevent apposition of the opposing walls [4]. Moreover, it is theorized to have other indirect effects on attenuating the fibrosis via immunomodulation. Hyaluronic acid interacts with CD44 (found on endometrial stromal and epithelial cells) and other receptors, such as TLR-2, TLR-4, RHAMM, and LYVE1; it also may inhibit the function of TGF-β1, as it has been shown to decrease the degree of fibrosis in mice induced with AS and treated with HA [4].
Current treatment for Asherman syndrome
Primary prevention of IUA is key since once the pathological cascade begins, the reversal becomes much more difficult. AAGL’s guideline for primary prevention of IUA states: the application of an adhesion barrier following surgery that may lead to endometrial damage significantly reduces the development of IUAs in the short term, although limited fertility data are available following this intervention, grading the evidence as level A. The second guideline with level B evidence emphasizes the key point: that operative procedures that disrupt the endometrial basalis, especially postpartum, more likely contribute to IUA than those that resect intracavitary pathology, e.g., polypectomy and myomectomy of type 0 fibroids [8].
Treatment of IUA should be considered only for patients with AS, as the risk of treating asymptomatic IUA outweighs the benefit. Because of the risk of uterine perforation and false tract formation, blind cervical probing and uterine curettage is discouraged now with the advent of ultrasound and hysteroscopy [8]. Hysteroscopic adhesiolysis has been performed using scissors, needles, blunt dissection, and electrosurgery. Monopolar and bipolar electrosurgical instruments as well as the Nd-YAG laser have been used for precision cutting and hemostasis, but using them risks damaging the endometrium further [8]. Fluoroscopically guided blunt dissection with a hysteroscopically directed Tuohy needle is another technique, but it is limited by its high cost, technical difficulty, and use of ionizing radiation [8]. The use of intraoperative ultrasound, fluoroscopy, or concomitant laparoscopy with hysteroscopy has been used for guidance to decrease the risk of perforation, yet the AAGL guideline states that these adjunctive interventions have not been shown to prevent perforation or improve outcomes.
The biggest challenge to treating Asherman syndrome is preventing the recurrence of adhesions after initial treatment, which is as high as 66% [8]. Treatment with estrogen has historically been used following adhesiolysis to stimulate regeneration and re-epithelization of the endometrium. The AAGL Practice Guideline recommends postoperative hormone therapy with estrogen after adhesiolysis, yet there is no standard dosage or regimen [8]. Different regimens with and without opposing progesterone have been used. Although estrogen alone is beneficial for all stages of IUA, its combination with adjunctive treatments is necessary for maximal effect in patients with moderate to severe adhesions [9]. One RCT evaluated the efficacy of different doses of estrogen treatment after hysteroscopic adhesiolysis. The low-dose group received 2 mg estradiol daily while the high-dose group received 6 mg daily for three cycles after surgery. Hysteroscopy was performed twice for each group at 4 and 8 weeks after the initial surgery. Using the American Fertility Society’s (AFS) classification of IUA, the results revealed significantly lower scores in both groups at the subsequent two time points. In addition, no significant difference was found between the two groups, suggesting that the low-dose therapy is sufficient in preventing adhesion reformation [10]. Another study administered estradiol preoperatively for 2 to 8 weeks to enhance endometrial proliferation. Hysteroscopic adhesiolysis under ultrasound guidance was then performed using scissors along with a controlled flow pump for distention. A triangular balloon catheter was left inside the uterine cavity for 3–7 days, after which it was replaced with a copper IUD. Oral estradiol with 4–6 mg daily was supplemented for 4–10 weeks, after which the IUD was removed. The 12 women with severe IUA by the AFS classification had all resumed menses after this regimen [11]. Six of the nine women under age 39 subsequently became pregnant, with four of those delivering at or near term.
As the trend toward minimally invasive modalities flourishes, treatment of Asherman syndrome (AS) has shied away from the antiquated method of hysterotomy in favor of hysteroscopic resection, intrauterine devices, hormones, adhesion barriers, and most recently, stem cells [12, 13]. Prior to 2010, there had been no controlled comparative studies for the effectiveness of the different modalities [14]. Currently, the gold standard treatment modality for Asherman syndrome is hysteroscopic resection of the adhesions [13]. After hysteroscopic adhesiolysis, 88% of patients with menstrual abnormalities restored normal menstruation in one study [15]. Surgical treatment is useful in patients with mild or moderate disease severity but not with severe AS [16]. In 2019, an RCT examined the effects of an inert IUD relative to a hyaluronan gel. The three groups included (1) IUD only, (2) IUD + hyaluronan gel, and (3) gel only. The results revealed similar efficacy in all three groups in preventing adhesion reformation after hysteroscopic adhesiolysis in patients with moderate to severe IUA. However, thicker endometrium was found in the groups that received the gel, suggesting that the hyaluronan gel was more effective in improving the endometrial thickness than the hormonal IUD [12]. Ongoing pregnancy rates after IVF cycles were not statistically significantly different though.
Other adhesion barriers used include amnion grafts, stents and gels made of hyaluronic acid, or polyethylene oxide-sodium carboxymethylcellulose. Gan et al. experimented with a freeze-dried amnion graft covering a balloon catheter and concluded that it reduced the risk of adhesion reformation after hysteroscopic adhesiolysis compared to those patients that received the balloon alone [17]. Another study claimed that blunt adhesiolysis with a flexible hysteroscope after primary adhesiolysis is also effective in maintaining cavity patency [18]. Zhu et al. examined the effects of hyaluronic acid (HA) in mice with iatrogenic AS, and they concluded that high molecular weight HA attenuated fibrosis but low molecular weight HA did not [4]. These effects are due to HA’s regulatory interaction with CD44, thereby inhibiting TGF-β1. AAGL’s guidelines for secondary prevention of intrauterine adhesions state that intrauterine stents, balloons, or IUDs appears to reduce the risk of adhesion reformation (grade A evidence); the risk of infection with these devices is minimal (grade A evidence); the use of antibiotics for preoperative, intraoperative, or postoperative therapy is controversial (grade C evidence); inert IUDs are preferable to copper and hormonal ones (grade C evidence); semi-solid barriers, such as HA or crosslinked HA gel, reduce adhesion reformation (grade A evidence); postoperative hormonal therapy may reduce the risk of recurrence of IUA (grade B evidence); adjuvant medications to improve vascular flow are still experimental (grade C evidence); and stem cell therapy is still experimental (grade C evidence) [8].
Recently, there have been experiments transplanting autologous and allogeneic stem cells to treat AS in animal as well as human subjects. One study demonstrated that fertility outcomes improved after administration of a bone marrow-derived stem cell transplant in mice with iatrogenic AS [19]. Likewise, autologous bone marrow stem cell transplant in humans is the latest advancement in treatment for patients with refractory AS [20–22]. The pioneer studies include two case series in India, which have demonstrated efficacy in restoring menstruation and fertility in humans, whereby bone marrow-derived stem cells were injected into the uterus followed by administration of estrogen therapy [20, 23]. Similarly, one prospective clinical study in China restored fertility using autologous menstrual stem cell transplants [22]. However, stem cell transplantation for Asherman syndrome is far from becoming commonplace.
Endometrial regeneration with stem cells
The uterus, like most other organs in the body, has regenerative capacity. With each menstrual cycle, the endometrial lining undergoes a process of degeneration, cellular proliferation, differentiation, and regeneration. Stem cells in the basalis layer of the endometrium exhibit self-renewal as well as multipotency, thereby regenerating the functionalis layer, which sheds with every menses as well as postpartum [19, 21, 24]. Although slightly more differentiated than mesenchymal stem cells and not capable of self-cloning, stromal fibroblasts have a broad multilineage differentiation potential for mesodermal, endodermal, and ectodermal lineages and contribute to the endometrial regeneration as well [25, 26]. Stem cells, on the other hand, also exert a myriad of other effects on tissues, including trophic support, self-renewal, regeneration of endogenous cells, immunosuppression, regulatory interactions, and paracrine signaling with endogenous cells.
Stem cells are undifferentiated or partially differentiated cells with the potential to differentiate into more mature cells and self-replicate into daughter cells. They owe their regenerative capability to telomerase activity. Aside from their multi-lineage differentiation potential and clonogenicity, stem cells are defined by their capability to be cultured long-term, their cell-surface markers, and their capacity for in vivo reconstruction. There are two types of stem cells: embryonic, originating from blastocysts, and adult stem cells, located in regenerating tissues. Embryonic stem cells are highly proliferative but also tumorigenic. Adult stem cells have a low malignant potential and thus are the type used for cell therapy. Stem cell potency denotes the degree of cell-lineage possibilities, whereby totipotent cells originating in blastocysts can differentiate into whole embryonic germ layers, including trophoblastic tissue. The disadvantage of using embryonic stem cells for research is the ethical dilemma since the embryo will be destroyed. The next category on the spectrum of differentiation is pluripotent stem cells, which can differentiate into any cell of the three germ layers; however, they cannot develop into trophoblasts. Further along, the multipotent stem cells can differentiate into a confined number of cell types limited to the same lineage, such as mesenchymal stem cell (MSC). For example, menstrual-derived stem cells can differentiate into neurons, osteoblasts, chondrocytes, adipocytes, and endotheliocytes. Unipotent stem cells, with the narrowest differentiation potential, can only become one cell type. MSC are advantageous for transplantation because they are easily extracted, highly proliferative, weakly immunogenic, immunomodulatory, and highly trophic. For this reason, they are most commonly used for cell therapy and regenerative medicine. Somatic cells can undergo dedifferentiation as well to become induced pluripotent stem cells (iPSC) by introduction of Myc, Sox2, Oct3/4, and KLF genes, which encode transcription factors to reprogram the cells into embryonic-like cells.
Stem cells are harbored in specialized microenvironments, or niches, which regulate their functions. Uterine sources of stem cells include endometrial epithelial, stromal, and endothelial cells, all of which contribute to endometrial regeneration [27]. The endometrium consists of two cell types: epithelium and supporting mesenchyme/stroma. The epithelium is categorized by glandular (GE) and luminal epithelium (LE), which are the majority of the functionalis layer but the minority of the basalis layer; the basalis mostly comprises stroma and harbors most of the putative endogenous stem cells. Stem cells are found in both basalis and functionalis layers as perivascular cells and pericytes, mural cells that wrap around the endothelium lining the capillaries and venules. The LE and GE (comprising leukocytes and vasculature) are derived from Müllerian duct epithelial cells, whereas the stromal cells derive from mesenchyme. Mesenchymal stromal cells are multipotent somatic stem cells that have the potential to differentiate into mesodermal and non-mesodermal lineages. In Asherman syndrome, the LE is largely lost, the stroma is replaced by fibrous tissue, and the endometrium thins and loses responsiveness to estrogen and progesterone [26]. The surface epithelium develops from proliferating epithelium near the tips of the GE. Epithelial stem cells are CD44+, which is a cell surface marker present on label-retaining cells (LRC). LRC are mostly quiescent and retain DNA labels over a longer period of time than more mature cells, which have a more rapid turnover [26].
Stem cells found in the uterus are also derived from non-uterine sources. These sources, such as bone marrow-derived stem cells (BMDSC), human embryonic stem cells (hES), and induced pluripotent stem cells (iPS), also partake in endometrial restoration [19, 28, 29]. BMDSC engraft into the uterine stroma after ischemia/reperfusion injury. Their mechanism of action is most likely via the production of trophic factors for recruitment of other cells. However, there is conflicting evidence on the role of these extra-uterine sources of stem cells in the natural regenerative processes. The HSC derived from bone marrow provide a supportive microenvironment, or niche, for hematopoiesis; they also help develop, stabilize, and maintain the sinusoidal network [30]. The bone marrow-derived MSC (bmMSC) are the subtype that contributes to endometrial regeneration. Recruitment of BMDSC to the endometrium is independent of hormonal stimulation; it is likely a reparative response to injury or pregnancy rather than for menstrual regeneration [19]. In the setting of AS, the quantity of stem cells is the limiting factor for repair. With loss of the uterine stem cell cohort, the recruitment of BMDSC may not suffice. Transplanting BMDSC has demonstrated a beneficial effect on the function of target organs, likely due to immunomodulation, recruitment of growth factors, and paracrine effects. Embryonic stem cells are totipotent but are tumorigenic when transplanted in mice. Using tissue recombination, embryoid bodies (3-D aggregates) of pluripotent stem cells generated from hES have been shown to regenerate the endometrium with differentiation by growth factors, such as BMP-4 and Activin A, which induce stem cells to differentiate into mesendoderm, an obligate intermediate during embryonic genital development; in response to estrogen, the epithelium proliferates and expresses glycodelin A (also known as progestogen-associated endometrial protein, PAEP) as well as other Müllerian markers, e.g., LIM1, PAX2, and HOXA10 [31]. This demonstrates induction of hES by the endometrial mesenchyme. Glycodelin A is secreted from the endometrial glands and decidual glandular epithelium in early pregnancy, and it has been shown to modulate the immune system by inducing apoptosis of T cells and monocytes, thereby stimulating production of IL-6 and IL-13 from NK cells and inducing immunologic tolerance of dendritic cells [32].
There are two subtypes of endometrial cells: main population (MP) and side population (SP). The SP phenotype is < 5% of the total, yet it identifies stem cells by way of rapid efflux of Hoechst dye 33342, which binds DNA via the ATP-Binding Cassette (ABC) G2 protein/Bcrp1 (breast cancer resistance protein) transporter [26, 28, 33]. This dye is commonly utilized when identifying the stem cells that localize to a certain tissue in a transplanted subject since the slow-cycling cells, i.e., stem cells, retain the dye for a longer period of time than other endometrial cells. SP cells exhibit preferential expression of endothelial cell markers compared to MP cells; the stem cells are located in the perivascular niche. Moreover, only SP cells exhibit telomerase activity and pluripotency markers, such as Oct4. SP cells are most abundant during the menstrual and proliferative phases, suggesting their crucial role in endometrial regeneration; they have greater clonogenicity than MP cells, and they differentiate into endothelial, stromal, and epithelial cells, whereas MP cells only differentiate into stromal cells [28, 34]. Despite their relationship to the menstrual cycle, SP cells do not contain steroid receptors, yet they demonstrate histologic changes at different points in the menstrual cycle. This suggests that sex steroids have no direct effect on the stem cells; however, the surrounding stromal cells respond to the hormones. Cervelló et al. hypothesizes that signals coming from the stem cell niche may maintain the somatic stem cell population in a naïve state but become responsive to steroids after differentiating into transient amplifying cells. Basalis glands contain ER-α throughout the menstrual cycle, whereas the functionalis layer only expresses it in the proliferative phase; neither eMSC (endometrial mesenchymal stem cells) nor SP cells contain ER-α or PR receptors [26]. During the decidualization process though, SP cells express the markers prolactin and IGFBP-1, and their cytoplasm expands, giving them a polygonal morphology. Moreover, the basalis expresses stage-specific embryonic antigen (SSEA-1), while the functionalis does not; SSEA-1 is also a marker of postmenopausal endometrial epithelium [35]. Markers such as Bcl-2, CD34, and c-kit/CD117 are also expressed in the stroma of the basalis. Bcl-2, an anti-apoptotic marker, is found at high levels during the proliferative phase and lower levels during the secretory phase. CD34 functions as a cell-cell adhesion factor that mediates the attachment of HSC to the ECM of bone marrow or directly to stromal cells; it also facilitates cell migration. C-kit/CD117 is a cytokine receptor expressed on many cells that binds to stem cell factor, activates intrinsic tyrosine kinase activity and activates signal transduction molecules to modulate cell proliferation and differentiation. N-cadherin, a marker of epithelial progenitor cells, plays a role in endometrial proliferative disorders, such as adenomyosis, endometriosis, and thin dysfunctional endometrium [35]. As part of the normal cycle of reproduction, these endometrial cells can be found in menstrual blood and are an easily acquired source of stem cells [26].
In contrast to epithelial stem cells, endometrial mesenchymal stem cells (eMSC) are similar to bone marrow stem cells in that they are multipotent, highly proliferative stem cells with immunomodulatory properties yet distinct from stromal fibroblasts [25]. However, cultured fibroblasts from the endometrium, bone marrow, and many organs also exhibit bone marrow-MSC (bmMSC) properties in vitro. Dissimilar to the ability of bmMSC to generate heterotropic bone or bone marrow organs in vivo, eMSC have not been able to generate a vascularized stroma with the capacity to differentiate into decidualized stroma when transplanted into an animal at the single-cell level [26].
Uterine ischemia or injury has been shown to recruit bone marrow stem cells to the uterus even in the absence of sex steroids [19, 36]. However, BMDSC do not seem to be involved in the monthly regeneration of estrous/menstrual cycles [36]. This concept has stimulated research regarding iatrogenic injury of the uterus in order to home stem cells to that region. Concomitantly, pluripotency transcription factors, e.g., Sox-2 and Oct-4, have been shown to increase expressivity after acute uterine injury [37]. The unproven theory is that the uterine-derived stem cells suffice for the monthly regeneration of the endometrium, but other sources are required in times of greater need [19, 36].
An important aspect of stem cell homing is the chemokine system that is secreted by the injured tissue in order to recruit stem cells. The CXCL12/CXCR4 protein-ligand is one chemokine-receptor complex that promulgates homing of stem cells to the uterus [38]. Stem cells generally express CXCR4 on their surface, which interacts with CXCL12, also known as stromal cell-derived factor-1 (SDF-1). ADM3100, an immunostimulant used to mobilize HSC into blood, is an antagonist of CXCR4. CXCL12 is also expressed on injured tissue [39]. SDF-1 plays a central role in the homing of bone marrow stem cells, which express CXCR4. In most of the experiments tracking the stem cells migration, a low number (< 5%) of those injected were actually found in the regenerating endometrium, implicating the importance of homing specifically to the site of injury as well as the strong effect of stem cells in regeneration despite their low concentration. As an example of homing in other organs, G-CSF has been shown to mobilize HSCs from the bone marrow to the blood, thereby reducing the amount of migration to the endometrium [36]. G-CSF also augments the expression of cytokeratin, vimentin, integrins, and leukemic-inhibiting factor (LIF), which all regulate endometrial function [39]. These stem cells then undergo trans-differentiation into endometrial stem cells after recruitment. Similarly, 17β-estradiol has been demonstrated to stimulate differentiation of bmMSC into epithelial cells in vitro [40].
Paracrine signaling and trophic factors play a key role in cell homing. Paracrine-signaling genes, such as BMP-6, PDGF-β, thrombospondin-1, TNF-α, and IGF-1, are markers of stem cell recruitment [24]. Cell damage due to radiation, chemotherapy, or hypoxia has been shown to induce expression and secretion of chemokines [41, 42]. Chemokines and cytokines are secreted by damaged tissue and are crucial for homing of stem cells to injured sites. For example, SDF-1, which is upregulated at sites of tissue injury, acts as a chemoattractant by binding to chemokine receptor-4 (CR-4) that is expressed on bone marrow stem cells [43]. There are many types of chemokine receptors; thence, knockout of CR-4 only is not sufficient to prevent homing of MSCs to the damaged site [41]. Decidualized SUSD2+ cells are a major source of several cytokines, including LIF and CCL7 [26]. More on the chemokine system is described in the immunomodulatory section that follows.
In animals, a labeling retention method can be used to identify the origin of stem cells. By injecting thymidine analog 5-bromo-2′-deoxyuridine (BrdU), one can identify its localization in the tissue, whereby the LRCs get incorporated into the newly replicated DNA. These newly formed cells identify the somatic stem cells. LRCs have been found in kidneys, hair, liver, and bone marrow as well as in the endometrium when injected with bmMSCs [21, 24, 42, 44]. This method allows for researchers to identify the stem cells that have homed to the damaged tissue.
Endometrial mesenchymal stem cell markers
According to the International Society for Cellular Therapy, MSCs are classified according to three criteria: (1) must be plastic-adherent in standard culture conditions; (2) must express CD105, CD73, and CD90 and cannot express CD45, CD34, CD14, CD11b, CD79α, CD19, and HLA-DR surface molecules; and (3) must be able to differentiate into osteoblasts, adipocytes, and chondroblasts in vitro [45]. Endometrial MSCs have demonstrated similar properties to other MSCs in vitro through clonogenicity assays, adherence to plastic, fibroblast-like morphology, and differentiation to adipogenic, chondrogenic, and osteogenic fates. They are located in perivascular sites in the basalis and functionalis layers; moreover, they contain the same surface markers as other MSCs, e.g., CD29, CD44, CD73, CD90, CD105, MSI1, NOTCH1 [26, 34]. Markers specific for eMSC include CD146 and CD140b (also known as PDGFR-β, platelet-derived growth factor receptor-β) as well as SUSD2 (Sushi domain containing-2, the epitope for the W5C5 antibody), SSEA-1, and LGR5 [26, 28]. Other markers that have been expressed in the stroma include Bcl-2, CD34, c-Kit/CD117, and Ki-67. Bcl-2 is limited to the embryonic endometrium, and it is expressed mostly in the proliferative phase. These markers are also used to detect MSC location in transplanted tissue. STRO-1, the most widely used marker for detecting bmMSC, is not found on eMSCs or stromal fibroblasts [26]. MSCA-1 is a bone marrow-derived MSC marker also known as tissue non-specific alkaline phosphatase (TNAP) that immunolocalizes to perivascular spaces in the endometrium; thus, it has been suggested as a specific eMSC marker [26]. However, TNAP also localizes to epithelium, so EpCAM is used to differentiate them. SSEA-1, or CD15, an epitope of a glycoprotein expressed in differentiating hES, is another surface marker commonly found in the endometrial basalis glands [26]. SSEA-1+ epithelial cells have greater telomerase activity and are more quiescent than SSEA-1− cells [26]. Cells with this marker express lower levels of ER-α and PR (progesterone receptor) than SSEA-1− cells, suggesting a less differentiated phenotype and lower reliance on growth factors to mediate estrogen-induced proliferation signals.
Genes involved in endometrial regeneration
There are many genes/proteins responsible for the development and regeneration of endometrium, of which Wnt, c-kit (CD117), Oct-4, CD34/KLF4, and Musashi-1 are best classified. The Wnt gene activates a signal cascade (Fig. 1) whereby secreted proteins (Wnts) bind to cell surface markers of the Frizzled family and direct the fate of the cell [28]. For example, Wnt7a is involved in endometrial regeneration, and its suppression by progesterone in the latter half of the menstrual cycle is likely the cause of transformation of the epithelium to a secretory phenotype [47]. Wnt4 is responsible for the structural development of internal genitalia in mice as well as stromal decidualization and proliferation during embryo implantation and growth [48]. Wnt signaling regulates Müllerian duct formation, differentiation, and regression [49]. Similarly, postmenopausal endometrium upregulates Wnt expression in the setting of high gonadotropin levels, suggesting the persistent presence of stem cells despite endometrial atrophy [28]. In addition, transcription factors (TFs) are critical to the activation of stem cells. Sex-determining region Y-box 2 (Sox2), Octamer-4 (Oct-4), and Nanog homeobox (Nanog) are three pluripotent TFs that are upregulated at times of uterine damage, which is itself an instigator of stem cell activation [37]. Oct-4 is a transcription factor involved in self-renewal of undifferentiated embryonic stem cells (ESCs). Xiao et al. demonstrated an increased expression of TFs after injection of lipopolysaccharide (LPS, an endotoxin) into mice uteri. CD34 and Sca-1 are expressed on vascular adventitial progenitor cells with broad differentiation capabilities. Sca1+CD34+ cells can be generated in situ by upregulating the reprogramming transcription factor Kruppel-like factor 4 (KLF4) in differentiated smooth muscle cells [50]. Additionally, post-translational modification by SUMOylation (small ubiquitin-like modifier) has an important effect on ESC renewal and differentiation [50]. Nanog is essential for maintaining the pluripotency and self-renewal of hES; Oct4 and Sox2 repress Nanog expression [50].
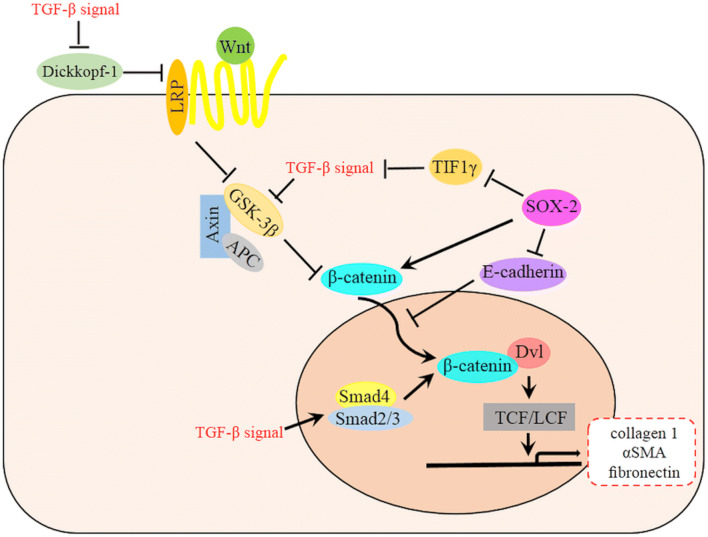
Fig. 1.
A schematic diagram of the relationship between Wnt and TGF-β signaling pathway in EMT. TGF-β signal is involved in many steps of Wnt signaling pathway, which ultimately accumulate β-catenin and then promote the EMT and fibrosis. TGF-β mediates decreased expression of the Wnt antagonist Dickkopf-1 via a p38-dependent manner while also stimulates Wnt signaling by inhibiting GSK-3β or promoting Smad4 and Smad2/3 complex to increase β-catenin in the cytoplasm and nucleus. TGF-β signal is also activated by SOX2 through the repression of TIF1γ. SOX2 could also promote the Wnt signaling pathway by transcriptional enhancement of β-catenin [46]
ΔNp63, a member of the p53 family that lacks the N-terminal transactivation domain, is hypothesized to regulate the development of ectoderm and epithelium through the balance of stemness, differentiation, and quiescence via the epithelial-mesenchymal transition [51, 52]. ΔNp63, a target of the PI3K pathway, is barely detectable in healthy endometrium yet is significantly upregulated in endometrial epithelial cells of patients with AS [52]. Mature epithelium primarily expresses ΔNp63, and Oh et al. demonstrated that retroviral transduction of ΔNp63α into rapidly proliferating human keratinocytes led to epithelial-mesenchymal transition and acquisition of stem-like properties [51]. Zhao et al. demonstrated that in patients with AS, Nanog, W5C5 (SUSD2), and SSEA-1 were downregulated on all ΔNp63+ cells in the endometria, while KLF4 and LGR5 were upregulated, suggesting that ΔNp63 plays a pathologic role by disrupting the normal regenerative properties of the endometrium and keeping it quiescent [52]. Hence, Sox2 and Oct4 were not found in these patients either. Their group investigated the effects of co-culturing bone marrow mononuclear cells (BMNCs) with ΔNp63+ epithelial cells of the endometrium. Expression of ΔNp63 mRNA was abated in the presence of BMNCs, suggesting that BMNCs have a modulatory effect to counteract the quiescence induced by ΔNp63 [52].
Mesenchymal-to-epithelial transition
One of the key capacities of stem cells is cellular trans-differentiation, whereby one somatic mature cell can transform into another without undergoing an intermediate pluripotent state. The mesenchymal-to-epithelial transition (MET) is one such example and plays another crucial role in endometrial epithelial repair. This well-described phenomenon is critical during embryogenesis, menstruation, and postpartum, the three periods of a woman’s life when uterine regeneration is significant [49, 53]. For instance, during embryonic development of genital tissues, stromal (mesenchymal) cells adopt an epithelial phenotype [49]. Patterson et al. proposes that prenatal developmental programming of the uterus remains intact in the adult for times in need of regeneration. Müllerian duct cells are known to have meso-epithelial properties as seen by their positive staining for cytokeratin, an epithelial marker, and vimentin, a mesenchymal marker. As the ducts develop, vimentin expression decreases, cytokeratin expression is maintained, and E-cadherin expression, an epithelial cell marker, increases. This change in expression of mesenchymal and epithelial cell markers is indicative of the MET in embryonic development [54]. Using genetic fate mapping, stromal cells have been identified in the endometrial epithelium in postpartum mice using LRC [54]. During endometrial regeneration, a unique population of pan-cytokeratin-expressing cells exists within the stroma and is shown to move from the stromal-myometrial border to the luminal interface where the LE will re-form. These cells do not appear to originate from the GE, as they do not connect to glands at any point during the transition.
Epithelial-to-mesenchymal transition (EMT), the counterpart to MET, is implicated in tumor metastases and dedifferentiation, whereby cancer cells transition from an epithelial to mesenchymal phenotype [55]. This involves signal transduction pathways, such as receptor tyrosine kinases and TGF-β receptors, and transcriptional repressors of the E-cadherin gene allow for the cancer cells to lose the epithelial phenotype. Similarly, altering p63 level in normal human epidermal keratinocytes has been described as a method of inducing mesenchymal multipotency [51]. Zhao et al. showed that the EMT was accelerated when cells transduced with a vector expressing ΔNp63 were treated with TGF-β. The TGF-β signaling cascade leads to EMT through activation of ERK MAPK, Rho GTPases, and the PI3K/AKT pathways via upregulation of Snail and ZEB family proteins, key TFs involved in EMT [52]. The EMT may be induced by other pathways too, including the Smad cascade, whereby TGF-β leads to phosphorylation and nuclear translocation of Smad 2/3 [52].
Immunomodulatory and antimicrobial effects of stem cells
Besides for trans-differentiation and paracrine signaling through chemokines, stem cells also aid in the regenerative process by immunomodulation. Mesenchymal stem cells suppress both the innate and adaptive immune systems. In the innate system, MSCs inhibit NK cell activation, proliferation, and cytotoxicity [34]. In the adaptive system, MSCs inhibit B and T cell proliferation and differentiation into pro-inflammatory Th1 and Th17 cells as well as to “tolerogenic” Treg cells, which are suppressor T cells that downregulate effector T cells [34]. On the contrary, MSCs secrete a multitude of cytokines and signaling molecules that promote growth, such as IGF-1, TGF-β, bFGF, HGF, IL-6, SDF-1, M-CSF, VEGF, PIGF, and MCP-1 [34]. They cause mature type I dendritic cells (DC) to decrease TNF-α secretion and type II DCs to increase IL-10 secretion; they cause Th1 and NK cells to decrease IFN-γ and Th2 cells to increase IL-4; and they cause an increase in the proportion of Treg cells when co-cultured with immune cells [56]. Interestingly, MSCs have been shown to have the capacity to polarize into a pro-inflammatory phenotype, pending interaction with IFN-γ and LPS, or they can exhibit an anti-inflammatory phenotype, pending interaction with IL-4 [57]. MSCs express toll-like receptors (TLR-3,4) as well, and their migration, invasion, and secretion of immune-modulating cytokines depend on the ligand that binds the TLR. When MSCs bind TLR4, pro-inflammatory cytokines are secreted, yet anti-inflammatory cytokines are secreted when MSCs bind TLR3 [57]. Moreover, co-culturing TLR3-primed MSCs with peripheral blood mononuclear cells led to anti-inflammatory markers being expressed and vice versa with TLR4-primed MSCs [57].
Stem cells have been shown to upregulate anti-inflammatory cytokines, e.g., IL-2, IL-4, IL-10, and TGF-β, as well as downregulate pro-inflammatory cytokines, e.g., IL-1, IL-6, IL-17, and TNF-α, depending on their surrounding niche [39]. In a pro-inflammatory environment with high concentration of TNF-α and IFN-γ, MSC secrete anti-inflammatory mediators, e.g., IL-10, TGF-β, PGE2, HLA-G, IDO, and become MSC-2, which suppress dendritic, T, B, and NK cell activation [26]. On the other hand, when LPS activates TLR-4 on MSC, the MSC adopts a pro-inflammatory phenotype (MSC-1) and recruits immune effector cells [57]. Zhao et al. corroborated the immunomodulatory effects of stem cells by injecting bmMSCs directly into the uterine cavity of mice. This induced the same effects as the study by Gan et al.: (1) higher expression of cytokeratin, vimentin, integrin αγβ3, and leukemic-inhibiting factor (LIF); (2) a greater amount of anti-inflammatory cytokines (bFGF, IL-6); and (3) a lower expression of pro-inflammatory cytokines (TNF-α, IL-1) [52, 58]. Another study noted a significantly decreased concentration of macrophages in the endometrium of patients with AS, which was hypothesized to be due to decreased levels of CSF-1 [59].
Menstrual stem cells have demonstrated immunomodulatory effects via inhibiting the generation and maturation of monocytes into dendritic cells. Dendritic cells (DC), or antigen-presenting cells, play a key role in directing the T cell response toward a Th1, Th2, Th17, or Treg pathway [60]. Menstrual stem cells prevent monocytes from maturing into immature DCs, thereby inhibiting an allogeneic mixed lymphocyte reaction. This is partly due to increased IL-6 and IL-10 levels. Additionally, one of the main reasons menstrual stem cells are not immunogenic when transplanted is due to the weak expression of HLA-DR.
G-CSF, or stem cell releasing factor, has also been studied for its stimulatory effect on stem cell recruitment as well as immunomodulation in injured tissue. Schneider et al. demonstrated the immunomodulatory effects of filgrastim, a recombinant human G-CSF analog, in 60 patients undergoing elective surgery. They demonstrated a blunted response to trauma: a greater increase was noted in anti-inflammatory mediators, such as TNF-R p55/p75 and IL-1ra (antagonists to TNF-α and IL-1), than in the pro-inflammatory cytokines, such as their ligands [61]. As such, studies then assessed these effects in the endometrium of mice after injection of G-CSF. Sabry et al. compared MSC to G-CSF and estrogen and assessed the extent of fibrosis, vascularization, and inflammation. The mice that received a combination of MSCs and G-CSF had the greatest attenuation of fibrosis and stimulation of angiogenesis, as measured by IL-1, IL-6, TNF-α, VEGF, RUNX, TGF-β, and CTGF. The effects were significantly improved compared to all the other groups, including those that received MSCs and estrogen together, MSC alone, or G-CSF alone [62]. G-CSF modulates the inflammatory cascade, shifting the response toward the anti-inflammatory state.
In addition to immunomodulatory effects, stem cells also have antimicrobial activity. MSCs secrete the antimicrobial factors lipocalin-2 and indoleamine 2,3-dioxygenase (IDO) in response to LPS [63–65]. MSC in equine endometrium expressed lipocalin-2 at higher concentrations than MSC in bone marrow or adipose tissue [64]. Unstimulated MSCs have been shown to inhibit the growth of gram-negative and gram-positive bacteria by secreting the cathelicidin peptide LL-37, which disrupts the bacterial cell membrane [66]. The antibacterial properties of umbilical cord and bone marrow-derived MSC have been validated via their secretion of β-defensin-2 and LL-37, respectively [64]. Besides for killing microorganisms, LL-37 also has chemotactic, immunomodulatory, angiogenic, reparative, and anti-apoptotic effects [66]. MSCs stimulated with IFN-γ have been found to secrete the tryptophan-catabolizing enzyme IDO, which depletes tryptophan, leading to accumulation of toxic kynurenines, thereby inhibiting bacterial growth [65]. Domnina et al. demonstrated an enhanced immunomodulatory effect with MSCs organized in spheroids compared to monolayers. The spheroids revealed an enhanced paracrine secretion of TNF-α-stimulated gene (TSG-6, an anti-inflammatory protein), hepatocyte growth factor (HGF, an anti-apoptotic and pro-angiogenic protein), and prostaglandin receptor 2 (EP2) [67]. Menstrual blood stem cells also suppress inflammation by secreting immunomodulatory factors, e.g., IL-6, IL-8, monocyte chemoattractant protein (MCP-1), chemokine ligand-5 (CCL5), and TLR-4, thereby decreasing proliferation and activation of CD4+ T cells, downregulating central memory T cells, and upregulating effector memory T cells [64, 68]. They also inhibit monocyte differentiation to immature dendritic cells, likely due to upregulation of IL-6 and IL-10 [60].
Menstrual blood-derived stem cells
Menstrual blood stem cells are a source of endometrial mesenchymal stem cells and have the added benefits of being easily and ethically attainable, genetically stable, and highly proliferative without being tumorigenic or immunogenic [67]. Menstrual blood stromal fibroblasts exhibit stem cell-associated anti-inflammatory and immunomodulatory properties [26]. These MSCs are a potential autologous source of therapy for uterine injuries, i.e., Asherman syndrome, and have been studied in both preclinical models and clinical trials.
Menstrual stem cells (menSC) are also known as endometrial regenerative cells but are phenotypically different than endometrial stem cells. They are broadly multipotent, with the capability of becoming endothelial, cardiomyocytic, neurocytic, cartilaginous, myocytic, respiratory epithelial, pancreatic, hepatic, adipocytic, and osteogenic lines as well as germ cells and endometrial cells [69, 70]. They are defined by these characteristics: (1) must be derived from menstrual fluid rather than the endometrium; (2) express the cell-surface markers CD9, CD29, CD44, CD49f, CD73, CD90, CD105, and CD166, HLA-ABC, and lack hematopoietic/endothelial markers; and (3) can be cultured and passaged in plastic-adherent containers and differentiate into adipocytes, chondrocytes, and osteocytes [71]. MenSC also have embryonic stem cell markers Oct-4 and SSEA-3/4 and eMSC markers CD146 and PDGFR-β [22, 26]. Expression of the embryonic markers c-Kit (CD117) and SSEA-4 is controversial though as some studies have not found expression of these markers on menSC [71]. They exert their effects on injured tissue in a similar manner to other stem cells: differentiation, immunomodulation, paracrine signaling, and homing and engrafting into target tissue. MenSC have been shown to inhibit cell death of neurons in rats through secretion of VEGF, BDNF, and NT-3 in vitro [71]. They can differentiate into various cells, and they can be reprogrammed to become iPSC to have a broader differentiation capacity [72] (Fig. 2). MenSC have demonstrated the capacity to differentiate into endometrial cells in vitro when cultured with TGF-β, 17β-estradiol, PDGF, and EGF; moreover, they create endometrial tissue in NOD-SCID mice in vivo [73]. They have also been found to have strong effects on inhibition of fibrosis. Zhu et al. extracted menSC from the proliferative phase of three healthy premenopausal females and cultured them with endometrial stromal cells. Their group noted an inhibition of myofibroblast activity by downregulating α-SMA and collagen I, thereby resulting in more rapid proliferation of endometrial stromal cells [74]. They concluded that menSC suppress endometrial myofibroblast differentiation by inducing the Hippo/TAZ pathway, which exports TAZ, a transcriptional regulator, from the nucleus. Subsequently, rapid proliferation of endometrial stromal cells and inhibition of TAZ led to attenuation of TGF-β and restoration of the cell’s natural wound healing ability [74].
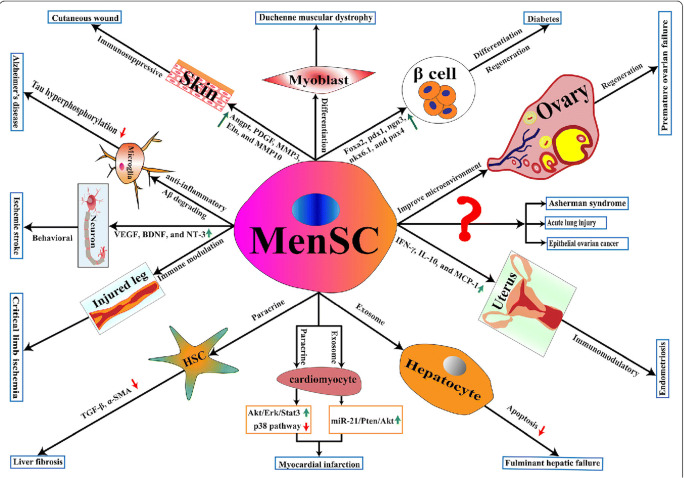
Fig. 2.
The effect of menstrual blood-derived stem cells on different tissues. Adopted from: Chen, Lijun et al. “The multi-functional roles of menstrual blood-derived stem cells in regenerative medicine.” Stem Cell Research & Therapy (2019) [71]. The Creative Commons license: https://creativecommons.org/publicdomain/zero/1.0/legalcode
MenSC isolated from humans express lower levels of Oct-4 and have worse cloning efficiency in patients with IUA compared to controls. When transplanted into NOD-SCID mice induced with AS, menSC have rebuilt the endometrium. Differentiation was tracked by detecting cytokeratin and vimentin expression by immunocytochemistry, which was significantly greater after induction with cytokines [73].
Autologous menSC transplantation has been studied in humans with AS. Tan et al. experimented with menSC to determine their effect on pregnancy and endometrial growth in patients with severe AS. MenSC were extracted from the menstrual effluent of seven infertile women with severe IUA who had already failed traditional management with adhesiolysis and hormone therapy. The stem cells were cultured and injected into the uterus. Subsequently, hormone therapy with estradiol was administered; progesterone was added if the endometrial thickness (ET) was < 7 mm. All seven patients had a significant increase of endometrial thickness after menSC transplant; in regard to pregnancy outcome, one patient with infertility for 3.5 years and grade V IUA had conceived spontaneously after transplant. Two others had conceived with IVF, one of which ended in a viable birth [22].
Amniotic fluid-derived stem cells
Stem cells from amniotic fluid have also been extracted and studied for the treatment of Asherman syndrome in preclinical models. Similar to menstrual blood stem cells, human amniotic fluid stem cells (AFSC) have the benefit of being ethically unrestricted (since they are not embryonic), easily extracted, multipotent, non-tumorigenic, and immunomodulatory. They are described as an “intermediate” stem cell as they express markers of both embryonic (Oct-4, c-Myc, Sox2, Nanog, ALP, SSEA3/4, CD117) and adult (CD29, CD90, CD105, CD44, CD73, CD324) stem cells, and they secrete transcription factors of pluripotency (Oct-4, Nanog, SSEA-3/4, ALP, SCF, Sox2) as well as mesenchymal commitment (vimentin, keratin, fibronectin); they can also be reprogrammed to become iPSC [75, 76]. AFSC can differentiate into mesodermal (bone, fat, cartilage, hematopoietic) and non-mesodermal (endothelial, hepatic, neuronal) lineages [76]. Early pregnancy (first trimester) AFSC have a less differentiated phenotype than AFSC extracted in the latter portion of pregnancy, as demonstrated by their embryonic surface markers (SSEA3/4, Tra-1-60, Tra-1-81, ALP) and expression of TFs, i.e., Oct-4, c-Myc, Sox2, and Nanog [76]. CD117/SSEA3+ AFSC have similarities to primordial germ cells, such as expression of c-Kit, FGF-8, Sox17, STELLA, DAZL, NANOS, VASA, SSEA1, FRAGILIS, and PUM2 [76]. First trimester AFSC also express high levels of several MSC markers, i.e., CD29, CD44, CD73, CD90, and CD105. In contrast, AFSC extracted in the latter portion of pregnancy express c-Myc and Oct-4 but not Nanog or KLF4. They are also positive for SSEA4 but not for SSEA3, Tra-1-60, Tra-1-81, or ALP. This suggests that second and third trimester AFSC are more mesodermally committed, yet they retain some embryonic stem cell-like properties [76].
Stem cells from amniotic fluid have been used in preclinical models for tissue engineering and regeneration. Some examples of their effects include shortened healing time in fetal lambs with intentional skin wounds, improved epidermal regeneration at intentional excisional wounds in mice, clustering of neural placodes in fetal lambs with neural tube defects, as well as tissue engineering for body-wall defects, myelomeningocele, bladder exstrophy, and congenital diaphragmatic hernia [77]. To date, however, there are no clinical data examining the effects of amniotic fluid stem cells in humans for the prevention or treatment of AS.
Bone marrow-derived stem cells
The role of bone marrow stem cells on uterine regeneration has been elucidated by many studies seemingly due to their extensive investigation in other disorders. Bone marrow-derived stem cells are the most common source for clinical transplantation. However, the process of extraction, the risks of complications, and the age-dependent decline of their self-renewal capacity limits their use and hinders clinical trials. Bone marrow-derived stem/progenitor cells include HSCs and MSCs, which migrate to injured sites and participate in tissue regeneration via engraftment, secretion of cytokines, recruitment of other cells, and trans-differentiation. It is known that irradiation, chemotherapy, and hypoxia all stimulate stem cell recruitment for tissue repair. Moreover, SDF-1, the ligand for CXCR-4 expressed on BMDSC, is a chemoattractant that plays a central role in the homing process. When transplanted into uteri, BMDSCs can be found in the uterine stroma as well as the epithelia. Gil-Sanchis et al. experimented with different types of bone marrow stem cells in order to compare each type with its effect on the endometrium.
The types of stem cells revealed different effects on the endometrial histology, morphology, proliferation, and apoptosis. Freshly isolated MSCs and endothelial progenitor cells (EPCs) induced a greater degree of endometrial regeneration than cultured MSCs and multipotent adult progenitor cells (MAPCs), with the EPCs contributing most to the epithelia and the MSCs contributing most to the stroma and endothelium. The mice that received cultured MSCs and Oct4− BM-MAPCs had lower proliferation indices in the uteri than the mice that received the other BMDCs. In addition, the uteri from animals treated with fresh unfractionated bone marrow (UBM), MSC, EPC, and hematopoietic progenitor cell (HPC) subpopulations and mOct4+ BM-MAPCs displayed thicker endometrium than those that received the cultured MSCs and mOct4− BM-MAPCs [42]. Similar results were seen in regard to gland concentration. In terms of engraftment into the uterus, a smaller proportion of the cultured stem cells reached the target tissue than the fresh ones. The loss of cell surface receptors in cultured cells is likely the reason for this difference [42, 78].
Overcoming the hurdle of stem cell engraftment in damaged tissue has been a major barrier to transplants for endometrial regeneration. Preparing MSCs in a 3D culture, or spheroid, has been shown to increase the survival and efficacy of stem cells as well as their expression of angiogenic and anti-inflammatory factors [63]. Domnina et al. demonstrated similarities and differences between spheroids and monolayers. Both express CD140b, CD105, CD73, CD90, CD44, HLA-1, and lack CD34, CD45, and HLA-DR. However, spheroids lack CD146. Spheroids upregulate pluripotency genes Nanog and Sox2 but less so than hES [63]. Their group also demonstrated improved fertility outcomes in the mice transplanted with spheroid eMSCs compared to those that received bone marrow or monolayer eMSC transplantation.
Many preclinical models have demonstrated significant improvements in mice with iatrogenic AS treated with stem cells. Cervelló et al. investigated the engraftment and proliferation of human bmMSC in mice. CD133+ bmMSC were extracted from humans with Asherman syndrome and labeled with super-paramagnetic iron oxide nanoparticles (SPIO). CD133, also known as VEGF-R2, is a marker expressed in immature hematopoietic and progenitor cells with high proliferative activity as well as circulating cells with endothelial regenerative capacity [24]. These stem cells were injected into mice either directly into their uterus or systemically, with the thought that direct injection may improve stem cell homing to the injured tissue. Quantitative PCR was performed to indicate paracrine-signaling genes, e.g., BMP-6, PDGF-β, thrombospondin-1, TNF-α, and IGF-1. Four parameters were measured: (1) the accumulation of collagen and glycosaminoglycan deposits detected by trichrome staining, (2) the percentage and localization of engrafted bmMSC by Prussian blue stain, (3) cell proliferation using Ki-67, and (4) paracrine factors by RT-PCR. The induction of Asherman syndrome was confirmed with visualization of the fibrotic factors, i.e., collagen and glycosaminoglycan. As a result, there was a significant increase in cell proliferation and paracrine factors in the treated damaged horns relative to the untreated damaged horns, regardless of administration modality. This corroborates the notion that uterine injury recruits stem cells following an increase in paracrine signals. Furthermore, these stem cells were not found in other organs. This suggests three key points: (1) the paracrine factors are responsible for recruitment of stem cells to the site of injury, (2) the stem cells induce proliferation of the neighboring endometrial cells indirectly, and (3) the stem cells are directed to the damaged tissue [24]. The engrafted cells were mostly localized around the endometrial blood vessels, thereby inducing proliferation of the surrounding cells through paracrine signals. The limitation of this study was its use of NOD/SCID mice, so research is needed to see if the same effects would occur in mice with a functional immune system.
Liu et al. experimented with two types of stem cells in mice with a mild uterine injury but no adhesions. Stem cells were obtained from the uterus and bone marrow of GFP-labeled mice. A comparison was done between local and systemic administration after injuring only one of the uterine horns. Mice that were injected intravenously with bone marrow-derived stem cells (BMDSCs) or uterine-derived stem cells (UDCs) had an increased recruitment of GFP+ cells to either an injured or non-injured horn compared to those that were injected directly into the uterus. Less than 1% of the endometrial cells were labeled with GFP, implicating the strong effect these cells have despite their paucity [79]. Systemic injection of BMDSCs led to greater recruitment of GFP+ cells to both the non-injured and injured horns compared with local injection at 2 and 3 weeks post-injection. Systemic injection of UDCs demonstrated greater recruitment of GFP+ cells compared to local injection at 3 weeks post-injection. Their group concluded that systemic injection is superior to local injection in homing stem cells to the uterus. Additionally, there was no difference in cell recruitment between the injured and non-injured horns. This implies that homing of stem cells occurs diffusely in the uterus rather than specifically to the site of injury [79].
Another study by Zhao et al. demonstrated encouraging effects of bmMSC transplantation not only on endometrial regeneration but also on its receptivity. Rats were administered ethanol in the uterine cavity to induce thin endometrium. Bone marrow mesenchymal stem cells were transplanted directly into the uterus; saline was injected into the control. The expression of integrin αγβ3 and LIF was significantly greater in rats with thin endometrium that received bone marrow stem cells compared to those that did not [80]. In addition, the endometrial thickness was significantly thicker, pro-inflammatory cytokines (IL-1β, TNF-α) were attenuated, anti-inflammatory cytokines (IL-6, bFGF) were upregulated, and markers of endometrial regeneration (cytokeratin, vimentin) were significantly greater in the treatment group compared to the control.
Many factors play a role in stem cell recruitment and engraftment to the endometrium, but the reasons for the difference between UDCs and BMDSCs have not been clearly elucidated. Santamaria et al. proposes that UDCs are partially differentiated toward an endometrial cell line and thus have a narrower potency than BMDSCs, which likely have a broader potency [21]. In regard to the mode of injection, the engraftment of the stem cells requires homing to their niche environment, and this is propagated via the circulation due to the strong effect of paracrine signals. Homing is the key aspect to stem cell efficacy since their effect is substantiated only if appropriately located. Hence, systemic injection of BMDSCs led to a greater recruitment of GFP+ cells compared to UDCs [21]. It is hypothesized that blood provides the stem cells with various trophic factors, which may enhance their survival when injected systemically as compared to local injection into the uterus [79].
BMDSCs have been used in clinical trials for regenerative therapy and have been shown to contribute to neo-angiogenesis during wound healing and limb ischemia, post-myocardial infarction, atherosclerosis, and tumor growth [21]. Santamaria et al. piloted the first clinical study of BMDSC transplantation in patients with AS. Eleven patients with refractory AS and five with endometrial atrophy had their own peripheral blood bone marrow-derived stem cells extracted using G-CSF for hematopoietic mobilization. CD133+ cells were isolated through apheresis. These autologous BMDSCs were then injected into the spiral arterioles by catheterization via interventional radiology into the 11 patients with AS. All patients were prescribed HRT before and after the transplantation. Endometrial effects were then assessed by resumption of menses, endometrial thickness, adhesion score, neo-angiogenesis, and ongoing pregnancy rate. Menstrual history was recorded with a diary; endometrial cavity was assessed by hysteroscopy 3 and 6 months later; transvaginal ultrasound and endometrial biopsies were used for endometrial thickness and histology; lastly, patients were invited to undergo ART. Neo-angiogenesis was assessed using immunofluorescence for CD31 and α-SMA, markers of angiogenesis. After transplantation, menstrual cycles resumed with HRT within 1 month in all the patients, except for one with endometrial atrophy. All patients with stage III IUA improved to stage I, one patient with stage II showed a normal cavity, and the other with stage II improved to stage I post-transplant. There also was a significant increase in the total number of mature blood vessels as detected by CD31+/α-SMA+ cells. In regard to functional status, 3 patients became pregnant spontaneously and 7 pregnancies resulted from 14 embryo transfers.
Umbilical cord-derived stem cells
Mesenchymal stem cells have also been derived from the umbilical cord (UC-MSC), and they express similar properties to placental MSCs: intermediate differentiation potential between embryonic and adult stem cells. They share common surface markers as other MSCs, i.e., CD29, CD44, CD73, CD90, and CD105, and lack CD31, CD45, and HLA-DR85. They express the pluripotency markers Sox2 and SSEA-4. Similar to other adult stem cells, UC-MSC are ethically unrestricted as they are normally discarded after birth. They are easy to harvest, and they are non-tumorigenic and non-immunogenic because they lack MHC-II and other co-stimulatory molecules. However, they are primarily hematopoietic lineage cells with < 1% multipotent cells [77]. They do express HLA-G though, which is a protein that induces the expansion of Treg cells and suppresses cytotoxic T cells and NK cells [81]. They have demonstrated stronger proliferation, differentiation, and migration abilities than bmMSC [82]. MSC can be isolated from the umbilical cord lining, subendothelial layer, perivascular zone, and Wharton’s jelly, the gelatinous matrix. UC-MSC have been used for therapy in animal models with ovarian and endometrial dysfunction [83]. Yang et al. first demonstrated the effects of UC-MSC on the proliferation, fibrosis, angiogenesis, and apoptosis of damaged endometrial stromal cells via secretion of VEGF. They noted a significant increase in proliferation and decrease in apoptosis in the stromal cells damaged by mifepristone that were co-cultured with UC-MSC compared to the controls [84]. This suggests that the UC-MSC can potentially prevent the damage to the endometrium by mifepristone. The UC-MSC also increased expression of VEGF and decreased expression of caspase-3,8,9 [84]. Zhang et al. examined fertility outcomes, endometrial morphology, angiogenesis, and cell proliferation in mice induced with AS. UC-MSC were injected systemically, and their group noted decreased fibrosis markers (α-SMA, TGF-β), increased cell proliferation (Ki-67), increased stromal and epithelial markers (vimentin, cytokeratin), increased vascular markers (CD-31, VEGF, MMP9), and decreased pro-inflammatory markers (IFN-γ, TNF-α, and IL-2) [85]. Shi et al. demonstrated the capacity of UC-MSC to differentiate into endometrial epithelial and stromal cells via different induction systems. The cAMP/PKA pathway was significantly involved in the differentiation of UC-MSC into endometrial stromal-like cells [82].
Recently, extracellular vesicles (EV), vesicles discharged from the cell membrane into the environment, have been found to play a role in paracrine signaling. Ebrahim et al. looked at the effect that vesicles derived from human UC-MSC (hUCMSC-EV) had on endometrial regeneration. Rats induced with IUA were treated with estrogen alone, hUCMSC-EV alone or the combination. The inflammatory cytokines (TGF-β, IL-1, IL-6, and TNF-α) were downregulated, and the stem cell marker (CD140b) was upregulated in the mice that received hUCMSC-EV alone or in combination with estrogen compared to the control mice with IUA but no treatment [86]. In addition, RUNX2, an anti-fibrotic factor, was significantly increased in the groups that received hUCMSC-EV, either alone or in combination. Estrogen treatment alone had no effect on the expression of inflammatory cytokines and fibrotic factors.
In humans, a landmark prospective phase I clinical trial has been performed in 26 women with recurrent IUA. UC-MSCs loaded onto a collagen scaffold were transplanted into the uterus following adhesiolysis. Three months later, the maximum endometrial thickness increased, the intrauterine adhesion score decreased, markers of proliferation (ER-α, vimentin, Ki-67, vWF) increased, ΔNp63 decreased, and fertility outcomes improved (10 of the 26 patients became pregnant, 8 live births were documented) [41]. This suggests that UC-MSC can be used as an adjunctive treatment in patients with refractory AS in a safe and efficacious manner.
Placenta-derived stem cells
Similar to other regenerating organs, the placenta is a rich source of stem cells. There are four types of placenta-derived stem cells: human amniotic epithelial (hAEC), amniotic mesenchymal stromal (hAMSC), chorionic mesenchymal stromal (hCMSC), and chorionic trophoblastic (hCTC) [87]. Some studies also include stem cells in the umbilical cord blood and tissue as well as amniotic fluid as derivations of the placenta. The amniotic membrane consists of two layers: a single layer of amniotic epithelial cells attached to the basement membrane and an outer mesenchymal layer containing fibroblasts and MSC. Stem cells have been isolated from both layers, and there is no ethical restriction in their use. Both fetal and maternal stem cells have been found in terminal chorionic villi. Amniotic epithelial stem cells can differentiate into the three germ lines and have demonstrated a higher proliferative rate than adult MSCs. They express the embryonic antigen SSEA-3/4 in addition to the pluripotency markers Sox2, Oct4, and Nanog. The chorion consists of the mesenchyme and trophoblast layers that encapsulate the amniotic tissue and embed into the maternal decidual layer. Human CMSC show greater immunosuppressive and angiogenic potential compared to umbilical cord tissue and decidual cells [87]. Mesenchymal cells isolated from fetal membranes (amnion, chorion, umbilical cord) should be termed mesenchymal stromal cells, either amniotic or chorionic, and should be defined by the same parameters as other MSC, i.e., adherence to plastic, formation of fibroblast CFUs, differentiation potential toward one or more lineages (osteocyte, chondrocyte, adipocyte, endothelial), and expression of specific cell-surface markers (positive for CD73, CD90, and CD105; negative for CD14, CD34, CD45, and HLA-DR) [87]. They have also been shown to differentiate into neurogenic, myogenic, cardiogenic, hepatic, and pancreatic lineages using specific induction methods.
MSC have been cultured from chorionic villi as early as the first trimester. Early pregnancy chorionic villi show marked differences in cell marker expression, proliferation, differentiation potential, and functional activity compared to term placenta MSC [88]. Placental MSC contribute to the development and function of the placenta through vasculogenesis as soon as 2 weeks post-fertilization when HSCs aggregate into cell cords [88]. The HSCs then differentiate into CD34+ endothelial cell cords and form a network of vascular structures during the seventh week of gestation, which is known as angiogenesis. Endothelial progenitor cells deriving from the bone marrow contribute to angiogenesis in adults but have not been detected in the placenta. Rather, placental MSC (PMSC) form the non-trophoblast cells, and trophoblast stem cells (TSC) form the differentiated lineages of the placenta [88]. The process of vasculogenesis (de novo synthesis of blood vessels) is mediated by chemokines secreted from the trophoblast, e.g., VEGF, FGF, PDGF, and angiopoietins, whereby the placental MSCs differentiate into endothelial cells [88]. TSCs in mice express the TFs CDX2, ELF5, and EOMES. In humans though, a different gene profile is expressed. CDX2, a totipotent marker, and GATA3, a transcription factor, are specifically expressed in the pre-implantation trophectoderm of human embryos, with Oct4 concomitantly overlapping expression in the inner cell mass; CLDN10, PLAC8, and TRIML1 are also only found in humans [89]. Studies have demonstrated enhanced endothelial cell viability, migration, and tube formation when incubated in the conditioned medium from cultured PMSCs via upregulation of pro-angiogenic proteins, e.g., angiogenin, angiopoietin-1/2, GRO, IL-6, IL-8, MCP-1, thrombopoietin, TIE-2 (angiopoietin receptor), TIMP-1/2 (tissue inhibitor of matrix metalloproteinase), and VEGF [90].
PMSCs demonstrated stronger effects on angiogenesis, endothelial cell migration, and network formation in comparison to amnion-derived avascular MSCs [90]. One study analyzed the effect of exosomes secreted by PMSCs and noted their incorporation into endothelial cells and subsequent stimulation of angiogenic gene expression [91]. The exosomes upregulated the transcriptional activity and mRNA expression of Oct4 in fibroblasts in vitro as well as the blood flow of mice with auricular wounds in vivo [91].
Considering that the placenta is hypothesized to account for the maternal immunologic tolerance toward the developing allogeneic fetus, PMSCs demonstrate significant immunomodulatory effects. They suppress the proliferation and secretion of IFN-γ from activated T lymphocytes [92]. In addition, they secrete PDL1, an immunosuppressive chemokine that inhibits T cell proliferation, as well as SDF-1 and SCF (stem cell factor, the antigen binding the c-kit receptor CD117), two chemokines that recruit CD34+ cells [92]. PMSCs also inhibit the proliferation of peripheral blood mononuclear cells when induced by CD3 or by mixed lymphocyte reaction (MLR) [90]. A subpopulation of chorionic villi-derived MSCs can be identified by VCAM-1 (vascular cell adhesion molecule, also known as CD106), a biomarker with unique immunosuppressive activity [93, 94]. VCAM-1 is extensively expressed on endothelial cells as well as some stromal cells existing in a vascular niche. It has been shown to regulate embryonic development, as mice deficient with VCAM-1 die early or display placental dysfunction. VCAM-1+ MSCs express angiogenic genes (CXCL-1,3,5,6 and CCL-7) and proteases (MMP-1,2) as well as secrete angiogenic cytokines and growth factors, e.g., IL-6 and IL-8, HGF, VEGF-A, FGF, TGF-β1,3, HIF1A, and ANGPTL2 [94].
In preclinical models, the role of PMSCs in tissue regeneration and wound healing in mice has been studied extensively. Transplantation of PMSCs in mice has demonstrated functional improvement on hindlimb ischemia. Gan et al. transplanted human amniotic mesenchymal stem cells (hAMSC) into mice and analyzed the endometrial thickness, glandular concentration, and areas of fibrosis. Engraftment of hAMSC was detected using anti-human nuclear antigen-positive cells in the endometrial glands. The mice that received the transplant showed significantly decreased levels of pro-inflammatory cytokines (TNF-α, IL-1β), increased levels of anti-inflammatory cytokines (bFGF, IL-6), and an increase in endometrial epithelial cells compared to the controls [57].
Li et al. experimented with human amniotic epithelial cells (hAEC) in mice and found increased expression of VEGF, proliferating cell nuclear antigen (PCNA), and ER, as well as thicker endometrium, greater concentration of glands, decreased amount of fibrosis, and increased microvasculature in the treatment group. Fecundity and pregnancy outcomes were also improved in the mice with iatrogenic Asherman syndrome [95]. Microvessel density (MVD) using vWF (von Willebrand factor, an endothelial marker) was lower in the IUA group compared to the group without IUA. MVD using vWF and VEGF was increased in the hAEC-treated group compared to the untreated group with IUA. PCNA expression, noting cell proliferation, was decreased in the IUA group and increased in the hAEC-treated group. Moreover, ER and PR expression were significantly increased in the hAEC-treated group compared to the control. HAECs also had improved effects on autophagy, programmed cell death. LC3, an indicator of autophagy, was significantly upregulated in the hAEC-treated group. Contrastingly, p62, an indicator of autophagy flux inhibition, was significantly downregulated in the hAEC-treated group [93]. The hAEC were then co-cultured with menSC and restored their normal morphology after exposure to hydrogen peroxide. The protein level of p62 was significantly higher in the menSC exposed to H2O2 but lower in those co-cultured with hAEC. These results indicate that hAEC could at least partly recover the damaged endometrium through autophagy induction.
In humans, placenta-derived stem cells have been used for clinical application in orthopedics [96], gastroenterology [97], cardiology [88, 98], neurology [97, 99], and recently, obstetrics [100]. In one study, patients with Crohn’s syndrome treated with placental stem cells have shown clinical improvement. In patients with sports medicine injuries, PMSCs have demonstrated benefit in regard to tibial and Achilles tendonitis, plantar fasciosis, and osteoarthritis [96]. There are ongoing clinical trials investigating the effects of PMSC for intrauterine adhesions but there have been no data published as of February 2020.
Adipose-derived stem cells
Similar to bone marrow-derived stem cells, adipose-derived stem cells are scarce and require invasive procedures for extraction. However, adipose stem cells can be extracted by liposuction, a minimally invasive method. Mesenchymal stem cells derived from adipose tissue have similar gene expression profiles and tri-lineage differentiation potential to bone marrow-derived stem cells but are more easily accessible and more proliferative [43, 101]. Kilic et al. evaluated the capacity of adipose tissue-derived mesenchymal stem cells (adMSC) to induce endometrial proliferation and angiogenesis in mice with Asherman syndrome. Forty mice were injected with trichloroacetic acid into the uterine horn to induce fibrosis. The first group was a control. In the second, adMSCs were injected into the uterus followed by three intra-peritoneal injections as a systemic modality. The third group was given oral estrogen 1 week after stem cell transplantation, and the fourth group was administered the adMSC and estrogen simultaneously. The amount of fibrosis, vascularization, inflammation, cell proliferation, and angiogenesis was evaluated using IHC staining of VEGF, PCNA, and Ki-67. In all the treatment groups, the amount of fibrosis was diminished and the vascularization and IHC staining were increased, suggesting an increase in proliferating cells, pro-angiogenic signals, and regeneration of healthy tissue [43]. The amount of fibrosis and vascularization were roughly the same in the second and third groups, suggesting that there is not a marked difference between the group that received adMSC and the group that received oral estrogen in fibrosis, inflammation, and angiogenesis. Also, the group that received simultaneous injection of adMSCs and oral estrogen had less fibrosis and higher concentration of VEGF, PCNA, and Ki-67 than the group that only received adMSC injection. This suggests that combining adMSCs with estrogen has a synergistic effect on the regrowth of the endometrium [43].
The first clinical trial using adipose-derived stem cells investigated the efficacy of autologous stromal vascular fraction (SVF) in regenerating the endometrium of patients with severe AS [102]. Liposuction was performed on six infertile women with severe AS classified by the AFS criteria who had thin endometrium after hysteroscopic adhesiolysis followed by hormone therapy. The SVF is extracted from adipose tissue and contains heterogeneous cell populations, including MSCs, endothelial cells, pericytes, T cells, and macrophages. The patients had autologous adipose SVF transplanted into their uteri via transcervical instillation followed by a dose of steroids and antibiotics. Hormone therapy with oral estradiol valerate 6 mg for 25 days followed by medroxyprogesterone acetate 10 mg/day for 5 days was administered after transplantation to support endometrial growth. The outcomes examined were return of menstruation, endometrial thickness, and pregnancy rate with IVF. Two women who previously had amenorrhea resumed menstruation with scant bleeding, and the other three with oligomenorrhea prior to treatment had increased menstrual flow after. The endometrial thickness was significantly greater post-therapy with a mean of 6.9 mm compared to 3.0 mm. Fresh or frozen-thawed embryo transfer was then performed. One woman conceived but miscarried at 9 weeks gestation. This study demonstrates clinical efficacy of adipose-derived stem cells for increasing endometrial thickness and increasing menstrual flow in patients with severe AS. More investigation, however, is needed to qualify the improvement in pregnancy outcomes.
Somatic cell nuclear transfer-derived stem cells
Somatic stem cells can be reprogrammed to induce pluripotency via Yamanaka factors or somatic cell nuclear transfer (SCNT). In one study, mesenchymal progenitor cells (MPC) were developed using single cell-derived clonal expansion from human fibroblasts reprogrammed to pluripotent stem cells using SCNT. Jun et al. experimented with these MPCs on mice induced with AS [103]. Their group noted a significant increase in angiogenic factors during repair and remodeling, resulting in better pregnancy outcomes. They hypothesize that the therapeutic effects relate to an increase in angiogenic cytokines, e.g. HGF, IGF, ANG-1 and VEGF, as well as a diminution of pro-inflammatory cytokines, e.g., TGF-β, TNF-α, TIMP-1, and COL1A1. The advantages of using these SCNT-MPCs were clonality and proliferation resembling ESCs, low immunogenicity, and no teratoma formation. Induced pluripotent stem cells also show gene expression, genomic integrity, and DNA methylation similar to ESCs.
Conclusion
Stem cells are a promising therapeutic modality for endometrial regeneration in patients with refractory Asherman syndrome. There have been many studies demonstrating their therapeutic potential in preclinical models as well as in clinical trials. Stem cells are involved in the natural regeneration of the endometrium every menstrual cycle as well as postpartum, post-abortion, and after iatrogenic procedures that disrupt the basalis layer. Stem cells have many important functions in regeneration, including but not limited to clonality, differentiation, paracrine signaling, angiogenesis, immunomodulation, and inhibition of fibrosis. The stem cells involved in tissue repair not only originate from the injured tissue but also from other organs. Experimentation with stem cell therapy in patients and animals with Asherman syndrome is still in its infancy but has alluded to a few conclusions: (1) less differentiated stem cells may be more effective than more differentiated ones in regenerating the endometrium, (2) systemic administration of stem cells may have a greater effect on recruitment to the injured site than does local administration, (3) stem cells can be isolated from different tissues with each type having a different capacity and effect on endometrial regeneration, and (4) the stem cells exert their effects on the injured tissues via a multitude of systems, each contributing to the physiologic regeneration and inhibition of pathologic scarring. In conclusion, stem cells may be a reasonable therapy for humans with recalcitrant Asherman syndrome. More research is needed on the long-term safety profile and effectiveness for patients with Asherman syndrome.
Funding information
This work did not receive any funding.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ariel Benor, Email: ariel.benor@gmail.com.
Steven Gay, Email: steven.gay@nih.gov.
Alan DeCherney, Email: decherna@mail.nih.gov.
References
- 1.Soares SR, Barbosa dos Reis MM, Camargos AF. Diagnostic accuracy of sonohysterography, transvaginal sonography, and hysterosalpingography in patients with uterine cavity diseases. Fertil Steril. 2000;73(2):406–411. doi: 10.1016/s0015-0282(99)00532-4. [DOI] [PubMed] [Google Scholar]
- 2.AAGL Practice report: practice guidelines for management of intrauterine synechiae. J Minim Invasive Gynecol. 2010;17(1):1–7. [DOI] [PubMed]
- 3.Hu J, Zeng B, Jiang X, Hu L, Meng Y, Zhu Y, Mao M. The expression of marker for endometrial stem cell and fibrosis was increased in intrauterine adhesious. Int J Clin Exp Pathol. 2015;8(2):1525–1534. [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu Y, Hu J, Yu T, Ren Y, Hu L. High molecular weight hyaluronic acid inhibits fibrosis of endometrium. Med Sci Monit. 2016;22:3438–3445. doi: 10.12659/MSM.896028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bai X, Liu J, Cao S, Wang L. Mechanisms of endometrial fibrosis and the potential application of stem cell therapy. Discov Med. 2019;27(150):267–279. [PubMed] [Google Scholar]
- 6.Liu D, Ha C, Zhang X, Zhang Z, Liu P. Molecular implication of ADAM-15 and -17 in intrauterine adhesions. Eur J Obstet Gynecol Reprod Biol. 2013;170(1):264–269. doi: 10.1016/j.ejogrb.2013.06.036. [DOI] [PubMed] [Google Scholar]
- 7.Farhi J, Bar-Hava I, Homburg R, Dicker D, Ben-Rafael Z. Induced regeneration of endometrium following curettage for abortion: a comparative study. Hum Reprod. 1993;8(7):1143–1144. doi: 10.1093/oxfordjournals.humrep.a138208. [DOI] [PubMed] [Google Scholar]
- 8.AAGL Elevating Gynecologic Surgery AAGL practice report: practice guidelines on intrauterine adhesions developed in collaboration with the European Society of Gynaecological Endoscopy (ESGE) Gynecol Surg. 2017;14(1):6. doi: 10.1186/s10397-017-1007-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johary J, Xue M, Zhu X, Xu D, Velu PP. Efficacy of estrogen therapy in patients with intrauterine adhesions: systematic review. J Minim Invasive Gynecol. 2014;21(1):44–54. doi: 10.1016/j.jmig.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 10.Guo J, Li TC, Liu Y, Xia E, Xiao Y, Zhou F, Yang X. A prospective, randomized, controlled trial comparing two doses of oestrogen therapy after hysteroscopic adhesiolysis to prevent intrauterine adhesion recurrence. Reprod BioMed Online. 2017;35(5):555–561. doi: 10.1016/j.rbmo.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 11.Myers EM, Hurst BS. Comprehensive management of severe Asherman syndrome and amenorrhea. Fertil Steril. 2012;97(1):160–164. doi: 10.1016/j.fertnstert.2011.10.036. [DOI] [PubMed] [Google Scholar]
- 12.Pabuccu R, Atay V, Orhon E, Urman B, Ergun A. Hysteroscopic treatment of intrauterine adhesions is safe and effective in the restoration of normal menstruation and fertility. Fertil Steril. 1997;68(6):1141–1143. doi: 10.1016/s0015-0282(97)00375-0. [DOI] [PubMed] [Google Scholar]
- 13.Conforti A, Alviggi C, Mollo A, De Placido G, Magos A. The management of Asherman syndrome: a review of literature. Reprod Biol Endocrinol. 2013;11:118. doi: 10.1186/1477-7827-11-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deans R, Abbott J. Review of intrauterine adhesions. J Minim Invasive Gynecol. 2010;17(5):555–569. doi: 10.1016/j.jmig.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 15.Valle RF, Sciarra JJ. Intrauterine adhesions: hysteroscopic diagnosis, classification, treatment, and reproductive outcome. Am J Obstet Gynecol. 1988;158(6 Pt 1):1459–1470. doi: 10.1016/0002-9378(88)90382-1. [DOI] [PubMed] [Google Scholar]
- 16.Yu D, Wong YM, Cheong Y, Xia E, Li TC. Asherman syndrome--one century later. Fertil Steril. 2008;89(4):759–779. doi: 10.1016/j.fertnstert.2008.02.096. [DOI] [PubMed] [Google Scholar]
- 17.Gan L, Duan H, Sun FQ, Xu Q, Tang YQ, Wang S. Efficacy of freeze-dried amnion graft following hysteroscopic adhesiolysis of severe intrauterine adhesions. Int J Gynaecol Obstet. 2017;137(2):116–122. doi: 10.1002/ijgo.12112. [DOI] [PubMed] [Google Scholar]
- 18.Robinson JK, Colimon LM, Isaacson KB. Postoperative adhesiolysis therapy for intrauterine adhesions (Asherman's syndrome) Fertil Steril. 2008;90(2):409–414. doi: 10.1016/j.fertnstert.2007.06.034. [DOI] [PubMed] [Google Scholar]
- 19.Alawadhi F, Du H, Cakmak H, Taylor HS. Bone marrow-derived stem cell (BMDSC) transplantation improves fertility in a murine model of Asherman's syndrome. PLoS One. 2014;9(5):e96662. doi: 10.1371/journal.pone.0096662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh N, Mohanty S, Seth T, Shankar M, Bhaskaran S, Dharmendra S. Autologous stem cell transplantation in refractory Asherman's syndrome: a novel cell based therapy. J Hum Reprod Sci. 2014;7(2):93–98. doi: 10.4103/0974-1208.138864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Santamaria X, Cabanillas S, Cervello I, Arbona C, Raga F, Ferro J, et al. Autologous cell therapy with CD133+ bone marrow-derived stem cells for refractory Asherman's syndrome and endometrial atrophy: a pilot cohort study. Hum Reprod. 2016;31(5):1087–1096. doi: 10.1093/humrep/dew042. [DOI] [PubMed] [Google Scholar]
- 22.Tan J, Li P, Wang Q, Li Y, Li X, Zhao D, Xu X, Kong L. Autologous menstrual blood-derived stromal cells transplantation for severe Asherman's syndrome. Hum Reprod. 2016;31(12):2723–2729. doi: 10.1093/humrep/dew235. [DOI] [PubMed] [Google Scholar]
- 23.Nagori CB, Panchal SY, Patel H. Endometrial regeneration using autologous adult stem cells followed by conception by in vitro fertilization in a patient of severe Asherman's syndrome. J Hum Reprod Sci. 2011;4(1):43–48. doi: 10.4103/0974-1208.82360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cervelló I, Gil-Sanchis C, Santamaria X, Cabanillas S, Diaz A, Faus A, et al. Human CD133(+) bone marrow-derived stem cells promote endometrial proliferation in a murine model of Asherman syndrome. Fertil Steril. 2015;104(6):1552–60.e1–3. doi: 10.1016/j.fertnstert.2015.08.032. [DOI] [PubMed] [Google Scholar]
- 25.Gargett CE, Ye L. Endometrial reconstruction from stem cells. Fertil Steril. 2012;98(1):11–20. doi: 10.1016/j.fertnstert.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 26.Gargett CE, Schwab KE, Deane JA. Endometrial stem/progenitor cells: the first 10 years. Hum Reprod Update. 2016;22(2):137–163. doi: 10.1093/humupd/dmv051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santamaria X, Mas A, Cervelló TH, Simón C. Uterine stem cells: from basic research to advanced cell therapies. Hum Reprod Update. 2018;24(6):673–693. doi: 10.1093/humupd/dmy028. [DOI] [PubMed] [Google Scholar]
- 28.Deane JA, Gualano RC, Gargett CE. Regenerating endometrium from stem/progenitor cells: is it abnormal in endometriosis, Asherman's syndrome and infertility? Curr Opin Obstet Gynecol. 2013;25(3):193–200. doi: 10.1097/GCO.0b013e32836024e7. [DOI] [PubMed] [Google Scholar]
- 29.Taylor HS. Endometrial cells derived from donor stem cells in bone marrow transplant recipients. JAMA. 2004;292(1):81–85. doi: 10.1001/jama.292.1.81. [DOI] [PubMed] [Google Scholar]
- 30.Bianco P, Robey PG, Simmons PJ. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell. 2008;2(4):313–319. doi: 10.1016/j.stem.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ye L, Mayberry R, Lo CY, Britt KL, Stanley EG, Elefanty AG, Gargett CE. Generation of human female reproductive tract epithelium from human embryonic stem cells. PLoS One. 2011;6(6):e21136. doi: 10.1371/journal.pone.0021136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee CL, Lam KK, Koistinen H, Seppala M, Kurpisz M, Fernandez N, et al. Glycodelin-a as a paracrine regulator in early pregnancy. J Reprod Immunol. 2011;90(1):29–34. doi: 10.1016/j.jri.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 33.Masuda H, Maruyama T, Gargett CE, Miyazaki K, Matsuzaki Y, Okano H, Tanaka M. Endometrial side population cells: potential adult stem/progenitor cells in endometrium. Biol Reprod. 2015;93(4):84. doi: 10.1095/biolreprod.115.131490. [DOI] [PubMed] [Google Scholar]
- 34.Mutlu L, Hufnagel D, Taylor HS. The endometrium as a source of mesenchymal stem cells for regenerative medicine. Biol Reprod. 2015;92(6):138. doi: 10.1095/biolreprod.114.126771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nguyen HPT, Xiao L, Deane JA, Tan KS, Cousins FL, Masuda H, Sprung CN, Rosamilia A, Gargett CE. N-cadherin identifies human endometrial epithelial progenitor cells by in vitro stem cell assays. Hum Reprod. 2017;32(11):2254–2268. doi: 10.1093/humrep/dex289. [DOI] [PubMed] [Google Scholar]
- 36.Du H, Naqvi H, Taylor HS. Ischemia/reperfusion injury promotes and granulocyte-colony stimulating factor inhibits migration of bone marrow-derived stem cells to endometrium. Stem Cells Dev. 2012;21(18):3324–3331. doi: 10.1089/scd.2011.0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xiao L, Song Y, Huang W, Yang S, Fu J, Feng X, Zhou M. Expression of SOX2, NANOG and OCT4 in a mouse model of lipopolysaccharide-induced acute uterine injury and intrauterine adhesions. Reprod Biol Endocrinol. 2017;15(1):14. doi: 10.1186/s12958-017-0234-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sahin Ersoy G, Zolbin MM, Cosar E, Moridi I, Mamillapalli R, Taylor HS. CXCL12 promotes stem cell recruitment and uterine repair after injury in Asherman's syndrome. Mol Ther Methods Clin Dev. 2017;4:169–177. doi: 10.1016/j.omtm.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Azizi R, Aghebati-Maleki L, Nouri M, Marofi F, Negargar S, Yousefi M. Stem cell therapy in Asherman syndrome and thin endometrium: stem cell-based therapy. Biomed Pharmacother. 2018;102:333–343. doi: 10.1016/j.biopha.2018.03.091. [DOI] [PubMed] [Google Scholar]
- 40.Zhang Y, Lin X, Dai Y, Hu X, Zhu H, Jiang Y, Zhang S. Endometrial stem cells repair injured endometrium and induce angiogenesis via AKT and ERK pathways. Reproduction. 2016;152(5):389–402. doi: 10.1530/REP-16-0286. [DOI] [PubMed] [Google Scholar]
- 41.Wright LM, Maloney W, Yu X, Kindle L, Collin-Osdoby P, Osdoby P. Stromal cell-derived factor-1 binding to its chemokine receptor CXCR4 on precursor cells promotes the chemotactic recruitment, development and survival of human osteoclasts. Bone. 2005;36(5):840–853. doi: 10.1016/j.bone.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 42.Cao Y, Sun H, Zhu H, Zhu X, Tang X, Yan G, et al. Allogeneic cell therapy using umbilical cord MSCs on collagen scaffolds for patients with recurrent uterine adhesion: a phase I clinical trial. Stem Cell Res Ther. 2018;9(1):192. doi: 10.1186/s13287-018-0904-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gil-Sanchis C, Cervello I, Khurana S, Faus A, Verfaillie C, Simon C. Contribution of different bone marrow-derived cell types in endometrial regeneration using an irradiated murine model. Fertil Steril. 2015;103(6):1596–605.e1. doi: 10.1016/j.fertnstert.2015.02.030. [DOI] [PubMed] [Google Scholar]
- 44.Kilic S, Yuksel B, Pinarli F, Albayrak A, Boztok B, Delibasi T. Effect of stem cell application on Asherman syndrome, an experimental rat model. J Assist Reprod Genet. 2014;31(8):975–982. doi: 10.1007/s10815-014-0268-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 46.Yang, Yan-Meng, Wan-Xi Yang. Epithelial-to-mesenchymal transition in the development of endometriosis. Oncotarget 2017;8(25):41679–89. [DOI] [PMC free article] [PubMed]
- 47.Fan D, Wu S, Ye S, Wang W, Guo X, Liu Z. Umbilical cord mesenchyme stem cell local intramuscular injection for treatment of uterine niche: protocol for a prospective, randomized, double-blinded, placebo-controlled clinical trial. Medicine (Baltimore) 2017;96(44):e8480. doi: 10.1097/MD.0000000000008480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Franco HL, Dai D, Lee KY, Rubel CA, Roop D, Boerboom D, Jeong JW, Lydon JP, Bagchi IC, Bagchi MK, DeMayo FJ. WNT4 is a key regulator of normal postnatal uterine development and progesterone signaling during embryo implantation and decidualization in the mouse. FASEB J. 2011;25(4):1176–1187. doi: 10.1096/fj.10-175349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kobayashi A, Stewart CA, Wang Y, Fujioka K, Thomas NC, Jamin SP, Behringer RR. β-Catenin is essential for Müllerian duct regression during male sexual differentiation. Development. 2011;138(10):1967–1975. doi: 10.1242/dev.056143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yin Q, Jin P, Liu X, Wei H, Lin X, Chi C, Liu Y, Sun C, Wei Y. SDF-1alpha inhibits hypoxia and serum deprivation-induced apoptosis in mesenchymal stem cells through PI3K/Akt and ERK1/2 signaling pathways. Mol Biol Rep. 2011;38(1):9–16. doi: 10.1007/s11033-010-0071-9. [DOI] [PubMed] [Google Scholar]
- 51.Oh JE, Kim RH, Shin KH, Park NH, Kang MK. DeltaNp63α protein triggers epithelial-mesenchymal transition and confers stem cell properties in normal human keratinocytes. J Biol Chem. 2011;286(44):38757–38767. doi: 10.1074/jbc.M111.244939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao J, Chen Q, Cai D, Duan Z, Li X, Xue X. Dominant factors affecting reproductive outcomes of fertility-desiring young women with intrauterine adhesions. Arch Gynecol Obstet. 2017;295(4):923–927. doi: 10.1007/s00404-017-4314-z. [DOI] [PubMed] [Google Scholar]
- 53.Huang H, Cheng C, Johnson G, Wang R, Xue M, Zhang A, et al. Hysteroscopic intrauterine adhesiolysis using a blunt spreading dissection technique with double-action forceps. J Minim Invasive Gynecol. 2018;25(4):583–584. doi: 10.1016/j.jmig.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 54.Patterson AL, Zhang L, Arango NA, Teixeira J, Pru JK. Mesenchymal-to-epithelial transition contributes to endometrial regeneration following natural and artificial decidualization. Stem Cells Dev. 2013;22(6):964–974. doi: 10.1089/scd.2012.0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139(5):871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 56.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105(4):1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 57.Waterman RS, Tomchuck SL, Henkle SL, Betancourt AM. A new mesenchymal stem cell (MSC) paradigm: polarization into a pro- inflammatory MSC1 or an immunosuppressive MSC2 phenotype. PLoS One. 2010;5(4):e10088. doi: 10.1371/journal.pone.0010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gan L, Duan H, Xu Q, Tang YQ, Li JJ, Sun FQ, Wang S. Human amniotic mesenchymal stromal cell transplantation improves endometrial regeneration in rodent models of intrauterine adhesions. Cytotherapy. 2017;19(5):603–616. doi: 10.1016/j.jcyt.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 59.Liu D, Wang J, Zhao G, Jiang P, Song M, Ding H, Wang Z, Lv H, Hu Y. CSF1-associated decrease in endometrial macrophages may contribute to Asherman's syndrome. Am J Reprod Immunol. 2020;83(1):e13191. doi: 10.1111/aji.13191. [DOI] [PubMed] [Google Scholar]
- 60.Bozorgmehr M, Moazzeni SM, Salehnia M, Sheikhian A, Nikoo S, Zarnani AH. Menstrual blood-derived stromal stem cells inhibit optimal generation and maturation of human monocyte-derived dendritic cells. Immunol Lett. 2014;162(2 Pt B):239–246. doi: 10.1016/j.imlet.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 61.Schneider C, von Aulock S, Zedler S, Schinkel C, Hartung T, Faist E. Perioperative recombinant human granulocyte colony-stimulating factor (Filgrastim) treatment prevents immunoinflammatory dysfunction associated with major surgery. Ann Surg. 2004;239(1):75–81. doi: 10.1097/01.sla.0000103062.21049.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sabry D, Mostafa A, Marzouk S, Ibrahim W, Ali HHM, Hassan A, Shamaa A. Neupogen and mesenchymal stem cells are the novel therapeutic agents in regeneration of induced endometrial fibrosis in experimental rats. Biosci Rep. 2017;37(5):BSR20170794. doi: 10.1042/BSR20170794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Meisel R, Brockers S, Heseler K, Degistirici O, Bulle H, Woite C, et al. Human but not murine multipotent mesenchymal stromal cells exhibit broad- spectrum antimicrobial effector function mediated by indoleamine 2,3-dioxygenase. Leukemia. 2011;25(4):648–654. doi: 10.1038/leu.2010.310. [DOI] [PubMed] [Google Scholar]
- 64.Cortes-Araya Y, Amilon K, Rink BE, Black G, Lisowski Z, Donadeu FX, et al. Comparison of antibacterial and immunological properties of Mesenchymal stem/stromal cells from equine bone marrow, endometrium, and adipose tissue. Stem Cells Dev. 2018;27(21):1518–1525. doi: 10.1089/scd.2017.0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Balan A, Lucchini G, Schmidt S, Schneider A, Tramsen L, Kuci S, et al. Mesenchymal stromal cells in the antimicrobial host response of hematopoietic stem cell recipients with graft-versus-host disease—friends or foes? Leukemia. 2014;28(10):1941–1948. doi: 10.1038/leu.2014.127. [DOI] [PubMed] [Google Scholar]
- 66.Krasnodembskaya A, Song Y, Fang X, Gupta N, Serikov V, Lee JW, Matthay MA. Antibacterial effect of human mesenchymal stem cells is mediated in part from secretion of the antimicrobial peptide LL-37. Stem Cells. 2010;28(12):2229–2238. doi: 10.1002/stem.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Domnina A, Novikova P, Obidina J, Fridlyanskaya I, Alekseenko L, Kozhukharova I, et al. Human mesenchymal stem cells in spheroids improve fertility in model animals with damaged endometrium. Stem Cell Res Ther. 2018;9(1):50. doi: 10.1186/s13287-018-0801-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Queckborner S, Syk Lundberg E, Gemzell-Danielsson K, Davies LC. Endometrial stromal cells exhibit a distinct phenotypic and immunomodulatory profile. Stem Cell Res Ther. 2020;11(1):15. doi: 10.1186/s13287-019-1496-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lai D, Wang F, Yao X, Zhang Q, Wu X, Xiang C. Human endometrial mesenchymal stem cells restore ovarian function through improving the renewal of germline stem cells in a mouse model of premature ovarian failure. J Transl Med. 2015;13:155. doi: 10.1186/s12967-015-0516-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Patel AN, Park E, Kuzman M, Benetti F, Silva FJ, Allickson JG. Multipotent menstrual blood stromal stem cells: isolation, characterization, and differentiation. Cell Transplant. 2008;17(3):303–311. doi: 10.3727/096368908784153922. [DOI] [PubMed] [Google Scholar]
- 71.Chen L, Qu J, Xiang C. The multi-functional roles of menstrual blood-derived stem cells in regenerative medicine. Stem Cell Res Ther. 2019;10(1):1. doi: 10.1186/s13287-018-1105-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li Y, Li X, Zhao H, Feng R, Zhang X, Tai D, An G, Wen J, Tan J. Efficient induction of pluripotent stem cells from menstrual blood. Stem Cells Dev. 2013;22(7):1147–1158. doi: 10.1089/scd.2012.0428. [DOI] [PubMed] [Google Scholar]
- 73.Zheng SX, Wang J, Wang XL, Ali A, Wu LM, Liu YS. Feasibility analysis of treating severe intrauterine adhesions by transplanting menstrual blood-derived stem cells. Int J Mol Med. 2018;41(4):2201–2212. doi: 10.3892/ijmm.2018.3415. [DOI] [PubMed] [Google Scholar]
- 74.Zhu H, Pan Y, Jiang Y, Li J, Zhang Y, Zhang S. Activation of the Hippo/TAZ pathway is required for menstrual stem cells to suppress myofibroblast and inhibit transforming growth factor beta signaling in human endometrial stromal cells. Hum Reprod. 2019;34(4):635–645. doi: 10.1093/humrep/dez001. [DOI] [PubMed] [Google Scholar]
- 75.Hamid AA, Joharry MK, Mun-Fun H, Hamzah SN, Rejali Z, Yazid MN, Thilakavathy K, Nordin N. Highly potent stem cells from full-term amniotic fluid: a realistic perspective. Reprod Biol. 2017;17(1):9–18. doi: 10.1016/j.repbio.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 76.Loukogeorgakis SP, De Coppi P. Concise review: amniotic fluid stem cells: the known, the unknown, and potential regenerative medicine applications. Stem Cells. 2017;35(7):1663–1673. doi: 10.1002/stem.2553. [DOI] [PubMed] [Google Scholar]
- 77.Dziadosz M, Basch RS, Young BK. Human amniotic fluid: a source of stem cells for possible therapeutic use. Am J Obstet Gynecol. 2016;214(3):321–327. doi: 10.1016/j.ajog.2015.12.061. [DOI] [PubMed] [Google Scholar]
- 78.Rombouts WJ, Ploemacher RE. Primary murine MSC show highly efficient homing to the bone marrow but lose homing ability following culture. Leukemia. 2003;17(1):160–170. doi: 10.1038/sj.leu.2402763. [DOI] [PubMed] [Google Scholar]
- 79.Liu Y, Tal R, Pluchino N, Mamillapalli R, Taylor HS. Systemic administration of bone marrow-derived cells leads to better uterine engraftment than use of uterine-derived cells or local injection. J Cell Mol Med. 2018;22(1):67–76. doi: 10.1111/jcmm.13294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhao J, Zhang Q, Wang Y, Li Y. Uterine infusion with bone marrow mesenchymal stem cells improves endometrium thickness in a rat model of thin endometrium. Reprod Sci. 2015;22(2):181–188. doi: 10.1177/1933719114537715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Watson N, Divers R, Kedar R, Mehindru A, Mehindru A, Borlongan MC, Borlongan CV. Discarded Wharton jelly of the human umbilical cord: a viable source for mesenchymal stromal cells. Cytotherapy. 2015;17(1):18–24. doi: 10.1016/j.jcyt.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shi Q, Gao J, Jiang Y, Sun B, Lu W, Su M, et al. Differentiation of human umbilical cord Wharton's jelly-derived mesenchymal stem cells into endometrial cells. Stem Cell Res Ther. 2017;8(1):246. doi: 10.1186/s13287-017-0700-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhao YX, Chen SR, Su PP, Huang FH, Shi YC, Shi QY, et al. Using Mesenchymal stem cells to treat female infertility: an update on female reproductive diseases. Stem Cells Int. 2019;2019:9071720. doi: 10.1155/2019/9071720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yang ZX, Han ZB, Ji YR, Wang YW, Liang L, Chi Y, Yang SG, Li LN, Luo WF, Li JP, Chen DD, du WJ, Cao XC, Zhuo GS, Wang T, Han ZC. CD106 identifies a subpopulation of mesenchymal stem cells with unique immunomodulatory properties. PLoS One. 2013;8(3):e59354. doi: 10.1371/journal.pone.0059354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang L, Li Y, Guan CY, Tian S, Lv XD, Li JH, et al. Therapeutic effect of human umbilical cord-derived mesenchymal stem cells on injured rat endometrium during its chronic phase. Stem Cell Res Ther. 2018;9(1):36. doi: 10.1186/s13287-018-0777-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ebrahim N, Mostafa O, El Dosoky RE, Ahmed IA, Saad AS, Mostafa A, Sabry D, Ibrahim KA, Farid AS. Human mesenchymal stem cell-derived extracellular vesicles/estrogen combined therapy safely ameliorates experimentally induced intrauterine adhesions in a female rat model. Stem Cell Res Ther. 2018;9(1):175. doi: 10.1186/s13287-018-0924-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Parolini O, Alviano F, Bagnara GP, Bilic G, Bühring HJ, Evangelista M, Hennerbichler S, Liu B, Magatti M, Mao N, Miki T, Marongiu F, Nakajima H, Nikaido T, Portmann-Lanz CB, Sankar V, Soncini M, Stadler G, Surbek D, Takahashi TA, Redl H, Sakuragawa N, Wolbank S, Zeisberger S, Zisch A, Strom SC. Concise review: isolation and characterization of cells from human term placenta: outcome of the first international workshop on placenta derived stem cells. Stem Cells. 2008;26(2):300–311. doi: 10.1634/stemcells.2007-0594. [DOI] [PubMed] [Google Scholar]
- 88.James JL, Srinivasan S, Alexander M, Chamley LW. Can we fix it? Evaluating the potential of placental stem cells for the treatment of pregnancy disorders. Placenta. 2014;35(2):77–84. doi: 10.1016/j.placenta.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 89.Chang CW, Parast MM. Human trophoblast stem cells: real or not real? Placenta. 2017;60(Suppl 1):S57–S60. doi: 10.1016/j.placenta.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.König J, Weiss G, Rossi D, Wankhammer K, Reinisch A, Kinzer M, Huppertz B, Pfeiffer D, Parolini O, Lang I. Placental mesenchymal stromal cells derived from blood vessels or avascular tissues: what is the better choice to support endothelial cell function? Stem Cells Dev. 2015;24(1):115–131. doi: 10.1089/scd.2014.0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Komaki M, Numata Y, Morioka C, Honda I, Tooi M, Yokoyama N, Ayame H, Iwasaki K, Taki A, Oshima N, Morita I. Exosomes of human placenta-derived mesenchymal stem cells stimulate angiogenesis. Stem Cell Res Ther. 2017;8(1):219. doi: 10.1186/s13287-017-0660-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Luan X, Li G, Wang G, Wang F, Lin Y. Human placenta-derived mesenchymal stem cells suppress T cell proliferation and support the culture expansion of cord blood CD34+ cells: a comparison with human bone marrow-derived mesenchymal stem cells. Tissue Cell. 2013;45(1):32–38. doi: 10.1016/j.tice.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 93.Wu M, Zhang R, Zou Q, Chen Y, Zhou M, Li X, Ran R, Chen Q. Comparison of the biological characteristics of Mesenchymal stem cells derived from the human placenta and umbilical cord. Sci Rep. 2018;8(1):5014. doi: 10.1038/s41598-018-23396-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Du W, Li X, Chi Y, Ma F, Li Z, Yang S, Song B, Cui J, Ma T, Li J, Tian J, Yang Z, Feng X, Chen F, Lu S, Liang L, Han ZB, Han ZC. VCAM-1+ placenta chorionic villi-derived mesenchymal stem cells display potent pro-angiogenic activity. Stem Cell Res Ther. 2016;7:49. doi: 10.1186/s13287-016-0297-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li B, Zhang Q, Sun J, Lai D. Human amniotic epithelial cells improve fertility in an intrauterine adhesion mouse model. Stem Cell Res Ther. 2019;10(1):257. doi: 10.1186/s13287-019-1368-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.McIntyre JA, Jones IA, Danilkovich A, Vangsness CT., Jr The placenta: applications in orthopaedic sports medicine. Am J Sports Med. 2018;46(1):234–247. doi: 10.1177/0363546517697682. [DOI] [PubMed] [Google Scholar]
- 97.Trounson A, McDonald C. Stem cell therapies in clinical trials: progress and challenges. Cell Stem Cell. 2015;17(1):11–22. doi: 10.1016/j.stem.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 98.Balbi C, Bollini S. Fetal and perinatal stem cells in cardiac regeneration: moving forward to the paracrine era. Placenta. 2017;59:96–106. doi: 10.1016/j.placenta.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 99.Pischiutta F, Sammali E, Parolini O, Carswell HVO, Zanier ER. Placenta-derived cells for acute brain injury. Cell Transplant. 2018;27(1):151–167. doi: 10.1177/0963689717732992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vanover M, Wang A, Farmer D. Potential clinical applications of placental stem cells for use in fetal therapy of birth defects. Placenta. 2017;59:107–112. doi: 10.1016/j.placenta.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 101.Heo JS, Choi Y, Kim HS, Kim HO. Comparison of molecular profiles of human mesenchymal stem cells derived from bone marrow, umbilical cord blood, placenta and adipose tissue. Int J Mol Med. 2016;37(1):115–125. doi: 10.3892/ijmm.2015.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lee SY, Shin JE, Kwon H, Choi DH, Kim JH. Effect of autologous adipose-derived stromal vascular fraction transplantation on endometrial regeneration in patients of Asherman's syndrome: a pilot study. Reprod Sci. 2020;27(2):561–568. doi: 10.1007/s43032-019-00055-y. [DOI] [PubMed] [Google Scholar]
- 103.Jun SM, Park M, Lee JY, Jung S, Lee JE, Shim SH, Song H, Lee DR. Single cell-derived clonally expanded mesenchymal progenitor cells from somatic cell nuclear transfer-derived pluripotent stem cells ameliorate the endometrial function in the uterus of a murine model with Asherman's syndrome. Cell Prolif. 2019;52(3):e12597. doi: 10.1111/cpr.12597. [DOI] [PMC free article] [PubMed] [Google Scholar]