Abstract
Neonatal shock and necrotizing enterocolitis (NEC) are leading causes of morbidity and mortality in premature infants. NEC is a life-threatening gastrointestinal illness, the precise etiology of which is not well understood, but is characterized by an immaturity of the intestinal barrier, altered function of the adaptive immune system, and intestinal dysbiosis. The complexities of NEC and shock in the neonatal population necessitates relevant clinical modeling using newborn animals that mimic the disease in human neonates to better elucidate the pathogenesis and provide an opportunity for the discovery of potential therapeutics. A wide variety of animal species—including rats, mice, piglets, and primates—have been used in developing experimental models of neonatal diseases such as NEC and shock. This review aims to highlight the immunologic differences in neonates compared to adults and provide an assessment of the advantages and drawbacks of established animal models of both NEC and shock using enteral or intraperitoneal induction of bacterial pathogens. The selection of a model has benefits unique to each type of animal species and provides individual opportunities for the development of targeted therapies. This review discusses the clinical and physiologic relevance of animal models and the insight they contribute to the complexities of the specific neonatal diseases: NEC and shock.
Keywords: sepsis, animal models, necrotizing enterocolitis, rodent, piglet, primate, shock
Introduction
Sepsis and necrotizing enterocolitis (NEC) are the most frequent acute life-threatening inflammatory conditions among preterm neonates. Premature neonates (born before completion of 37 weeks’ completed gestation) experience the highest incidence and impact of sepsis among all age groups with a frequency of bacteremia during the birth hospitalization that may be as high as 62% (1–3). In a cohort of 5,100 extremely preterm infants born before 29 weeks of gestation in the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network, 34% experienced sepsis (defined a positive blood culture with a bacterial or fungal organism), which was associated with 18% mortality (4). In neonates, the bloodstream is the most common site of infection that leads to a diagnosis of sepsis and is typically primary or central line-associated in origin (5). Multiple studies have demonstrated that the source of neonatal bacteremia originates from the intestine (6,7). One such intestinal disease is necrotizing enterocolitis (NEC), an important primary abdominal source of sepsis in preterm neonates (8,9). The incidence and mortality of NEC in very low birth weight infants is 7% and 28% respectively, with up to 35% of affected infants requiring surgical intervention (10).
Both sepsis and NEC are preceded by nonspecific clinical signs and can rapidly progress to shock and death even with timely recognition and treatment. Despite technological advances in neonatal intensive care within high-resource countries over the last five decades, management of these acute life-threatening neonatal inflammatory conditions is limited to supportive intensive care including antimicrobial treatment, parenteral nutrition, and surgical resection of the necrotic bowel in NEC. Death or major disability occurs in two of every five infants affected by sepsis, and up to 30% of infants affected by NEC (11,12).
A protracted birth hospitalization complicated by sepsis and critical illness superimposed on developmental immaturity increases associated intensive care costs; however, the significant financial and societal impact of neonatal sepsis and NEC begins once the surviving infant is discharged from the hospital. Preterm neonates that develop and survive sepsis at the beginning of their lives and prior to the normal completion of in utero development, suffer decades of consequences secondary to sepsis-induced brain injury. In addition, sepsis-survivors suffer prolonged hospitalization and poor growth (weight and length), as well as an increased risk for comorbid conditions at discharge including chronic lung disease, neurologic injury, and retinopathy of prematurity (1). When evaluated at two years of age, neonatal sepsis survivors have a greater risk of mental and psychomotor impairment, brain atrophy (microcephaly), hearing and vision impairment, and cerebral palsy (13,14).
Studies of neonatal sepsis survivors into the second decade of life reveal persistent motor impairment, cognitive impairment (reduced intellectual quotient), and a greater risk of attention-deficit hyperactivity disorder even when compared to healthy prematurely-born peers, who carry a greater risk of these complications as compared to infants born at term (15,16). The incidence of infants with acquired sepsis is expected to rise with the global rate of premature birth, which now accounts for greater than 11% of the 135 million annual births internationally (17,18), and the successful resuscitation of infants at the edge of viability (22–24 weeks’ completed gestation) (19). Similar to the outcomes observed in septic adults treated with immune-modifying pharmacologic interventions, attempts at immunomodulatory treatments have also been ineffective in improving neonatal sepsis survival (12,20). These failures illustrate that a greater understanding of the pathobiology is needed. This review aims to describe established animal models of sepsis, shock, and NEC. A thorough PubMed search was performed on these topics and the advantages and limitations of the animal models were identified. Developing a relevant and reliable model of neonatal disease is necessary, as the impacts of early-life septic insults on long-term outcomes cannot be accurately discerned without studying a developing organism.
Animals Models for Sepsis
In the last several decades, a variety of animal models of sepsis and shock have been used to mimic the clinical presentation in humans. Animal models have been developed to simulate disease in both adults and neonates, as key differences in the host immune response occur during the transition over a lifetime from the newborn period to adulthood (8,9,20–26). These differences in immune capabilities emphasize the need for studies in neonatal animals rather than placing implication that adult animals challenged with pathogens respond equivalently to affected neonates.
Immune deficits and altered immune function, as compared to immunity in children and adults, is central to the neonate’s increased susceptibility to develop and succumb to a fulminant infection (Figure 1) (21). Multiple clinical and preclinical investigations of both innate and adaptive immunologic function have demonstrated that developmental age strongly influences immune responses following stimulation (21,27–30). Therefore, highlighting clinically-relevant modeling must extend beyond the type of specific pathogen challenge and include studies in neonatal animals.
Figure 1. Characteristics of Neonatal Innate and Adaptive Immune Responses.
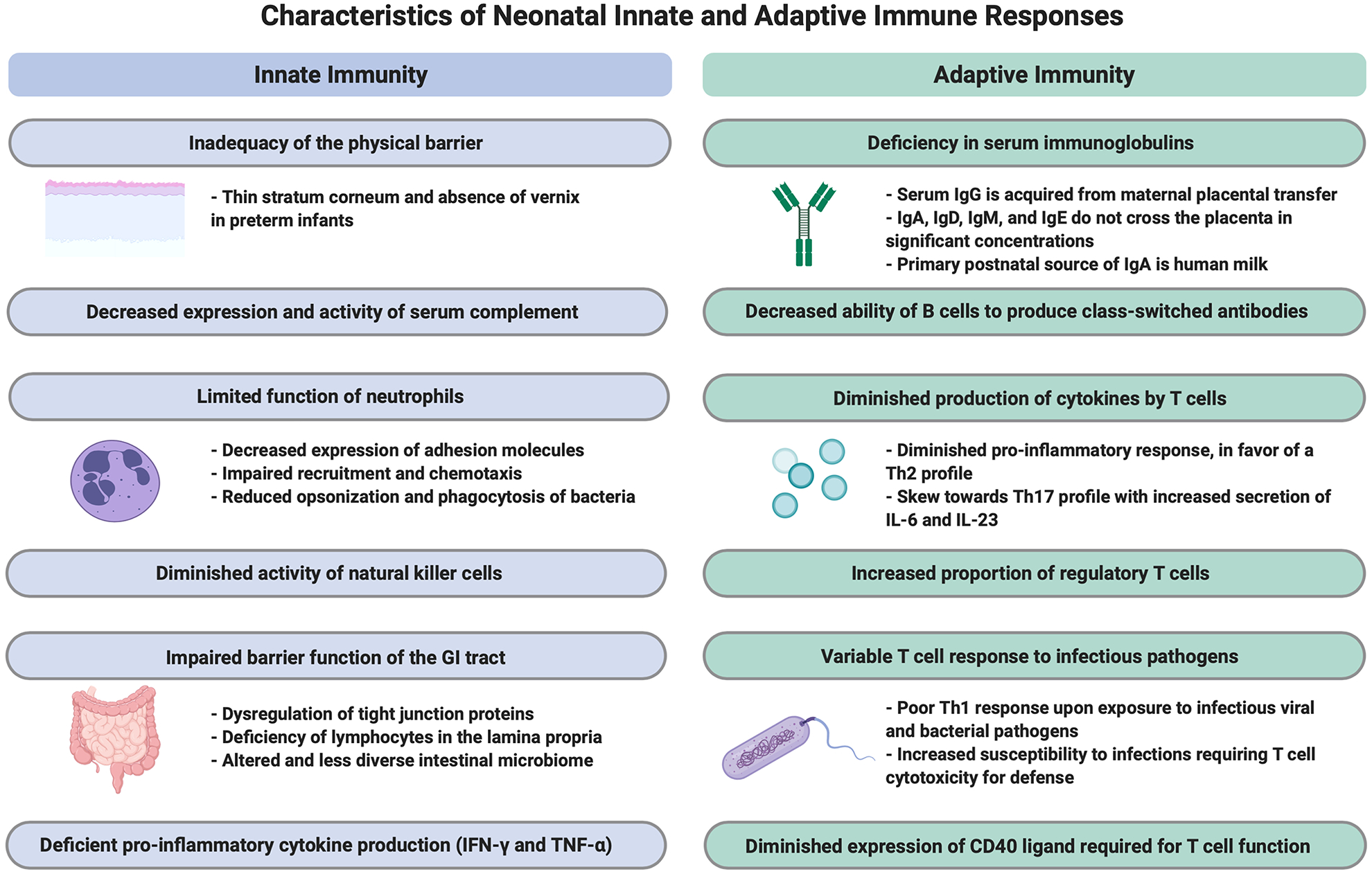
Newborn infants have distinctive features of the innate and adaptive immune responses when compared with adults. These characteristics result in immune deficits in the infant, particularly in those born preterm. Figure created with Biorender.com.
In this manuscript, we selected single-pathogen challenges that represent the many different approaches to effectively model infection in neonates. The majority of neonatal single-pathogen modeling approaches involve common bacterial pathogens that affect this age group, including Group B Streptococcus (GBS), Escherichia coli (E. coli), Klebsiella pneumoniae, Pseudomonas aeruginosa, Staphylococcus aureus, and Coagulase-negative Staphylococcus (31–37). The specific pathogen, severity of challenge (colony counts), and host site provide multiple opportunities to characterize the neonatal-specific host response to each bacterial challenge and to assess the response to potential treatments (31,32,34–36,38–41).
Of note, an international consensus initiative identified preclinical sepsis modeling guidelines (Minimum Quality Threshold in Preclinical Sepsis Studies), which specifically recommended against the use of lipopolysaccharide to be used as a model of “sepsis,” as this approach may not adequately replicate the clinical scenario (42). Therefore, we will not discuss those or other pathogen-associated molecular pattern (PAMP)-driven approaches. Therapies derived from modeling sepsis using a PAMP-based approach have unequivocally failed in adult trials. A comprehensive review of all possible pathogens and model organisms in a single manuscript is not feasible. Towards this end, we present commonly utilized approaches for both neonatal sepsis/shock and NEC along with their strengths and weaknesses with respect to the clinical condition.
Rodents (Mice and Rats)
Sepsis
Rodents are the most commonly studied mammalian model system for neonatal sepsis. There are several advantages to the study of rodents, including the availability of reagents, large litter sizes, short gestations, and a known genome. As several studies have shown, rodents serve as imperfect models for humans, and as a result, findings derived using rodent-based modeling approaches should ideally be accompanied by a demonstration of overlapping human pathobiology (43–45). A notable limitation of the majority of murine neonatal infectious models of both sepsis and NEC is the exclusion of antimicrobial treatment and supportive intensive care that would accompany the disease in the human neonate. The size of the model animal limits the possibilities for supportive care; however, the increasing availability of depot antimicrobials with long half-lives along with very fine gauge needles should facilitate the inclusion of antimicrobials in future studies.
Modeling of sepsis in neonatal rodents has been performed using polymicrobial (intraperitoneal) and single-pathogen (bacterial, fungal, or viral) challenges via a number of sites of administration including brain, lung, blood, gastrointestinal, and peritoneum. Although there is debate regarding the clinical relevance of any bolus administration of a pure single pathogen to a single body site, the use of single pathogen models may help to identify pathogen-specific and compartment-specific host response capabilities that may lead to novel therapeutic opportunities. Neonatal adaptive immune system function is impaired and immature when compared with adults as mothers do not transfer T-cell-mediated cellular immunity to their infants. In contrast to adults, neonatal adaptive immune system function differs due to limited antigenic exposure before birth, underdeveloped secondary lymphoid tissues, abundant and potent T-regulatory population, poor CD4+ T cell function, an increased requirement for CD4+ T-cell stimulation, weak immunoglobulin production (predominantly IgM), poor antibody class switching, poor response to polysaccharide antigens, and poor T-cell–dependent B-cell stimulation. The altered function of the neonatal adaptive immune system implies that the neonate heavily depends on the innate immune system for defense against pathogenic challenges.
Polymicrobial Challenge: A Gold Standard of Neonatal Sepsis
In stark contrast to findings in adult mice, neonatal mice that lack an adaptive immune system showed no difference in polymicrobial sepsis survival when compared with wild-type neonatal mice (28). Kazarian and colleagues examined the response of adult Yucatan minipigs to two peritoneal sepsis challenges: a “fecal inoculum” and a pure culture of E. coli (46). Both substrates were administered via a direct injection through a surgically-opened abdomen, and it was observed that a pure E. coli challenge resulted in a rapid systemic response very similar to that which was observed with intravenous administration of E. coli or lipopolysaccharide alone. In contrast, the fecal inoculum generated a systemic response akin to a slower developing septic process (over three days) with the development of multiple abdominal abscesses, pyogenic granulomas, and adhesions. Subsequently, Sam et al. maintained the approach of a direct injection of a fecal inoculum through a surgical incision, but transitioned the model into adult rats using a “cecal slurry” as the injection substrate (47,48). The slurry, prepared fresh for each experiment and used within two hours of preparation, consisted of cecal contents of donor rats mixed in 5% dextrose. The cecal slurry model was later modified for use in mice with a focus on neonatal pups (29). The slurry was prepared similarly to previous models by Sam et al., but was administered by Wynn et al. via a simple needle-based intraperitoneal injection rather than by direct visualization through an open abdomen (29,47) (Figure 2 and Video). The use of mice and syringe-based injection for the cecal slurry was associated with several advantages, including no requirement for anesthesia or survival surgery; broad access to mouse reagents, assays, and genetic modifications (global, conditional, tissue-specific); and a fully sequenced mouse genome. In addition, neonatal-specific advantages include a short gestation, production of large litters of pups, a significantly reduced risk of cannibalization (compared to the surgically-altered neonate), and, perhaps most importantly, the ability to perform a direct comparison of neonatal and adult host responses to sepsis (29). In addition to the technical advantages associated with the cecal slurry model in mice, the practice of exclusive use of cecal “donor mice” of a specific gender (female), purchased at a particular age (6 weeks), from a single vendor and location, and used within 2 weeks of arrival, yields predictable consistency for neonatal mortality and the host response across time, institution, and performing scientist (25,28,29,43,44,49–51). As such, the cecal slurry model has become a cornerstone of neonatal polymicrobial sepsis studies and has also been employed to study juvenile and adult responses (28,29,43,44,49,50,52–62). Although there are advantages and disadvantages to each approach of sepsis modeling (Table 1), it is worth noting that results derived using other polymicrobial models of sepsis including cecal ligation and puncture (CLP) may be affected by variables, including differences in microbiome composition, research center, diet, age, gender, and individual surgical or anesthetic skill of the technician performing the procedure. These variations can limit the utility of comparisons and findings between research groups. Using the cecal slurry model, many of these formidable challenges are ameliorated or circumvented entirely.
Figure 2.
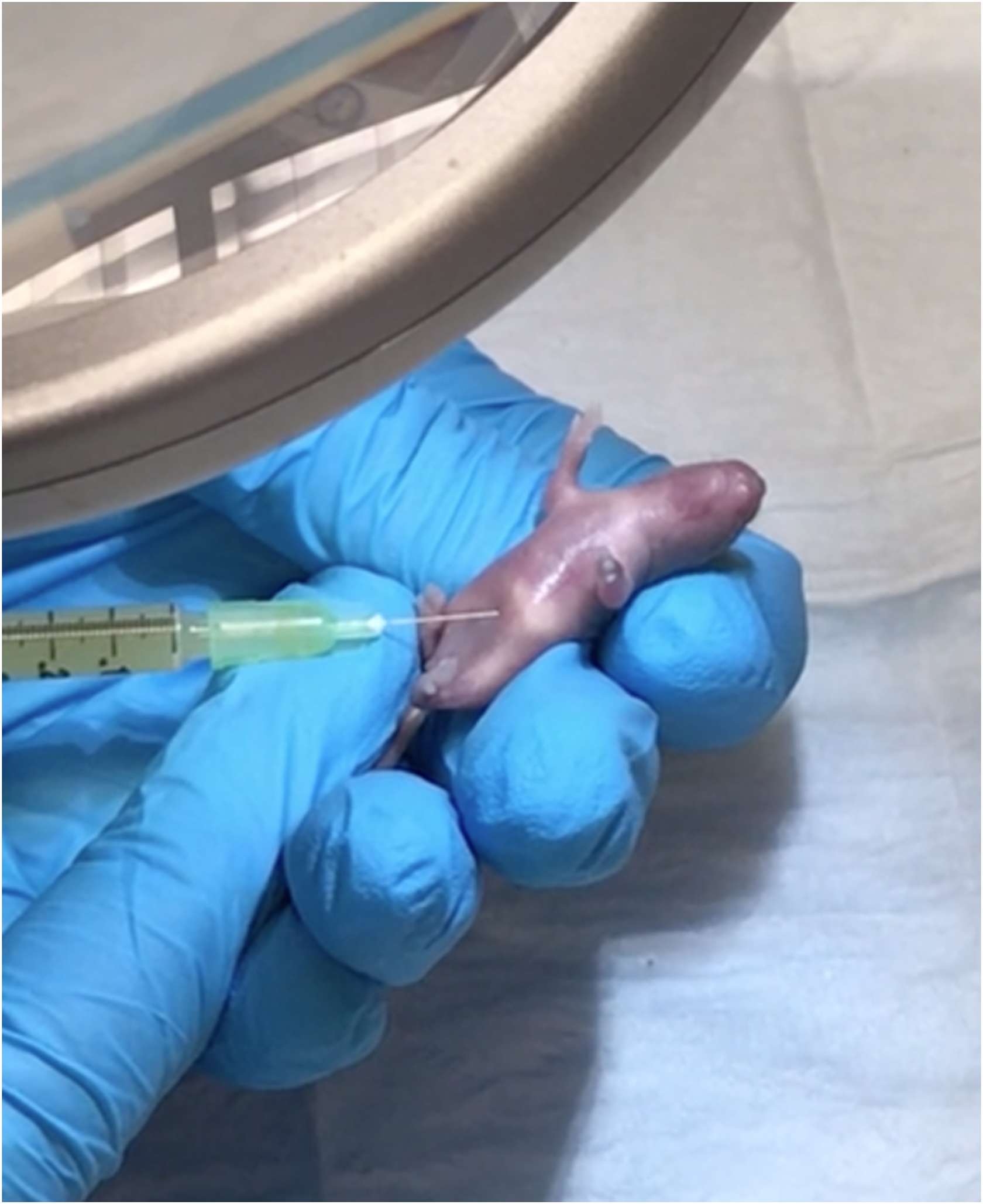
Demonstration of a simple needle-based intraperitoneal injection of a bacterial slurry to induce sepsis in a neonatal mouse.
Table 1.
Approach to inducing and simulating necrotizing enterocolitis and neonatal sepsis in animal models.
| Approach to Inducing Disease | Advantages | Disadvantages | References |
|---|---|---|---|
| Enteral gavage of a slurry of commercial formula ± enteric bacteria, often followed by exposure to hypoxia | Does not necessitate injection or surgical alteration. | Technical challenges involved in feeding newborn pups. | 70, 71, 72, 73, 74, 75, 88, 89, 90, 93 |
| Polymicrobial or single pathogen injection through a surgical incision | Induces a prompt state of sepsis and septic shock. | Increased risk of cannibalization with surgical alteration. Requires anesthesia and survival surgery. |
47, 48 |
| Polymicrobial (Cecal Slurry Model) or single pathogen intraperitoneal injection | Procedure does not necessitate anesthesia or survival surgery. Reduced risk of cannibalization. |
Technical challenges involved in injecting newborn pups and avoiding trauma to abdominal organs. | 28, 29, 37, 43, 44, 45, 46, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 66, 67, 69, 78 |
| Cecal ligation and puncture (CLP) | Widely accepted and effective model of sepsis in adult animals. | Associated with high mortality of the animal due to the rapid state of sepsis. Technical differences in the location of the placement of the ligating suture prevents quality reproduction of studies. Technically challenging in small animal models. Increased risk of cannibalization. |
29, 57, 63, 81, 82, 83 |
| Intravenous injection of a bacterial pathogen | Provides a direct inoculum of bacteria to the bloodstream. | Intravenous access may be challenging to obtain in small animal models. | 33, 84, 85, 94 |
The cecal slurry model of polymicrobial sepsis is representative of the human neonatal disease, whereas CLP remains the gold standard for adult sepsis studies (63). The kinetics of mortality in response to cecal slurry in murine neonates begins at 12–16 hours following substrate challenge and typically occurs within 72 hours after the challenge. This timeline mirrors the progression to mortality in human neonatal sepsis (5,64). Adult mice exposed to cecal slurry demonstrate earlier death and a more severe early inflammatory response compared to CLP (29). As is required with any preclinical model of sepsis (42,65), the cecal slurry model leads to bacteremia with end-organ colonization (29) as well as gross and histologic organ injury with subsequent organ dysfunction and mortality (28,29,43,44,49,50,52–62,66–69).
Necrotizing enterocolitis
The enteral induction of bacterial pathogens, as an adjunct to human commercial formula, has been studied as a means of simulating NEC-like injury in experimental models of rat or mouse pups (70,71). The literature describes many variations in the chosen composition of enteral gavage feeds used in murine NEC models. Previous studies described a unique humanized model of experimental NEC in neonatal mice induced by using a formula composed of human commercial formula, canine milk replacer, and a slurry of enteric bacteria isolated from a human infant with NEC. Thereafter, ischemia and reperfusion injury in NEC is modeled with exposure to hypoxia (5% O2-95% N2) in a hypoxic chamber (72). Murine NEC models have been modified by various researchers to suit their experimental questions. For instance, enteral gavage of Klebsiella to neonatal mouse pups along with intraperitoneal injection of the zinc chelator, dithizone, has been shown to induce intestinal injury and Paneth cell disruption along with a bloom of Enterobacteriaceae species in the small intestine resembling human NEC (73,74). Hunter and colleagues have studied enteral exposure to Cronobacter sakazakii (previously known as Enterobacter sakazakii) in neonatal rats subjected to intestinal epithelial injury in experimental NEC, providing implications for the pathogenic etiology in humans (75). Histological assessment of the intestine in human and murine NEC has demonstrated similarities that allow for murine NEC models to provide the ability to study the molecular mechanisms involved in the pathogenesis of NEC (76). The use of rodents in NEC modeling has clear advantages (Table 2), although there are technical challenges involved in the care and gavage feeding of newborn pups when compared with models using larger animals.
Table 2.
Summary of advantages and limitations of modeling of necrotizing enterocolitis and neonatal sepsis.
| Organism | Advantages | Limitations |
|---|---|---|
| Rodents (Mice and Rats) | Reagents are widely available. Low cost. Large litter size with short gestation. Known genome with genetic similarities to humans and available knockout strains. Similarities of the gastrointestinal tract function including in response to intestinal injury. |
Technical difficulties with maintenance and feeding newborn pups. Inadequate for assessing response to antibiotics or supportive care following induction of disease. Inability to study disease in prematurity as rodents have poor survival with preterm birth. |
| Piglets | Size comparable to the human infant. Preterm birth and disease in the setting of prematurity can be supported and studied. Similarities in anatomy, development and immune response. Able to monitor response to supportive care measures such as parenteral nutrition and mechanical ventilation. Ability to monitor hemodynamics, pulmonary function, and cerebral perfusion in response to disease. |
Long gestational period. High cost of maintenance and housing. Reduced availability of reagents. |
| Nonhuman Primates | High degree of physiologic and anatomic similarity. Ability to study long-term outcomes and neurodevelopmental deficits. Preterm birth and congenital or acquired infections can be studied and supported. |
Controversial and ethical concerns in the use of nonhuman primates in research. High cost of maintenance and housing. |
Piglets
Although the neonatal rodent and human immune systems share central similarities, it is important to note that rodents do not survive when born very prematurely, and premature birth is a substantial risk factor for sepsis and NEC in humans. The size limitations of neonatal rodents prevent complete mirroring of the clinical presentation of sepsis in human neonates. Specifically, the ability to maintain intravascular monitoring, perform serial blood sampling, and provide vasoactive medications and intravenous nutrition is not presently feasible in neonatal rodents. In contrast, preterm piglets can be successfully supported after preterm birth and may exhibit comparable organ immaturity of the lungs, gastrointestinal tract, brain, as well as impairment in thermoregulation.
Sepsis
Newborn preterm pigs exhibit similar immature systemic immune responses to preterm human neonates that may predispose to sepsis (77). The neonatal innate immune system is limited by altered mucosal immunity due to decreased barrier integrity and hyper-reactivity of intestinal epithelium (9). Reduced production of complement and antimicrobial peptide production, distinct Toll-like receptor responses, increased baseline serum IL-18, decreased neutrophil storage, reduced neutrophil amplification and mobilization, and impaired antigen presenting cell function and co-stimulatory molecule production have also been observed (20–22,25,43). Piglet models offer the possibility to model similar supportive care, such as parenteral nutrition and mechanical ventilation.
Similar to rodents, single pathogen and polymicrobial challenges (such as CLP and cecal slurry) to induce infection have been explored using piglets (33,77–84). The ability to evaluate hemodynamic parameters in piglets has proved useful in studies modeling neonatal septic shock. Lobe et al. used an infant pig model to monitor hemodynamics of shock as a potential early detector of disease (78). In this method, a slurry of pig feces and E. coli mixed in saline and administered to the animals by intraperitoneal injection, induced an E. coli peritonitis with septic shock. Measures of hemodynamics were monitored via arterial and venous femoral catheters and serial determinations of certain parameters were associated with an early ability to predict Gram-negative septic shock (78). As GBS is a common pathogen that is associated with sepsis in neonates, a study by Gibson et al. used a neonatal piglet model to study nitric oxide (NO) production in GBS sepsis and the effect of a nitric oxide synthase inhibitor on the pulmonary and systemic hemodynamics. The piglets underwent cannulation of the aorta, external jugular vein, and pulmonary artery to monitor hemodynamic parameters. GBS sepsis was induced via GBS infusion, in contrast to saline infusion in the control group. The piglets then received a treatment infusion of a NO synthase inhibitor, which ultimately resulted in increased pulmonary arterial pressures and pulmonary vascular resistance, implicating that a NO synthase inhibitor is likely a contraindicated treatment option in human neonatal GBS sepsis (33). Respiratory distress and sepsis secondary to GBS pneumonia are common in the preterm neonatal population and replicating this clinical scenario along with an assessment of the benefits of treatment with exogenous surfactant have been successfully implemented in piglet models (80).
Evaluation of cerebral perfusion, with the risk of cerebral ischemia and neuronal damage as seen in neonatal sepsis or bacterial meningitis, is also feasible for study in a newborn piglet model. Cisternal suboccipital puncture with needle maintained in situ allows for continuous ICP monitoring and sampling of cerebrospinal fluid (79). In a study in which bacterial meningitis was induced by injecting E. coli into the cisternal magna and intravascularly, followed by dopamine infusion, adequate cerebral perfusion pressure was observed when compared with controls without dopamine infusion (79). In a recent piglet model of endotoxic shock with placement on extracorporeal membrane oxygenation (ECMO), cerebral perfusion and hemodynamics were studied when endotoxic shock was induced with E. coli intravenous injection (85). The ability to evaluate the effect of ECMO on changes in cerebral perfusion in endotoxin exposure is a feature that is unique and unable to be performed in a rodent model. It is worth noting, however, the potential drawbacks to consider for the use of piglets to model neonatal sepsis include costs of animals and housing, long gestation (approximately four months), fecundity, and reagent availability as reviewed in Table 2.
Necrotizing enterocolitis
Dysfunction of the gastrointestinal tract is implicated in sepsis and the piglet gastrointestinal tract has been described as similar to humans in anatomic and physiologic structure and microbiome composition, permitting the study of preterm intestinal function and NEC (37,70,71,86–88). Similar to murine models, piglet models of NEC have been produced using hypoxia, hypothermia, and enteral feedings as an adjunct to human commercial formula. One experimental model involved premature delivery of piglets with the induction of NEC using gavaged enteral formula feeds composed of commercial infant formula, MCT oil, whey protein additive, and a slurry of enteral bacteria from a human infant with NEC (72). In another study, Nguyen and colleagues challenged premature piglets with intraperitoneal Staphylococcus epidermidis, a causative organism of late-onset sepsis in preterm human infants, and minimal enteral nutrition to induce experimental NEC. They demonstrated the presence of circulating cell-free DNA levels in the plasma of neonatal piglets with NEC, indicating a potential blood biomarker to be evaluated in human NEC (37). In seeking to develop therapeutics for NEC, the anti-inflammatory benefits of human milk oligosaccharides (HMOs) have been studied in a preterm piglet model of NEC (87). Most recently, Roy and colleagues fed newborn female piglets with human infant formula and E. coli isolated from the stool of human infants with NEC to investigate the implications of bacterial fermentation of non-hydrolyzed lactose (89). They found newborn piglets that received the bacteria-fermented formula developed an increased production of short-chain fatty acids (SCFA) and exaggerated intestinal inflammation compared to the bacteria only or formula fed only groups, suggesting that SCFA may contribute to the intestinal inflammation seen in human NEC (89).
Rhesus
The involvement of nonhuman primates (NHP) has been vital in scientific advancement due to the high degree of physiologic and anatomical similarity to humans, permitting replication of human disease. Specifically, rhesus macaque monkeys are an established model of human disease, and the neonatal rhesus macaque model has maintained a significant role in the study of congenital or acquired infections. The similarities between nonhuman primates and humans in reproductive regulation permits investigation of the intrauterine environment, maternal-fetal relationships, and neonatal outcomes. Preterm baboons delivered at 67% gestation have a similar clinical course to preterm human infants delivered at 25–27 weeks of gestation (90,91). Kelleher et al. developed a NHP model of infection-induced premature birth via induction of intra-amniotic infection with Ureaplasma parvum and thereafter, assessed postnatal outcomes in the preterm rhesus macaque infant (92). The study permitted the assessment of inflammatory brain injury following exposure to intrauterine infection, which in the human preterm neonate can increase their risk of long-term neurodevelopmental deficits and cerebral palsy diagnosis (92).
Sepsis
Similar to the aforementioned animal models, neonatal GBS infection has also been studied in a rhesus monkey model, in which GBS suspended in amniotic fluid was deposited intratracheally into the carina or via nasogastric tube into the stomach to induce bacteremia, sepsis, or pneumonia (93). GBS immunoglobulin was then administered, which subsequently proved the ability to prevent serious bacteremia and sepsis in the infected monkeys, indicating a possible therapeutic for human neonatal GBS infection (93).
The presence of bacterial endotoxin in the blood of bacteremic patients has been an essential observation in the correlation in septic shock and is associated with high mortality. In a macaque model of septic shock, E. coli endotoxin of two different doses was injected intravenously to two groups and hemodynamic parameters and plasma concentrations of endotoxin were monitored (94). A biphasic clearance pattern of endotoxin was noted, which has the potential for therapeutic targeting (94).
Necrotizing enterocolitis
NHPs are useful in the understanding of NEC pathogenesis, as preterm baboon NEC can clinically and radiologically resemble human NEC. In one study, intestinal tissue samples from preterm baboons with NEC-like intestinal injury were noted to demonstrate features similar to human NEC, as the samples were deficient in transforming growth factor-β2 (TGF-β2) expression and exhibited similar inflammatory changes with increased expression of cytokines such as interleukin (IL)-1β and IL-8 (90). This same study provided further insight into NEC pathogenesis, as the cases of NEC were correlated with increased tissue expression of Smad7, which was also noted to suppress TGF-β2 expression upon exposure to LPS in cell culture (90). This insight into the inflammatory changes in NEC using a preterm baboon model provides an opportunity for future study of Smad7 as a potential therapeutic focus in NEC management.
Despite the vital role of NHPs in the advancement of human biomedical research, several core ethical issues require consideration. Primate research remains controversial due to the high cognitive capacity and social behavior of NHPs, which has influenced guidelines for experimental use of NHPs in research to reduce suffering and pain and promote psychological well-being (95). The use of NHPs as a relevant model of diseases, such as sepsis and shock, is valuable in understanding biological processes that are unique to humans. Still, feasibility is limited by the resources required to maintain primate models, due to the expense, large size, and moral obligation to reduce undue stress and suffering of the animals.
Summary
The understanding of the mechanisms involved in neonatal sepsis, shock, and NEC continue to evolve. Experimental animal models have transformed scientists’ ability to mimic the complex human physiology of the immature intestinal epithelium, gastrointestinal microbial community, and inflammatory pathways, particularly in neonatal disease. The selection of a clinically-relevant animal has advantages unique to each mammal and provide individual opportunities for the development of targeted therapies. Alternative techniques have since emerged that seek to replicate the physiologic aspects of disease in an in vitro microenvironment using stem cells in conventional organoid cultures or in the more complex organ-on-a-chip models. These novel platforms of disease modeling may provide innovative insights into disease pathogenesis as well as patient-derived microphysiological systems of neonatal sepsis, shock and NEC.
Supplementary Material
Using a very small gauge needle (such as a tuberculin syringe), a bacterial slurry is injected into the intraperitoneal cavity of a neonatal mouse to induce sepsis, carefully avoiding trauma to the abdominal organs during the injection process.
Acknowledgements
The Institutional Animal Care and Use Committee at the University of Florida gave permission to video record and photograph the animals.
JLW is supported by R01GM128452, R01HD089939, R01HD088541 from the National Institutes of Health. MG is supported by R01DK118568 from the National Institutes of Health, the Children’s Discovery Institute of Washington University and St. Louis Children’s Hospital, the St. Louis Children’s Hospital Foundation and the Department of Pediatrics at Washington University School of Medicine, St. Louis. MG has previously received sponsored research agreement funding from Astarte Medical Partners and participated in a neonatal microbiome advisory board for Abbott Laboratories. None of the above sources had any role in this study.
References
- 1.Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, Hale EC, Newman NS, Schibler K, Carlo WA, et al. Neonatal outcomes of extremely preterm infants from the NICHD neonatal research network. Pediatrics 126(3):443–56, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin GS, Mannino DM, Moss M. The effect of age on the development and outcome of adult sepsis. Crit Care Med 34(1):15–21, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Cohen-Wolkowiez M, Moran C, Benjamin DK, Cotten CM, Clark RH, Benjamin DK, Smith PB. Early and late onset sepsis in late preterm infants. Pediatr Infect Dis J 28(12):1052–6, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greenberg RG, Kandefer S, Do BT, Smith PB, Stoll BJ, Bell EF, Carlo WA, Laptook AR, Sánchez PJ, Shankaran S, et al. Late-onset sepsis in extremely premature infants: 2000–2011. Pediatr Infect Dis J 36(8):774–9, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giannoni E, Agyeman PKA, Stocker M, Posfay-Barbe KM, Heininger U, Spycher BD, Bernhard-Stirnemann S, Niederer-Loher A, Kahlert CR, Donas A, et al. Neonatal sepsis of early onset, and hospital-acquired and community-acquired late onset: a prospective population-based cohort study. J Pediatr 201:106–114.e4, 2018. [DOI] [PubMed] [Google Scholar]
- 6.Carl MA, Ndao IM, Springman AC, Manning SD, Johnson JR, Johnston BD, Burnham CAD, Weinstock ES, Weinstock GM, Wylie TN, et al. Sepsis from the gut: the enteric habitat of bacteria that cause late-onset neonatal bloodstream infections. Clin Infect Dis 58(9):1211–8, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tamburini FB, Andermann TM, Tkachenko E, Senchyna F, Banaei N, Bhatt AS. Precision identification of diverse bloodstream pathogens in the gut microbiome. Nat Med 24(12):1809–14, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mara MA, Good M, Weitkamp JH. Innate and adaptive immunity in necrotizing enterocolitis. Semin Fetal Neonatal Med 23(6):394–9, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hodzic Z, Bolock AM, Good M. The role of mucosal immunity in the pathogenesis of necrotizing enterocolitis. Front Pediatr 5(March):1–17, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jammeh ML, Adibe OO, Tracy ET, Rice HE, Clark RH, Smith PB, Greenberg RG. Racial/ethnic differences in necrotizing enterocolitis incidence and outcomes in premature very low birth weight infants. J Perinatol 38(10):1386–90, 2018. [DOI] [PubMed] [Google Scholar]
- 11.Brocklehurst P, Farrell B, King A, Juszczak E, Darlow B, Haque K, Salt A, Stenson B, TarnowMordi W. Treatment of neonatal sepsis with intravenous immune globulin. N Engl J Med 365(13):1201–11, 2011. [DOI] [PubMed] [Google Scholar]
- 12.Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med 364(3):255–64, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stoll BJ, Hansen NI, Adams-Chapman I, Fanaroff AA, Hintz SR, Vohr B, Higgins RD. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. J Am Med Assoc 292(19):2357–65, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Adams-Chapman I, Stoll BJ. Neonatal infection and long-term neurodevelopmental outcome in the preterm infant. Curr Opin Infect Dis 19(3):290–7, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Bright HR, Babata K, Allred EN, Erdei C, Kuban KCK, Joseph RM, O’Shea TM, Leviton A, Dammann O, Ware J, et al. Neurocognitive outcomes at 10 years of age in extremely preterm newborns with late-onset bacteremia. J Pediatr 187:43–49.e1, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rand KM, Austin NC, Inder TE, Bora S, Woodward LJ. Neonatal infection and later neurodevelopmental risk in the very preterm infant. J Pediatr 170:97–104, 2016. [DOI] [PubMed] [Google Scholar]
- 17.Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE, Rudan I, Campbell H, Cibulskis R, Li M, et al. Global, regional, and national causes of child mortality: An updated systematic analysis for 2010 with time trends since 2000. Lancet 379(9832):2151–61, 2012. [DOI] [PubMed] [Google Scholar]
- 18.Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, Narwal R, Adler A, Vera Garcia C, Rohde S, Say L, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet 379(9832):2162–72, 2012. [DOI] [PubMed] [Google Scholar]
- 19.Rysavy MA, Li L, Bell EF, Das A, Hintz SR, Stoll BJ, Vohr BR, Carlo WA, Shankaran S, Walsh MC, et al. Between-hospital variation in treatment and outcomes in extremely preterm infants. N Engl J Med 372(19):1801–11, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wynn JL, Neu J, Moldawer LL, Levy O. Potential of immunomodulatory agents for prevention and treatment of neonatal sepsis. J Perinatol 29(2):79–88, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wynn J, Cornell TT, Wong HR, Shanley TP, Wheeler DS. The host response to sepsis and developmental impact. Pediatrics 125(5):1031–41, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Collins A, Weitkamp JH, Wynn JL. Why are preterm newborns at increased risk of infection? Arch Dis Child Fetal Neonatal Ed 103(4):F391–4, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kollmann TR, Kampmann B, Mazmanian SK, Marchant A, Levy O. Protecting the newborn and young infant from infectious diseases: lessons from immune ontogeny. Immunity 46(3):350–63, 2017. [DOI] [PubMed] [Google Scholar]
- 24.Lawrence SM, Corriden R, Nizet V. Age-appropriate functions and dysfunctions of the neonatal neutrophil. Front Pediatr 5(February):23, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raymond SL, Stortz JA, Mira JC, Larson SD, Wynn JL, Moldawer LL. Immunological defects in neonatal sepsis and potential therapeutic approaches. Front Pediatr 5(February):1–8, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cuenca AG, Wynn JL, Moldawer LL, Levy O. Role of innate immunity in neonatal infection. Am J Perinatol 30(2):105–12, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strunk T, Currie A, Richmond P, Simmer K, Burgner D. Innate immunity in human newborn infants: prematurity means more than immaturity. J Matern Neonatal Med 24(1):25–31, 2011. [DOI] [PubMed] [Google Scholar]
- 28.Wynn JL, Scumpia PO, Winfield RD, Delano MJ, Kelly-Scumpia K, Barker T, Ungaro R, Levy O, Moldawer LL. Defective innate immunity predisposes murine neonates to poor sepsis outcome but is reversed by TLR agonists. Blood 112(5):1750–8, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wynn JL, Scumpia PO, Delano MJ, O’Malley KA, Ungaro R, Abouhamze A, Moldawer LL. Increased mortality and altered immunity in neonatal sepsis produced by generalized peritonitis. Shock 28(6):675–83, 2007. [DOI] [PubMed] [Google Scholar]
- 30.PrabhuDas M, Adkins B, Gans H, King C, Levy O, Ramilo O, Siegrist C-A. Challenges in infant immunity: implications for responses to infection and vaccines. Nat Immunol 12(3):189–94, 2011. [DOI] [PubMed] [Google Scholar]
- 31.Martin TR, Rubens CE, Wilson CB, Martin TR. Lung antibacterial defense mechanisms in infant and adult rats: Implications for the pathogenesis of group b Streptococcal infections in the neonatal lung. J Infect Dis 157(1):91–100, 1988. [DOI] [PubMed] [Google Scholar]
- 32.Andrade EB, Magalhães A, Puga A, Costa M, Bravo J, Portugal CC, Ribeiro A, Correia-Neves M, Faustino A, Firon A, et al. A mouse model reproducing the pathophysiology of neonatal group B streptococcal infection. Nat Commun 9(1):3138, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gibson RL, Berger JI, Redding GJ, Standaert TA, Mayock DE, Truog WE. Effect of nitric oxide synthase inhibition during group B streptococcal sepsis in neonatal piglets. Pediatr Res 36(6):776–83, 1994. [DOI] [PubMed] [Google Scholar]
- 34.Deshmukh HS, Liu Y, Menkiti OR, Mei J, Dai N, O’Leary CE, Oliver PM, Kolls JK, Weiser JN, Worthen GS. The microbiota regulates neutrophil homeostasis and host resistance to Escherichia coli K1 sepsis in neonatal mice. Nat Med 20(5):524–30, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dalgakiran F, Witcomb LA, McCarthy AJ, Birchenough GMH, Taylor PW. Non-invasive model of neuropathogenic Escherichia coli infection in the neonatal rat. J Vis Exp (92):1–10, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fitzpatrick EA, You D, Shrestha B, Siefker D, Patel VS, Yadav N, Jaligama S, Cormier SA. A neonatal murine model of MRSA pneumonia. PLoS One 12(1):e0169273, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nguyen DN, Stensballe A, Lai JCY, Jiang P, Brunse A, Li Y, Sun J, Mallard C, Skeath T, Embleton ND, et al. Elevated levels of circulating cell-free DNA and neutrophil proteins are associated with neonatal sepsis and necrotizing enterocolitis in immature mice, pigs and infants. Innate Immun 23(6):524–36, 2017. [DOI] [PubMed] [Google Scholar]
- 38.Zhu Y, Xu L, Collins JJP, Vadivel A, Cyr-Depauw C, Zhong S, Mense L, Möbius MA, Thébaud B. Human umbilical cord mesenchymal stromal cells improve survival and bacterial clearance in neonatal sepsis in rats. Stem Cells Dev 26(14):1054–64, 2017. [DOI] [PubMed] [Google Scholar]
- 39.Jaillon S, Mancuso G, Hamon Y, Beauvillain C, Cotici V, Midiri A, Bottazzi B, Nebuloni M, Garlanda C, Frémaux I, et al. Prototypic long pentraxin PTX3 is present in breast milk, spreads in tissues, and protects neonate mice from Pseudomonas aeruginosa lung infection. J Immunol 191(4):1873–82, 2013. [DOI] [PubMed] [Google Scholar]
- 40.Kronforst KD, Mancuso CJ, Pettengill M, Ninkovic J, Power Coombs MR, Stevens C, Otto M, Mallard C, Wang X, Goldmann D, et al. A neonatal model of intravenous Staphylococcus epidermidis infection in mice <24 h old enables characterization of early innate immune responses. PLoS One 7(9):e43897, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cole BK, Scott E, Ilikj M, Bard D, Akins DR, Dyer DW, Chavez-Bueno S. Route of infection alters virulence of neonatal septicemia Escherichia coli clinical isolates. PLoS One 12(12):1–22, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Osuchowski MF, Ayala A, Bahrami S, Bauer M, Boros M, Cavaillon JM, Chaudry IH, Coopersmith CM, Deutschman CS, Drechsler S, et al. Minimum quality threshold in pre-clinical sepsis studies (mqtipss): An international expert consensus initiative for improvement of animal modeling in sepsis. Shock 50(4):377–80, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wynn JL, Wilson CS, Hawiger J, Scumpia PO, Marshall AF, Liu JH, Zharkikh I, Wong HR, Lahni P, Benjamin JT, et al. Targeting IL-17A attenuates neonatal sepsis mortality induced by IL-18. Proc Natl Acad Sci U S A 113(19):E2627–35, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Benjamin JT, Moore DJ, Bennett C, van der Meer R, Royce A, Loveland R, Wynn JL. Cutting edge: IL-1α and not IL-1β drives IL-1R1-dependent neonatal murine sepsis lethality. J Immunol 201(10):2873–8, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brook B, Amenyogbe N, Ben-Othman R, Cai B, Harbeson D, Francis F, Liu AC, Varankovich N, Wynn J, Kollmann TR. A controlled mouse model for neonatal polymicrobial sepsis. J Vis Exp 2019(143):1–13, 2019. [DOI] [PubMed] [Google Scholar]
- 46.Kazarian KK, Perdue PW, Lynch W, Dziki A, Nevola J, Lee CH, Hayward I, Williams T, Law WR. Porcine peritoneal sepsis: modeling for clinical relevance. Shock 1(3):201–12, 1994. [PubMed] [Google Scholar]
- 47.Sam AD, Sharma AC, Law WR, Ferguson JL. Splanchnic vascular control during sepsis and endotoxemia. Front Biosci 2:e72–92, 1997. [DOI] [PubMed] [Google Scholar]
- 48.Sam AD, Sharma AC, Lee LY, Hales DB, Law WR, Ferguson JL, Bosmann HB. Sepsis produces depression of testosterone and steroidogenic acute regulatory (StAR) protein. Vol. 11, Shock. p. 298–3011999. [DOI] [PubMed] [Google Scholar]
- 49.Rincon JC, Cuenca AL, Raymond SL, Mathias B, Nacionales DC, Ungaro R, Efron PA, Wynn JL, Moldawer LL, Larson SD. Adjuvant pretreatment with alum protects neonatal mice in sepsis through myeloid cell activation. Clin Exp Immunol 191(3):268–78, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wynn JL, Scumpia PO, Stocks BT, Romano-Keeler J, Alrifai MW, Liu J-H, Kim AS, Alford CE, Matta P, Weitkamp J-H, et al. Neonatal CD71 + erythroid cells do not modify murine sepsis mortality. J Immunol 195(3):1064–70, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cuenca AG, Cuenca AL, Gentile LF, Efron PA, Islam S, Moldawer LL, Kays DW, Larson SD. Delayed emergency myelopoiesis following polymicrobial sepsis in neonates. Innate Immun 21(4):386–91, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li Y, Ke J, Peng C, Wu F, Song Y. microRNA-300/NAMPT regulates inflammatory responses through activation of AMPK/mTOR signaling pathway in neonatal sepsis. Biomed Pharmacother 108(August):271–9, 2018. [DOI] [PubMed] [Google Scholar]
- 53.Fallon EA, Chun TT, Young WA, Gray C, Ayala A, Heffernan DS. Program cell death receptor-1-mediated invariant natural killer T-cell control of peritoneal macrophage modulates survival in neonatal sepsis. Front Immunol 8(Nov):1–10, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Young WA, Fallon EA, Heffernan DS, Efron PA, Cioffi WG, Ayala A. Improved survival after induction of sepsis by cecal slurry in PD-1 knockout murine neonates. Surgery 161(5):1387–93, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Laitano O, Van Steenbergen D, Mattingly AJ, Garcia CK, Robinson GP, Murray KO, Clanton TL, Nunamaker EA. Xiphoid surface temperature predicts mortality in a murine model of septic shock. Shock 50(2):226–32, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Steele AM, Starr ME, Saito H. Late therapeutic intervention with antibiotics and fluid resuscitation allows for a prolonged disease course with high survival in a severe murine model of sepsis. Shock 47(6):726–34, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.O’Brien AJ, Terala D, Orie NN, Davies NA, Zolfaghari P, Singer M, Clapp LH. BK large conductance Ca2+-activated K+ channel-deficient mice are not resistant to hypotension and display reduced survival benefit following polymicrobial sepsis. Shock 35(5):485–91, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ganatra HA, Varisco BM, Harmon K, Lahni P, Opoka A, Wong HR. Zinc supplementation leads to immune modulation and improved survival in a juvenile model of murine sepsis. Innate Immun 23(1):67–76, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gentile LF, Nacionales DC, Lopez MC, Vanzant E, Cuenca A, Cuenca AG, Ungaro R, Szpila BE, Larson S, Joseph A, et al. Protective immunity and defects in the neonatal and elderly immune response to sepsis. J Immunol 192(7):3156–65, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bolognese AC, Yang W-L, Hansen LW, Sharma A, Nicastro JM, Coppa GF, Wang P. Activation of invariant natural killer T cells redirects the inflammatory response in neonatal sepsis. Front Immunol 9(APR):833, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hansen LW, Jacob A, Yang WL, Bolognese AC, Prince J, Nicastro JM, Coppa GF, Wang P. Deficiency of receptor-interacting protein kinase 3 (RIPK3) attenuates inflammation and organ injury in neonatal sepsis. J Pediatr Surg 53(9):1699–705, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fujioka K, Kalish F, Zhao H, Wong RJ, Stevenson DK. Heme oxygenase-1 deficiency promotes severity of sepsis in a non-surgical preterm mouse model. Pediatr Res 84(1):139–45, 2018. [DOI] [PubMed] [Google Scholar]
- 63.Gentile LF, Nacionales DC, Lopez MC, Vanzant E, Cuenca A, Szpila BE, Cuenca AG, Joseph A, Moore FA, Leeuwenburgh C, et al. Host responses to sepsis vary in different low-lethality murine models. PLoS One 9(5):1–10, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wynn JL, Kelly MS, Benjamin DK, Clark RH, Greenberg R, Benjamin DK, Smith PB. Timing of multiorgan dysfunction among hospitalized infants with fatal fulminant sepsis. Am J Perinatol 34(7):633–9, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Remick DG, Ayala A, Chaudry IH, Coopersmith CM, Deutschman C, Hellman J, Moldawer L, Osuchowski MF. Premise for standardized sepsis models. Shock 51(1):4–9, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hansen LW, Yang WL, Bolognese AC, Jacob A, Chen T, Prince JM, Nicastro JM, Coppa GF, Wang P. Treatment with milk fat globule epidermal growth factor-factor 8 (MFG-E8) reduces inflammation and lung injury in neonatal sepsis. Surgery 162(2):349–57, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fujioka K, Kalish F, Zhao H, Lu S, Wong S, Wong RJ, Stevenson DK. Induction of heme oxygenase-1 attenuates the severity of sepsis in a non-surgical preterm mouse model. Shock 47(2):242–50, 2017. [DOI] [PubMed] [Google Scholar]
- 68.Veach RA, Liu Y, Zienkiewicz J, Wylezinski LS, Boyd KL, Wynn JL, Hawiger J. Survival, bacterial clearance and thrombocytopenia are improved in polymicrobial sepsis by targeting nuclear transport shuttles. PLoS One 12(6):1–17, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Atkinson SJ, Varisco BM, Sandquist M, Daly MN, Klingbeil L, Kuethe JW, Midura EF, Harmon K, Opoka A, Lahni P, et al. Matrix metalloproteinase-8 augments bacterial clearance in a juvenile sepsis model. Mol Med 22:455–63, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ares GJ, McElroy SJ, Hunter CJ. The science and necessity of using animal models in the study of necrotizing enterocolitis. Semin Pediatr Surg 27(1):29–33, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lu P, Sodhi CP, Jia H, Shaffiey S, Good M, Branca MF, Hackam DJ. Animal models of gastrointestinal and liver diseases. Animal models of necrotizing enterocolitis: pathophysiology, translational relevance, and challenges. Am J Physiol - Gastrointest Liver Physiol 306(11)2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Good M, Sodhi CP, Ozolek JA, Buck RH, Goehring KC, Thomas DL, Vikram A, Bibby K, Morowitz MJ, Firek B, et al. Lactobacillus rhamnosus HN001 decreases the severity of necrotizing enterocolitis in neonatal mice and preterm piglets: evidence in mice for a role of TLR9. Am J Physiol - Gastrointest Liver Physiol 306(11)2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang C, Sherman MP, Prince LS, Bader D, Weitkamp JH, Slaughter JC, McElroy SJ. Paneth cell ablation in the presence of Klebsiella pneumoniae induces necrotizing enterocolitis (NEC)-like injury in the small intestine of immature mice. DMM Dis Model Mech 5(4):522–32, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lueschow SR, Stumphy J, Gong H, Kern SL, Elgin TG, Underwood MA, Kalanetra KM, Mills DA, Wong MH, Meyerholz DK, et al. Loss of murine Paneth cell function alters the immature intestinal microbiome and mimics changes seen in neonatal necrotizing enterocolitis. PLoS One 13(10):1–18, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hunter CJ, Singamsetty VK, Chokshi NK, Boyle P, Camerini V, Grishin AV, Upperman JS, Ford HR, Prasadarao NV. Enterobacter sakazakii enhances epithelial Cell injury by inducing apoptosis in a rat model of necrotizing enterocolitis. J Infect Dis 198(4):586–93, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Barlow B, Santulli TV, Heird WC, Pitt J, Blanc WA, Schullinger JN. An experimental study of acute neonatal enterocolitis-the importance of breast milk. J Pediatr Surg 9(5):587–95, 1974. [DOI] [PubMed] [Google Scholar]
- 77.Nguyen DN, Jiang P, Frøkiær H, Heegaard PMH, Thymann T, Sangild PT. Delayed development of systemic immunity in preterm pigs as a model for preterm infants. Sci Rep 6:36816, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lobe TE, Woodall DL, Griffin MP. Early hemodynamic indicators of gram-negative sepsis and shock in an infant pig model. J Pediatr Surg 26(9):1051–7, 1991. [DOI] [PubMed] [Google Scholar]
- 79.Park WS, Chang YS, Shim JW, Kim MJ, Ko SY, Kim SS, Hwang JH, Choi CW, Lee M. Effects of dopamine infusion on cerebral blood flow, brain cell membrane function and energy metabolism in experimental Escherichia coli meningitis in the newborn piglet. J Korean Med Sci 18(6):869–75, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.van Kaam AH, Lachmann RA, Herting E, De Jaegere A, van Iwaarden F, Noorduyn LA, Kok JH, Haitsma JJ, Lachmann B. Reducing atelectasis attenuates bacterial growth and translocation in experimental pneumonia. Am J Respir Crit Care Med 169(9):1046–53, 2004. [DOI] [PubMed] [Google Scholar]
- 81.Kato T, Hussein MH, Sugiura T, Suzuki S, Fukuda S, Tanaka T, Kato I, Togari H. Development and characterization of a novel porcine model of neonatal sepsis. Shock 21(4):329–35, 2004. [DOI] [PubMed] [Google Scholar]
- 82.Daoud GA, Kato T, Sugiura T, Nobata M, Suzuki S, Togari H. Edaravone, a novel free radical scavenger, reduces high-mobility group box 1 and prolongs survival in a neonatal sepsis model. Shock 32(6):586–92, 2009. [DOI] [PubMed] [Google Scholar]
- 83.Goto T, Hussein MH, Kato S, Daoud GA, Kato T, Sugiura T, Kakita H, Nobata M, Kamei M, Mizuno H, et al. Endothelin receptor antagonist attenuates oxidative stress in a neonatal sepsis piglet model. Pediatr Res 72(6):600–5, 2012. [DOI] [PubMed] [Google Scholar]
- 84.Pors SE, Skovgaard K, Brunse A, Sangild PT. Oral supplementation with bovine colostrum prevents septic shock and brain barrier disruption during blood stream infection in preterm newborn pigs. Shock 51(3):337–47, 2019. [DOI] [PubMed] [Google Scholar]
- 85.Batts SG, Mu TS, Uyehara-Lock JH, Murata L-A, Uyehara CFT. ECMO maintains cerebral blood flow during endotoxic shock in piglets. ASAIO J 62(6):732–6, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Levesque CL, Turner J, Li J, Wizzard P, St Pierre B, Lim D, Wales P. In a neonatal piglet model of intestinal failure, administration of antibiotics and lack of enteral nutrition have a greater impact on intestinal microflora than surgical resection alone. JPEN J Parenter Enteral Nutr 41(6):938–45, 2017. [DOI] [PubMed] [Google Scholar]
- 87.Rasmussen SO, Martin L, Østergaard MV, Rudloff S, Roggenbuck M, Nguyen DN, Sangild PT, Bering SB. Human milk oligosaccharide effects on intestinal function and inflammation after preterm birth in pigs. J Nutr Biochem 40:141–54, 2017. [DOI] [PubMed] [Google Scholar]
- 88.Call L, Stoll B, Oosterloo B, Ajami N, Sheikh F, Wittke A, Waworuntu R, Berg B, Petrosino J, Olutoye O, et al. Metabolomic signatures distinguish the impact of formula carbohydrates on disease outcome in a preterm piglet model of NEC. Microbiome 6(1):1–15, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Roy SK, Meng Q, Sadowitz BD, Kollisch-Singule M, Yepuri N, Satalin J, Gatto LA, Nieman GF, Cooney RN, Clark D. Enteral administration of bacteria fermented formula in newborn piglets: a high fidelity model for necrotizing enterocolitis (NEC). PLoS One 13(7):e0201172, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Namachivayam K, Blanco CL, MohanKumar K, Jagadeeswaran R, Vasquez M, McGill-Vargas L, Garzon SA, Jain SK, Gill RK, Freitag NE, et al. Smad7 inhibits autocrine expression of TGF-β2 in intestinal epithelial cells in baboon necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol 304(2):G167–80, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kerecman J, Mehrotra A, Goodman Z. Liver disease after intensive care of premature baboons: histopathologic observations. J Pediatr Gastroenterol Nutr 57(2):172–9, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kelleher MA, Liu Z, Wang X, Kroenke CD, Houser LA, Dozier BL, Martin LD, Waites KB, McEvoy C, Schelonka RL, et al. Beyond the uterine environment: a nonhuman primate model to investigate maternal-fetal and neonatal outcomes following chronic intrauterine infection. Pediatr Res 82(2):244–52, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hemming VG, London WT, Fischer GW, Curfman BL, Baron PA, Gloser H, Bachmayer H, Wilson SR. Immunoprophylaxis of postnatally acquired group B streptococcal sepsis in neonatal rhesus monkeys. J Infect Dis 156(4):655–8, 1987. [DOI] [PubMed] [Google Scholar]
- 94.Premaratne S, May ML, Nakasone CK, McNamara JJ. Pharmacokinetics of endotoxin in a rhesus Macaque septic shock model. J Surg Res 59(4):428–32, 1995. [DOI] [PubMed] [Google Scholar]
- 95.Phillips KA, Bales KL, Capitanio JP, Conley A, Czoty PW, ‘t Hart BA, Hopkins WD, Hu S, Miller LA, Nader MA, et al. Why primate models matter. Am J Primatol 76(9):801–27, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Using a very small gauge needle (such as a tuberculin syringe), a bacterial slurry is injected into the intraperitoneal cavity of a neonatal mouse to induce sepsis, carefully avoiding trauma to the abdominal organs during the injection process.


