Abstract
The chronic variable stress (CVS) paradigm is frequently used to model the changes in hypothalamic pituitary adrenal (HPA) axis function characteristic of many stress-related diseases. However, male C57BL/6 mice are typically resistant to CVS’s effects, making it difficult to determine how chronic stress exposure may alter acute HPA function and regulation in these mice. As social support in rodents can profoundly influence physiological and behavioral processes, including the HPA axis, we sought to characterize the effects of CVS exposure on basal and acute stress-induced HPA axis function in pair- and single- housed adult male mice. Despite all subjects exhibiting decreased body weight gain after six weeks of CVS, the corticosterone response to a novel, acute restraint stressor was enhanced by CVS exclusively in single-housed males. CVS also significantly increased arginine vasopressin (AVP) mRNA in the hypothalamic paraventricular nucleus (PVN) in single-housed males only. Moreover, in single-, but not pair-housed mice, CVS attenuated decreases in circulating OT found following acute restraint. Only the effect of CVS to elevate PVN corticotropin releasing hormone (CRH) mRNA levels after an acute stressor was restricted to pair-housed mice. Collectively, our findings suggest that social isolation reveals effects of CVS on the HPA axis in male C57BL/6 mice.
Keywords: chronic variable stress (CVS), social environment, paraventricular nucleus (PVN), corticotropin releasing hormone (CRH), vasopressin (AVP), oxytocin (OT)
Introduction
Although acute activation of the hypothalamic pituitary adrenal (HPA) axis enables survival-oriented responses to stressful situations, (Tsigos and Chrousos, 2002) its prolonged activation by chronic stressors can increase risk for cardiometabolic and psychiatric disorders (De Kloet et al, 2005; Sapolsky et al, 2000). Thus, understanding HPA axis regulation under chronic stress conditions has potential to shed light on the pathology of some stress-related diseases. Neuropeptides, including corticotropin-releasing hormone (CRH), arginine vasopressin (AVP) and oxytocin (OT), play a fundamental role in controlling the HPA axis, as they are directly synthesized and secreted by neuroendocrine neurons located in the hypothalamic paraventricular nucleus (PVN) in response to stressors (Gibbs et al, 1984; Whitnall, 1993). Their secretion into the hypothalamo-hypophyseal portal circulation allows for the release of adrenocorticotropic hormone (ACTH) from the anterior pituitary and subsequent glucocorticoid secretion by the adrenal cortex. Glucocorticoids (e.g. cortisol in humans and corticosterone (CORT) in rats and mice), in turn, mobilize energy stores to facilitate appropriate body wide responses (Sapolsky et al, 2000). Chronic stressors can act at any of these levels of the HPA axis to disrupt its normal functioning (Flak et al, 2011; Herman et al, 1995; Ostrander et al, 2006).
Notably, social environment can also influence behavioral and physiological processes in rodents, including the HPA axis (DeVries et al, 2007; Kappel et al, 2017). Previous studies have shown altered basal and acute stress-induced CORT secretion in isolated versus socially housed rodents (Berry et al, 2012; Ros-Simó and Valverde, 2012). Furthermore, limbic regulation of the HPA axis may vary in male rats depending on housing condition, as a distinct pattern of neuronal activation has been found in group- versus single-housed males (Westenbroek et al, 2003a). Although few in number, studies have also begun to investigate the effect of social environment on HPA axis function following chronic stress, revealing adrenal hypertrophy and unique limbic neuronal activation in group- versus single-housed male rats (Westenbroek et al, 2003b, 2003a).
In the current studies, we investigated whether social support influences the HPA response to chronic stress in C57BL/6 mice, a strain frequently used to study HPA axis regulation, but typically resistant to the effects of chronic variable stress (CVS) (Chan et al, 2017). The CVS paradigm involves exposure to a variety of mild stressors applied in an unpredictable manner over a prolonged period of time and effectively reproduces behavioral and physiological changes found in patients with stress-related diseases (Willner, 2017). Thus, it is advantageous for studying changes in HPA activity following chronic stress that may eventually increase stress-related disease risk. Using the CVS paradigm, we examined the effect of social versus isolation housing on the basal and stress-induced activity of the HPA axis in CVS- and control-treated male mice. Results show that predominantly single-housed males exhibited altered HPA axis activity following CVS, indicated by a sensitized CORT response and attenuated circulating OT response to acute stress, as well as elevated PVN AVP mRNA levels. Thus, social isolation unmasks the effects of CVS on the HPA axis in male C57BL/6 mice.
Methods
Subjects
Two-month-old male C57BL/6N mice were purchased from Charles Rivers Laboratories (Wilmington, MA) and maintained on a 12:12 light cycle (lights on at 0600 h) in the Colorado State University Laboratory Animal Research facility. Subjects were pair- or single- housed in one of two colony rooms and allowed to equilibrate to the Laboratory Animal Research facility for at least one week prior to the start of experiments. Because of fighting between cage mates, 12 pair-housed animals were immediately separated and used to supplement the number of animals (38) in the single-housed cohorts. No significant differences between mice who were separated because of aggression and those who were not were found for any of the parameters examined in these studies. Access to food and water was available ad libitum. All animal protocols were approved by the Colorado State University Institutional Animal Care and Use Committee and were performed in accordance with the guidelines of Colorado State University, the National Institutes of Health, and the Association for Assessment and Accreditation of Laboratory Animal Care International.
CVS and novel, acute restraint exposure
Adult male mice were exposed to CVS daily, over a six-week period, as previously described (Borrow et al, 2018). Briefly, subjects experienced an average of two semi-random stressors per day, including a morning and/or evening stressor administered at random times before 1200 h or after 1200 h, respectively. Stressors included three hours of occupying a cage with damp bedding, no bedding, or bedding soiled by same-sex unfamiliar mice, three hours of cage tilt (approximately 45°), one hour of exposure to cat odor, eight hours of white noise (85 dB) and overnight exposure to overhead light. Control subjects were housed in a separate colony room to prevent unintended stress exposure. Subjects were weighed at baseline and at six weeks after the onset of CVS or control conditions.
The morning (0900 h-1100 h) after the last CVS stressor, CVS-treated and control single- (n=10-11) or pair- (n=8-10) housed subjects were exposed to 20 minutes of restraint stress in a plexiglass tube placed inside their home cages, or they were left undisturbed. For the pair-housed animals, one cage mate was restrained while the other non-stressed cage mate was killed within one minute of first cage disturbance. All subjects were anesthetized with isoflurane, weighed, and decapitated immediately following the first cage disturbance or the termination of restraint. Upon decapitation, trunk blood was collected from all animals in chilled tubes containing 0.5 M EDTA and aprotinin (4 mg/ml; Sigma-Aldrich, St. Louis, MO) and centrifuged at 2000 rpm for 10 min in a Beckman J6 centrifuge. Plasma was isolated and stored at −20°C until assayed for CORT and OT by radioimmunoassay (RIA). Brains and adrenal glands were also collected shortly following decapitation, flash-frozen in 2 methyl butane at −40°C, and stored at −80°C.
Radioimmunoassays
Plasma CORT was measured as previously described (Weiser and Handa, 2009). Briefly, plasma was diluted (1:25) in PBS and plasma binding proteins were denatured at 65°C for one hour. Diluted plasma samples were incubated overnight at 4°C with rabbit anti-CORT antiserum (1:1200, Cat# 7120016, RRID:AB_2801269, MP Biomedicals, Sonon OH) and 3H-CORT (PerkinElmer, Boston, MA) in 0.01 M PBS containing 0.1% gelatin. Dextran coated charcoal was used to separate antibody bound CORT from free CORT. Standard curves were constructed from dilutions of CORT (4-pregnen-11β, 21-diol-3, 20-dione; Steraloids, Wilton, NH; 5–500 ng/ml). The intra-assay coefficient of variation was less than 10%. The lower limit of quantitation, set at 90% of total binding, was 3.34 ng/ml. Three nonstressed control single-housed mice were excluded from analyses due to technical issues with the CORT radioimmunoassay.
To measure circulating OT levels, plasma was subjected to an extraction protocol that was adapted from a previous report (Moenter et al, 1991) using methanol. Briefly, 2.0 ml methanol was added to all samples and following vortex and centrifugation the supernatant was further subjected to cold precipitation (−20°C) for 24 hours. After three rounds of cold precipitation, the supernatant was dried under nitrogen at 45°C and resuspended in RIA buffer to the same volume as the starting amount. This product was then used in the protocol for an Oxytocin RIA kit (RK-051-01; Phoenix Pharmaceuticals, Burlingame, CA) following manufacturer’s instructions. The extraction procedure resulted in the recovery of 69.1% of the starting material as determined by spiking samples with known amounts of 125I-oxytocin. The intra-assay coefficient of variation was 4.5%.
In situ hybridization
16-μm brain sections were cut into five series in the coronal plane using a CM3050 S cryostat (Leica, Wetzlar). Sections containing the supraoptic nucleus (SON) and/or PVN were cut at −20°C, thaw mounted onto positively charged slides (Superfrost Plus, VWR Scientific, West Chester, PA), and stored at −80°C. In situ hybridization was performed as previously described (Borrow et al, 2018). Briefly, tissue was thawed to room temperature, fixed, acetylated, delipidated, dehydrated in graded ethanols and air-dried. 48-bp oligonucleotide mRNA probes for Crh (5’CAGTTTCCTGTTGCTGTGAGCTTGCTGAGCTAACTGCTCTGCCCGGGC-3’), Ot(5’-AAGCAGGCAGCAAGCGAGACTGGGGCAGGCCATGGCGATGGTGCTCAG-3‘) and Avp (5’GTAGACCCGGGGCTTGGCAGAATCCACGGAC TCCCGTGTCCCAGCCAG-3’) were end-labeled with [35S] dATP using terminal deoxynucleotidyl transferase (Thermo Scientific, Waltham, MA) and added to hybridization solution at a concentration of 20 × 106 cpm/mL. Brain sections were incubated in this hybridization solution at 37°C overnight and then washed and dehydrated in a series of solutions with increasing levels of ethanol. To examine hybridization intensity, slides were exposed to X-ray film (Carestream Kodak Biomax MR, Carestream, Rochester, NY) for 4 days (CRH), or overnight (AVP and OT) to generate autoradiograms. Analysis of film autoradiograms was conducted using ImageJ software (version 1.51r). Optical density was quantified bilaterally throughout the rostral-caudal extent of the PVN in three (for CRH), four (for AVP), or six (for OT) adjacent and anatomically matched sections using a template of fixed size (1.8 μm2). Optical density representing AVP or OT mRNA was also quantified bilaterally in two anatomically matched sections containing the SON using the same sized template. The density of exposed pixels in each half of the PVN or SON for all sections was expressed as arbitrary density units (ADUs). Background density in an adjacent area without labeling was subtracted from each measurement and resulting ADUs were averaged to obtain a single value per animal for statistical analysis.
Statistical Analysis
All statistical analyses were performed using the Prism statistical program (GraphPad Software, La Jolla, CA). The effects of CVS treatment on body weights and adrenal weights were examined using two-way ANOVAs (CVS X housing condition). All other statistical comparisons were made using three-way ANOVAs (CVS X housing X restraint). Because the studies in this manuscript were designed based on the hypothesis that single versus pair housing would influence the HPA axis response to CVS or restraint, we performed post hoc analyses to examine effects of CVS and restraint stress within pair- and single-housed groups. Post hoc analyses were performed with two-way ANOVAs (CVS X restraint) and the Fisher’s Least Significant Differences test. Significance was set at p<0.05. Additionally, all data were analyzed using the Extreme Studentized Deviate method (GraphPad) to detect significant outliers. 7 outliers, were detected in the following data sets and excluded from further analyses: for plasma CORT, 1 stressed control single-housed male; for PVN AVP mRNA, 1 stressed CVS pair-housed male and 1 nonstressed CVS single-housed male; for SON OT mRNA, 1 nonstressed CVS single-housed male; for plasma OT: 1 nonstressed control pair-housed male, 1 stressed control single-housed male, and 1 stressed CVS single-housed male.
Results
Effects of CVS exposure on physiological measures in pair- versus single-housed mice
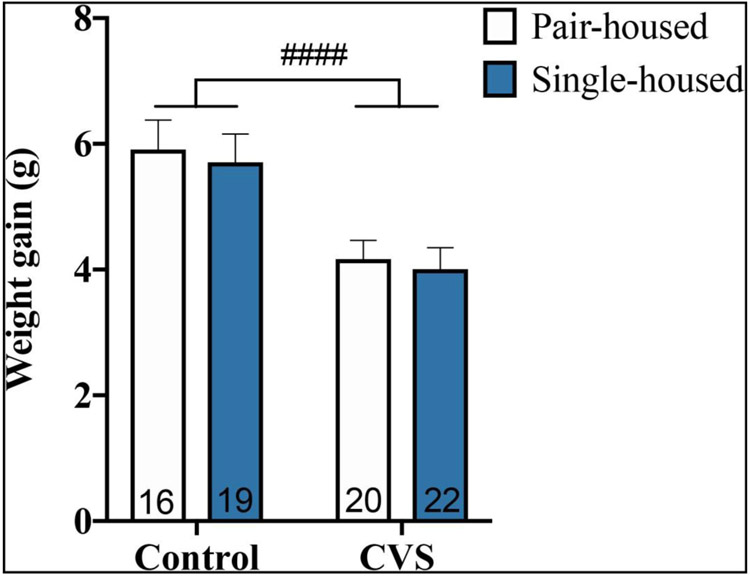
To initially assess the efficacy of our CVS model, we examined changes in body weight gain relative to baseline at six weeks following the start of the CVS protocol in control- and CVS-treated subjects (Figure 1). A two-way ANOVA (CVS X housing condition) revealed a main effect of CVS to decrease weight gain, regardless of housing condition (F(1,73)=19.58; p<0.0001).
Figure 1.
Weight gain relative to baseline weight of pair- and single-housed male mice after six-week exposure to chronic variable stress (CVS) or control conditions. Each bar represents mean ± SEM, and numbers within each bar indicate the number of animals used for each group. ####=p<0.0001 for a main effect of CVS.
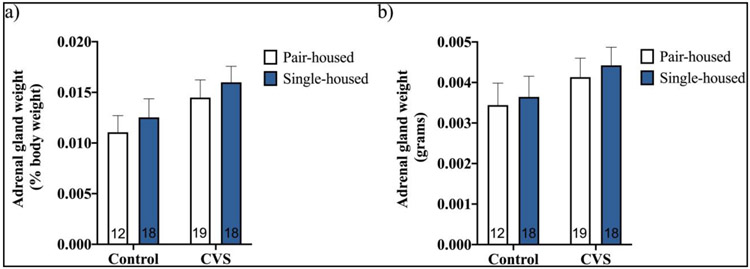
We also compared weights of adrenal glands collected from CVS-treated versus control pair- and single-housed subjects to begin our examination of CVS’s effects on the activity of the HPA axis (Figure 2). No significant effects of CVS, housing condition, or interaction on adrenal weight expressed as a percentage of body weight (Figure 2a) or on total adrenal weight (Figure 2b) were found by two-way ANOVA (CVS X housing condition).
Figure 2.
Adrenal weights (% of body weight (a); total (b)) of pair- or single-housed male mice exposed to six weeks of chronic variable stress (CVS) or control conditions. Each bar represents mean ± SEM, and numbers within each bar indicate the number of animals used for each group.
Effect of housing condition on the CORT response to restraint stress and/or CVS
Plasma CORT levels immediately following a novel 20-minute restraint stressor were elevated in CVS- and control-treated subjects in a housing condition-dependent fashion (Figure 3). A three-way ANOVA (CVS X housing X restraint) accordingly showed significant effects of CVS (F(1,64)=4.080; p<0.05), restraint (F(1,64)=745.6; p<0.0001), CVS by housing interaction (F(1,64)=5.154; p<0.05), and CVS by restraint interaction (F(1,64)=7.751; p<0.01). Because we originally hypothesized that single versus pair housing would influence the CORT response to restraint and/or CVS, we performed two-way ANOVAs (CVS X restraint) to examine effects of CVS and restraint stress within pair- and single-housed groups. Whereas only a main effect of restraint stress to increase plasma CORT levels was found in pair-housed males (F(1,32)=464.1; p<0.0001), main effects of CVS (F(1,32)=7.815; p<0.01), restraint (F(1,32)=307.9; p<0.0001) and CVS by restraint interaction (F(1,32)=7.359; p<0.05) were found in single-housed males. Specifically, single-housed males exhibited significantly higher restraint-induced CORT levels following CVS exposure (p<0.001).
Figure 3.
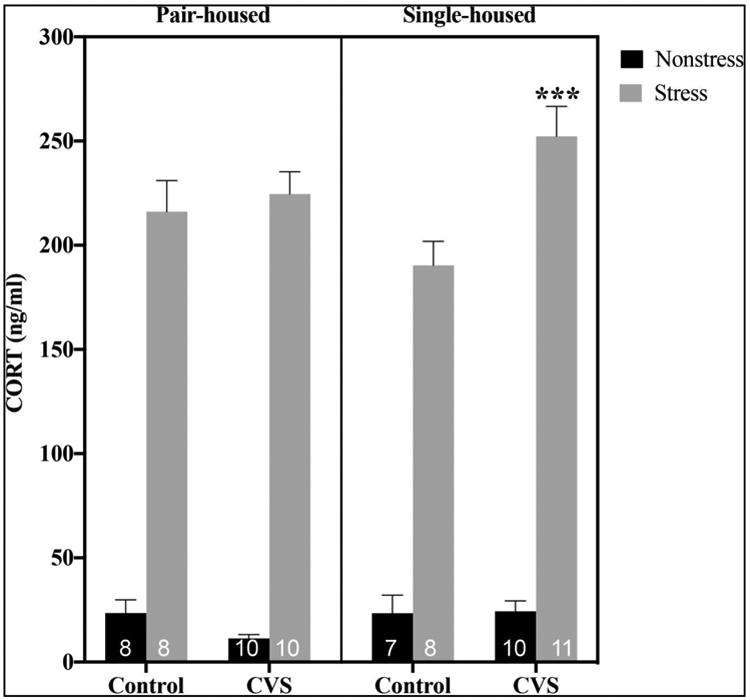
Plasma corticosterone (CORT) levels at baseline and 20 minutes after the initiation of restraint stress in control or chronic variable stress (CVS)-treated pair- versus single-housed male mice. Each bar represents mean ± SEM, and numbers within each bar indicate the number of animals used for each group. ***=p<0.001 for restrained single-housed CVS-treated versus restrained single-housed control animals.
Effect of housing condition on hypothalamic neuropeptide responses to restraint stress and/or CVS
AVP
Depending on housing conditions, Avp gene expression in the PVN was altered following CVS in male mice (Figure 4a and b). Although no significant CVS, housing, restraint or interaction effects were found by three-way ANOVA (CVS X housing X restraint), planned comparisons using two-way ANOVAs revealed a significant main effect of CVS to increase PVN AVP mRNA levels in single- (F(1,34)=4.999; P<0.05), but not pair-, housed mice.
Figure 4.
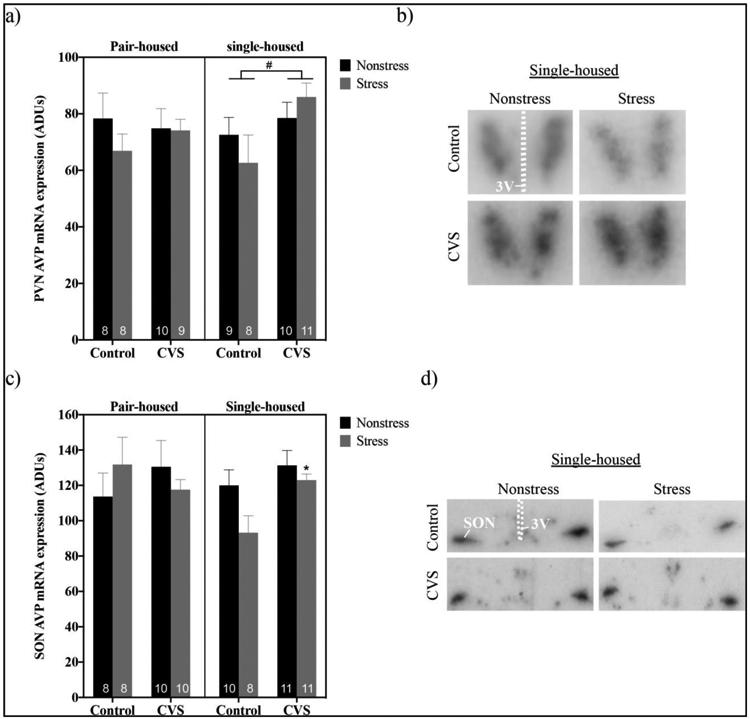
Levels of arginine vassopressin (AVP) mRNA at baseline and after 20 minutes of restraint stress within the paraventricular nucleus (PVN) (a,b) and supraoptic nucleus (SON) (c,d) of pair- versus single-housed male mice exposed to chronic variable stress (CVS) or control conditions. Arbitrary density units (ADUs) calculated from in situ hybridization autoradiograms of AVP mRNA are shown in panels a and c. Each bar represents mean ± SEM, and numbers within each bar indicate the number of animals used for each group. #=p<0.05 for a main effect of CVS present in single-, but not pair-, housed mice. *=p<0.05 for stressed CVS-treated versus stressed control-treated single-housed males. Panels b and d show representative autoradiograms of AVP hybridization in the PVN (b) and SON (d) of single-housed mice. 3V=third ventricle.
To determine if the housing-dependent effect of CVS we observed on Avp gene expression was unique to the PVN, we also examined AVP mRNA in the SON following CVS and acute restraint in pair- versus single-housed males (Figure 4c and d). A three-way ANOVA (CVS x housing x restraint) showed a trend toward a significant effect of CVS by housing by restraint interaction (F(1,68)=2.891; p=0.0936; and main effects of CVS (F(1,37)=7.099; p<0.05) and restraint (F(1,37)=5.175; p<0.05) were found by planned two-way ANOVAs (CVS x restraint) in single-, but not pair-, housed mice. Specifically, there was a trend toward a significant effect of restraint stress to decrease SON AVP mRNA in control- (p=0.0777), but not CVS-treated, single-housed males. Moreover, stressed CVS-treated single-housed males had elevated AVP mRNA levels versus stressed control-treated ones (p<0.05).
OT
PVN and SON OT mRNA levels were unaltered by restraint and/or CVS, regardless of housing condition (Figure 5a and b). Accordingly, no significant effects of CVS, restraint stress, housing condition, or interaction were found by three-way ANOVAs (CVS X housing X restraint). Planned comparisons examining effects of CVS and restraint in single-housed and pair-housed mice by two-way ANOVAs also revealed no significant effects.
Figure 5.
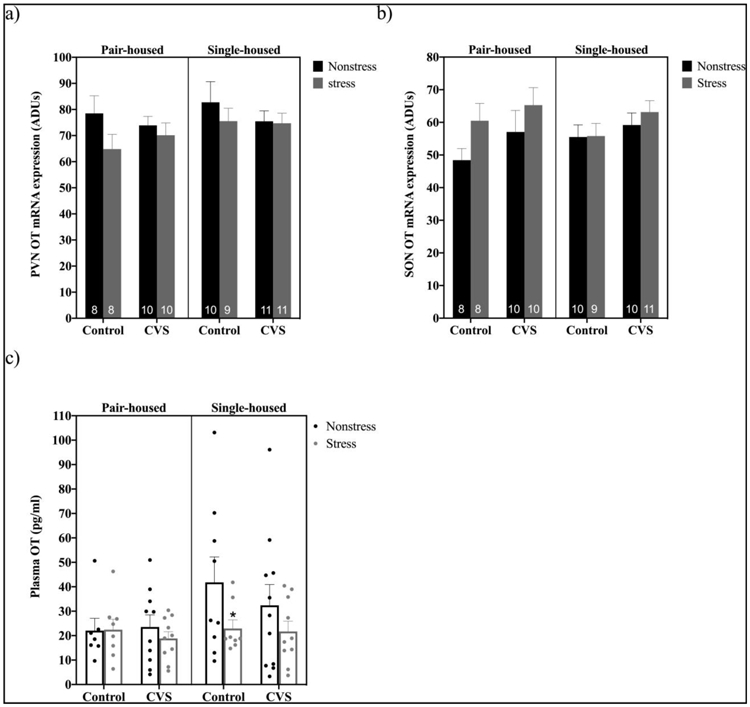
Levels of hypothalamic oxytocin (OT) mRNA and plasma OT at baseline and after 20 minutes of restraint stress in pair- versus single-housed male mice exposed to chronic variable stress (CVS) or control conditions. Panels a and b show mean ± SEM arbitrary density units (ADUs) calculated from in situ hybridization autoradiograms of OT mRNA within the paraventricular nucleus (PVN) (a) and supraoptic nucleus (SON) (b). Panel c shows mean ± SEM plasma OT levels in pair- versus single-housed mice. *= p<0.05 for stressed versus nonstressed control single-housed males. Numbers within each bar indicate the number of animals used for each group.
However, plasma levels of OT were decreased by restraint stress in a housing condition and CVS-dependent fashion (Figure 5c). A three-way ANOVA (CVS X housing X restraint) showed trends toward effects of restraint stress (F(1,65)=3.630; p=0.0612) and of housing condition (F(1,65)=3.219; p=0.0774) on circulating OT levels. Additionally, planned comparisons by two-way ANOVAs (CVS x restraint) demonstrated a strong trend toward a main effect of restraint stress to decrease plasma OT levels in single- (F(1,34)=3.857; p=0.0577), but not pair-, housed males. Based on the hypothesis that CVS exposure would alter hypothalamic neuropeptide responses to acute stress, we further examined the effects of restraint stress in control versus CVS treated pair- and single-housed mice. A significant decrease in plasma OT levels after restraint stress was present in control-, but not CVS-, treated single-housed males (p<0.05).
CRH
PVN CRH mRNA levels were enhanced by restraint stress depending on housing conditions and exposure to CVS (Figure 6). A three-way ANOVA (CVS X housing X restraint) showed a main effect of restraint stress (F(1,61)=21.71; p<0.0001) on CRH mRNA levels, as did planned comparisons by two-way ANOVAs (CVS x restraint) within pair- (F(1,23)=8.740; p<0.01) and single-housed (F(1,38)=15.82; p<0.001) males. Because we originally hypothesized that CVS exposure would alter hypothalamic neuropeptide expression, we also examined the effects of restraint stress in control versus CVS treated pair- and single-housed mice. PVN CRH mRNA levels were elevated following restraint stress in CVS- (p<0.01), but not control-, treated pair-housed mice. In single-housed mice, alternatively, restraint stress increased PVN CRH mRNA in both control- (p<0.05) and CVS-treated (p<0.01) males.
Figure 6.
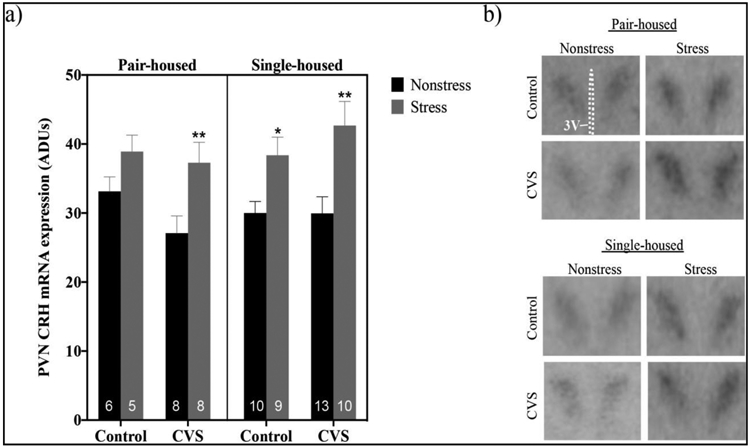
Levels of corticotropin releasing hormone (CRH) mRNA at baseline or after 20 minutes of restraint stress within the paraventricular nucleus (PVN) of pair- versus single-housed male mice exposed to chronic variable stress (CVS) or control conditions. Arbitrary density units (ADUs) calculated from in situ hybridization autoradiograms of CRH mRNA are shown in panel a. Each bar represents mean ± SEM, and numbers within each bar indicate the number of animals used for each group. *=p<0.05 and **=p<0.01 for restraint stressed males versus non-stressed males of the same treatment and housing condition. Panel b shows representative autoradiograms of CRH hybridization in the PVN of pair- and single-housed male mice. 3V= third ventricle.
Discussion
Male C57BL/6 mice are typically resistant to CVS’s effects, making it difficult to determine how chronic stress may alter the acute HPA function and regulation often examined in this mouse strain (Chan et al, 2017). As social support in male rodents can alter HPA axis activity (Berry et al, 2012; Ros-Simó and Valverde, 2012), we sought to determine if housing condition could influence the HPA response to CVS in C57BL/6 mice. Specifically, we characterized the effects of CVS exposure on basal and acute stress-induced HPA axis function in pair- and single-housed male mice. The results of these studies indicate that CVS alters HPA axis activity predominately in single-housed males. Although subjects exhibited decreased body weight gain, irrespective of housing condition, the CORT response to a novel, acute stressor was enhanced by CVS exclusively in single-housed males. Likewise, we found that CVS elevated PVN AVP mRNA and prevented the SON AVP mRNA response to an acute stressor only in single-housed males. Furthermore, CVS attenuated the circulating OT response to acute restraint in single-, but not pair-, housed mice. Interestingly, only the effects of CVS on PVN CRH mRNA levels after an acute stressor were restricted to pair-housed mice. Thus, social isolation ultimately leaves C57BL/6 male mice more sensitive to CVS-induced changes in the HPA axis.
Typically, the C57BL/6 strain is thought to be relatively stress-resistant, exhibiting lesser behavioral and physiological responses to stressors, including reduced HPA responses (Chan et al, 2017). Compared to BALB/cByJ mice, C57BL/6ByJ mice, for example, have lesser stress-induced ACTH and CORT levels, as well as decreased secretion of CRH and AVP at the median eminence (Anisman and Matheson, 2005; Shanks et al, 1990). However, one study did report an effect of CVS to increase adrenal weight and serum CORT in group-housed male C57BL/6 mice (Monteiro et al, 2015). Because an eight-week protocol rather than a four-week protocol was necessary to induce these changes (Monteiro et al, 2015), the limited CVS effects on HPA activity observed in pair-housed male C57BL/6 mice after six weeks in our studies is not unusual. As previously reported in C57BL/6 mice (Castañeda et al, 2011), we also observed decreased body weight gain in mice post CVS, supporting the efficacy of our CVS protocol.
Despite their relative stress-resistance, C57BL/6 mice often exhibit altered HPA activity following social isolation. In male C57BL/6 mice, reduced plasma CORT levels and a reduced difference between the right and left adrenal glands have been reported after chronic social isolation stress, suggesting a state of isolation-provoked HPA hyporeactivity (Ieraci et al, 2016). Conversely, other studies suggest a more hyperactive state of the HPA axis in socially deprived versus group-housed C57BL/6 mice indicated by increased adrenal weight (Berry et al, 2012). Higher CORT levels in response to a social challenge were also found in these socially deprived C57BL/6 mice (Berry et al, 2012). Although other studies have indicated no effect of social isolation on plasma CORT levels after four (Berry et al, 2012) or eight weeks (Sun et al, 2014) of single housing, social isolation remains well positioned to influence the HPA response to CVS in C57BL/6 mice.
Studies previously examining the effect of social environment on HPA activity under chronic stress conditions are limited, especially in C57BL/6 mice. However, one group demonstrated adrenal hypertrophy in group-housed, but not isolated male rats, as well as housing-dependent limbic c-Fos expression (Westenbroek et al, 2003a, 2003b). Westenbroek et al. 2003 notably suggest that group housing enhances the effects of chronic stress in rats, whereas our findings suggest that isolation may have a greater effect. In the present studies, CVS induced sensitization of the CORT response to acute stress in single-, but not pair-housed mice. Although the two housing groups could have different temporal dynamics for observing sensitization after CVS, these findings support the possibility that, at least when socially isolated, male C57BL/6 mice exhibit altered HPA activity as is often reported in CVS-treated rats (Herman et al, 1995; Ostrander et al, 2006; Ulrich-Lai et al, 2007). Importantly, discrepancies between our findings and those of Westenbroek et al. 2003 could be related to the caveat that pairing rodents may not be the same as group housing rodents, which may lead to increased competition for resources and a subsequent changes in the HPA axis (Beery and Kaufer, 2015; Brown and Grunberg, 1995). Nonetheless, our findings suggest that housing condition greatly influences the HPA axis response to chronic stress in C57BL/6 mice.
We also observed an effect of CVS to elevate PVN AVP mRNA and to oppose the SON Avp response to a novel, acute stressor exclusively in single-housed mice. If or how these changes in AVP mRNA influence the activity of the HPA axis remains to be determined. Although magnocellular AVP neurons in both the PVN and SON have been implicated in HPA axis control, the PVN, unlike the SON, also contains parvocellular AVP neurons, which directly regulate HPA activity, synergizing with CRH to stimulate ACTH production by the anterior pituitary (Herman et al, 2008; Lolait et al, 2007). Thus, the fact that CVS differentially affected PVN and SON AVP mRNA levels may highlight varying functions for magnocellular versus parvocellular AVP neurons following CVS exposure. Accordingly, in the PVN, changes in AVP mRNA in parvocellular neurons after CVS may contribute to the greater levels of AVP peptide available to stimulate the CORT response to restraint. AVP mRNA in magnocellular PVN neurons, like in those of the SON, may exhibit a differential response following CVS due to their greater involvement in regulating other physiological functions, such as body fluid homeostasis (Armstrong, 2015).
In these studies, we further examined effects of CVS on OT expression due to OT’s known involvement in regulating HPA axis activity. Peripherally, OT can act on the anterior pituitary to stimulate ACTH production and enhance HPA output (Gibbs et al, 1984). An important role for PVN OT neurons to locally inhibit PVN CRH neurons and thereby limit HPA activity has also been described (Neumann et al, 2000). Although we observed no changes in hypothalamic OT mRNA following CVS, we found CVS-induced alterations in circulating levels of OT, but only in single-housed subjects. Specifically, CVS attenuated the decrease in plasma OT found following restraint stress in control, single-housed males. This effect of restraint notably contrasts with studies in rats demonstrating an effect of acute stress to increase OT secretion from PVN neurons, elevating levels in plasma (Lang et al, 1983; Torner et al, 2017). Our findings may indicate a substantial effect of social environment to influence HPA axis activity, as previous studies examined socially housed rodents. Nonetheless, the effect of CVS to attenuate the plasma OT response to restraint in single-housed males could reflect an increase in OT acting at the level of the pituitary during restraint, leading to increased ACTH and ultimately CORT secretion under acute stress conditions (Gibbs et al, 1984). This may partly explain the augmented CORT response to a novel, acute stressor we observed in CVS-treated, isolated males. Unfortunately, the volume of plasma obtained from mice was insufficient to assay other hormones. Future studies will examine changes in circulating AVP, CRH, and ACTH following restraint stress and CVS under varying housing conditions to provide a more complete picture of how social environment influences the HPA axis.
Whereas CVS altered Avp and OT expression exclusively in single-housed male mice, it altered PVN CRH mRNA levels only in pair-housed males. Specifically, PVN CRH mRNA levels were increased by acute restraint stress in CVS-treated, but not control, pair-housed males. Single-housed males, in contrast, exhibited elevated CRH mRNA after acute restraint, irrespective of previous CVS exposure. Whether the effect of CVS in pair-housed males influences the activity of the HPA axis remains to be determined, yet it suggests that CVS treated pair-housed males, like single-housed males, are more responsive to acute restraint. Although acute stress-induced CORT levels in pair-housed males were not changed by CVS, the possibility remains that ACTH levels would have been increased due to an eventual increase in circulating CRH. If such increases in ACTH ultimately increased glucocorticoid synthesis, then this would further support the possibility that different temporal dynamics exist for observing sensitization after CVS in pair versus single-housed animals.
Notably, the changes in PVN CRH mRNA and SON AVP mRNA we observed after 20 minutes of restraint are surprisingly rapid changes for mRNA (Herman, 1995; Ma et al, 1997). Thus, further investigation is certainly necessary to determine mechanisms for the changes in mRNA levels we observed. To influence the pool of mRNA present at any given time, restraint stress and CVS can either influence mRNA synthesis or degradation. It is possible, therefore, that, for SON AVP, 20-minute restraint enhanced mRNA degradation to rapidly decrease mRNA levels in control single-housed mice and that CVS reprogrammed the degradation and/or transcriptional machinery to eliminate the Avp response to restraint. For PVN CRH mRNA, 20-minute restraint more likely enhanced mRNA synthesis in single-house mice, as well as in pair—housed CVS-treated controls.
Conclusions
Collectively, the results of these studies have demonstrated CVS-invoked changes predominantly in the HPA axis of single- rather than pair-, housed male mice. Thus, at least when socially isolated, C57BL/6 male mice can be used to examine HPA function and regulation in times of chronic stress using a physiological model that is thought to be etiologically relevant for human stress-related disease risk.
Acknowledgements
This research was supported by the National Institutes of Health (Grant RO1-DK105826 to R.J.H.)
Footnotes
Disclosure Summary: The authors have nothing to disclose.
References
- Anisman H, Matheson K (2005). Stress, depression, and anhedonia: Caveats concerning animal models. Neurosci Biobehav Rev 29: 525–546. [DOI] [PubMed] [Google Scholar]
- Armstrong WE (2015). Hypothalamic supraoptic and paraventricular nuclei. Rat Nerv Syst Fourth Ed 295–21doi: 10.1016/B978-0-12-374245-2.00014-0. [DOI] [Google Scholar]
- Beery AK, Kaufer D (2015). Stress, social behavior, and resilience: Insights from rodents. Neurobiol Stress 1: 116–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry A, Bellisario V, Capoccia S, Tirassa P, Calza A, Alleva E, et al. (2012). Social deprivation stress is a triggering factor for the emergence of anxiety- and depression-like behaviours and leads to reduced brain BDNF levels in C57BL/6J mice. Psychoneuroendocrinology 37: 762–772. [DOI] [PubMed] [Google Scholar]
- Borrow AP, Bales NJ, Stover SA, Handa RJ (2018). Chronic variable stress induces sex-specific alterations in social behavior and neuropeptide expression in the mouse. Endocrinology 159:2803–2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KJ, Grunberg NE (1995). Effects of housing on male and female rats: Crowding stresses males but calms females. Physiol Behav 58: 1085–1090. [DOI] [PubMed] [Google Scholar]
- Castañeda TR, Nogueiras R, Müller TD, Krishna R, Grant E, Jones A, et al. (2011). Decreased glucose tolerance and plasma adiponectin: resistin ratio in a mouse model of post-traumatic stress disorder. Diabetologia 54: 900–909. [DOI] [PubMed] [Google Scholar]
- Chan JC, Houghton AB, Bale TL (2017). Strained in planning your mouse background? Using the HPA stress axis as a biological readout for backcrossing strategies. Neuropsychopharmacology 42: 1749–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet ER, Joëls M, Holsboer F (2005). Stress and the brain: from adaptation to disease. Nat Rev Neurosci 6: 463–475. [DOI] [PubMed] [Google Scholar]
- DeVries AC, Craft TK, Glasper ER, Neigh GN, Alexander JK (2007). 2006 Curt P. Richter award winner. Social influences on stress responses and health. Psychoneuroendocrinology 32: 587–603. [DOI] [PubMed] [Google Scholar]
- Flak JN, Jankord R, Solomon MB, Krause EG, Herman JP (2011). Opposing effects of chronic stress and weight restriction on cardiovascular, neuroendocrine and metabolic function. Physiol Behav 104: 228–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs DM, Vale W, Rivier J, Yen SS (1984). Oxytocin potentiates the ACTH-releasing activity of CRF(41) but not vasopressin. Life Sci 34: 2245–2249. [DOI] [PubMed] [Google Scholar]
- Herman JP (1995). In situ hybridization analysis of vasopressin gene transcription in the paraventricular and supraoptic nuclei of the rat: Regulation by stress and glucocorticoids. J Comp Neurol 363: 15–27. [DOI] [PubMed] [Google Scholar]
- Herman JP, Adams D, Prewit C (1995). Regulatory changes in neuroendocrine stress-integrative circuitry produced by a variable stress paradigm. Neuroendocrinology 61:180: 180–190. [DOI] [PubMed] [Google Scholar]
- Herman JP, Flak J, Jankord R (2008). Chronic stress plasticity in the hypothalamic paraventricular nucleus. Prog Brain Res 170: 353–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ieraci A, Mallei A, Popoli M (2016). Social isolation stress induces anxious-depressive-like behavior and alterations of neuroplasticity-related genes in adult male mice. Neural Plast 2016:6212983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappel S, Hawkins P, Mendl MT (2017). To group or not to group? Good practice for housing male laboratory mice. Animals 7: E88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang RE, Heil JWE, Ganten D, Hermann K, Unger T, Rascher W (1983). Oxytocin unlike vasopressin is a stress hormone in the rat. Neuroendocrinology 37: 314–316. [DOI] [PubMed] [Google Scholar]
- Lolait SJ, Stewart LQ, Jessop DS, Young WS 3rd, O’Carroll AM (2007). The hypothalamic-pituitary-adrenal axis response to stress in mice lacking functional vasopressin V1b receptors. Endocrinology 148: 849–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma XM, Levy A, Lightman SL (1997). Rapid changes in heteronuclear RNA for corticotrophin-releasing hormone and arginine vasopressin in response to acute stress. J Endocrinol 152: 81–89. [DOI] [PubMed] [Google Scholar]
- Moenter SM, Caraty A, Locatelli A, Karsch FJ (1991). Pattern of gonadotropin-releasing hormone (GnRH) secretion leading up to ovulation in the ewe: Existence of a preovulatory GnRH surge. Endocrinology 129: 1175–1182. [DOI] [PubMed] [Google Scholar]
- Monteiro S, Roque S, de Sá-Calçada D, Sousa N, Correia-Neves M, Cerqueira JJ (2015). An efficient chronic unpredictable stress protocol to induce stress-related responses in C57BL/6 mice. Front Psychiatry 6: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann ID, Krömer SA, Toschi N, Ebner K (2000). Brain oxytocin inhibits the (re)activity of the hypothalamo-pituitary-adrenal axis in male rats: Involvement of hypothalamic and limbic brain regions. Regul Pept 96: 31–38. [DOI] [PubMed] [Google Scholar]
- Ostrander MM, Ulrich-Lai YM, Choi DC, Richtand NM, Herman JP (2006). Hypoactivity of the hypothalamo-pituitary-adrenocortical axis during recovery from chronic variable stress. Endocrinology 147: 2008–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ros-Simó C, Valverde O (2012). Early-life social experiences in mice affect emotional behaviour and hypothalamic-pituitary-adrenal axis function. Pharmacol Biochem Behav 102: 434–441. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU (2000). How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev 21: 55–89. [DOI] [PubMed] [Google Scholar]
- Shanks N, Griffiths J, Zalcman S, Zacharko RM, Anisman H (1990). Mouse strain differences in plasma corticosterone following uncontrollable footshock. Pharmacol Biochem Behav 36: 515–519. [DOI] [PubMed] [Google Scholar]
- Sun M, Choi E, Magee D, Stets C, During M, Lin E (2014). Metabolic effects of social isolation in adult C57BL/6 mice. Int Sch Res Not doi: 10.1155/2014/690950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torner L, Plotsky PM, Neumann ID, de Jong TR (2017). Forced swimming-induced oxytocin release into blood and brain: Effects of adrenalectomy and corticosterone treatment. Psychoneuroendocrinology 77: 165–174. [DOI] [PubMed] [Google Scholar]
- Tsigos C, Chrousos GP (2002). Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J Psychosom Res 53: 865–871. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Ostrander MM, Thomas IM, Packard BA, Furay AR, Dolgas CM, et al. (2007). Daily limited access to sweetened drink attenuates hypothalamic-pituitary- adrenocortical axis stress responses. Endocrinology 148: 1823–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser MJ, Handa RJ (2009). Estrogen impairs glucocorticoid dependent negative feedback on the hypothalamic-pituitary-adrenal axis via estrogen receptor alpha within the hypothalamus. Neuroscience 159: 883–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westenbroek C, Den Boer JA, Ter Horst GJ (2003). Gender-specific effects of social housing on chronic stress-induced limbic FOS expression. Neuroscience 121: 189–199. [DOI] [PubMed] [Google Scholar]
- Westenbroek C, Ter Horst GJ, Roos MH, Kuipers SD, Trentani A, den Boer JA (2003). Gender-specific effects of social housing in rats after chronic mild stress exposure. Prog Neuro-Psychopharmacology Biol Psychiatry 27: 21–30. [DOI] [PubMed] [Google Scholar]
- Whitnall MH (1993). Regulation of the hypothalamic corticotropin-releasing hormone neurosecretory system. Prog Neurobiol 40: 573–629. [DOI] [PubMed] [Google Scholar]
- Willner P (2017). The chronic mild stress (CMS) model of depression: History, evaluation and usage. Neurobiol Stress 6: 78–93. [DOI] [PMC free article] [PubMed] [Google Scholar]