Abstract
Purpose
To assess if the ovarian response of FMR1 premutated women undergoing preimplantation genetic testing (PGT) for Fragile X syndrome is lower compared with fully mutated patients, due to their frequent premature ovarian failure.
Methods
In a retrospective cohort study from January 2009 to March 2019, we compared PGT outcomes in 18 FMR1 premutated women and 12 fully mutated women and aimed to identify predictive factors of stimulation outcomes.
Results
Eighty-six IVF/PGT-M cycles for FMR1 PGT were analyzed. Premutation and full mutation patients were comparable in terms of age, body mass index (BMI), basal FSH, antral follicular count, and cycle length. However, premutation carriers had significantly lower AMH (1.9 versus 4.0 ng/mL, p = 0.0167). Premutated patients required higher doses of FSH (2740 versus 1944 IU, p = 0.0069) but had similar numbers of metaphase II oocytes (7.1 versus 6.6, p = 0.871) and embryos (5.6 versus 4.9, p = 0. 554). Pregnancy rates (37.1% versus 13.3%, p = 0.1076) were not statistically different in both groups.
Conclusion
In spite of lower ovarian reserve and thanks to an increased total dose of FSH, FMR1 premutated selected patients seem to have similar ovarian response as fully mutated patients. Neither the number of CGG repeats in FMR1 gene nor FMR1 mutation status was good predictors of the number of retrieved oocytes.
Keywords: Fragile X syndrome, Ovarian reserve, Ovarian stimulation, IVF, Preimplantation genetic testing
Introduction
Fragile X syndrome (FRAX) is the most common cause of inherited mental retardation, affecting about 1 in 4000 males and 1 in 8000 females [1]. In 1969, Herbert Lubs first observed a “marker X chromosome” with a secondary constriction near the end of the long arm—an apparently “broken” chromosome—in some mentally retarded males and their relatives [2]. A few decades later, the gene involved in the “fragile X” syndrome was sequenced on the X chromosome: FMR1 (Fragile X mental retardation 1) [3].
The mutation implicated in FRAX is a dynamic expansion of a CGG repeat in the 5′ untranslated region of the FMR1 [4] which is subsequently hypermethylated in mutated individuals with more than 200 repeats, hence inactivating FMR1 gene [5]. The absence of FMR1 gene product (FMRP) leads to the characteristics of FRAX in male individuals, and to a lesser extent in some female individuals because of X chromosome inactivation. In the general population, the normal range of CGG repeats varies from 6 to 52 [6]. A premutation is defined by the presence of 52 to 200 repeats. Premutated women are at risk for expansion from premutated to fully mutated status over generations and thus transmission of FRAX to their offspring [7].
Nowadays, preimplantation genetic testing for monogenic disease (PGT-M) provides the opportunity to achieve a pregnancy with a healthy baby and to avoid the burden of prenatal genetic diagnosis and the risk of pregnancy termination. However, carrying out PGT-M for FRAX is challenging for two reasons: genetic analysis is arduous since standard PCR amplification frequently fails to identify abnormally long CGG repeats. Therefore, normal alleles with shorter CGG repeats inherited from both parents need to be identified [8]. This might require resorting to additional analysis based on multiple genetic markers to identify parental haplotypes [9–14]. Moreover, female carriers of the FMR1 premutation—unlike full mutation carriers—are at higher risk of premature ovarian failure (POF) [15] with a relative risk above 20 [16]. Interestingly, premutation carriers with a middle range of repeats (80–100 or 80–110) are at the highest risk of ovarian dysfunction [17–19].
If the impact of FRAX premutation on ovarian reserve is generally admitted, the repercussion of FRAX premutation and FRAX mutation on ovarian stimulation response and subsequent PGT-M outcomes remains under debate. While some authors considered PGT-M for FRAX patients as an “unrealistic option” due to its inefficiency [10], various PGT centers nowadays offer this strategy for FRAX mutation or premutation carriers and several reports have been published with contradictory results. On the one hand, Platteau and colleagues as well as Avraham and his team showed that premutation carriers required a higher dose of FSH for ovarian stimulation and were at higher risk of cancellation for low ovarian response [20, 21]; on the other hand, Tsafrir and colleagues reported that premutation carriers necessitated less FSH and had more retrieved oocytes than mutation carriers [22]. Moreover, some studies suggested that the number of CGG repeats is correlated with ovarian response [23] and that the lowest response is observed in women with mid-size CGG repeats (80–120) [24]. Still, some authors did not reproduce such observation.
How should we counsel our patients about PGT-M for FRAX? Which patients should we accept in our PGT program with reasonable chances of ovarian response and pregnancy? Should mutation/premutation status or number of CGG repeats be decisive factors when selecting our patients for ovarian stimulation? We report here our experience in performing PGT-M for FRAX mutation and premutation carriers over a 10-year period. We compare clinical outcomes in both groups and aim to identify predictive factors of ovarian response.
Materials and methods
Patients
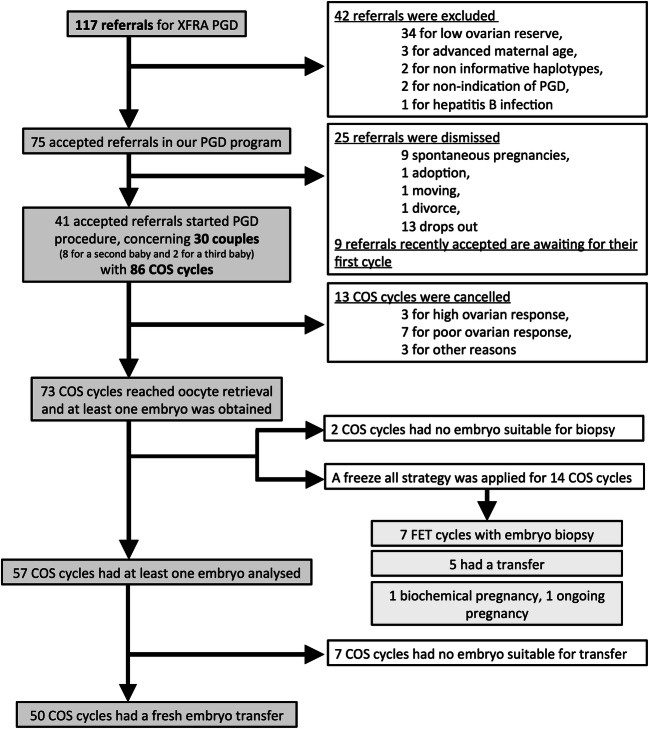
From January 2009 until March 2019, 117 FRAX premutation or full mutation female carriers were referred to our department for PGT-M. To be enrolled in our PGT program, informative haplotypes and sufficient ovarian reserve were required. Access to our PGT program was denied to patients with both AMH < 1ng/mL and AFC < 3. Our retrospective study included 30 women who actually started ovarian controlled stimulation (see flowchart on Fig. 1): 18 women were premutated and 12 had the full mutation for XFRA.
Fig. 1.
Study flowchart
PGT-M clinical procedure and laboratory practice
Ovarian stimulation protocol and initial dose of gonadotropins were chosen based on patient age, AFC, AMH, BMI, and previous ovarian stimulation. Ovarian stimulation dose was not based on FMR1 status. The agonist protocol was achieved using a gonadotropin-releasing hormone (GnRH) analogue (triptorelin 0.1 mg or 3 mg—Decapeptyl, Ipsen Pharma, France; or nafarelin 200 μg—Synarel twice daily, Pfizer, France) started from day 1 or day 21. When desensitization was achieved about 2 weeks later (no ovarian cyst on vaginal ultrasound examination, endometrial thickness < 5 mm, estradiol level < 50 pg/mL), daily subcutaneous injection of recombinant FSH (follitropin alpha—Gonal F, Merck Serono, Geneva, Switzerland; Bemfola, Gedeon Richter, France; follitropin beta—Puregon, MSD Organon, OSS The Netherlands;) or urinary FSH (menotropin—Menopur, Ferring Pharmaceuticals, Copenhagen, Denmark; Fertistartkit, Genevrier, France; urofollitropin—Fostimon, Genevrier, France) was initiated. Initial daily gonadotropin dose ranged from 100 to 300 IU. In the antagonist protocol, patients were scheduled using sex-steroid pretreatment: either estradiol 4 mg (Provames, Sanofi Aventis, France) from day 21 of preceding cycle and held at the maximum 3 days after bleeding started or an estroprogestative pill (Minidril, Pfizer, France) for 10 to 40 days. Daily subcutaneous injection of similar recombinant FSH or urinary FSH was commenced either 24 h after estradiol cessation or 5 days after pill cessation. Initial daily gonadotropin dose ranged from 100 to 300 IU. A GnRH antagonist (ganirelix—Orgalutran 0.25 mg, MSD, France) was introduced after a first evaluation 6 days after ovarian stimulation initiation. Ovarian stimulation was monitored in both groups by transvaginal ultrasound and blood assessment of E2, progesterone, and LH level from day 6 of stimulation and on a regular basis until hCG administration. Ovulation was triggered with hCG (Ovitrelle 250 μg, Serono, Switzerland) or triptorelin 0.2 mg (Decapeptyl, Ipsen, France) subcutaneous injection administrated when at least 3 follicles of > 17 mm in diameter developed. Oocyte retrieval was achieved transvaginally with local anesthesia or sedation 35 to 36 h after ovulation trigger by hCG. ICSI procedure was performed in our unit as previously described [25]. Blastomere biopsy and analysis were performed on day 3 as previously described for PGT procedure [26]. Up to two embryos were transferred on day 4 of in vitro culture and extra healthy embryos were cryopreserved on days 4–5 or 6, if available. Luteal support was achieved with vaginal progesterone 600 mg daily (Utrogestan, Laboratoires Besins International, SA, France) for a minimum of 15 days. Pregnancy (clinical pregnancy) was defined as a pregnancy diagnosed by ultrasonographic visualization of one or more gestational sacs.
Frozen embryo transfers (FET) were performed on natural cycle, stimulated cycle, or with hormonal replacement treatment. FET cycles were not included in the present analysis.
Genetic analysis
PrePGT-M work-up was performed on genomic DNA from the families requesting PGT-M for FRAX (couples and relatives). A panel of microsatellite markers both intragenic and on each side of the FMR1 gene (from 7 to 30, according to the date of inclusion of the couples in the PGT program) were amplified on 100 ng of genomic DNA. Informative markers were selected for each couple and studied in a mutiplex PCR protocol. The amelogenin gene (present on both X and Y chromosomes) and two Y chromosome-specific markers were also studied. PCR conditions were developed and validated on single lymphocytes isolated from each couple members. As CGG repeats could not be amplified at the single cell level using this multiplex PCR approach, an indirect genetic analysis strategy was used to trace inheritance of both normal and mutant haplotypes, as already performed for other genetic disorders [27, 28]. Transferable embryos are the ones harboring the maternal haplotype linked to the normal gene.
Statistical analysis
The main outcome assessed was the number of retrieved oocytes. The secondary outcome was the pregnancy rate. A descriptive analysis was performed. The continuous variables were summarized as mean ± standard deviation and categorical data were expressed as number and percentages. To avoid bias, the mean number of retrieved oocytes, the mean number of metaphase II oocytes, and the mean number of day 3 embryos in premutated patients and fully mutated patients were compared in all cycles including cancelled cycles. In cancelled cycles, numbers of oocytes or embryos were set to zero.
Comparisons of baseline characteristics were performed and putative risk factors between the 2 groups (FRAX permutation versus FRAX full mutation) were identified using the Wilcoxon test and the chi-square or exact test of Fisher as appropriate. This was subsequently confirmed by exploring all controlled ovarian stimulation (COS) cycles with a univariate normal mixed model to account for the non-random nature of the COS cycles analyzed. Then a univariate analysis was performed to study the link between the number of retrieved oocytes and the clinical and biological risk factors. All variables with a p value lower than 0.20 were considered as potential risk factor and were submitted to the multivariate analysis. A Poisson mixed model was proposed as multivariate analysis to select determinants of the ovarian response. Therefore, the outcome was adjusted for clustering within COS cycles. For continuous variables when the linearity was not verified, data was categorized according to the median. All variables with a p value threshold of 5% were not significant and were therefore excluded from the model. We also inquired if the number of CGG repeats in FMR1 gene (as continuous variable) was associated with the number of retrieved oocytes. All statistical analysis was performed with R Development Core Team (2008) software (version R-3.5.0).
Ethical approval
Patients were informed of the investigations and gave their consent before participation in the study, which was approved by the internal ethical board of the Montpellier University Hospital.
Results
Eigthy-six cycles of COS were initiated for FRAX PGT-M: 49 according to a long agonist protocol (57.0%), 36 with an antagonist protocol (41.9%), and 1 with a short agonist protocol (1.1%).
Ovarian reserve and characteristics of FRAX patients
We observed patient characteristics in both FRAX premutation and mutation groups (Table 1). Premutated and mutated patients were comparable for each of their studied features (mean age, BMI, FSH at day 3, E2 at day 3, antral follicular count (AFC), cycle duration) except for their AMH serum levels, which were lower in premutation group.
Table 1.
Baseline characteristics for carrier of the FRAX premutation and the FRAX mutation
| All patients (n = 30) Median [IQ] | FRAX premutation (n = 18) Mean (SD) |
FRAX full mutation (n = 12) Mean (SD) | p value | |
|---|---|---|---|---|
| Woman age at 1st PGD cycle (years) | 32.5 [22–40] | 31.0 (4.7) | 33.3 (4.4) | 0.1609 |
| Man age at 1st PGD cycle (years) | 35 [22–45] | 32.5 (6.6) | 36.1 (6.0) | 0.1372 |
| Woman BMI at 1st PGD cycle (kg/m2) | 21.3 [18.7–32.1] | 21.9 (3.2) | 23.8 (3.9) | 0.2358 |
| Serum AMH at 1st PGD cycle (ng/mL) | 1.9 [0.27–9.6] | 1.9 (1.3) | 4.0 (2.6) | 0.0167 |
| Serum E2 at D3 at 1st PGD cycle (pg/mL) | 42 [12–118] | 38.9 (24.3) | 45.8 (11.9) | 0.0537 |
| Serum FSH at D3 at 1st PGD cycle (IU/L) | 7.3 [2.9–20.4] | 8.1 (3.5) | 6.6 (1.9) | 0.2192 |
| AFC at 1st PGD cycle | 13 [5–33] | 13.7 (5.5) | 17.4 (7.5) | 0.1889 |
| Cycle duration (days) | 28 [24–33] | 28.2 (1.9) | 28.0 (2.2) | 0.8944 |
AFC, antral follicular count; AMH, anti-mullerian hormone; BMI, body mass index; D3, day 3; E2, estradiol; SD, standard deviation
Compared with Wilcoxon test
PGT-M cycle outcomes in FRAX premutated and mutated patients
We inquired about ovarian response and PGT-M cycle outcomes in premutated patients with a reduced ovarian reserve compared with mutated patients. Treatment outcomes are reported in Table 2. Premutation carriers required significantly higher doses of FSH for ovarian stimulation than full mutation carriers to yield the same number of oocytes. The mean initial daily gonadotropin dose for premutation carriers was 269 ± 53 IU (ranging from 125 to 300 IU). The initial daily gonadotropin dose was increased in 3 cycles (5.4%), reduced in 5 cycles (8.9%), and maintained in 48 cycles (87.5%). The mean initial daily gonadotropin dose for full mutation carriers was 208 ± 75 IU (ranging from 100 to 300 IU). The initial daily gonadotropin dose was reduced in 5 cycles (16.7%) and maintained in 25 cycles (83.3%).
Table 2.
Clinical outcome of IVF-PGT-M in carriers of the FRAX premutation and the FRAX mutation
| FRAX premutation cycles | FRAX full mutation cycles | p value | ||
|---|---|---|---|---|
| (n = 56) | (n = 30) | |||
| Protocol (%) |
Long Agonist Antagonist Short agonist |
34 (60.7) 21 (37.5) 1 (1.9) |
15 (50) 15 (50) |
- |
| Stimulation outcome |
Satisfying ovarian response (%) Cycle cancellation (%) |
48 (85.7%) 8 (14.5%) |
25 (83.3%) 5 (16.7%) |
0.7703 |
| Mean ovarian stimulation duration (days) (SD) | 10.1 (2.1) | 9.7 (1.1) | 0.2364 | |
| Mean total dose of FSH/cycle (IU)(SD) | 2740 (857) | 1944 (857) | 0.0069 | |
| Mean estradiol peak (pg/mL) (SD) | 1996 (1242) | 2529 (1595) | 0.1065 | |
| Mean number of retrieved oocytes (SD) | 10.9 (4.9) | 10.6 (5.1) | 0.765 | |
| Mean number of metaphase II oocytes (SD) | 7.1 (5.0) | 6.6 (4.2) | 0.871 | |
| Mean number of day 3 embryos (SD) | 5.6 (4.1) | 4.9 (3.5) | 0.554 | |
| Mean number of embryos to transfer after genetic analysis (SD) | 1.7 (1.1) | 2 (1.5) | 0.4253 | |
| Mean number of transferred embryos after genetic analysis (SD) | 1.5 (0.5) | 1.6 (0.5) | 0.5020 | |
| Pregnancy rate/started cycle (%) | 13/56 (23.2%) | 2/30 (6.7%) | 0.0787 | |
| Pregnancy rate/oocyte retrieval (%) | 13/48 (27.1%) | 2/25 (8.0%) | 0.0851 | |
| Pregnancy rate/embryo transfer (%) | 13/35 (37.1%) | 2/15 (13.3%) | 0.1076 | |
FRAX premutation carriers had similar treatment outcomes in terms of ovarian stimulation duration, maximal serum E2 peak, number of metaphase II oocytes, number of embryos available for biopsy and for transfer, and cancellation rate.
In the premutation group, 73 embryos were healthy among 221 analyzed embryos (33.03%) while 28 embryos were healthy among 75 analyzed embryos in the full mutation group (37.33%) (p value = 0.5906). In the premutation group, there was no healthy embryo for transfer in 5 cycles among 40 cycles with embryo analysis (12.50%) versus 2 cycles among 17 cycles in the full mutation group (11.76%) (p value = 0.9999).
We reported 20 pregnancies after fresh embryo transfers (among which 13 live births and 3 on-going pregnancy). Seven FET cycles were achieved with supernumerary embryos: 4 pregnancies were reported (among which 2 live births and 1 on-going pregnancy). Implantation rate was not statistically different between FMR1 premutation and full mutation patients (30.3% versus 13.3% respectively, p = 0.401).
Pregnancy rates per started cycle, per oocyte retrieval, or per embryo transfer were not statistically different between FMR1 premutation and full mutation patients.
Identification of predictive factors of ovarian response
We explored which factors were decisive in the number of retrieved oocytes. There was no statistical link between the number of retrieved oocytes and factors such as age, BMI, FSH and estradiol at day 3, cycle length, and total dose of FSH. A higher AMH serum level and a higher AFC were significantly correlated with an increased number of retrieved oocytes (p = 0.0155 and p = 0.0292, respectively). FRAX status (10.9 ± 4.9 oocytes in FMR1 premutation versus 10.6 ± 5.1 in full mutation, p = 0.765) was not associated with number of retrieved oocytes.
The variables subsequently submitted to the model were as follows: AMH serum level and AFC. The multivariate mixed model analysis showed no association between the number of retrieved oocytes and neither FRAX mutation status (p = 0.9751) nor number of CGG repeats (p = 0.2690). Figure 2 graphically confirms that there was no link between the number of retrieved oocytes and the FRAX premutation (star dots) or full mutation (white dots) status (Kendall correlation coefficient = 0.11). The boxplots representing the number of retrieved oocytes according to FMR1 mutation status (on Fig. 2, right panel) corroborate this finding.
Fig. 2.
Number of retrieved oocytes according to CGG repeats and to FMR1 mutation status. Premutation cycles are represented with star dots and clear background, and full mutation cycles are represented with white dots and dark background (number of CGG repeats for full mutation are accounted > 200)
Discussion
We report our experience in PGT-M for patients carrying fragile X mutation and premutation, compiling data of 86 IVF cycles in 30 patients. To the best of our knowledge, it is the first study demonstrating that increasing FSH dose for ovarian stimulation in selected FRAX premutation patients compensates their lower ovarian reserve and allows similar ovarian response as full mutation patients.
FRAX premutated women appeared to have a reduced ovarian reserve. It is indeed well-established that FMR1 premutated patients display a lower ovarian reserve [29] as suggested by an elevation in the serum FSH level [30] reflecting a decreased follicle number [31]. We reported a lower AFC in premutated patients compared with mutated patients. However, this difference was not significant. This might be due to a lack of power of our study, own to the reduced size of our population. FRAX mutation or premutation remains infrequent and previous published studies focusing on this genetic condition are scarce and with few patients.
AMH was significantly lower in premutation carriers than in full mutation carriers. AMH is a better marker than FSH in identifying an early decline in ovarian function in FRAX premutation patients [32] and it was previously reported to be lower in premutation carriers [33]. Several hypotheses were raised regarding the mechanisms involved in such ovarian dysfunction. First, no significant correlation could be stressed between age of menopause and the percentage of active X chromosomes that carried the premutation [34]. However, X chromosome inactivation (XCI) was studied on peripheral blood cells and it is well admitted that, due to diverse patterns of XCI mosaicism in females, XCI can selectively occur in a cell type and thus be tissue-specific [35]. Secondly, the number of CGG repeats in FMR1 gene was incriminated but the question remains polemic. While Murray et al. demonstrated that CGG repeat number was not correlated with the age of menopause [34], Spath et al. advocated that CGG repeat size had the highest association with menopausal age among FRAX premutation carriers [36]. This might be due to the non-homogenous distribution of number of CGG repeats in premutation carriers included in these studies. Moreover, it was recently suggested that the CGG repeat size might not be the only element to determine ovarian reserve in premutation carriers: the presence of AGG interruptions within the CGG repeat sequence could alleviate the noxious effect of long CGG repeats [37]. Besides, it has been suggested that the relationship between the number of CGG repeats and the age of menopause is non-linear with an increasing risk up to 100 CGG repeats and thereafter a plateau or even a decrease for carriers over 100 CGG repeats [15, 17, 18].
The mechanism of the impaired ovarian function related to the FMR1 premutation is not fully unveiled. In full mutation female carriers, expansion of CGG repeats over 200 is hypermethylated and FMR1 gene is hence transcriptionally silenced. Thus, the absence of the FMRP is not accountable for POF. In premutation female carriers, FMRP displays normal or slightly reduced levels. However, there is an accumulation of FMR1 mRNA [38] that might be toxic to the ovary and responsible for POF in a RNA gain-of-function toxicity mechanism [19, 24]. Elizur et al. suggested that this substantial accumulation of FMR1 mRNA in granulosa cells for “mid-range” premutated carriers (80 to 120 repeats) was responsible for this non-linear association between the number of CGG repeats and ovarian function. Buijsen et al. recently proposed that repeat-associated non-AUG translation might play a role in ovarian dysfunction in FRAX premutation carriers: accumulated FMR1 premutated mRNA could be translated in polyglycine-containing protein (FMRpolyG) that accumulates in toxic intranuclear inclusions in ovarian tissue [39]. Important progress in understanding ovarian dysfunction in animal models notwithstanding the mechanisms underlying premature ovarian failure in FMR1 premutated women is still being unraveled.
Premutated patients required significantly higher doses of FSH for ovarian stimulation. This is consistent with previous observations [20, 21, 23]. However, Tsafrir et al. reported opposite observation with a lower dose of FSH required in FMR1 premutated patients [22]; as a matter of fact, their premutated patients displayed unusually higher ovarian reserve than their mutated patients.
It has been suggested that premutation patients experiment normal implantation. As long as oocytes and embryos are obtained, premutation carriers might have good chance of pregnancy [40]. Pregnancy rates per started cycle, per oocyte retrieval, or per embryo transfer were not statistically different between FRAX pre mutation and full mutation patients. There was however a trend for pregnancy rates to be superior in premutation group, without reaching statistical significance (37.1% versus 13.3%, NS). To our knowledge, there is no published study mentioning implantation difficulties in FMR1 full mutation carriers. However, in a multicenter survey of 131 fragile X carriers, Schwartz et al. observed a trend for a lower average number of pregnancies per women above 18 years old in full mutation carriers (1.7 (63/37) versus 2.8 (263/91) in premutation carriers) [41]. The absence of FMRP in full mutation patients might be involved in reproductive impairment, albeit unrelated to POF. Based on these observations, it would be interesting to explore endometrial receptivity and embryo quality in order to identify a possible implantation issue in full mutation patients.
On the contrary, Loesch and Hay reported a larger number of offspring in fragile X female carriers with borderline intellectual disabilities, that are most likely mutation carriers [42]. Besides, some authors hinted that both full mutation and premutation patients tend to have more pregnancies than the general obstetric population, without reporting any imbalance between the two FMR1 populations [43]. A mouse mutant carrying a human premutation with 90 CGG repeats in FMR1 gene was studied for its reproductive proficiency. A decreased number of pups per litter related to diminish ovarian reserve were reported, along with an increased age at first litter [44]. This questions about the quality of implantation not only in full mutation carriers but also in premutated animal model.
The variability observed in both animal and human, full mutation and premutation carriers, raises question about the physiopathological mechanism underlying implantation ability in FRAX women. An individual-specific XCI skewing in endometrial tissue could account for such discrepancies and could easily be explored through an endometrial biopsy. We can imagine that this may become one day an interesting and accessible tool to predict endometrial receptivity in FMR1 premutation or full mutation patients.
Thus, the question of implantation in premutation and full mutation carriers is far from being uncovered and further investigation is required.
AMH and AFC appeared as predictive factors of ovarian response. In poor ovarian responders, AMH was previously shown to predict ovarian response along with AFC [45, 46], menstrual cycle length [46], and maternal age [46, 47]. No correlation between the number of retrieved oocytes and the number of CGG repeats could be demonstrated in this study. Our study has a few limitations. It is a retrospective study with a small sample size: this may have resulted in failure to establish significance of some variables. Results should thus be interpreted carefully. We may also propose the hypothesis that ovarian dysfunction associated with mid-range of CGG repeats is counterbalanced by increased FSH dosage. Based on our results, we believe that PGT-M should be offer for fragile X women, without regard for neither their FRAX mutation status (premutation or full mutation) nor their number of CGG repeats, but simply after considering their ovarian reserve. As a matter of fact, ovarian reserve markers such as AFC and AMH appeared to be good predictor factors for ovarian response in our patients, unlike characteristics related to FMR1 gene. Access to our PGT program is denied to patients with both AMH < 1 ng/mL and AFC < 3. It has indeed been shown that successful PGT is related to the number of retrieved oocytes and that the chances of achieving a pregnancy are drastically reduced with a low ovarian response [48, 49]. Therefore, the ovarian response of FRAX patients with extremely low ovarian reserve could not be studied here. The criteria used in our center to refuse patients into our PGD program occurred to be relevant as cancellation rate was low in both mutation and premutation groups (respectively 16.7% and 14.5%).
Thus, once patients at risk of ovarian dysfunction were properly dismissed based on their AMH and AFC, using higher dose of FSH was sufficient to allow similar treatment outcomes in both groups. High dose of FSH appeared as an efficient approach to amend the presumed toxicity of intranuclear inclusions of FMRpolyG that accumulate in premutated ovarian tissue. It is worth reminding that 34 out of 117 referrals for FRAX mutation or premutation (29.1%) were denied access to PGT program owing to excessively poor ovarian reserve (see Fig. 1). It appears essential for premutation patients to be informed about their diminished ovarian reserve in order to consider fertility preservation or to proceed to pregnancy as soon as possible.
To the best of our knowledge, this is the first study that inquires about implantation issues in FMR1 full mutation women. Studies about implantation and pregnancy rates in larger FMR1 population could strengthen our observations. Further research on endometrial receptivity in FMR1 patients may help developing useful tools to predict implantation in daily practice.
All together, FRAX premutation patients, although having a lower ovarian reserve, had similar ovarian response as full mutation patients, after increasing FSH dose for ovarian stimulation, with possibly higher pregnancy rates. This observation will improve our strategy in assisting premutated and mutated women in having a healthy baby, both in refining our ovarian stimulation offer and in exploring their implantation potential through new endometrial receptivity assessment.
Acknowledgments
The authors thank Nelly Guigue for her contribution to the data management of the study. We are grateful to the University Hospital of Montpellier for supporting the PGT activity.
Compliance with ethical standards
Patients were informed of the investigations and gave their consent before participation in the study, which was approved by the internal ethical board of the Montpellier University Hospital.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Turner G, Webb T, Wake S, Robinson H. Prevalence of fragile X syndrome. Am J Med Genet. 1996;64:196–197. doi: 10.1002/(SICI)1096-8628(19960712)64:1<196::AID-AJMG35>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 2.Lubs HA. A marker X chromosome. Am J Hum Genet. 1969;21:231–244. [PMC free article] [PubMed] [Google Scholar]
- 3.Verkerk AJ, Pieretti M, Sutcliffe JS, Fu YH, Kuhl DP, Pizzuti A, et al. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991;65:905–914. doi: 10.1016/0092-8674(91)90397-h. [DOI] [PubMed] [Google Scholar]
- 4.Yu S, Pritchard M, Kremer E, Lynch M, Nancarrow J, Baker E, Holman K, Mulley J, Warren S, Schlessinger D. Fragile X genotype characterized by an unstable region of DNA. Science. 1991;252:1179–1181. doi: 10.1126/science.252.5009.1179. [DOI] [PubMed] [Google Scholar]
- 5.Oberlé I, Rousseau F, Heitz D, Kretz C, Devys D, Hanauer A, et al. Instability of a 550-base pair DNA segment and abnormal methylation in fragile X syndrome. Science. 1991;252:1097–1102. doi: 10.1126/science.252.5009.1097. [DOI] [PubMed] [Google Scholar]
- 6.Fu YH, Kuhl DP, Pizzuti A, Pieretti M, Sutcliffe JS, Richards S, et al. Variation of the CGG repeat at the fragile X site results in genetic instability: resolution of the Sherman paradox. Cell. 1991;67:1047–1058. doi: 10.1016/0092-8674(91)90283-5. [DOI] [PubMed] [Google Scholar]
- 7.Nolin SL, Lewis FA, Ye LL, Houck GE, Glicksman AE, Limprasert P, et al. Familial transmission of the FMR1 CGG repeat. Am J Hum Genet. 1996;59:1252–1261. [PMC free article] [PubMed] [Google Scholar]
- 8.Sermon K, Seneca S, Vanderfaeillie A, Lissens W, Joris H, Vandervorst M, van Steirteghem A, Liebaers I. Preimplantation diagnosis for fragile X syndrome based on the detection of the non-expanded paternal and maternal CGG. Prenat Diagn. 1999;19:1223–1230. [PubMed] [Google Scholar]
- 9.Burlet P, Frydman N, Gigarel N, Kerbrat V, Tachdjian G, Feyereisen E, Bonnefont JP, Frydman R, Munnich A, Steffann J. Multiple displacement amplification improves PGD for fragile X syndrome. Mol Hum Reprod. 2006;12:647–652. doi: 10.1093/molehr/gal069. [DOI] [PubMed] [Google Scholar]
- 10.Apessos A, Abou-Sleiman PM, Harper JC, Delhanty JD. Preimplantation genetic diagnosis of the fragile X syndrome by use of linked polymorphic markers. Prenat Diagn. 2001;21:504–511. doi: 10.1002/pd.111. [DOI] [PubMed] [Google Scholar]
- 11.Malcov M, Naiman T, Yosef DB, Carmon A, Mey-Raz N, Amit A, Vagman I, Yaron Y. Preimplantation genetic diagnosis for fragile X syndrome using multiplex nested PCR. Reprod BioMed Online. 2007;14:515–521. doi: 10.1016/s1472-6483(10)60901-7. [DOI] [PubMed] [Google Scholar]
- 12.Lee HS, Kim MJ, Lim CK, Cho JW, Song IO, Kang IS. Multiple displacement amplification for preimplantation genetic diagnosis of fragile X syndrome. Genet Mol Res. 2011;10:2851–2859. doi: 10.4238/2011.November.17.3. [DOI] [PubMed] [Google Scholar]
- 13.Kieffer E, Nicod JC, Gardes N, Kastner C, Becker N, Celebi C, Pirrello O, Rongières C, Koscinski I, Gosset P, Moutou C. Improving preimplantation genetic diagnosis for Fragile X syndrome: two new powerful single-round multiplex indirect and direct tests. Eur J Hum Genet. 2016;24:221–227. doi: 10.1038/ejhg.2015.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen M, Zhao M, Lee CG, Chong SS. Identification of microsatellite markers <1 Mb from the FMR1 CGG repeat and development of a single-tube tetradecaplex PCR panel of highly polymorphic markers for preimplantation genetic diagnosis of fragile X syndrome. Genet Med. 2016;18:869–875. doi: 10.1038/gim.2015.185. [DOI] [PubMed] [Google Scholar]
- 15.Wittenberger MD, Hagerman RJ, Sherman SL, McConkie-Rosell A, Welt CK, Rebar RW, Corrigan EC, Simpson JL, Nelson LM. The FMR1 premutation and reproduction. Fertil Steril. 2007;87:456–465. doi: 10.1016/j.fertnstert.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 16.Sherman SL. Premature ovarian failure in the fragile X syndrome. Am J Med Genet. 2000;97:189–194. doi: 10.1002/1096-8628(200023)97:3<189::AID-AJMG1036>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 17.Ennis S, Ward D, Murray A. Nonlinear association between CGG repeat number and age of menopause in FMR1 premutation carriers. Eur J Hum Genet. 2006;14:253–255. doi: 10.1038/sj.ejhg.5201510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sullivan AK, Marcus M, Epstein MP, Allen EG, Anido AE, Paquin JJ, Yadav-Shah M, Sherman SL. Association of FMR1 repeat size with ovarian dysfunction. Hum Reprod. 2005;20:402–412. doi: 10.1093/humrep/deh635. [DOI] [PubMed] [Google Scholar]
- 19.Allen EG, Sullivan AK, Marcus M, Small C, Dominguez C, Epstein MP, Charen K, He W, Taylor KC, Sherman SL. Examination of reproductive aging milestones among women who carry the FMR1 premutation. Hum Reprod. 2007;22:2142–2152. doi: 10.1093/humrep/dem148. [DOI] [PubMed] [Google Scholar]
- 20.Platteau P, Sermon K, Seneca S, Van Steirteghem A, Devroey P, Liebaers I. Preimplantation genetic diagnosis for fragile Xa syndrome: difficult but not impossible. Hum Reprod. 2002;17:2807–2812. doi: 10.1093/humrep/17.11.2807. [DOI] [PubMed] [Google Scholar]
- 21.Avraham S, Almog B, Reches A, Zakar L, Malcov M, Sokolov A, Alpern S, Azem F. The ovarian response in fragile X patients and premutation carriers undergoing IVF-PGD: reappraisal. Hum Reprod. 2017;32:1508–1511. doi: 10.1093/humrep/dex090. [DOI] [PubMed] [Google Scholar]
- 22.Tsafrir A, Altarescu G, Margalioth E, Brooks B, Renbaum P, Levy-Lahad E, Rabinowitz R, Varshaver I, Eldar-Geva T. PGD for fragile X syndrome: ovarian function is the main determinant of success. Hum Reprod. 2010;25:2629–2636. doi: 10.1093/humrep/deq203. [DOI] [PubMed] [Google Scholar]
- 23.Bibi G, Malcov M, Yuval Y, Reches A, Ben-Yosef D, Almog B, Amit A, Azem F. The effect of CGG repeat number on ovarian response among fragile X premutation carriers undergoing preimplantation genetic diagnosis. Fertil Steril. 2010;94:869–874. doi: 10.1016/j.fertnstert.2009.04.047. [DOI] [PubMed] [Google Scholar]
- 24.Elizur SE, Lebovitz O, Derech-Haim S, Dratviman-Storobinsky O, Feldman B, Dor J, Orvieto R, Cohen Y. Elevated levels of FMR1 mRNA in granulosa cells are associated with low ovarian reserve in FMR1 premutation carriers. PLoS One. 2014;9:e105121. doi: 10.1371/journal.pone.0105121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Girardet A, Hamamah S, Anahory T, Déchaud H, Sarda P, Hédon B, Demaille J, Claustres M. First preimplantation genetic diagnosis of hereditary retinoblastoma using informative microsatellite markers. Mol Hum Reprod. 2003;9:111–116. doi: 10.1093/molehr/gag014. [DOI] [PubMed] [Google Scholar]
- 26.Girardet A, Ishmukhametova A, Willems M, Coubes C, Hamamah S, Anahory T, Des Georges M, Claustres M. Preimplantation genetic diagnosis for cystic fibrosis: the Montpellier center’s 10-year experience. Clin Genet. 2015;87:124–132. doi: 10.1111/cge.12411. [DOI] [PubMed] [Google Scholar]
- 27.Girardet A, Fernandez C, Claustres M. Efficient strategies for preimplantation genetic diagnosis of spinal muscular atrophy. Fertil Steril. 2008;90:443.e7–443.12. doi: 10.1016/j.fertnstert.2007.07.1305. [DOI] [PubMed] [Google Scholar]
- 28.Zhao M, Chen M, Tan ASC, Cheah FSH, Mathew J, Wong PC, Chong SS. Single-tube tetradecaplex panel of highly polymorphic microsatellite markers < 1 Mb from F8 for simplified preimplantation genetic diagnosis of hemophilia A. J Thromb Haemost. 2017;15:1473–1483. doi: 10.1111/jth.13685. [DOI] [PubMed] [Google Scholar]
- 29.Allingham-Hawkins DJ, Babul-Hirji R, Chitayat D, Holden JJ, Yang KT, Lee C, et al. Fragile X premutation is a significant risk factor for premature ovarian failure: the International Collaborative POF in Fragile X study--preliminary data. Am J Med Genet. 1999;83:322–325. [PMC free article] [PubMed] [Google Scholar]
- 30.Murray A, Webb J, MacSwiney F, Shipley EL, Morton NE, Conway GS. Serum concentrations of follicle stimulating hormone may predict premature ovarian failure in FRAXA premutation women. Hum Reprod. 1999;14:1217–1218. doi: 10.1093/humrep/14.5.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Welt CK, Smith PC, Taylor AE. Evidence of early ovarian aging in fragile X premutation carriers. J Clin Endocrinol Metab. 2004;89:4569–4574. doi: 10.1210/jc.2004-0347. [DOI] [PubMed] [Google Scholar]
- 32.Rohr J, Allen EG, Charen K, Giles J, He W, Dominguez C, Sherman SL. Anti-Mullerian hormone indicates early ovarian decline in fragile X mental retardation (FMR1) premutation carriers: a preliminary study. Hum Reprod. 2008;23:1220–1225. doi: 10.1093/humrep/den050. [DOI] [PubMed] [Google Scholar]
- 33.Spath MA, Feuth TB, Allen EG, Smits AP, Yntema HG, van Kessel AG, et al. Intra-individual stability over time of standardized anti-Mullerian hormone in FMR1 premutation carriers. Hum Reprod. 2011;26:2185–2191. doi: 10.1093/humrep/der146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murray A, Ennis S, MacSwiney F, Webb J, Morton NE. Reproductive and menstrual history of females with fragile X expansions. Eur J Hum Genet. 2000;8:247–252. doi: 10.1038/sj.ejhg.5200451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deng X, Berletch JB, Nguyen DK, Disteche CM. X chromosome regulation: diverse patterns in development, tissues and disease. Nat Rev Genet. 2014;15:367–378. doi: 10.1038/nrg3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spath MA, Feuth TB, Smits AP, Yntema HG, Braat DD, Thomas CM, et al. Predictors and risk model development for menopausal age in fragile X premutation carriers. Genet Med. 2011;13:643–650. doi: 10.1097/GIM.0b013e31821705e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lekovich J, Man L, Xu K, Canon C, Lilienthal D, Stewart JD, Pereira N, Rosenwaks Z, Gerhardt J. CGG repeat length and AGG interruptions as indicators of fragile X-associated diminished ovarian reserve. Genet Med. 2018;20:957–964. doi: 10.1038/gim.2017.220. [DOI] [PubMed] [Google Scholar]
- 38.Allen EG, He W, Yadav-Shah M, Sherman SL. A study of the distributional characteristics of FMR1 transcript levels in 238 individuals. Hum Genet. 2004;114:439–447. doi: 10.1007/s00439-004-1086-x. [DOI] [PubMed] [Google Scholar]
- 39.Buijsen RA, Visser JA, Kramer P, Severijnen EA, Gearing M, Charlet-Berguerand N, et al. Presence of inclusions positive for polyglycine containing protein, FMRpolyG, indicates that repeat-associated non-AUG translation plays a role in fragile X-associated primary ovarian insufficiency. Hum Reprod. 2016;31:158–168. doi: 10.1093/humrep/dev280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nayot D, Chung JT, Son WY, Ao A, Hughes M, Dahan MH. Live birth following serial vitrification of embryos and PGD for fragile X syndrome in a patient with the premutation and decreased ovarian reserve. J Assist Reprod Genet. 2013;30:1439–1444. doi: 10.1007/s10815-013-0079-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schwartz CE, Dean J, Howard-Peebles PN, Bugge M, Mikkelsen M, Tommerup N, Hull C, Hagerman R, Holden JJA, Stevenson RE. Obstetrical and gynecological complications in fragile X carriers: a multicenter study. Am J Med Genet. 1994;51:400–402. doi: 10.1002/ajmg.1320510419. [DOI] [PubMed] [Google Scholar]
- 42.Loesch DZ, Hay DA. Clinical features and reproductive patterns in fragile X female heterozygotes. J Med Genet. 1988;25:407–414. doi: 10.1136/jmg.25.6.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kallinen J, Korhonen K, Kortelainen S, Heinonen S, Ryynänen M. Pregnancy outcome in carriers of fragile X. BJOG. 2000;107:969–972. doi: 10.1111/j.1471-0528.2000.tb10398.x. [DOI] [PubMed] [Google Scholar]
- 44.Lu C, Lin L, Tan H, Wu H, Sherman SL, Gao F, Jin P, Chen D. Fragile X premutation RNA is sufficient to cause primary ovarian insufficiency in mice. Hum Mol Genet. 2012;21:5039–5047. doi: 10.1093/hmg/dds348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Polyzos NP, Tournaye H, Guzman L, Camus M, Nelson SM. Predictors of ovarian response in women treated with corifollitropin alfa for in vitro fertilization/intracytoplasmic sperm injection. Fertil Steril. 2013;100:430–437. doi: 10.1016/j.fertnstert.2013.04.029. [DOI] [PubMed] [Google Scholar]
- 46.Oehninger S, Nelson SM, Verweij P, Stegmann BJ. Predictive factors for ovarian response in a corifollitropin alfa/GnRH antagonist protocol for controlled ovarian stimulation in IVF/ICSI cycles. Reprod Biol Endocrinol. 2015;13:117. doi: 10.1186/s12958-015-0113-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Galey-Fontaine J, Cédrin-Durnerin I, Chaïbi R, Massin N, Hugues JN. Age and ovarian reserve are distinct predictive factors of cycle outcome in low responders. Reprod BioMed Online. 2005;10:94–99. doi: 10.1016/s1472-6483(10)60808-5. [DOI] [PubMed] [Google Scholar]
- 48.Vandervorst M, Liebaers I, Sermon K, Staessen C, De Vos A, Van de Velde H, et al. Successful preimplantation genetic diagnosis is related to the number of available cumulus-oocyte complexes. Hum Reprod. 1998;13:3169–3176. doi: 10.1093/humrep/13.11.3169. [DOI] [PubMed] [Google Scholar]
- 49.Ben-Nagi J, Jones B, Naja R, Amer A, Sunkara S, SenGupta S, Serhal P. Live birth rate is associated with oocyte yield and number of biopsied and suitable blastocysts to transfer in preimplantation genetic testing (PGT) cycles for monogenic disorders and chromosomal structural rearrangements. Eur J Obstet Gynecol Reprod Biol X. 2019;4:100055. doi: 10.1016/j.eurox.2019.100055. [DOI] [PMC free article] [PubMed] [Google Scholar]