Abstract
Background
To study the role of the long-chain noncoding RNA (lncRNA) metastasis-related lung adenocarcinoma transcript 1 (MALAT1), microRNA-503 (miR-503), Toll-like receptor 4 (TLR4) signal axis in the pathogenesis of pulmonary arterial hypertension (PAH).
Material/Methods
Total RNA was extracted from the plasma of 45 PAH patients and 45 healthy subjects, and the expression of lncRNA MALAT1 and miR-503 was measured by quantitative real-time polymerase chain reaction (qRT-PCR). The effects of lncRNA MALAT1 and miR-503 on Toll-like receptor 4 (TLR4) and the proliferation, migration, and apoptosis of human pulmonary artery smooth muscle cells (hPASMCs) were tested following in vitro transfection of hPASMCs.
Results
lncRNA MALAT1 was highly expressed in the plasma of PAH patients and in hypoxia-induced hPASMCs. Silencing lncRNA MALAT1 inhibited the proliferation and migration of hPASMC cells while promoting their apoptosis. MiR-503 is underexpressed in plasma and hPASMCs of patients with PAH. TLR4 was a target gene of miR-503 and was highly expressed in peripheral blood mononuclear cells (PBMCs) of PAH patients. lncRNA MALAT1 was a “molecular sponge” of miR-503, regulating the expression of TLR4 and the proliferation, migration, and apoptosis of hPASMCs through miR-503.
Conclusions
lncRNA MALAT1 promotes the proliferation and migration of hPASMCs and inhibits their apoptosis by inhibiting the miR-503/TLR4 signal axis.
MeSH Keywords: Persistent Fetal Circulation Syndrome; RNA, Long Noncoding; Toll-Like Receptor 4
Background
Cardiovascular diseases (CVDs) have a significant effect on human health [1], with high morbidity and mortality rates and a heavy economic burden on public health. Pulmonary arterial hypertension (PAH) is a common CVD; although its cause is currently unknown, it may be associated with genes [2], the environment, and metabolism. The pathogenesis of PAH is closely related to pulmonary vasoconstriction, vascular wall remodeling, and in situ thrombosis. Owing to a variety of factors, pulmonary artery pressure (PAP) and pulmonary vascular resistance have been increasing annually [3], as have rates of sudden death due to these disorders. Pulmonary vascular remodeling is the main mechanism underlying the development of PAH, and can include dysfunction in fibroblasts, pulmonary endothelial cells, and pulmonary smooth muscle cells [4–6]. In addition, an increase in the extracellular matrix, stiffening the pulmonary vessel walls and/or narrowing the diameter of pulmonary vessels, can lead to an irreversible increase in pulmonary vessel pressure and the eventual development of PAH [4–6].
Long-chain noncoding RNA (lncRNA) is a type of noncoding RNA >200 bp long involved in the basic process of gene regulation. LncRNA was shown to be involved in chromatin modification and direct transcriptional regulation, as well as the regulation of posttranscriptional splicing, editing, localization, translation, and degradation [7–10]. LncRNA metastasis-related lung adenocarcinoma transcript 1 (MALAT1) is a highly conserved lncRNA in mammals [11] and is encoded on chromosome 11q13. LncRNA MALAT1 has been associated with the pathogenesis of non-small cell lung cancer [12], cervical cancer [13], liver cancer [14], esophageal squamous cell carcinoma [15], metastasis and recurrence of colorectal carcinoma [16], gastric cancer [17], and breast cancer [18,19]. This lncRNA was also shown to play a crucial role in CVD. For example, hypoxia has been found to promote the angiogenesis of endothelial cells (ECs) through vascular endothelial growth factor (VEGF) signaling and can induce the expression of MALAT1 [20–23]. To date, however, the role, if any, of lncRNA MALAT1 in the pathogenesis of PAH has not been fully determined.
Toll-like receptors (TLRs) are pattern recognition receptors first identified in Drosophila [24]. TLRs have been shown to regulate innate and adaptive immunity and to play important roles in recognizing exogenous and endogenous ligands [24]. TLR4 is mainly expressed in immune system cells [25], as well as in myocardial and microvascular ECs. TLR4 has been shown to play an important role in innate immunity, activating inflammatory pathways and mediating the occurrence of CVDs [26].
Bioinformatics technology can determine targeted binding sites for lncRNA MALAT1 and miR-503 and for miR-503 and the 3’ untranslated region (UTR) of TLR4. We hypothesized that lncRNA MALAT1, miR-503, and TLR4 may interact in a cascade signal regulation axis that may be associated with the pathogenesis of PAH. The present study therefore investigated the role of the lncRNA MALAT1/miR-503/TLR4 signal axis in the pathogenesis of PAH through in vitro cell transfection experiments. These findings may suggest new markers for the diagnosis and treatment of PAH.
Material and methods
Subjects
Peripheral blood samples were obtained from 45 patients with PAH who underwent treatment at Shaoxing People’s Hospital from June 2018 to August 2019. PAH was diagnosed in accordance with the 2015 criteria of the European College of Cardiology and European Respiratory Society, in that the mean pulmonary artery pressure (PAPm), as measured by a right heart catheter in the resting state, of ≥25 mmHg (1 mmHg=0.133 kPa) [27]. Patients with a history of malignancy, severe chronic obstructive pulmonary disease, or drug addiction were excluded. As controls, peripheral blood samples were obtained from 45 healthy subjects, all of whom had a mean PAPm, as measured by a right heart catheter, of <25 mmHg. Subjects were included in the control group if they had no history of chest trauma or smoking (<ten packs of cigarettes per year), if chest radiographs showed clear lung areas, and if bronchoscopy showed clear bronchial tubes. The study protocol was approved by the Ethics Committee of Shaoxing People’s Hospital, and all subjects provided written informed consent.
Cell culture
Human pulmonary artery smooth muscle cells (hPASMCs) were purchased from the American Type Culture Collection (ATCC; Manassas, VA, USA), cultured for 24 hours, transferred to 6-well plates and cultured at 37°C in an atmosphere containing 5% CO2 in Smooth Muscle Growth medium-2 BulletKit (Lonza, Basel, Switzerland) containing 10% (v/v) fetal bovine serum (FBS, Gibco; Grand Island, NY, USA), 0.5 ng/ml human recombinant epidermal growth factor (EGF), 2 ng/ml human reconstituted fibroblast growth factor (FGF), 5 µg/ml insulin, and 50 µg/ml gentamicin. hPASMC were passaged four times prior to transfection and detection of lncRNA MALAT1 and miR-503. HEK-293 cells (ATCC) were cultured in Dulbecco’s modified Eagle medium containing 10% FBS at 37°C and 5% CO2.
Cell transfection
Short hairpin RNAs (shRNA) targeting lncRNA MALAT1, a plasmid overexpressing of lncRNA MALAT1 (pcDNA-MALAT1), pcDNA vector (NC), an miR-503 mimic, and anmiR-503 inhibitor were synthesized by Shanghai Integrated Biotechnology Solutions Co., Ltd. hPASMC cells were transfected with sh-MALAT1, pcDNA-MALAT1, and NC using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA), according to the manufacturer’s supplier’s instructions. In addition, pcDNA-MALAT1 hPASMC cells were transfected with miR-503 mimic and co-transfected with sh-MALAT1 and miR-503 inhibitor. After culture for 24 and 48 hours, the cells were collected for further experiments.
Quantitative reverse transcription- polymerase chain reaction (RT-qPCR)
Total RNA was isolated from peripheral blood mononuclear cells (PBMCs), hPASMCs, and subject plasma using TRIzol reagent (Thermo Fisher Scientific, Shanghai, China). The RNA was reverse transcribed to cDNA using All-in-One miRNA First-Strand cDNA Synthesis Kits (GeneCopoeia, Rockville, MD, USA). Sequences of interest were amplified using FastFire qPCR PreMix (Tiangen Biotech, Co., Beijing, China) on an ABI StepOne Real-Time PCR System (Thermo Fisher Scientific, Shanghai, China), and using primers for MALAT1 (forward, 5’-GCA GGC GTT GTG CGT AGA G-3’; reverse, 5’-TTG CCG ACC TCA CGG ATT-3’), miR-503 (forward, 5’-TGC CCT AGC AGC GGG AAC-3’; reverse, 5’-ACC CTG GCA GCG GAA ACA ATA-3’) TLR4 (forward, 5’-GAT AGC GAG CCA CGC ATT CA-3’; reverse. 5’-TTA GGA ACC ACC TCC ACG CA-3’), U6 (forward, 5’-CTC GCT TCG GCA GCA CA-3’; reverse, 5’-TGG TGT CGT GGA GTC G-3’), and GAPDH (forward, 5’-GGG TGA TGC AGG TGC TAC TT-3’; reverse, 5’-GGC AGG TTT CTC AAG ACG GA-3’). The expression of lncRNA MALAT1 and miR-503 relative to U6, and the expression of TLR4 mRNA relative to GAPDH mRNA were calculated using the 2−Ct method.
Clonal formation
Transfected hPASMCs were seeded at a density of 5×105 cells/well in 6-well plates, cultured for 24 h and digested with 0.25% trypsin. About 500 cells were seeded in each well of a 24-well plate, using triplicate samples from each group, and the culture medium was changed once per day. After incubation for 7 days, the colonies formed were counted to evaluate cell proliferation ability.
Wound healing assay
Transfected hPASMCs were seeded at a density of 5×105/well in 6-well plates and cultured until the cells covered the bottom of the well. The cells in each well were scratched with a 20-μl sterile pipette tip with visualization using an inverted microscope. The wells were washed three times with phosphate-buffered saline (PBS) to remove non-adherent cells and cultured in serum-free medium and in a 37°C, 5% CO2 incubator. The wells were photographed using an inverted microscope at 0, 24, and 48 h to record the migration of hPASMC cells.
Cell Counting Kit-8 (CCK-8) assays
Transfected hPASMCs were suspended in 100 μl of medium, added to wells of a 96-well plate, and cultured for 24 h at 37°C in 5% CO2. To each well was added 10 μl of CCK-8 reagent (Dojindo Laboratories, Kumamoto, Japan), with three auxiliary wells in each group. The, absorbance of each well at 450 nm was measured after 0, 24, 48, 72, and 96 h.
Detection of apoptosis by flow cytometry
hPASMC cells were collected by centrifugation at 500–1000 rpm for 5 min and the culture medium was discarded. After washing the cells once with 3 ml PBS, ice-cold 70% ethanol was added, and cells were fixed at 4°C for 1–2 h. After centrifugation to remove the fixation solution, the cells were resuspended in 3 ml PBS for 5 min, filtered through a 400-mesh screen, and centrifuged at 500–1000 rpm for 5 min. The cell pellets were resuspended in 1 ml PI staining solution and incubated at 4°C in the dark for 30 min. The cell suspensions were sorted on an Accuri C6 flow cytometer (BD Biosciences, San Jose, CA, USA), and the results were analyzed using Cflow software (BD Biosciences).
Dual luciferase reporter experiment
A wild-type MALAT1 sequence and a TLR4 sequence containing miR-503 binding sites, along with mutant MALAT1 sequences and TLR4 sequences with binding site mutations were synthesized by Shanghai Integrated Biotech Solutions Co., Ltd. The four sequences (MALAT1 WT, MALAT1 MUT, TLR4 WT, and TLR4 MUT) were each co-transfected in triplicate with miR-503 mimic, miR-503 inhibitor, and NC into HEK-293 cells. Luciferase activity was measured using a dual-luciferase reporter assay (Promega, Madison, WI, USA).
Western blot
Proteins were extracted from hPASMC cells using RIPA buffer (Beyotime Biotechnology Shanghai, China), separated by 15% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), and transferred to polyvinylidene fluoride (PVDF) membranes (Millipore Inc., Bedford, MA, USA). The membranes were incubated with solution containing 5% skim milk powder for 2 h at room temperature and then with anti-TLR4 (1: 1000; ab13867, Abcam, Cambridge, MA, USA) and anti-β-actin (1: 1000; ab179467, Abcam) at 4°C for 12–16 h. The membranes, were washed and incubated with secondary antibody for 2 h at 26°C, followed by incubation with an enhanced chemiluminescence (ECL) reagent. The expression of TLR4 relative to β-actin was analyzed in triplicate samples.
Statistical analysis
All statistical analyses were was performed using GraphPad Prism7 (GraphPad Software, Inc., USA) and SPSS20.0 (IBM, Armonk, NY) software. Groups were compared by Student’s t-test and one-way analysis of variance. Cutoffs for plasma lncRNA MALAT1 and miR-503 associated with the diagnosis of PAH were determined by receiver operating curve (ROC) analysis. All tests were two-tailed, with p<0.05 indicating statistical significance.
Results
lncRNA MALAT1 is highly expressed in the plasma of PAH patients and hypoxia-induced hPASMC cells
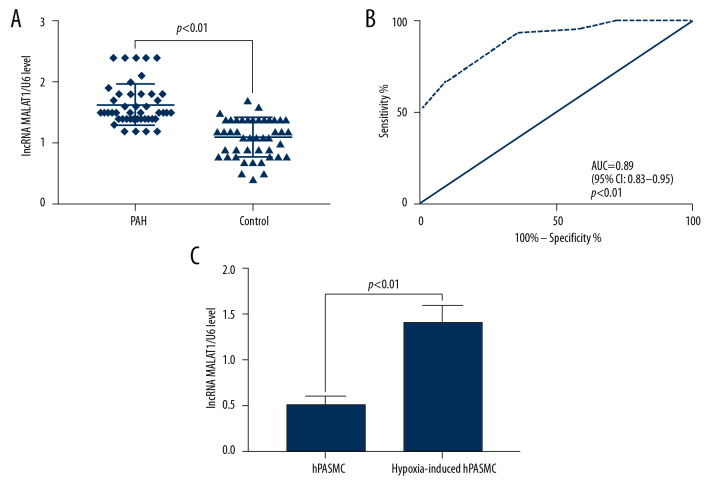
Peripheral blood samples were obtained from 45 patients with PAH, consisting of 28 men and 17 women, of mean age 56.31±8.73 years (range, 35–74 years). As controls, peripheral blood samples were obtained from 45 healthy subjects, 25 men and 20 women, of mean age 56.42±11.64 years (range. 34–77 years). The plasma levels of lncRNA MALAT1 were significantly higher in the 45 PAH patients than in the 45 controls (p<0.01, Figure 1A). ROC analysis showed that the area under the curve (AUC) of plasma lncRNA MALAT1 diagnostic of PAH was 0.89 (95% confidence interval [CI]: 0.83–0.95, p<0.01, Figure 1B), indicating that the plasma lncRNA MALAT1 levels are potential molecular biomarkers for the diagnosis of PAH. In addition, the level of lncRNA MALAT1 was higher in hypoxia-induced hPASMC cells than in non-hypoxic hPASMC cells (p<0.01, Figure 1C).
Figure 1.
Long-chain non-coding RNA metastasis-related lung adenocarcinoma transcript 1 (lncRNA MALAT1) is highly expressed in the plasma and hypoxic human pulmonary artery smooth muscle cells (hPASMCs) of patients with PAH. (A) Quantitative reverse transcription-PCR (qRT-PCR) detection of plasma lncRNA MALAT1 levels. (B) Receiver operating curve (ROC) for the diagnosis of PAH based on plasma lncRNA MALAT1 level. (C) Comparison of lncRNA MALAT1 levels in hypoxic and non-hypoxic hPASMC cells by RT-qPCR
Silencing MALAT1 inhibit the proliferation and migration of hPASMCs and promote their apoptosis
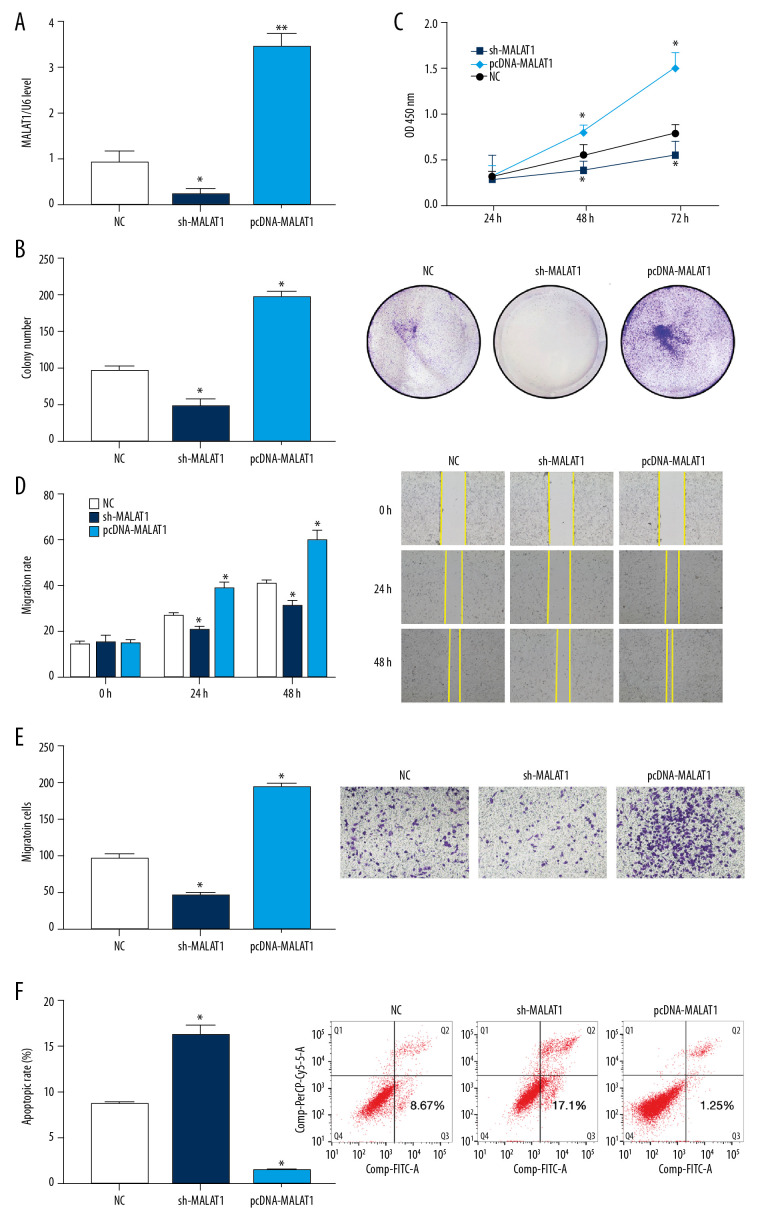
qRT-PCR showed that the expression of lncRNA MALAT1 in hPASMCs was reduced following transfection with sh-MALAT1 but was significantly increased after transfection with pcDNA-MALAT1 compared with the NC group (p<0.05, Figure 2A). The number of hPASMC clones was also significantly lower after transfection with sh-MALAT1, whereas the number of clones was significantly higher after transfection with pcDNA-MALAT1, when compared with the NC group (p<0.05, Figure 2B). In addition, the CCK-8 assay showed that knockdown of lncRNA MALAT1 significantly inhibited the proliferation of hPASMCs, whereas overexpression of lncRNA MALAT1 significantly enhanced the proliferation of these cells (p<0.05, Figure 2C).
Figure 2.
Silencing of long-chain non-coding RNA metastasis-related lung adenocarcinoma transcript 1 (lncRNA MALAT1) significantly inhibits the proliferation and migration of hPASMCs. (A) Levels of lncRNA MALAT1 expression in hPASMCs with silenced and overexpressed lncRNA MALAT1. (B) Numbers of clones formed by hPASMCs with silenced and overexpressed lncRNA MALAT1. (C) Cell counting kit-8 (CCK-8) assays of the proliferation of hPASMCs with silenced and overexpressed lncRNA MALAT1. (D) Wound healing assay assessing the effect of lncRNA MALAT1 silencing and overexpression on the migration ability of hPASMC cells. (E) Transwell assays assessing the effect of lncRNA MALAT1 silencing and overexpressed on the migration of hPASMC cells. (F) Flow cytometry assays of the effect of lncRNA MALAT1 silencing and overexpression on hPASMC cell apoptosis.* p<0.05, ** p<0.01 compared with the no template control (NC) group.
Wound healing assays performed to assess the migration of hPASMC cells after transfection found That cell migration was significantly lower after transfection with sh-MALAT1, but was significantly greater higher after transfection with pcDNA-MALAT1, when compared with the NC group (p<0.05 each, Figure 2D). Transwell tests showed similar results, with the migration ability of hPASMCs decreasing significantly after transfection with sh-MALAT1 but increasing significantly after transfection with pcDNA-MALAT1 (p<0.05, Figure 2E).
Flow cytometry assessments of hPASMC apoptosis after transfection showed that transfection with sh-MALAT1 significantly increased the apoptosis rate of hPASMCs, whereas transfection with pcDNA-MALAT1 significantly reduced the apoptosis rate of these cells, when compared with the NC group (p<0.05 each, Figure 2F). Taken together, these results indicate that silencing lncRNA MALAT1 inhibits the proliferation and migration of hPASMCs while promoting their apoptosis.
Low expression of miR-503 in plasma and hPASMC cells of patients with PAH
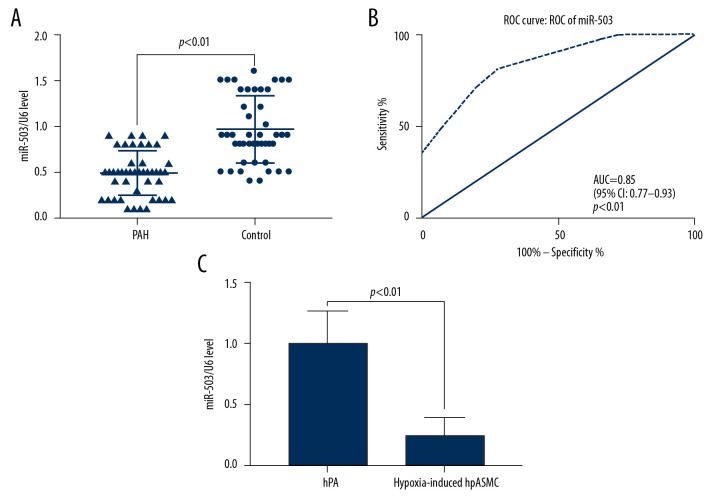
qRT-PCR measurements of miR-503 expression in plasma showed that the level of expression of miR-503 in plasma was lower in patients with PAH than in control subjects (p<0.05, Figure 3A). ROC analysis showed that the AUC for the diagnosis of PAH based on plasma miR-503 levels was 0.85 (95% CI: 0.77–0.93, p<0.01, Figure 3B). Moreover analysis of the effect of hypoxia on miR-503 expression in hPASMC cells showed that expression of miR-503 was significantly lower in hypoxic than in non-hypoxic hPASMC cells (p<0.01, Figure 3C).
Figure 3.
Low expression of microRNA-503 (miR-503) in plasma and hypoxic hPASMCs in patients with pulmonary hypertension (PAH). (A) Plasma miR-503 levels in plasma samples from patients with PAH and healthy controls. (B) Receiver operating curve (ROC) analysis for the diagnosis of PAH based on plasma miR-503 level. (C) miR-503 levels in hypoxic and non-hypoxic hPASMC cells.
lncRNA MALAT1 can be directly targeted to miR-503 as ceRNA
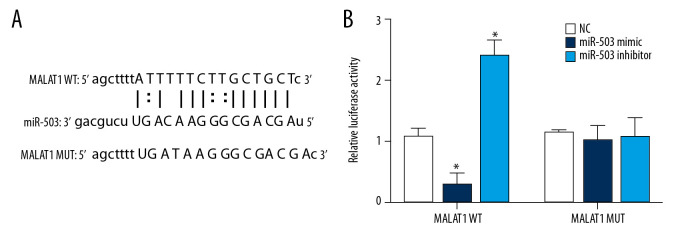
Using an online lncRNA-microRNA binding site prediction tool (starBase v2.0: http://starbase.sysu.edu.cn/) [28], we found an interaction site between lncRNA MALAT1 and miR-503 (Figure 4A). A double luciferase reporter assay was performed to verify whether lncRNA MALAT1 and miR-503 bind at this predicted binding site. This assay confirmed the interaction between lncRNA MALAT1 and miR-503, consistent with the predicted binding site (Figure 4B). This shows that lncRNA MALAT1 can directly target miR-503 as ceRNA.
Figure 4.
Long-chain non-coding RNA metastasis-related lung adenocarcinoma transcript 1 (lncRNA MALAT1) can directly target microRNA-503 (miR-503) as a competing endogenous RNAs (ceRNA). (A) Prediction of the binding site of lncRNA MALAT1 and miR-503. (B) Double luciferase reporter gene test results. * p<0.05 compared with the no template control (NC) group.
TLR4 is a target gene of miR-503 and is highly expressed in PBMCs of PAH patients
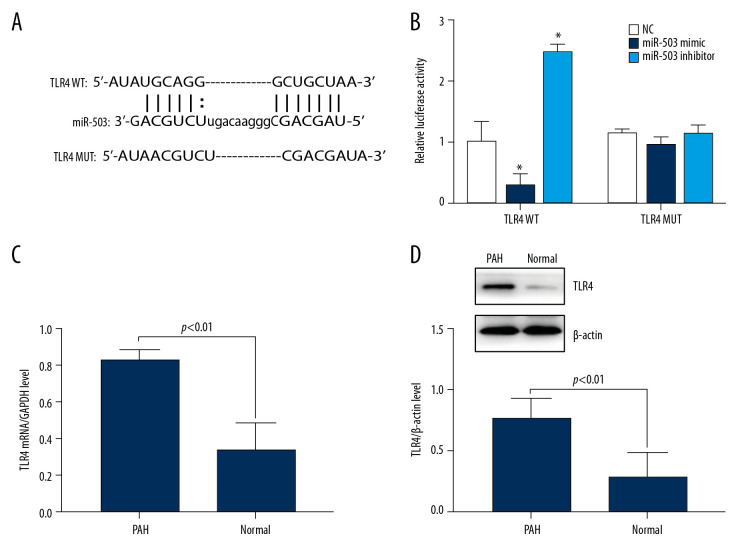
Bioinformatics predicted that the 3’ UTR region of TLR4 is present at the binding site of miR-503 (Figure 5A). A dual luciferase reporter assay tested whether miR-503 binds to this predicted target site. Co-transfection of miR-503 mimic with TLR4 wild type (WT) into hPASMC cells resulted in reduced luciferase activity, whereas co-transfection of miR-503 inhibitor with TLR4 WT resulted in an increase in luciferase activity. Transfection of either miR-503 mimic or miR-503 inhibitor with TLR4 mutant (MUT), however, had no effect on luciferase activity (Figure 5B), indicating that TLR4 is a target gene of miR-503.
Figure 5.
Long-chain non-coding RNA metastasis-related lung adenocarcinoma transcript 1 (lncRNA MALAT1) can directly target microRNA-503 (miR-503) as a competing endogenous RNAs (ceRNA). (A) Prediction of the binding site of lncRNA MALAT1 and miR-503. (B) Double luciferase reporter gene test results. * p<0.05, compared with the no template control (NC) group. (C) Toll like receptor-4 (TLR-4) mRNA expression in peripheral blood mononuclear cells (PBMCs) of PAH patients and healthy controls. (D) TLR4 protein expression in PBMCs of PAH patients and healthy controls.
To assess the role of TLR4 in patients with PAH, the expression of TLR4 mRNA in PBMCs of PAH patients and healthy subjects was evaluated qRT-PCR. We found that the level of expression of TLR4 mRNA was significantly higher in PAH patients than in healthy controls (p<0.01, Figure 5C). Similarly, Western blotting showed that the expression of TLR4 protein was higher in PAH patients than in healthy subjects (p<0.01, Figure 5D), indicating an association between high TLR4 expression and the development of PAH.
lncRNA MALAT1 is a “molecular sponge” for miR-503, which regulates the expression of TLR4
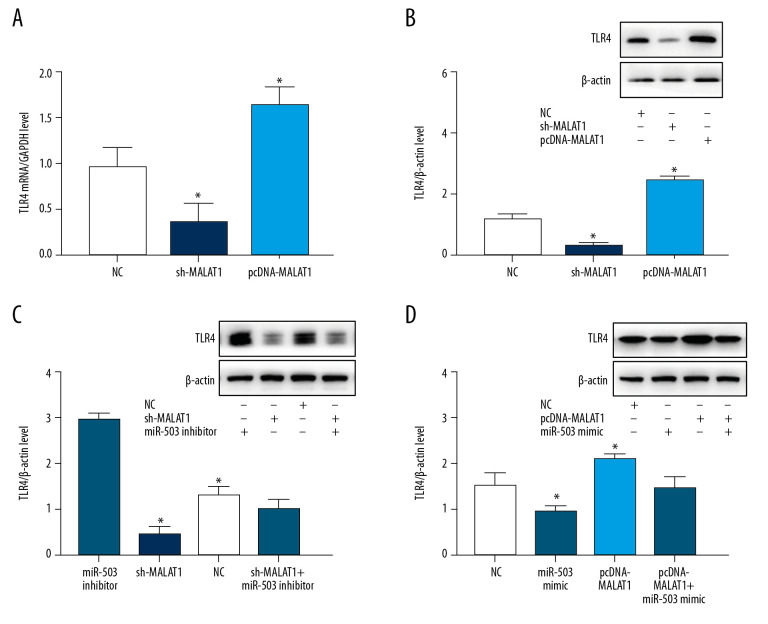
To further evaluate the interactions of lncRNA MALAT1, miR-503, and TLR4, hPASMC cells were transfected with sh-MALAT1, pcDNA-MALAT1, miR-503 mimic, and miR-503 inhibitor, and the effects of transfection on cell growth and TLR4 expression were evaluated. qRT-PCR showed that sh-MALAT1 significantly inhibited TLR4 mRNA expression, whereas pcDNA-MALAT1 enhanced TLR4 mRNA expression (Figure 6A). In addition, Western blotting showed that sh-MALAT1 significantly inhibited TLR4 protein expression; whereas pcDNA-MALAT1 enhanced TLR4 protein expression (Figure 6B). Transfection of miR-503 inhibitor increased the levels of TLR4 protein in hPASMCs, an upregulation significantly reversed by transfection of sh-MALAT1 (Figure 6C). In contrast, transfection of miR-503 mimic reduced the expression of TLR4 protein in hPASMCs, with this downregulation of expression being significantly reversed by transfection of pcDNA-MALAT1 (Figure 6D). These findings indicated that lncRNA MALAT1 is a “molecular sponge” of miR-503 and regulates the expression of TLR4.
Figure 6.
Long-chain non-coding RNA metastasis-related lung adenocarcinoma transcript 1 (lncRNA MALAT1) and microRNA-503 (miR-503) regulate the expression of toll like receptor-4 (TLR-4). (A) Levels of TLR4 mRNA expression on hPASMCs with silenced or overexpressed lncRNA MALAT1. (B) Effect of TLR4 protein expression on hPASMCs cells with silenced or overexpressed by lncRNA MALAT1. (C) TLR4 protein expression levels in hPASMC cells transfected with sh-MALAT1, miR-503 inhibitor, sh-MALAT1+miR-503 inhibitor, or no template control (NC). (D) TLR4 protein expression levels in hPASMC cells transfected with miR-503 mimic, pcDNA-MALAT1, pcDNA-MALAT1+miR-503 mimic, or NC. * p<0.05 compared with the NC group.
lncRNA MALAT1 regulates the proliferation, migration, and apoptosis of hPASMC cells through miR-503
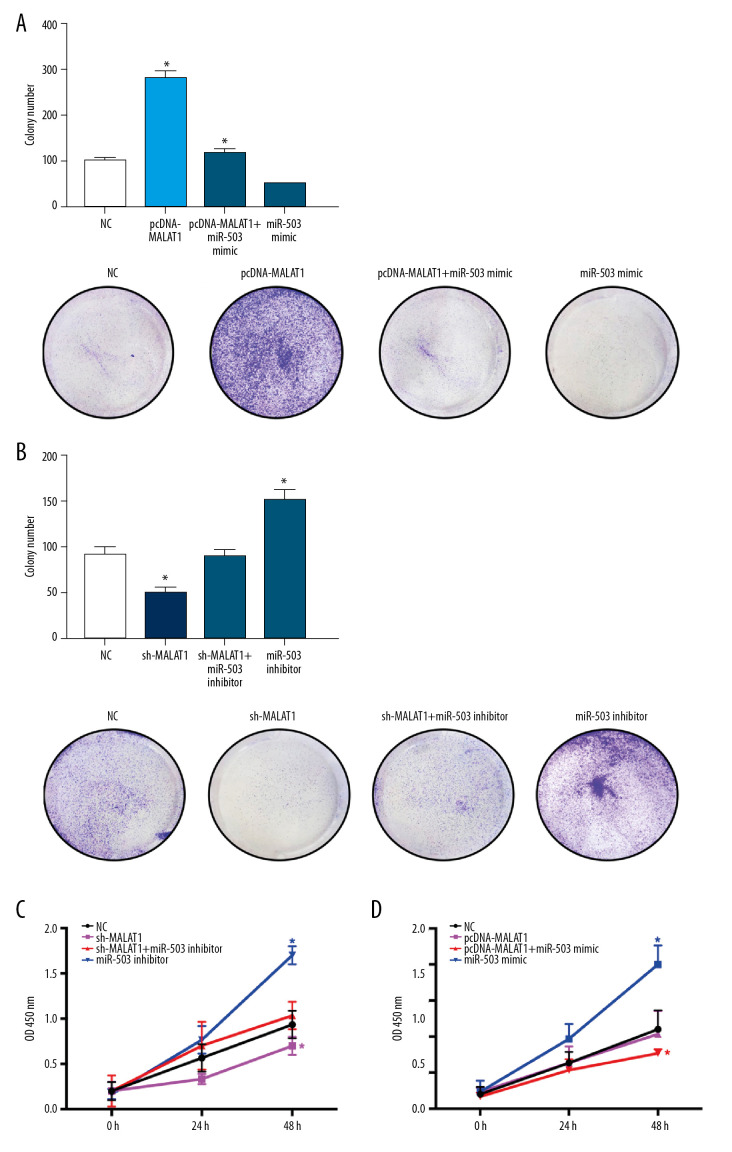
Clone formation experiments showed that the number of hPASMC clones increased significantly after transfection of pcDNA-MALAT1 and decreased significantly after transfection of miR-503 mimic. Co-transfection of both pcDNA-MALAT1 and miR-503 mimic significantly reversed the effects of either alone (p<0.05, Figure 7A). The number of hPASMC clones decreased significantly after transfection of sh-MALAT1 and increased significantly after transfection of miR-503 inhibitor. Again, co-transfection of both sh-MALAT1 and miR-503 inhibitor significantly reversed the effect of either alone on the number of hPASMC clones (p<0.05, Figure 7B).
Figure 7.
Long non-coding RNA metastasis-related lung adenocarcinoma transcript 1 (lncRNA MALAT1) regulates the proliferation of hPASMCs through microRNA-503 (miR-503). (A) Number of clones of hPASMCs after transfection with pcDNA-MALAT1, miR-503 mimic, pcDNA-MALAT1+miR-503 mimic, or no template control (NC). (B) Number of clones of hPASMCs after transfection with miR-503 inhibitor, sh-MALAT1, sh-MALAT1+miR-503 inhibitor, or NC. (C, D) Cell counting kit assays showing the proliferation of hPASMCs (C) after transfection with miR-503 inhibitor, sh-MALAT1, sh-MALAT1+miR-503 inhibitor, or NC, and (D) after transfection with pcDNA-MALAT1, miR-503 mimic, pcDNA-MALAT1+miR-503 mimic, or NC.
CCK-8 assays of cell proliferation showed similar results, in that the number of hPASMCs decreased significantly after transfection of sh-MALAT1 and increased significantly after transfection of miR-503 inhibitor, with co-transfection of sh-MALAT1 and miR-503 inhibitor significantly reversing the effect of either alone on hPASMC cell proliferation (p<0.05, Figure 7C). The number of hPASMC cells increased significantly after transfection of pcDNA-MALAT1 and decreased significantly after transfection of miR-503 mimic. Co-transfection of both pcDNA-MALAT1 and miR-503 mimic significantly reversed the effects of either alone on hPASMC cell proliferation (p<0.05, Figure 7D).
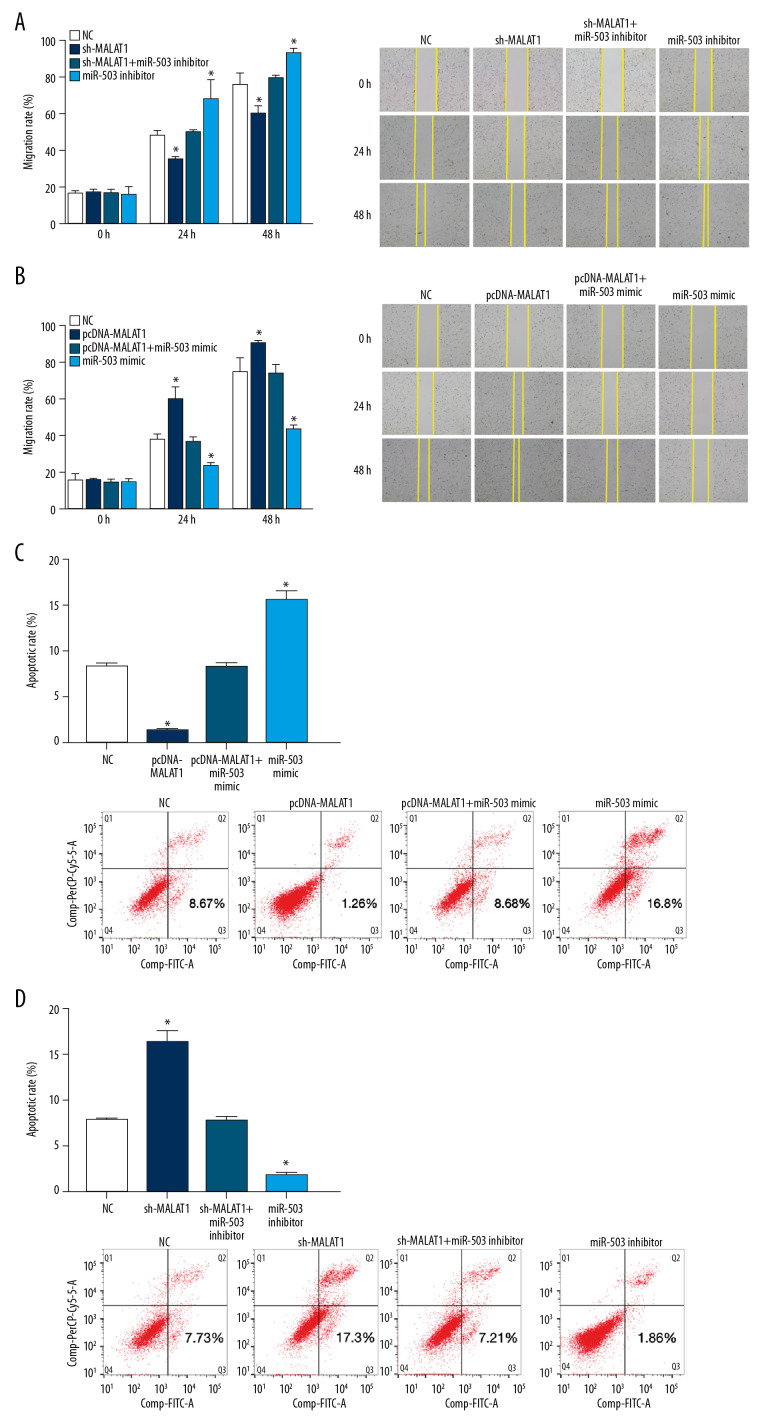
We also tested the effects of transfection on the migration of hPASMCs in wound healing assays. The migration rate of hPASMCs after transfection with sh-MALAT1 was slower than that of the NC group at 24 h and 48 h. In contrast, the migration rate was higher after transfection with miR-503 inhibitor than that of the NC group at 24 h and 48 h. Co-transfection with sh-MALAT1 and miR-503 inhibitor significantly reversed the effects of either alone on hPASMC migration (p<0.05, Figure 8A). Transfection of pcDNA-MALAT1 increased the migration rate of hPASMCs after 24 h and 48 h when compared with the NC group,. whereas transfection with miR-503 mimic reduced the hPASMC migration rate after 24 h and 48 h when compared with the NC group. Co-transfection with pcDNA-MALAT1 and miR-503 mimic significantly reversed the effect either alone on hPASMC cell migration (p<0.05, Figure 8B).
Figure 8.
Long non-coding RNA metastasis-related lung adenocarcinoma transcript 1 (lncRNA MALAT1) regulates the migration and apoptosis of hPASMCs through microRNA-503 (miR-503). (A, B) Wound healing assays showing the migration of hPASMCs (A) after transfection with sh-MALAT1, miR-503 inhibitor sh-MALAT1+miR-503 inhibitor, or no template control (NC), and (B) after transfection with pcDNA-MALAT1, miR-503 mimic, pcDNA-MALAT1+miR-503 mimic, or NC. (C, D) Flow cytometry, showing the apoptosis of hPASMCs after (C) transfection with pcDNA-MALAT1, miR-503 mimic, pcDNA-MALAT1+miR-503 mimic, or NC, and (D) after transfection with, sh-MALAT1, miR-503 inhibitor, sh-MALAT1+miR-503 inhibitor, or NC.
Flow cytometry results showed that hPASMCs transfected with pcDNA-MALAT1 had a lower rate of apoptosis, whereas hPASMCs transfected with miR-503 mimic had a higher rate of apoptosis than the NC group. Co-transfection of both pcDNA-MALAT1 and miR-503 mimic significantly reversed the effect of either alone on hPASMC cell apoptosis (p<0.05, Figure 8C). Compared with the NC group, transfection with sh-MALAT1 increased the apoptosis rate of hPASMCs, whereas transfection with miR-503 inhibitor reduced the apoptosis rate of hPASMC cells. Co-transfection of sh-MALAT1 and miR-503 inhibitor significantly reversed the effect of either alone on hPASMC cell apoptosis (p<0.05, Figure 8D). Taken together, these results indicate that lncRNA MALAT1 regulates the proliferation, migration, and apoptosis of hPASMCs through miR-503.
Discussion
This study analyzed the expression of lncRNA MALAT1 and miR-503 in PBMCs of PAH patients and healthy subjects. ROC curve analysis showed that plasma lncRNA MALAT1 and miR-503 levels were potential diagnostic markers of PAH. Sites on lncRNA MALAT1 and TLR4 mRNA were found to bind to miR-503. Silencing of lncRNA MALAT1 was shown to inhibit the proliferation and migration of hPASMCs, while promoting their apoptosis. These findings indicate that lncRNA MALAT1 is a “molecular sponge” for miR-503 that regulates the expression of TLR4, enhances the proliferation and migration of hPASMCs, and inhibits the apoptosis of these cells. Therse findings therefore suggest that the lncRNA MALAT1/miR-503/TLR4 signal axis plays a crucial role in the development of PAH.
PAH is histologically characterized by dysregulation of endothelial cell function, stimulation of inflammation, and increased proliferation and reduced apoptosis of hPASMC cells [29,30]. Pulmonary vascular remodeling is an important pathological basis for the continuous progression of PAH and is a compensatory response to changes in the external environment [31,32]. The process of vascular remodeling includes abnormal endothelial cell apoptosis, smooth muscle cell proliferation, and fibroblast proliferation [33]. The abnormal proliferation and migration of hPASMC cells and the suppression of their apoptosis are important features of pulmonary vascular remodeling. Therefore, agents that inhibit vascular remodeling are particularly important in the treatment of PAH, and finding appropriate targets is necessary.
lncRNA has been shown to play a significant role in vascular remodeling. For example, lncRNA MALAT was found to regulate endothelial cell function and vascular growth, with silencing of lncRNA MALAT inhibiting blood vessel growth in vivo [34]. Silencing of MALAT in the present study inhibited the proliferation and migration of hPASMCs, while enhancing their rate of apoptosis. These findings indicate that lncRNA MALAT1 promotes the proliferation and migration of hPASMC s and inhibits their senescence. Moreover, these findings are consistent with results showing that the expression of lncRNA MALAT1 is higher in PBMCs of PAH patients than of healthy subjects.
The specific mechanism by which lncRNA MALAT1 regulates PAH occurrence remains unknown. The starBase tool (http://starbase.sysu.edu.cn/agoClipRNA.php?source=lncRNA) predicted the presence of miR-503 binding sites on lncRNA MALAT1, and the TargetScan tool predicted that the presence of miR-503 binding sites on the TLR4 gene. Several studies have confirmed that lncRNA MALAT1 acts as a “molecular sponge” of microRNA [35,36]. Moreover, lncRNA MALAT1 was shown to act as a “molecular sponge” of miR-124-3p.1, to regulate the expression of KLF5, and to promote the vascular remodeling and cell cycle process of PAH [37]. These findings suggested that lncRNA MALAT1 may also act as a “molecular sponge” of miR-503, regulating TLR4 expression and participating in vascular remodeling.
To verify this hypothesis, a dual luciferase reporter gene assay was performed to confirm that lncRNA MALAT1 is a “molecular sponge” of miR-503 and that miR-503 targets the TLR4 gene. lncRNA MALAT1, which has high transfection efficiency in HEK 293 cells, was shown to regulate TLR4 expression through miR-503 in this cell line. Consistent with previous findings [38], we found that the levels of miR-503 in plasma were lower in patients with PAH than in healthy controls. In addition to being downregulated in PAH, miR-503 acts as a receptor targeting fibroblast growth factor 2 (FGF2). This receptor is a key factor for endothelial FGF signaling, and increase expression of this receptor is related to the excessive proliferation of hPASMCs.
We also found that co-transfection with sh-MALAT1 and miR-503 inhibitor, as well as co-transfection with pcDNA-MALAT1 and miR-503 mimic, significantly reversed the effects of either construct alone on hPASMC cell proliferation and migration. These findings further indicate that lncRNA MALAT1 acts as a “molecular sponge” of miR-503, promoting the proliferation and migration of hPASMCs and inhibiting their apoptosis.
Enhancement and activation of TLR4 expression are positively related to vascular inflammation, oxidative stress, and vasoconstriction, factors closely associated with the homeostasis of vascular function. Interestingly, the mechanism by which TLR4 induces vascular remodeling remains unclear. For example, TLR4 was found to induce vascular remodeling by generating reactive oxygen species (ROS) [39]. Specifically, TLR4 inhibits the expression and activity of the antioxidant enzyme ecSOD and simultaneously activates NADPH oxidase to regulate Ang II-induced vascular ROS levels. Activation of NADPH oxidase can therefore enhance inflammation and promote vascular remodeling. In contrast, TLR4 was shown to play a crucial role in maintaining normal pulmonary blood vessels and hypoxia-induced PAH [40]. TLR4 deficiency can lead to vascular hypertrophy and vascular remodeling. Although the results of the present study indicate that TLR4 promotes vascular remodeling, they have not yet been validated in vivo, so direct evidence remains lacking.
This study had several limitations. First, only the hPASMC cell line was analyzed. Other types of cells, such as vascular endothelial cells, should be studied simultaneously. Second, lncRNA MALAT1 may be involved in the pathogenesis of PAH through other signal-regulating axes or signal regulation networks. It is unclear whether there cross-reactions occur, with further research needed to assess this possibility. In addition, the results obtained in vitro require validation in vivo. Finally, all PAH patients in this study were from a single center, requiring confirmation in other cohorts of patients with PAH.
Conclusions
The present study found that lncRNA MALAT1 promotes the proliferation and migration of hPASMC cells, and inhibit their apoptosis, by inhibiting the miR-503/TLR4 signal axis. These results suggest that the lncRNA MALAT1/miR-503/TLR4 signal axis may play a crucial role in the development of PAH.
Footnotes
Conflict of interest
None.
Source of support: This work was supported by grants (Nos. 2019KY226 and 2020KY322) from the Department of Health of Zhejiang Province, China and by a grant (No. Q20H010011) from the Zhejiang Provincial Natural Science Foundation of China
References
- 1.Widmer RJ, Flammer AJ, Lerman LO, Lerman A. The Mediterranean diet, its components, and cardiovascular disease. Am J Med. 2015;128:229–38. doi: 10.1016/j.amjmed.2014.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sheu CC, Chang WA, Tsai MJ, et al. Gene expression changes associated with nintedanib treatment in idiopathic pulmonary fibrosis fibroblasts: A next-generation sequencing and bioinformatics study. J Clin Med. 2019;8:308. doi: 10.3390/jcm8030308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lannan KL, Phipps RP, White RJ. Thrombosis, platelets, microparticles and PAH: More than a clot. Drug Discov Today. 2014;19:1230–35. doi: 10.1016/j.drudis.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pietra GG, Capron F, Stewart S, et al. Pathologic assessment of vasculopathies in pulmonary hypertension. J Am Coll Cardiol. 2004;43:25–32S. doi: 10.1016/j.jacc.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 5.Abe K, Toba M, Alzoubi A, et al. Formation of plexiform lesions in experimental severe pulmonary arterial hypertension. Circulation. 2010;121:2747–54. doi: 10.1161/CIRCULATIONAHA.109.927681. [DOI] [PubMed] [Google Scholar]
- 6.Stenmark KR, Davie N, Frid M, et al. Role of the adventitia in pulmonary vascular remodeling. Physiology (Bethesda) 2006;21:134–45. doi: 10.1152/physiol.00053.2005. [DOI] [PubMed] [Google Scholar]
- 7.Jathar S, Kumar V, Srivastava J, Tripathi V. Technological developments in lncRNA biology. Adv Exp Med Biol. 2017;1008:283–323. doi: 10.1007/978-981-10-5203-3_10. [DOI] [PubMed] [Google Scholar]
- 8.Lu W, Cao F, Wang S, et al. LncRNAs: The regulator of glucose and lipid metabolism in tumor cells. Front Oncol. 2019;9:1099. doi: 10.3389/fonc.2019.01099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mishra K, Kanduri C. Understanding long noncoding RNA and chromatin interactions: What we know so far. Noncoding RNA. 2019;5:54. doi: 10.3390/ncrna5040054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Z, Lei T, Chen X, et al. Long non-coding RNA in lung cancer. Clin Chim Acta. 2020;504:190–200. doi: 10.1016/j.cca.2019.11.031. [DOI] [PubMed] [Google Scholar]
- 11.Ji P, Diederichs S, Wang W, et al. MALAT-1, a novel noncoding RNA, and thymosin beta4 predict metastasis and survival in early-stage non-small cell lung cancer. Oncogene. 2003;22:8031–41. doi: 10.1038/sj.onc.1206928. [DOI] [PubMed] [Google Scholar]
- 12.Li S, Mei Z, Hu HB, Zhang X. The lncRNA MALAT1 contributes to non-small cell lung cancer development via modulating miR-124/STAT3 axis. J Cell Physiol. 2018;233:6679–88. doi: 10.1002/jcp.26325. [DOI] [PubMed] [Google Scholar]
- 13.Peng L, Yuan X, Jiang B, et al. LncRNAs: Key players and novel insights into cervical cancer. Tumour Biol. 2016;37:2779–88. doi: 10.1007/s13277-015-4663-9. [DOI] [PubMed] [Google Scholar]
- 14.He Y, Meng XM, Huang C, et al. Long noncoding RNAs: Novel insights into hepatocelluar carcinoma. Cancer Lett. 2014;344:20–27. doi: 10.1016/j.canlet.2013.10.021. [DOI] [PubMed] [Google Scholar]
- 15.Yao W, Bai Y, Li Y, et al. Upregulation of MALAT-1 and its association with survival rate and the effect on cell cycle and migration in patients with esophageal squamous cell carcinoma. Tumour Biol. 2016;37:4305–12. doi: 10.1007/s13277-015-4223-3. [DOI] [PubMed] [Google Scholar]
- 16.Wu C, Zhu X, Tao K, et al. MALAT1 promotes the colorectal cancer malignancy by increasing DCP1A expression and miR203 downregulation. Mol Carcinog. 2018;57:1421–31. doi: 10.1002/mc.22868. [DOI] [PubMed] [Google Scholar]
- 17.YiRen H, YingCong Y, Sunwu Y, et al. Long noncoding RNA MALAT1 regulates autophagy associated chemoresistance via miR-23b-3p sequestration in gastric cancer. Mol Cancer. 2017;16:174. doi: 10.1186/s12943-017-0743-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soudyab M, Iranpour M, Ghafouri-Fard S. The role of long non-coding RNAs in breast cancer. Arch Iran Med. 2016;19:508–17. [PubMed] [Google Scholar]
- 19.Gutschner T, Hammerle M, Eissmann M, et al. The noncoding RNA MALAT1 is a critical regulator of the metastasis phenotype of lung cancer cells. Cancer Res. 2013;73:1180–89. doi: 10.1158/0008-5472.CAN-12-2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salle-Lefort S, Miard S, Nolin MA, et al. Hypoxia upregulates Malat1 expression through a CaMKK/AMPK/HIF-1alpha axis. Int J Oncol. 2016;49:1731–36. doi: 10.3892/ijo.2016.3630. [DOI] [PubMed] [Google Scholar]
- 21.Lelli A, Nolan KA, Santambrogio S, et al. Induction of long noncoding RNA MALAT1 in hypoxic mice. Hypoxia (Auckl) 2015;3:45–52. doi: 10.2147/HP.S90555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tee AE, Liu B, Song R, et al. The long noncoding RNA MALAT1 promotes tumor-driven angiogenesis by up-regulating pro-angiogenic gene expression. Oncotarget. 2016;7:8663–75. doi: 10.18632/oncotarget.6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang C, Qu Y, Suo R, Zhu Y. Long non-coding RNA MALAT1 regulates angiogenesis following oxygen-glucose deprivation/reoxygenation. J Cell Mol Med. 2019;23:2970–83. doi: 10.1111/jcmm.14204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–97. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 25.de Kleijn D, Pasterkamp G. Toll-like receptors in cardiovascular diseases. Cardiovasc Res. 2003;60:58–67. doi: 10.1016/s0008-6363(03)00348-1. [DOI] [PubMed] [Google Scholar]
- 26.Song Y, Hou M, Li Z, et al. TLR4/NF-kappaB/ceramide signaling contributes to Ox-LDL-induced calcification of human vascular smooth muscle cells. Eur J Pharmacol. 2017;794:45–51. doi: 10.1016/j.ejphar.2016.11.029. [DOI] [PubMed] [Google Scholar]
- 27.Humbert M, Galie N. The Ten Commandments for 2015 European Society of Cardiology – European Respiratory Society Guidelines on Pulmonary Hypertension. Eur Heart J. 2016;37:5. [PubMed] [Google Scholar]
- 28.Li JH, Liu S, Zhou H, et al. starBase v2.0: decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res. 2014;42:D92–97. doi: 10.1093/nar/gkt1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vonk Noordegraaf A, Groeneveldt JA, Bogaard HJ. Pulmonary hypertension. Eur Respir Rev. 2016;25:4–11. doi: 10.1183/16000617.0096-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoeper MM, Ghofrani HA, Grunig E, et al. Pulmonary hypertension. Dtsch Arztebl Int. 2017;114:73–84. doi: 10.3238/arztebl.2017.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tuder RM. Pulmonary vascular remodeling in pulmonary hypertension. Cell Tissue Res. 2017;367:643–49. doi: 10.1007/s00441-016-2539-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thompson AAR, Lawrie A. Targeting vascular remodeling to treat pulmonary arterial hypertension. Trends Mol Med. 2017;23:31–45. doi: 10.1016/j.molmed.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 33.Baeyens N, Schwartz MA. Biomechanics of vascular mechanosensation and remodeling. Mol Biol Cell. 2016;27:7–11. doi: 10.1091/mbc.E14-11-1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Michalik KM, You X, Manavski Y, et al. Long noncoding RNA MALAT1 regulates endothelial cell function and vessel growth. Circ Res. 2014;114:1389–97. doi: 10.1161/CIRCRESAHA.114.303265. [DOI] [PubMed] [Google Scholar]
- 35.Dai X, Liang Z, Liu L, et al. Silencing of MALAT1 inhibits migration and invasion by sponging miR-1-3p in prostate cancer cells. Mol Med Rep. 2019;20:3499–508. doi: 10.3892/mmr.2019.10602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yuan X, Guo Y, Chen D, et al. Long non-coding RNA MALAT1 functions as miR-1 sponge to regulate connexin 43-mediated ossification of the posterior longitudinal ligament. Bone. 2019;127:305–14. doi: 10.1016/j.bone.2019.06.019. [DOI] [PubMed] [Google Scholar]
- 37.Wang D, Xu H, Wu B, et al. Long non-coding RNA MALAT1 sponges miR-124-3p.1/KLF5 to promote pulmonary vascular remodeling and cell cycle progression of pulmonary artery hypertension. Int J Mol Med. 2019;44:871–84. doi: 10.3892/ijmm.2019.4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim J, Kang Y, Kojima Y, et al. An endothelial apelin-FGF link mediated by miR-424 and miR-503 is disrupted in pulmonary arterial hypertension. Nat Med. 2013;19:74–82. doi: 10.1038/nm.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakashima T, Umemoto S, Yoshimura K, et al. TLR4 is a critical regulator of angiotensin II-induced vascular remodeling: the roles of extracellular SOD and NADPH oxidase. Hypertens Res. 2015;38:649–55. doi: 10.1038/hr.2015.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ma L, Ambalavanan N, Liu H, et al. TLR4 regulates pulmonary vascular homeostasis and remodeling via redox signaling. Front Biosci (Landmark Ed) 2016;21:397–409. doi: 10.2741/4396. [DOI] [PMC free article] [PubMed] [Google Scholar]