Abstract
Increasing evidence has indicated that the dysregulation of microRNA (miRNA) occur in the pathogenesis of retinoblastoma (RB). Aim of the present study was to investigate the possible role of miR-494 (miR-494-3p) in RB. It was demonstrated that miR-494 expression was increased in RB tissue samples and cell lines. Also, it was prominently associated with clinicopathological features. Functional assays showed that RB cell proliferation, invasion and migration can be promoted by miR-494 overexpression. Besides, phosphatase and tensin homolog (PTEN) was verified as a possible target of miR-494 by a luciferase assay, western blot and qRT-PCR assay in RB. miR-494 and PTEN expression was negatively related in a correlation analysis on tumor tissues of 66 patients. In addition, PTEN was proved to reverse miR-494 effect on RB cell progression. Moreover, PI3K/AKT signaling pathway was validated to take part in RB progression. Taken together, the current study proposes that miR-494 might function as a tumor promoter and regulates RB progression through targeting PTEN.
Keywords: retinoblastoma, miR-494, PTEN, PI3K/AKT pathway
Introduction
Retinoblastoma (RB) is the most prevalent type of primary inherited intraocular malignant tumor in infancy and children, affecting their life, vision, facial appearance and psychological development (1,2). The disease is prone to intracranial and distant metastasis, often endangering the life of the child, particularly in children aged <5 years (3,4). The predominant treatment for patients includes chemotherapy, enucleation, laser therapy or cryotherapy, the most common treatment protocol includes chemotherapy combined with immunotherapy, which is cytotoxic to RB cells (1). Therefore, early detection, early diagnosis and early treatment are the key to improving the cure rate and reducing the mortality rate.
MiRNAs, a family of small and noncoding RNA molecules consisting of 21–25 nucleotides, regulate the expression of their target genes through suppressing the translation or promoting the RNA degradation (5,6). They are identified as tumor suppressors or oncogenes in many types of tumors and thus regulate tumor progression and metastasis. Mounting evidence has indicated that miRNAs were identified in RB and regulated cell migration, invasion, proliferation and apoptosis (7–11). Sun et al (12) showed that suppression of miR-492 inhibited RB cell viability and invasion by targeting LATS2. miR-183 may be involved in RB progression by targeting LRP6 (13). Also, miR-34a exhibited inhibitory effect on RB cell growth and promoting effect on cell apoptosis (14). Moreover, miR-494 (miR-494-3p) was identified to be highly expressed in RB cells and associated with its tumorigenesis (15). However, the mechanism regulating RB progression by miR-494 expression is yet to be fully elucidated.
Phosphatase and tensin homolog (PTEN) has been reported to regulate the PI3K/AKT signaling pathway negatively (16). Studies have confirmed that PTEN was disordered in multiple cancers, and was considered to be one of the potential factors in tumorigenesis (17,18). Previous studies have summarized the role of miRNAs in regulating PTEN in cancers and have determined that the miRNA/PTEN pathway is involved in tumor cell growth, invasion and migration (19–21). For instance, miR-296/PTEN axis facilitated gastric cancer tumorigenesis in vitro and in vivo (22). miR-548 promoted NSCLC cell invasion by regulating PTEN (23). Moreover, miR-182 promoted breast cancer cell viability and invasion via targeting PTEN (24). A previous study has shown that miR-494-3p can promote the progression of endometrial cancer by regulating the PTEN/PI3K/AKT pathway (25). However, whether PTEN acted as the target of miR-494 in regulating RB progression is unclear.
Herei, it is suggested that miR-494 expression was upregulated in RB tissues, and cells and miR-494 enhanced RB cell viability, migration and invasion. PTEN was verified as the target of miR-494 in RB cells. I was shown that miR-494 facilitated RB cell progression by targeting PTEN through PI3K/AKT signaling pathway.
Patients and methods
Tissue samples
Ten normal retina tissues and RB tissues were provided by Jinan Zhangqiu District Hospital of TCM (Jinan, China). None of the patients in this study received preoperative radiotherapy or chemotherapy, and all patients were diagnosed by three pathologists. The patients consisted of 6 males and 4 females aged 1.2 to 9.9 years (mean, 5.4). According to ICRB, the RB patients were divided into phase I (3 cases), phase II (5 cases) and phase III (2 cases). There was no significant difference in age, sex and clinical stages of the 10 patients. The adjacent healthy tissues were set as the control. The fresh samples were stored in −80°C refrigerator for further analysis. Parents of the patients provided a written informed consent prior to surgical excision. The Ethics Committee of Jinan Zhangqiu District Hospital of TCM approved this study.
Cell culture
Two RB cell lines (Y79, SO-RB50) and normal retina epithelium cell line (APRE-19) were obtained from ATCC. All the cells were cultured in RPMI-1640 medium with 10% FBS. Cells were then left to grow in a humidified incubator containing 5% CO2 at 37°C.
qRT-PCR
Total RNA was extracted with the help of TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.). The concentration and purity of RNA were tested via NanoDrop 2000 instrument (Thermo Fisher Scientific, Inc.). To perform the quantitative detection of miR-18a or mRNA expression, cDNAs were synthesized through the PrimeScript RT reagent (Takara Biotechnology Co.). GAPDH was used as the control for the normalization of expression levels of genes. The snRNA U6 was used as the control of miRNA. The primers were as follows: miR-494-F, 5′-GATACTCGAAGGAGAGGTTGTC-3′ and miR-494-R, 5′-GAGGTTTCCCGTGTATGTTTCAT-3′; PTEN-F, 5′-CGGCAGCATCAAATGTTTCAG-3′ and PTEN-R, 5′-AACTGGCAGGTAGAAGGCAACTC-3′; GAPDH-F, 5′-CTCTGCTCCTCCTGTTCGAC-3′ and GAPDH-R, 5′-CGACCAAATCCGTTGACTCC-3′; U6-F, 5′-ATTGGAACGATACAGAGAAGATT-3′ and U6-R, 5′-GGAACGCTTCACGAATTTG-3′. The data were expressed as 2−ΔΔCt, indicating the target mRNA relative level.
Western blot analysis
Total protein was extracted with RIPA lysis buffer. The protein concentration was determined using the BCA method (Beyotime Institute of Biotechnology). The assay was performed as follow: 50 µg of total proteins were loaded onto the gel and electrophoresed. After transferring to the NC membranes, the membranes were blocked with 5–10% skim milk. Then, the membranes were incubated with the primary antibodies at 4°C overnight. Subsequently, the second antibodies were added for incubating at room temperature for 2 h. Finally, ECL agents were applied for observing the protein bands. ImageJ software (version 1.48; National Institutes of Health) was used for densitometry. GAPDH was employed as an internal control.
MTT assay
RB cells (5×103) were placed onto 96-well plates. When the cells were cultured for 0, 1, 2, 3 and 4 days, MTT solution (20 µl) was added and cultured for 4 h at 37°C. A Bio-Rad microplate reader (Bio-Rad Laboratories, Inc.) was used to read the absorbance at 490 nm.
Overexpression of miR-494 or silence miR-494 and overexpression of PTEN
miR-494 mimic or inhibitor was purchased from RiboBio Co., Ltd. SiRNA PTEN was obtained from Genechem Co., Ltd. The transfection was performed with the help of Lipofectamine 2000 for 48 h.
Transwell assay
The Transwell assay was applied to perform cell migration and invasion using Transwell inserts (8 µm pores; BD Biosciences). The differences between cell migration and invasion were whether the top chamber was pre-coated with or without Matrigel. Firstly, the cells were placed into the top chamber to incubate for 24 h. The lower chamber was added with complete medium with 10% FBS. The cells in the lower chamber that migrated or invaded from the top chamber were then fixed with 4% paraformaldehyde and stained with 0.5% crystal violet. Finally, the number of migrated or invaded cell was counted using a microscope (magnification, ×200).
Luciferase assay
The wild-type (wt) and mutant (mut) 3′UTR of PTEN was first inserted into the pGL3 reporter vector (Promega Corporation). Then, the PTEN 3′UTR-pGL3 reporter vector and miR-494 mimic were co-transfected into Y79 cells and plated in 24-well plates for 48 h, the cells were harvested and lysed, and the luciferase activity was detected by a Dual-Luciferase Reporter System (Promega) according to the protocol of the manufacturer. Relative firefly luciferase activity was normalized to Renilla luciferase activity.
Statistical analysis
Results are represented as the mean ± SD of at least triplicates. SPSS 22.0 Software (IBM Corp.) was applied for performing statistical analyses. Student's t-test was carried out for determining the statistical significance of differences in two groups and one-way analysis of variance with Tukey's post hoc test was applied in more than two groups. P<0.05 indicates statistical significance.
Results
Increase of miR-494 in RB tissues
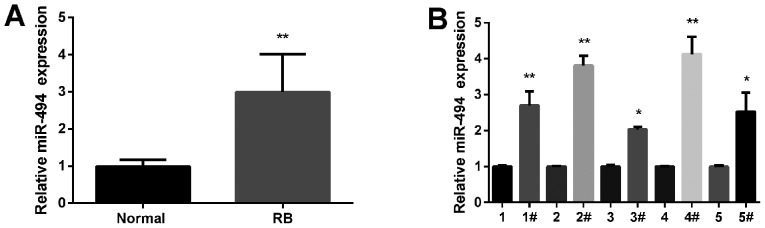
The average expression level of miR-494 in RB tissues was analyzed by qRT-PCR and the findings indicated that it was obviously increased in RB tissue samples compared with normal tissues (Fig. 1A). Then miR-494 expression level was evaluated in 5 clinical RB specimens versus 5 normal tissues. Results showed that its expression was obviously increased in RB tissues (Fig. 1B). Moreover, the correlation between miR-494 expression and the clinicopathological features of 66 RB patients was analysed. The median value of miR-494 expression was used as the cut-off point to divide miR-494 expression into high and low expression of miR-494. Results demonstrated that the high miR-494 expression was associated with N classification and differentiation in patients with RB (Table I). Collectively, these results indicated that miR-494 might play important roles in RB progression.
Figure 1.
Upregulation of miR-494 in RB tissue samples. (A) Average expression level of miR-494 tested in RB tissue samples by qRT-PCR (n=10). (B) Expression level of miR-494 in 5 RB tissue specimens by qRT-PCR. *P<0.05, **P<0.01. RB, retinoblastoma.
Table I.
Association study, and correlation between miR-494 expression and clinicopathological characteristics.
| miR-494 | ||||
|---|---|---|---|---|
| Characteristics | N=66 | Low expression | High expression | P-value |
| Age (years) | 0.569 | |||
| ≤5 | 52 | 18 | 34 | |
| >5 | 14 | 6 | 8 | |
| Sex | 0.930 | |||
| Male | 39 | 14 | 25 | |
| Female | 27 | 10 | 17 | |
| Clinical stage | 0.478 | |||
| I–II | 20 | 6 | 14 | |
| III–IV | 46 | 18 | 28 | |
| N classification | 0.019a | |||
| N0 | 29 | 6 | 23 | |
| N1+2 | 37 | 18 | 19 | |
| Differentiation | 0.047a | |||
| Well and moderate | 24 | 5 | 19 | |
| Poorly | 42 | 19 | 23 | |
| Largest tumor (mm) | 0.070 | |||
| ≤15 | 40 | 18 | 22 | |
| >15 | 26 | 6 | 20 | |
Statistical analyses were performed by the χ2 test.
P<0.05 was considered significant.
miR-494 enhances RB cell viability, invasion and migration
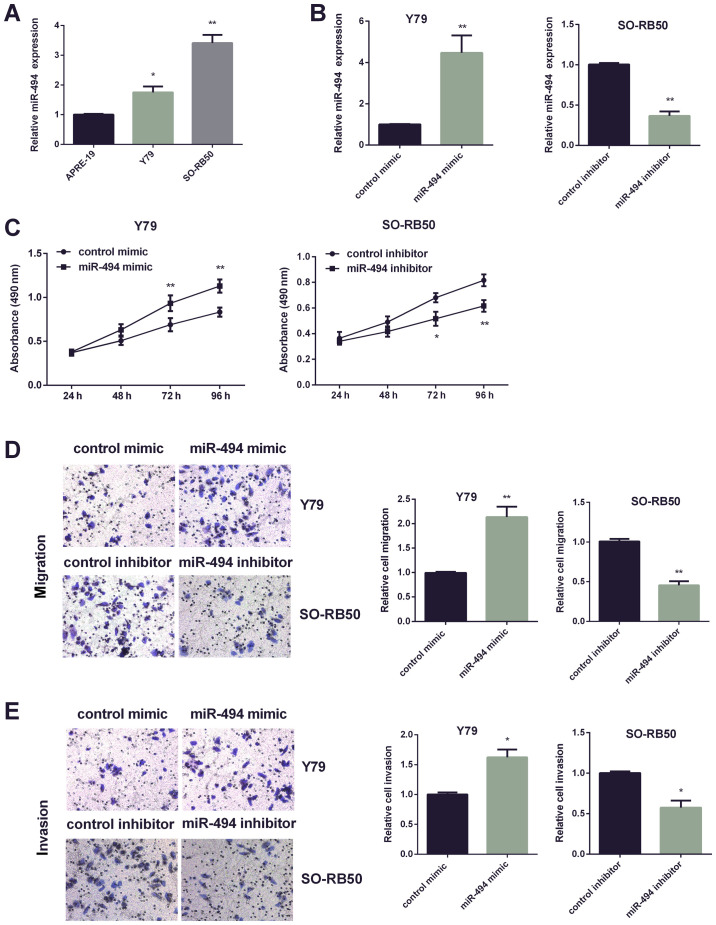
Two RB cell lines (Y79, SO-RB50) were applied to investigate miR-494 function in RB progression. Due to the higher expression of miR-494 in Y79 cells, miR-494 expression was increased in Y79 cells, and miR-494 expression in SO-RB50 cells was suppressed (Fig. 2A). qRT-PCR was applied for confirming efficiency of miR-494 transfection in the two cell lines respectively (Fig. 2B). Then, MTT assay was performed to detect cell viability affected by altered miR-494. It was found that increasing miR-494 enhanced Y79 cell paoliferation, while decreasing miR-494 inhibited SO-RB50 cells viability (Fig. 2C). Furthermore, Transwell assay was used to investigate miR-494 effect on cell invasion and migration. Data showed dramatically increased mobility and invasiveness of Y79 cells by miR-494 mimic, whereas it was markedly reduced by miR-494 inhibitor in SO-RB50 cells (Fig. 2D and E).
Figure 2.
Promotion effect of miR-494 on RB cell progression. (A) qRT-PCR analysis of miR-494 expression level in Y79 and SO-RB50 cells. (B) The expression level of miR-494 tested in Y79 cells after increasing miR-494 and in SO-RB50 cells after decreasing miR-494 by qRT-PCR. (C) Y79 cell viability was measured after increasing miR-494 or SO-RB50 cell viability after inhibiting miR-494 expression by MTT assay. (D) The ability of Y79 cell migration was detected after increasing miR-494 or SO-RB50 cell migration was detected after inhibiting miR-494 expression by Transwell assay. (E) The ability of Y79 cell invasion was measured after increasing miR-494 or SO-RB50 cell invasion and was detected after decreasing miR-494 expression by Transwell assay. *P<0.05, **P<0.01. RB, retinoblastoma.
PTEN is the miR-494 target gene in RB cells
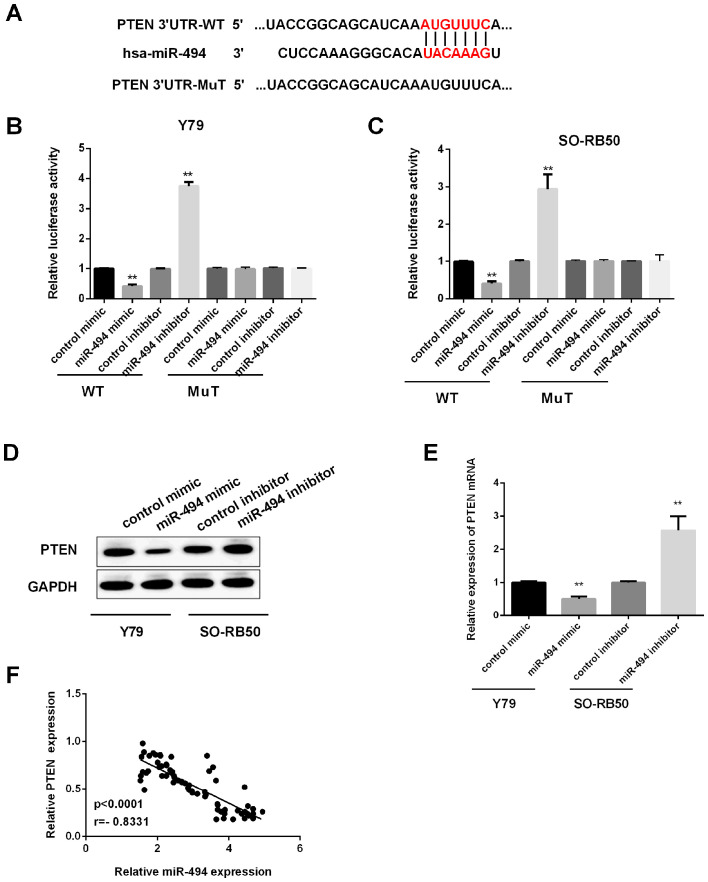
We used the algorithm provided by TargetScan Human to predict the candidate targets of miR-494 and to determine the mechanisms responsible for the effects of miR-494 in RB cells. PTEN was temporarily selected for further validation among the predicted targets, because it has a potential role in the regulation of RB malignancies (Fig. 3A). To further verify whether PTEN was the direct target of miR-494, Luciferase-based assay was carried out to perform this experiment. The findings showed that miR-494 mimic dramatically inhibited PTEN 3′UTR luciferase activity in Y79 cells (Fig. 3B). Consistently, miR-494 inhibitor dramatically enhanced PTEN 3′UTR luciferase activity in SO-RB50 cells (Fig. 3C). Apart from this, western blot analysis and qRT-PCR were performed to test PTEN expression. Results indicated that PTEN protein (Fig. 3D) and mRNA (Fig. 3E) levels were remarkably inhibited by miR-494 overexpression in Y79 cells, and increased by miR-494 suppression in SO-RB50 cells. Fig. 3F shows the negative relationship between miR-494 and PTEN expression by Spearman's Rank correlation.
Figure 3.
PTEN was the target of miR-494 in RB cells. (A) Schematic representation of the binding sites of miR-494 with PTEN 3′UTR. (B) Relative luciferase activity was tested after overexpression of miR-494 in wild-type (WT) or mutated (MuT) Y79 cells. (C) Relative luciferase activity detected in SO-RB50 cells after silence miR-494 in WT or MuT. (D) Relative protein level of PTEN by western blotting in Y79 cells or SO-RB50 cells. (E) Relative PTEN mRNA expression in Y79 cells or SO-RB50 cells by qRT-PCR. (F) The association of miR-494 expression and PTEN expression was measured by Spearman's Rank correlation. **P<0.01. RB, retinoblastoma; PTEN, phosphatase and tensin homolog.
miR-494 promotes RB cell progression via PTEN
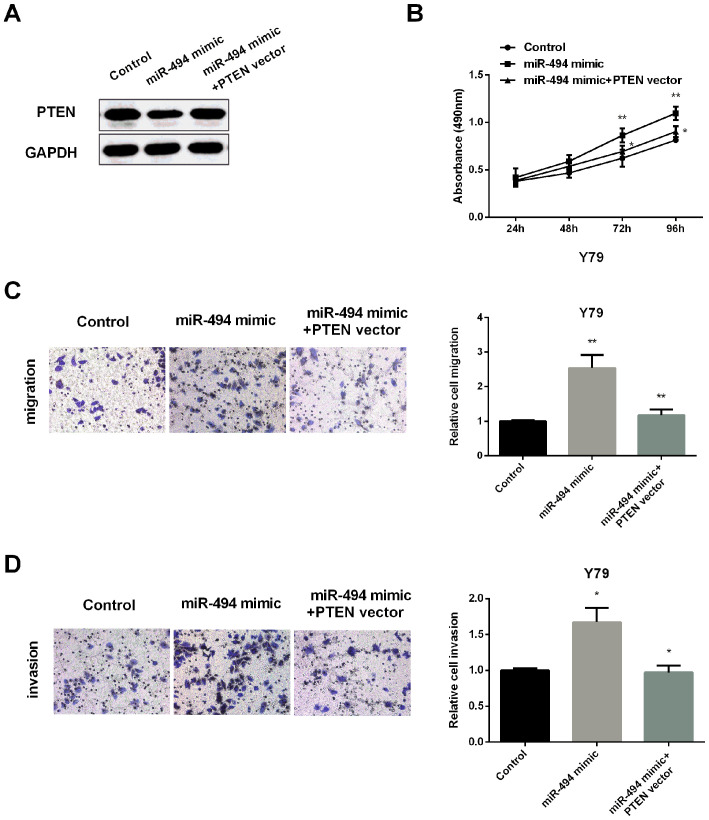
To further detect whether PTEN regulates miR-494 effect on RB cell viability, invasion and metastasis, PTEN level was restored in Y79 cells inhibited by miR-494 overexpression (Fig. 4A). qRT-PCR was applied for confirming efficiency of PTEN transfection in Y79 cells. Then, MTT assay was performed to test cell viability. As expected, miR-494 mimic increased the cell proliferation, but its effect was overturned by overexpression of PTEN (Fig. 4B). Similarly, increasing the expression of PTEN also reversed the increased migration (Fig. 4C) and invasion (Fig. 4D) regulated by miR-494 mimic. All the results demonstrated that miR-494 promoted RB cell proliferation, invasion and migration via inhibiting PTEN expression.
Figure 4.
miR-494 regulates RB cell progression via PTEN. (A) Detection of PTEN protein in Y79 cells by western blotting. (B) Cell viability was measured in Y79 cells after increasing miR-494 expression or both miR-494 and PTEN expression. (C) Cell migration was detected in Y79 cells after upregulation of miR-494 or with miR-494 and PTEN. (D) Cell invasion was detected in Y79 cells after upregulation of miR-494 or both miR-494 and PTEN. *P<0.05, **P<0.01. RB, retinoblastoma; PTEN, phosphatase and tensin homolog.
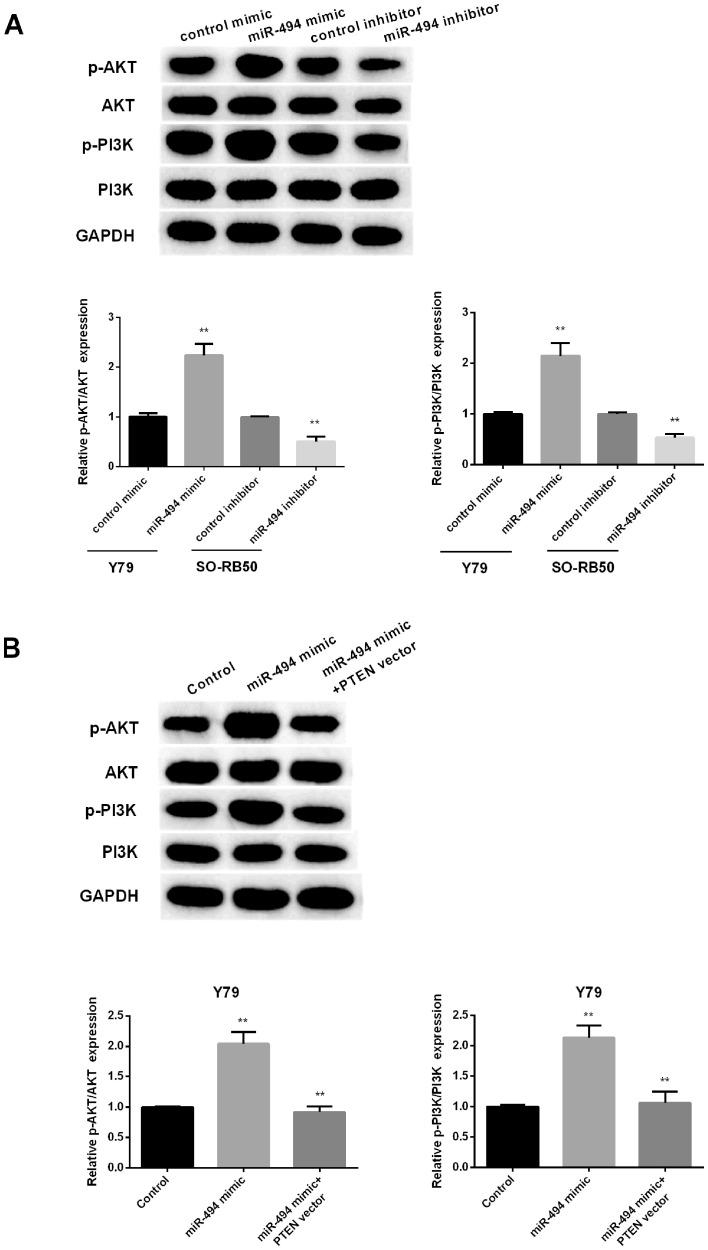
miR-494 regulates PI3K/AKT signaling pathway via PTEN
PTEN has been reported to negatively regulate PI3K/AKT signaling pathway.
To investigate whether a change in miR-665 expression affects the PI3K/AKT pathway in RB cells, the expression levels of several important molecules in the pathway, including p-AKT, AKT, p-PI3K and PI3K, were measured in RB cells following upregulation or downregulation of miR-494. As presented in Fig. 5A, miR-494 overexpression remarkably promoted the phosphorylation of PI3K and AKT in Y79 cells. However, silencing miR-494 obviously inhibited the phosphorylation of PI3K and AKT in SO-RB50 cells. Restoration of PTEN reversed effect of miR-494 mimic on PI3K/AKT pathway (Fig. 5B). The results suggested that miR-494 promoted RB cell progression via PTEN/PI3K/AKT signaling pathway.
Figure 5.
miR-494 regulated PTEN/PI3K/AKT signaling pathway. (A) The expression of p-PI3K/PI3K or p-AKT/AKT in Y79 cells after increasing miR-494 or in SO-RB50 cells after inhibiting miR-494. (B) Quantitative expression of p-PI3K/PI3K or p-AKT/AKT in Y79 cells after overexpression of miR-494 or both miR-494 and PTEN. PTEN, phosphatase and tensin homolog. **P<0.01.
Discussion
Herein, we displayed that miR-494 was increased in RB tissue samples and cells, and it was related to the clinicopathological features of RB patients. Moreover, increasing miR-494 facilitated, while inhibiting miR-494, repressed RB cell viability, invasion and migration. Furthermore, PTEN was verified as the target of miR-494 in RB. In addition, it was demonstrated that miR-494 regulated PI3K/AKT signaling pathway by suppressing PTEN in RB cells. Collectively, our findings suggested that overexpression of miR-494 promoted RB cell progression through PTEN/PI3K/AKT axis.
In previous studies, miR-494 has been implicated in the development and progression of various types of tumors. Zhang et al (26) stated that miR-494 played an oncomiR role in lung cancer progression. Zhu et al (25) proved that miR-494 overexpression obviously promoted endometrial cancer cell viability, invasion and migration. Besides, miR-494 mimic significantly enhanced nasopharyngeal carcinoma cell proliferation, invasion and migration (27). In our study, it was shown that miR-494 functioned as an oncogene in RB progression by enhancing cell viability, invasion and migration. In general, miR-494 played oncomiR roles in multiple cancers via targeting different mRNA genes. For example, miR-494 took part in gastric cancer cell survival by suppressing BAG-1 (28). Cheng et al (29) found that miR-494 repressed cervical cancer cell proliferation, invasion via promoting SOCS6. Zhang et al (30) stated that miR-494 was higher in colorectal cancer and promoted cell progression by targeting APC. In this study we first showed that miR-494 regulated RB development by targeting PTEN, which was the novelty of this research.
PTEN is essential for the maintenance of normal cells and acts as a tumor suppressor in human cancers (31). A large number of studies have reported that PTEN played important roles in tumor development and progression, including lung cancer, ovarian cancer, and prostate cancer (23,32,33). Furthermore, in the RB development, PTEN showed an inhibitory effect and it served as the target of multiple miRNAs. For instance, it was the directly target of miR-93, and miR-198 in promoting human RB progression, and the decreased PTEN expression was also found, which was consists with our findings (34,35). In the present study, it was shown that miR-494 targeted PTEN in regulating RB cell progression, and PTEN overexpression can reverse the effect of miR-494 upregulation in RB cells.
The PI3K/Akt axis is well known to participate in the cell growth and survival of various types of cancers, including RB (36–38). A previous study reported that the ethanol extracts of R. japonica radix (ERJR) could play an inhibitory effect on hepatocellular carcinoma metastasis via PI3K/Akt signaling pathways (39). PTEN is reported to act as an antagonist of PI3K action, it regulates PI3K/Akt signaling negatively (40). For instance, microRNA-155-5p promoted hepatocellular carcinoma progression by suppressing PTEN through the PI3K/Akt pathway (41). Our findings demonstrated that overexpression of miR-494 promoted PI3K/Akt signaling pathways by suppressing PTEN, and PTEN overexpression can reverse the effect of miR-494 upregulation in PI3K/Akt signaling pathways.
Potential limitation of this study is that though miR-494 promoted proliferation of retinoblastoma in our study, this was only verified in RB cell lines, and mouse model might be more convincing. For the experiments using cells transfected with miR-494, using an untransfected control cell line to show that transfection alone did not affect the behavior would be more convincing. Because miR-494 is upregulated and promoted cell viability, invasion and migration in RB, it might be a possible biomarker for predicting progression. In summary, our study indicated that miR-494 acted as an oncogenic microRNA in RB cells by suppressing the tumor suppressor PTEN. miR-494 regulated PI3K/AKT signaling pathway via PTEN.
The present study revealed the important role of miR-494 in promoting the progression of retinoblastoma, although further studies are required to confirm this. Taken together, our research stated that miR-494 overexpression enhanced RB cell progression through PTEN/PI3K/AKT signaling pathway.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
FX conceived the study and drafted the manuscript. FX, GL and LW were responsible for cell culture, qRT-PCR and western blot analysis. XW, XJ and WB performed MTT assay and luciferase assay. All authors read and approved the final manuscript.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Jinan Zhangqiu District Hospital of TCM (Jinan, China). Patients who participated in this research had complete clinical data. Parents of the child patients signed the informed consent form.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Dimaras H, Dimba EA, Gallie BL. Challenging the global retinoblastoma survival disparity through a collaborative research effort. Br J Ophthalmol. 2010;94:1415–1416. doi: 10.1136/bjo.2009.174136. [DOI] [PubMed] [Google Scholar]
- 2.Kivelä T. The epidemiological challenge of the most frequent eye cancer: Retinoblastoma, an issue of birth and death. Br J Ophthalmol. 2009;93:1129–1131. doi: 10.1136/bjo.2008.150292. [DOI] [PubMed] [Google Scholar]
- 3.Narang S, Mashayekhi A, Rudich D, Shields CL. Predictors of long-term visual outcome after chemoreduction for management of intraocular retinoblastoma. Clin Exp Ophthalmol. 2012;40:736–742. doi: 10.1111/j.1442-9071.2012.02757.x. [DOI] [PubMed] [Google Scholar]
- 4.Shinohara ET, DeWees T, Perkins SM. Subsequent malignancies and their effect on survival in patients with retinoblastoma. Pediatr Blood Cancer. 2014;61:116–119. doi: 10.1002/pbc.24714. [DOI] [PubMed] [Google Scholar]
- 5.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, et al. European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 6.Holland EC. Gliomagenesis: Genetic alterations and mouse models. Nat Rev Genet. 2001;2:120–129. doi: 10.1038/35052535. [DOI] [PubMed] [Google Scholar]
- 7.Liu S, Hu C, Wang Y, Shi G, Li Y, Wu H. miR-124 inhibits proliferation and invasion of human retinoblastoma cells by targeting STAT3. Oncol Rep. 2016;36:2398–2404. doi: 10.3892/or.2016.4999. [DOI] [PubMed] [Google Scholar]
- 8.Wang L, Lyu X, Ma Y, Wu F, Wang L. MicroRNA 504 targets AEG 1 and inhibits cell proliferation and invasion in retinoblastoma. Mol Med Rep. 2019;19:2935–2942. doi: 10.3892/mmr.2019.9923. [DOI] [PubMed] [Google Scholar]
- 9.Guo L, Bai Y, Ji S, Ma H. MicroRNA 98 suppresses cell growth and invasion of retinoblastoma via targeting the IGF1R/k Ras/Raf/MEK/ERK signaling pathway. Int J Oncol. 2019;54:807–820. doi: 10.3892/ijo.2019.4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Golabchi K, Soleimani-Jelodar R, Aghadoost N, Momeni F, Moridikia A, Nahand JS, Masoudifar A, Razmjoo H, Mirzaei H. MicroRNAs in retinoblastoma: Potential diagnostic and therapeutic biomarkers. J Cell Physiol. 2018;233:3016–3023. doi: 10.1002/jcp.26070. [DOI] [PubMed] [Google Scholar]
- 11.Yu F, Pang G, Zhao G. ANRIL acts as onco-lncRNA by regulation of microRNA-24/c-Myc, MEK/ERK and Wnt/β-catenin pathway in retinoblastoma. Int J Biol Macromol. 2019;128:583–592. doi: 10.1016/j.ijbiomac.2019.01.157. [DOI] [PubMed] [Google Scholar]
- 12.Sun Z, Zhang A, Zhang L. Inhibition of microRNA 492 attenuates cell proliferation and invasion in retinoblastoma via directly targeting LATS2. Mol Med Rep. 2019;19:1965–1971. doi: 10.3892/mmr.2018.9784. [DOI] [PubMed] [Google Scholar]
- 13.Wang J, Wang X, Li Z, Liu H, Teng Y. MicroRNA-183 suppresses retinoblastoma cell growth, invasion and migration by targeting LRP6. FEBS J. 2014;281:1355–1365. doi: 10.1111/febs.12659. [DOI] [PubMed] [Google Scholar]
- 14.Dalgard CL, Gonzalez M, deNiro JE, O'Brien JM. Differential microRNA-34a expression and tumor suppressor function in retinoblastoma cells. Invest Ophthalmol Vis Sci. 2009;50:4542–4551. doi: 10.1167/iovs.09-3520. [DOI] [PubMed] [Google Scholar]
- 15.Zhao JJ, Yang J, Lin J, Yao N, Zhu Y, Zheng J, Xu J, Cheng JQ, Lin JY, Ma X. Identification of miRNAs associated with tumorigenesis of retinoblastoma by miRNA microarray analysis. Childs Nerv Syst. 2009;25:13–20. doi: 10.1007/s00381-008-0701-x. [DOI] [PubMed] [Google Scholar]
- 16.Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 17.Tay Y, Kats L, Salmena L, Weiss D, Tan SM, Ala U, Karreth F, Poliseno L, Provero P, Di Cunto F, et al. Coding-independent regulation of the tumor suppressor PTEN by competing endogenous mRNAs. Cell. 2011;147:344–357. doi: 10.1016/j.cell.2011.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu W, Yang Z, Zhou SF, Lu N. Posttranslational regulation of phosphatase and tensin homolog (PTEN) and its functional impact on cancer behaviors. Drug Des Devel Ther. 2014;8:1745–1751. doi: 10.2147/DDDT.S71061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu W, Yang J, Feng X, Wang H, Ye S, Yang P, Tan W, Wei G, Zhou Y. MicroRNA-32 (miR-32) regulates phosphatase and tensin homologue (PTEN) expression and promotes growth, migration, and invasion in colorectal carcinoma cells. Mol Cancer. 2013;12:30. doi: 10.1186/1476-4598-12-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang ZX, Lu BB, Wang H, Cheng ZX, Yin YM. MicroRNA-21 modulates chemosensitivity of breast cancer cells to doxorubicin by targeting PTEN. Arch Med Res. 2011;42:281–290. doi: 10.1016/j.arcmed.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 21.Ma F, Zhang J, Zhong L, Wang L, Liu Y, Wang Y, Peng L, Guo B. Upregulated microRNA-301a in breast cancer promotes tumor metastasis by targeting PTEN and activating Wnt/β-catenin signaling. Gene. 2014;535:191–197. doi: 10.1016/j.gene.2013.11.035. [DOI] [PubMed] [Google Scholar]
- 22.Rong D, Lu C, Zhang B, Fu K, Zhao S, Tang W, Cao H. CircPSMC3 suppresses the proliferation and metastasis of gastric cancer by acting as a competitive endogenous RNA through sponging miR-296-5p. Mol Cancer. 2019;18:25. doi: 10.1186/s12943-019-0958-6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Akgun S, Kucuksayan H, Ozes ON, Can O, Alikanoglu AS, Yildiz M, Akca H. NF-κB-induced upregulation of miR-548as-3p increases invasion of NSCLC by targeting PTEN. Anticancer Agents Med Chem. 2019;19:1058–1068. doi: 10.2174/1871520619666190206165215. [DOI] [PubMed] [Google Scholar]
- 24.Zhao YS, Yang WC, Xin HW, Han JX, Ma SG. MiR-182-5p knockdown targeting PTEN inhibits cell proliferation and invasion of breast cancer cells. Yonsei Med J. 2019;60:148–157. doi: 10.3349/ymj.2019.60.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu L, Wang X, Wang T, Zhu W, Zhou X. miR-494-3p promotes the progression of endometrial cancer by regulating the PTEN/PI3K/AKT pathway. Mol Med Rep. 2019;19:581–588. doi: 10.3892/mmr.2018.9649. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Q, Li Y, Zhao M, Lin H, Wang W, Li D, Cui W, Zhou C, Zhong J, Huang C. MiR-494 acts as a tumor promoter by targeting CASP2 in non-small cell lung cancer. Sci Rep. 2019;9:3008. doi: 10.1038/s41598-019-39453-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He H, Liao X, Yang Q, Liu Y, Peng Y, Zhong H, Yang J, Zhang H, Yu Z, Zuo Y, et al. MicroRNA-494-3p promotes cell growth, migration, and invasion of nasopharyngeal carcinoma by targeting Sox7. Technol Cancer Res Treat. 2018 Jan 1; doi: 10.1177/1533033818809993. (Epub ahead of print). doi: 10.1177/1533033818809993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shen Z, Li Y, Zhao C, Wang F, Zhou R, Chen G. miR-494 BAG 1 axis is involved in cinobufacini induced cell proliferation and apoptosis in gastric cancer. Mol Med Rep. 2018;17:7435–7441. doi: 10.3892/mmr.2018.8788. [DOI] [PubMed] [Google Scholar]
- 29.Cheng L, Kong B, Zhao Y, Jiang J. miR-494 inhibits cervical cancer cell proliferation through upregulation of SOCS6 expression. Oncol Lett. 2018;15:3075–3080. doi: 10.3892/ol.2017.7651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y, Guo L, Li Y, Feng GH, Teng F, Li W, Zhou Q. MicroRNA-494 promotes cancer progression and targets adenomatous polyposis coli in colorectal cancer. Mol Cancer. 2018;17:1. doi: 10.1186/s12943-017-0753-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee YR, Chen M, Pandolfi PP. The functions and regulation of the PTEN tumour suppressor: New modes and prospects. Nat Rev Mol Cell Biol. 2018;19:547–562. doi: 10.1038/s41580-018-0015-0. [DOI] [PubMed] [Google Scholar]
- 32.Schroeder C, Grell J, Hube-Magg C, Kluth M, Lang D, Simon R, Höflmayer D, Minner S, Burandt E, Clauditz TS, et al. Aberrant expression of the microtubule-associated protein tau is an independent prognostic feature in prostate cancer. BMC Cancer. 2019;19:193. doi: 10.1186/s12885-019-5390-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim TH, Park JH, Woo JS. Resveratrol induces cell death through ROS dependent downregulation of Notch1/PTEN/Akt signaling in ovarian cancer cells. Mol Med Rep. 2019;19:3353–3360. doi: 10.3892/mmr.2019.9962. [DOI] [PubMed] [Google Scholar]
- 34.Cao Y, Xia F, Wang P, Gao M. MicroRNA 93-5p promotes the progression of human retinoblastoma by regulating the PTEN/PI3K/AKT signaling pathway. Mol Med Rep. 2018;18:5807–5814. doi: 10.3892/mmr.2018.9573. [DOI] [PubMed] [Google Scholar]
- 35.Wei D, Miao Y, Yu L, Wang D, Wang Y. Downregulation of microRNA-198 suppresses cell proliferation and invasion in retinoblastoma by directly targeting PTEN. Mol Med Rep. 2018;18:595–602. doi: 10.3892/mmr.2018.8979. [DOI] [PubMed] [Google Scholar]
- 36.Lin A, Piao HL, Zhuang L, Sarbassov D, Ma L, Gan B. FoxO transcription factors promote AKT Ser473 phosphorylation and renal tumor growth in response to pharmacologic inhibition of the PI3K-AKT pathway. Cancer Res. 2014;74:1682–1693. doi: 10.1158/0008-5472.CAN-13-1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spangle JM, Roberts TM, Zhao JJ. The emerging role of PI3K/AKT-mediated epigenetic regulation in cancer. Biochim Biophys Acta Rev Cancer. 2017;1868:123–131. doi: 10.1016/j.bbcan.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xie C, Lu H, Nomura A, Hanse EA, Forster CL, Parker JB, Linden MA, Karasch C, Hallstrom TC. Co-deleting Pten with Rb in retinal progenitor cells in mice results in fully penetrant bilateral retinoblastomas. Mol Cancer. 2015;14:93. doi: 10.1186/s12943-015-0360-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim BR, Ha J, Lee S, Park J, Cho S. Anti-cancer effects of ethanol extract of Reynoutria japonica Houtt. radix in human hepatocellular carcinoma cells via inhibition of MAPK and PI3K/Akt signaling pathways. J Ethnopharmacol. 2019;245:112179. doi: 10.1016/j.jep.2019.112179. [DOI] [PubMed] [Google Scholar]
- 40.Haddadi N, Lin Y, Travis G, Simpson AM, Nassif NT, McGowan EM. PTEN/PTENP1: ‘Regulating the regulator of RTK-dependent PI3K/Akt signalling’, new targets for cancer therapy. Mol Cancer. 2018;17:37. doi: 10.1186/s12943-018-0803-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fu X, Wen H, Jing L, Yang Y, Wang W, Liang X, Nan K, Yao Y, Tian T. MicroRNA-155-5p promotes hepatocellular carcinoma progression by suppressing PTEN through the PI3K/Akt pathway. Cancer Sci. 2017;108:620–631. doi: 10.1111/cas.13177. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.