Abstract
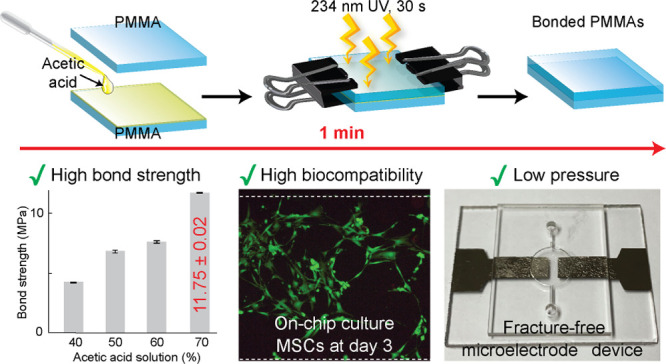
In the present study, we introduce a new approach for rapid bonding of poly(methyl methacrylate) (PMMA)-based microdevices using an acetic acid solvent with the assistance of UV irradiation. For the anticipated mechanism, acetic acid and UV irradiation induced free radicals on the PMMA surfaces, and acrylate monomers subsequently formed cross-links to create a permanent bonding between the PMMA substrates. PMMA devices effectively bonded within 30 s at a low pressure using clamps, and a clogging-free microchannel was achieved with the optimized 50% acetic acid. For surface characterizations, contact angle measurements and bonding performance analyses were conducted using predetermined acetic acid concentrations to optimize bonding conditions. In addition, the highest bond strength of bonded PMMA was approximately 11.75 MPa, which has not been reported before in the bonding of PMMA. A leak test was performed over 180 h to assess the robustness of the proposed method. Moreover, to promote the applicability of this bonding method, we tested two kinds of microfluidic device applications, including a cell culture-based device and a metal microelectrode-integrated device. The results showed that the cell culture-based application was highly biocompatible with the PMMA microdevices fabricated using an acetic acid solvent. Moreover, the low pressure required during the bonding process supported the integration of metal microelectrodes with the PMMA microdevice without any damage to the metal films. This novel bonding method holds great potential in the ecofriendly and rapid fabrication of microfluidic devices using PMMA.
Introduction
In recent years, lab-on-a-chip technology has attracted considerable interest in various fields such as biomedical research, DNA analysis, and healthcare. Because of the advantages such as low-cost and rapid bonding methods at the industrial level, thermoplastics have attracted much attention for fabricating microdevices. Among several thermoplastics, poly(methyl methacrylate) (PMMA) is one of the essential thermoplastics for microdevice fabrication because of its cost-effectiveness, optical transparency, and biocompatibility.1,2 In addition, PMMA microfluidics have been widely used in applications in the various biorelated fields.3 There are different types of bonding used for sealing PMMA–PMMA, such as thermal bonding, solvent bonding, adhesive bonding, and chemical bonding.4−7 Although thermal bonding is commonly used for fabricating PMMA devices allowing for simple and uniform surface bonding, the use of bulky press machines and heaters can lead to the deformation of the microchannel.4 Adhesive and chemical bonding require low pressure and low temperature; however, adhesives can clog the microchannel, while chemical surface modifications are time-consuming, involving multistep processes.6,7 Among these methods, solvent bonding generally possesses a robust and straightforward bonding process as it achieves a high bond strength with a relatively simple bonding strategy.8 However, as a strong solvent could dissolve PMMA, microchannel clogging is a universal drawback of solvent bonding.9,10 As an alternative, a solvent with a moderate dissolution rate was applied for bonding PMMA with the help of heat, and the overall process took more than 10 min.8 In our previous studies, PMMAs were successfully bonded using 90% ethanol as a solvent and UV light, and the bond strength was approximately 6.17 MPa.11 Although it did not require any bulky press machine or heater, it needed to control the solvent concentration to avoid clogging of the microchannel. Moreover, we recently demonstrated the room-temperature bonding of PMMA using acetic acid as a solvent.12 However, it took around 20 min to seal PMMAs using a press machine. Considering these limitations, there is an urgent need for a new solvent bonding approach for the rapid fabrication of microdevices.
Over the last decade, microsensor-based microfluidic devices have attracted wide attention for the detection of various markers in the environment, healthcare, temperature, biomedical, and chemical parameters.13 These devices have a significantly increased sensitivity and accuracy than commercial sensors. In particular, biosensor-based devices containing a thin metal film as a microelectrode have been used for temperature measurement, separation, and detection.14,15 Mainly, thermal bonding and glue-assisted bonding were used to create microdevices integrated with electrodes.16−19 Although the thermal bonding method is widely applicable, the risk of fractures on the integrated metal electrode still remains because of the high bonding temperature and pressure,20 whereas glue-assisted bonding faces the possibility of microchannel clogging. As an alternative, an elastomeric substrate such as poly(dimethylsiloxane) (PDMS) was used as a part of microdevices integrated with metal electrodes. However, the pressure applied during the bonding process had to be finely controlled to prevent the deformation or collapse of the PDMS microchannel.21,22 Therefore, there is an urgent demand for a new method to integrate thermoplastic devices with microelectrodes that can improve the biosensor performance and further advance the biomedical fields.
To overcome these problems, in this study, we introduce a simple and robust bonding method for fabricating a PMMA microdevice using acetic acid as a solvent with the assistance of UV irradiation at low pressure (see Figure 1). In a previous study, we introduced the use of acetic acid as a solvent for bonding PMMA, which was performed at room temperature for 20 min under mild pressure conditions.12 In the present study, acetic acid was still used as a solvent for bonding PMMA; however, the overall bonding process was superior in terms of time and pressure, requiring only 30 s of the UV irradiation with the clamp-assisted low pressure. Based on the proof of concept, we suggest that the highly energetic UV irradiation has reinforced the activation of the PMMA surfaces in the presence of acetic acid, and then the activated monomers of the acrylate functionalities on two surfaces of PMMA were possibly re-cross-linked to realize a permanent bonding. To demonstrate the bonding performance, various strength measurement tests, such as bond strength analysis and leak test, were performed. The water contact angle and Fourier transform infrared spectroscopy (FTIR) were also analyzed. The tightly assembled microdevice was used for two major applications—cell culture and integration of a platinum-microelectrode into the device. The results showed that the cells added into the PMMA microchannels showed a high viability and decent adhesion to the surface even without postbonding surface treatments. In addition, the integrated microelectrodes device was successfully fabricated without the leak and fractures of the microelectrodes.
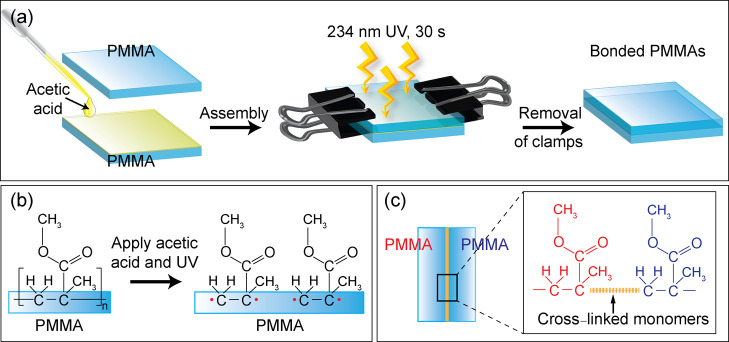
Figure 1.
(a) Overall procedure for bonding two PMMA substrates using acetic acid treatment, followed by UV irradiation. (b,c) Chemical reaction expected to occur on the surfaces of PMMA substrates after treating with acetic acid and UV irradiation.
Results and Discussion
Contact Angle Measurement
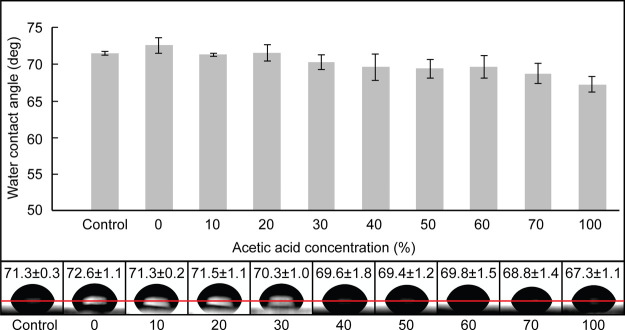
The change in surface wettability was analyzed by measuring the water contact angle, as shown in Figure 2. Prior to the measurement, PMMA substrates were treated with acetic acid and UV irradiation. Figure 2 shows the changes in water contact angles of PMMA before and after treatment with varying acetic acid concentrations from 0 to 100%. When PMMA was treated with 0% acetic acid and irradiated with UV for 30 s, the contact angle of PMMA was around 72.6 ± 1.1°, which did not significantly differ from that of pristine PMMA. By increasing the concentration of the acetic acid solution, the contact angle values slightly decreased to approximately 67.3 ± 1.1° when the acetic acid concentration reached 100%. The use of acetic acid barely altered the surface property of PMMA, which was also observed in our previous study,12 indicating that the addition of UV treatment did not affect the surface chemistry of the PMMA surface. Based on this result, we can conclude that acetic acid is a preferable solvent because of its minimal disturbance to the microchannel’s surface, which reduces the risk of clogging frequently encountered by using solvents with a high dissolution rate. In addition, by preserving the original PMMA surface, the attractive properties of PMMA can be readily used.
Figure 2.
Water contact angle change of PMMA with varying acetic acid concentrations, followed by UV irradiation.
Bonding Performance
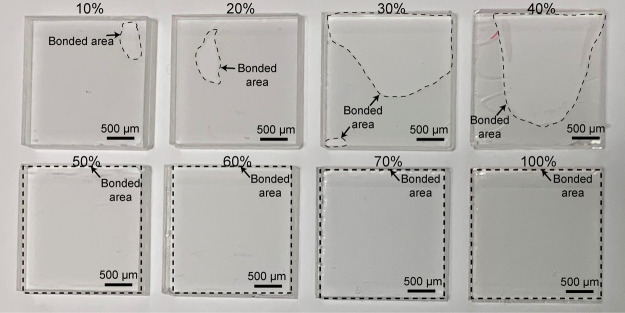
Figure 3 shows the bonding performance of PMMA substrates using different concentrations of acetic acid. An increase in the acetic acid concentration resulted in the expansion of the bonded area. Particularly, with 10–40% acetic acid, PMMA substrates were only partially sealed, whereas using 50–100% acetic acid, complete sealing was achieved. Based on these findings, 50% of acetic acid was found to be the optimum condition to achieve the successful homogeneous bonding of PMMA after 30 s of the UV irradiation.
Figure 3.
Results of bonding with different acetic acid concentrations (10–100%) at 30 s of UV irradiation.
FTIR Measurement
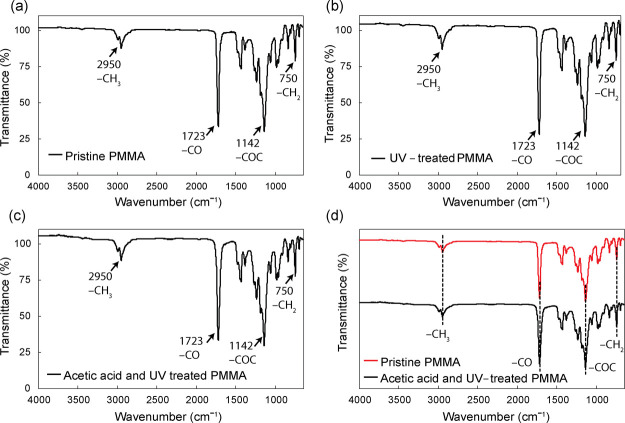
Figure 4 shows the FTIR spectra of three samples, including pristine PMMA (Figure 4a), UV-treated PMMA (Figure 4b), and acetic acid- and UV-treated PMMA (Figure 4c). Figure 4d compares the peaks for pristine PMMA, and acetic acid- and UV-treated PMMA. According to the FTIR spectrum in Figure 4a, pristine PMMA shows spectra regions at around 2950, 1723, 1142, and 750 cm–1, which corresponded to the presence of CH3, CO stretching, COC, and CH2 twisting modes, respectively. The FTIR spectrum obtained from pristine PMMA is consistent with our previous results.23−26 As shown in Figure 4b,c, IR spectra of PMMA after the UV treatment and PMMA after acetic acid and UV treatment displayed the same absorption peaks exhibited by pristine PMMA, indicating that UV irradiation and acetic acid did not change the chemical property of PMMA. This result differs from our previous study on PMMA bonding mediated by acetic acid at room temperature, where we hypothesized that an acetic acid molecule directly participated in the reaction to bridge two PMMA substrates in 20 min.12 In the current study, however, an additional UV irradiation was applied to reduce the bonding time down to 30 s. It is well-known that UV irradiation causes chain scissions of PMMA, creating unstable radicals. Here, we hypothesized that though acetic acid works to partially dissolve the PMMA surface, it does not directly participate in the chemical reaction because the reaction between broken PMMA chains was greater than that between acetic acid and PMMA. Therefore, we speculate that when acetic acid was removed immediately after UV irradiation, there was no sufficient time provided for acetic acid to modify the PMMA surface.
Figure 4.
FTIR spectra of (a) pristine PMMA, (b) UV-treated PMMA, and (c) acetic acid- and UV-treated PMMA. (d) Comparative FTIR spectra of pristine PMMA and acetic acid- and UV-treated PMMA.
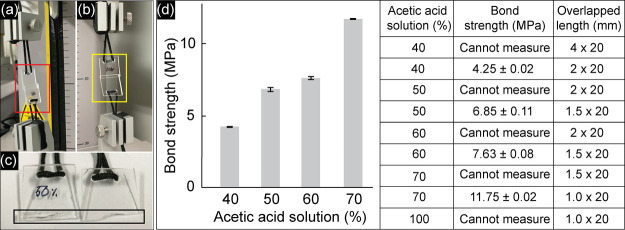
Bond Strength Measurement
Figure 5 shows the results of bond strength measurements of the PMMA substrates (20 mm × 20 mm × 2 mm). All bonded PMMAs only partially overlapped and were sealed using acetic acid and immediately treated with UV irradiation, as described above. Figure 5a,b shows the real images of bonded PMMAs in which the overlapped lengths were 4 and 2 mm, respectively; the bonded area after the detachment is shown in Figure 5c. According to the results, when PMMA substrates were bonded with the overlapped length of 4 mm, the two substrates were unable to detach; thus, the bond strengths could not be measured for PMMAs bonded with 40–100% acetic acid. In our previous study, where the PMMAs were bonded without UV irradiation, the bond strength measurement was still possible even with an overlapped length of 5 mm.12 Therefore, the bond strength in the present study was significantly increased as compared to the previous bonding strategies.1,11,12 To measure the strength, the overlapped length was decreased correspondingly with the reduction of the acetic acid concentration (see Figure 5d). In detail, when 40% acetic acid was used and the overlapped length was 2 mm, the bond strength was around 4.25 ± 0.02 MPa. However, for 50 and 60% acetic acid, the overlapped length was 1.5 mm, and the bond strength systematically increased to 6.85 ± 0.11 and 7.63 ± 0.08 MPa, respectively. For 70 and 100% acetic acid, the overlapped length was 1 mm. As a result, the bond strength could not be measured because of the undetached PMMAs in the case of 100% acetic acid. From these results, the highest possible measure for bond strength was exhibited to be approximately 11.75 ± 0.02 MPa using 70% acetic acid, which is considered the strongest bonding method for PMMAs (see Table 1). Based on the results, shown in Figures 3 and 5d, 50% acetic acid, which is considered to be the optimized bonding condition, was used in the further test.
Figure 5.
Results of bond strength measurement. Bonded PMMAs with the overlapped length: (a) 4; (b) 2 mm. (c) Enlarged photo showing the bonded area after the detachment. (d) Results of bond strength measured at different acetic acid concentrations. All experiments were performed in triplicate.
Table 1. Comparison Showing Several Solvent Bonding Methods for Bonding PMMAsa.
| bonding method | bonding conditions | bond strength | applications | reference |
|---|---|---|---|---|
| solvent-assisted thermal bonding | •acid-, amine-, and plasma-modified | 5.5 MPa | •not mentioned | (27) |
| •85 °C for 30 min | ||||
| •pressure (0.005 MPa) | ||||
| plasticizer-assisted thermal bonding | •dibutyl phthalate | not mentioned | •capillary electrophoresis | (28) |
| •90 °C for 10 min | ||||
| •pressure (0.6 MPa) | ||||
| solvent bonding | •1,2-dichloroethane and ethanol (2:8) | 3.8 MPa | •micromixer | (29) |
| •RT for 5 min | •capillary electrophoresis | |||
| •pressure (0.1 MPa) | ||||
| solvent bonding | •acetonitrile | not mentioned | •electrophoresis | (30) |
| •solvent exposure for 8 min | ||||
| •pressure (0.5 MPa) | ||||
| solvent bonding | •chloroform and ethanol | 2.675 MPa | •micromixer | (31) |
| •40 °C for 10 min | ||||
| •pressure (using quartz glass fixture) | ||||
| solvent bonding | •acetic acid 50% | 8.55 MPab | •polymerase chain reaction | (12) |
| •RT for 20 min | •human cell culture | |||
| •pressure (0.4 MPa) | ||||
| microwave-assisted solvent bonding | •methanol, ethanol, and isopropanol | not mentioned | •electrophoresis | (32) |
| •microwave (90 s) | ||||
| •pressure (using clamp) | ||||
| ultrasonic-assisted solvent bonding | •isopropanol 100% | 2.25 MPa | •not mentioned | (33) |
| •ultrasonic (8 s) | ||||
| •pressure (0.32 MPa) | ||||
| UV-assisted solvent bonding | •ethanol 90% | 6.17 MPa | •not mentioned | (11) |
| •UV irradiation (1 min) | ||||
| •pressure (using clamp) | ||||
| UV-assisted solvent bonding | •acetic acid 50% | 11.75 MPab | •integrated microelectrode device | this work |
| •UV irradiation (30 s) | •human cell culture | |||
| •pressure (using clamp) |
RT: room temperature.
Maximum bond strength could not be measured.
Leak Test
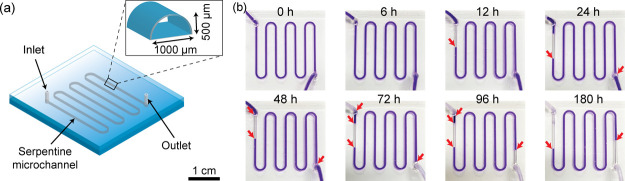
Figure 6 shows the results of the leak test performed to confirm the stability of the bonded PMMA microdevice. The PMMA microdevice (40 × 40 mm2) with a serpentine microchannel (500 × 1000 μm2) was fabricated using the introduced bonding method (see Figure 6a). To perform the test, the ink solution was introduced into the microchannel, and the leak phenomena were continuously observed from 0 to 180 h at room temperature (see Figure 6b). There was no leak during the test period in which the ink was maintained inside the microchannel. However, the red arrows indicated the evaporation phenomena observed at 12 h after the introduction of the ink solution into the microchannel. This could be explained by the permeability of the silicone tubes used for the introduction of the ink solution at the inlet and outlet of the microdevice. From those results, we can conclude that using the introduced bonding method, a highly stable bond between two PMMAs was formed without a leak.
Figure 6.
(a) Design of the microdevice used for the leak test. (b) Results of the leak test performed when observed over a 0–180 h period. The red arrows indicate ink which was lost because of natural evaporation.
Channel Profile: Cross-Sectional View of the Microchannel
Figure 7 shows the cross-sectional view of the bonded PMMAs fabricated by the method introduced in this study. The engraved microchannels possessed curved morphologies as the ball mills were used for computer numerical control (CNC) milling. Microchannels with various dimensions were fabricated, whose width and depth were identical—300, 500, and 1000 μm. The results showed that the bonding process did not significantly deform the microchannel morphology, verified by almost identical channel morphologies. We can speculate that the acetic acid in the optimized conditions influences only the surface of the PMMA substrate without melting the bulk itself. Moreover, only a mild pressure exerted by the use of clamps was applied for the bonding. Therefore, we can conclude that channel deformations often caused by PMMA swelling during other solvent bonding did not occur when treated with acetic acid followed by UV irradiation. Acetic acid and UV irradiation can sufficiently activate the PMMA surface to produce monomers to participate in a permanent bonding, without any adverse effect on the morphology of the microchannel. Therefore, the introduced bonding method can be used for rapid bonding of PMMA, particularly having small microchannels.
Figure 7.
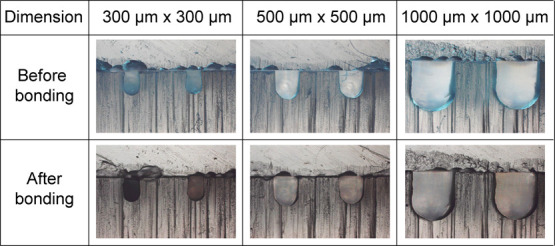
Bright-field photos of the cross-sectional view of the microchannels engraved on the PMMA substrate before and after the PMMA–PMMA bonding based on the introduced method.
Bonded PMMA Microdevice for Human Cell Culture
The feasibility of the bonded PMMA microdevice for human cell culture was assessed by directly seeding mesenchymal stem cells (MSCs) into the channels and incubating for 3 days. Figure 8a illustrates the design of the microdevice. The device consists of four straight semicircular microchannels with inlets and outlets. The biocompatibility of the acetic acid- and the UV-treated PMMA was confirmed by performing a cell viability test using calcein-AM/ethidium homodimer-1 (EthD-1) on day 3 of the incubation period (see Figure 8b). It was observed that the attached MSCs were highly viable and, judging from the cell stretching, showed a decent adhesion to the PMMA surface. This result indicates that, without further surface treatment to induce cell viability and adhesion, the acetic acid- and UV-treated PMMA microdevice can be directly used for cell-related applications.
Figure 8.
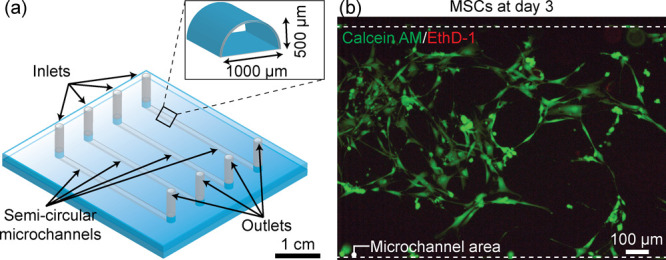
PMMA microdevice for human cell culture (MSCs). (a) Illustration of the bonded microdevice having semicircular straight microchannels. (b) Fluorescent image of the MSCs cultured inside the semicircular microchannel at day 3. Cell viability was characterized by the calcein AM/EthD-1 staining kit distinguishing live cells (green) and dead cells (red). The cell density was 5 × 105 cells mL–1.
PMMA Microdevice Integrated with Platinum Microelectrodes
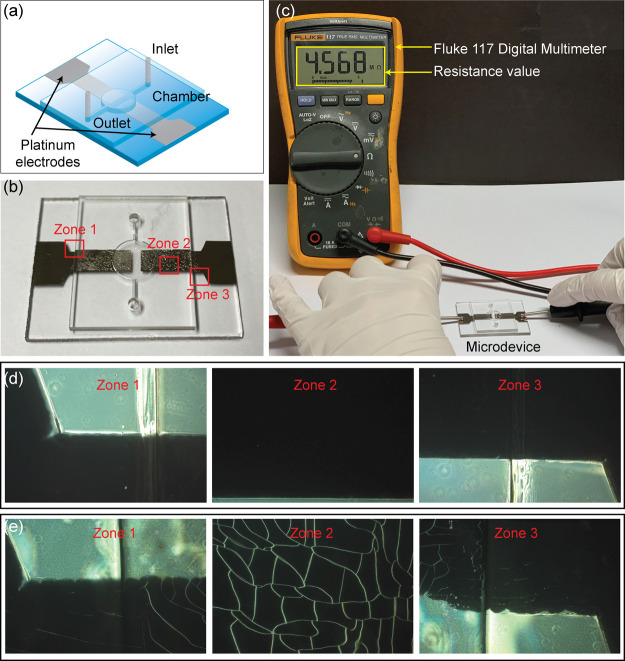
Figure 9 shows the results of fabricating a PMMA microdevice integrated with a platinum microelectrode. Close-up images confirmed that microelectrode was not fractured, ensuring the ability to bond the metal electrode layer and the microchannel plate while maintaining the electrode intact. Figure 9a,b shows the illustration and real image of the integrated microdevice using the introduced bonding method, respectively. To validate the operation of the microelectrode, the overall experiment was set up as shown in Figure 9c. The microchamber was first filled by the electrolyte solution, and then the resistance was measured using a Fluke 117 digital multimeter. The presented 4.568 MΩ value indicated that the device retained an excellent electric conductivity between platinum electrodes inside the microchamber; therefore, the proposed bonding strategy led to the successful fabrication of the platinum-integrated microdevice. This bonding strategy possesses potential applications in microanalytical systems such as separation, detection, and electrical lysis. Moreover, the fracture phenomenon of platinum microelectrodes was observed. As shown in Figure 9d, no fracture or minimal fracture was observed at the edge of the PMMA plate adjoined to the metal film (zone 1 and 3) as well as the center of the electrode (zone 2). In contrast, by using thermal bonding, the fractured platinum electrode was indicated at the edge of the cover layer, and extensive cracks appeared on the metal electrode (see Figure 9e) induced by thermal stress (105 °C, 20 min, and 0.1 MPa). Thermal bonding was achieved by following the conditions specified in a previous study.34 The difference in the thermal expansion coefficient between the microelectrode and PMMA may lead to a larger thermal plastic deformation. Consequently, the plastic deformation because of a high bonding temperature is mainly responsible for the fracture of the microelectrode.14 Based on the results, as compared with other bonding strategies, the proposed bonding approach showed many improvements and advantages for bonding the integrated microelectrode PMMA device in terms of a simple and robust process at mild pressure (see Table 2). Therefore, to prevent the cracking of electrodes during the bonding process, the proposed method holds high potential for the rapid and straightforward fabrication of metal-electrode-integrated microdevices.
Figure 9.
Results of the bonding method for a rapid prototype PMMA microdevice with integrated metal microelectrodes. (a) Illustration of the PMMA microdevice containing the platinum microelectrodes. (b) Real image of the microdevice using the proposed bonding method. (c) Setup and performance of resistance measurement using the fabricated microdevice. (d) Enlarged photos of three squares marked in (b) showing fracture-free platinum electrodes at the edge of the microdevice. (e) Fractured platinum electrodes in the same zones of (b) using thermal bonding as a control experiment.
Table 2. Comparison of Some Bonding Methods for Fabricating Integrated Microelectrode PMMA Devices.
| bonding method | bonding conditions | metallic microelectrode | reference |
|---|---|---|---|
| thermal bonding | •108 °C for 25 min | gold | (20) |
| •pressure (tightly clamped) | |||
| plasma-assisted thermal bonding | •oxygen plasma | copper | (14) |
| •85 °C for 15 min | |||
| •pressure (0.002 MPa) | |||
| UV-assisted thermal bonding | •UV irradiation (8 min) | gold | (21) |
| •80 °C for 3 min | |||
| •pressure (0.00135 MPa) | |||
| UV/O3-assisted thermal bonding | •UV/O3 treatment (8 min) | gold | (35) |
| •80 °C for 5 min | |||
| •pressure (0.02 MPa) | |||
| solvent-assisted thermal bonding | •isopropanol | gold | (36) |
| •80 °C for 10 min | |||
| •pressure (0.0025 MPa) | |||
| UV-assisted solvent bonding | •acetic acid 50% | platinum | this work |
| •UV irradiation (30 s) | |||
| •pressure (using clamp) |
Conclusions
In the present study, a novel bonding strategy for bonding PMMA using acetic acid as a solvent followed by UV irradiation for 30 s was introduced. This approach is based on the use of acetic acid as a solvent for the partial dissolution of PMMA, which is followed by UV irradiation for re-cross-linking monomers of PMMA during the bonding process. Also, the introduced method is superior to other solvent bonding methods in terms of simplicity in the bonding process, biocompatibility, and integrity of the microchannel (see Table S1). Of note, the bonded PMMAs have achieved a bond strength as high as 11.75 MPa without the use of external hot embossing instruments, and with the mild conditions applied, clogging-free microchannels were attained. When the device was applied to the cell culture, the microchannels facilitated cells with high viability and decent adhesion after 3 days of culture. Moreover, the bond of the microelectrode-integrated PMMA device was strong, and the electrodes stayed fracture-free after the bonding process, which helped to maintain the performance of the microelectrodes. Therefore, the introduced bonding method is a powerful approach for the rapid fabrication of PMMA microdevices as well as to expand the applications for biosensing to advance the healthcare system.
Experimental Section
Materials
Acetic acid (99.7%) was purchased from Sigma-Aldrich (MO, USA). PMMA substrates (2 mm thicknesses) were obtained from Goodfellow (Coraopolis, USA). Acetic acid solutions diluted in deionized water to the appropriate concentrations were used for all bonding experiments and analytical measurements. MSC and MSC medium were acquired from ScienCell (CA, USA). Calcein AM and EthD-1 for the cell viability/cytotoxicity test were purchased from Thermo Fisher Scientific (MA, USA). Silicone tubes (i.d. 1.0 mm, o.d. 2.0 mm) were obtained from Dow Corning (MI, USA). Platinum 99.95% was acquired from Vacuum Thin Film Materials (Incheon, Korea).
Bonding Mechanism
Figure 1a shows the overall scheme for bonding two PMMA substrates using acetic acid treatment, followed by UV irradiation. First, acetic acid was placed on the surface of one PMMA substrate and then covered by the other PMMA. With the help of two clamps, the two PMMA substrates were tightly fastened. Then, the pair of PMMA was irradiated with 234 nm UV light for 30 s. Finally, the two clamps were removed from the bonded PMMAs after the UV treatment process. Figure 1b shows the PMMA substrates producing free radicals on the surfaces when treated with the acetic acid solution under UV irradiation. Subsequently, acrylate monomers of PMMA substrates were formed (see Figure 1b), which possibly formed cross-links to create a permanent bond between the two PMMA substrates (see Figure 1c).
Surface Characterization (1): Water Contact Angle Measurement
The surface wettability of the acetic acid-treated PMMA followed by the UV irradiation was evaluated by the water contact angle measurement. For the measurements, PMMA substrates (20 mm × 20 mm) were covered with 0, 10, 20, 30, 40, 50, 60, 70, and 100% acetic acid solution and immediately treated with a 234 nm UV lamp for 30 s using a UV light curing system. After the UV treatment, the PMMA substrates were carefully dried by compressed air before the measurement of water contact angles. By using a Phoenix 300 contact angle analyzer, the water contact angle was measured by depositing static water droplets onto the substrates. The results were analyzed with the Image-Pro 300 software. Five measurements were made and averaged for further assessment. For comparison, the water contact angle was also measured on the surface of a pristine PMMA as a control.
Surface Characterization (2): FTIR Analysis
PMMA samples (pristine and acetic acid-treated PMMAs, followed by UV light) were analyzed by FTIR (JASCO 4700 FTIR spectrophotometer), which offers quantitative and qualitative analyses to characterize the chemical nature of the modified surfaces. The FTIR indicated He–Ne as the reference at the resolution of 4 cm–1 and the mode of diffuse reflectance with scanning from 4500 to 400 cm–1 of resolution.
Bond Strength Analysis and Leak Tests
For the bond strength analyses, partially bonded PMMAs were measured using a texture analyzer (QTS 25, Brookfield, Middleborough, MA, USA). All samples used for strength measurements were treated with acetic acid solution (40, 50, 60, 70, and 100%), and the UV exposure time was fixed at 30 s. Before bond strength analysis, the samples with through-holes, which were punctured on each PMMA substrate using a drilling machine for the insertion of twines, were prepared. Then, the partially overlapped PMMA substrates were fixed in the strength measurement analyzer using the inserted twines and then pulled apart at the speed of 100 mm min–1. All experiments were triplicated to examine bonding reproducibility. For the leak test analysis, the PMMA microdevice (40 mm × 40 mm × 4 mm) was used to observe the ink solution inside the microchannel in 0, 6, 12, 24, 48, 72, 96, and 180 h, successively.
Bonded PMMA Microdevice for Human Cell Culture
In order to test the biocompatibility of the microdevice with human cell culture, semicircular channels with a diameter of 1 mm were engraved on the PMMA and bonded with another flat PMMA substrate using the method mentioned above. The cell culture chips were sterilized by placing them in a clean bench under UV light for 1 h. The trypsinized MSCs (passage 4) were collected from a cell-culture plate and directly inserted to the channel after dilution. After incubating in a 5% humidified CO2 incubator at 37 °C for 3 days, cell viability was examined by staining them with 2 μM calcein AM and 4 μM EthD-1 following the manufacturer’s instruction to indicate the live and dead cells in green and red fluorescence, respectively. The captured microscopic images were analyzed using the ProgRes CapturePro 2.8 software (Jenoptik).
PMMA Microdevice Integrated with Platinum Microelectrodes
The microelectrode-integrated microdevice consisted of channel and electrode layers. The overall schematic of the fabrication of the PMMA microdevice integrated with microelectrodes is shown in Figure S1. Briefly, the patterns were engraved on the PMMA substrate by using a CNC machine.12 After ultrasonic cleaning, the engraved PMMA was dried by compressed air. A paper mask with electrode patterns was cut by a laser cutter and placed on the PMMA substrate. The platinum microelectrode on the PMMA plate was created by sputter deposition, with a 100 nm thick platinum layer coated on a 0.1 mm platinum target using a Cressington sputter coater 108 auto machine. Subsequently, two halves of the device were carefully aligned and bonded using acetic acid assisted with UV irradiation. Particularly, 50% acetic acid was covered between the channel and the electrode layer. The assembled device was clamped and treated with a 234 nm UV for 30 s to realize bonding. After bonding, the microdevice was washed to remove any excess acid inside the channel. At each end of the channel, a 2 mm diameter hole was bored and connected with a silicon tube to form the inlet/outlet. The fracture phenomenon, as well as the electric conductivity of the microelectrode of the complete microdevice, was later assessed.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (no. NRF-2020R1A2B5B01001971).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c01770.
Comparison of different solvent bonding methods and a schematic illustration of a PMMA microdevice integrated with microelectrodes (PDF)
Author Contributions
The manuscript was written through the contributions of all the authors.
The authors declare no competing financial interest.
Supplementary Material
References
- Matellan C.; del Río Hernández A. E. Cost-effective rapid prototyping and assembly of poly(methyl methacrylate) microfluidic devices. Sci. Rep. 2018, 8, 6971. 10.1038/s41598-018-25202-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liga A.; Morton J. A. S.; Kersaudy-Kerhoas M. Safe and cost-effective rapid-prototyping of multilayer PMMA microfluidic devices. Microfluid. Nanofluid. 2016, 20, 164. 10.1007/s10404-016-1823-1. [DOI] [Google Scholar]
- Chen Y.; Zhang L.; Chen G. Fabrication, modification, and application of poly(methyl methacrylate) microfluidic chips. Electrophoresis 2008, 29, 1801–1814. 10.1002/elps.200700552. [DOI] [PubMed] [Google Scholar]
- Mathur A.; Roy S. S.; Tweedie M.; Mukhopadhyay S.; Mitra S. K.; McLaughlin J. A. Characterisation of PMMA microfluidic channels and devices fabricated by hot embossing and sealed by direct bonding. Curr. Appl. Phys. 2009, 9, 1199–1202. 10.1016/j.cap.2009.01.007. [DOI] [Google Scholar]
- Tennico Y. H.; Koesdjojo M. T.; Kondo S.; Mandrell D. T.; Remcho V. T. Surface modification-assisted bonding of polymer-based microfluidic devices. Sens. Actuators, B 2010, 143, 799–804. 10.1016/j.snb.2009.10.001. [DOI] [Google Scholar]
- Song I.-H.; Park T. PMMA solution assisted room temperature bonding for PMMA–PC hybrid devices. Micromachines 2017, 8, 284. 10.3390/mi8090284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T.; Jung S. H.; Lee M. S.; Park T.-E.; Ahn S.-K.; Kang J. H. Robust chemical bonding of PMMA microfluidic devices to porous PETE membranes for reliable cytotoxicity testing of drugs. Lab Chip 2019, 19, 3706–3713. 10.1039/c9lc00338j. [DOI] [PubMed] [Google Scholar]
- Kelly R. T.; Pan T.; Woolley A. T. Phase-Changing Sacrificial Materials for Solvent Bonding of High-Performance Polymeric Capillary Electrophoresis Microchips. Anal. Chem. 2005, 77, 3536–3541. 10.1021/ac0501083. [DOI] [PubMed] [Google Scholar]
- Ng S. P.; Wiria F. E.; Tay N. B. Low distortion solvent bonding of microfluidic chips. Procedia Eng. 2016, 141, 130–137. 10.1016/j.proeng.2015.09.212. [DOI] [Google Scholar]
- Shah J. J.; Geist J.; Locascio L. E.; Gaitan M.; Rao M. V.; Vreeland W. N. Capillarity induced solvent-actuated bonding of polymeric microfluidic devices. Anal. Chem. 2006, 78, 3348–3353. 10.1021/ac051883l. [DOI] [PubMed] [Google Scholar]
- Tran H. H.; Wu W.; Lee N. Y. Ethanol and UV-assisted instantaneous bonding of PMMA assemblies and tuning in bonding reversibility. Sens. Actuators, B 2013, 181, 955–962. 10.1016/j.snb.2012.11.060. [DOI] [Google Scholar]
- Trinh K. T. L.; Pham Q. N.; Lee N. Y. Clog-free and reliable solvent bonding of poly(methyl methacrylate) microdevice mediated by eco-friendly acetic acid at room temperature and its application for polymerase chain reaction and human cell culture. Sens. Actuators, B 2019, 282, 1008–1017. 10.1016/j.snb.2018.10.077. [DOI] [Google Scholar]
- Koudelka-Hep M.; van der Wal P. D. Microelectrode sensors for biomedical and environmental applications. Electrochim. Acta 2000, 45, 2437–2441. 10.1016/s0013-4686(00)00331-5. [DOI] [Google Scholar]
- Liu J.; Qiao H.; Liu C.; Xu Z.; Li Y.; Wang L. Plasma assisted thermal bonding for PMMA microfluidic chips with integrated metal microelectrodes. Sens. Actuators, B 2009, 141, 646–651. 10.1016/j.snb.2009.07.032. [DOI] [Google Scholar]
- Cheng E.; Xing B.; Li S.; Yu C.; Li J.; Wei C.; Cheng C. Pressure-driven micro-casting for electrode fabrication and its applications in wear grain detections. Materials 2019, 12, 3710. 10.3390/ma12223710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graß B.; Neyer A.; Jöhnck M.; Siepe D.; Eisenbeiß F.; Weber G.; Hergenröder R. A new PMMA-microchip device for isotachophoresis with integrated conductivity detector. Sens. Actuators, B 2001, 72, 249–258. 10.1016/s0925-4005(00)00643-2. [DOI] [Google Scholar]
- Liu J.-S.; Liu C.; Guo J.-H.; Wang L.-D. Electrostatic bonding of a silicon master to a glass wafer for plastic microchannel fabrication. J. Mater. Process. Technol. 2006, 178, 278–282. 10.1016/j.jmatprotec.2006.04.009. [DOI] [Google Scholar]
- Ueno K.; Kitagawa F.; Kim H.-B.; Tokunaga T.; Matsuo S.; Misawa H.; Kitamura N. Fabrication and characteristic responses of integrated microelectrodes in polymer channel chip. Chem. Lett. 2000, 29, 858–859. 10.1246/cl.2000.858. [DOI] [Google Scholar]
- Nugen S. R.; Asiello P. J.; Connelly J. T.; Baeumner A. J. PMMA biosensor for nucleic acids with integrated mixer and electrochemical detection. Biosens. Bioelectron. 2009, 24, 2428–2433. 10.1016/j.bios.2008.12.025. [DOI] [PubMed] [Google Scholar]
- Ueno K.; Kim H.-B.; Kitamura N. Characteristic Electrochemical Responses of Polymer Microchannel–Microelectrode Chips. Anal. Chem. 2003, 75, 2086–2091. 10.1021/ac0264675. [DOI] [PubMed] [Google Scholar]
- Liu J.; Qin J.; Li J.; Li D.; Xu Z.; Zhang X.; Du L.; Liu C. Monolithic integration of three-material microelectrodes for electrochemical detection on PMMA substrates. Electrochem. Commun. 2013, 31, 20–23. 10.1016/j.elecom.2013.02.024. [DOI] [Google Scholar]
- Ko J. S.; Yoon H. C.; Yang H.; Pyo H.-B.; Chung K. H.; Kim S. J.; Kim Y. T. A polymer-based microfluidic device for immunosensing biochips. Lab Chip 2003, 3, 106–113. 10.1039/b301794j. [DOI] [PubMed] [Google Scholar]
- Rajendran S.; Sivakumar M.; Subadevi R. Investigations on the effect of various plasticizers in PVA–PMMA solid polymer blend electrolytes. Mater. Lett. 2004, 58, 641–649. 10.1016/s0167-577x(03)00585-8. [DOI] [Google Scholar]
- Trinh K. T. L.; Zhang H.; Kang D.-J.; Kahng S.-H.; Tall B. D.; Lee N. Y. Fabrication of polymerase chain reaction plastic lab-on-a-chip device for rapid molecular diagnoses. Int. Neurourol. J. 2016, 20, S38–S48. 10.5213/inj.1632602.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H.; Wen Y.; Peng H.; Zheng C.; Li Y.; Wang S.; Sun S.; Xie X.; Zhou X. Grafting polytetrafluoroethylene micropowder via in situ electron beam irradiation-induced polymerization. Polymers 2018, 10, 503. 10.3390/polym10050503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H.; Chong Z. Z.; Tor S. B.; Liu E.; Loh N. H. Low temperature and deformation-free bonding of PMMA microfluidic devices with stable hydrophilicity via oxygen plasma treatment and PVA coating. RSC Adv. 2015, 5, 8377–8388. 10.1039/c4ra12771d. [DOI] [Google Scholar]
- Brown L.; Koerner T.; Horton J. H.; Oleschuk R. D. Fabrication and characterization of poly(methylmethacrylate) microfluidic devices bonded using surface modifications and solvents. Lab Chip 2006, 6, 66–73. 10.1039/b512179e. [DOI] [PubMed] [Google Scholar]
- Duan H.; Zhang L.; Chen G. Plasticizer-assisted bonding of poly(methyl methacrylate) microfluidic chips at low temperature. J. Chromatogr. A 2010, 1217, 160–166. 10.1016/j.chroma.2009.11.018. [DOI] [PubMed] [Google Scholar]
- Lin C.; Chao C.; Lan C. Low azeotropic solvent for bonding of PMMA microfluidic devices. Sens. Actuators, B 2007, 121, 698–705. 10.1016/j.snb.2006.04.086. [DOI] [Google Scholar]
- Sun X.; Peeni B. A.; Yang W.; Becerril H. A.; Woolley A. T. Rapid prototyping of poly(methyl methacrylate) microfluidic systems using solvent imprinting and bonding. J. Chromatogr. A 2007, 1162, 162–166. 10.1016/j.chroma.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H.; Liu X.; Li T.; Han X. Miscible organic solvents soak bonding method use in a PMMA multilayer microfluidic device. Micromachines 2014, 5, 1416–1428. 10.3390/mi5041416. [DOI] [Google Scholar]
- Rahbar M.; Chhina S.; Sameoto D.; Parameswaran M. Microwave-induced, thermally assisted solvent bonding for low-cost PMMA microfluidic devices. J. Micromech. Microeng. 2009, 20, 015026. 10.1088/0960-1317/20/1/015026. [DOI] [Google Scholar]
- Zhang Z.; Luo Y.; Wang X.; Zheng Y.; Zhang Y.; Wang L. A low temperature ultrasonic bonding method for PMMA microfluidic chips. Microsyst. Technol. 2010, 16, 533–541. 10.1007/s00542-010-1027-7. [DOI] [Google Scholar]
- Trinh K. T. L.; Zhang Y.; Lee N. Y. One-step DNA purification and amplification on an integrated plastic microdevice for on-site identification of foodborne pathogens. Anal. Chim. Acta 2018, 1040, 63–73. 10.1016/j.aca.2018.06.042. [DOI] [PubMed] [Google Scholar]
- Wongkaew N.; He P.; Kurth V.; Surareungchai W.; Baeumner A. J. Multi-channel PMMA microfluidic biosensor with integrated IDUAs for electrochemical detection. Anal. Bioanal. Chem. 2013, 405, 5965–5974. 10.1007/s00216-013-7020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda M.; Slouka Z.; Schrott W.; Červenka P.; Přibyl M.; Šnita D. Fabrication of plastic microchips with gold microelectrodes using techniques of sacrificed substrate and thermally activated solvent bonding. Microelectron. Eng. 2010, 87, 1590–1593. 10.1016/j.mee.2009.11.010. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.