Abstract

8-Oxo-7,8-dihydro-2′-deoxyguanosine (8-oxo-dG), an oxidized form of guanosine residues, is a critical biomarker for various cancers. Herein, a sensitive citrate-capped gold nanoparticle-based aptasensor device has been developed for the detection of 8-oxo-dG in urine. We previously designed a 38-nt anti-8-oxo-dG-aptamer by a computer simulation and the experimental validation has been performed in the present work. The analytical performance of the 38-nt aptamer from the in silico design was compared with the parent 66-nt aptamer. This assay is based on the principle of salt-induced aggregation of citrate-capped gold nanoparticles. Based on this sensing mechanism, the difference between the absorbance in the presence and absence of 8-oxo-dG at λ = 525 nm (ΔA525) increased linearly as a function of 8-oxo-dG concentrations in the ranges of 10–100 and 15–100 nM for 38-nt and 66-nt aptasensors, respectively. This method can provide detection limits of 6.4 nM for 8-oxo-dG in the 38-nt aptasensor and 13.2 nM in the 66-nt aptasensor. Similar to the 66-nt aptamer, the shortened aptamer, 38-nt long, can provide high sensitivity and selectivity with rapid detection time. In addition, using the 38-nt aptamer as a recognition component in the developed portable low-cost device showed high sensitivity in the detection range of 15–100 nM with a detection limit of 12.9 nM, which is much lower than the threshold value (280 nM) for normal human urine. This easy-to-use device could effectively and economically be utilized for monitoring 8-oxo-dG in real urine samples and potentially serve as a prototype for a commercial device.
Introduction
8-Oxo-7,8-dihydro-2′-deoxyguanosine (8-oxo-dG) is a crucial product of DNA oxidation. It is typically used as a critical biomarker of oxidative stress-derived DNA damage.1−4 The excess 8-oxo-dG is removed from DNA by various self-protection mechanisms and subsequently excreted into urine without further metabolism. Significantly elevated levels of 8-oxo-dG in serum or urine are associated with highly increased risks of several cancers5 such as breast,6,7 colorectal,8,9 and bile duct cancers.10 However, the quantification of 8-oxo-dG in serum or urine is not an easy task in bioanalytical chemistry due to its low concentrations and the complexity of the coexisting substances in biosamples.11−14 Therefore, developing an efficient approach to detect urinary 8-oxo-dG molecules is a particularly challenging issue.
Analytical techniques that have been broadly used for urinary 8-oxo-dG detection include enzyme-linked immunosorbent assay (ELISA),4,15−17 high-performance liquid chromatography with electrochemical detection (HPLC-ECD),18,19 gas chromatography-mass spectrometry (GC-MS),20−22 liquid chromatography-tandem mass spectrometry (LC-MS/MS),12,23−29 and electrochemical aptasensors.30 ELISA is easy to perform in most laboratories with low cost, high sensitivity, and simple equipment; nevertheless, sample preparation is tedious and time-consuming. For HPLC, even though they are sensitive and specific for 8-oxo-dG detection, these techniques are costly and highly specialized. Electrochemical aptasensors provide highly sensitive detection with a wide detection range. However, they are a time-consuming tool. Due to these limitations, there is an urgent need to develop a highly sensitive, cost-efficient, and easy-to-use method for urinary 8-oxo-dG detection.
Herein, a redesigned single-stranded DNA (ssDNA) aptamer with improved specificity and sensitivity and a portable low-cost device based on salt-induced citrate-capped gold nanoparticle (AuNP) aggregation have been developed. Highly accurate, easy-to-use, low-cost, and rapid detection method and device can be obtained. This assay is based on the principle of salt-induced AuNP aggregation, which can be controlled by a specific aptamer on the surface of AuNPs. The anti-8-oxo-dG aptamers can be adsorbed onto the synthesized citrate-capped AuNPs and enhance the stability of AuNPs by electrostatic and steric stabilization31,32 under high salt concentrations, as illustrated in Figure 1. Upon specific binding with 8-oxo-dG, the aptamer can form a complex with the target molecule. By forming such complexes, the aptamer can no longer prevent AuNPs from aggregation under high salt concentrations. Thus, the color of the solution changes from red to purple. Despite various applications based on this reaction, to date, a portable low-cost device for urinary 8-oxo-dG detection has not yet been developed.
Figure 1.

Schematic illustration of the sensing mechanism for the detection of 8-oxo-dG molecules via assemblies of citrate-capped AuNPs under high salt (NaCl) conditions. Nitrogenous bases of the anti-8-oxo-dG aptamer are color-coded as follows: blue, adenine (A); yellow, thymine (T); green, cytosine (C); and purple, guanine (G). The color of the solution with 8-oxo-dG molecules is purple and red without.
In our earlier works,33−35 we used computational simulations to redesign the anti-8-oxo-dG-aptamer to enhance sensitivity and reduce the cost of the sensor fabrication. In the present work, we experimentally validate our simulated results by comparing the performances of two detection systems: one based on a regularly used 66-nt aptamer36 and the other on our redesigned 38-nt aptamer.36 In the first system, the ssDNA sequence is as follows: 5′-GCG GGC GAT CGG CGG GGG GTG CGT GCG CTC TGT GCC AGG GGG TGG GAC AGA TCA TAT GGG GGT GCT-3′, whereas our second system employs the following sequence: 5′-TGC GTG CGC TCT GTG CCA GGG GGT GGG ACA GAT CAT AT-3′. We have also successfully developed a portable, low-cost device whose sensitivity is comparable to the well-established UV-Vis spectrophotometry-based instrument; which, however, is unsuitable for point-of-care detection. Our low-cost device can be effectively used for the determination of 8-oxo-dG in urine samples and can be further extended into the commercial stage.
Results and Discussion
Design Strategy and Sensing Mechanism
The working principle of the colorimetric aptasensor is basically based on the optical property of AuNPs. The detection sensitivity of the aptasensor can be improved by the use of relatively large nanoparticles; however, the long-term stability of sensors tends to be inversely proportional to nanoparticle sizes. Therefore, smaller nanoparticles (e.g., 10–20 nm in diameter) have been typically used to achieve a compromise between sensitivity and stability.37,38 In the present work, the diameter of the synthesized citrate-capped AuNPs was 17.22 ± 4.23 nm, as shown in Figure S1. The concentration of the AuNP solution was calculated to be 12 nM according to the Beer-Lambert law. The citrate-stabilized AuNPs were wine red in color and showed a signature absorption peak at 525 nm.
To improve the analytical performance of the aptasensor, two ssDNA aptamer sequences were comparatively studied in the present study. The 66-nt aptamer was obtained from the literature36 (5′-GCG GGC GAT CGG CGG GGG GTG CGT GCG CTC TGT GCC AGG GGG TGG GAC AGA TCA TAT GGG GGT GCT-3′), while the 38-nt aptamer (a shortened sequence of the 66-nt aptamer was computationally designed; 5′-TGC GTG CGC TCT GTG CCA GGG GGT GGG ACA GAT CAT AT-3′) has been reported by our group.33 Comparative study of the 66-nt aptamer (Figure 2a–e) and the 38-nt aptamer (Figure 2f–j) was performed. Upon addition of 45 mM NaCl into the AuNP solution, the absorption of the citrate-capped AuNPs significantly decreased (Figure 2a,f). The solution color changed from red to blue-purple due to the screening of the electrostatic repulsion, leading to the AuNP aggregation (Figure 2c,h). After addition of the aptamer into the AuNP solution, the aptamer can be adsorbed onto the surface of the AuNPs, and it can protect the AuNPs from aggregation under high salt concentrations (Figure 2d,i). This was due to coordination interactions between the nitrogen and oxygen atoms of the exposed nucleobases and the AuNPs,34 which can increase the repulsion between the AuNPs, resulting in enhanced AuNPs stability under high salt concentrations. In effect, in the presence of 100 nM aptamers, the absorption peak (Figure 2a,f with green dashed line) and the dispersion of the AuNPs were retained (Figure 2d,i). The addition of 50 nM 8-oxo-dG, which has a high specificity to the aptamer, resulted in the formation of a complex between 8-oxo-dG and the aptamer. The mechanisms of the 8-oxo-dG-aptamer complex formation have been recently reported.39 The 8-oxo-dG can form a complex with the 66-nt aptamer, and the structural change as a result of a G-quadruplex structure was confirmed by circular dichroism. In contrast, the G-quadruplex structure is not found during the binding between the 38-nt aptamer and 8-oxo-dG.33 The stacking interactions of the 8-oxo-dG molecules during the stabilization stage play an important role in the specific binding interaction.35 The complex formation between the truncated aptamer and the target molecule was observed by a molecular dynamics (MD) simulation.33 The mechanism by which the 38-nt aptamer specifically binds with the 8-oxo-dG molecule can be divided into the following characteristic stages: (i) adsorption, (ii) binding, and (iii) complex stabilization stages.33 During the complex formation, up to eight hydrogen bonds are found. After the complex formation, the aptamer can no longer be adsorbed onto the surface of AuNPs to prevent AuNPs from aggregating at a NaCl concentration of 45 mM (Figure 2e,j).
Figure 2.

Comparison of absorption spectra of AuNPs in different systems: (a–e) with 66-nt aptamer and (f–j) with 38-nt aptamer. (b, g) AuNPs. (c, h) AuNPs after addition of NaCl. (d, i) AuNPs after addition of anti-8-oxo-dG-aptamer and NaCl. (e, j) AuNPs after addition of anti-8-oxo-dG-aptamer, 8-oxo-dG, and NaCl. The distribution of AuNPs in the solution was observed by transmission electron microscopy (scale bars, 200 nm).
In addition, as can be seen in Figure S2, a control experiment without the aptamer in a range of 8-oxo-dG concentrations of 20–100 nM was conducted to rule out unspecific adsorption of the target to the AuNPs that can induce color change through aggregation. Similarly, the use of irrelevant ssDNA sequences (5′-AAC AGT AAA GGC AAC GTC CA-3′ and 5′-TCT TAC CGG TAA AAA GCC GAA GTC ATG GGT CGA CGA ACG ACT AGA GAC-3′) in our assay instead of the anti-8-oxo-dG aptamer was also performed, indicating that irrelevant ssDNA cannot be used in our sensing platform (Figure S3). These data suggest that both the 38-nt and 66-nt aptamers can be used in the colorimetric aptasensor. Comparison of the selectivity and sensitivity of both aptamers is discussed in the following sections.
Optimization of Detection Conditions
Based on the detection principle shown in Figure 1, the developed aptasensor shows a characteristic peak of the UV–Vis spectrum at λ = 525 nm. The absorption of the colorimetric aptasensor at this signature wavelength decreases with increasing concentration of 8-oxo-dG. This is due to the salt-induced aggregation of AuNPs resulting in the red shift of the absorption spectrum. The color of the AuNP solution therefore changes from red to purple-blue. To optimize parameters affecting the analytical figure of merit of the aptasensor, the difference between the absorbance in the presence and absence of 8-oxo-dG at λ = 525 nm (ΔA525) was optimized for all related parameters including the concentrations of AuNPs, aptamer, NaCl, pH, and incubation time. To obtain the calibration curve, the concentrations of 8-oxo-dG were finally varied in the range of 10–100 nM. It is worth noting that instead of using A525/A650, ΔA525 was employed as an analytical signal in the present work as the absorbance at ca. 650 nm depended on the shape of aggregated AuNPs, which was an uncontrollable factor. Therefore, using a ratio of A620 to A525 caused a reproducibility problem and a narrow detection range was obtained.
To optimize the concentration of AuNPs, it was varied in the range of 1–3 nM, while the concentrations of aptamer, 8-oxo-dG, and NaCl were 133, 50, and 33 mM, respectively. As a result, the optimization of ΔA525 was obtained at the AuNP concentration of 2 nM for both 66-nt and 38-nt aptamer systems, as shown in Figure S4. Hence, the concentration of AuNPs at 2 nM was used for further studies. As shown in Figure S5, the effect of pH in the range of 2–8 was evaluated in phosphate buffer. It was found that, during the monitoring of 8-oxo-dG, the absorbance of the AuNP solution was not significantly different while varying the pH from 2 to 8. Thus, the pH of the solution at 7 was selected for the aptasensor as it was close to the pH of normal urine.40
Subsequently, the optimization of the aptamer concentrations in the present study was performed in the range of 0–170 nM, in which the concentrations of AuNPs, NaCl, and 8-oxo-dG were fixed at 2, 33, and 50 nM, respectively. As shown in Figure S6, the optimal concentrations of aptamers for the 38-nt and 66-nt aptasensors were 83 and 100 nM, respectively.
Salts can induce the aggregation of AuNPs due to the screening of electrostatic repulsion between the negatively charged AuNPs that caused the aggregation of AuNPs, leading to decreased absorption at λ = 525 nm. The optimization of NaCl concentration is shown in Figure S7. The concentration of NaCl was varied in the range of 0–57 mM. It was found that the ΔA525 was optimized at the concentrations of 45 and 26 mM for 66-nt and 38-nt aptasensors, respectively.
Finally, the reaction time between the anti-8-oxo-dG-aptamer and AuNPs was optimized. The binding time between the aptamer and 8-oxo-dG was observed in the time range of 0–18 min. For the reaction time between the aptamer and AuNPs in the 38-nt and 66-nt aptasensors, it was found that A525 gradually increased for both systems and became constant at t = 12 min, as shown in Figure S8. In addition to the reaction time, the binding time between the aptamers and 8-oxo-dG was also studied, as shown in Figure S9. The optimal binding time for the 66-nt aptamer was obtained at t = 14 min. The binding of the aptamer to 8-oxo-dG required a slightly longer time than that of the 38-nt (12 min). To sum up, the reaction and binding time of the 66-nt aptamer were 12 and 14 min, respectively, while those of the 38-nt aptamer were 12 and 12 min, respectively. Taken into account of all these factors, both 38-nt and 66-nt aptamers can be used in rapid 8-oxo-dG detection. However, to develop a low-cost, portable device, the truncated aptamer was chosen for better cost-effectiveness in the determination of 8-oxo-dG in real samples.
Sensitivity for 8-Oxo-dG Detection
Under the optimum experimental conditions, the calibration curves of the developed aptasensors were obtained by varying the concentrations of 8-oxo-dG in the range of 10–100 nM. The calibration curves of both 66-nt and 38-nt aptamers are shown in Figure 3. As can be seen, the absorption at λ = 525 nm decreased with increasing concentrations of 8-oxo-dG. As illustrated in Figure 3, the absorption of AuNP solution at λ = 525 nm exhibited a linear correlation to 8-oxo-dG concentrations within the range of 10–100 nM. The linear regression equation of the 38-nt aptamer was fitted as y = (0.00239 ± 0.00004)x – (0.00228 ± 0.00277) with a correlation coefficient of 0.9966, as illustrated in Figure 3b. The limit of detection (LOD) is 6.4 nM according to the equation LOD = (3 × S.D.)/m, where S.D. is the standard deviation of the blank measurements (n = 15) and m is the slope of the calibration curve. In the case of the 66-nt aptasensor (Figure 3c,d), the decreasing absorption of AuNP solution at λ = 525 nm (Figure 3c) was linearly proportional to the concentrations of 8-oxo-dG in the range of 15–100 nM (Figure 3d). The linear equation was y = (0.00181 ± 0.00010)x – (0.00035 ± 0.00513) with a correlation coefficient of 0.9713. The corresponding detection limit was calculated to be 13.2 nM. It is worth noting that there was an improvement in the LOD of the 38-nt aptasensor, being slightly lower than that of the 66-nt aptasensor.
Figure 3.
Absorption spectra and calibration curves of the AuNPs-based aptasensors using different concentrations of 8-oxo-dG (10–100 nM) after addition of NaCl and anti-8-oxo-dG-aptamers: (a, b) 38-nt aptamer and (c, d) 66-nt aptamer.
Selectivity of the Developed Aptasensor
The selectivity of the proposed aptasensors was examined by challenging the 38-nt and 66-nt aptasensors against other structural analogues, including guanosine, guanine, 8′-hydroxyguanosine, 8′-hydroxyguanine, and 2′-deoxyguanosine, under the optimum conditions. The selectivity of the developed aptasensors was examined for both the 66-nt and 38-nt aptamers. As shown in Figure 4, solely 8-oxo-dG responded to the developed aptasensors. There was no obvious analytical signal change with addition of various 8-oxo-dG analogues and uric acid. The interference ratios of 38-nt aptamer to 66-nt aptamer for uric acid and the structural analogues were 0.89 (uric acid), 0.41 (guanosine), 0.45 (guanine), 2.85 (8′-hydroxyguanosine), 0.83 (8′-hydroxyguanine), and 0.24 (2′-deoxyguanosine). These data demonstrate that the 38-nt aptamer has slightly less interference than the 66-nt aptamer with highly specific binding to 8-oxo-dG.
Figure 4.

Comparison of the responses of the developed aptasensors using 66-nt (blue) and 38-nt (purple) aptamers to 8-oxo-dG and its structural analogues.
To investigate the selectivity of the 38-nt aptamer, we previously elucidated the strength of the binding interactions of 8-oxo-dG and the 66-nt aptamer at different recognition sites by MD simulation.33 It was revealed that the central region of the 66-nt aptamer was more favorable than other recognition sites and up to eight hydrogen bonds could be formed in the complex stabilization stage. We therefore drew the conclusion that strong binding occurs at the central region of the 66-nt aptamer. Eliminating nucleobases outside this binding site has been demonstrated that the 38-nt aptamer did not change the specificity of the aptamer. Therefore, the truncated aptamer can be effectively applied in the colorimetric aptasensor for 8-oxo-dG detection.
Recovery Studies and Urine Analysis
The applications of the proposed detection method were evaluated for the determination of 8-oxo-dG in both phosphate buffer solution (pH = 7) and spiked urine samples. As shown in Table 1, three different concentrations of 8-oxo-dG (55, 75, and 95 nM) were spiked into phosphate buffer solutions (pH = 7), and the effect of the aptamer length on the analytical performance was observed. With the 38-nt aptasensor, the quantities of 8-oxo-dG measured in the spiked samples were 56.7 ± 1.3, 72.7 ± 1.3, and 94.3 ± 1.1 nM, while those for the 66-nt aptasensor were 50.7 ± 2.2, 69.7 ± 2.5, and 101.2± 2.0 nM, respectively. The percentage recoveries of the 38-nt aptasensor for the concentrations of 55.0, 75.0, and 95.0 nM were 103.1 ± 2.2, 97.0 ± 1.8, and 99.3 ± 1.2%, respectively, while those of the 66-nt aptasensor were 92.3 ± 4.4, 93.0 ± 3.6, and 106.5 ± 2.0%, respectively. In addition to the detection in spiked urine samples, the determination of 8-oxo-dG content in urine samples of healthy people and some patients was also demonstrated, as shown in Table S1. It shows the concentrations of 8-oxo-dG in urine samples of healthy people and a couple of patients with bile duct cancer. This illustrates the feasibility of using the proposed platform to differentiate healthy people and patients. These data suggest that the highly specific binding of the 38-nt aptamer, designed by a computer simulation, can be effectively applied in the colorimetric aptasensor, compared with the analytical performance of the 66-nt aptamer obtained from the literature via the systematic evolution of ligands by the exponential enrichment (SELEX) technique.
Table 1. Measurement of 8-Oxo-dG in Phosphate Buffer Solution (pH = 7) by 66-nt and 38-nt Aptasensorsa.
| 38-nt
aptasensor (n = 3) |
66-nt aptasensor (n = 3) |
|||
|---|---|---|---|---|
| amount added (nM) | amount found ± S.D. (nM) | recovery ± RSD (%) | amount found ± S.D. (nM) | recovery ± RSD (%) |
| 55 | 56.7 ± 1.3 | 103.1 ± 2.2 | 50.7 ± 2.2 | 92.3 ± 4.4 |
| 75 | 72.7 ± 1.3 | 97.0 ± 1.8 | 69.7 ± 2.5 | 93.0 ± 3.6 |
| 95 | 94.3 ± 1.1 | 99.3 ± 1.2 | 101.2 ± 2.0 | 106.5 ± 2.0 |
Note that S.D. and RSD are the standard deviation and relative standard deviation, respectively.
Development of a Portable Device
For both systems,
the aptasensors were developed based on the use of a UV–Vis
spectrophotometer, unsuitable for point-of-care detection. We, therefore,
developed a portable, low-cost device for 8-oxo-dG detection. The
3D model of the device is presented in Figure 5. This device was easily fabricated by 3D
printing. A light-emitting diode (LED) in which λ = 525 nm (size
= 40 × 40 mm2, 1 W, 60–70 LM, and 3.2–3.4
V) was used as a light source. Optical fiber was used to control the
direction of the light. The light intensity of the solution was measured
and analyzed by the sensor module for light intensity measurement
(BH1750 LUX Sensor). The absorbance (A) of the solution
was calculated from the light intensity: A = −log(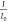 ), where A is the absorbance, I0 is the intensity of the incident radiation,
and I is the intensity of the transmitted radiation.
As illustrated in Figure 6, a linear range of 8-oxo-dG detection by the developed portable
device was obtained in the range of 15–100 nM for both 38-mer
and 66-mer aptasensors. The linear equations for both aptasensors
were y = (0.00169 ± 0.00004)x + (0.02180 ± 0.00244) (R2 = 0.9958)
and y = (0.00174 ± 0.00004)x + (0.02213 ± 0.00158) (R2 = 0.9961),
respectively. To verify the accuracy of the developed portable device,
both 66-nt and 38-nt aptasensors were used to detect 8-oxo-dG in the
spiked urine samples. It was revealed that the device based on either
aptasensor was effectively used to detect 8-oxo-dG, as shown in Table 2. The limits of detection
obtained from our portable device were as low as 12.9 nM (38-nt aptasensor)
and 9.8 nM (66-nt aptasensor), demonstrating its ability to detect
8-oxo-dG in real human urine as both LODs are much lower than the
threshold value (280 nM) for normal human urine.41 Our 38-nt aptasensor was compared with previously reported
methods using the 66-nt aptamer, as shown in Table 3. Our method surpassed Rayleigh light scattering
(RLS)39 and chiroplasmic42 techniques in a wide linear range. Electrochemical methods30 can provide a wider detection range than our
portable device but a longer detection time. Compared with the 66-nt
aptamer, the use of the computationally redesigned 38-nt aptamer would
lead to the reduction of the cost for developing an aptasensor. In
addition, the costs of a portable device and one urine test are lower
than $50 and $1, respectively, proving that our proposed method could
potentially serve as an economically viable replacement.
), where A is the absorbance, I0 is the intensity of the incident radiation,
and I is the intensity of the transmitted radiation.
As illustrated in Figure 6, a linear range of 8-oxo-dG detection by the developed portable
device was obtained in the range of 15–100 nM for both 38-mer
and 66-mer aptasensors. The linear equations for both aptasensors
were y = (0.00169 ± 0.00004)x + (0.02180 ± 0.00244) (R2 = 0.9958)
and y = (0.00174 ± 0.00004)x + (0.02213 ± 0.00158) (R2 = 0.9961),
respectively. To verify the accuracy of the developed portable device,
both 66-nt and 38-nt aptasensors were used to detect 8-oxo-dG in the
spiked urine samples. It was revealed that the device based on either
aptasensor was effectively used to detect 8-oxo-dG, as shown in Table 2. The limits of detection
obtained from our portable device were as low as 12.9 nM (38-nt aptasensor)
and 9.8 nM (66-nt aptasensor), demonstrating its ability to detect
8-oxo-dG in real human urine as both LODs are much lower than the
threshold value (280 nM) for normal human urine.41 Our 38-nt aptasensor was compared with previously reported
methods using the 66-nt aptamer, as shown in Table 3. Our method surpassed Rayleigh light scattering
(RLS)39 and chiroplasmic42 techniques in a wide linear range. Electrochemical methods30 can provide a wider detection range than our
portable device but a longer detection time. Compared with the 66-nt
aptamer, the use of the computationally redesigned 38-nt aptamer would
lead to the reduction of the cost for developing an aptasensor. In
addition, the costs of a portable device and one urine test are lower
than $50 and $1, respectively, proving that our proposed method could
potentially serve as an economically viable replacement.
Figure 5.

3D model of a portable, low-cost device for monitoring 8-oxo-dG in urine.
Figure 6.

Calibration curves of the (a) 38-nt and (b) 66-nt aptasensors obtained from the developed portable device.
Table 2. Analytical Results of 8-Oxo-dG Detection in the Spiked Urine Samples from our Portable Low-Cost Device Based on the 38-nt and 66-nt Aptasensors.
| spiked 8-oxo-dG (nM) | amount found ± S.D. (nM) | recovery ± RSD (%) |
|---|---|---|
| 38-nt aptasensor | ||
| 0 | 24.2 ± 3.0 | |
| 20 | 43.5 ± 4.9 | 96.3 ± 11.3 |
| 40 | 68.6 ± 2.7 | 110.9 ± 4.0 |
| 66-nt aptasensor | ||
| 0 | 24.3 ± 2.2 | |
| 20 | 48.2 ± 4.0 | 119.7 ± 8.3 |
| 40 | 63.1 ± 3.4 | 97.2 ± 5.4 |
Table 3. Comparison of Different Methods for 8-Oxo-dG Analysisa.
| detection method | LOD | detection range | detection time |
|---|---|---|---|
| Rayleigh light scattering*39 | 27.3 pM | 90.8 pM–14.1 nM | 25 min |
| resonance light scattering43 | 11 pM | 32 pM–12 nM | 45 min |
| fluorescence*44 | 1.19 nM | 3.96–211 nM | 65 min |
| fluorescence45 | 4 pM | 0.02–70 nM | 9 h 25 min |
| chiroplasmonic*42 | 33 pM | 0.05–2 nM | 60 min |
| high-performance liquid chromatography-mass spectrometry (HPLC-MS)*46 | 0.35 aM | 0.18 fM–0.706 pM | 11 min |
| electrochemical*30 | 2.5 pM | 10 pM–100 μM | 2 h 40 min |
| electrochemical47 | 36.67 nM | 0.05–536.5 μM | |
| electrochemiluminescence48 | 25 fM | 100 fM–10 nM | |
| fluorometric and colorimetric*49 | ∼350 pM | 0.5–500 nM | 30 min |
| colorimetric*50 | 141 pM | 466 pM–247 nM | 1.50 h |
| colorimetric*51 | 1.7 nM | 5.6–282 nM | 25 min |
| present work (66-nt aptasensor with a portable device) | 9.8 nM | 15–100 nM | 30 min |
The detection methods using the same aptamer as the present work (66-nt aptamer) are denoted with asterisks (*).
Conclusions
We have developed aptasensors for the detection of 8-oxo-dG by using ssDNA aptamers and citrate-capped AuNPs. We have conducted a comparative study to investigate the effect of the length of the aptamers (38-nt and 66-nt aptamers) on their analytical performances. The 66-nt 8-oxo-dG aptamer was established by the SELEX method and reported in the literature.36,44,50 Based on the parent 66-nt aptamer sequence, we previously designed and reported a shortened aptamer sequence of 38-nt.33 Both aptamers have been applied in the development of 66-nt and 38-nt aptasensors. It was found that 8-oxo-dG can specifically bind to both 66-nt and 38-nt aptamers and they can be effectively utilized in the aptasensors. The analytical signal (ΔA525) exhibits a linear relation to the increasing concentrations of 8-oxo-dG in the range of 10–100 nM for the 38-nt aptasensor and 15–100 nM for the 66-nt aptasensor. In comparison to the analytical performance of the 66-nt aptasensor, the redesigned 38-nt aptamer shows promising potential to be used as a main component of the aptasensor. More interestingly, a point-of-care device has been developed and it showed high accuracy for urinary 8-oxo-dG detection. Therefore, our proposed portable device could be effectively and economically used to detect 8-oxo-dG in urine samples and this point-of-care device could also be extended into the commercial stage.
Materials and Methods
Materials
8-Oxo-dG was obtained from Merck KGaA. The anti-8-oxo-dG-aptamers used in the present work (5′-GCG GGC GAT CGG CGG GGG GTG CGT GCG CTC TGT GCC AGG GGG TGG GAC AGA TCA TAT GGG GGT GCT-3′ and 5′-TGC GTG CGC TCT GTG CCA GGG GGT GGG ACA GAT CAT AT-3′) were synthesized by Ward Medic Ltd. All reagents were of analytical grade and used without any further purification. Gold(III) chloride trihydrate (HAuCl4·3H2O), sodium citrate dibasic sesquihydrate, 2-deoxyguanosine monohydrate (99%), 8-hydroxy-2′-deoxyguanosine (98%), 8-hydroxy-2′-deoxyguanine (98%) were purchased from Sigma Aldrich. Sodium phosphate dibasic (Na2HPO4), sodium dihydrogen phosphate anhydrous (NaH2PO4), and sodium chloride (NaCl) were purchased from Quality Reagent Chemicals.
Apparatus
Absorbance measurements were recorded on a UV–Vis spectrophotometer (Shimadzu, UV-1800). Transmission electron microscopy (TEM) measurements were made on a Tecnai (FEI 5022/22 Tecnai G2 20 S-Twin). The pH values of the solutions were measured using a Mettler Toledo LE438.
Synthesis of AuNPs
AuNPs were prepared by reducing HAuCl4 with sodium citrate.52 HAuCl4 solution (0.1 M, 2 mL) was heated until boiling with vigorous stirring in a 200 mL round-bottom flask. After stirring for 15 min, 20 mL of 38.8 mM sodium citrate was rapidly added into the solution, and the reaction continued for 2 min. Subsequently, the solution was cooled down to room temperature. The resulting wine-red solution was filtered by a 0.45 μm filter paper and stored at 4 °C until used. The concentration of AuNPs was characterized by UV–Vis spectroscopy based on an extinction coefficient of 3.67 × 108 M–1 cm–1 at 525 nm for 17.22 nm diameter particles, and it was estimated to be 12 nM. TEM was used to characterize the morphology of the AuNPs.
8-Oxo-dG Detection
To detect 8-oxo-dG with 66-nt and 38-nt aptasensors, all necessary parameters were optimized. After completing the optimization, 8-oxo-dG detection was performed under the optimum conditions. For the 66-nt aptasensor, in a 2 mL centrifuge tube, 156.5 μL of 1 mM phosphate buffer (pH = 7.0) was mixed with 50 μL of 12 nM AuNPs in 30 μ L of 1 μM 66-nt aptamer. Then, the mixture was incubated for 12 min at room temperature. To obtain the calibration curve, 50 μL of a range of concentrations of 8-oxo-dG (15–100 nM) was mixed in the mixture solution and incubated for another 14 min at room temperature. Subsequently, 13.5 μL of 1 M NaCl solution was added into the mixture to reach a final volume of 300 μL. After equilibrating for 5 min, the absorption was measured with a 1 cm path length cuvette and the UV–Vis absorbance at 525 nm was observed.
To detect 8-oxo-dG with the 38-nt aptasensor, 50 μL of 12 nM AuNPs and 25 μL of 1 μM 38-np aptamer were added into 167 μL of 1 mM phosphate buffer (pH = 7). The mixture solution was incubated for 12 min at room temperature and then mixed with 50 μL of 8-oxo-dG in the concentration range of 10–100 nM. The mixture was incubated for 12 min at room temperature. Then, 8 μL of 1 M NaCl solution was added into the mixture to reach a final volume of 300 μL and incubated for another 5 min. The calibration curve was observed at the absorption wavelength of 525 nm.
For the urinary 8-oxo-dG detection, urine samples were collected and stored at −20 °C until used. The present study was approved by the Khon Kaen University Ethics Committee for Human Research (no. HE621151). To detect 8-oxo-dG, the urine was centrifuged and it required a 32-fold dilution with phosphate buffer (pH = 7). In spike and recovery, a known amount of 8-oxo-dG was added into the urine matrix. Then, the assay was run to measure the recovery of the spiked sample matrix.
Acknowledgments
This work was financially supported by the Cholangiocarcinoma Screening and Care Program (CASCAP), Khon Kaen University (CASCAP-08) to C.S. and T.P. P.M. would also like to express her gratitude to the research capability enhancement program through graduate student scholarship, Faculty of Science, Khon Kaen University (SCG-2016-1) for the financial support throughout her study.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c01834.
TEM image and size distribution, optimization of the concentration of AuNPs, optimization of pH, optimization of the aptamer concentration, optimization of the NaCl concentration, reaction time optimization, binding time optimization, and urine analysis (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Korkmaz K. S.; Butuner B. D.; Roggenbuck D. Detection of 8-OHdG as a Diagnostic Biomarker. J. Lab. Precis. Med. 2018, 3, 1–8. 10.21037/jlpm.2018.11.01. [DOI] [Google Scholar]
- Kaneko K.; Kimata T.; Tsuji S.; Ohashi A.; Imai Y.; Sudo H.; Kitamura N. Measurement of ?Urinary 8-Oxo-7,8-dihydro-2-deoxyguanosine in a Novel Point-of-care Testing Device to Assess Oxidative Stress in Children. Clin. Chim. Acta 2012, 413, 1822–1826. 10.1016/j.cca.2012.07.009. [DOI] [PubMed] [Google Scholar]
- Li P.; Ramm G. A.; Macdonald G. A. Value of the 8-oxodG/dG Ratio in Chronic Liver Inflammation of Patients with Hepatocellular Carcinoma. Redox Biol. 2016, 8, 259–270. 10.1016/j.redox.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganapathy V.; Manyanga J.; Brame L.; McGuire D.; Sadhasivam B.; Floyd E.; Rubenstein D. A.; Ramachandran I.; Wagener T.; Queimado L. Electronic Cigarette Aerosols Suppress Cellular Antioxidant Defenses and Induce Significant Oxidative DNA Damage. PLoS One 2017, 12, e0177780. 10.1371/journal.pone.0177780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirstein M. M.; Vogel A. Epidemiology and Risk Factors of Cholangiocarcinoma. Visc. Med. 2016, 32, 395–400. 10.1159/000453013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo H.-W.; Chou S.-Y.; Hu T.-W.; Wu F.-Y.; Chen D.-J. Urinary 8-Hydroxy-2′-deoxyguanosine (8-OHdG) and Genetic Polymorphisms in Breast Cancer Patients. Mutat. Res., Genet. Toxicol. Environ. Mutagen. 2007, 631, 62–68. 10.1016/j.mrgentox.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Lee J. D.; Cai Q.; Shu X. O.; Nechuta S. J. The Role of Biomarkers of Oxidative Stress in Breast Cancer Risk and Prognosis: A Systematic Review of the Epidemiologic Literature. J. Women’s Health 2017, 26, 467–482. 10.1089/jwh.2016.5973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C.; Li X.; Wang R.; Yu J.; Ye M.; Mao L.; Zhang S.; Zheng S. Association between Oxidative DNA Damage and Risk of Colorectal Cancer: Sensitive Determination of Urinary 8-Hydroxy-2′-deoxyguanosine by UPLC-MS/MS Analysis. Sci. Rep. 2016, 6, 32581. 10.1038/srep32581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziaman T.; et al. Characteristic Profiles of DNA Epigenetic Modifications in Colon Cancer and Its Predisposing Conditions–Benign Adenomas and Inflammatory Bowel Disease. Clin. Epigenet. 2018, 10, 72. 10.1186/s13148-018-0505-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wangboon C.; et al. Elevated Levels of Urinary 8-oxodG Correlate with Persistent Periductal Fibrosis after Praziquantel Treatment in Chronic Opisthorchiasis. Am. J. Trop. Med. Hyg. 2018, 98, 1763–1769. 10.4269/ajtmh.17-0971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei S.; Yao Q.; Wu C.; Xu G. Determination of urinary 8-hydroxy-2′-deoxyguanosine by two approaches—capillary electrophoresis and GC/MS: An assay for in vivo oxidative DNA damage in cancer patients. J. Chromatogr., B 2005, 827, 83–87. 10.1016/j.jchromb.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Wang C.-J.; Yang N.-H.; Chang C.-C.; Liou S.-H.; Lee H.-L. Rapid and simple one-step membrane extraction for the determination of 8-hydroxy-2′-deoxyguanosine in human plasma by a combination of on-line solid phase extraction and LC–MS/MS. J. Chromatogr., B 2011, 879, 3538–3543. 10.1016/j.jchromb.2011.09.038. [DOI] [PubMed] [Google Scholar]
- Barrera G. Oxidative Stress and Lipid Peroxidation Products in Cancer Progression and Therapy. ISRN Oncol. 2012, 2012, 1–21. 10.5402/2012/137289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Q.-H.; Mei S.-R.; Weng Q.-F.; Zhang P.-D.; Yang Q.; Wu C.-Y.; Xu G.-W. Determination of Urinary Oxidative DNA Damage Marker 8-Hydroxy-2′-deoxyguanosine and The Association with Cigarette Smoking. Talanta 2004, 63, 617–623. 10.1016/j.talanta.2003.12.024. [DOI] [PubMed] [Google Scholar]
- Rossner P. Jr.; et al. Urinary 8-Oxo-7,8-dihydro-2′-deoxyguanosine Analysis by An Improved ELISA: An Inter-Laboratory Comparison Study. Free Radical Biol. Med. 2016, 95, 169–179. 10.1016/j.freeradbiomed.2016.03.016. [DOI] [PubMed] [Google Scholar]
- Rossner P. Jr.; Mistry V.; Singh R.; Sram R. J.; Cooke M. S. Urinary 8-oxo-7,8-dihydro-2′-deoxyguanosine values determined by a modified ELISA improves agreement with HPLC–MS/MS. Biochem. Biophys. Res. Commun 2013, 440, 725–730. 10.1016/j.bbrc.2013.09.133. [DOI] [PubMed] [Google Scholar]
- Kobayashi S.; et al. Urinary 8-Hydroxy-2′-deoxyguanosine as a Novel Biomarker of Inflammatory Activity in Patients with Cardiac Sarcoidosis. Int. J. Cardiol. 2015, 190, 319–328. 10.1016/j.ijcard.2015.04.144. [DOI] [PubMed] [Google Scholar]
- Cooke M. S.; Olinski R.; Loft S. Measurement and Meaning of Oxidatively Modified DNA Lesions in Urine. Cancer Epidemiol. Biomarkers Prev. 2008, 17, 3–14. 10.1158/1055-9965.EPI-07-0751. [DOI] [PubMed] [Google Scholar]
- Loft S.; Svoboda P.; Kawai K.; Kasai H.; Sørensen M.; Tjønneland A.; Vogel U.; Møller P.; Overvad K.; Raaschou-Nielsen O. Association Between 8-Oxo-7,8-dihydroguanine Excretion and Risk of Lung Cancer in a Prospective Study. Free Radical Biol. Med. 2012, 52, 167–172. 10.1016/j.freeradbiomed.2011.10.439. [DOI] [PubMed] [Google Scholar]
- Lin H.-S.; Jenner A. M.; Ong C. N.; Huang S. H.; Whiteman M.; Halliwell B. A High-Throughput and Sensitive Methodology for the Quantification of Urinary 8-Hydroxy-2′-deoxyguanosine: Measurement with Gas Chromatography-Mass Spectrometry After Single Solid-Phase Extraction. Biochem. J. 2004, 380, 541–548. 10.1042/bj20040004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seet R. C.; et al. Oxidative Damage in Parkinson Disease: Measurement using Accurate Biomarkers. Free Radical Biol. Med. 2010, 48, 560–566. 10.1016/j.freeradbiomed.2009.11.026. [DOI] [PubMed] [Google Scholar]
- Rozalski R.; Gackowski D.; Siomek-Gorecka A.; Starczak M.; Modrzejewska M.; Banaszkiewicz Z.; Olinski R. Urinary 5-hydroxymethyluracil and 8-oxo-7,8-dihydroguanine as potential biomarkers in patients with colorectal cancer. Biomarkers 2015, 20, 287–291. 10.3109/1354750X.2015.1068860. [DOI] [PubMed] [Google Scholar]
- Hu C.-W.; Huang Y.-J.; Li Y.-J.; Chao M.-R. Correlation between Concentrations of 8-Oxo-7,8-dihydro-2′-deoxyguanosine in Urine, Plasma and Saliva Measured by On-line Solid-phase Extraction LC-MS/MS. Clin. Chim. Acta 2010, 411, 1218–1222. 10.1016/j.cca.2010.04.029. [DOI] [PubMed] [Google Scholar]
- Lee K.-F.; Chung W.-Y.; Benzie I. F. F. Urine 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxodG), a specific marker of oxidative stress, using direct, isocratic LC–MS/MS: Method evaluation and application in study of biological variation in healthy adults. Clin. Chim. Acta 2010, 411, 416–422. 10.1016/j.cca.2009.12.013. [DOI] [PubMed] [Google Scholar]
- Mesaros C.; Arora J. S.; Wholer A.; Vachani A.; Blair I. A. 8-Oxo-2′-deoxyguanosine as a Biomarker of Tobacco-Smoking-Induced Oxidative Stress. Free Radical Biol. Med. 2012, 53, 610–617. 10.1016/j.freeradbiomed.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam P. M. W.; Mistry V.; Marczylo T. H.; Konje J. C.; Evans M. D.; Cooke M. S. Rapid measurement of 8-oxo-7,8-dihydro-2′-deoxyguanosine in human biological matrices using ultra-high-performance liquid chromatography–tandem mass spectrometry. Free Radical Biol. Med. 2012, 52, 2057–2063. 10.1016/j.freeradbiomed.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan R.; Wang D.; Ramage R.; She J. Fast and Simultaneous Determination of Urinary 8-Hydroxy-2′-deoxyguanosine and Ten Monohydroxylated Polycyclic Aromatic Hydrocarbons by Liquid Chromatography/Tandem Mass Spectrometry. Chem. Res. Toxicol. 2012, 25, 491–499. 10.1021/tx200517h. [DOI] [PubMed] [Google Scholar]
- Kuligowski J.; et al. Assessment of Oxidative Damage to Proteins and DNA in Urine of Newborn Infants by a Validated UPLC-MS/MS Approach. PLoS One 2014, 9, 1–9. 10.1158/1055-9965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C.-W.; Cooke M. S.; Tsai Y.-H.; Chao M.-R. 8-Oxo-7,8-dihydroguanine and 8-Oxo-7,8-dihydro-2-deoxyguanosine Concentrations in Various Human Body Fluids: Implications for Their Measurement and Interpretation. Arch. Toxicol. 2015, 89, 201–210. 10.1007/s00204-014-1255-1. [DOI] [PubMed] [Google Scholar]
- Jia L.-P.; Wang L.-J.; Ma R.-N.; Shang L.; Zhang W.; Xue Q.-W.; Wang H.-S. An Electrochemical Aptasensor for the Highly Sensitive Detection of 8-Hydroxy-2′-deoxyguanosine Based on the Hybridization Chain Reaction. Talanta 2018, 179, 414–419. 10.1016/j.talanta.2017.11.036. [DOI] [PubMed] [Google Scholar]
- Arkhipova V. V.; Apyari V. V.; Dmitrienko S. G. A Colorimetric Probe Based on Desensitized Ionene-Stabilized Gold Nanoparticles for Single-Step Test for Sulfate Ions. Spectrochim. Acta A 2015, 139, 335–341. 10.1016/j.saa.2014.12.090. [DOI] [PubMed] [Google Scholar]
- Terenteva E. A.; Arkhipova V. V.; Apyari V. V.; Volkov P. A.; Dmitrienko S. G. Simple and Rapid Method for Screening of Pyrophosphate Using 6,6-Ionene-Stabilized Gold and Silver Nanoparticles. Sens. Actuators B Chem. 2017, 241, 390–397. 10.1016/j.snb.2016.10.093. [DOI] [Google Scholar]
- Choodet C.; Toomjeen P.; Phanchai W.; Matulakul P.; Thanan R.; Sakonsinsiri C.; Puangmali T. Combinedin silicoandin vitrostudy of an aptasensor based on citrate-capped AuNPs for naked-eye detection of a critical biomarker of oxidative stress. RSC Adv 2019, 9, 17592–17600. 10.1039/C9RA01497G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phanchai W.; Srikulwong U.; Chompoosor A.; Sakonsinsiri C.; Puangmali T. Insight Into the Molecular Mechanisms of AuNP-Based Aptasensor for Colorimetric Detection: A Molecular Dynamics Approach. Langmuir 2018, 34, 6161–6169. 10.1021/acs.langmuir.8b00701. [DOI] [PubMed] [Google Scholar]
- Toomjeen P.; Phanchai W.; Choodet C.; Chompoosor A.; Thanan R.; Sakonsinsiri C.; Puangmali T. Designing an Aptasensor Based on Cysteamine-Capped AuNPs for 8-oxo-dG Detection: A Molecular Dynamics Approach and Experimental Validation. J. Phys. Chem. B 2019, 123, 1129–1138. 10.1021/acs.jpcb.8b10436. [DOI] [PubMed] [Google Scholar]
- Miyachi Y.; Shimizu N.; Ogino C.; Fukuda H.; Kondo A. Selection of a DNA Aptamer That Binds 8-OHdG Using GMP-agarose. Bioorg. Med. Chem. Lett. 2009, 19, 3619–3622. 10.1016/j.bmcl.2009.04.130. [DOI] [PubMed] [Google Scholar]
- Krpetić Z̆.; Guerrini L.; Larmour I. A.; Reglinski J.; Faulds K.; Graham D. Importance of Nanoparticle Size in Colorimetric and SERS-Based Multimodal Trace Detection of Ni(II) Ions with Functional Gold Nanoparticles. Small 2012, 8, 707–714. 10.1002/smll.201101980. [DOI] [PubMed] [Google Scholar]
- Nath N.; Chilkoti A. Label-Free Biosensing by Surface Plasmon Resonance of Nanoparticles on Glass: Optimization of Nanoparticle Size.. Anal. Chem. 2004, 76, 5370–5378. 10.1021/ac049741z. [DOI] [PubMed] [Google Scholar]
- Wang J.-C.; Wang Y.-S.; Xue J.-H.; Zhou B.; Qian Q.-M.; Wang Y.-S.; Yin J.-C.; Zhao H.; Liu H.; Liu S.-D. An Ultrasensitive Label-Free Assay of 8-Hydroxy-2′-deoxyguanosine Based on the Conformational Switching of Aptamer. Biosens. Bioelectron. 2014, 58, 22–26. 10.1016/j.bios.2014.02.047. [DOI] [PubMed] [Google Scholar]
- Kanbara A.; Miura Y.; Hyogo H.; Chayama K.; Seyama I. Effect of Urine pH Changed by Dietary Intervention on Uric Acid Clearance Mechanism of pH-dependent Excretion of Urinary Uric Acid. Nutrition 2012, 11, 39. 10.1186/1475-2891-11-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao L.; Yue Q.; Hou Y.; Wang Y.; Chen C.; Li C.-Z. Resonance light scattering aptasensor for urinary 8-hydroxy-2′-deoxyguanosine based on magnetic nanoparticles: a preliminary study of oxidative stress association with air pollution. Microchim. Acta 2018, 185, 419. 10.1007/s00604-018-2937-9. [DOI] [PubMed] [Google Scholar]
- Liu H.; Wang Y.-S.; Tang X.; Yang H.-X.; Chen S.-H.; Zhao H.; Liu S.-D.; Zhu Y.-F.; Wang X.-F.; Huang Y.-Q. A Novel Fluorescence Aptasensor for 8-Hydroxy-2′-deoxyguanosine Based on the Conformational Switching of K+-Stabilized G-quadruplex. J. Pharm. Biomed. Anal. 2016, 118, 177–182. 10.1016/j.jpba.2015.10.035. [DOI] [PubMed] [Google Scholar]
- Wei W.; Wei M.; Yin L.; Pu Y.; Liu S. Improving the fluorometric determination of the cancer biomarker 8-hydroxy-2′-deoxyguanosine by using a 3D DNA nanomachine. Microchim. Acta 2018, 185, 494. 10.1007/s00604-018-3036-7. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Wei M.; Zhang L.; Zhang Y.; Wei W.; Yin L.; Pu Y.; Liu S. Chiroplasmonic Assemblies of Gold Nanoparticles for Ultrasensitive Detection of 8-Hydroxy-2′-deoxyguanosine in Human Serum Sample. Anal. Chem. 2016, 88, 6509–6514. 10.1021/acs.analchem.6b01258. [DOI] [PubMed] [Google Scholar]
- Gan H.; Xu H. A Novel Aptamer-Based Online Magnetic Solid Phase Extraction Method for the Selective Determination of 8-Hydroxy-2′-deoxyguanosine in Human Urine. Anal. Chim. Acta 2018, 1008, 48–56. 10.1016/j.aca.2017.12.032. [DOI] [PubMed] [Google Scholar]
- Govindasamy M.; Wang S.-F.; Subramanian B.; Ramalingam R. J.; Al-lohedan H.; Sathiyan A. A Novel Electrochemical Sensor for Determination of DNA Damage Biomarker (8-hydroxy-2′-deoxyguanosine) in Urine Using Sonochemically Derived Graphene Oxide Sheets Covered Zinc Oxide Flower Modified Electrode. Ultrason. Sonochem. 2019, 58, 104622. 10.1016/j.ultsonch.2019.104622. [DOI] [PubMed] [Google Scholar]
- Zhao R.-N.; Jia L.-P.; Feng Z.; Ma R.-N.; Zhang W.; Shang L.; Xue Q.-W.; Wang H.-S. Ultrasensitive electrochemiluminescence aptasensor for 8-hydroxy-2′-deoxyguanosine detection based on target-induced multi-DNA release and nicking enzyme amplification strategy. Biosens. Bioelectron 2019, 144, 111669. 10.1016/j.bios.2019.111669. [DOI] [PubMed] [Google Scholar]
- Ammanath G.; Yildiz U. H.; Palaniappan A.; Liedberg B. Luminescent Device for the Detection of Oxidative Stress Biomarkers in Artificial Urine. ACS Appl. Mater. Interfaces 2018, 10, 7730–7736. 10.1021/acsami.7b17252. [DOI] [PubMed] [Google Scholar]
- Liu H.; et al. A Colorimetric Aptasensor for the Highly Sensitive Detection of 8-Hydroxy-2′-deoxyguanosine Based on G-quadruplex–hemin DNAzyme. Anal. Biochem. 2014, 458, 4–10. 10.1016/j.ab.2014.04.031. [DOI] [PubMed] [Google Scholar]
- Wang J.-C.; Wang Y.-S.; Rang W.-Q.; Xue J.-H.; Zhou B.; Liu L.; Qian Q.-M.; Wang Y.-S.; Yin J.-C. Colorimetric determination of 8-hydroxy–2′-deoxyguanosine using label-free aptamer and unmodified gold nanoparticles. Microchim. Acta 2014, 181, 903–910. 10.1007/s00604-014-1173-1. [DOI] [Google Scholar]
- Hao J.-N.; Li Y. Concurrent Modulation of Competitive Mechanisms to Design Stimuli-Responsive Ln-MOFs: A Light-Operated Dual-Mode Assay for Oxidative DNA Damage. Adv. Funct. Mater. 2019, 29, 1903058. 10.1002/adfm.201903058. [DOI] [Google Scholar]
- Yang C.; Wang Y.; Marty J.-L.; Yang X. Aptamer-Based Colorimetric Biosensing of Ochratoxin A Using Unmodified Gold Nanoparticles Indicator. Biosens. Bioelectron. 2011, 26, 2724–2727. 10.1016/j.bios.2010.09.032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



