Abstract
PD-L1 inhibitors are widely used in tumor immunotherapy, but their mechanism in colorectal cancer remains unclear. The present study aimed to investigate the mechanisms underlying programmed death ligand 1 (PD-L1) regulation via the interferon-γ (IFN-γ)/janus kinase (JAK)/STAT signaling pathway, and its prognostic value in patients with colorectal cancer (CRC). A cohort of 181 patients were recruited to determine the association between PD-L1 expression and CRC prognosis; the patients were newly diagnosed with colorectal adenocarcinoma and had also undergone a physical tumorectomy. Immunohistochemical staining and survival analysis were used to evaluate the predictive value of PD-L1 protein expression in CRC. Gene set enrichment analysis, RT-qPCR and western blotting, etc were performed to confirm that PD-L1 is regulated by the IFN-γ/JAK/STAT signaling pathway. PD-L1 up-regulation was more frequently observed in patients with larger tumors, positive vascular or lymphatic infiltration and a poorly differentiated stage in addition to being associated with a poor survival in patients with CRC. Following the stimulation with IFN-γ, PD-L1 expression levels were revealed to be increased via the JAK2/STAT1 signaling pathway. In conclusion, the findings of the present study indicated that the expression levels of PD-L1 may be associated with a poor prognosis in patients with CRC. In addition, the results suggested that the IFN-γ-mediated overexpression of PD-L1 in CRC cells may be regulated by the JAK2/STAT1 signaling pathway.
Keywords: programmed death 1, colorectal cancer, janus kinase/STAT, prognosis
Introduction
Colorectal cancer (CRC) represents a major threat to global health, with an estimated 1,096,601 new cases and 551,269 CRC-associated mortalities predicted worldwide in 2018 (1). The prognosis of CRC remains poor, particularly in the advanced stages; this is due to the symptoms frequently appearing in the later stages of disease, resulting in delayed diagnosis and treatment (2). Chemotherapy is still the preferred adjuvant therapy for patients with CRC undergoing radical resection (3). In addition, an increased range of targeted therapies, such as EGFR or KRAS targeted therapies are being applied in clinical practice (4), of which the suppression of immune checkpoint pathways may represent the most promising approach (5). However, the discovery of reliable biomarkers for screening the target population remains an essential factor for successful targeted therapy.
Programmed death 1 (PD-1) and programmed death ligand 1 (PD-L1) are important regulators of immune checkpoints that induce tumor cell immune escape (6). The expression levels of PD-L1 were discovered to be increased in various types of tumor, including non-small cell lung cancer, gastric cancer, breast cancer and colorectal adenocarcinoma (7–10). The PD-1/PD-L1 blockade has been used as a novel oncotherapy for multiple types of cancer, including CRC (11). However, studies assessing the prognostic significance of PD-L1 expression in CRC remain controversial (10).
Additionally, various pathways are involved in the upregulation of PD-L1 in several cancers of the digestive system, such as the epidermal growth factor receptor/ERK signaling pathway in esophageal squamous cell carcinoma (12), the janus kinase (JAK)/STAT signaling pathway in gastric cancer (13) and the ERK/mitogen-activated protein kinase pathway in hepatocellular carcinoma (14). Nonetheless, the mechanisms by which PD-L1 expression is regulated in CRC are yet to be fully elucidated. Notably, the interferon-γ (IFN-γ)/JAK/STAT signaling pathway has been confirmed to induce PD-L1 expression in myeloid leukemia cells, and pancreatic and gastric cancer (13,15,16). However, the roles of the IFN-γ/JAK/STAT signaling pathway in regulating PD-L1 expression in CRC remain to be determined. Therefore, the present study aimed to determine the predictive value of PD-L1 in the prognosis of CRC and the mechanisms of action of PD-L1 regulation, with a focus on the IFN-γ/JAK/STAT signaling pathway in vitro and in patients with CRC.
Materials and methods
Patient studies
The present study was approved by the Institutional Review Board of China-Japan Union Hospital of Jilin University (Changchun, China) and written informed consent was provided by all patients. Patients with colorectal adenocarcinoma were randomly recruited from the Department of Gastric and Colorectal Surgery in the China-Japan Union Hospital of Jilin University between January 2010 and December 2015. Patients enrolled in the present study adhered to the following inclusion criteria: i) Initially diagnosed with colorectal adenocarcinoma; ii) had undergone tumorectomy; and iii) had not received chemotherapy or radiotherapy before surgery. The exclusion criteria were as follows: i) Patients with distant metastases and positive surgical margins; and ii) patients who had succumbed to postoperative complications within 30 days following surgery. Patient diagnosis was independently confirmed by two pathologists. Finally, 183 patients were randomly selected from the patients that meet the inclusion and exclusion criteria above.
Clinicopathological data
The following principal clinicopathological parameters were obtained from the patients: Sex, age, World Health Organization classification (17), the primary tumor, tumor size, vascular lymphatic infiltration, perineurium invasion, tumor location, tumor differentiation and tumor-node-metastasis (TNM) stage according to the American Joint Committee on Cancer/Leading the global fight against cancer 2010 classifications (18). All patients underwent follow-up after surgery in the first, third and sixth month in the first year, and every year by phone until death or the last scheduled follow-up. Survival time was defined as the duration between the date of surgery to the date of death or the final successful follow-up date. Patients who succumbed to surgical complications during the perioperative period or who were lost to follow-up at the time of the first interview were excluded from the survival analysis. A total of 181 patients were included in the survival analysis.
Gene set enrichment analysis (GSEA)
RNA-sequencing data (level 3 with RPKM files) were downloaded from The Cancer Genome Atlas (TCGA; http://gdc-portal.nci.nih.gov). This data set comprised the gene expression data from cancerous and healthy normal tissue of 276 patients with colorectal adenocarcinoma (19). These data were preprocessed using TCGA biolinks and annotated with Entrez ID v.17.0 (https://cancergenome.nih.gov/). The co-expression of PD-L1 with other genes whose sequences were present in this database was determined using the cBioPortal for Cancer Genomics v.3.2.13 (20,21). Signaling pathway enrichment was performed using the Kyoto Encyclopedia of Genes and Genomes (KEGG) database (http://www.genome.jp/kegg) (22).
Cell culture and treatment
The HCT 116 human CRC cell line (cat. no. CBP60028, Cobioer) was cultured in DMEM (HyClone; GE Healthcare Life Sciences), supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.) and 100 U/ml penicillin/streptomycin. The cells were maintained at 37°C (5% CO2) in a humidified incubator. Recombinant human IFN-γ (R&D Systems, Inc.) was diluted with PBS to a concentration of 0.2 mg/ml and stored at 70°C. Cells were seeded into 6-well plates at 2×105 cells/well, incubated overnight and then treated with 10 or 20 ng/ml IFN-γ for 24 h at 37°C.
Immunohistochemistry (IHC)
Cancer tissue and paired normal tissue were obtained from the all of the 181 patients included in the survival analysis following surgery. Tissue microarray slides of embedded tumor specimens from patients with colorectal adenocarcinoma were used for IHC staining. Briefly, tissues were fixed in 10% formalin for 24 h and embedded in paraffin at 65°C. Paraffin-embedded tissues were subsequently cut to a thickness of 5 µm. After washing with xylene for 20 min twice at room temperature and rehydration in descending alcohol series for 5 min in different concentrations (100, 90 and 80%), the slides were boiled for 20 min in ethylenediamine tetraacetic acid without high pressure for antigen retrieval. Endogenous peroxidase activity was blocked using 3% H2O2. The sections were blocked with 10% normal goat serum (cat. no. SP-B5; Fuzhou Maixin Biotech Co., Ltd) for 10 min at room temperature prior to incubation with primary antibody. The sections were incubated with a primary monoclonal antibody against PD-L1 (1:200; cat. no. 13684; Cell Signaling Technology, Inc.) at room temperature for 90 min. Following the primary antibody incubation, the sections were incubated with a horseradish peroxidase (HRP)-conjugated secondary antibody (cat. no. 5020; Fuzhou Maixin Biotech Co., Ltd) for 15 min at room temperature. The level of protein expression was determined using the 3,3-diaminobenzidine (DAB) (cat. no. DAB-0031; Maixin Biotech, Co., Ltd) and a light microscope (magnification ×100) was used to visualize the slides. PD-L1 expression was observed in the cell membrane and cytoplasm. The staining intensity was defined as: i) 0, no immunostaining (<5% expression); ii) 1 weak staining (5–19% expression); iii) 2, moderate staining (20–49% expression); or iv) 3, strong staining (≥50% expression). The sum of the intensity and percentage scores resulted in an immunoreactive score value ranging from 0–6 and a total score of >2 was defined as positive PD-L1 expression.
Western blotting
Total protein from HTC116 cell lines was extracted using a mammalian protein extraction kit (cat. no. CW0891M; CoWin Biosciences) and used according to the manufacturer's instructions. Total protein was quantified using a bicinchoninic acid assay kit (cat. no. CW0014S; CoWin Biosciences). The mass of protein loaded per lane were 10–40 µg on a 12.5% SDS-PAGE gel. The proteins were transferred to PVDF membranes. The membranes were blocked with 5% no-fat milk at room temperature for 1 h. The membranes were incubated with the following primary antibodies overnight at 4°C: Anti-PD-L1 (1:2,000; cat. no. 13684; Cell Signaling Technology, Inc.), anti-JAK2 (1:2,000; cat. no. ab39636; Abcam), anti-phosphorylated (p)-JAK2 (1:2,000; cat. no. ab32101; Abcam), anti-STAT1 (1:2,000; cat. no. ab31369; Abcam), anti-p-STAT1 (1:1,000; cat. no. ab109461; Abcam) and anti-GAPDH (1:1,000; cat. no. ab181602; Abcam). The membranes were incubated with an HRP-conjugated goat anti-rabbit IgG secondary antibody at room temperature for 25 min (1:10,000; cat. no. ab6721; Abcam). Protein bands were visualized using ECL reagents (iBright cat. no. CL750; Thermo Fisher Scientific, Inc.) and a ChemiDoc XRS + imaging system (Bio-Rad Laboratories, Inc.).
Reverse transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from the tumor tissues of 45 patients from whom fresh tissues were available using RNA extraction buffer from the Promega SV Total RNA Isolation System kit, (cat. no. Z3100; Promega Corporation) according to the manufacturer's instructions. Total RNA was reverse transcribed into cDNA using the cDNA synthesis kit Roche Transcriptor cDNA Synth. Kit 2 (cat. no. 4897030001; Roche Diagnostics) according to the manufacturer's instructions (first step 65°C for 10 min, then 65°C for 30 min and finally 85°C for 5 min). qPCR was subsequently performed using SYBR Master Mix (Roche Diagnostics) on a LightCycler 480 Real Time PCR system (Roche Diagnostics). The thermocycling conditions were as follows: 94°C for 3 min, followed by 30 cycles of 94°C for 30 sec, 55°C for 30 sec, 72°C for 1 min and 72°C for 1 min with a final extension step at 72°C for 10 min. The primers used for RT-qPCR were as follows: PD-L1, forward: 5′-GTACCGCTGCATGATCAGCTAT-3′ and reverse: 5′-GGCATTGACTTTCACAGTAATTCG-3′; IFN-γ, forward: 5′-TCTGGATCCATGAACGCTACACACTGC-3′ and reverse: 5′-ACTAAGCTTTCAGCAGCGACTCCTTTTCC-3′; JAK2, forward: 5′-CTGCAGGAAGGAGAGAGGAAGAGGA-3′ and reverse: 5′-GAATGTTATTGGCAGTCAG-3′; STAT1, forward: 5′-CCACTGAGACATCCTGCCACC-3′ and reverse: 5′-CCACTGAGACATCCTGCCACC-3′ and GAPDH, forward: 5′-ACCACAGTCCATGCCATCACT-3′ and reverse: 5′-ACTGTGCCGTTGAATTTGCC-3′. GAPDH was used as the endogenous reference gene. Expression levels of PD-L1, IFNG, JAK2 and STAT1 were quantified using the 2−ΔΔCq method (23).
Statistical analysis
Statistical analysis was performed using SPSS version 17.0 software (SPSS, Inc.) or GraphPad Prism 5.0 software (GraphPad Software, Inc.). Experiments were repeated 3 times and the data are presented as the mean ± SD. Statistical differences between groups were determined using one-way ANOVA and a Fisher's Least Significant Difference post-hoc test. Categorical variables are presented as frequencies (percentages) and were compared using a χ2 test. The log-rank test was used to determine the significance between the Kaplan-Meier survival curves generated by SPSS v.17.0 (SPSS Inc.). The variables with P<0.1 in log-rank test were included in the subsequent multivariate analysis. Multivariate Cox regression analysis was performed to assess the hazard ratio (HR) and 95% CI of possible prognostic factors. The correlations between mRNA expression levels in the tumor tissues were calculated using Spearman's rank correlation analysis. P<0.05 was considered to indicate a statistically significant difference.
Results
Association between PD-L1 expression levels and the clinicopathological features of patients with CRC
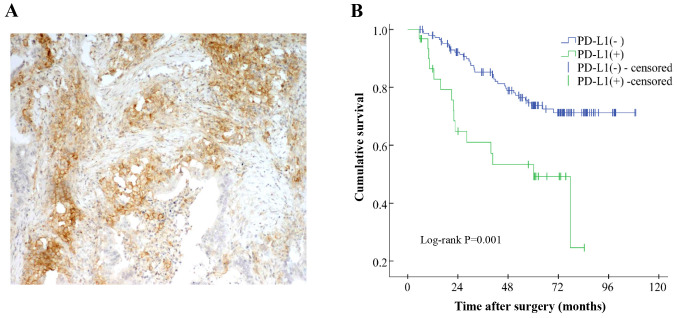
Among the 181 patients with CRC, 17.1% exhibited PD-L1 expression in the tumor tissues (Fig. 1A). The association between PD-L1 expression levels and clinicopathological features are presented in Table I. The expression levels of PD-L1 were found to be significantly associated with a tumor size of >5 cm, positive vascular or lymphatic infiltration and a poorly differentiated stage in the patients (all P<0.01; Table I).
Figure 1.
PD-L1 expression is associated with a poor prognosis in patients with CRC. (A) Immunohistochemical analysis was used to determine the expression levels of PD-L1 in CRC tissues. A representative tissue section is presented. Magnification ×400. (B) Survival plots for PD-L1 expression levels in patients with CRC. PD-L1, programmed death ligand 1; CRC, colorectal cancer.
Table I.
Association between PD-L1 expression levels and clinicopathological variables of patients with colorectal cancer.
| Variable | PD-L1 (+), n (%) | PD-L1 (−), n (%) | χ2-value | P-value |
|---|---|---|---|---|
| Age, years | ||||
| >65 | 13 (41.9) | 59 (39.3) | 0.073 | 0.788 |
| ≤65 | 18 (58.1) | 91 (60.7) | ||
| Sex | ||||
| Male | 13 (41.9) | 86 (57.3) | 2.458 | 0.117 |
| Female | 18 (58.1) | 64 (42.7) | ||
| Tumor size | ||||
| >5 cm | 10 (32.3) | 92 (61.3) | 8.830 | 0.003 |
| ≤5 cm | 21 (67.7) | 58 (38.7) | ||
| Tumor classification | ||||
| Tubular adenocarcinoma | 22 (71.0) | 121 (80.7) | 4.002 | 0.135 |
| Mucinous adenocarcinoma | 5 (16.1) | 23 (15.3) | ||
| Other | 4 (12.9) | 6 (4.0) | ||
| Vascular and lymphatic infiltration | ||||
| No | 11 (35.5) | 95 (63.3) | 8.221 | 0.004 |
| Yes | 20 (64.5) | 55 (36.7) | ||
| Perineurium invasion | ||||
| No | 21 (67.7) | 119 (79.3) | 1.970 | 0.160 |
| Yes | 10 (32.3) | 31 (20.7) | ||
| Tumor location | ||||
| Rectum | 12 (38.7) | 74 (49.3) | 1.163 | 0.281 |
| Colon | 19 (61.3) | 76 (50.7) | ||
| Tumor differentiation | ||||
| Medium and high differentiation | 13 (41.9) | 134 (89.3) | 37.831 | <0.001 |
| Poorly differentiated | 18 (58.1) | 16 (10.7) | ||
| Tumor-node-metastasis stage | ||||
| I–II | 11 (35.5) | 85 (56.7) | 4.628 | 0.031 |
| III | 20 (64.5) | 65 (43.3) |
PD-L1, programmed death ligand 1.
PD-L1 expression levels are associated with a poor prognosis in patients with CRC
The median follow-up time of the patients was 72.9 months (range, 5.4–109.0 months). At the end of the follow-up period, 105 (58.0%) patients remained alive, 49 (27.1%) patients had died and 27 (14.9%) were lost to follow-up. A log-rank test demonstrated that PD-L1 expression levels were significantly associated with a poor prognosis in patients with CRC (Fig. 1B and Table SI). In addition, the tumor classification as a mucinous adenocarcinoma, positive vascular and lymphatic infiltration, positive perineurium invasion and higher TNM stages were also associated with a poor prognosis of patients with CRC (P<0.05; Table SI). Variables with a P-value of <0.1 in the log-rank test were subsequently included in the multivariate analysis. Multivariate Cox regression with a stepwise method analysis revealed that the PD-L1 expression levels in tumor cells was an independent risk factor for the poor survival of the patients with CRC (HR=1.937; 95% CI=1.038–3.616; P=0.038; Table II). In addition, positive perineural invasion (P=0.004) and higher TNM stages (P=0.013) were also discovered to be independently associated with a poor survival of patients with CRC. The variables, tumor classification (P=0.391) and vascular & lymphatic infiltration (P=0.368), were excluded from the final multivariate analysis in Table II.
Table II.
Multivariate Cox's regression analysis for PD-L1 expression levels and the prognosis of patients with colorectal cancer.
| Variable | Hazard ratio | 95% CI | P-value |
|---|---|---|---|
| PD-L1 | |||
| Positive vs. Negative | 1.937 | 1.038–3.616 | 0.038 |
| Perineural invasion | |||
| Positive vs. Negative | 2.416 | 1.330–4.389 | 0.004 |
| TNM stage | |||
| III vs. I–II | 2.206 | 1.181–4.123 | 0.013 |
P-values were calculated using multivariate Cox's regression analysis and the stepwise selection method for tumor classification, vascular & lymphatic infiltration, perineurium invasion, TNM stage and PD-L1 expression. PD-L1, programmed death ligand 1; TNM, tumor-node-metastasis.
PD-L1 expression levels are correlated with IFNG, JAK2 and STAT1 expression levels at the mRNA level
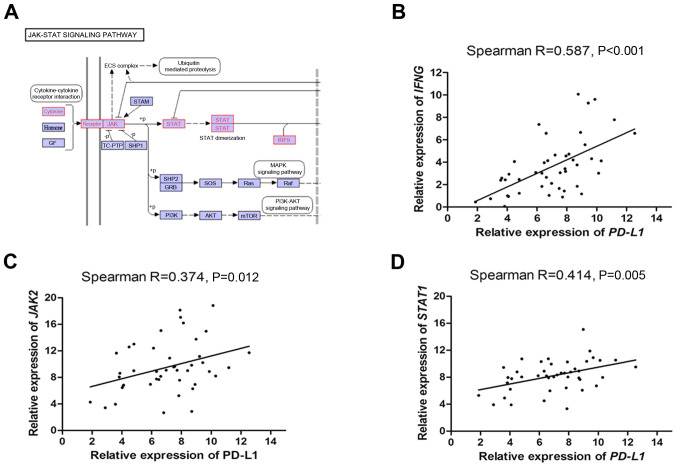
Based on the dataset from TCGA, the genes that were co-expressed with PD-L1 were sorted using Spearman's rank correlation analysis (Table SII). Based on r >0.6, 164 genes were selected for pathway enrichment analysis using the KEGG pathway database. The first 13 pathways sorted by the number of hits from the 164 aforementioned genes are presented in Table SIII. Following pathway enrichment analysis, 9 of the 164 genes were discovered to be enriched in the JAK/STAT signaling pathway (Fig. 2A and Table III).
Figure 2.
PD-L1 mRNA expression levels are correlated the expression levels of IFNG, JAK2 and STAT1. (A) Kyoto Encyclopedia of Genes and Genomes analysis revealed nine genes that were enriched in the JAK/STAT signaling pathway. Spearman's rank correlation analysis between the mRNA expression levels of (B) PD-L1 and IFNG, (C) PD-L1 and JAK2 and (D) PD-L1 and STAT1. PD-L1, programmed death ligand 1; JAK, janus kinase; GF, galphaf; STAM, signal transducing adaptor molecule; TC-PTP, protein tyrosine phosphatase non-receptor; SHP1, the protein-tyrosine phosphatase Shp1; SHP2, the protein-tyrosine phosphatase SHP2; GRB, growth factor receptor; IRF9, interferon regulatory factor 9; GRB, growth factor receptor bound protein; SOS, SOS Ras/Rac guanine nucleotide exchange factor.
Table III.
Gene enrichment in the JAK/STAT signaling pathway.
| Correlated gene | Cytoband | Spearman's r-value | P-value |
|---|---|---|---|
| STAT1 | 2q32.2 | 0.7929 | 5.00×10−54 |
| Interferon γ | 12q15 | 0.7021 | 1.50×10−37 |
| IL2RA | 22q12.3 | 0.6837 | 5.71×10−35 |
| IL2RB | 22q12.3 | 0.6837 | 5.71×10−35 |
| STAT2 | 12q13.3 | 0.6530 | 4.84×10−31 |
| JAK2 | 9p24.1 | 0.6516 | 7.08×10−31 |
| STAT4 | 2q32.2-q32.3 | 0.6445 | 4.94×10−30 |
| IL10RA | 11q23.3 | 0.6060 | 7.56×10−26 |
| IRF9 | 14q12 | 0.6039 | 1.22×10−25 |
STAT1, signal transducer and activator of transcription 1; IL2RA, interleukin 2 receptor subunit α; IL2RB, interleukin 2 receptor subunit β; STAT2, signal transducer and activator of transcription 2; JAK2, janus kinase 2; STAT4, signal transducer and activator of transcription 4; IL10RA, interleukin 10 receptor subunit α; IRF9, interferon regulatory factor 9.
Tumors from 45 randomly selected patients with colorectal adenocarcinoma (among the 181 recruited patients) were investigated to confirm the correlation between PD-L1 and IFNG, JAK2 and STAT1 mRNA expression levels. The results revealed that PD-L1 expression levels were significantly positively correlated with the expression levels of IFNG, JAK2 and STAT1 (Fig. 2B-D).
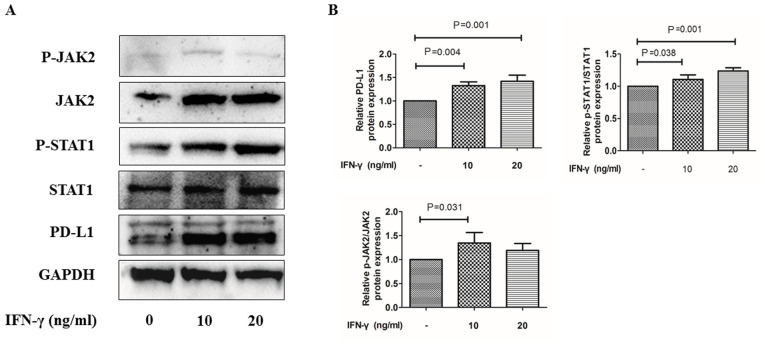
PD-L1 expression levels are increased by treatment with IFN-γ via the JAK2/STAT1 signaling pathway using the HCT 116 cell line
Following treatment with 10 or 20 ng/ml IFN-γ for 24 h, HCT 116 cells exhibited significantly increased expression levels of PD-L1 expression compared with the untreated group (Fig. 3A and B). Consistent with these results, the expression levels of JAK2 and p-STAT1 were also significantly increased in the IFN-γ treatment groups compared with the untreated group (Fig. 3A and B). The p-JAK2/JAK2 and p-STAT1/STAT1 ratios were also increased following the treatment with IFN-γ compared with the untreated group. These findings indicated that the JAK2/STAT1 signaling pathway may influence IFN-γ-mediated upregulation of PD-L1 (Fig. 3).
Figure 3.
Treatment with IFN-γ induces a significant increase in PD-L1 protein expression levels in colorectal cancer cells. HCT 116 cells were treated with 10 or 20 ng/ml IFN-γ for 24 h. (A) Western blotting was used to determine the relative protein expression levels of PD-L1, JAK2, p-JAK2, STAT1 and p-STAT1. (B) Relative protein expression of PD-L1, p-JAK2/JAK2, p-STAT1/STAT1 following IFN-γ treatment. IFN, interferon; PD-L1, programmed death ligand 1; JAK, janus kinase; p-, phosphorylated.
Discussion
In the present study, increased expression levels of PD-L1 were discovered to be associated with a poor prognosis in patients with CRC. This result was consistent with the studies of Zhu et al (24) and Enkhbat et al (25), but paradoxical to the findings reported by Liu et al (26). However, in the latter study, the subjects were patients with metastatic CRC, whereas in the present study, patients with distant metastases were excluded. PD-L1 expression was also observed in a large proportion of patients with tumors of >5 cm, the presence of positive vascular or lymphatic infiltration and poorly differentiated tumors. These three clinicopathological variables were all associated with a poor prognosis in patients with CRC, which suggests that a poor patient prognosis may be associated with the expression levels of PD-L1.
Anti-PD-L1 therapy works by blocking the binding of PD-1 to PD-L1 to inhibit negative signaling transmission, eliminating the T cell immune inhibitory effects and activating the immune microenvironment, and thus, antitumor cells (27). Immunotherapy targeting the PD-1/PD-L1 checkpoint pathway is effective in a variety of tumors, such as non-small cell lung cancer, Hodgkin's lymphoma and malignant melanoma; in fact, the FDA has reported an objective remission rate of >65% following treatment with approved PD-L1 inhibitors in advanced or metastatic hepatocellular carcinoma (28). Notably, CRC treatment with PD-L1 inhibitors was reported to be ineffective (29). Furthermore, a proportion of patients with CRC are PD-L1-negative and even those with PD-L1-positive CRC do not always respond to checkpoint inhibitors (30). Therefore, continued investigations into the molecular mechanisms underlying the action of PD-L1 in CRC are essential.
The JAK/STAT signaling pathway regulates the upregulation of PD-L1 in pancreatic, gastric, and head and neck cancers (13,16,31). However, the role of the JAK/STAT signaling pathway in CRC remains unclear. In the present study, PD-L1 expression in a cohort of 181 patients was discovered to be significantly positively correlated with the expression levels of IFNG, JAK2 and STAT1, which was consistent with the data extracted from TCGA database. Additionally, IFN-γ was demonstrated to increase the expression levels of PD-L1 at the protein level, which was suggested to be mediated via the increased expression levels of p-JAK2 and p-STAT1. The current findings indicate that the activation of the JAK2/STAT1 signaling pathway may regulate PD-L1 expression in CRC.
Previous studies have reported that PD-L1 expression on tumor-infiltrating immune cells is correlated with the survival of patients with CRC (32–34). Moreover, the tumor microenvironment in CRC becomes infiltrated with T lymphocytes, which have the potential to activate the JAK/STAT signaling pathway following IFN-γ stimulation (35). Thus, it was hypothesized that in CRC, IFN-γ released from tumor-infiltrating T lymphocytes may activate the JAK2/STAT1 signaling pathway and promote the expression of PD-L1. Other studies have indicated that dysregulated JAK/STAT signaling represents a promising therapeutic target for modulating immune responses (36). Furthermore, JAK1/JAK2 mutations were revealed to block PD-L1 induction, protecting cancer cells from immune attack (37). In concordance with the findings of the present study, these findings suggested that blocking the JAK/STAT signaling pathway may affect the efficacy of PD-L1 inhibitors in tumor immunotherapy, though further research is required to confirm this hypothesis.
There are several limitations to the current study. Firstly, previous studies have reported that PD-L1 expression in tumor-infiltrating immune lymphocytes has a prognostic value in CRC (32,38). However, it was difficult to determine PD-L1 expression levels in the tumor-infiltrating immune cells using the tissue microarray slides in the present IHC staining experiments. Furthermore, comparing the current IHC data with paired, normal healthy tissues for PD-L1 staining is required to validate our findings in further studies. Secondly, previous studies have suggested that PD-L1 inhibitors demonstrate good efficacy in patients with microsatellite instability (MSI) and cancers of the digestive system (39,40); however, information on the patient MSI was not collected in the present study and a relative analysis could therefore not be conducted. Thirdly, experiments using pharmacological inhibitors of JAK/STAT in the presence of IFN-γ are required to be performed in future research to validate the IFN-γ/JAK/STAT/PD-L1 hypothesis. Finally, according to the GSEA, STAT2 and STAT4 are also enriched in the PD-L1-related pathway; however, whether they regulate PD-L1 expression in CRC requires further clarification.
In conclusion, the findings of the present study suggest that increased expression levels of PD-L1 may be associated with a poor prognosis in patients with CRC. Furthermore, the IFN-γ-mediated upregulation of PD-L1 expression may be regulated via the JAK2/STAT1 signaling pathway in CRC cells.
Supplementary Material
Acknowledgements
Not applicable.
Glossary
Abbreviations
- PD-L1
programmed death ligand 1
- CRC
colorectal cancer
- GSEA
gene set enrichment analysis
- TCGA
The Cancer Genome Atlas
- KEGG
Kyoto Encyclopedia of Genes and Genomes
Funding
The present study was supported by the Jilin Province Department of Finance (grant nos. 2018sc2006 and sczsyz01506), the Health Commission of Jilin Province (grant no. 2018Q021) and The Education Department of Jilin Province (grant no. JJKH20190077KJ).
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
BZ conceived and designed the study and guaranteed its integrity; TCZ contributed to the design of the experiment, performed the literature research and conducted the statistical analysis; and YZL and JYZ performed the clinical and experimental studies. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by the Institutional Review Board of China-Japan Union Hospital of Jilin University (approval number, 2018-NFSC-046, Changchun, China) and written informed consent provided by from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Maida M, Macaluso FS, Ianiro G, Mangiola F, Sinagra E, Hold G, Maida C, Cammarota G, Gasbarrini A, Scarpulla G. Screening of colorectal cancer: Present and future. Expert Rev Anticancer Ther. 2017;17:1131–1146. doi: 10.1080/14737140.2017.1392243. [DOI] [PubMed] [Google Scholar]
- 3.Binefa G, Rodriguez-Moranta F, Teule A, Medina-Hayas M. Colorectal cancer: From prevention to personalized medicine. World J Gastroenterol. 2014;20:6786–6808. doi: 10.3748/wjg.v20.i22.6786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tapia Rico G, Townsend AR, Broadbridge V, Price TJ. Targeted therapies in elderly patients with metastatic colorectal cancer: A review of the evidence. Drugs Aging. 2017;34:173–189. doi: 10.1007/s40266-017-0439-9. [DOI] [PubMed] [Google Scholar]
- 5.Passardi A, Canale M, Valgiusti M, Ulivi P. Immune checkpoints as a target for colorectal cancer treatment. Int J Mol Sci. 2017;18:E1324. doi: 10.3390/ijms18061324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci USA. 2002;99:12293–12297. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu Y, Cao D, Qu L, Cao X, Jia Z, Zhao T, Wang Q, Jiang J. PD-1 and PD-L1 co-expression predicts favorable prognosis in gastric cancer. Oncotarget. 2017;8:64066–64082. doi: 10.18632/oncotarget.19318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song P, Cui X, Bai L, Zhou X, Zhu X, Zhang J, Jin F, Zhao J, Zhou C, Zhou Y, et al. Molecular characterization of clinical responses to PD-1/PD-L1 inhibitors in non-small cell lung cancer: Predictive value of multidimensional immunomarker detection for the efficacy of PD-1 inhibitors in Chinese patients. Thorac Cancer. 2019;10:1303–1309. doi: 10.1111/1759-7714.13078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li S, Chen L, Jiang J. Role of programmed cell death ligand-1 expression on prognostic and overall survival of breast cancer: A systematic review and meta-analysis. Medicine (Baltimore) 2019;98:e15201. doi: 10.1097/MD.0000000000015201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Y, He M, Zhou Y, Yang C, Wei S, Bian X, Christopher O, Xie L. The prognostic and clinicopathological roles of PD-L1 expression in colorectal cancer: A systematic review and meta-analysis. Front Pharmacol. 2019;10:139. doi: 10.3389/fphar.2019.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yaghoubi N, Soltani A, Ghazvini K, Hassanian SM, Hashemy SI. PD-1/PD-L1 blockade as a novel treatment for colorectal cancer. Biomed Pharmacother. 2019;110:312–318. doi: 10.1016/j.biopha.2018.11.105. [DOI] [PubMed] [Google Scholar]
- 12.Ng HY, Li J, Tao L, Lam AK, Chan KW, Ko JMY, Yu VZ, Wong M, Li B, Lung ML. Chemotherapeutic treatments increase PD-L1 expression in esophageal squamous cell carcinoma through EGFR/ERK activation. Transl Oncol. 2018;11:1323–1333. doi: 10.1016/j.tranon.2018.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mimura K, Teh JL, Okayama H, Shiraishi K, Kua LF, Koh V, Smoot DT, Ashktorab H, Oike T, Suzuki Y, et al. PD-L1 expression is mainly regulated by interferon gamma associated with JAK-STAT pathway in gastric cancer. Cancer Sci. 2018;109:43–53. doi: 10.1111/cas.13424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qin X, Liu C, Zhou Y, Wang G. Cisplatin induces programmed death-1-ligand 1(PD-L1) over-expression in hepatoma H22 cells via Erk/MAPK signaling pathway. Cell Mol Biol (Noisy-le-grand) 2010;11:OL1366–OL1372. [PubMed] [Google Scholar]
- 15.Bellucci R, Martin A, Bommarito D, Wang K, Hansen SH, Freeman GJ, Ritz J. Interferon-γ-induced activation of JAK1 and JAK2 suppresses tumor cell susceptibility to NK cells through upregulation of PD-L1 expression. Oncoimmunology. 2015;4:e1008824. doi: 10.1080/2162402X.2015.1008824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Imai D, Yoshizumi T, Okano S, Itoh S, Ikegami T, Harada N, Aishima S, Oda Y, Maehara Y. IFN-γ promotes epithelial-mesenchymal transition and the expression of PD-L1 in pancreatic cancer. J Surg Res. 2019;240:115–123. doi: 10.1016/j.jss.2019.02.038. [DOI] [PubMed] [Google Scholar]
- 17.Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA, WHO Classification of Tumours Editorial Board The 2019 WHO classification of tumours of the digestive system. Histopathology. 2019;76:182–188. doi: 10.1111/his.13975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cuccurullo V, Mansi L. AJCC Cancer staging Handbook: From the AJCC cancer staging manual (7th edition) Eur J Nucl Med Mol Imaging. 2011;38:408–408. doi: 10.1007/s00259-010-1693-9. [DOI] [Google Scholar]
- 19.Cancer Genome Atlas Network: Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330–337. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanehisa M, Furumichi M, Tanabe M, Sato Y, Morishima K. KEGG: New perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017;45:D353–D361. doi: 10.1093/nar/gkw1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 24.Zhu H, Qin H, Huang Z, Li S, Zhu X, He J, Yang J, Yu X, Yi X. Clinical significance of programmed death ligand-1 (PD-L1) in colorectal serrated adenocarcinoma. Int J Clin Exp Pathol. 2015;8:9351–9359. [PMC free article] [PubMed] [Google Scholar]
- 25.Enkhbat T, Nishi M, Takasu C, Yoshikawa K, Jun H, Tokunaga T, Kashihara H, Ishikawa D, Shimada M. Programmed cell death ligand 1 expression is an independent prognostic factor in colorectal cancer. Anticancer Res. 2018;38:3367–3373. doi: 10.21873/anticanres.12603. [DOI] [PubMed] [Google Scholar]
- 26.Liu R, Peng K, Yu Y, Liang L, Xu X, Li W, Yu S, Liu T. Prognostic value of immunoscore and PD-L1 expression in metastatic colorectal cancer patients with different RAS status after palliative operation. Biomed Res Int. 2018;2018:5920608. doi: 10.1155/2018/5920608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, Sharpe AH. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206:3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carretero-Gonzalez A, Lora D, Ghanem I, Zugazagoitia J, Castellano D, Sepúlveda JM, López-Martin JA, Paz-Ares L, de Velasco G. Analysis of response rate with ANTI PD1/PD-L1 monoclonal antibodies in advanced solid tumors: A meta-analysis of randomized clinical trials. Oncotarget. 2018;9:8706–8715. doi: 10.18632/oncotarget.24283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Asaoka Y, Ijichi H, Koike K. PD-1 Blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;373:1979. doi: 10.1056/NEJMc1510353. [DOI] [PubMed] [Google Scholar]
- 30.O'Neil BH, Wallmark J, Lorente D, Elez E, Raimbourg J, Gomez-Roca C, Ejadi S, Piha-Paul SA, Moss RA, Siu LL, et al. Pembrolizumab (MK-3475) for patients (pts) with advanced colorectal carcinoma (CRC): Preliminary results from KEYNOTE-028. Eur J Cancer. 2015;51:433. doi: 10.1016/S0959-8049(16)30304-5. [DOI] [Google Scholar]
- 31.Concha-Benavente F, Srivastava RM, Trivedi S, Lei Y, Chandran U, Seethala RR, Freeman GJ, Ferris RL. Identification of the cell-intrinsic and -extrinsic pathways downstream of EGFR and IFNγ that induce PD-L1 expression in head and neck cancer. Cancer Res. 2016;76:1031–1043. doi: 10.1158/0008-5472.CAN-15-2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Y, Liang L, Dai W, Cai G, Xu Y, Li X, Li Q, Cai S. Prognostic impact of programed cell death-1 (PD-1) and PD-ligand 1 (PD-L1) expression in cancer cells and tumor infiltrating lymphocytes in colorectal cancer. Mol Cancer. 2016;15:55. doi: 10.1186/s12943-016-0539-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kong P, Wang J, Song Z, Liu S, He W, Jiang C, Xie Q, Yang L, Xia X, Xia L. Circulating lymphocytes, PD-L1 expression on tumor-infiltrating lymphocytes, and survival of colorectal cancer patients with different mismatch repair gene status. J Cancer. 2019;10:1745–1754. doi: 10.7150/jca.25187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Calik I, Calik M, Turken G, Ozercan IH, Dagli AF, Artas G, Sarikaya B. Intratumoral cytotoxic T-lymphocyte density and PD-L1 expression are prognostic biomarkers for patients with colorectal cancer. Medicina (Kaunas) 2019;55:E723. doi: 10.3390/medicina55110723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sudoyo AW, Kurniawan AN, Kusumo GD, Putra TP, Rexana FA, Yunus M, Budiyati AD, Kurniawan D, Utama A, Utomo AR. Increased CD8 tumor infiltrating lymphocytes in colorectal cancer microenvironment supports an adaptive immune resistance mechanism of PD-L1 expression. Asian Pac J Cancer Prev. 2019;20:3421–3427. doi: 10.31557/APJCP.2019.20.11.3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meyer SC. Mechanisms of resistance to JAK2 inhibitors in myeloproliferative neoplasms. Hematol Oncol Clin North Am. 2017;31:627–642. doi: 10.1016/j.hoc.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 37.Gotthardt D, Putz EM, Grundschober E, Prchal-Murphy M, Straka E, Kudweis P, Heller G, Bago-Horvath Z, Witalisz-Siepracka A, Cumaraswamy AA, et al. STAT5 is a key regulator in NK cells and acts as a molecular switch from tumor surveillance to tumor promotion. Cancer Discov. 2016;6:414–429. doi: 10.1158/2159-8290.CD-15-0732. [DOI] [PubMed] [Google Scholar]
- 38.Valentini AM, Di Pinto F, Cariola F, Guerra V, Giannelli G, Caruso ML, Pirrelli M. PD-L1 expression in colorectal cancer defines three subsets of tumor immune microenvironments. Oncotarget. 2018;9:8584–8596. doi: 10.18632/oncotarget.24196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oliveira AF, Bretes L, Furtado I. Review of PD-1/PD-L1 inhibitors in metastatic dMMR/MSI-H colorectal cancer. Front Oncol. 2019;9:396. doi: 10.3389/fonc.2019.00396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Angell HK, Lee J, Kim KM, Kim K, Kim ST, Park SH, Kang WK, Sharpe A, Ogden J, Davenport A, et al. PD-L1 and immune infiltrates are differentially expressed in distinct subgroups of gastric cancer. Oncoimmunology. 2019;8:e1544442. doi: 10.1080/2162402X.2018.1544442. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.