Abstract
Although the prognostic value of the neutrophil/lymphocyte ratio (NLR), platelet/lymphocyte ratio (PLR) and lymphocyte/white blood cell ratio (LWR) has been described in advanced non-small cell lung cancer (NSCLC), the association between complete blood cell parameters prior to disease treatment and NSCLC have yet to be identified. The aim of the present study was to assess the complete blood cell parameters prior to disease treatment in patients with advanced NSCLC. A total of 268 patients with advanced NSCLC were enrolled in this study. Clinical and laboratory data of the patients were acquired through medical records. Receiver operating characteristic curve analysis was used to determine the optimal cut-off values of the neutrophil/white blood cell ratio (NWR), NLR, platelet/white blood cell ratio (PWR), PLR, monocyte/white blood cell ratio (MWR), monocyte/lymphocyte ratio (MLR) and LWR. Kaplan-Meier univariate and multivariate Cox regression analyses were used to evaluate the effect of complete blood parameters on progression-free survival (PFS) and overall survival (OS). The optimal cut-off values were identified as 0.67 for NWR, 2.85 for NLR, 37.23 for PWR, 166.56 for PLR, 0.074 for MWR, 0.31 for MLR and 0.24 for LWR. Univariate analysis revealed that sex (P=0.038), histological type (P<0.0001), NWR (P=0.026), NLR (P=0.044) and MLR (P=0.012) were all associated with PFS, whereas histological type (P=0.003), NWR (P=0.003), NLR (P=0.015), MLR (P=0.006) and LWR (P=0.043) were significantly associated with OS in patients with advanced NSCLC. Histological type (P=0.002) was an independent prognostic factor for PFS in patients with advanced NSCLC. Whereas histological type (P=0.005), NWR (P=0.005), NLR (P=0.014), MLR (P=0.006), and LWR (P=0.034) were independent prognostic factors for OS. Taken together, the present study identified high NWR, NLR and MLR, and low LWR as independent prognostic factors for poor OS in patients with NSCLC.
Keywords: complete blood cell parameters, prognostic factors, non-small cell lung cancer, progression-free survival, overall survival
Introduction
Lung cancer is a major cause of cancer-associated mortality worldwide, with non-small cell lung cancer (NSCLC) accounting for ~85% of all cases (1). Despite the improvements that have been made in the early detection of NSCLC, the majority of patients are initially diagnosed at an advanced stage, and the median survival rate is <13 months (2,3). Therefore, valuable prognostic factors are urgently required for the diagnosis of patients with NSCLC. The aim of the present study was to investigate the clinical significance of complete blood cell parameter values prior to disease treatment, and their association with the progression-free survival (PFS) and overall survival (OS) of Chinese patients with advanced NSCLC.
The hypothesis proposed by Rudolf Virchow in 1863 on cancer and inflammation is now widely accepted (4). An emerging body of evidence has confirmed that inflammation of the microenvironment serves a pivotal role in the development and progression of malignancies by inhibiting apoptosis and promoting angiogenesis (5). Numerous studies have reported on the association between the inflammatory index and prognosis of patients with NSCLC, including the complete blood count parameters, neutrophil/lymphocyte ratio (NLR), platelet/lymphocyte ratio (PLR) and lymphocyte/white blood cell ratio (LWR) (6–8). Yuan et al (9) reported on the association between the complete blood cell parameter values prior to disease treatment and prognosis in patients with curatively resected NSCLC, and suggested that elevated neutrophil/white blood cell ratio (NWR) and monocyte/lymphocyte ratio (MLR) may be independent prognostic factors in curatively resected NSCLC. Feng et al (10) examined the association between various blood test parameters and prognosis in patients with gastric cancer, and revealed that high MLR, NLR, PLR, NWR and monocyte/white blood cell ratio (MWR), and low LWR, were associated with poor prognosis in patients with gastric cancer.
To the best of our knowledge, the prognostic value of complete blood cell parameters in advanced NSCLC has not yet been investigated. Therefore, the present study aimed to investigate the prognostic value of various blood test parameters in patients with advanced NSCLC. Since the main pathological types of NSCLC are adenocarcinoma and squamous cell carcinoma, these two types were primarily investigated in this study.
Materials and methods
Patients
A total of 268 patients diagnosed with unresectable NSCLC at The Affiliated Hospital of Qingdao University (Qingdao, China) between January 2009 and December 2015 were retrospectively analyzed. Clinicopathological information and laboratory parameters of the patients were obtained from electronic records, including sex, age, smoking history, tumor location, histological type, Eastern Cooperative Oncology Group (ECOG) performance status (11), tumor-node-metastasis (TNM) staging and blood results (12). Laboratory blood tests from patients were obtained within 7 days prior to treatment. The blood sample results were obtained via the electronic medical system and patient consent was provided by the participants or their families via telephone. ‘Pre-treatment’ is used to represent the blood parameter results that were collected before treatment. The major inclusion criterion was pathological confirmation of NSCLC at an advanced stage (stage IIIB-IV). Patients with infection, inflammation-associated disease, other malignant tumors, insufficient blood test data or that were lost to follow-up were excluded. The present study was approved by the Ethics Committee of The Affiliated Hospital of Qingdao University (approval no. QYFYW2LL 25620). All patients were restaged according to the 7th International Classification System for Lung Cancer (12). The last follow-up visit occurred in November 2018.
The following parameters were assessed: NWR, NLR, platelet/white blood cell ratio (PWR), platelet/lymphocyte ratio (PLR), MWR, monocyte/lymphocyte ratio (MLR) and LWR. MWR is calculated by dividing the monocyte count by the white blood cell count. NLR is calculated by dividing the neutrophil count by the lymphocyte count. PWR is calculated by dividing the platelet count by the white blood count. PLR is calculated by dividing the platelet count by the lymphocyte count. MWR is calculated by dividing the monocyte count by the white blood cell count. MLR is calculated by dividing the monocyte count by the lymphocyte count. LWR is calculated by dividing the lymphocyte count by the white blood cell count.
Statistical analysis
Receiver operating characteristic (ROC) curves were used to assess the optimal cut-off values. Kaplan-Meier survival curves were generated to assess PFS and OS, and differences among the curves were determined using the log-rank test. Variables that were identified to be statistically significant at the level of univariate analysis were then submitted to the Cox proportional hazards regression model for multivariate analysis. Meaningless variables were also further analyzed to determine the values of all indicators following multivariate analysis. Categorical variables were compared using the χ2 test or Fisher's exact tests. OS was defined as the period from the date of the first diagnosis to the date of mortality or the last follow-up. PFS was calculated from the date of the first diagnosis to the date of disease progression, or the last follow-up if the disease had not progressed. Continuous variable is presented as the average value (minimum to maximum). SPSS version 20.0 (IBM Corp.) was used to perform the statistical analysis. P<0.05 was considered to indicate a statistically significant value.
Results
Optimal cut-off values for the blood test parameters
ROC curve analysis was used to determine the most appropriate cut-off values for the complete blood cell parameters. According to the ROC curve analysis, the cut-off point for NWR was 0.67. Therefore, 0.67 was selected as the cut-off value for NWR. Similarly, the optimal points based on the ROC curves revealed cut-off values of 2.85 for NLR, 37.23 for PWR, 166.56 for PLR, 0.074 for MWR, 0.31 for MLR and 0.24 for LWR. Consequently, these parameters were categorized as optimal cut-off values.
Basic characteristics of patients
Clinical characteristics of all the patients are shown in Table I. The median (range) age of patients was 59.10 (33–79) years, and 128(47.8%) of them were <60 years old. The study was comprised of 161 (60.1%) male patients and 107 (39.9%) female patients. A total of 46 (17.2%) and 222 (82.8%) patients presented with TNM stages IIIB and IV, respectively. Out of the total patients 225 (84.0%) were diagnosed with adenocarcinoma and 43 (16.0%) were diagnosed with squamous cell carcinoma. Of the 268 patients, 133 (49.6%) had never smoked compared with 135 patients (50.4%) who were former or current smokers. Patients with performance status 0, 1 or 2 accounted for 10.8, 78.0 and 11.2% of the patients, respectively. With 112 (41.8%) of the patients, the tumor was located on the left, whereas with 156 (58.2%) of the patients, the tumor was located on the right.
Table I.
Characteristics of patients with advanced non-small cell lung cancer.
| Characteristics | Patients (%) |
|---|---|
| Total number | 268 |
| Age | |
| <60 years | 128 (47.8) |
| ≥60 years | 140 (52.2) |
| Sex | |
| Male | 161 (60.1) |
| Female | 107 (39.9) |
| Stage | |
| III | 46 (17.2) |
| IV | 222 (82.8) |
| Histological type | |
| Adenocarcinoma | 225 (84.0) |
| Sqcc | 43 (16.0) |
| Smoking history | |
| Never | 133 (49.6) |
| Current/Previous | 135 (50.4) |
| Performance status | |
| 0 | 29 (10.8) |
| 1 | 209 (78.0) |
| 2 | 30 (11.2) |
| Tumor location | |
| Left | 112 (41.8) |
| Right | 156 (58.2) |
Sqcc, squamous cell carcinoma.
Univariate and multivariate analysis for PFS and OS
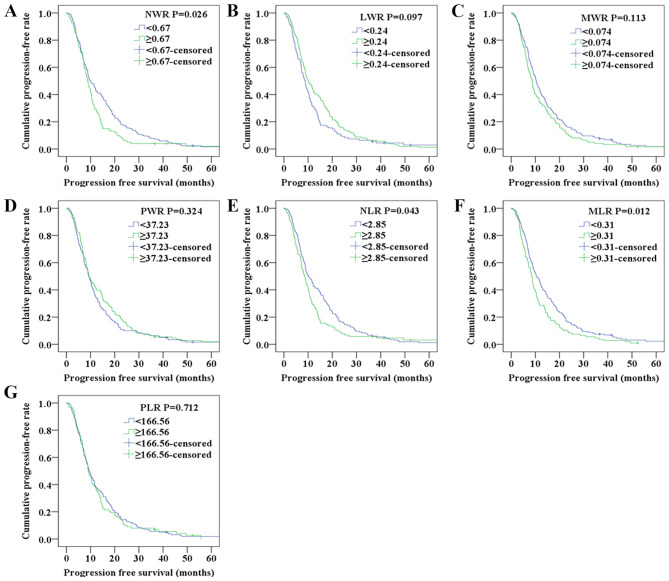
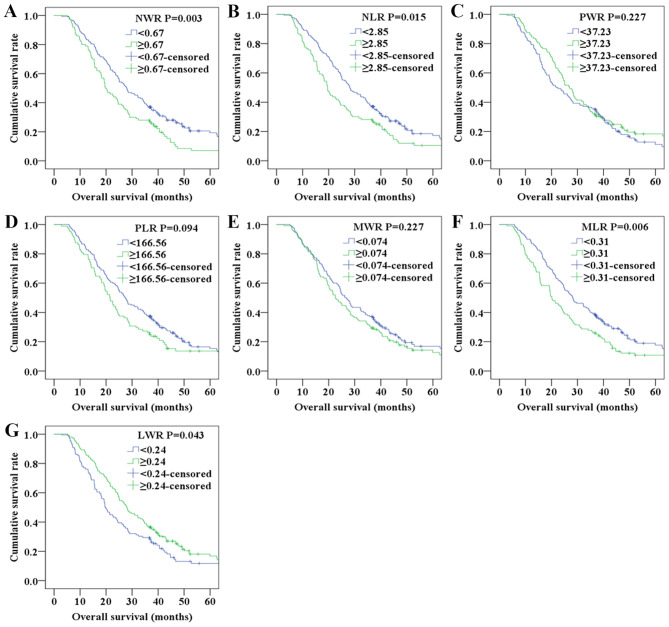
Kaplan-Meier analyses were performed to determine the differences in PFS and OS among the blood test parameters classified by the optimal cut-off values. The Kaplan-Meier survival curves shown in Fig. 1 indicated that the NWR and MLR values were associated with PFS, whereas the survival curves shown in Fig. 2 indicated that elevated NWR, NLR, MLR and decreased LWR were associated with poor OS.
Figure 1.
Progression-free survival of patients with non-small cell lung cancer according to (A) NWR, (B) LWR, (C) MWR, (D) PWR, (E) NLR, (F) MLR and (G) PLR.LWR, lymphocyte/white blood cell ratio; MLR, monocyte/lymphocyte ratio; MWR, monocyte/white blood cell ratio; NLR, neutrophil/lymphocyte ratio; NWR, neutrophil/white blood cell ratio; PLR, platelet/lymphocyte ratio; PWR, platelet/white blood cell ratio.
Figure 2.
Overall survival of patients with NSCLC according to (A) NWR, (B) NLR, (C) PWR, (D) PLR, (E) MWR, (F) MLR and (G) LWR.LWR, lymphocyte/white blood cell ratio; MLR, monocyte/lymphocyte ratio; MWR, monocyte/white blood cell ratio; NLR, neutrophil/lymphocyte ratio; NSCLC, non-small cell lung cancer; NWR, neutrophil/white blood cell ratio; PLR, platelet/lymphocyte ratio; PWR, platelet/white blood cell ratio.
As shown in Table II, sex (P=0.038), histological type (P<0.0001), NWR (P=0.026), NLR (P=0.044) and MLR (P=0.012) of the patients were significantly associated with PFS according to the univariate analysis. As presented in Table III, histological type (P=0.003), NWR (P=0.003), NLR (P=0.015), MLR (P=0.006) and LWR (P=0.043) were significantly associated with OS in the univariate analysis. To determine the independent predictors, further Cox multivariate analyses were performed. Multivariate analysis demonstrated that histological type [hazard ratio (HR)=0.577; 95% confidence interval (CI)=0.404–0.822; P=0.002] was an independent factor for PFS (Table II). Correspondingly, histological type (HR=0.582; 95% CI=0.401–0.846; P=0.005), NWR (HR=0.673; 95% CI=0.511–0.888; P=0.005), NLR (HR=0.703; 95% CI=0.530–0.931; P=0.014), MLR (HR=0.669; 95% CI=0.504–0.889; P=0.006) and LWR (HR=1.351; 95% CI=1.022–1.785; P=0.034) were independent prognostic factors for OS (Table III).
Table II.
Univariate and multivariate analyses of clinical characteristics for progression-free survival of patients with advanced non-small-cell lung cancer.
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
| Age (<60/≥60 years) | 1.181 | 0.925–1.507 | 0.182 | 1.224 | 0.955–1.569 | 0.111 |
| Sex (Male/female) | 0.766 | 0.595–0.985 | 0.038 | 0.786 | 0.566–1.092 | 0.152 |
| Stage (III/IV) | 0.866 | 0.628–1.194 | 0.380 | 0.854 | 0.615–1.186 | 0.346 |
| Histological type (Adeno/sqcc) | 0.540 | 0.387–0.753 | 0.000 | 0.577 | 0.404–0.822 | 0.002 |
| Smoking history (None/yes) | 0.815 | 0.639–1.041 | 0.101 | 0.999 | 0.715–1.395 | 0.995 |
| ECOG PS (0+1/2) | 0.856 | 0.581–1.262 | 0.433 | 0.874 | 0.588–1.298 | 0.504 |
| Tumor location (Left/right) | 0.977 | 0.765–1.249 | 0.855 | 1.000 | 0.780–1.280 | 0.997 |
| NWR (≥0.67/<0.67) | 0.750 | 0.582–0.967 | 0.026 | 0.793 | 0.609–1.032 | 0.085 |
| NLR (≥2.85/<2.85) | 0.772 | 0.601–0.993 | 0.044 | 0.835 | 0.643–1.084 | 0.175 |
| PWR (≥37.23/<37.23) | 1.129 | 0.886–1.439 | 0.325 | 1.124 | 0.867–1.456 | 0.378 |
| PLR (≥166.56/<166.56) | 0.953 | 0.736–1.234 | 0.713 | 1.060 | 0.806–1.394 | 0.676 |
| MWR (≥0.074/<0.074) | 0.822 | 0.645–1.048 | 0.114 | 0.901 | 0.698–1.162 | 0.422 |
| MLR (≥0.31/<0.31) | 0.728 | 0.568–0.933 | 0.012 | 0.798 | 0.611–1.042 | 0.098 |
| LWR (≥0.24/<0.24) | 1.233 | 0.962–1.579 | 0.098 | 1.135 | 0.876–1.472 | 0.338 |
Adeno, adenocarcinoma; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance score; HR, hazard ratio; LWR, lymphocyte/white blood cell ratio; MLR, monocyte/lymphocyte ratio; MWR, monocyte/white blood cell ratio; NLR, neutrophil/lymphocyte ratio; NWR, neutrophil/white blood cell ratio; PLR, platelet/lymphocyte ratio; PWR, platelet/white blood cell ratio; Sqcc, squamous cell carcinoma.
Table III.
Univariate and multivariate analyses of clinical characteristics for overall survival of patients with advanced non-small cell lung cancer.
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
| Age (<60/≥60 years) | 1.172 | 0.902–1.523 | 0.234 | 1.248 | 0.955–1.632 | 0.105 |
| Sex (Male/female) | 0.837 | 0.641–1.093 | 0.192 | 0.827 | 0.560–1.222 | 0.340 |
| Stage (III/IV) | 0.975 | 0.682–1.393 | 0.889 | 1.042 | 0.724–1.500 | 0.824 |
| Histological type (Adeno/sqcc) | 0.577 | 0.415–0.801 | 0.003 | 0.582 | 0.401–0.846 | 0.005 |
| Smoking history (None/yes) | 0.882 | 0.680–1.145 | 0.345 | 1.116 | 0.753–1.653 | 0.586 |
| ECOG PS (0+1/2) | 1.223 | 0.806–1.854 | 0.344 | 1.316 | 0.861–2.012 | 0.204 |
| Tumor location (Left/right) | 1.003 | 0.769–1.308 | 0.982 | 0.997 | 0.763–1.302 | 0.981 |
| NWR (≥0.67/<0.67) | 0.669 | 0.512–0.875 | 0.003 | 0.673 | 0.511–0.888 | 0.005 |
| NLR (≥2.85/<2.85) | 0.718 | 0.549–0.938 | 0.015 | 0.703 | 0.530–0.931 | 0.014 |
| PWR (≥37.23/<37.23) | 1.174 | 0.904–1.525 | 0.228 | 1.172 | 0.889–1.544 | 0.260 |
| PLR (≥166.56/<166.56) | 0.790 | 0.599–1.042 | 0.095 | 0.789 | 0.594–1.047 | 0.101 |
| MWR (≥0.074/<0.074) | 0.852 | 0.656–1.106 | 0.228 | 0.863 | 0.657–1.132 | 0.287 |
| MLR (≥0.31/<0.31) | 0.692 | 0.531–0.901 | 0.006 | 0.669 | 0.504–0.889 | 0.006 |
| LWR (≥0.24/<0.24) | 1.318 | 1.008–1.723 | 0.043 | 1.351 | 1.022–1.785 | 0.034 |
Adeno, adenocarcinoma; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance score; HR, hazard ratio; LWR, lymphocyte/white blood cell ratio; MLR, monocyte/lymphocyte ratio; MWR, monocyte/white blood cell ratio; NLR, neutrophil/lymphocyte ratio; NWR, neutrophil/white blood cell ratio; PLR, platelet/lymphocyte ratio; PWR, platelet/white blood cell ratio; Sqcc, squamous cell carcinoma.
PFS and OS according to histological type
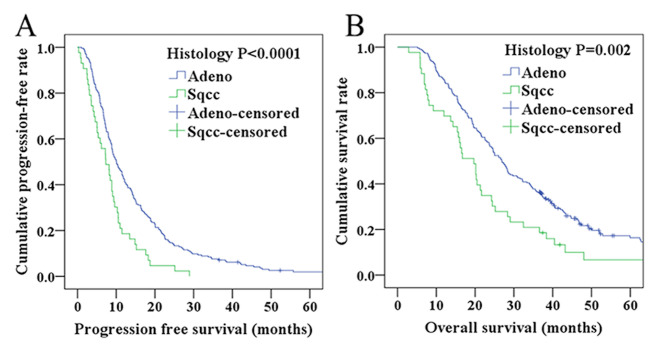
As shown in Tables II and III, multivariate analysis revealed that histological type was significantly associated with PFS and OS (HR=0.577; 95% CI=0.404–0.822; P=0.002 for PFS, and HR=0.582; 95% CI=0.401–0.846, and P=0.005 for OS). To analyze these results further, graphical representations of the PFS and OS of different pathological types were prepared according to histology. In the squamous cell carcinoma group, the 1-, 2-and 3-year PFS rates were 18.6, 4.7 and 0.0% respectively, whereas in the adenocarcinoma group, the PFS rates were 42.7, 14.7 and 7.4% (Fig. 3A). Correspondingly, the 1-, 2- and 3-year OS rates were 69.8, 34.9 and 20.9% in the squamous cell carcinoma group, and 85.8, 56.4 and 36.4% in the adenocarcinoma group (Fig. 3B). Taken together, these results demonstrated that the PFS and OS rates in the adenocarcinoma group were longer compared with patients in the squamous cell carcinoma group.
Figure 3.
Survival curves of patients according to different histological types. (A) Progression-free survival curve of patients with Adeno and Sqcc (P<0.0001). (B) Overall survival curve of patients with Adeno and Sqcc (P=0.002). Adeno, adenocarcinoma; Sqcc, squamous cell carcinoma.
Prognostic factors of patients with adenocarcinoma according to NWR, NLR, MLR and LWR
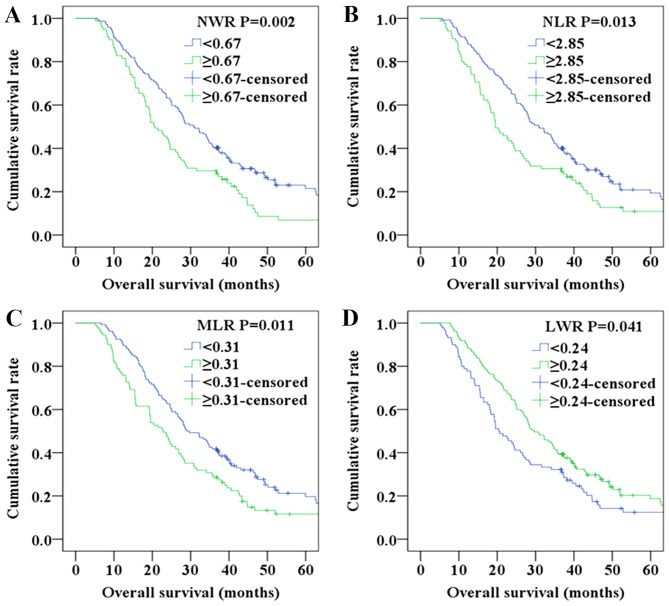
Further analyses were performed in subgroups (adenocarcinoma and squamous cell carcinoma). Since the number of patients with squamous cell carcinoma was relatively small, only adenocarcinoma was analyzed. Patients with NWR<0.67, NLR<2.85, MLR<0.31 and LWR≥0.24,were found to exhibit a higher OS compared with those with NWR≥0.67, NLR≥2.85, MLR≥0.31 and LWR<0.24 in the adenocarcinoma subgroup (P=0.002 for NWR, Fig. 4A; P=0.013 for NLR, Fig. 4B; P=0.011, Fig. 4C; P=0.041, Fig. 4D).
Figure 4.
Survival curves of patients with adenocarcinoma. (A) OS curve of patients with NWR<0.67 and NWR≥0.67 (P=0.002). (B) OS curve of patients with NLR<2.85 and NLR≥2.85 (P=0.013). (C) OS curve of patients with MLR<0.31 and MLR≥0.31 (P=0.011). (D) OS curve of patients with LWR<0.24 and LWR≥0.24 (P=0.041). LWR, lymphocyte/white blood cell ratio; MLR, monocyte/lymphocyte ratio; NLR, neutrophil/lymphocyte ratio; NWR, neutrophil/white blood cell ratio; OS, overall survival.
Association between blood test parameters and clinicopathological variables
The associations between NWR, NLR, MLR, LWR and clinical factors of the patients with NSCLC are shown in Tables IV and V. A total of 168 (62.7%) patients were in the NWR<0.67 group and 100 (37.3%) patients were in the NWR≥0.67 group, whereas 165 (61.6%) patients were in the NLR<2.85 group and 103 (38.4%) patients were in the NLR≥2.85 group. In addition, 160 (59.7%) patients were in the MLR<0.31 group and 108 (40.3%) patients were in the MLR≥0.31 group, and 109 (40.7%) patients were in the LWR<0.24 group, whereas 159 (59.3%) patients were in the LWR≥0.24 group. The present study revealed that NLR and MLR were markedly associated with sex, whereas LWR was closely associated with sex and ECOG performance status.
Table IV.
Association between NWR, NLR and clinical parameters of patients with non-small cell lung cancer.
| NWR | NLR | |||||
|---|---|---|---|---|---|---|
| Variables | <0.67 | ≥0.67 | P-value | <2.85 | ≥2.85 | P-value |
| Age | ||||||
| <60 years | 77 | 51 | 75 | 53 | ||
| ≥60 years | 91 | 49 | 0.413 | 90 | 50 | 0.330 |
| Sex | ||||||
| Male | 95 | 66 | 91 | 70 | ||
| Female | 73 | 34 | 0.156 | 74 | 33 | 0.041 |
| Stage | ||||||
| III | 26 | 20 | 28 | 18 | ||
| IV | 142 | 80 | 0.403 | 137 | 85 | 0.915 |
| Histological type | ||||||
| Adeno | 144 | 81 | 140 | 85 | ||
| Sqcc | 24 | 19 | 0.308 | 25 | 18 | 0.612 |
| Smoking history | ||||||
| None | 86 | 47 | 85 | 48 | ||
| Yes | 82 | 53 | 0.507 | 80 | 55 | 0.434 |
| ECOG PS | ||||||
| 0 | 153 | 85 | 151 | 87 | ||
| Others | 15 | 15 | 0.161 | 14 | 16 | 0.110 |
| Tumor location | ||||||
| Left | 70 | 42 | 67 | 45 | ||
| Right | 98 | 58 | 0.957 | 98 | 58 | 0.619 |
Adeno, adenocarcinoma; ECOG PS, Eastern Cooperative Oncology Group performance score; NLR, neutrophil/lymphocyte ratio; NWR, neutrophil/white blood cell ratio; Sqcc, squamous cell carcinoma.
Table V.
Association between MLR, LWR and clinical parameters of patients with non-small cell lung cancer.
| MLR | LWR | |||||
|---|---|---|---|---|---|---|
| Variables | <0.31 | ≥0.31 | P-value | <0.24 | ≥0.24 | P-value |
| Age | ||||||
| <60 years | 72 | 56 | 54 | 74 | ||
| ≥60 years | 88 | 52 | 0.271 | 55 | 85 | 0.629 |
| Sex | ||||||
| Male | 86 | 75 | 75 | 86 | ||
| Female | 74 | 33 | 0.011 | 34 | 73 | 0.016 |
| Stage | ||||||
| III | 30 | 16 | 18 | 28 | ||
| IV | 130 | 92 | 0.509 | 91 | 131 | 0.870 |
| Histological type | ||||||
| Adeno | 134 | 91 | 90 | 135 | ||
| Sqcc | 26 | 17 | 1.000 | 19 | 24 | 0.615 |
| Smoking history | ||||||
| None | 83 | 50 | 49 | 84 | ||
| Yes | 77 | 58 | 0.370 | 60 | 75 | 0.205 |
| ECOG PS | ||||||
| 0 | 146 | 92 | 91 | 147 | ||
| Others | 14 | 16 | 0.166 | 18 | 12 | 0.022 |
| Tumor location | ||||||
| Left | 60 | 52 | 49 | 63 | ||
| Right | 100 | 56 | 0.083 | 60 | 96 | 0.385 |
Adeno, adenocarcinoma; ECOG PS, Eastern Cooperative Oncology Group performance score; LWR, lymphocyte/white blood cell ratio; MLR, monocyte/lymphocyte ratio; Sqcc, squamous cell carcinoma.
Discussion
Although all 268 patients with advanced NSCLC in this study undergo active anticancer treatment, including chemotherapy, radiotherapy, targeted therapy and immunotherapy, recurrence and metastasis are inevitable, thus leading to treatment failure. Of the 268 patients recruited in the present study, all developed recurrence and metastasis. The purpose of the present study was to reveal the association between blood test parameters prior to disease treatment and the prognosis of patients with advanced NSCLC. Previously, significant attention has been paid to the underlying mechanism that links malignancies with inflammation (4). NLR, PLR and LWR, as cancer-associated inflammatory variables, have been widely studied, and are regarded as important prognostic factors in multiple types of malignancy, including breast (13), lung (8,14), gastric (15,16) and colorectal cancer (17). Recently, an increasing number of studies has evaluated the association between complete blood parameters and patient prognosis. Moreover, high NWR and MLR have been identified as independent prognostic factors in curatively resected NSCLC (9,10,18,19). The present study is, to the best of our knowledge, the first attempt to address the issue of the prognostic significance of complete blood parameters in patients with advanced NSCLC.
Lymphocytes fulfill a crucial role in host immune response and possess potent anticancer activities that lead to inhibition of tumor cell proliferation and metastasis (20,21). A previous study demonstrated that a decrease in the level of lymphocytes was able to induce the release of several inhibitory immunological mediators, such as transforming growth factor-bandinterleukin-10 (22). It is now widely considered that increased lymphocyte levels are associated with improved clinical outcomes in various types of cancer (20,23). Consistent with these results, high NLR and MLR, and low LWR, were associated with poor prognosis in patients with advanced NSCLC in the present study.
It has previously been reported that high levels of monocytes are associated with poor prognosis of various tumor types, including rectal, breast and prostate cancer (24–26). Monocytes are an important component in the inflammatory microenvironment that stimulate tumor cell growth, promote angiogenesis and suppress the host anticancer immune response (27,28). Monocytes also influence the development of malignant cells by producing pro-inflammatory cytokines, including tumor necrosis factor, interleukin-1 and interleukin-6 (28). On the other hand, cytokines and chemokines produced by tumor cells may induce the differentiation of monocytes into tumor-associated macrophages (29). Tumor-associated macrophages are able to weaken the antitumor immune response, stimulate migration and promote metastasis of tumor cells (30). In the present study, elevated MLR was identified as an independent factor for poor prognosis in advanced NSCLC, a finding that was consistent with previous research.
Peripheral neutrophils are recognized as markers of acute and chronic inflammation (31). It has been reported that neutrophils are able to produce vascular endothelial growth factor and matrix metalloproteinase-9, which can promote tumor angiogenesis and progression (32,33). In addition, elevated neutrophil levels may inhibit the antitumor system by influencing the activity of natural killer cells, lymphocytes and activated T cells (34–37). The combination of neutrophilia and lymphocytopenia could be considered as a useful marker that reflects the balance between inflammation and immune reaction. A high NLR level has long been demonstrated to be associated with poor prognosis of a variety of tumor types, such as colorectal cancer and renal cell carcinoma (38). Meta-analysis studies have revealed that elevated NLR may be associated with poor prognosis in NSCLC (6,39). In the present study, it was also demonstrated that NLR was an independent prognostic factor in advanced NSCLC; however, the underlying mechanisms require further study.
Previous studies have suggested that thrombocytosis may be linked to poor clinical outcomes in various types of cancer, such as gastric cancer (40,41). Platelets are involved in the proliferation and adhesion of tumor cells by activating and secreting growth factors, thereby promoting the occurrence and invasion of tumors (42). In studies concerned with NSCLC, PLR was found to be an independent risk factor influencing the prognosis of patients (43). In the present study, PLR did not achieve statistical significance, and this may be attributed to a relatively small sample size, although the underlying reasons still need to be elucidated.
The current study demonstrated that sex, histological type, NWR, NLR and MLR were associated with PFS in patients with advanced NSCLC. However, only histological type was an independent prognostic factor for PFS. In addition, this study revealed that histological type, NWR, NLR, MLR and LWR were independent prognostic factors for OS in patients with advanced NSCLC. In addition, these four indicators (NWR, NLR, MLR and LWR) were associated with OS in patients with adenocarcinoma. It should be noted that it was not possible to analyze these in squamous cell carcinoma due to the insufficient number of patients in this study. Therefore, the association between these four indicators and squamous cell carcinoma requires further study in the future. It was also observed that the prognosis of adenocarcinoma was better compared with that of squamous cell carcinoma, with regards to PFS and OS.
However, there were certain limitations associated with the present study. Firstly, this study was performed in a single medical center and only 268 patients were included. Therefore, analyzing a large sample associated with a clinical multicenter is required to confirm the predictive value of the parameters measured in this study. Secondly, the cut-off values of the present and previous studies were different (9,14). Thus, a reasonable cut-off value should be identified to predict the outcomes of advanced NSCLC. Finally, the prognosis of advanced NSCLC is affected by a variety of factors, and the influence of those factors should be excluded as far as possible in subsequent studies.
In conclusion, in the present study, high NWR, NLR and MLR values, and a low LWR value, were associated with poor prognosis in patients with advanced NSCLC. Furthermore, these indicators were identified to be independent prognostic factors in advanced NSCLC.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Natural Science Foundation of Shandong Province (grant no. ZR2017MH062) and the Science and Technology for People's Livelihood Project of Qingdao (grant no. 17-3-3-33-nsh).
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author upon reasonable request.
Authors' contributions
LW, JW, LF and ZY acquired the data, performed the literature review and designed the present study. HS, WZ and SD analyzed the data. All authors were involved in writing the initial manuscript. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by the Ethics Committee of The Affiliated Hospital of Qingdao University (approval no. QYFYW2LL 25620). Consent to participate was provided from patients or their families via telephone.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Dela Cruz CS, Tanoue LT, Matthay RA. Lung cancer: Epidemiology, etiology, and prevention. Clin Chest Med. 2011;32:605–644. doi: 10.1016/j.ccm.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med. 2008;359:1367–1380. doi: 10.1056/NEJMra0802714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moro-Sibilot D, Smit E, de Castro Carpeño J, Lesniewski- Kmak K, Aerts J, Villatoro R, Kraaij K, Nacerddine K, Dyachkova Y, Smith KT, et al. Outcomes and resource use of non-small-cell lung cancer (NSCLC) patients treated with first-line platinum-based chemotherapy across Europe: FRAME prospective observational study. Lung Cancer. 2015;88:215–222. doi: 10.1016/j.lungcan.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 4.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McMillan DC. Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care. 2009;12:223–226. doi: 10.1097/MCO.0b013e32832a7902. [DOI] [PubMed] [Google Scholar]
- 6.Gu XB, Tian T, Tian XJ, Zhang XJ. Prognostic significance of neutrophil-to-lymphocyte ratio in non-small-cell lung cancer: A meta-analysis. Sci Rep. 2015;5:12493. doi: 10.1038/srep12493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang H, Gao L, Zhang B, Zhang L, Wang C. Prognostic value of platelet to lymphocyte ratio in non-small-cell lung cancer: A systematic review and meta-analysis. Sci Rep. 2016;6:22618. doi: 10.1038/srep22618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li W, Ma G, Wu Q, Deng Y, Liu Y, Wang J. Prognostic value of lymphocyte-to-monocyte ratio among Asian lung cancer patients: A systematic review and meta-analysis. Oncotarget. 2017;8:110606–110613. doi: 10.18632/oncotarget.20574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yuan C, Li N, Mao X, Liu Z, Ou W, Wang SY. Elevated pretreatment neutrophil/white blood cell ratio and monocyte/lymphocyte ratio predict poor survival in patients with curatively resected non-small-cell lung cancer: Results from a large cohort. Thorac Cancer. 2017;8:350–358. doi: 10.1111/1759-7714.12454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng F, Sun L, Zheng G, Liu S, Liu Z, Xu G, Guo M, Lian X, Fan D, Zhang H. Low lymphocyte-to-white blood cell ratio and high monocyte-to-white blood cell ratio predict poor prognosis in gastric cancer. Oncotarget. 2017;8:5281–5291. doi: 10.18632/oncotarget.14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. doi: 10.1097/00000421-198212000-00014. [DOI] [PubMed] [Google Scholar]
- 12.Groome PA, Bolejack V, Crowley JJ, Kennedy C, Krasnik M, Sobin LH, Goldstraw P, IASLC International Staging Committee; Cancer Research and Biostatistics; Observers to the Committee; Participating Institutions The IASLC Lung Cancer Staging Project: Validation of the proposals for revision of the T, N, and M descriptors and consequent stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol. 2007;2:694–705. doi: 10.1097/JTO.0b013e31812d05d5. [DOI] [PubMed] [Google Scholar]
- 13.Losada B, Guerra JA, Malón D, Jara C, Rodriguez L, Del Barco S. Pretreatment neutrophil/lymphocyte, platelet/lymphocyte, lymphocyte/monocyte, and neutrophil/monocyte ratios and outcome in elderly breast cancer patients. Clin Transl Oncol. 2019;21:855–863. doi: 10.1007/s12094-018-1999-9. [DOI] [PubMed] [Google Scholar]
- 14.Wang L, Liang D, Xu X, Jin J, Li S, Tian G, Gao Z, Liu C, He Y. The prognostic value of neutrophil to lymphocyte and platelet to lymphocyte ratios for patients with lung cancer. Oncol Lett. 2017;14:6449–6456. doi: 10.3892/ol.2017.6660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma JY, Liu Q. Clinicopathological and prognostic significance of lymphocyte to monocyte ratio in patients with gastric cancer: A meta-analysis. Int J Surg. 2018;50:67–71. doi: 10.1016/j.ijsu.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Lu JJ, Du YP, Feng CX, Wang LQ, Chen MB. Prognostic value of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in gastric cancer. Medicine (Baltimore) 2018;97:e0144. doi: 10.1097/MD.0000000000010144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ying HQ, Deng QW, He BS, Pan YQ, Wang F, Sun HL, Chen J, Liu X, Wang SK. The prognostic value of preoperative NLR, d-NLR, PLR and LMR for predicting clinical outcome in surgical colorectal cancer patients. Med Oncol. 2014;31:305. doi: 10.1007/s12032-014-0305-0. [DOI] [PubMed] [Google Scholar]
- 18.Cananzi FCM, Minerva EM, Samà L, Ruspi L, Sicoli F, Conti L, Fumagalli Romario U, Quagliuolo VL. Preoperative monocyte-to-lymphocyte ratio predicts recurrence in gastrointestinal stromal tumors. J Surg Oncol. 2019;119:12–20. doi: 10.1002/jso.25412. [DOI] [PubMed] [Google Scholar]
- 19.Feng F, Tian Y, Liu S, Zheng G, Liu Z, Xu G, Guo M, Lian X, Fan D, Zhang H. Combination of PLR, MLR, MWR, and tumor size could significantly increase the prognostic value for gastrointestinal stromal tumors. Medicine (Baltimore) 2016;95:e3248. doi: 10.1097/MD.0000000000003248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quigley DA, Kristensen V. Predicting prognosis and therapeutic response from interactions between lymphocytes and tumor cells. Mol Oncol. 2015;9:2054–2062. doi: 10.1016/j.molonc.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang SC, Chou JF, Strong VE, Brennan MF, Capanu M, Coit DG. Pretreatment neutrophil to lymphocyte ratio independently predicts disease-specific survival in resectable gastroesophageal junction and gastric adenocarcinoma. Ann Surg. 2016;263:292–297. doi: 10.1097/SLA.0000000000001189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salazar-Onfray F, López MN, Mendoza-Naranjo A. Paradoxical effects of cytokines in tumor immune surveillance and tumor immune escape. Cytokine Growth Factor Rev. 2007;18:171–182. doi: 10.1016/j.cytogfr.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 23.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 24.Zhang LN, Xiao W, OuYang PY, You K, Zeng ZF, Ding PR, Pan ZZ, Xu RH, Gao YH. The prognostic impact of preoperative blood monocyte count in pathological T3N0M0 rectal cancer without neoadjuvant chemoradiotherapy. Tumour Biol. 2015;36:8213–8219. doi: 10.1007/s13277-015-3560-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wen J, Ye F, Huang X, Li S, Yang L, Xiao X, Xie X. Prognostic significance of preoperative circulating monocyte count in patients with breast cancer: Based on a Large Cohort Study. Medicine (Baltimore) 2015;94:e2266. doi: 10.1097/MD.0000000000002266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindholm PF, Sivapurapu N, Jovanovic B, Kajdacsy-Balla A. Monocyte-induced prostate cancer cell invasion is mediated by chemokine ligand 2 and nuclear factor-κB activity. J Clin Cell Immunol. 2015;6:308. doi: 10.4172/2155-9899.1000308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mantovani A, Schioppa T, Porta C, Allavena P, Sica A. Role of tumor-associated macrophages in tumor progression and invasion. Cancer Metastasis Rev. 2006;25:315–322. doi: 10.1007/s10555-006-9001-7. [DOI] [PubMed] [Google Scholar]
- 29.Ikemoto S, Sugimura K, Yoshida N, Wada S, Yamamoto K, Kishimoto T. TNF alpha, IL-1 beta and IL-6 production by peripheral blood monocytes in patients with renal cell carcinoma. Anticancer Res. 2000;20:317–321. [PubMed] [Google Scholar]
- 30.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 31.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13:159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 32.Tan KW, Chong SZ, Wong FH, Evrard M, Tan SM, Keeble J, Kemeny DM, Ng LG, Abastado JP, Angeli V. Neutrophils contribute to inflammatory lymphangiogenesis by increasing VEGF-A bioavailability and secreting VEGF-D. Blood. 2013;122:3666–3677. doi: 10.1182/blood-2012-11-466532. [DOI] [PubMed] [Google Scholar]
- 33.Bausch D, Pausch T, Krauss T, Hopt UT, Fernandez-del- Castillo C, Warshaw AL, Thayer SP, Keck T. Neutrophil granulocyte derived MMP-9 is a VEGF independent functional component of the angiogenic switch in pancreatic ductal adenocarcinoma. Angiogenesis. 2011;14:235–243. doi: 10.1007/s10456-011-9207-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kay HD, Smith DL. Regulation of human lymphocyte-mediated natural killer (NK) cell activity. I. Inhibition in vitro by peripheral blood granulocytes. J Immunol. 1983;130:475–483. [PubMed] [Google Scholar]
- 35.Petrie HT, Klassen LW, Kay HD. Inhibition of human cytotoxic T lymphocyte activity in vitro by autologous peripheral blood granulocytes. J Immunol. 1985;134:230–234. [PubMed] [Google Scholar]
- 36.el-Hag A, Clark RA. Immunosuppression by activated human neutrophils. Dependence on the myeloperoxidase system. J Immunol. 1987;139:2406–2413. [PubMed] [Google Scholar]
- 37.Shau HY, Kim A. Suppression of lymphokine-activated killer induction by neutrophils. J Immunol. 1988;141:4395–4402. [PubMed] [Google Scholar]
- 38.Templeton AJ, McNamara MG, Šeruga B, Vera-Badillo FE, Aneja P, Ocaña A, Leibowitz-Amit R, Sonpavde G, Knox JJ, Tran B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: A systematic review and meta-analysis. J Natl Cancer Inst. 2014;106:dju124. doi: 10.1093/jnci/dju124. [DOI] [PubMed] [Google Scholar]
- 39.Peng B, Wang YH, Liu YM, Ma LX. Prognostic significance of the neutrophil to lymphocyte ratio in patients with non-small-cell lung cancer: A systemic review and meta-analysis. Int J Clin Exp Med. 2015;8:3098–3106. [PMC free article] [PubMed] [Google Scholar]
- 40.Xin-Ji Z, Yong-Gang L, Xiao-Jun S, Xiao-Wu C, Dong Z, Da-Jian Z. The prognostic role of neutrophils to lymphocytes ratio and platelet count in gastric cancer: A meta-analysis. Int J Surg. 2015;21:84–91. doi: 10.1016/j.ijsu.2015.07.681. [DOI] [PubMed] [Google Scholar]
- 41.Rachidi S, Metelli A, Riesenberg B, Wu BX, Nelson MH, Wallace C, Paulos CM, Rubinstein MP, Garrett-Mayer E, Hennig M, et al. Platelets subvert T cell immunity against cancer via GARP-TGFβ axis. Sci Immunol. 2017;2:eaai7911. doi: 10.1126/sciimmunol.aai7911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Majeti BK, Lee JH, Simmons BH, Shojaei F. VEGF is an important mediator of tumor angiogenesis in malignant lesions in a genetically engineered mouse model of lung adenocarcinoma. BMC Cancer. 2013;13:213. doi: 10.1186/1471-2407-13-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qiang G, Liang C, Xiao F, Yu Q, Wen H, Song Z, Tian Y, Shi B, Guo Y, Liu D. Prognostic significance of platelet-to-lymphocyte ratio in non-small-cell lung cancer: A meta-analysis. Onco Targets Ther. 2016;9:869–876. doi: 10.2147/OTT.S96804. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author upon reasonable request.