Abstract
The rising trend in pregnancy-related deaths during the past 2 decades in the United States stands out among other high-income countries where pregnancy-related deaths are declining. Cardiomyopathy and other cardiovascular conditions, hemorrhage, and other chronic medical conditions are all important causes of death. Unintentional death from violence, overdose, and self-harm are emerging causes that require medical and public health attention. Significant racial/ethnic inequities exist in pregnancy care with non-Hispanic black women incurring 3 to 4 times higher rates of pregnancy-related death than non-Hispanic white women. Varied terminology and lack of standardized methods for identifying maternal deaths in the United States have resulted in nuanced data collection and interpretation challenges. State maternal mortality review committees are important mechanisms for capturing and interpreting data on cause, timing, and preventability of maternal deaths. Importantly, a thorough standardized review of each maternal death leads to recommendations to prevent future pregnancy-associated deaths. Key interventions to improve maternal health outcomes include 1) integrating multidisciplinary care for women with high-risk comorbidities during preconception care, pregnancy, postpartum, and beyond; 2) addressing structural racism and the social determinants of health; 3) implementing hospital-wide safety bundles with team training and simulation; 4) providing patient education on early warning signs for medical complications of pregnancy; and 5) regionalizing maternal levels of care so that women with risk factors are supported when delivering at facilities with specialized care teams.
INTRODUCTION
Maternal death during pregnancy, childbirth, or postpartum is a tragedy with catastrophic impact on families and serves as an important indicator of the quality of a health system. The World Health Organization (WHO) has defined maternal mortality ratio (MMR) as “the number of maternal deaths per 100,000 live births, [where] maternal death is the death of a woman while pregnant or within 42 days of termination of pregnancy,” regardless of whether the cause was related to or aggravated by pregnancy. (1) However, according to the WHO definition, maternal deaths do not include those from accidental or incidental causes. Although the MMR has been the most common indicator for international comparisons of maternal health, it does not specify the cause of death in relation to pregnancy.
The MMR in the United States has decreased drastically in the last century because of advances in surgical technique, safer anesthesia, antisepsis, and overall improved living conditions. Although the MMR dropped from 900 deaths per 100,000 live births in the 1900s to 12.7 in 2007, the US rate of MMR has seen a rise over the past several decades. (2) In 2014, complications during pregnancy, childbirth, and the postpartum period ranked as the 6th greatest cause of death among women aged 20 to 34 in the United States. (3) The MMR in the United States has more than doubled from 9.8 per 100,000 live births in 2000 to 21.5 in 2014, (4) and this trend stands out among high-income countries; maternal mortality has decreased in other high-income countries, such as Canada and the United Kingdom, during the same time period. According to the WHO, the MMR has fallen by 44% from 1990 to 2015 in low- and middle-income countries. (5)
When interpreting these trends, however, we must also account for improvements in data ascertainment. The addition of a pregnancy question on the US death certificate in 2003 coincided with increased mortality rate, which suggests improved detection and reporting as part of the story. (4) Increasing maternal mortality rates vary by state and have generated public attention on family planning availability and the growing prevalence of chronic medical conditions including obesity, diabetes, and heart disease. Delayed childbearing leading to more advanced age during pregnancy, higher cesarean delivery rates, the opioid epidemic, and fragmented and limited access to care during and after pregnancy have also been identified as potential contributors. (6)
In the United States, the racial/ethnic inequities in maternal deaths are troubling; most notably, non-Hispanic black women carry a 3- to 4-fold risk of pregnancy-related deaths compared with non-Hispanic white women. (7) While the medical and public health communities have made strides to reduce infant mortality with strategies such as the Safe to Sleep campaign, (8) it is time to focus on solutions to improve maternal health as well, particularly for those at highest risk for adverse outcomes. This review summarizes the data collection challenges, causes of maternal mortality and severe maternal morbidity, inequities in maternal health outcomes, and solutions to reduce maternal morbidity and mortality.
DEFINITIONS AND DATA COLLECTION CHALLENGES
In the United States, the Centers for Disease Control and Prevention (CDC) has put forward 3 classifications of pregnancy-associated death (death of a woman while pregnant or within 1 year of termination of pregnancy, irrespective of the cause)(9):
Pregnancy-related: “The death of a woman while pregnant or within 1 year of termination of pregnancy, from any cause related to or aggravated by her pregnancy or its management, but not from accidental or incidental causes.” (Example: the death of a woman from postpartum hemorrhage or amniotic fluid embolism).
Pregnancy-associated but not pregnancy-related: “The death of a woman while pregnant or within 1 year of termination of pregnancy due to a cause unrelated to pregnancy.” (Example: the death of a pregnant woman from an earthquake).
Pregnancy-associated but undetermined if pregnancy-related: “The death of a woman while pregnant or within 1 year of termination of pregnancy from a cause that cannot be determined or conclusively categorized as either pregnancy-related or not pregnancy related.” (Example: a woman with an unknown mental health history dies at 6 months postpartum from a self-inflicted cause).
The CDC manages the 2 national data sources of maternal deaths: 1) the National Vital Statistics System compiled annually by the National Center for Health Statistics (NCHS), and 2) the Pregnancy Mortality Surveillance System (PMSS), a flagship program run by the Division of Reproductive Health at the National Center for Chronic Disease Prevention and Health Promotion (Table). (10) The NCHS relies exclusively on International Classification of Diseases, 10th Revision (ICD-10) codes assigned to causes of death listed on maternal death certificates and publishes the maternal mortality rate, consistent with the WHO definition of maternal death. The PMSS relies on epidemiologists to classify deaths according to the aforementioned definitions of pregnancy-related and pregnancy-associated deaths and allocate the causes of death into 10 categories: hemorrhage, infection/sepsis, amniotic fluid embolism, thrombotic pulmonary or other embolism, hypertensive disorders of pregnancy, anesthesia complications, cerebrovascular accidents, cardiomyopathy, cardiovascular disease, and noncardiovascular medical conditions.
TABLE.
Sources of Maternal Mortality Information in the United States
| NATIONAL CENTER FOR HEALTH STATISTICS (NCHS) | PREGNANCY MORTALITY SURVEILLANCE SYSTEM (PMSS) | |
|---|---|---|
| Data source | Death certificates | Death certificates linked to fetal death and birth certificates |
| Time frame | During pregnancy to 42 days postpartum | During pregnancy to 365 days postpartum |
| Source of classification | ICD-10 codes | Medical epidemiologists assign PMSS codes |
| Terms | Maternal death | Pregnancy-associated death |
| Pregnancy-related death | ||
| Associated but not pregnancy-related death | ||
| Measure | Maternal Mortality Rate | Pregnancy-Related Mortality Ratio |
| = # of maternal deaths per 100,000 live births | = # of pregnancy-related deaths per 100,000 live births | |
| Purpose(s) | Show national trends and provide basis for international comparison | Analyze clinical factors associated with deaths, publish information that may lead to prevention strategies |
| Strengths | Best source of historical data (back to 1900) | Most clinically relevant national measure of the burden of maternal deaths |
| Reliable basis for international comparison | ||
| Based on readily available data (death certificates) | ||
| Challenges | Constrained by ICD-10 codes | Constrained by information available on death and birth certificates |
| Lacks sufficient detail to inform prevention strategies | Lacks detailed information on contributors to death | |
ICD-10=International Classification of Diseases, 10th Revision.
Adapted from St Pierre et al. (80)
Measurement challenges include the limitations of ICD code accuracy and significant variation in the statewide implementation of the pregnancy checkbox on death certificates, which began in 2003, but was not fully implemented in all states until 2016. (10) Multiple studies have concluded that improvements in reporting and case ascertainment explain some of the recent increase in maternal mortality in the United States. (7)(11)(12)(13) One study estimated that about 80% of the reported increase in maternal mortality between 2000 and 2014 could be attributed to improvements in data linkages and the pregnancy box. (10) Another study found that the addition of the checkbox may have increased case identification but also misclassification, particularly among women aged 40 years or older. (14) However, after correcting for improved ascertainment of maternal deaths from implementation of the pregnancy question, the adjusted average MMR across 48 US states is still estimated to have risen by 27% from 18.8 to 23.8 per 100,000 live births from 2000 to 2014 (4); the smaller adjusted increase in MMR is because of significant under-reporting during the early time point. (4) The MMR in Texas doubled from 2011 to 2014, suggesting gaps in data quality rather than a true doubling of maternal death rates. (13) The study team used an enhanced method with full review of medical records for identifying pregnancy-associated deaths and found that more than half of the obstetric-coded deaths were inaccurately labeled. (13) However, even accounting for these data collection challenges, the MMR in the United States has not decreased substantially in the recent decades, as it has in other high-income countries.
State-based maternal mortality review committees (MMRCs) are the gold standard in identifying and reviewing pregnancy-associated and pregnancy-related deaths because they are made of a multidisciplinary team that reviews all available data, including prenatal records, hospital records, and autopsy reports. (10) MMRCs are now functional in approximately two-thirds of states and are best positioned to classify deaths as preventable or not and to make recommendations to prevent similar deaths in the future. (15)(16)(17)(18)(19)(20) The CDC has developed a standardized data collection system for state MMRCs called the Maternal Mortality Review Information Application (MMRIA). (21) The MMRIA is a publicly available set of standardized forms for abstracting data and recording MMRC decisions on 6 key questions: 1) Was the death pregnancy-related? 2) What was the cause of death? 3) Was the death preventable? 4) What were the factors that contributed to this death? 5) What are the recommendations and actions that address those contributing factors? 6) What is the anticipated impact of those actions if implemented?”(21) MMRCs play a critical role in evaluating all information about maternal deaths to identify systems solutions to improve care delivery for those that are deemed preventable.
CAUSES OF MATERNAL MORTALITY AND MORBIDITY
The 2018 report from 9 state MMRCs concluded that around 50% of all pregnancy-related deaths were caused by hemorrhage, cardiovascular/coronary conditions, cardiomyopathy, or infection. For non-Hispanic black women, the most common underlying causes of death included preeclampsia, eclampsia, and embolism. For non-Hispanic white women, mental health conditions were the leading cause of death. (22)
The most recent CDC report on maternal mortality from May 2019 also identified cardiovascular conditions (including cardiomyopathy, myocardial infarction, and cerebrovascular accidents) as the cause for more than 33% of pregnancy-related deaths (Fig 1). (22)(23)(24) From 2003 to 2012 there was a 25% increase in the number of women entering pregnancy with preexisting heart disease. Most of these women have congenital heart disease or valvular heart disease. However, the prevalence of cardiomyopathy and pulmonary hypertension increased significantly and demonstrated the highest in-hospital mortality, most often because of heart failure, arrhythmia, respiratory failure, shock, renal failure, and preeclampsia. (25) It is unclear why the incidence of cardiomyopathy is increasing, but it could be associated with increases in maternal age, multifetal pregnancies, or improved recognition.
Figure 1.
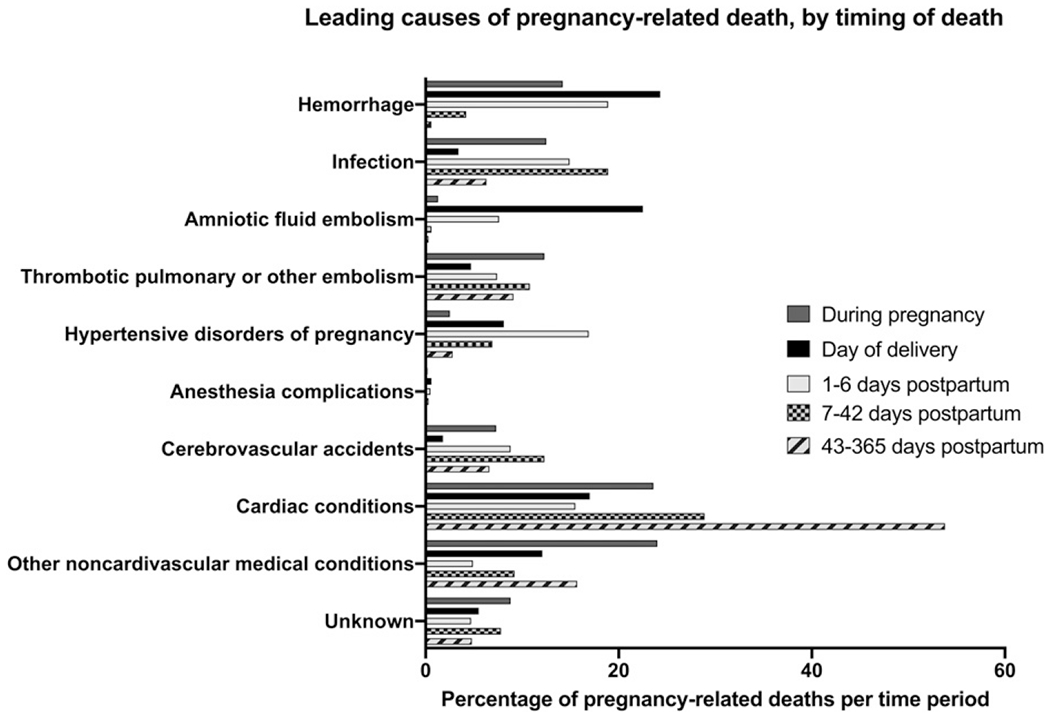
Leading causes of pregnancy-related death, by timing of death. Adapted from Petersen EE, Davis NL, Goodman D,etal. Vital signs: pregnancy-related deaths, United States, 2011–2015, and strategies for prevention, 13 states, 2013–2017. MMWR Morb Mortal Wkly Rep. 2019;68:423–429. Note: Cardiac conditions include both cardiomyopathy and other cardiovascular disease.
In a study of the National Inpatient Sample from 2002 to 2013, the incidence of cardiogenic shock (the most extreme of cardiovascular disease associated with mortality) increased over 3-fold; the mortality rate for pregnant women with cardiogenic shock was 19% compared with 0.02% of women without cardiogenic shock. (26) More than 80% of pregnant and postpartum women with cardiogenic shock had peripartum cardiomyopathy, a pregnancy-associated diagnosis that has also increased during the same period. (27) Both acute and chronic renal failure were significantly associated with mortality in women with cardiogenic shock. (26) Commonly identified risk factors for cardiovascular death include increasing maternal age, obesity, and hypertensive disorders. (27)(28) Strategies aimed at reducing modifiable chronic conditions such as obesity, hypertension, and diabetes in women of reproductive age may help reduce the incidence of cardiovascular disease and major adverse events. In addition, multidisciplinary care of pregnant women with cardiovascular disease, beginning with adequate risk assessment and evaluation for known and unknown cardiac disease is necessary to eliminate preventable maternal deaths. (29)(30)(31)
Obstetric hemorrhage was the cause of 11.5% of pregnancy-related deaths from 2011 to 2014 and is usually preventable. (32) Hemorrhage also accounts for the overwhelming majority of severe maternal morbidity, with blood product transfusions rising from 25 per 10,000 delivery hospitalizations in 1993 to 122 per 10,000 delivery hospitalizations in 2014. (24) This is a critical area for focused efforts to implement hospital-based national safety bundles, multidisciplinary team training, simulation, and reporting systems so that cases can be reviewed to ensure continuous improvement in safety and quality. (33) Successful implementation of state-wide bundles across 99 diverse hospitals in California has demonstrated reductions in severe morbidity rates from hemorrhage by 20.8%. (34) Some of the increase in prevalence of hemorrhage can be attributed to skyrocketing rates of cesarean delivery. Although cesarean can be a life-saving intervention, the US average annual cesarean delivery rate has risen from 23% in 1996 to 33% in 2011 without a corresponding reduction in maternal and neonatal morbidity or mortality. (35) Cesarean delivery is associated with increased maternal mortality and morbidity (particularly hemorrhage, infection, and thromboembolism) compared with vaginal birth and leads to future risks for abnormal placentation such as placenta previa and placenta accreta in subsequent pregnancies. The American College of Obstetricians and Gynecologists (ACOG) has taken steps to reduce the number of unnecessary cesarean deliveries by creating guidelines for the safe prevention of the primary cesarean. (35)
Another important emerging contributor to maternal death is self-harm (suicide or accidental overdose). In Colorado, 30% of the 211 maternal deaths over a 9-year study period were related to self-harm, with the majority occurring in the postpartum period. Prior psychiatric history and psychopharmacotherapy use during pregnancy were documented in over half of these women. (36) In Philadelphia, over a 4-year period, 49% of maternal deaths had nonmedical causes, including unintentional injury (overdose, motor vehicle crash, or other), homicide, and suicide; overdose composed 40% of nonmedical causes of maternal deaths. (37) The authors caution againstnarrowing the focus of maternal mortality on medical causes because nonmedical causes, particularly unintentional overdose, are important contributors to pregnancy-associated mortality. Mental illness, substance use, and intimate partner violence are common risk factors among women who died of both medical and nonmedical causes, reinforcing the importance of screening and providing interdisciplinary perinatal management of substance use disorders and psychobehavioral interventions. (37)
Severe maternal morbidity (SMM) is defined by the CDC as an index of 18 indicators of significant events (such as blood transfusion, hysterectomy, heart failure, eclampsia, respiratory distress, and sepsis) corresponding to ICD-10 diagnoses during delivery admission. (24) These indicators were chosen because they can be life-threatening, and are associated with short- or long-term morbidity, prolonged hospitalization, and high health care costs. (2) Many studies of smaller datasets use SMM as a surrogate for maternal mortality because SMM is thought to include the sentinel events that lead to significantly increased risk of death. SMM rates have increased 200% from 1993 to 2014, and this increase is primarily driven by increasing blood transfusions in response to postpartum hemorrhage. After removing blood transfusion, SMM has increased 20% over this period, with hysterectomy and temporary ventilatory support accounting for the next most common complications. (24)
The prevalence of reproductive-aged women with chronic conditions continues to rise, and pregnant women with multiple chronic conditions are at 276% higher risk of SMM and mortality than women with no chronic conditions. (38) Women with multiple chronic conditions are often older than women with a single chronic condition or no chronic conditions. Noncardiovascular medical conditions accounted for 14.3% of pregnancy-related deaths during the period from 2011 to 2015. (23)
Prepregnancy obesity has been associated with SMM and mortality in a cohort of women delivering in Washington State between 2004 and 2013, suggesting that the obesity epidemic was an important contributing factor to adverse maternal health outcomes. (39) An analysis of California births found that the incidence of SMM increased 65% from 2007 to 2014 and the prevalence of prepregnancy obesity, maternal age older than or equal to 35 years, and comorbid conditions also increased during the same period, but were estimated to contribute only 13% of the increasing morbidity. (40) Cesarean delivery was estimated to contribute 37% of the SMM in California during these 7 years. Increasing prevalence of maternal chronic medical conditions and cesarean delivery accounts for only half of the SMM, leaving room for investigation of other contributing factors. Although national efforts to reduce unnecessary cesarean deliveries may be promising interventions for reducing SMM, this study underscores the need to also address other contributing factors. (40)
In a study using delivery data from Washington State from 2000 to 2008, the rate of early-onset preeclampsia before 34 weeks’ gestation increased by 33% and was associated with a 10-fold greater risk of maternal death compared with women without preeclampsia. (41) Preeclampsia is also associated with significantly higher rates of SMM, particularly from cardiovascular, respiratory, or renal failure. Hypertensive disease in pregnancy has long-term health implications, including a 2-fold increased risk for all-cause mortality before age 50 years, and increased mortality related to diabetes, ischemic heart disease, and stroke. These long-term sequelae occurred more frequently in the group of women who had 2 or more pregnancies complicated by hypertensive disease. (42) Identifying women at risk for cardiovascular disease during their childbearing years offers an opportunity to intervene and optimize long-term health.
Access to abortion care has been curtailed in multiple states because of legislative restrictions on physician and facility requirements, medication abortion restrictions, gestational age limits, funding cuts, mandatory waiting periods, and parental consent laws, which can place women at increased risk of harm if they attempt self-induced abortions. (43) Improved insurance coverage for abortion care through state Medicaid has been associated with 16% fewer cases of SMM, suggesting that increased coverage for abortion care reduces complications associated with pregnancy. (44)
INEQUITIES IN MATERNAL MORBIDITY AND MORTALITY
Inequities—differences that are systematic, avoidable, and unjust—in maternal health outcomes have persisted and are a cause for concern. Although multiple social conditions confer increased risk of adverse outcomes, the most prominent examples of inequities in maternal health in the United States are rooted in the social constructs of race and ethnicity. One conceptual model from Dr Elizabeth Howell demonstrates the ecosystem of factors (patient, community/neighborhood, provider, and systems) that contribute to adverse health outcomes throughout the continuum of reproductive health care, particularly for women of color. (45)
Black women experience maternal deaths at a rate 3 to 4 times that of white women in the United States, (2)(7) regardless of age. (46) According to the CDC, the pregnancy-related mortality ratios between 2011 and 2014 were as follows: 12.4 deaths per 100,000 live births for white women, 40.0 deaths per 100,000 live births for black women, and 17.8 deaths per 100,000 live births for women of other races (32); this inequity remains unchanged in the 2019 report of pregnancy-related deaths from 2015. (23) The 2018 report of 9 MMRCs revealed that greater proportions of pregnancy-related deaths of all pregnancy-associated deaths occurred among non-Hispanic black women compared with white women (Fig 2). (22)
Figure 2.
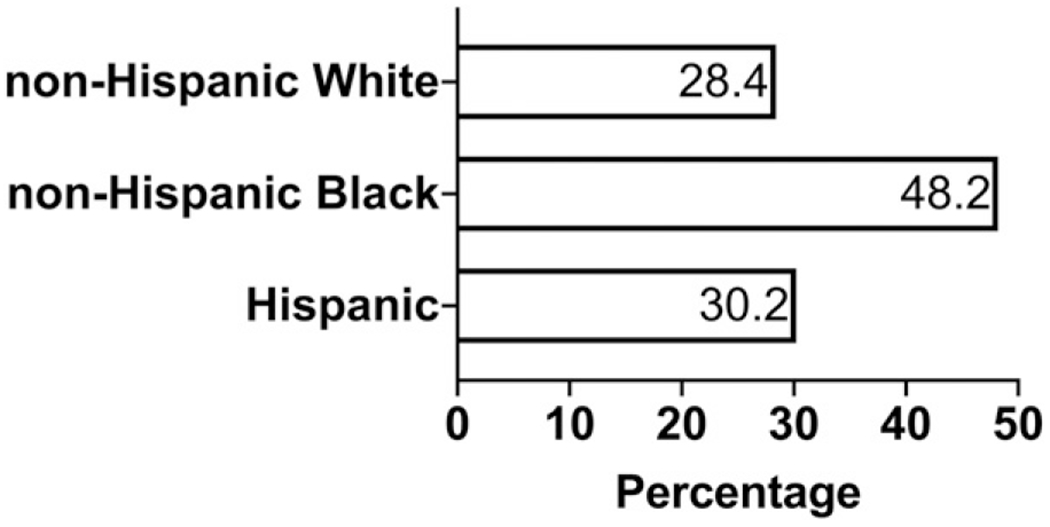
Proportion of pregnancy-associated deaths determined to be pregnancy-related based on race and ethnicity. Adapted from Building U.S. Capacity to Review and Prevent Maternal Deaths. (2018). Report from 9 maternal mortality review committees. http://reviewtoaction.org/Report_from_Nine_MMRCs. (22)
Black and Native American women also experience increased complications from pregnancy compared with white women, regardless of socioeconomic status and comorbidities. (47) Using 7 state inpatient databases from 2008 to 2010, Creanga et al identified race/ethnicity as an important predictor of maternal morbidity. (48) Although other factors were also identified as significant predictors of SMM, such as age less than 20 years or more than or equal to 35 years, self-pay or Medicaid coverage for delivery, low socioeconomic status, and presence of chronic medical conditions, they did not fully explain the observed racial/ethnic disparities in SMM. (48) Using the National Inpatient Sample from 2012 to 2015, Admon et al also found that SMM during delivery hospitalization was higher among all racial/ethnic groups compared with non-Hispanic whites. (49) The most common SMM was blood transfusion, which accounted for nearly 75% of all morbidity across racial/ethnic groups. The incidence of SMM was highest among women with multiple chronic conditions and particularly among those of color, suggesting increased case morbidity. (49) Grobman et al found that racial/ethnic disparities in maternal morbidity persisted even after controlling for patient-level factors and hospital of delivery. (50) In addition, racial/ethnic disparities in obstetric care delivery exist. Non-Hispanic blacks, Hispanics, and Asians all had lower odds of labor induction than non-Hispanic whites. The odds of receiving an episiotomy was increased in Asians but decreased in non-Hispanic blacks and Hispanics compared with non-Hispanic whites, (50) indicating that there may be biases in care delivery. Women of color also experience pregnancy-related complications that are associated with higher rates of death, such as tuberculosis, (51) or experience higher case fatality with conditions such as ectopic pregnancy. (52)
The root cause of racial/ethnic inequities is the legacy of structural racism that permeates people’s lived experiences, including experiences in the health care system. One important driver of inequities in maternal health is distrust in the health system as a result of historical and contemporary discrimination, which often manifests as lower prenatal care utilization and adherence with treatment plans. An analysis of more than 2,000 responses to the Listening to Mothers III survey found that more than 40% of participants reported communication challenges in prenatal care and 24% perceived discrimination during birth hospitalization, predominantly among black or Hispanic women and uninsured women. (53)
Another important driver of inequities is the variation in hospital quality of care during childbirth, in which women of color more commonly go to hospitals with higher risk-adjusted morbidity compared with the hospitals where white women go more commonly. (54) In an analysis of the black-white differences in hospital of delivery in New York City, Howell et al estimated that as much as 48% of the racial disparity in SMM could be attributed to differences in care quality at the hospital level. (55) A similar analysis among Hispanic women also showed that up to 37% of the ethnic disparity could be attributed to differences in care quality at the hospital level. (55)
Solutions to reduce inequities span all levels, from policies to address racism and social determinants of health to improving quality of care delivery and experience of care during childbirth, to mobilizing community-based organizations to support women before, during, and after pregnancy. Some examples include standardizing and improving quality of care in hospitals, particularly among facilities that have higher risk-adjusted morbidity rates and also care for a disproportionate number of women of color. (56) In addition, developing disparity dashboards to track outcomes among specific groups is important for monitoring, evaluation, and quality improvement. (57) Training on implicit bias is critical across the health care workforce to sensitize individuals to the role implicit bias may play in their interactions with patients. Lastly, there are innovative antenatal and postnatal care delivery models that are being evaluated as strategies to close disparities, such as group antenatal care and postpartum home visits with integrated interdisciplinary health teams. (58)
IMPROVING SAFETY AND QUALITY OF CHILDBIRTH CARE
With the increases in both maternal mortality and morbidity, there has been an increasing focus on quality of care at the hospital level in the days before and after childbirth. In addition to wide variation in SMM across hospitals, there is also substantial variation within hospitals. (56) The imperative to standardize and improve safety and quality is the foundation for reducing adverse maternal outcomes in the hospital setting. The most common mechanisms to achieve this end are 1) a focus on team communication and team training; 2) implementation of evidence-based safety bundles or toolkits to manage obstetric complications that are most likely to cause SMM and/or death; and 3) data-driven MMRCs that can provide specific recommendations for systems improvement to prevent future maternal deaths.
According to a review of sentinel events reported to the Joint Commission, failures in communication were the second leading root cause of SMM and maternal mortality and the leading root cause of perinatal deaths and injuries. (59) Key facilitators include leadership champions to build and support a culture of safety that encourages open communication among all team members and transparent, nonpunitive reporting of safety-critical events with a focus on systems improvement. (59) In addition, structured communication tools, such as safety huddles, safety checklists, “SBAR” (Situation, Background, Assessment, Recommendation) approach, and team training simulation such as TeamSTEPPS facilitate interprofessional communication and teamwork, particularly in acute situations that require coordination of resources and expertise. The maternal early warning triggers or criteria facilitate communication between bedside nurses and clinicians through increased clinical surveillance and responsiveness to patients with abnormal vital signs who may require prompt evaluation and treatment to prevent morbidity. (60)(61)(62)
Safety bundles and/or toolkits that facilitate adherence with evidence-based guidelines have been critical efforts to reduce maternal morbidity and mortality in the United States. At a national level, multiple professional organizations and stakeholders came together to form the National Partnership for Maternal Safety, which developed safety bundles for obstetric hemorrhage, severe hypertension in pregnancy, and peripartum venous thromboembolism. (63) Since the introduction of universal pneumatic compression devices at the time of cesarean delivery and rapid treatment of severe hypertension, there was a reduction in maternal deaths from postcesarean pulmonary embolism and in deaths and morbidity related to hypertensive disorders. (64)(65)(66)
The California Maternal Quality Care Collaborative (CMQCC) was developed as a public-private partnership and leveraged data from the Department of Public Health to support data-driven large-scale quality improvement initiatives to reduce maternal deaths and morbidity. (67) CMQCC-affiliated hospitals have demonstrated reductions in maternal morbidity from postpartum hemorrhage after implementing a comprehensive hemorrhage bundle compared with non–CMQCC-affiliated hospitals in California (20.8% vs 1.2%). (34)
With federal funding from the Health Resources and Services Administration Maternal Child Health Bureau, the Alliance for Innovation on Maternal Health (AIM) was formed and led by partners from the ACOG Council on Patient Safety and Women’s Health Care and the National Partnership for Maternal Safety. (68) AIM is a state-based program that develops and provides implementation support for safety bundles. Each bundle has 4 components: readiness, recognition, response, and reporting and systems learning. (68) AIM operationalizes the bundle implementation and reporting systems through state-based teams, often organized as state perinatal quality collaboratives (PQCs). PQCs are defined as “state or multistate networks of multidisciplinary teams, working to improve measurable population outcomes for maternal and infant health by advancing evidence-informed clinical practices and processes using quality improvement principles.”(69) The anchoring structure of PQCs relies on the department of public health, state hospital association, and clinician leadership. Additional members may include the state MMRC, community health organizations, patient advocacy groups, risk management, payers, and purchasers. (69) A critical function of the PQCs is to develop and sustain a robust data collection system for maternal health indicators at the state level.
Adjunct health system solutions to improve maternal outcomes include developing an obstetrics hospitalist workforce that may be best positioned to respond to uncommon emergencies (4)(70) and improve patient education at the time of discharge from the hospital. Although the obstetrics hospitalist workforce is growing across the United States, there remains wide variation regarding work models and scope of practice. (71) More data are needed to assess maternal outcomes in hospitals where a robust obstetrics hospitalist workforce manages intrapartum care. Similar to the integrated regional neonatal levels of care to improve perinatal mortality, maternal levels of care provide risk-appropriate care for those who would benefit from additional expertise and infrastructure because of preexisting comorbidities in pregnancy. (72) Implementation of regionalized maternal levels of care would allow for patients with high-risk conditions to be cared for at higher-volume hospitals with greater access to subspecialists. In addition, patient communication and education about danger signs, such as the POST-BIRTH tool, have been additional areas of focus to improve prompt recognition of symptoms and medical evaluation, especially once patients return home after childbirth. (73)
The 2018 report of 9 MMRCs found that more than 60% of pregnancy-related deaths were preventable and the leading factors contributing to death were patient/family factors (namely, lack of knowledge about early warning signs to seek care), provider factors (misdiagnosis or ineffective treatments), and factors related to systems of care (lack of coordination between providers) (Fig 3). (22) Most importantly, the MMRCs provided recommendations to prevent future maternal deaths, such as adopting levels of maternal care, (72) improving and enforcing policies and procedures on obstetric hemorrhage, and addressing health equity. Specifically, a California review of pregnancy-related cardiovascular deaths identified both patient and provider contributing factors, particularly a delay in patients seeking care as well as in provider response, which suggests an opportunity to improve patient education and standardize response protocols. (28)
Figure 3.
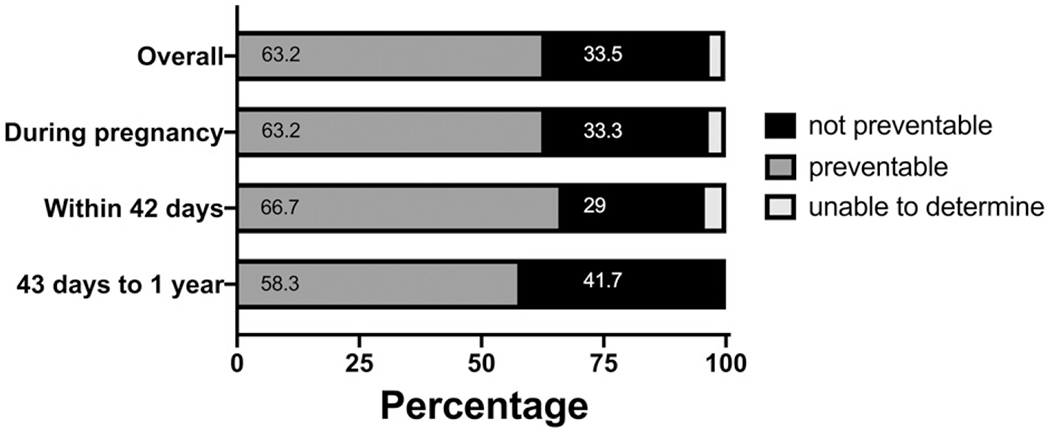
Preventability of pregnancy-related deaths by time of death in analysis of 9 state maternalmortality review committees. Adapted from Building U.S. Capacity to Review and Prevent Maternal Deaths. (2018). Report from 9 maternalmortality review committees. http://reviewtoaction.org/Report_from_Nine_MMRCs. (22)
CURRENT HEALTH CARE POLICY TO ADDRESS MATERNAL MORTALITY
On December 21, 2018, the Preventing Maternal Deaths Act (HR 1318) was signed into law. This legislation allocates federal funding to support states in establishing and sustaining MMRCs. (74) One challenge will be standardizing data collection and reporting systems among the various MMRCs.
Other maternal health bills in Congress at this time are the Maternal Care Access and Reducing Emergencies (CARE) Act, the Rural Maternal and Obstetric Modernization of Services (MOMS) Act, and the Mothers and Offspring Mortality and Morbidity Awareness (MOMMA) Act. The CARE Act, introduced by California Senator Kamala Harris, focuses on dismantling structural racism through training programs around implicit bias for clinicians. The MOMS Act, introduced by North Dakota Senator Heidi Heitkamp, addresses the disparities in access to obstetric care for women in rural communities by creating regional networks and increasing workforce capacity in rural areas. The MOMMA Act, introduced by Illinois Representative Robin Kelly, seeks to expand Medicaid through 1 year after delivery, standardize data collection through the CDC, establish and enforce national emergency obstetric protocols, and improve culturally competent care. In addition, there are multiple bills at the state and federal levels around expanding Medicaid through 1 year after delivery and providing Medicaid reimbursement for doula services.
PREPREGNANCY AND INTERPREGNANCY HEALTH
Pregnancy conditions, such as hypertensive disease and gestational diabetes, are known risk factors for cardiovascular disease later in life and increased early mortality. (42)(75) Other adverse pregnancy outcomes such as preterm labor and fetal growth restriction may also be associated with increased lifelong cardiovascular risk. (76) The postpartum period is a time of significant biological, psychological, and social transition with increased risk of complications, yet 10% to 40% of women do not attend any postpartum visit between 4 and 12 weeks. (77) During pregnancy and postpartum care, obstetricians have an opportunity to provide anticipatory guidance; arrange for appropriate follow-up for chronic health conditions, mental health, and substance use disorders; and identify and educate patients about early warning signs of SMM. According to the most recent CDC report, the majority of unintentional deaths occurred between 6 weeks and 1 year after delivery, highlighting the importance of the continuum of care that extends beyond the traditional 6-week postpartum period. (23) ACOG has revised recommendations for postpartum care to become an ongoing process of addressing recovery from birth, newborn care, psychosocial and sexual well-being, contraception, chronic disease management, health maintenance, and a transition to ongoing well-woman care, rather than a single encounter. (78) The prepregnancy and interpregnancy periods are windows of opportunity for implementing risk-reducing interventions for women with multiple medical conditions, mental health issues, or substance use disorders to optimize outcomes for future pregnancies and long-term well-being. Improving health systems to allow longitudinal continuous care from pregnancy and beyond is critical to reduce maternal mortality. (79)
CONCLUSION
Pregnancy-related deaths have been steadily rising in the United States and are not just a result of improved data acquisition. Cardiovascular conditions, obstetric hemorrhage, and self-harm or unintentional harm are important causes of pregnancy-related deaths; significant inequities exist between non-Hispanic black and non-Hispanic white women. The majority of pregnancy-related deaths are preventable. Implementation of safety bundles, team training, integrated multidisciplinary care for high-risk patients, risk-stratified levels of maternal care, improvements in communication between providers and patients regarding early warning signs, and addressing structural racism and the social determinants of health are all strategies for improving maternal safety, quality and equity. Health care policy to improve funding and resources for standardized, state-based review of pregnancy-related deaths are important steps to reverse rising rates and close persistent inequities in maternal morbidity and mortality.
Practice Gaps.
In contrast to other high-income countries, the maternal death rate in the United States has been rising for over 20 years with persistent racial/ethnic inequities.
Initiatives to improve safety, quality, and equity of care during pregnancy, delivery, and beyond are essential to optimize maternal health outcomes.
Objectives After completing this article, readers should be able to:
Explain the definitions of pregnancy-related and pregnancy-associated deaths and the data challenges in the United States.
Recognize key contributors to rising maternal deaths and persistent inequities in the United States.
Identify key strategies and solutions for improving maternal health.
American Board of Pediatrics Neonatal-Perinatal Content Specifications.
Know the effects on the fetus and/or newborn infant of maternal cardiac disease and its management.
Know the essentials of prenatal care, including risk assessment, perinatal referral, screening, and standard monitoring.
Know how maternal obesity may influence pregnancy and pregnancy outcome.
Know the components of pre- and periconceptional health care (including nutritional requirements during pregnancy) that influence pregnancy outcomes.
Know the issues in the organization of perinatal care (e.g., regionalization, transport, practice guidelines, benchmarking data, quality improvement).
Acknowledgments
AUTHOR DISCLOSURE Dr Collier is supported by the Reproductive Scientist Development Program (K12HD000849), the Eunice Kennedy Shriver National Institute of Child Health & Human Development, and Burroughs Wellcome Fund as part of the Reproductive Scientist Development Program. Dr Molina has disclosed no financial relationships relevant to this article. This commentary does not contain a discussion of an unapproved/investigative use of a commercial product/device.
ABBREVIATIONS
- ACOG
American College of Obstetricians and Gynecologists
- AIM
Alliance for Innovation on Maternal Health
- CARE Act
Maternal Care Access and Reducing Emergencies Act
- CDC
Centers for Disease Control and Prevention
- CMQCC
California Maternal Quality Care Collaborative
- ICD-10
International Classification of Diseases, 10th Revision
- MMR
maternal mortality ratio
- MMRC
maternal mortality review committee
- MMRIA
Maternal Mortality Review Information Application
- MOMMA Act
Mothers and Offspring Mortality and Morbidity Awareness Act
- MOMS Act
Maternal and Obstetric Modernization of Services Act
- NCHS
National Center for Health Statistics
- PMSS
Pregnancy Mortality Surveillance System
- PQC
perinatal quality collaborative
- SMM
severe maternal morbidity
- WHO
World Health Organization
References
- 1.World Health Organization. Health statistics and information systems: maternal mortality ratio. 2019. Available at: https://www.who.int/healthinfo/statistics/indmaternalmortality/en/. Accessed April 29, 2019.
- 2.Callaghan WM. Overview of maternal mortality in the United States. Semin Perinatol. 2012;36(1):2–6 [DOI] [PubMed] [Google Scholar]
- 3.Heron M Deaths: leading causes for 2014. Natl Vital Stat Rep. 2016;65(5):1–96 [PubMed] [Google Scholar]
- 4.MacDorman MF, Declercq E, Cabral H,Morton C. Recent increases in the U.S. maternal mortality rate: disentangling trends from measurement issues. Obstet Gynecol. 2016;128(3):447–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. Trends in maternal mortality: 1990 to 2015: estimates by WHO, UNICEF, UNFPA, World Bank Group and the United Nations Population Division. Geneva, Switzerland: World Health Organization; 2015 [Google Scholar]
- 6.Carroll AE. Why is US maternal mortality rising? JAMA. 2017;318(4):321. [DOI] [PubMed] [Google Scholar]
- 7.MacDorman MF, Declercq E, Thoma ME. Trends in maternal mortality by sociodemographic characteristics and cause of death in 27 states and the District of Columbia. Obstet Gynecol. 2017;129(5):811–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moon RY; Task Force on Sudden Infant Death Syndrome. SIDS and other sleep-related infant deaths: evidence base for 2016 updated recommendations for a safe infant sleeping environment. Pediatrics. 2016;138(5):e20162940. [DOI] [PubMed] [Google Scholar]
- 9.Review to action, CDC Foundation. https://reviewtoaction.org/learn/definitions. Accessed July 1, 2019
- 10.Creanga AA. Maternal mortality in the United States: a review of contemporary data and their limitations. Clin Obstet Gynecol. 2018;61(2):296–306 [DOI] [PubMed] [Google Scholar]
- 11.Joseph KS, Lisonkova S, Muraca GM, et al. Factors underlying the temporal increase in maternal mortality in the United States. Obstet Gynecol. 2017;129(1):91–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MacDorman MF, Declercq E, Thoma ME. Trends in Texas maternal mortality bymaternal age, race/ethnicity, and cause of death, 2006-2015. Birth. 2018;45(2):169–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baeva S, Saxton DL, Ruggiero K, et al. Identifying maternal deaths in Texas using an enhanced method, 2012. Obstet Gynecol. 2018;131(5):762–769 [DOI] [PubMed] [Google Scholar]
- 14.Davis NL, Hoyert DL, Goodman DA, Hirai AH, Callaghan WM. Contribution of maternal age and pregnancy checkbox on maternal mortality ratios in the United States, 1978-2012. Am J Obstet Gynecol. 2017;217(3):352.e1–352.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geller SE, Koch AR, Martin NJ, Rosenberg D, Bigger HR; Illinois Department of Public Health Maternal Mortality Review Committee Working Group. Assessing preventability of maternal mortality in Illinois: 2002-2012. Am J Obstet Gynecol. 2014;211(6):698.e1–698.e11 [DOI] [PubMed] [Google Scholar]
- 16.Geller SE, Koch AR, Martin NJ, Prentice P, Rosenberg D; Illinois Department of PublicHealthMaternalMortality Review Committee Working Group. Comparing two review processes for determination of preventability of maternal mortality in Illinois. Matern Child Health J. 2015;19(12):2621–2626 [DOI] [PubMed] [Google Scholar]
- 17.Hernandez LE, Sappenfield WM, Harris K, et al. Pregnancy-related deaths, Florida, 1999-2012: opportunities to improve maternal outcomes. Matern Child Health J. 2018;22(2):204–215 [DOI] [PubMed] [Google Scholar]
- 18.Mitchell C, Lawton E, Morton C, McCain C, Holtby S, Main E. California pregnancy-associated mortality review: mixed methods approach for improved case identification, cause of death analyses and translation of findings. Matern Child Health J. 2014;18(3):518–526 [DOI] [PubMed] [Google Scholar]
- 19.Shellhaas C, Conrey E. State-based review of maternal deaths: the Ohio experience. Clin Obstet Gynecol. 2018;61(2):332–339 [DOI] [PubMed] [Google Scholar]
- 20.Shellhaas CS, Zaharatos J, Clayton L, Hameed A. Examination of a death due to cardiomyopathy by a maternal mortality review committee. Am J Obstet Gynecol. 2019;221(1):1–8 [DOI] [PubMed] [Google Scholar]
- 21.Maternal Mortalty Review Information Application (MMRIA). 2019. http://mmria.org/. Accessed July 1, 2019
- 22.Review to action, CDC Foundation. Capacity to review and prevent maternal deaths. report from nine maternal mortality review committees. 2018. http://reviewtoaction.org/Report_from_Nine_MMRCs. Accessed July 1, 2019.
- 23.Petersen EE, Davis NL, Goodman D, et al. Vital signs: pregnancyrelated deaths, United States, 2011-2015, and strategies for prevention, 13 States, 2013-2017. MMWR Morb Mortal Wkly Rep. 2019;68(18):423–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention. Severe maternal morbidity indicators and corresponding ICD codes during delivery hospitalizations. https://www.cdc.gov/reproductivehealth/maternalinfanthealth/smm/severe-morbidity-ICD.htm. Accessed July 1, 2019
- 25.Lima FV, Yang J, Xu J, Stergiopoulos K. National trends and in-hospital outcomes in pregnant women with heart disease in the United States. Am J Cardiol. 2017;119(10):1694–1700 [DOI] [PubMed] [Google Scholar]
- 26.Banayan J, Rana S,Mueller A, et al. Cardiogenic shock in pregnancy: analysis from the National Inpatient Sample. Hypertens Pregnancy. 2017;36(2):117–123 [DOI] [PubMed] [Google Scholar]
- 27.Kolte D, Khera S, Aronow WS, et al. Temporal trends in incidence and outcomes of peripartum cardiomyopathy in the United States: a nationwide population-based study. J Am Heart Assoc. 2014;3(3):e001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hameed AB, Lawton ES, McCain CL, et al. Pregnancy-related cardiovascular deaths in California: beyond peripartum cardiomyopathy. Am J Obstet Gynecol. 2015;213(3):379.e1–379.e10 [DOI] [PubMed] [Google Scholar]
- 29.Brown HL, Warner JJ, Gianos E, et al. ; American Heart Association and the American College of Obstetricians and Gynecologists. Promoting risk identification and reduction of cardiovascular disease in women through collaboration with obstetricians and gynecologists: a presidential advisory from the American Heart Association and the American College of Obstetricians and Gynecology. Circulation. 2018;37(24):e843–e852 [DOI] [PubMed] [Google Scholar]
- 30.American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 212: pregnancy and heart disease. Obstet Gynecol. 2019;133(5):e320–e356 [DOI] [PubMed] [Google Scholar]
- 31.Wolfe DS, Hameed AB, Taub CC, Zaidi AN, Bortnick AE. Addressing maternal mortality: the pregnant cardiac patient. Am J Obstet Gynecol. 2019;220(2):167.e1–167.e8 [DOI] [PubMed] [Google Scholar]
- 32.Centers for Disease Control and Prevention. Reproductive health: pregnancy mortality surveillance system. https://www.cdc.gov/reproductivehealth/maternalinfanthealth/pregnancy-mortalitysurveillance-system.htm. Accessed July 1, 2019
- 33.Main EK, Goffman D, Scavone BM, et al. ; National Parternship for Maternal Safety; Council for Patient Safety in Women’s Health Care. National Partnership for Maternal Safety: consensus bundle on obstetric hemorrhage. Anesth Analg. 2015;121(1):142–148 [DOI] [PubMed] [Google Scholar]
- 34.Main EK, Cape V, Abreo A, et al. Reduction of severe maternal morbidity from hemorrhage using a state perinatal quality collaborative. Am J Obstet Gynecol. 2017;216(3):298.e1–298.e11 [DOI] [PubMed] [Google Scholar]
- 35.American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine. Obstetric care consensus no. 1: safe prevention of the primary cesarean delivery. Obstet Gynecol. 2014;123(3):693–711 [DOI] [PubMed] [Google Scholar]
- 36.Metz TD, Rovner P, Hoffman MC, Allshouse AA, Beckwith KM, Binswanger IA. Maternal deaths from suicide and overdose in Colorado, 2004-2012. Obstet Gynecol. 2016;128(6):1233–1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mehta PK, Bachhuber MA, Hoffman R, Srinivas SK. Deaths from unintentional injury, homicide, and suicide during or within 1 year of pregnancy in Philadelphia. Am J Public Health. 2016;106(12):2208–2210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Admon LK, Winkelman TNA, Heisler M, Dalton VK. Obstetric outcomes and delivery-related health care utilization and costs among pregnant women with multiple chronic conditions. Prev Chronic Dis. 2018;15:E21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lisonkova S, Muraca GM, Potts J, et al. association between prepregnancy body mass index and severe maternal morbidity. JAMA. 2017;318(18):1777–1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leonard SA, Main EK, Carmichael SL. The contribution of maternal characteristics and cesarean delivery to an increasing trend of severe maternal morbidity. BMC Pregnancy Childbirth. 2019;19(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lisonkova S, Sabr Y, Mayer C, Young C, Skoll A, Joseph KS. Maternal morbidity associated with early-onset and late-onset preeclampsia. Obstet Gynecol. 2014;124(4):771–781 [DOI] [PubMed] [Google Scholar]
- 42.Theilen LH, Meeks H, Fraser A, Esplin MS, Smith KR, Varner MW. Long-term mortality risk and life expectancy following recurrent hypertensive disease of pregnancy. Am J Obstet Gynecol. 2018;219(1):107.e1–107.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Conti JA, Brant AR, Shumaker HD, Reeves MF. Update on abortion policy. Curr Opin Obstet Gynecol. 2016;28(6):517–521 [DOI] [PubMed] [Google Scholar]
- 44.Jarlenski M,Hutcheon JA, Bodnar LM, Simhan HN. State Medicaid coverage of medically necessary abortions and severe maternal morbidity and maternal mortality. Obstet Gynecol. 2017;129(5):786–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Howell EA. Reducing disparities in severe maternal morbidity and mortality. Clin Obstet Gynecol. 2018;61(2):387–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Creanga AA, Berg CJ, Syverson C, Seed K, Bruce FC, Callaghan WM. Race, ethnicity, and nativity differentials in pregnancy-related mortality in the United States: 1993-2006. Obstet Gynecol. 2012;120(2 Pt 1):261–268 [DOI] [PubMed] [Google Scholar]
- 47.Howland RE, Angley M, Won SH, et al. Determinants of severe maternal morbidity and its racial/ethnic disparities in New York City, 2008-2012. Matern Child Health J. 2019;23(3):346–355 [DOI] [PubMed] [Google Scholar]
- 48.Creanga AA, Bateman BT, Kuklina EV, Callaghan WM. Racial and ethnic disparities in severematernal morbidity: a multistate analysis, 2008-2010. Am J Obstet Gynecol. 2014;210(5):435.e1–435.e8 [DOI] [PubMed] [Google Scholar]
- 49.Admon LK, Winkelman TNA, Zivin K, Terplan M, Mhyre JM, Dalton VK. Racial and ethnic disparities in the incidence of severe maternal morbidity in the United States, 2012-2015. Obstet Gynecol. 2018;132(5):1158–1166 [DOI] [PubMed] [Google Scholar]
- 50.Grobman WA, Bailit JL, Rice MM, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units (MFMU) Network. Racial and ethnic disparities in maternal morbidity and obstetric care. Obstet Gynecol. 2015;125(6):1460–1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dennis EM, Hao Y, Tamambang M, et al. Tuberculosis during pregnancy in theUnited States:Racial/ethnic disparities in pregnancy complications and in-hospital death. PLoS One. 2018;13(3):e0194836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stulberg DB, Cain L, Dahlquist IH, Lauderdale DS. Ectopic pregnancy morbidity and mortality in low-income women, 2004-2008. Hum Reprod. 2016;31(3):666–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Attanasio L, Kozhimannil KB. Patient-reported communication quality and perceived discrimination in maternity care. Med Care. 2015;53(10):863–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Howell EA, Egorova NN, Balbierz A, Zeitlin J, Hebert PL. Site of delivery contribution to black-white severe maternal morbidity disparity. Am J Obstet Gynecol. 2016;215(2):143–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Howell EA, Egorova NN, Janevic T, Balbierz A, Zeitlin J, Hebert PL. Severe maternal morbidity among hispanic women in New York City: investigation of health disparities. Obstet Gynecol. 2017;129(2):285–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Howell EA, Zeitlin J. Improving hospital quality to reduce disparities in severe maternal morbidity and mortality. Semin Perinatol. 2017;41(5):266–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Council on Patient Safety in Women’s Health Care. Reduction of peripartum racial/ethnic disparities (+ AIM). https://safehealthcareforeverywoman.org/patient-safety-bundles/reduction-of-peripartum-racialethnic-disparities/#1472748392134-776d4866-a0fb0f3f-74eb. Accessed July 1, 2019
- 58.Howell EA, Padrón NA, Beane SJ, et al. Delivery and payment redesign to reduce disparities in high risk postpartum care. Matern Child Health J. 2017;21(3):432–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brennan RA, Keohane CA. How communication among members of the health care team affects maternal morbidity and mortality. J Obstet Gynecol Neonatal Nurs. 2016;45(6):878–884 [DOI] [PubMed] [Google Scholar]
- 60.Hedriana HL, Wiesner S, Downs BG, Pelletreau B, Shields LE. Baseline assessment of a hospital-specific early warning trigger system for reducing maternal morbidity. Int J Gynaecol Obstet. 2016;132(3):337–341 [DOI] [PubMed] [Google Scholar]
- 61.Mhyre JM, D’Oria R,Hameed AB, et al. The maternal early warning criteria: a proposal from the national partnership for maternal safety. J Obstet Gynecol Neonatal Nurs. 2014;43(6):771–779 [DOI] [PubMed] [Google Scholar]
- 62.Zuckerwise LC, Lipkind HS. Maternal early warning systems: towards reducing preventable maternal mortality and severe maternal morbidity through improved clinical surveillance and responsiveness. Semin Perinatol. 2017;41(3):161–165 [DOI] [PubMed] [Google Scholar]
- 63.D’Alton ME, Main EK, Menard MK, Levy BS. The National Partnership for Maternal Safety. Obstet Gynecol. 2014;123(5):973–977 [DOI] [PubMed] [Google Scholar]
- 64.Clark SL, Christmas JT, Frye DR, Meyers JA, Perlin JB. Maternal mortality in the United States: predictability and the impact of protocols on fatal postcesarean pulmonary embolism and hypertension-related intracranial hemorrhage. Am J Obstet Gynecol. 2014;211(1):32.e1–32.e9 [DOI] [PubMed] [Google Scholar]
- 65.Gupta M, Greene N, Kilpatrick SJ. Timely treatment of severe maternal hypertension and reduction in severe maternal morbidity. Pregnancy Hypertens. 2018;14:55–58 [DOI] [PubMed] [Google Scholar]
- 66.Shields LE, Wiesner S, Klein C, Pelletreau B, Hedriana HL. Early standardized treatment of critical blood pressure elevations is associated with a reduction in eclampsia and severe maternal morbidity. Am J Obstet Gynecol. 2017;216(4):415.e1–415.e5 [DOI] [PubMed] [Google Scholar]
- 67.Main EK, Markow C, Gould J. Addressing maternal mortality and morbidity In California through public-private partnerships. Health Aff (Millwood). 2018;37(9):1484–1493 [DOI] [PubMed] [Google Scholar]
- 68.Mahoney J The alliance for innovation in maternal health care: a way forward. Clin Obstet Gynecol. 2018;61(2):400–410 [DOI] [PubMed] [Google Scholar]
- 69.Main EK. Reducing maternal mortality and severe maternal morbidity through state-based quality improvement initiatives. Clin Obstet Gynecol. 2018;61(2):319–331 [DOI] [PubMed] [Google Scholar]
- 70.Stevens TA, Swaim LS, Clark SL. The role of obstetrics/gynecology hospitalists in reducing maternal mortality. Obstet Gynecol Clin North Am. 2015;42(3):463–475 [DOI] [PubMed] [Google Scholar]
- 71.Srinivas SK. Potential impact of obstetrics and gynecology hospitalists on safety of obstetric care. Obstet Gynecol Clin North Am. 2015;42(3):487–491 [DOI] [PubMed] [Google Scholar]
- 72.American College of Obstetricians and Gynecologists. Obstetric care consensus no. 2: levels of maternal care. Obstet Gynecol. 2015;125(2):502–515 [DOI] [PubMed] [Google Scholar]
- 73.Suplee PD, Kleppel L, Santa-Donato A, Bingham D. improving postpartum education about warning signs of maternal morbidity and mortality. Nurs Womens Health. 2017;20(6):552–567 [DOI] [PubMed] [Google Scholar]
- 74. [Accessed July 1, 2019];H.R. 1318: Preventing Maternal Deaths Act of 2018. https://www.congress.gov/bill/115th-congress/house-bill/1318.
- 75.England L, Kotelchuck M, Wilson HG, et al. Estimating the recurrence rate of gestational diabetes mellitus (GDM) in Massachusetts 1998-2007: methods and findings. Matern Child Health J. 2015;19(10):2303–2313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bohrer J, Ehrenthal DB. Other adverse pregnancy outcomes and future chronic disease. Semin Perinatol. 2015;39(4):259–263 [DOI] [PubMed] [Google Scholar]
- 77.Spelke B,Werner E. the fourth trimester of pregnancy: committing to maternal health and well-being postpartum. R I Med J (2013). 2018;101(8):30–33 [PubMed] [Google Scholar]
- 78.American College of Obstetricians and Gynecologists. ACOG committee opinion no. 736: optimizing postpartum care. Obstet Gynecol. 2018;131:e140–e150 [DOI] [PubMed] [Google Scholar]
- 79.Lu MC, Highsmith K, de la Cruz D, Atrash HK. Putting the “M” back in the Maternal and Child Health Bureau: reducing maternal mortality and morbidity. Matern Child Health J. 2015;19(7):1435–1439 [DOI] [PubMed] [Google Scholar]
- 80.St Pierre A, Zaharatos J, Goodman D, Callaghan WM. Challenges and opportunities in identifying, reviewing, and preventing maternal deaths. Obstet Gynecol. 2018;131(1):138–142 [DOI] [PMC free article] [PubMed] [Google Scholar]


