Abstract
Background:
The role of inflammatory states in cardiometabolic risks among patients with type 2 diabetes mellitus (T2DM) with similar degrees of obesity is unknown. The study aimed to compare cardiometabolic risk factors in inflammatory obesity phenotypes with regard to the role of the FTO rs9939609 gene polymorphism.
Materials and Methods:
This study was performed on 155 patients with T2DM (77 men and 78 women) in Ahvaz, Iran. Participants were grouped into four groups based on the presence of obesity and inflammation (high-sensitivity C-reactive protein ≥3.9 mg/L): low inflammatory normal weight (LINW), high inflammatory normal weight (HINW), low inflammatory obese (LIO), and high inflammatory obese (HIO). The genotypes of FTO rs9939609, including homozygous carriers of the FTO risk allele (AA), heterozygous carriers (AT), and carrying no risk allele (TT), were studied. The cardiometabolic risk factors, including anthropometric status, hypertension, lipid and glycemic profile, and inflammatory markers, were evaluated. The waist–hip ratio (WHR), mean arterial pressure (MAP), and atherogenic index of plasma (AIP) were calculated.
Results:
The patients in inflammatory groups (HINW and HIO) have significantly higher levels in AIP when compared to inflammatory healthy groups (LINW and LIO). No significant differences between any of the four group means were detected in WHR, blood pressure, MAP, glycemic status (fasting blood sugar and insulin), homeostatic model assessment, lipid profile (triglyceride, very low-density lipoprotein, high-density lipoprotein, low-density lipoprotein, and cholesterol), interleukin-6, and total antioxidant capacity. The most frequent of high-risk genotype (AA) of FTO rs9939609 was in HIO, LIO, HINW, and LINW.
Conclusion:
T2DM patients with inflammatory condition have similar degree of increased atherogenic risk irrespective of obesity. The obesity-risk genotype AA of FTO gene was associated with an increased risk for inflammatory obesity in T2DM patients.
Keywords: C-reactive protein, diabetes mellitus, FTO, inflammation, obesity
INTRODUCTION
Type 2 diabetes mellitus (T2DM) is ninth major cause of death and accounts for approximately 451 million (age 18–99 years) cases in worldwide.[1,2] Obesity, sedentary lifestyle, dyslipidemia, unhealthy dietary pattern, and nutrients deficiency are important risk factors for the T2DM development and its complications.[3,4] The cardiometabolic risk factors, including hypertension, insulin resistance, dyslipidemia, and cardiovascular disease (CVD), are greater in patients with T2DM compared to healthy people. However, T2DM-related complications are not same for all patients with T2DM.[5] Previous studies showed that the vascular atherosclerotic risks were higher in abdominally obese diabetic patients than in the diabetics without abdominal obesity.[6] It is recognized that obesity-related inflammatory cytokines (adipocytokines) are risk factors for the development of T2DM and its complications.[7] Excess adipose tissue in obese patients by activation of the immune system leads to an increase in interferon-γ-producing Th1 cells and a decrease in anti-inflammatory regulatory T cells, which induces insulin resistance.[8] However, various forms of obesity do not have the same adverse effects on health status. Not all obese people display inflammatory unhealthy state and not all normal-weight people have inflammatory healthy state.[9] In addition, studies have shown that C-reactive protein (CRP) is an inflammatory marker independent of obesity associated with glycemic status.[10]
Therefore, it is possible that inflammatory or noninflammatory obesity could be helpful in explaining the variation in the cardiometabolic risk factors in T2DM. It seems that noninflammatory obesity phenotype is a low-risk subgroup and inflammatory nonobesity phenotype is a high-risk subgroup for CVD. Therefore, the assessment of cardiometabolic risk factors in inflammatory subgroup among obese people needs more research.[11]
Finding out the etiology of inflammatory obesity in T2DM is important. FTO (fat mass and obesity associated) gene is one of genetic factors for predisposing to T2DM, obesity, and probably inflammatory obesity.[12] The previous studies have shown that the FTO-rs9939609 risk variant (A allele) was associated with increased risk of T2DM and indices of obesity such as body mass index (BMI), hip circumference (HC), waist circumference (WC), and waist–hip ratio (WHR) compared to wild type (TT).[13,14] In addition, evidence suggests that there is a significant relationship between FTO rs9939609 polymorphism A allele and more high-sensitivity CRP (hs-CRP) levels.[15]
Currently, there is no consistent evidence regarding differences in terms of cardiovascular risk factors between inflammatory and noninflammatory obese diabetic patients. In addition, no study has been evaluated the role of FTO rs9939609 polymorphism in inflammatory obese patients. Thus, the aim of our study was to compare cardiometabolic risk factors in inflammatory obesity phenotypes with regard to the role of the FTO rs9939609 gene polymorphism.
MATERIALS AND METHODS
Participants
A cross-sectional study was performed in Ahvaz, Southwest of Iran, during 2018–2019. In the present study, 165 patients with T2DM using simple random sampling were screened from an endocrine clinic. Patients with T2DM were screened based on fasting blood sugar (FBS) >126 mg/dl and recruited based on the inclusion and exclusion criteria. Inclusion criteria were as follows: 20–65 years of age and BMI between 18.5 and 35 kg/m2. Exclusion criteria were as follows: pregnancy, lactation and estrogen therapy in women, insulin therapy, inflammatory disease and use of anti-inflammatory agents, liver dysfunction, adrenal or thyroid dysfunction, and cancer. Obesity was defined based on BMI >30 kg/m2. The inflammation condition was defined as a serum hs-CRP level >3.9 mg/L based on the optimal cutoff point of hs-CRP for inflammatory state in diabetic patients. Participants were grouped into four groups: (1) low inflammatory normal weight (LINW), (2) high inflammatory normal weight (HINW), (3) low inflammatory obese (LIO), and (4) high inflammatory obese (HIO).
Ethical approval
The study protocol conforms to the ethical guidelines of the Declaration of Helsinki, and all procedures involving patients were approved by the Ethics Committee of Baqiyatallah University of Medical Sciences, Tehran, Iran. Furthermore, informed consent form was obtained from the participants.
Anthropometric and blood pressure measurement
Weight and height of individuals were determined in an overnight fasting status using a standard scale (Seca). BMI was calculated using the formula: (weight [kg])/(height2 [m]). WC and HC were measured. WHR was calculated. The systolic blood pressure (SBP), diastolic blood pressure (DBP), and heart rate (HR) were measured. To evaluate the patient's blood pressure, the participants sit on a chair and have no physical activity 1 h. The mean arterial pressure (MAP) was calculated: ([2DBP + SBP]/3).[16]
Dietary intake and physical activity measurement
The usual dietary intake of participants in the previous year was collected by the interviewer using the valid and reliable food frequency questionnaire. Physical activity level was questioned. Physical activity defined as: ≥3 times/week and each time >30 min.
Biochemical measurements
The blood samples were collected in the fasting status. The blood samples were centrifuged. Part of the serum was used to evaluate the lipid profiles. The concentrations of total cholesterol (TC), triglyceride (TG), high-density lipoprotein-cholesterol (HDL-C), and low-density lipoprotein-cholesterol (LDL-C) were measured by an autoanalyzer. The atherogenic index of plasma (AIP) was calculated as the logarithm of molar ratio of TG/HDL-C.[17] FBS was immediately measured using enzymatic method by Pars–Azmoon kits (Tehran, Iran). The serum hs-CRP, insulin, interleukin-6 (IL-6), and total antioxidant capacity (TAC) concentrations were assessed by ELISA kits. The insulin resistance in the homeostasis model (homeostatic model assessment-insulin resistance [HOMA-IR]) was calculated as follows: FBS (mg/dL) × fasting serum insulin (mU/mL)/405.
Genotyping of the FTO rs9939609
The genomic DNA extraction from whole blood was done by the DNA purification Kit according to the instructions of the manufacturer (Sinaclon, Iran). We used PCR-RFLP (polymerase chain reaction-restriction fragment length polymorphism) of FTO rs9939609 gene for genotyping the single-nucleotide polymorphisms (SNPs).
The primers F: 5′-AACTGGCTCTTGAATGAAATAGGA TTCAGA-3′) and (R: 5′-AGAGTAACAGAGACTAT CCAAGTGCAGTAC-3′) were used for amplifying a DNA fragment (containing rs9939609 polymorphism).
The PCR product of the FTO rs9939609 was digested by restriction enzyme (ScaI), which recognized the SNP T to A in the first intron of FTO gene. The RFLP products were resolved by performing electrophoresis on a 2.5% agarose gel. The T allele produced a 182 bp band and the A allele produced 154 bp and 28 bp bands. Hence, homozygous wild-type TT genotype has the 182 bp band only; homozygous mutated AA genotype has the 154 and 28 bp bands; and heterozygous TA genotype has the 182, 154, and 28 bp bands.
Statistical analysis
The software SPSS 20.0 (SPSS Inc., Chicago, IL, USA) was used to analyze data. The data normality was checked by Kolmogorov–Smirnov test. The Chi-squared test, Student's t-test, and ANOVA were used to evaluate differences within groups, followed by the Tukey's post hoc test. P <0.05 was considered statistically significant. Odds ratios for AIP-based obesity and FTO phenotypes were measured using logistic regression using low-risk groups as the reference.
RESULTS
Out of the 165 patients with T2DM recruited, ten had incomplete data (genotyping, blood results, dietary, and medical information). Consequently, the data were analyzed for 155 patients consisting of 77 (49.7%) males and 78 (50.3%) females, with a mean age of 53.03 ± 9.98 years (range: 20–65 years). Table 1 displays anthropometric and biochemical characteristics between males and females. The optimal cutoff point for hs-CRP to detect obese T2DM was 3.9 mg/L. The inflammation health was defined as a serum hs-CRP level <3.9 mg/L (sensitivity of 52% and specificity of 78.0%). Based on obesity and inflammatory state, the patients were grouped into four groups: 47 LINW (30.3%), 44 HINW (28.4%), 29 LIO (18.7%), and 35 HIO (22.6%).
Table 1.
General characteristics of the participants
| Variables | Total (n=155) | Male (n=77) | Female (n=78) | P |
|---|---|---|---|---|
| Age (years) | 53.03±9.98 | 52.39±10.42 | 53.64±9.55 | 0.44 |
| Weight (kg) | 80.46±12.47 | 85.93±11.86 | 75.21±10.71 | <0.001 |
| BMI (kg/m2) | 29.64±3.80 | 28.67±3.37 | 30.57±3.97 | 0.002 |
| WC (cm) | 101.08±7.48 | 99.23±6.02 | 102.70±8.26 | 0.006 |
| WHR | 0.97±0.05 | 0.98±0.04 | 0.96±0.05 | 0.07 |
| SBP (mmHg) | 13.42±2.23 | 13.78±1.97 | 13.06±2.41 | 0.04 |
| DBP (mmHg) | 8.52±1.36 | 8.86±1.34 | 8.19±1.31 | 0.002 |
| HR (n) | 84.66±11.98 | 82.83±12.40 | 86.44±11.36 | 0.07 |
| MAP | 10.15±1.55 | 10.50±1.49 | 9.81±1.55 | 0.006 |
| TG (mg/dl) | 190.11±104.89 | 199.10±120.20 | 181.23±87.08 | 0.29 |
| HDL (mg/dl) | 48.77±9.49 | 45.87±6.54 | 51.63±11.02 | <0.001 |
| LDL (mg/dl) | 76.49±28.54 | 75.24±28.66 | 77.68±28.56 | 0.60 |
| Cholesterol (mg/dl) | 161.63±38.70 | 157.19±38.28 | 166.01±38.87 | 0.15 |
| VLDL (mg/dl) | 38.35±21.96 | 40.48±25.59 | 36.25±17.59 | 0.23 |
| AIP | 0.54±0.25 | 0.57±0.25 | 0.51±0.25 | 0.08 |
| FBS (mg/l) | 176.79±65.97 | 169.52±57.91 | 183.97±72.73 | 0.17 |
| Insulin (ng/dl) | 13.09±6.04 | 13.20±5.55 | 12.99±6.50 | 0.83 |
| HOMA-IR | 5.62±3.31 | 5.56±3.18 | 5.67±3.43 | 0.85 |
| hs-CRP (mg/l) | 3.88±0.84 | 3.81±0.63 | 3.95±0.99 | 0.32 |
| IL-6 (ng/dl) | 2.02±1.53 | 1.99±1.65 | 2.04±1.42 | 0.85 |
| TAC | 0.39±0.12 | 0.42±0.10 | 0.38±0.14 | 0.03 |
BMI=Body mass index; WC=Waist circumference; WHR=Waist-to-hip ratio; SBP=Systolic blood pressure; DBP=Diastolic blood pressure; HR=Heart rat; MAP=Mean arterial pressure; TG=Triglyceride; HDL=High-density lipoprotein; LDL=Low-density lipoprotein; VLDL=Very-LDL; AIP=Atherogenic index of plasma; FBS=Fasting blood sugar; HOMA-IR=Homeostatic model assessment of insulin resistance; hs-CRP=High-sensitivity C-reactive protein; IL-6: Interleukin-6; TAC: Total antioxidant capacity
Table 2 shows that there were no significant differences in dietary intake and demographic characteristics (age, gender, physical activity, dietary intake, education levels, and taking medication) between groups.
Table 2.
Dietary intake and demographic characteristics according to the inflammatory condition
| Variables | LINW (n=47) | HINW (n=44) | LIO (n=29) | HIO (n=35) | P |
|---|---|---|---|---|---|
| Age | 53.57±9.58 | 53.01±12.11 | 51.38±8.08 | 53.71±9.24 | 0.78 |
| Female/male | 26/21 | 19/25 | 12/17 | 21/14 | 0.31 |
| Physical activity, n (%) | |||||
| Inactive | 44 (94) | 42 (95) | 28 (97) | 34 (97) | 0.60 |
| Active | 3 (6) | 2 (5) | 1 (3) | 1 (3) | |
| Education levels, n (%) | |||||
| Diploma or less | 45 (96) | 40 (91) | 24 (83) | 32 (91) | 0.30 |
| University | 2 (4) | 4 (9) | 5 (17) | 3 (9) | |
| Medication, n (%) | |||||
| Anti-glycemic | 42 (89) | 40 (90) | 27 (93) | 30 (86) | 0.79 |
| Lipid-lowering | 17 (36) | 23 (52) | 11 (38) | 21 (60) | 0.11 |
| Dietary intake | |||||
| Energy (Kcal) | 2155.45±83.92 | 2172.24±72.01 | 2186.23±60.31 | 2206.36±110.39 | 0.09 |
| Fat (gr) | 99.52±12.66 | 96.71±15.64 | 99.14±13.36 | 101.48±12.31 | 0.53 |
| Protein (gr) | 58.16±10.95 | 58.68±9.71 | 60.14±7.89 | 59.27±10.97 | 0.87 |
| Carbohydrates (gr) | 268.35±31.41 | 279.01±31.32 | 275.47±27.65 | 275.52±20.78 | 0.40 |
| Fiber (gr) | 14.68±4.42 | 16.42±8.49 | 15.39±6.34 | 13.94±4.57 | 0.38 |
| Cholesterol (mg) | 344.54±117.49 | 340.13±101.79 | 351.87±123.27 | 391.25±109.33 | 0.79 |
| SFA (gr) | 39.01±9.61 | 39.27±8.70 | 41.03±9.72 | 42.35±9.92 | 0.95 |
| MUFA | 31.53±5.91 | 31.02±5.75 | 31.13±5.25 | 31.73±5.59 | 0.94 |
| PUFA | 14.43±5.57 | 15.29±6.15 | 13.73±4.43 | 14.53±6.82 | 0.63 |
| Vitamin A (mgr) | 284.81±199.46 | 261.55±152.20 | 307.08±183.45 | 265.08±138.69 | 0.71 |
| Vitamin D (µg) | 6.21±6.52 | 6.21±7.61 | 8.09±6.24 | 5.61±5.86 | 0.53 |
| Vitamin E (mg) | 14.71±10.20 | 13.09±7.25 | 12.54±6.89 | 13.71±11.50 | 0.08 |
| Vitamin K (mg) | 70.02±35.10 | 67.33±34.74 | 63.06±35.74 | 53.92±28.04 | 0.22 |
| Vitamin C (mg) | 83.57±35.50 | 78.87±29.29 | 78.85±35.84 | 76.41±34.99 | 0.83 |
| Folate (µg) | 299.48±120.93 | 307.26±110.51 | 300.40±133.02 | 275.61±121.73 | 0.73 |
| Magnesium (mg) | 213.72±61.17 | 223.82±100.65 | 208.90±59.89 | 196.03±70.65 | 0.54 |
| Zinc (mg) | 8.27±2.63 | 8.01±2.35 | 7.64±1.12 | 8.35±2.22 | 0.10 |
| Beta-carotene (mg) | 1702.28±1249.96 | 1594.51±905.59 | 1805.54±1098.0 | 1505.12±864.49 | 0.70 |
| Lutein (µg) | 709.16±430.35 | 714.21±534.93 | 703.31±472.39 | 562.56±354.02 | 0.47 |
| Lycopene (µg) | 5625.97±3444.84 | 5847.70±4616.46 | 6533.01±5254.18 | 6527.84±4975.68 | 0.77 |
aSignificant difference between LINW compared to HINW; bSignificant difference between LINW compared to LIO; cSignificant difference between LINW compared to HIO; dSignificant difference between HINW compared to LIO; eSignificant difference between HINW compared to HIO; fSignificant difference between LIO compared to HIO. LINW=Low-inflammatory normal-weight; HINW=High-inflammatory normal-weight; LIO=Low-inflammatory obese; HIO=High-inflammatory obese; SFA=Saturated fatty acids; MUFA=Monounsaturated fatty acid; PUFA=Polyunsaturated fatty acid
Table 3 indicates comparison of anthropometric measures and biochemical variables between four groups according to the inflammatory condition. Based on within-group analysis, there were no significant differences in age between groups.
Table 3.
Anthropometric and biochemical characteristics according to the inflammatory condition
| Variables | LINW (n=47) | HINW (n=44) | LIO (n=29) | HIO (n=35) | P |
|---|---|---|---|---|---|
| BMI (kg/m2) | 26.82±1.84b,c | 27.50±1.79d,e | 33.80±3.59 | 32.37±2.53 | <0.001 |
| WC (cm) | 97.40±6.31b,c | 97.92±5.41d,e | 107.20±6.71 | 105.61±6.62 | <0.001 |
| WHR | 0.96±0.04 | 0.98±0.04 | 0.95±0.06 | 0.97±0.05 | 0.12 |
| SBP (mmHg) | 13.90±2.23 | 13.27±2.14 | 13.29±2.08 | 13.04±2.43 | 0.33 |
| DBP (mmHg) | 8.86±1.43 | 8.43±1.33 | 8.60±1.23 | 8.10±1.33 | 0.08 |
| HR (n) | 81.27±12.20 | 87.81±12.50 | 87.04±9.47 | 83.88±12.40 | 0.06 |
| MAP | 10.54±1.55 | 10.04±1.54 | 10.16±1.41 | 9.74±1.61 | 0.13 |
| TG (mg/dl) | 174.17±113.25 | 207.32±112.15 | 164.03±76.44 | 211.49±99.89 | 0.14 |
| HDL (mg/dl) | 49.36±9.06 | 47.84±10.58 | 50.01±9.08 | 48.11±9.19 | 0.74 |
| LDL (mg/dl) | 78.34±30.01 | 74.34±30.18 | 78.41±26.79 | 75.01±26.51 | 0.88 |
| Cholestrol (mg/dl) | 161.87±41.60 | 162.09±38.71 | 161.55±33.08 | 160.77±40.57 | 0.99 |
| VLDL (mg/dl) | 35.27±24.35 | 42.46±23.94 | 33.34±15.10 | 41.51±20.25 | 0.19 |
| AIP | 0.48±0.27a,c | 0.59±0.26d | 0.48±0.18f | 0.60±0.24 | 0.04 |
| FBS (mg/l) | 189.49±55.94 | 176.89±60.45 | 173.48±78.39 | 162.37±73.15 | 0.32 |
| Insulin (ng/dl) | 13.62±5.66 | 11.52±5.46 | 14.53±5.58 | 13.09±7.32 | 0.19 |
| HOMA-IR | 6.42±3.47 | 4.72±2.07 | 5.90±2.58 | 5.36±4.49 | 0.10 |
| hs-CRP (mg/l) | 3.40±0.74b,c | 4.45±0.47d | 3.26±0.83f | 4.37±0.49 | <0.001 |
| IL-6 (ng/dl) | 1.78±1.29 | 2.05±2.05 | 1.97±1.03 | 2.36±1.44 | 0.44 |
| TAC | 0.40±0.11 | 0.37±0.13 | 0.41±0.10 | 0.40±0.14 | 0.70 |
aSignificant difference between LINW compared to HINW; bSignificant difference between LINW compared to LIO, cSignificant difference between LINW compared to HIO; dSignificant difference between HINW compared to LIO; eSignificant difference between HINW compared to HIO; fSignificant difference between LIO compared to HIO. LINW=Low-inflammatory normal-weight; HINW=High-inflammatory normal-weight; LIO=Low-inflammatory obese; HIO=High-inflammatory obese; BMI=Body mass index; WC=Waist circumference; WHR=Waist-hip ratio; SBP=Systolic blood pressure; DBP=Diastolic blood pressure; HR=Heart rate; MAP=Mean arterial pressure; TG=Triglyceride; HDL=High-density lipoprotein; LDL=Low-density lipoprotein; VLDL=Very-LDL; AIP=Atherogenic index of plasma; FBS=Fasting blood sugar; HOMA-IR=Homeostatic model assessment of insulin resistance; hs-CRP=High-sensitivity C-reactive protein; IL-6: Interleukin-6; TAC: Total antioxidant capacity
As expected, the BMI and WC were more in obese groups (LIO and HIO) than normal-weight groups (LINW and HINW). The patients in the inflammatory unhealthy groups (HINW and HIO) have significantly higher levels in AIP [Figure 1] and hs-CRP when compared to the inflammatory healthy groups (LINW and LIO). There were no significant differences between any of the four group in WHR, SBP, DBP, MAP, HR, glycemic status (FBS, insulin, and HOMA-IR), profile lipid (TG, VLDL, HDL, LDL, and cholesterol), IL-6, and TAC.
Figure 1.
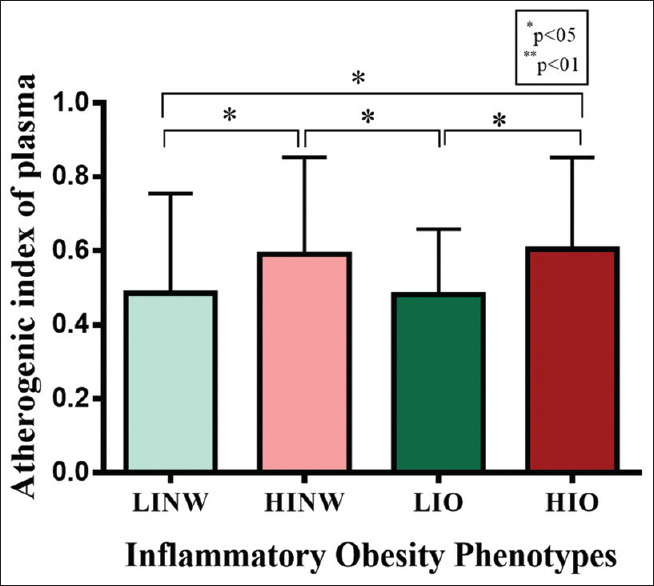
Comparison atherogenic index of plasma levels between groups
The genotype frequencies for FTO rs9939609 were 28.4%, 54.8%, and 16.8% for AA, AT, and TT genotype, respectively. Pearson's Chi-square test showed that there was a significant different between groups in the frequency of FTO rs9939609 genotype (AA, AT, and TT, P = 0.003). The most frequent of high-risk genotype (AA) was in HIO, LIO, HINW, and LINW, respectively [Table 4].
Table 4.
Fat mass and obesity associated (rs9939609) genotypes frequency in groups
| Genotype | Total (%) | LINW (%) | HINW (%) | LIO (%) | HIO (%) | P* |
|---|---|---|---|---|---|---|
| AA | 44 (28.4) | 7 (14.9) | 12 (27.3) | 9 (31.0) | 16 (45.7) | 0.003 |
| AT | 85 (54.8) | 36 (76.6) | 23 (52.3) | 11 (38.0) | 15 (42.9) | |
| TT | 26 (16.8) | 4 (8.5) | 9 (20.4) | 9 (31.0) | 4 (11.4) |
*Difference between TT, AT and AA between four groups. LINW=Low-inflammatory normal-weight; HINW=High-inflammatory normal-weight; LIO=Low-inflammatory obese; HIO=High-inflammatory obese
The odds ratios of AIP according to inflammatory obesity and FTO phenotypes are summarized in Table 5. The inflammatory unhealthy groups (HIO and HINW) had higher odds ratios than LINW as reference group. Patients with genotype AA (odds ratio = 1.34 [0.16–11.19]) and AA-AT (odds ratio = 1.22 [0.21–6.96]) have shown nonsignificantly higher AIP levels compared to TT individuals.
Table 5.
Odds ratios for atherogenic index of plasma based inflammatory obesity and FTO-rs9939609 Phenotypes
| Groups | Inflammatory obesity phenotypes | |||
|---|---|---|---|---|
| LINW | HINW | LIO | HIO | |
| OR | Reference | 6.97 (1.23-39.33) | 4.19 (0.43-55.43) | 16.44 (2.17-124.68) |
| P | 0.02 | 0.19 | 0.007 | |
| Groups | FTO-rs9939609 phenotypes | |||
| TT | AT | AA | AA-AT | |
| OR | Reference | 0.87 (0.13-5.72) | 1.34 (0.16-11.19) | 1.22 (0.21-6.96) |
| P | 0.89 | 0.78 | 0.81 | |
LINW=Low-inflammatory normal-weight; HINW=High-inflammatory normal-weight; LIO=Low-inflammatory obese; HIO=High-inflammatory obese; OR=Odd ratio; FTO=Fat mass and obesity associated
DISCUSSION
In this study, we found that irrespective of obesity, T2DM patients with inflammatory unhealthy condition have similar degree of increased atherogenic risk compared to patients with inflammatory healthy phenotypes. In fact, our findings suggest that the role of inflammatory status in developing atherogenic problems is stronger than obesity. To our knowledge, this is the first study that has evaluated the association between inflammatory obesity phenotypes with cardiometabolic risk factors in T2DM patients.
In accordance with our findings, Lin et al. reported that HINW men have higher carotid intima–media thickness as a risk factor for subclinical atherosclerosis compared to their noninflammatory counterparts. Furthermore, they suggested that LIO men have lower coronary artery calcium scores compared to their inflammatory counterparts. However, their results were not repeated among women.[18] A number of studies reported that high hs-CRP level is strongly associated with the number of CVD risk factors and metabolic syndrome components.[19,20] The study results of Zeba et al. showed that adults with hs-CRP >1 mg/L were more probable to have the cardiometabolic risk factors compared to adults with hs-CRP <1 mg/L.[21] The relative risks of cardiovascular events according to the highest quartile of hs-CRP demonstrated two times compared to the lowest quintile in women.[22]
The possible mechanisms of CRP role in plaque deposition and atherosclerosis are complex. CRP may facilitate monocyte adhesion and macrophage infiltration in atherosclerotic lesions.[23,24] Furthermore, CRP inhibited endothelial nitric oxide synthase and impaired vasoreactivity.[25] Evidences reported CRP found in lipid microdomains of endothelial cells and plaques.[26]
We did not find any different in glycemic profile between inflammatory obesity phenotypes. Previous studies indicated that elevated serum hs-CRP level was associated with an increased degree of glycemic variables.[27,28] On the other hand, some theories suggested that inflammation during obesity is not all bad for T2DM.[29]
Our results demonstrate that in T2DM patients, variants in the FTO rs9939609 gene predispose to inflammatory obesity. Fisher et al. reported that variation in the FTO rs9939609 gene may contribute to enhance inflammatory state independently of obesity. Their results suggested that each additional copy of the obesity-risk allele (AA) of FTO gene was associated with an increase in CRP level (1.14-fold in men and 1.12-fold in women).[30]
Saucedo et al. suggested that lower concentrations of adiponectin and higher concentrations of pro-inflammatory tumor necrosis factor-alpha were associated with the FTO rs9939609 risk allele A in women with gestational diabetes mellitus after adjusting for maternal pregestational body weight.[31] Furthermore, recently, a study observed that the carriers of risk allele A of FTO Polymorphism rs9939609 had increased CRP and insulin values.[32] However, some studies did not report an association between the FTO rs9939609 variant and inflammatory state.[28,29] Animal studies demonstrated that independent of body weight, FTO gene influences the metabolic outcomes by alteration of nuclear factor kappa B signaling in hypothalamus.[33]
CONCLUSION
T2DM patients with inflammatory condition are at high risk for atherogenic risks than patients with inflammatory healthy status. The results of our research show the more importance of inflammatory status in the development of atherosclerosis compared to obesity. The nonobese patients with inflammatory condition indicated atherogenic risks similar to patients with inflammatory obesity, therefore, they should be considered as a high risk group. The obesity-risk genotype AA of FTO gene was associated with an increased risk for inflammatory obesity in T2DM patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
This work was financially supported by a grant (No. 91002661) from Health Research Center, Life Style Institute, Baqiyatallah University of Medical Sciences, Tehran, Iran.
REFERENCES
- 1.Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, et al. IDF diabetes atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–81. doi: 10.1016/j.diabres.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 2.Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14:88–98. doi: 10.1038/nrendo.2017.151. [DOI] [PubMed] [Google Scholar]
- 3.Aljack HA, Abdalla MK, Idris OF, Ismail AM. Vitamin D deficiency increases risk of nephropathy and cardiovascular diseases in type 2 diabetes mellitus patients. J Res Med Sci. 2019;24:47. doi: 10.4103/jrms.JRMS_303_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rahimi N, Samavati Sharif MA, Goharian AR, Pour AH. The effects of aerobic exercises and 25(OH) D supplementation on GLP1 and DPP4 level in type II diabetic patients. Int J Prev Med. 2017;8:56. doi: 10.4103/ijpvm.IJPVM_161_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cox AJ, Hsu FC, Freedman BI, Herrington DM, Criqui MH, Carr JJ, et al. Contributors to mortality in high-risk diabetic patients in the diabetes heart study. Diabetes Care. 2014;37:2798–803. doi: 10.2337/dc14-0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lukich A, Gavish D, Shargorodsky M. Normal weight diabetic patients versus obese diabetics: Relation of overall and abdominal adiposity to vascular health. Cardiovasc Diabetol. 2014;13:141. doi: 10.1186/s12933-014-0141-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jaganathan R, Ravindran R, Dhanasekaran S. Emerging Role of adipocytokines in type 2 diabetes as mediators of insulin resistance and cardiovascular disease. Can J Diabetes. 2018;42:446–560. doi: 10.1016/j.jcjd.2017.10.040. [DOI] [PubMed] [Google Scholar]
- 8.Deng T, Liu J, Deng Y, Minze LJ, Xiao X, Wright V, et al. Adipocyte adaptive immunity mediates diet-induced adipose inflammation and insulin resistance by decreasing adipose Treg cells. Nature communications. 2017;8:15725. [Google Scholar]
- 9.Alam I, Ng TP, Larbi A. Does inflammation determine whether obesity is metabolically healthy or unhealthy? The aging perspective. Mediators Inflamm. 2012;2012:456456. doi: 10.1155/2012/456456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ebrahimi M, Heidari-Bakavoli AR, Shoeibi S, Mirhafez SR, Moohebati M, Esmaily H, et al. Association of Serum hs-CRP Levels With the Presence of Obesity, Diabetes Mellitus, and Other Cardiovascular Risk Factors. J Clin Lab Anal. 2016;30:672–6. doi: 10.1002/jcla.21920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim SH, Després JP, Koh KK. Obesity and cardiovascular disease: Friend or foe? Eur Heart J. 2016;37:3560–8. doi: 10.1093/eurheartj/ehv509. [DOI] [PubMed] [Google Scholar]
- 12.Kamura Y, Iwata M, Maeda S, Shinmura S, Koshimizu Y, Honoki H, et al. FTO gene polymorphism is associated with type 2 diabetes through its effect on increasing the maximum BMI in Japanese men. PLoS One. 2016;11:e0165523. doi: 10.1371/journal.pone.0165523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sabarneh A, Ereqat S, Cauchi S, AbuShamma O, Abdelhafez M, Ibrahim M, et al. Common FTO rs9939609 variant and risk of type 2 diabetes in palestine. BMC Med Genet. 2018;19:156. doi: 10.1186/s12881-018-0668-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tupikowska-Marzec M, Kolačkov K, Zdrojowy-Wełna A, Słoka NK, Szepietowski JC, Maj J. The Influence of FTO Polymorphism rs9939609 on Obesity, Some Clinical Features, and Disturbance of Carbohydrate Metabolism in Patients with Psoriasis. Biomed Res Int. 2019;2019:7304345. doi: 10.1155/2019/7304345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun Y, Sun J, Wang X, You W, Yang M. Variants in the fat mass and obesity associated (FTO) gene are associated with obesity and C-reactive protein levels in Chinese Han populations. Clin Invest Med. 2010;33:E405–12. doi: 10.25011/cim.v33i6.14592. [DOI] [PubMed] [Google Scholar]
- 16.Ashtary-Larky D, Lamuchi-Deli N, Milajerdi A, Salehi MB, Alipour M, Kooti W, et al. inflammatory and biochemical biomarkers in response to high intensity resistance training in trained and untrained men. Asian J Sports Med. 2017;8:e13739. [Google Scholar]
- 17.Karabay E, Karsiyakali N, Duvar S, Tosun C, Aslan AR, Yucebas OE. Relationship between plasma atherogenic index and final pathology of Bosniak III-IV renal masses: A retrospective, single-center study. BMC Urol. 2019;19:85. doi: 10.1186/s12894-019-0514-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin A, Lacy ME, Eaton C, Correa A, Wu WC. Inflammatory obesity phenotypes, gender effects, and subclinical atherosclerosis in African Americans: The Jackson heart study. Arterioscler Thromb Vasc Biol. 2016;36:2431–8. doi: 10.1161/ATVBAHA.116.307728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Razban MM, Eslami M, Bagherzadeh A. The relationship between serum levels of hs-CRP and coronary lesion severity. Clujul Med. 2016;89:322–6. doi: 10.15386/cjmed-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seyedian SM, Ahmadi F, Dabagh R, Davoodzadeh H. Relationship between high-sensitivity C-reactive protein serum levels and the severity of coronary artery stenosis in patients with coronary artery disease. ARYA Atheroscler. 2016;12:231–7. [PMC free article] [PubMed] [Google Scholar]
- 21.Zeba AN, Delisle HF, Rossier C, Renier G. Association of high-sensitivity C-reactive protein with cardiometabolic risk factors and micronutrient deficiencies in adults of Ouagadougou, Burkina Faso. Br J Nutr. 2013;109:1266–75. doi: 10.1017/S0007114512003182. [DOI] [PubMed] [Google Scholar]
- 22.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557–65. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 23.Badimon L, Peña E, Arderiu G, Padró T, Slevin M, Vilahur G, et al. C-reactive protein in atherothrombosis and angiogenesis. Front Immunol. 2018;9:430. doi: 10.3389/fimmu.2018.00430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kones R. Primary prevention of coronary heart disease: Integration of new data, evolving views, revised goals, and role of rosuvastatin in management. A comprehensive survey. Drug Des Devel Ther. 2011;5:325–80. doi: 10.2147/DDDT.S14934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jialal I, Verma S, Devaraj S. Inhibition of endothelial nitric oxide synthase by C-reactive protein: Clinical relevance. Clin Chem. 2009;55:206–8. doi: 10.1373/clinchem.2008.119206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McFadyen JD, Kiefer J, Braig D, Loseff-Silver J, Potempa LA, Eisenhardt SU, et al. Dissociation of C-reactive protein localizes and amplifies inflammation: Evidence for a direct biological role of c-reactive protein and its conformational changes. Front Immunol. 2018;9:1351. doi: 10.3389/fimmu.2018.01351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uemura H, Katsuura-Kamano S, Yamaguchi M, Bahari T, Ishizu M, Fujioka M, et al. Relationships of serum high-sensitivity C-reactive protein and body size with insulin resistance in a Japanese cohort. PLoS One. 2017;12:e0178672. doi: 10.1371/journal.pone.0178672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shaheer AK, Tharayil JK, Krishna PW. A comparative study of high sensitivity C-reactive protein and metabolic variables in type 2 diabetes mellitus with and without nephropathy. J Clin Diagn Res. 2017;11:BC01–BC04. doi: 10.7860/JCDR/2017/30272.10528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ye J, McGuinness OP. Inflammation during obesity is not all bad: Evidence from animal and human studies. Am J Physiol Endocrinol Metab. 2013;304:E466–77. doi: 10.1152/ajpendo.00266.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fisher E, Schulze MB, Stefan N, Häring HU, Döring F, Joost HG, et al. Association of the FTO rs9939609 single nucleotide polymorphism with C-reactive protein levels. Obesity (Silver Spring) 2009;17:330–4. doi: 10.1038/oby.2008.465. [DOI] [PubMed] [Google Scholar]
- 31.Saucedo R, Valencia J, Gutierrez C, Basurto L, Hernandez M, Puello E, et al. Gene variants in the FTO gene are associated with adiponectin and TNF-alpha levels in gestational diabetes mellitus. Diabetol Metab Syndr. 2017;9:32. doi: 10.1186/s13098-017-0234-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tupikowska-Marzec M, Kolačkov K, Zdrojowy-Wełna A, Słoka NK, Szepietowski JC, Maj J. The influence of FTO polymorphism rs9939609 on obesity, some clinical features, and disturbance of carbohydrate metabolism in patients with psoriasis. Biomed Res Int. 2019;2019:7304345. doi: 10.1155/2019/7304345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zimmermann E, Skogstrand K, Hougaard DM, Astrup A, Hansen T, Pedersen O, et al. Influences of the common FTO rs9939609 variant on inflammatory markers throughout a broad range of body mass index. PLoS One. 2011;6:e15958. doi: 10.1371/journal.pone.0015958. [DOI] [PMC free article] [PubMed] [Google Scholar]


