BACKGROUND
COVID19 is an emerging pandemic outbreak that is changing our life causing a big challenge worldwide.[1] A major concern is being the virus highly and rapidly contagious, very protean clinical features along with the poor preparedness of global armamentarium against this “gate-crasher.” Apart from proper preventive measures, we as clinicians should find a way to cope optimally with the inflicted victims. Given the new face of a wild disease in addition to the absence adequate data on the treatment of COVID19 no standard of care is proposed so far; a great burden on health care systems. Most of the international and local guidelines on treatments are based on empirical and/or anecdotal reports with overall disappointing results and rather high morbidity and mortality.[2]
These include both anti-viral and immunomodulatory modalities. Among the anti-viral agents, more populous drugs are lopinavir-ritonavir, remdesivir, favipiravir, umifenovir, and oseltamivir (abandoned to date) prescribed with different protocols. On the other hand, there are plenty of nonantiviral/supportive approaches; namely stem-cell therapy, plasma treatment, colchicine, methylprednisolone, intravenous (IV) immunoglobulin, antimalarials, interferons (alfa, beta), extracorporeal membrane oxygenation, ozonated autohemotherapy, mono-clonal antibodies (tocilizumab).[3] Considering the global burden of disease and treatment failures worldwide, this idea is to correct the proposed international guidelines,[4,5] that discourages administering glucocorticoids (GCs), due to the lack of evidence. We hope with further global investigations we would have better treatment protocols.
UNIQUE IMMUNE RESPONSE IN COVID19
In viral pneumonias, lung tissue reaction is usually mild and mostly natural killer (NK) cells, and cytotoxic T-cells are involved and interferons are secreted. Interferon Type-I is secreted by infected cells with viruses, while Type-II from T-cells, NK cells, and macrophages boost the immune system against viruses.[6]
A two-phase immune response for COVID19 is proposed by Yufang Shi; an initial immune defense-based protective phase in very early phase of clinical disease and postinitial inflammation-driven damaging phase. The adaptive immune response is the major mechanism for the former and the innate immune response for the latter.[7]
From clinical standpoint, a majority of patients with COVID19 have positive imaging findings on computed tomography (CT) images suggestive of tissue infiltrations, fibromyxoid exudation, hyaline membrane formation, and in later stages forthcoming damage and eventual fibrosis. The full-blown immune response is presented as cytokine storm.[7]
OTHER SIDE OF THE COIN
The treatment strategy in the initial phase (immune defense-based protective phase) of the viral attack is to combat viruses with specific antiviral and immune-boosting therapies, i.e., interferons. While as the patients deteriorate into later stages of disease, host immunological response damages outweigh its protective role that merit judicious use of immunosuppressive agents. Unfortunately, most of the patients (CT positive cases) have already entered the inflammatory phase of the disease, and we theoretically have lost the window of opportunity for anti-viral therapy. Hence, the cornerstone of therapy, should be targeted toward the suppression of host overwhelming inflammatory reactions to halt more and more tissue damage.
A common pitfall in caring patients with COVID19 is to intermix different phases of pathophysiology and overemphasizing the antiviral agents. There is huge controversy dealing with this important issue among different disciplines caring COVID19 patients, for example, infectious disease specialists, and pulmonologists. Most practicing physicians are prescribing an antiviral agent along with an antimalarial usually with azithromycin with or without naproxen or acetaminophen as rescue medications. In general, they are concerned about the risky approach of immunomodulation and the paradoxical negative effects of therapy on this viral disease.[8] Noteworthy, there are a lot of differences between immunomodulation versus immunosuppression on both basic and clinical grounds.[9] However, plenty of studies indicate that the main pathogenic event in respiratory failure and other organ impairment results from uncontrolled protracted immunity rather than the virus itself.[10] Resembling the “Trojan horse” story, in which the novel coronavirus is the wooden horse and invasive immune cells as the men inside. Considering the fact that most of the patients with COVID19 are successfully recovered, it could be postulated that handling of virus load in the immune-competent host is not a major problem in clinical COVID19. Instead, different immunological responses possibly based on genetic background (e.g., human leukocyte antigen) may be the case.[11,12]
CASE SELECTION FOR IMMUNOMODULATION/GLUCOCORTICOIDS IN COVID19
According to the mentioned notions, the best time for considering immunomodulatory measures could be just after first objective signs of organ involvement, prior to decompensated organ failure, without any concerns without any concerns of the wooden horse – the virus – in a previously immunocompetent host.
Up to this point, we have covered “when” to start immunomodulation, the critical question now would be “who” are the best candidates? In a short sentence, a typical candidate for immunomodulation with GCs in a rational manner could be an already healthy person with typical lung involvement (on CT) without any comorbid conditions or overt objective signs of frank infection. They should already have received appropriate antiviral, hydroxychloroquine, and also an antibiotic with bimodal effect on both bacterial superinfection and inflammation itself. Typically, these patients are those who have passed the early (viral) phase of disease, entering the inflammatory phase [Figure 1]. Oral tetracyclines may be the best choice that should be started upon diagnosing parenchymal lung involvement.[13] We think anosmia and ageusia, that are commonly seen in COVID19, are instances of organ damage, and should probably be considered for systemic GCs as mentioned earlier.
Figure 1.
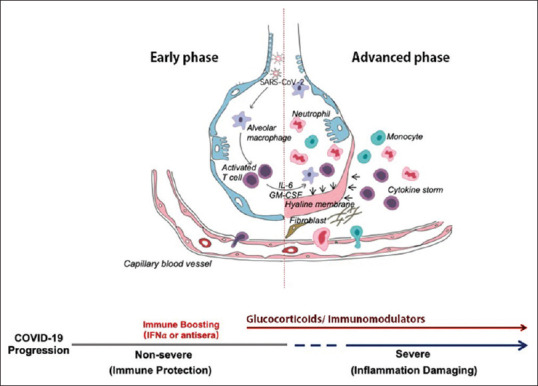
Schematic illustration depicting alveolar-capillary membrane changes in lung parenchyma in patients with COVID19 indicating a two-phase model theory addressing the proper timing of administering the two arms of treatment, antivirals and immunomodulators. Reconstructed with permission (Yufang Shi; doi: 10.1038/s41418-020-0530-3; March 26, 2020)
On the other hand, COVID19 patients who are not the best candidates (but not discouraged at all)–at the present time–waiting for more comprehensive results–are elderly people, patients with advanced acute respiratory distress syndrome and other organ failures impending to cardiovascular collapse, uncontrolled diabetes, preexisting cardiac, renal or hepatic impairment, and also morbid obese patients. Nonetheless, as a rule, we do not recommend GC prescription in patients with very mild disease.
THE ISSUE OF SAFE IMMUNOMODULATION
There are different drugs to modulate overactive immune response consisting of a wide range of drug categories, including glucocorticoids, cytotoxics, calcineurin inhibitors, and disease-modifying anti-rheumatic drugs.
Among them, GCs have unique action on immune system; with the advantage of blocking the inflammatory cascade from the origin (i.e., phospholipase A2 and most importantly on a nongenomic basis by transmembrane electrolyte changes using IV pulsed route of administration). Other benefits deal with global availability, very low cost, the prompt onset of action using the pulsed method, and finally–that is pivotal in this very setting, infection being a matter–its excellent titrability compared to the long-lasting biologic agents and monoclonal antibodies with rather long duration of immune blockage.
The major concern among physicians to use GCs is unwanted side effects like immunosuppression (which may theoretically lead to increased viral shedding), brittle diabetes, hypertension crisis, avascular necrosis, osteoporosis; that are generally seen when GCs with high-dosage, short-intervals, long-term, and long half-life ones (e.g., dexamethasone) are used. Hence, administering GCs with long intervals (IV pulsed and/or oral alternate doses), short-term use (two to three weeks), and recruiting short half-life GCs (e.g., prednisone), and proper timing according to our study and years of experience.[14,15]
Similar causal relationship between a viral trigger and the consequent immune damage is seen in clinical scenarios like polyarteritis nodosa associated with hepatitis B or C, and cryoglobulinemic vasculitis secondary to hepatitis C. The standard of care is to prescribe immnuosuppression before antiviral therapy.[16]
Authors’ experience on more than twenty-five Iranian COVID19 cases of moderate severity refractory to the conventional local/international protocols (tetracycline, oseltamivir/ Kaletra, and hydroxychloroquine) after 7–10 days; resulted in dramatic and long-lasted response from oral alternate prednisolone (25 mg) without any unwanted events up until 4 weeks after initiation of prednisolone.
CONCLUSION
Considering the two-phase pathophysiology of the COIVD19, immunomodulation with GCs has a crucial role in halting the pathologic process and should not be underemphasized. Proper case selection is the key.
REFERENCES
- 1.WHO Announces COVID-19 Outbreak a Pandemic. 2020 [Google Scholar]
- 2.Baud D, Qi X, Nielsen-Saines K, Musso D, Pomar L, Favre G. Real estimates of mortality following COVID-19 infection. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30195-X. published online ahead of print, 2020 Mar 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belhadi D, Peiffer-Smadja N, Yazdanpanah Y, Mentré F, Laouénan C. A brief review of antiviral drugs evaluated in registered clinical trials for COVID-19 Preprint from medRxiv, 20 Mar 2020. doi: 101101/2020031820038190. [Google Scholar]
- 4.Management of Patients with Confirmed 2019-nCoV | CDC. [Last accessed on 2020 Apr 10]. Available from: https://wwwcdcgov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patientshtml .
- 5.Case Management. [Last accessed on 2020 Apr 10]. Available from: https://wwwwhoint/emergencies/diseases/novel-coronavirus-2019/technical-guidance/patient-management .
- 6.Le Page C, Génin P, Baines MG, Hiscott J. Interferon activation and innate immunity. Rev Immunogenet. 2000;2:374–86. [PubMed] [Google Scholar]
- 7.Shi Y, Wang Y, Shao C, Huang J, Gan J, Huang X, et al. COVID-19 infection: The perspectives on immune responses. Cell Death Differ. 2020;27:1451–4. doi: 10.1038/s41418-020-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Mailhe M, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: Results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020:105949. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–2. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dutta M, Dutta P, Medhi S, Borkakoty B, Biswas D. Polymorphism of HLA class I and class II alleles in influenza A (H1N1) pdm09 virus infected population of Assam, Northeast India. J Med Virol. 2018;90:854–60. doi: 10.1002/jmv.25018. [DOI] [PubMed] [Google Scholar]
- 11.Matzaraki V, Kumar V, Wijmenga C, Zhernakova A. The MHC locus and genetic susceptibility to autoimmune and infectious diseases. Genome Biol. 2017;18:76. doi: 10.1186/s13059-017-1207-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kovarik J. From immunosuppression to immunomodulation: Current principles and future strategies. Pathobiology. 2013;80:275–81. doi: 10.1159/000346960. [DOI] [PubMed] [Google Scholar]
- 13.Sodhi M, Etminan M. Therapeutic potential for tetracyclines in the treatment of COVID-19. Pharmacotherapy. 2020 doi: 10.1002/phar.2395. published online ahead of print, 2020 Apr 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghasemian M, Owlia M. A different look at pulsed glucocorticoid protocols; is high dose oral prednisolone really necessary just after initiation of pulse therapy. J Case Rep Pract. 2015;3:1–3. [Google Scholar]
- 15.Owlia MB, Mehrpoor G, Modares Mosadegh M. Bedtime single-dose prednisolone in clinically stable rheumatoid arthritis patients. ISRN Pharmacol. 2012;2012:637204. doi: 10.5402/2012/637204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goglin S, Chung S. A. Current treatment of cryoglobulinemic vasculitis. Curr Treat Options Rheumatol. 2016;2:213–24. [Google Scholar]


