Abstract
Several microRNAs (miRNA/miR) have been reported to serve critical roles in tumorigenesis. The present study aimed to investigate miR-518b expression in non-small cell lung cancer (NSCLC), and determine its clinical significance and biological function in this malignancy. Reverse transcription-quantitative PCR was performed to assess miR-518b expression in NSCLC. The diagnostic value of miR-518b was determined via a receiver operating characteristic curve, while its prognostic value was assessed using the Kaplan-Meier method. Gain- and loss-of-function experiments were performed to determine the functional role of miR-518b in NSCLC progression. The results demonstrated that miR-518b expression was upregulated in NSCLC serum, tissues and cell lines compared with the corresponding normal controls. Furthermore, high miR-518b expression was significantly associated with larger tumor size, lymph node metastasis and advanced TNM stage, as well as poor overall survival in patients with NSCLC. Serum miR-518b expression was identified as a candidate diagnostic biomarker for NSCLC, with sensitivity of 88.1% and specificity of 81.7%. Furthermore, the cell experiments indicated that NSCLC cell proliferation, migration and invasion were enhanced following overexpression of miR-518b; however, these effects were reversed following miR-518b knockdown. Taken together, the results of the present study suggest that elevated miR-518b expression in NSCLC serves a potential oncogenic role by facilitating tumor cell proliferation, migration and invasion, and thus may serve as a candidate diagnostic and prognostic biomarker.
Keywords: microRNA-518b, non-small cell lung cancer, diagnosis, prognosis, proliferation, migration, invasion
Introduction
Lung cancer remains the most prevalent malignancy and a leading cause of global cancer-associated mortalities (1). According to the statistics from 2012, there were 1.8 million new lung cancer cases and 1.59 million deaths worldwide (2). Non-small cell lung cancer (NSCLC) is the most common subtype of lung cancer, accounting for ~80% of all cases (3). Metastasis and invasion are considered two major biological characteristics of NSCLC, which pose treatment challenges and continue to increase mortality in patients with NSCLC (4). A lack of typical clinical manifestations in patients with lung cancer further contributes to NSCLC mortality, as it is difficult to effectively diagnose NSCLC at the early stages (5). Despite major advancements in therapeutic strategies, such as surgery, chemotherapy and radiotherapy, the 5-year overall survival rate of patients with NSCLC remains <15% (6). Thus, it is critical to identify and develop novel biomarkers involved in tumor progression for effective NSCLC diagnosis, prognosis and treatment.
Currently, a number of tumor-associated molecules have been confirmed to be involved in NSCLC progression, such as coiled-coil domain containing 106, long non-coding RNA XIST and microRNA-16 (7,8). microRNAs (miRNAs/miR) are a group of non-coding small RNA molecules that function in regulating tumor initiation and progression (9). miRNAs regulate gene expression by directly binding to the 3′- untranslated region of target mRNAs (10), and influence several cellular processes, including cell proliferation, migration and invasion (11). Previous studies have reported the pivotal roles of miRNAs in different types of human cancer, such as glioma, breast cancer and NSCLC (12–15). Furthermore, the clinical significance of miRNAs has been highlighted through their effective diagnostic and prognostic values in different types of cancer (16,17). For example, serum elevated miR-191 and miR-425 levels are biomarkers for gastric cancer diagnosis and prognosis (18). The increased expression of miR-665 in patients with lung cancer has been reported to predict poor prognosis (19).
miR-518b is a member of the functional miRNAs (20), which has been investigated in hepatocellular carcinoma (21,22), chondrosarcoma (23) and esophageal squamous cell carcinoma (24). Regarding hepatocellular carcinoma, Zheng et al (21) and Wang et al (22) demonstrated that miR-518b expression is elevated in tumor samples compared with the normal controls, while miR-518b expression is downregulated in chondrosarcoma (23) and esophageal squamous cell carcinoma (24). These results suggest that miR-518b expression varies in different types of human cancer. In NSCLC, an in silico study reported that miR-518b expression was higher in tumor samples compared with the normal controls (25). However, the precise expression patterns of miR-518b in NSCLC clinical samples, as well as its role in tumor progression remain unclear.
The present study aimed to determine the biological role and clinical significance of miR-518b in patients with NSCLC, and investigate the regulatory effects of miR-518b on NSCLC cell proliferation, migration and invasion. Taken together, the results of the present study suggest that miR-518b may serve as a novel potential diagnostic and prognostic biomarker, and a candidate therapeutic target for NSCLC treatment.
Materials and methods
Patients and serum and tissue sample collection
The present study was approved by the Ethics Committee of Qilu Hospital Huantai Branch (Zibo, China), and written informed consent was provided by all participants prior to the study start. A total of 118 patients, including 48 females and 70 males with a mean age of 58.37±12.37 years (age range, 34–85 years), who were pathologically diagnosed with NSCLC at the Qilu Hospital Huantai Branch, were enrolled in the present study between January 2011 and December 2013. The inclusion criteria were as follows: i) All cases received their first surgical resection at the Qilu Hospital Huantai Branch and were pathologically diagnosed with NSCLC; ii) Patients who had not received any previous preoperative antitumor therapy; iii) Patients who had no history of exposure to asbestos; and iv) Patients with complete clinicopathological data and follow-up information. A total of 60 healthy volunteers, including 25 females and 35 males with a mean age of 57.62±12.06 years (age range, 35–83 years), who had no history of malignancy, were also enrolled in the present study as the controls. Blood samples were collected from the patients and healthy individuals, and immediately centrifuged at 1500 × g at 4°C for 10 min for serum extraction. Tumor tissues and adjacent normal tissues (controls; at least 3 cm from the edge of tumor) were collected from patients during surgical resection. The Tumor-Node-Metastasis (TNM) stage of the tumor tissues was determined using the criteria from the American Joint Committee on Cancer classification (26). All serum and tissue samples were stored at −80ºC until subsequent experimentation. The demographic and clinical characteristics of the patients and the survival information obtained from a 5-year follow-up survey were recorded for subsequent analyses. During the 5-year follow-up, the patients were followed up every 3 months in the first 2 years, then after every 6 months for the subsequent 2 years and annually for the last year.
Cell culture and transfection
A normal human lung epithelial cell line (BEAS-2B) and four NSCLC cell lines (A549, H1299, H1975 and PC9) were purchased from the Shanghai Institutes for Biological Sciences of the Chinese Academy of Sciences. Cells were cultured in DMEM supplemented with 10% FBS (Invitrogen; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin and 100 µg/ml streptomycin at 37 ºC in 5% CO2.
A549 and PC9 cells were seeded into 6-well plates at a density of 5×104 cells/well and transfected with 50 nM of miR-518b mimic, miR-518b inhibitor or non-targeting miRNA negative control (miR-NC) using Lipofectamine® 3000 reagent (Invitrogen; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol. Following were the sequences of the vectors: miR-518b mimic, 5′-CAAAGCGCUCCCCUUUAGAGGU-3′; miR-518b inhibitor, 5′-ACCUCUAAAGGGGAGCGCUUUG-3′; miR-NC, 5′-UUCUCCGAACGUGUCACGU-3′. All vectors were synthesized by Shanghai GenePharma Co., Ltd., and all the experiments were performed in triplicate. Subsequent experiments were performed 48 h post-transfection.
Reverse transcription-quantitative (RT-q)PCR
Total RNA was extracted from serum of patients and healthy controls, tissues of patients and NSCLC cell lines using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.). Total RNA was reverse transcribed into cDNA using the PrimeScript™ RT reagent kit (Takara Bio, Inc.). All the experiments were performed following manufacturer's protocols. qPCR was subsequently performed using the SYBR Green I Master mix kit (Invitrogen; Thermo Fisher Scientific, Inc.) and a 7500 Real-Time PCR System (Applied Biosystems; Thermo Fisher Scientific, Inc.). The thermocycling conditions were as follows: Initial denaturation at 95°C for 10 min; 40 cycles of denaturation at 95°C for 30 sec, annealing at 60°C for 20 sec and elongation at 72°C for 30 sec; and final extension at 72°C for 10 min. The following primer sequences were used for qPCR: miR-518b forward, 5′-GCCGAGCAAAGCGCTCCCCT-3′, and reverse, 5′-CTCAACTGGTGTCGTGGA-3′; and U6 forward, 5′-CTCGCTTCGGCAGCACA-3′ and reverse, 5′-AACGCTTCACGAATTTGCGT-3′. Relative miR-518b expression levels were measured using the 2−ΔΔCq method (27) and normalized to the internal reference gene U6.
Cell Counting Kit-8 (CCK-8) assay
The CCK-8 assay was performed to assess NSCLC cell proliferation. A549 and PC9 were seeded into 96-well plates at density of 5×103 cells/well (100 µl/well) and cultured at 37ºC for 72 h. A volume of 10 µl CCK-8 reagent (Sigma-Aldrich; Merck KGaA) was added to the plates at 0, 24, 48 and 72 h, and incubated at 37ºC for 2 h at each time point according to the manufacturer's instructions. Cell proliferation was subsequently analyzed at a wavelength of 450 nm, using a microplate reader (BioTek).
Migration and invasion assays
A549 and PC9 cells were plated in the upper chambers (cell density of 3×105 cells/well) of Transwell plates in serum-free DMEM medium (Invitrogen; Thermo Fisher Scientific, Inc.) and incubated at 37ºC for 24 h. Transwell membranes were pre-coated with Matrigel (Corning, Inc.) at 37°C for 1 h for the invasion assay. The DMEM medium supplemented with 10% FBS was plated in the lower chambers. After 24 h of incubation at 37°C, the migratory and invasive cells in the lower chambers were stained with 0.1% crystal violet at room temperature for 10 min and counted in five randomly-selected fields using a light microscope (magnification, ×200).
Statistical analysis
Statistical analysis was performed using SPSS (version 21.0; IBM Corp.) and GraphPad Prism (version 7.0; GraphPad Software, Inc.) software. Data are presented as the mean ± standard deviation and all experiments were performed in triplicate. Paired Student's t-test was used to compare differences of miR-518b expression between tumor tissues and non-tumor tissues, and unpaired Student's t-test was used to compared the serum expression of miR-518b between patients with NSCLC and healthy controls, while one-way ANOVA, followed by Tukey's post-hoc test was used to compare differences between multiple groups. The expression of miR-518b was divided into low and high expression group based on the mean expression value (1.20 for serum miR-518b expression; 3.45 for tissue miR-518b expression), then the association between miR-518b expression and clinicopathological characteristics of patients with NSCLC was determined using the χ2 test. A receiver operating characteristic (ROC) curve was plotted to determine the diagnostic value of miR-518b, while a Kaplan-Meier survival curve was generated to assess the prognostic value of miR-518b in patients with NSCLC and the log-rank test was used to compare the differences between the survival curves. A multivariate Cox regression analysis was performed to verify miR-518b as a prognostic indicator. P<0.05 was considered to indicate a statistically significant difference.
Results
miR-518b expression is upregulated in NSCLC serum, tissues and cell lines
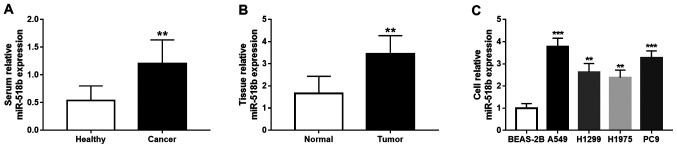
The results demonstrated that miR-518b expression levels in the serum and tissue samples significantly increased in patients with NSCLC compared with the healthy controls and adjacent normal tissues, respectively (both P<0.01; Fig. 1A and B). Similarly, miR-518b expression significantly increased in all four NSCLC cell lines compared with BEAS-2B cells (all P<0.01; Fig. 1C), and a greater significance was observed for the upregulation of miR-518b in A549 and PC9 cell lines (both P<0.001).
Figure 1.
miR-518b expression in NSCLC. (A) miR-518b serum expression levels significantly increased in patients with NSCLC compared with healthy volunteers (**P<0.01 compared to healthy controls). (B) miR-518b tissue expression levels were significantly higher in NSCLC tissues compared with adjacent normal tissues (**P<0.01 compared to normal controls). (C) miR-518b expression was significantly upregulated in all four NSCLC cell lines (A549, H1299, H1975 and PC9) compared with normal BEAS-2B cells (**P<0.01, ***P<0.001 compared to BEAS-2B). miR, microRNA; NSCLC, non-small cell lung cancer.
Association between miR-518b expression and clinicopathological characteristics of patients with NSCLC
The clinicopathological characteristics of patients with NSCLC are presented in Table I. Patients were divided into low (n=58) and high (n=60) miR-518b expression groups based on serum mean expression value of miR-518b. Meanwhile, according to the mean value of miR-518b in tumor tissues, patients were grouped into low miR-518b group (n=54) and high miR-518b group (n=64). The results demonstrated that serum miR-518b expression was significantly associated with tumor size (P=0.042), TNM stage (P=0.006) and lymph node metastasis (P=0.039). Similarly, tissue miR-518b expression was significantly associated with tumor size (P=0.014), TNM stage (P=0.006) and lymph node metastasis (P=0.031) in patients with NSCLC. However, no significant associations were observed between miR-518b expression and age, sex, smoking status, histological type and degree of differentiation (all P>0.05).
Table I.
Association between miR-518b expression and clinicopathological characteristics of patients with non-small cell lung cancer (n=118).
| Serum miR-518b expression | Tissue miR-518b expression | ||||||
|---|---|---|---|---|---|---|---|
| Characteristic | Patients, n | Low (n=58) | High (n=60) | P-value | Low (n=54) | High (n=64) | P-value |
| Age, years | 0.969 | 0.569 | |||||
| ≤60 | 47 | 23 | 24 | 20 | 27 | ||
| >60 | 71 | 35 | 36 | 34 | 37 | ||
| Sex | 0.879 | 0.265 | |||||
| Female | 48 | 24 | 24 | 19 | 29 | ||
| Male | 70 | 34 | 36 | 35 | 35 | ||
| Smoking status | 0.732 | 0.554 | |||||
| Never | 49 | 25 | 24 | 24 | 25 | ||
| Previous/Current | 69 | 33 | 36 | 30 | 39 | ||
| Histological type | 0.791 | 0.946 | |||||
| Adenocarcinoma | 70 | 34 | 36 | 32 | 38 | ||
| Squamous cell carcinoma | 36 | 19 | 17 | 16 | 20 | ||
| Othersa | 12 | 5 | 7 | 6 | 6 | ||
| Tumor size, cm | 0.042 | 0.014 | |||||
| ≤3 | 62 | 36 | 26 | 35 | 27 | ||
| >3 | 56 | 22 | 34 | 19 | 37 | ||
| Differentiation | 0.127 | 0.097 | |||||
| Well/Moderate | 69 | 38 | 31 | 36 | 33 | ||
| Poor | 49 | 20 | 29 | 18 | 31 | ||
| TNM stage | 0.006 | 0.006 | |||||
| I–II | 58 | 36 | 22 | 34 | 24 | ||
| III–IV | 60 | 22 | 38 | 20 | 40 | ||
| Lymph node metastasis | 0.039 | 0.031 | |||||
| Negative | 66 | 38 | 28 | 36 | 30 | ||
| Positive | 52 | 20 | 32 | 18 | 34 | ||
large cell carcinoma and adenosquamous carcinoma. miR, microRNA; TNM, tumor-node-metastasis.
Diagnostic value of miR-518b in patients with NSCLC
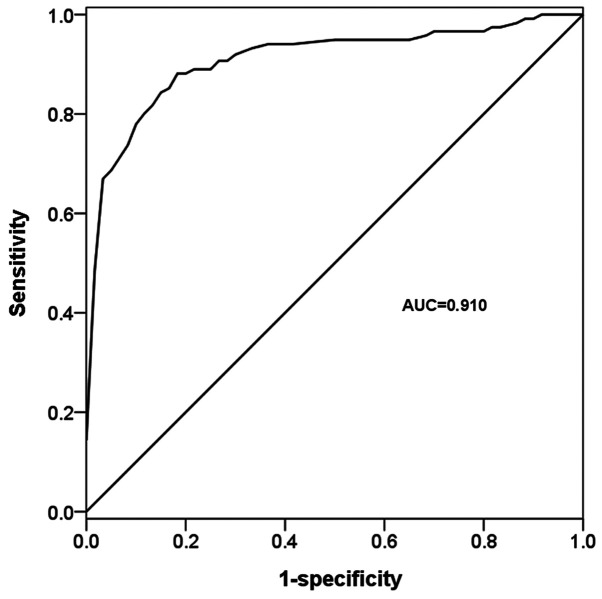
Molecules aberrantly expressed in the serum of patients with cancer are considered effective diagnostic tools (28). In the present study, the diagnostic value of serum miR-518b was determined by analyzing its deregulated expression in patients with NSCLC. The ROC curve based on serum miR-518b expression exhibited an area under the curve value of 0.910, with 88.1% sensitivity and 81.7% specificity, under the cut-off value of 0.745 (Fig. 2), which indicated the diagnostic accuracy of serum miR-518b in patients with NSCLC.
Figure 2.
Receiver operating characteristic curve according to microRNA-518b serum expression in patients with non-small cell lung cancer and healthy individuals. The AUC value was 0.910, with 88.1% sensitivity and 81.7% specificity. AUC, area under the curve.
Prognostic value of miR-518b in patients with NSCLC
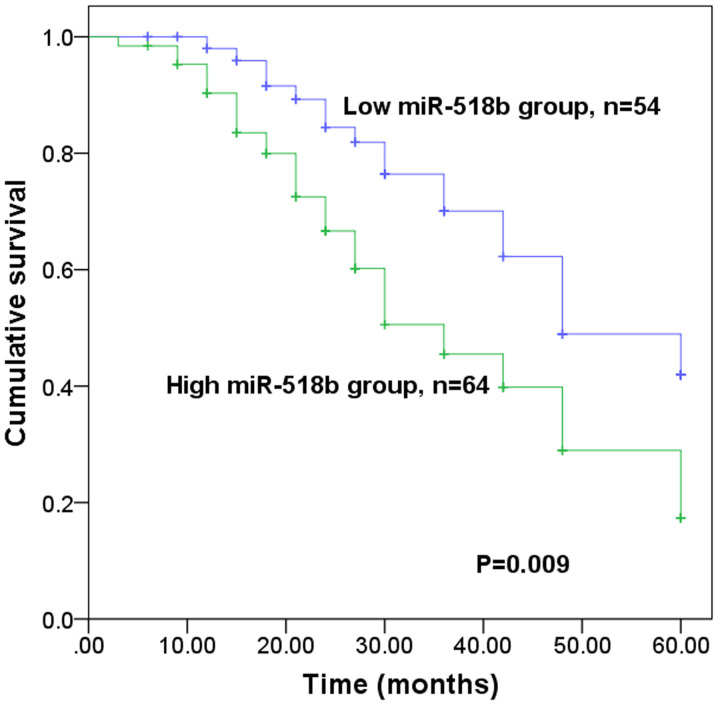
The present study further investigated the prognostic value of miR-518b in patients with NSCLC. The results demonstrated that patients with high miR-518b expression levels experienced a shorter survival time than those with low miR-518b expression levels (P=0.009; Fig. 3). Furthermore, Cox regression analysis indicated that miR-518b expression may serve as an independent prognostic indicator in patients with NSCLC (P=0.012; hazard ratio=2.270; 95% confidence interval=1.197–4.305; Table II).
Figure 3.
Kaplan-Meier survival curve for patients with non-small cell lung cancer, according to miR-518b expression levels. Low and high miR-518b expression was defined based on the mean expression value of miR-518b in tumor tissues and high miR-518b expression was associated with a shorter survival time. miR, microRNA.
Table II.
Multivariate Cox regression analysis for miR-518b in patients with non-small cell lung cancer.
| Cox regression analysis | |||
|---|---|---|---|
| Characteristic | HR | 95% CI | P-value |
| miR-518b | 2.270 | 1.197–4.305 | 0.012 |
| Age | 1.064 | 0.601–1.884 | 0.832 |
| Sex | 1.060 | 0.583–1.927 | 0.850 |
| Smoking status | 1.425 | 0.802–2.531 | 0.227 |
| Histological type | 2.408 | 0.562–10.326 | 0.495 |
| Tumor size | 1.123 | 0.610–2.068 | 0.710 |
| Differentiation | 1.198 | 0.652–2.198 | 0.560 |
| TNM | 5.359 | 1.177–24.389 | 0.030 |
| Lymph node metastasis | 5.957 | 1.374–25.829 | 0.017 |
miR, microRNA; TNM, tumor-node-metastasis; HR, hazard ratio; CI, confidence interval.
Overexpression of miR-518b facilitates NSCLC cell proliferation, migration and invasion
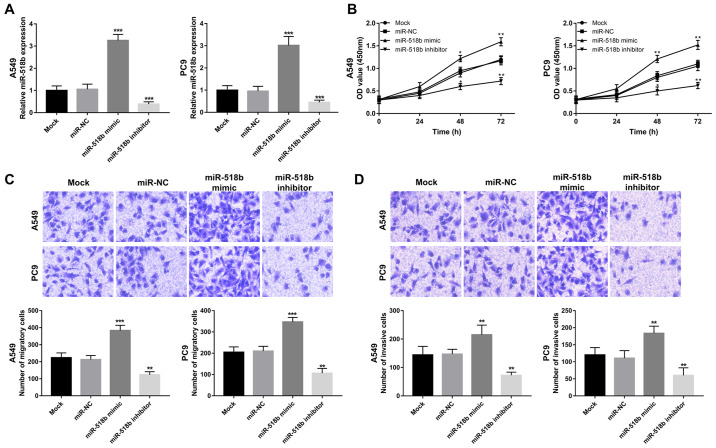
The biological function of miR-518b in NSCLC progression was further investigated in A549 and PC9 cells as the expression of miR-518b was significantly elevated compared with normal cells. miR-518b expression was successfully regulated in vitro via cell transfection, evidenced by increased miR-518b expression induced by miR-518 mimic, and decreased miR-518b expression induced by miR-518b inhibitor (all P<0.001; Fig. 4A). Results from the CCK-8 assay, and cell migration and invasion assays demonstrated that overexpression of miR-518b in NSCLC cells enhanced cell proliferation, migration and invasion, while miR-518b knockdown inhibited NSCLC cell proliferation, migration and invasion, respectively (all P<0.05; Fig. 4B-D).
Figure 4.
Effects of miR-518b on NSCLC cell proliferation, migration and invasion in A549 and PC9 cell lines. (A) miR-518b expression in A549 and PC9 cells was upregulated following transfection with miR-518b mimic and downregulated following transfection with miR-518b inhibitor. (B) NSCLC cell proliferation in A549 and PC9 cells was facilitated by overexpression of miR-518b and inhibited following knockdown of miR-518b. Overexpression of miR-518b enhanced (C) cell migration and (D) invasion in A549 and PC9 cells, while miR-518b knockdown reversed these effects. Magnification, ×200; *P<0.05, **P<0.01, ***P<0.001, compared to mock. miR, microRNA; NSCLC, non-small cell lung cancer; NC, negative control.
Discussion
Several aberrantly expressed miRNAs have been reported to serve crucial roles in tumor pathology of different types of human cancer, such as gastric (29), breast (30) and lung cancer (31). The present study aimed to determine the clinical significance and biological function of miR-518b in NSCLC. The results of the present study demonstrated significantly increased miR-518b expression in NSCLC serum, tissues and cell lines compared with the corresponding normal controls. Furthermore, elevated miR-518b expression levels in serum and tissues were associated with tumor size, lymph node metastasis and TNM stage of patients with NSCLC. Serum miR-518b expression had potential diagnostic value to distinguish patients with NSCLC from healthy individuals, and miR-518b expression in tumor tissues was identified as an independent prognostic indicator in patients with NSCLC. The gain- and loss-of-function experiments demonstrated that the cell proliferation, migration and invasion abilities of NSCLC cells were enhanced following overexpression of miR-518b, while miR-518b knockdown reversed these effects. Taken together, the results of the present study suggest that miR-518b may represent a novel molecule that can be used improve NSCLC diagnosis and prognosis. Furthermore, determining the biological function of miR-518b may help to better understand its underlying molecular mechanisms in the pathogenesis of NSCLC.
The significant roles of miRNAs have been highlighted in human malignancies in recent decades. For example, aberrantly expressed miRNAs are associated with tumorigenesis and have attracted considerable attention in their role as diagnostic and prognostic biomarkers in several types of cancer, such as bladder cancer and hepatocellular carcinoma (32,33). Thus, the expression profiles of miRNAs remain an important focus in the research field regarding the treatment of human malignancies. In patients with NSCLC, several aberrantly expressed miRNAs have been identified. For example, Du et al (34) demonstrated that miR-335-3p expression is downregulated in NSCLC tissues compared with normal tissues. Furthermore, downregulated miR-7-5p expression has been reported in NSCLC tissues and cell lines, which exerts regulatory effects on tumor cell biological processes (35). Overexpression of miR-100 in NSCLC tissues has been demonstrated to predict the poor prognosis of this malignancy (36). miR-518b expression has been demonstrated to be downregulated in chondrosarcoma (23) and esophageal squamous cell carcinoma (24), and upregulated in hepatocellular carcinoma (21,22). RT-qPCR analysis in the present study indicated that miR-518b expression levels were elevated in NSCLC serum and tissue samples compared with healthy control and normal tissues, respectively, which was consistent with a previous in silico study that reported increased miR-518b expression in NSCLC (25). Furthermore, miR-518b expression was demonstrated to be significantly associated with tumor size, lymph node metastasis and TNM stage in patients with NSCLC. Taken together, the results of the present study suggest that miR-518b may influence the progression of NSCLC.
A lack of typical clinical symptoms and the complexity of tumor pathogenesis means that diagnosis and prediction of prognosis are problematic, both of which are important for effective cancer management and treatment (37). miRNAs are a group of well-established biomarkers for cancer diagnosis and prognosis (38). Increased serum miR-484 expression has been identified as a potential diagnostic and prognostic biomarker for patients with NSCLC (39). Furthermore, upregulated miR-25 expression has been associated with poor overall survival of patients with NSCLC, and is considered to serve as an independent prognostic indicator (40). Li et al (41) reported that patients with NSCLC, with high miR-421 expression had a shorter overall survival time compared with low miR-421 expression levels. These previous findings indicate the significant clinical significance of miRNAs in the diagnosis and prognosis of NSCLC. In the present study, a ROC curve was plotted according to serum miR-518b expression, which demonstrated the diagnostic accuracy of miR-518b in differentiating between patients with NSCLC and healthy individuals. Furthermore, the sensitivity and specificity of serum miR-518b were 88.1 and 81.7%, respectively, indicating the potential of miR-518b as a novel candidate diagnostic biomarker of NSCLC. The survival analysis implied that miR-518b was associated with overall survival time, thus may function as an independent prognostic biomarker in patients with NSCLC. However, the present study is not without limitations. A small sample size was implemented, thus prospective studies will aim to use larger cohorts to validate the clinical significance of miR-518b, in order to determine whether it can be used as an early biomarker in NSCLC.
It has been reported that miRNAs serve critical regulatory functions in several biological processes, such as cell proliferation, migration and invasion (42). Previous studies have investigated the functional roles of miRNAs in tumorigenesis in different types of human cancer, including NSCLC (43–45). For example, miR-650 has been reported to be highly expressed in NSCLC tissues and cells, which promotes tumor cell proliferation and invasion (46). Furthermore, Tian et al (47) reported that overexpression of miR-16 in NSCLC cells suppresses cell proliferation, migration and invasion abilities, indicating the potential of miR-16 as a therapeutic target of NSCLC. In the present study, cell experiments were also performed, which provided evidence supporting the role of miR-518b as an oncogenic miRNA. The results demonstrated that overexpression miR-518b enhanced NSCLC cell proliferation, migration and invasion, while miR-518b knockdown resulted in the opposite effects. Although the present study provided novel insight into the functional role of miR-518b, the underlying molecular mechanisms remain unclear.
Rap1b has been identified as a target gene for miR-518b during its inhibiting effects on the cell proliferation and invasion of esophageal squamous cell carcinoma (24). Furthermore, Kushwaha et al (48) reported that miR-518b regulates epithelial lineage development by targeting forkhead box N1 (FOXN1). Notably, FOXN1 has been identified as a tumor suppressor in NSCLC cells and exerts its effects via inhibiting tumor cell proliferation and invasion (49). Thus, it is speculated that miR-518b may regulate tumor progression in NSCLC cells by targeting FOXN1. However, further studies are required to determine whether FOXN1 has the ability to mediate the biological function of miR-518b in NSCLC progression.
In conclusion, the results of the present study demonstrated that miR-518b expression was upregulated in serum, tissues and cell lines in NSCLC, thus miR-518b may serve as a candidate non-invasive biomarker for the diagnosis and prognosis of NSCLC. Furthermore, miR-518b may function as a potential oncogene in NSCLC tumorigenesis as its knockdown resulted in the inhibition of tumor cell proliferation, migration and invasion abilities, indicating that downregulated miR-518b expression may improve the treatment of NSCLC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
XZ and CZ designed the study, collected and analyzed the clinical data, drafted and revised the manuscript. YH and CG conducted the cell experiments and analyzed the data. All authors have read and approved the manuscript.
Ethics approval and consent to participate
The present study was approved by the Ethics Committee of Qilu Hospital Huantai Branch (Zibo, China; approval no. ZQH-001086), and written informed consent was provided by all participants prior to the study start.
Patients consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Torre LA, Siegel RL, Jemal A. Lung cancer statistics. Adv Exp Med Biol. 2016;893:1–19. doi: 10.1007/978-3-319-24223-1_1. [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 3.Akhurst T. Staging of Non-Small-Cell Lung Cancer. PET Clin. 2018;13:1–10. doi: 10.1016/j.cpet.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 4.Valentino F, Borra G, Allione P, Rossi L. Emerging targets in advanced non-small-cell lung cancer. Future Oncol. 2018;14:61–72. doi: 10.2217/fon-2018-0099. [DOI] [PubMed] [Google Scholar]
- 5.Hirsch FR, Scagliotti GV, Mulshine JL, Kwon R, Curran WJ, Jr., Wu YL, Paz-Ares L. Lung cancer: Current therapies and new targeted treatments. Lancet. 2017;389:299–311. doi: 10.1016/S0140-6736(16)30958-8. [DOI] [PubMed] [Google Scholar]
- 6.Heist RS, Engelman JA. SnapShot: Non-small cell lung cancer. Cancer Cell. 2012;21:448.e2. doi: 10.1016/j.ccr.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 7.Zhang X, Zheng Q, Wang C, Zhou H, Jiang G, Miao Y, Zhang Y, Liu Y, Li Q, Qiu X, Wang E. CCDC106 promotes non-small cell lung cancer cell proliferation. Oncotarget. 2017;8:26662–26670. doi: 10.18632/oncotarget.15792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou X, Xu X, Gao C, Cui Y. XIST promote the proliferation and migration of non-small cell lung cancer cells via sponging miR-16 and regulating CDK8 expression. Am J Transl Res. 2019;11:6196–6206. [PMC free article] [PubMed] [Google Scholar]
- 9.Peng H, Pan X, Su Q, Zhu LS, Ma GD. MiR-372-3p promotes tumor progression by targeting LATS2 in colorectal cancer. Eur Rev Med Pharmacol Sci. 2019;23:8332–8344. doi: 10.26355/eurrev_201910_19144. [DOI] [PubMed] [Google Scholar]
- 10.Shi C, Yang Y, Zhang L, Yu J, Qin S, Xu H, Gao Y. MiR-200a-3p promoted the malignant behaviors of ovarian cancer cells through regulating PCDH9. Onco Targets Ther. 2019;12:8329–8338. doi: 10.2147/OTT.S220339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Z, Zhang L, Wang B, Wei R, Wang Y, Wan J, Zhang C, Zhao L, Zhu X, Zhang Y, et al. MiR-337-3p suppresses proliferation of epithelial ovarian cancer by targeting PIK3CA and PIK3CB. Cancer Lett. 2019;469:54–67. doi: 10.1016/j.canlet.2019.10.021. [DOI] [PubMed] [Google Scholar]
- 12.Chen Y, Gao DY, Huang L. In vivo delivery of miRNAs for cancer therapy: Challenges and strategies. Adv Drug Deliv Rev. 2015;81:128–141. doi: 10.1016/j.addr.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu X, Ban Y, Zhao Z, Pan Q, Zou J. MicroRNA-1298-3p inhibits proliferation and invasion of glioma cells by downregulating Nidogen-1. Aging (Albany NY) 2020;12 doi: 10.18632/aging.103087. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu X. Expressions of miR-21 and miR-210 in breast cancer and their predictive values for prognosis. Iran J Public Health. 2020;49:21–29. [PMC free article] [PubMed] [Google Scholar]
- 15.Wang J, Shu H, Guo S. MiR-646 suppresses proliferation and metastasis of non-small cell lung cancer by repressing FGF2 and CCND2. Cancer Med. 2020 Apr 29; doi: 10.1002/cam4.3062. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qiu Z, Li H, Wang J, Sun C. miR-146a and miR-146b in the diagnosis and prognosis of papillary thyroid carcinoma. Oncol Rep. 2017;38:2735–2740. doi: 10.3892/or.2017.5994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yuan Z, Baker K, Redman MW, Wang L, Adams SV, Yu M, Dickinson B, Makar K, Ulrich N, Bohm J, et al. Dynamic plasma microRNAs are biomarkers for prognosis and early detection of recurrence in colorectal cancer. Br J Cancer. 2017;117:1202–1210. doi: 10.1038/bjc.2017.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bie LY, Li N, Deng WY, Lu XY, Guo P, Luo SX. Serum miR-191 and miR-425 as diagnostic and prognostic markers of advanced gastric cancer can predict the sensitivity of FOLFOX chemotherapy regimen. Onco Targets Ther. 2020;13:1705–1715. doi: 10.2147/OTT.S233086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xia J, Li D, Zhu X, Xia W, Qi Z, Li G, Xu Q. Upregulated miR-665 expression independently predicts poor prognosis of lung cancer and facilitates tumor cell proliferation, migration and invasion. Oncol Lett. 2020;19:3578–3586. doi: 10.3892/ol.2020.11457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xing Z, Li S, Liu Z, Zhang C, Bai Z. CTCF-induced upregulation of HOXA11-AS facilitates cell proliferation and migration by targeting miR-518b/ACTN4 axis in prostate cancer. Prostate. 2020;80:388–398. doi: 10.1002/pros.23953. [DOI] [PubMed] [Google Scholar]
- 21.Zheng J, Sadot E, Vigidal JA, Klimstra DS, Balachandran VP, Kingham TP, Allen PJ, D'Angelica MI, DeMatteo RP, Jarnagin WR, Ventura A. Characterization of hepatocellular adenoma and carcinoma using microRNA profiling and targeted gene sequencing. PLoS One. 2018;13:e0200776. doi: 10.1371/journal.pone.0200776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang W, Zhao LJ, Tan YX, Ren H, Qi ZT. MiR-138 induces cell cycle arrest by targeting cyclin D3 in hepatocellular carcinoma. Carcinogenesis. 2012;33:1113–1120. doi: 10.1093/carcin/bgs113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liang W, Li X, Li Y, Li C, Gao B, Gan H, Li S, Shen J, Kang J, Ding S, Lin X, Liao L. Gallic acid induces apoptosis and inhibits cell migration by upregulating miR-518b in SW1353 human chondrosarcoma cells. Int J Oncol. 2014;44:91–98. doi: 10.3892/ijo.2013.2155. [DOI] [PubMed] [Google Scholar]
- 24.Zhang M, Zhou S, Zhang L, Zhang J, Cai H, Zhu J, Huang C, Wang J. miR-518b is down-regulated, and involved in cell proliferation and invasion by targeting Rap1b in esophageal squamous cell carcinoma. FEBS Lett. 2012;586:3508–3521. doi: 10.1016/j.febslet.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 25.Xu C, Zheng Y, Lian D, Ye S, Yang J, Zeng Z. Analysis of microRNA expression profile identifies novel biomarkers for non-small cell lung cancer. Tumori. 2015;101:104–110. doi: 10.5301/tj.5000224. [DOI] [PubMed] [Google Scholar]
- 26.Singletary SE, Allred C, Ashley P, Bassett LW, Berry D, Bland KI, Borgen PI, Clark GM, Edge SB, Hayes DF, et al. Staging system for breast cancer: Revisions for the 6th edition of the AJCC cancer staging manual. Surg Clin North Am. 2003;83:803–819. doi: 10.1016/S0039-6109(03)00034-3. [DOI] [PubMed] [Google Scholar]
- 27.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 28.Chu GCW, Lazare K, Sullivan F. Serum and blood based biomarkers for lung cancer screening: A systematic review. BMC Cancer. 2018;18:181. doi: 10.1186/s12885-018-4024-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shin VY, Chu KM. MiRNA as potential biomarkers and therapeutic targets for gastric cancer. World J Gastroenterol. 2014;20:10432–10439. doi: 10.3748/wjg.v20.i30.10432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adhami M, Haghdoost AA, Sadeghi B, Malekpour Afshar R. Candidate miRNAs in human breast cancer biomarkers: A systematic review. Breast Cancer. 2018;25:198–205. doi: 10.1007/s12282-017-0814-8. [DOI] [PubMed] [Google Scholar]
- 31.Hashemi ZS, Khalili S, Forouzandeh Moghadam M, Sadroddiny E. Lung cancer and miRNAs: A possible remedy for anti-metastatic, therapeutic and diagnostic applications. Expert Rev Respir Med. 2017;11:147–157. doi: 10.1080/17476348.2017.1279403. [DOI] [PubMed] [Google Scholar]
- 32.Yang X, Wang P. MiR-188-5p and MiR-141-3p influence prognosis of bladder cancer and promote bladder cancer synergistically. Pathol Res Pract. 2019;215:152598. doi: 10.1016/j.prp.2019.152598. [DOI] [PubMed] [Google Scholar]
- 33.Ning S, Liu H, Gao B, Wei W, Yang A, Li J, Zhang L. miR-155, miR-96 and miR-99a as potential diagnostic and prognostic tools for the clinical management of hepatocellular carcinoma. Oncol Lett. 2019;18:3381–3387. doi: 10.3892/ol.2019.10606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Du W, Tang H, Lei Z, Zhu J, Zeng Y, Liu Z, Huang JA. miR-335-5p inhibits TGF-beta1-induced epithelial-mesenchymal transition in non-small cell lung cancer via ROCK1. Respir Res. 2019;20:225. doi: 10.1186/s12931-019-1184-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Q, Wu X, Guo L, Shi J, Li J. MicroRNA-7-5p induces cell growth inhibition, cell cycle arrest and apoptosis by targeting PAK2 in non-small cell lung cancer. FEBS Open Bio. 2019;9:1983–1993. doi: 10.1002/2211-5463.12738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma X, Zhou J, Mo H, Ying Y. Association of miR-100 expression with clinicopathological features and prognosis of patients with lung cancer. Oncol Lett. 2019;18:1318–1322. doi: 10.3892/ol.2019.10393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mott TF. Lung Cancer: Management. FP Essent. 2018;464:27–30. [PubMed] [Google Scholar]
- 38.Yang X, Zhang Q, Zhang M, Su W, Wang Z, Li Y, Zhang J, Beer DG, Yang S, Chen G. Serum microRNA signature is capable of early diagnosis for non-small cell lung cancer. Int J Biol Sci. 2019;15:1712–1722. doi: 10.7150/ijbs.33986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhuang Z, Sun C, Gong H. High serum miR-484 expression is associated with the diagnosis and prognosis of patients with non-small cell lung cancer. Exp Ther Med. 2019;18:4095–4102. doi: 10.3892/etm.2019.8010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang YL, Zhang ZL, Zhu XB, Xu L, Lu P, Xu M, Liu WJ, Zhang XY, Yao HM, Ye XW. Low plasma miR-25 expression is a favorite prognosis factor in non-small cell lung cancer. Eur Rev Med Pharmacol Sci. 2019;23:5251–5259. doi: 10.26355/eurrev_201906_18191. [DOI] [PubMed] [Google Scholar]
- 41.Li Y, Cui X, Li Y, Zhang T, Li S. Upregulated expression of miR-421 is associated with poor prognosis in non-small-cell lung cancer. Cancer Manag Res. 2018;10:2627–2633. doi: 10.2147/CMAR.S167432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hu C, Peng J, Lv L, Wang X, Zhou Y, Huo J, Liu D. miR-196a regulates the proliferation, invasion and migration of esophageal squamous carcinoma cells by targeting ANXA1. Oncol Lett. 2019;17:5201–5209. doi: 10.3892/ol.2019.10186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arabsorkhi Z, Gharib E, Yaghmoorian Khojini J, Farhadieh ME, Nazemalhosseini-Mojarad E, Zali MR. miR-298 plays a pivotal role in colon cancer invasiveness by targeting PTEN. J Cell Physiol. 2019;235:4335–4350. doi: 10.1002/jcp.29310. [DOI] [PubMed] [Google Scholar]
- 44.Han X, Du C, Chen Y, Zhong X, Wang F, Wang J, Liu C, Li M, Chen S, Li B. Overexpression of miR-939-3p predicts poor prognosis and promotes progression in lung cancer. Cancer Biomark. 2019;25:325–332. doi: 10.3233/CBM-190271. [DOI] [PubMed] [Google Scholar]
- 45.Huang T, Wang G, Yang L, Peng B, Wen Y, Ding G, Wang Z. MiR-186 inhibits proliferation, migration, and invasion of non-small cell lung cancer cells by downregulating Yin Yang 1. Cancer Biomark. 2017;21:221–228. doi: 10.3233/CBM-170670. [DOI] [PubMed] [Google Scholar]
- 46.Tang X, Ding Y, Wang X, Wang X, Zhao L, Bi H. miR-650 promotes non-small cell lung cancer cell proliferation and invasion by targeting ING4 through Wnt-1/β-catenin pathway. Oncol Lett. 2019;18:4621–4628. doi: 10.3892/ol.2019.10805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tian G, Wang SW, Song M, Hu YF, Cao XN, Ge JW. MicroRNA-16 inhibits the proliferation, migration and invasion of non-small cell lung carcinoma cells by down-regulating matrix metalloproteinase-19 expression. Eur Rev Med Pharmacol Sci. 2019;23:5260–5269. doi: 10.26355/eurrev_201906_18192. [DOI] [PubMed] [Google Scholar]
- 48.Kushwaha R, Thodima V, Tomishima MJ, Bosl GJ, Chaganti RS. miR-18b and miR-518b Target FOXN1 during epithelial lineage differentiation in pluripotent cells. Stem Cells Dev. 2014;23:1149–1156. doi: 10.1089/scd.2013.0262. [DOI] [PubMed] [Google Scholar]
- 49.Ji X, Ji Y, Wang W, Xu X. Forkhead box N1 inhibits the progression of non-small cell lung cancer and serves as a tumor suppressor. Oncol Lett. 2018;15:7221–7230. doi: 10.3892/ol.2018.8210. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.