Abstract
Background:
Current treatments for glioblastoma (GB), the most common and malignant primary brain tumor are inadequate and as such, the median survival for most patients with GB is on the order of months, even after cytoreductive surgery, radiation and chemotherapy.
Case Description:
Current study reports two cases of glioblastoma (GB) with subventricular zone (SVZ) involvement. SVZ biopsies demonstrated the presence of hypercellularity, nestin immunoreactivity, and a Ki-67 labeling index (LI) of 1-2%. Interestingly, tumor morphology and proliferative indices are different in the SVZ specimens than the hemispheric recurrences, which displayed similar nestin immunoreactivity, but a greater LI of 10%. Biopsy specimens demonstrated both intense nestin immunoreactivity and GFAP immunoreactivity in and around the GB recurrence. Nestin positive cells were more abundant closer to the SVZ nearest to the dorsolateral horn of the left lateral ventricle, while GFAP immunoreactivity was more intense closer to the center of the tumor recurrence. Additionally, co-labeling of cells with Ki67 and several different progenitor markers (CD133, CD140, TUJ-1, and nestin) demonstrated that these cells found in and around the GB recurrence were actively dividing. Having failed standard therapy with evidence of bi-hemispheric spread and progression to GB, we report a novel approach of using intraventricular liposomal encapsulated cytarabine (DepoCyt) for the treatment for GB by suppressing glial progenitor cells that surround the ventricular system in patients with GB.
Conclusions:
MRI and immunohistochemistry demonstrated that the SVZ is the incubator for future recurrences of GB and propose targeting SVZ progenitor cells with intraventricular liposomal encapsulated Ara-C. Two patients treated using this novel regimen have demonstrated partial radiographic responses warranting further studies looking at targeting the subventricular zone
Keywords: Glioblastoma, Subventricular Zone, Cytarabine, Intraventricular chemotherapy
Introduction
Neuro-oncology is currently in the midst of a paradigm shift in terms of our accepted understanding of the pathophysiology of gliomagenesis. Classic “dedifferentiation” hypotheses [1], modeling the cellular origin of gliomas after neoplastic transformation of differentiated glia, are currently being challenged by “cancer stem cell” hypotheses suggesting dysregulated glial progenitor cells are responsible for gliomagenesis.
Gliomas include tumors of presumed glial origin including astrocytic, oligodendroglial, and ependymal lineage. Although dedifferentiation and neoplastic transformation of glia has been assumed to be the mechanism of gliomagenesis, this hypothesis has never been proven [2, 3]. Additionally this “classic” view fails to adequately explain the origin of “mixed” gliomas such as gangliogliomas, which would be better explained by transformation of a pluripotent precursor cell. Growing evidence exists that glial progenitor cells persisting in the adult mammalian brain, lining the lateral ventricles in the subventricular zone (SVZ) and dentate gyrus, play a role in gliomagenesis [4, 5]. Gliomas frequently occur in close proximity to the ventricular system and SVZ with high-grade lesions like glioblastoma (GB) “spreading” to midline structures and crossing the corpus callosum to the contralateral hemisphere.
The lack of significant clinical advances in treating GB may be due to oversight of the cellular origin of this disease. Our current understanding of gliomagenesis has not yet been used clinically for the treatment of GB. Intraventricular (ITV) administration of the anti-mitotic agent cytosine-β-D-arabinofuranoside (Ara-C, cytarabine) has been shown to inhibit the proliferation and migration of SVZ precursor cells in adult mice [6]. Using this “bench-top” scientific concept, we hypothesize that the use of ITV Ara-C will slow or eliminate the migration of SVZ progenitors thought to be the cells of origin and recurrence of GB [2, 4, 7].
To test this hypothesis, we used a sustained release form of Ara-C, DepoCyt (Cytarabine Liposome Injection), for the ITV administration of this drug in two patients with GB. DepoCyt maintains cytotoxic concentrations of free Ara-C in the CSF for >14 days following a single injection [8]. Both patients treated with this agent demonstrated significant responses in the first patient, and a partial response in the second.
Case Reports
Case 1
History and Initial Examination
A 30-year-old female presented with headaches, diplopia and a Karnofsky Performance Scale (KPS) of 70. Magnetic resonance images demonstrated a 2.5 cm left frontal mass enhancing mass with extensive surrounding edema and 1 cm subfalcine herniation.
Treatments and Pathological Analyses
The patient underwent a left frontal craniotomy and gross total resection of the lesion. Pathology revealed an anaplastic gemistocytic astrocytoma. Surgery was followed by adjuvant external-beam radiation therapy (59 Gy) and concurrent temozolomide at a dose of 75 mg/m2for 6 weeks. She subsequently underwent 12 cycles of adjuvant temozolomide and KPS improved to 100.
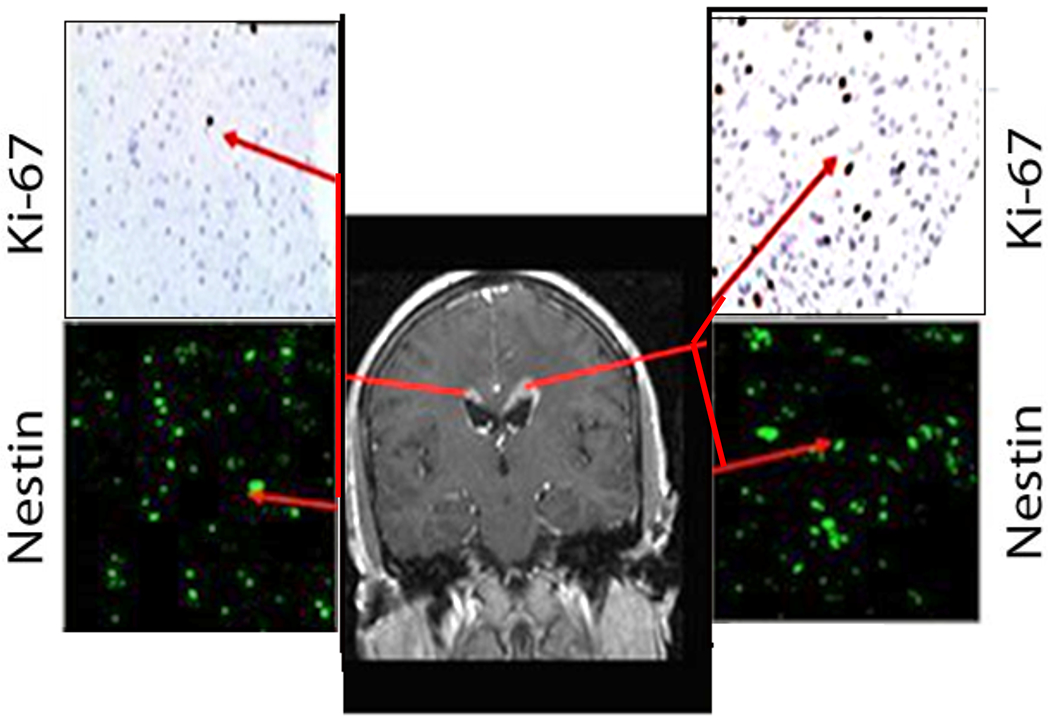
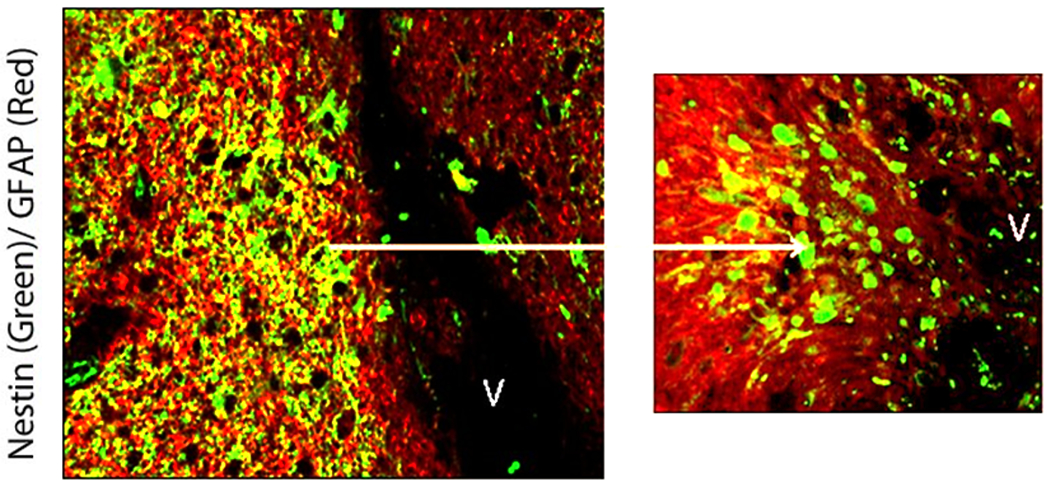
Fifty-four weeks later, MRI brain showed local tumor recurrence in the left frontal lobe and a new area of right-sided periventricular enhancement adjacent to the frontal horn of the lateral ventricle (Figure 1 and 4a). The patient’s KPS was 90 and underwent biopsy of both the left sided hemispheric lesion, right sided periventricular lesion, and placement of Ommaya reservoir. Pathology results revealed left frontal GB with a Ki-67 labeling index of 10%, and hypercellularity of the right frontal periventricular lesion with a Ki-67 labeling index of 1.5% (Figure 1). Both biopsy specimens demonstrated intense nestin immunoreactivity (Figures. 1, 2). Both nestin immunoreactivity (green) and GFAP immunoreactivity (red) were demonstrated in and around the GB recurrence (Figure 2). Nestin positive cells were more abundant closer to the SVZ nearest to the dorsolateral horn of the left lateral ventricle, while GFAP immunoreactivity was more intense closer to the center of the tumor recurrence (Figure 2). Additionally, co-labeling of cells with Ki67 and several different progenitor markers (CD133, CD140, TUJ-1, and nestin) demonstrated that these cells found in and around the GB recurrence were actively dividing (Figure 3).
Figure 1.
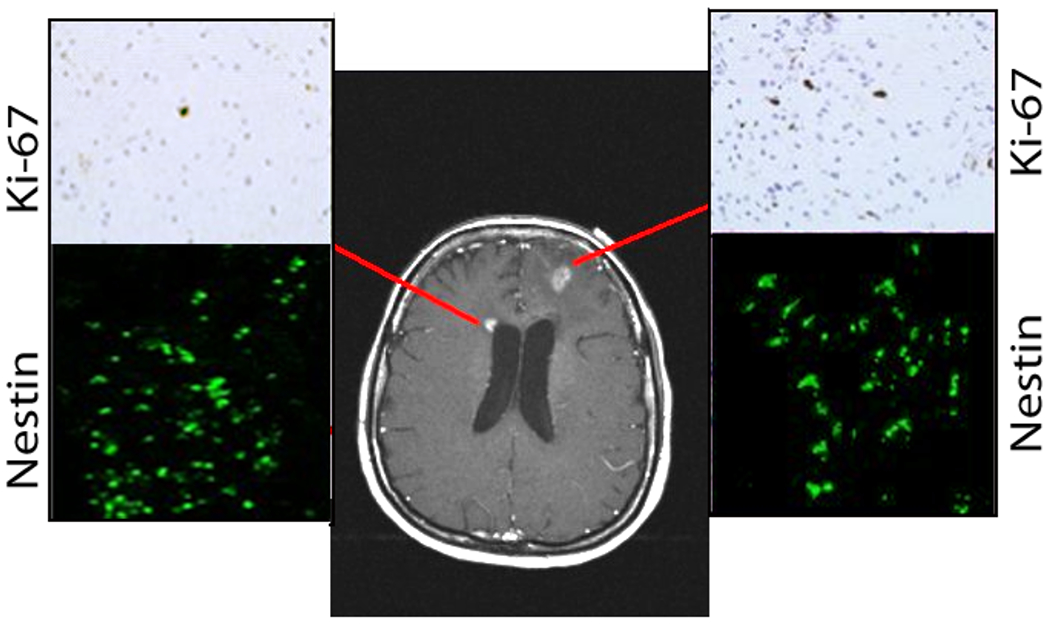
(a) Gadolinium enhanced MR image (Case 1) demonstrating recurrent left frontal GB and right periventricular enhancement. Ki-67 immunohistochemistry stains are shown from biopsies of both regions (upper insets), and nestin immunocytochemistry staining (lower insets).
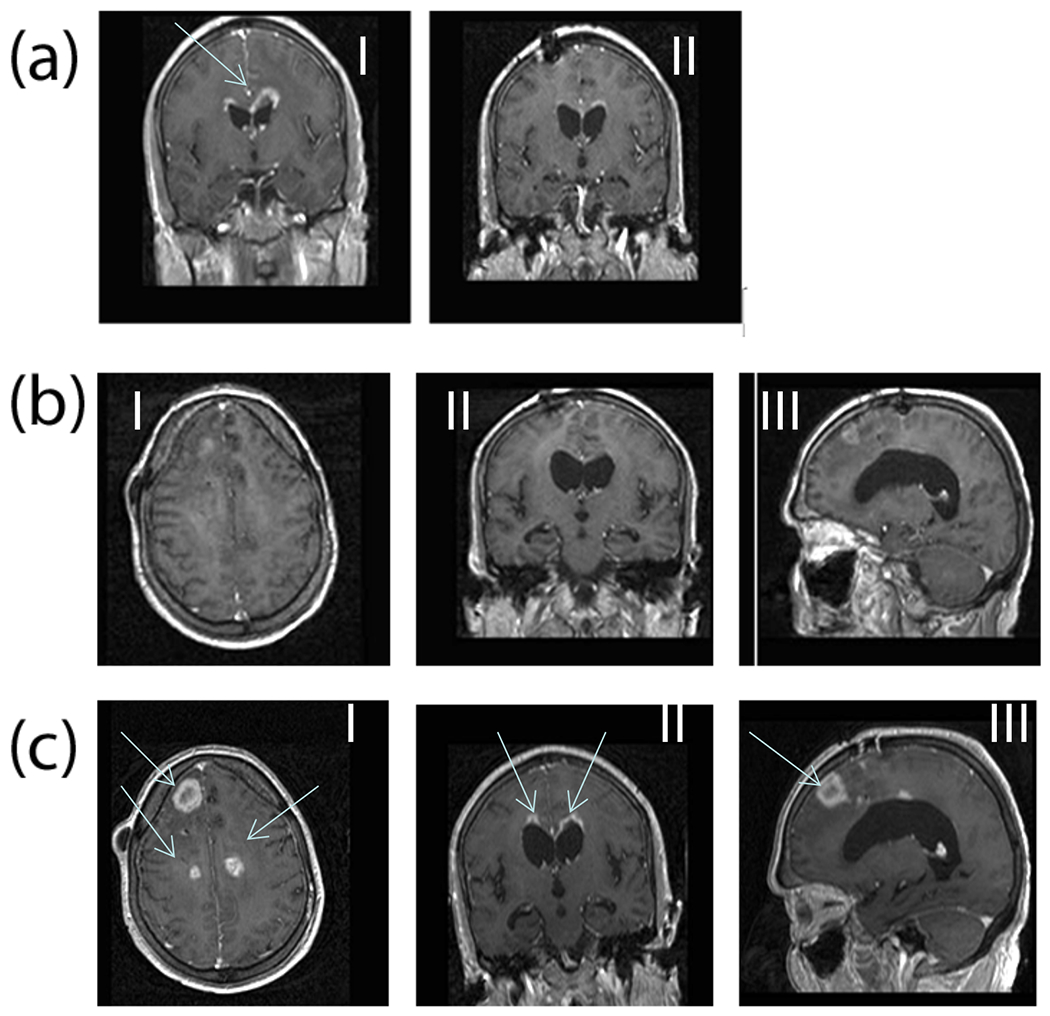
Figure 4.
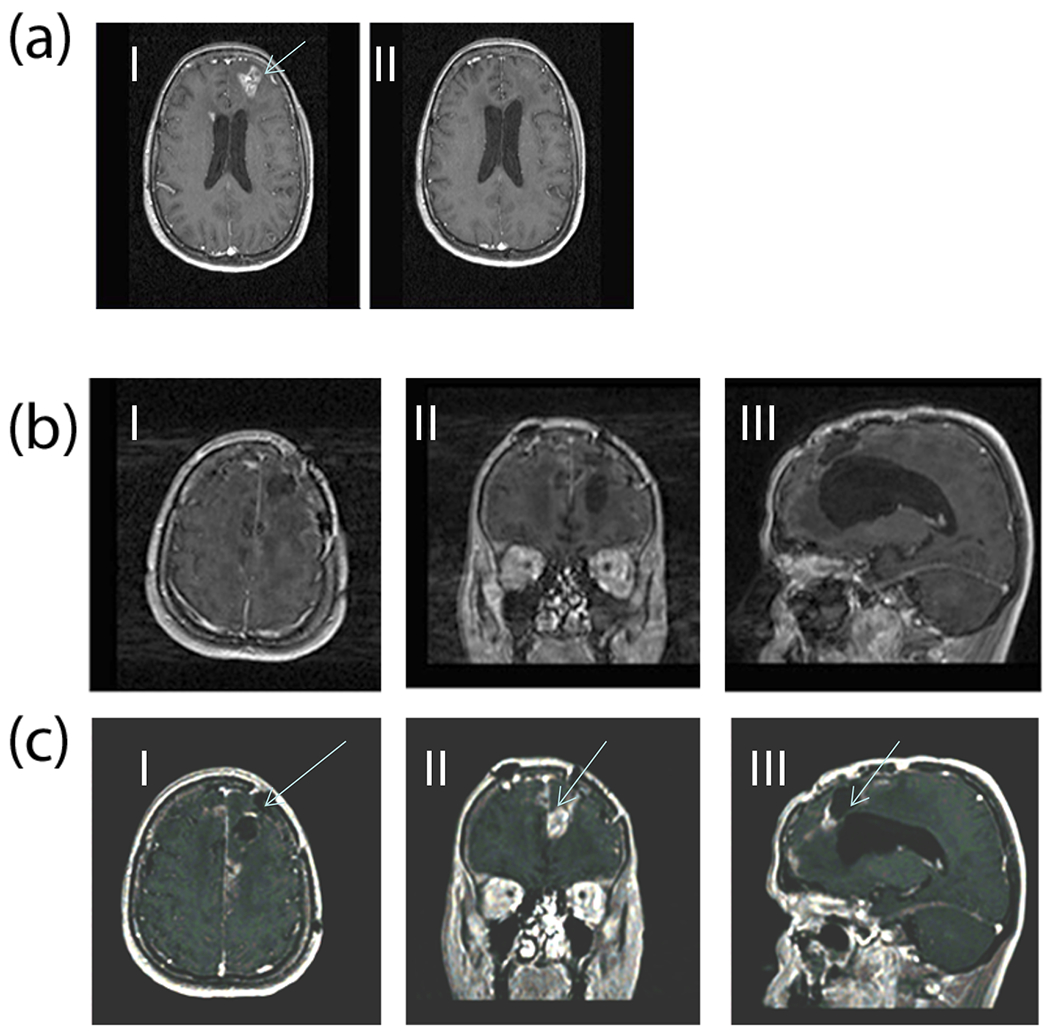
(a) Gadolinium enhanced MR images (Case 1) comparing pre- (I) and post-DepoCyt treatment (II) after completion of the consolidation phase (cycle 6). After four months of treatment, the original left frontal lesion, and periventricular enhancement has disappeared. (b) MRI 40 weeks after restarting ITV DepoCyt in patient (Case #1). Images (I, II, III) demonstrate a marked decrease in the bihemispheric tumor burden as compared to Figure 4c. (c) MRI 6 weeks after discontinuing ITV DepoCyt in patient (Case#1). Bihemispheric recurrent GB is demonstrated (I, II, III).
Figure 2.
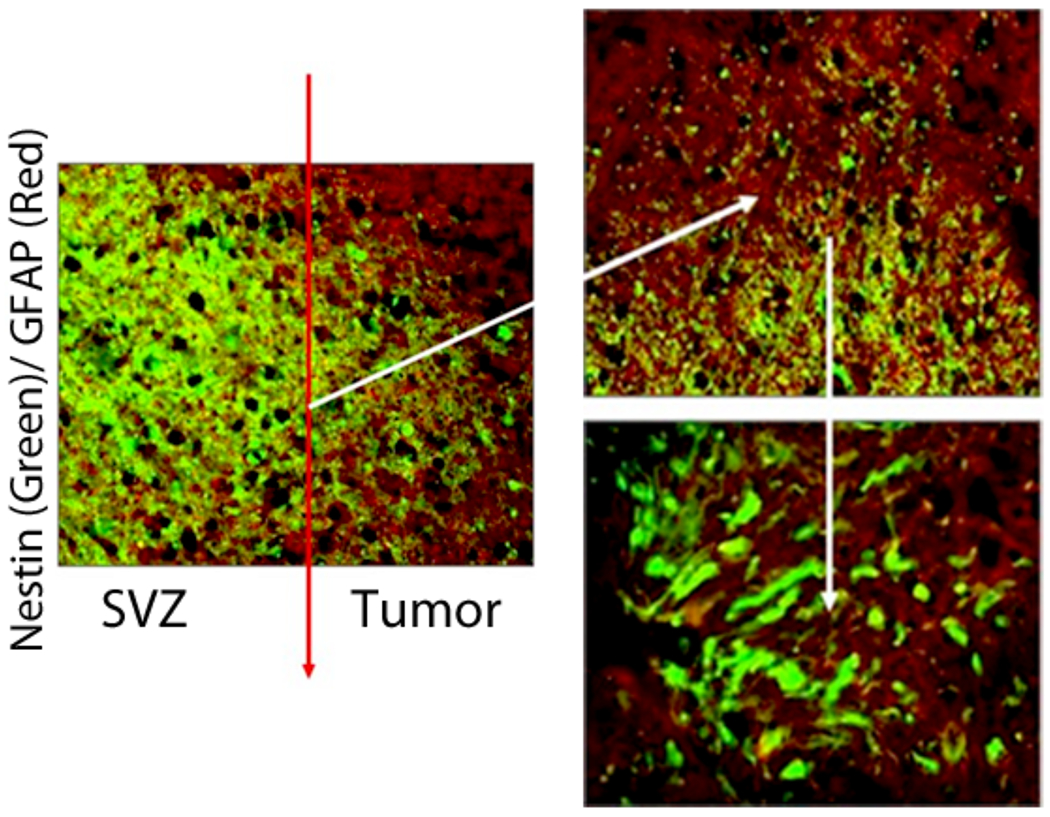
Confocal photomicrographs of left frontal GB recurrence (Case #1). Nestin immunoreactivity (green) and GFAP immunoreactivity (red) are demonstrated in and around the GB recurrence. Nestin positive cells are more abundant closer to the SVZ nearest to the dorsolateral horn of the left lateral ventricle, while GFAP immunoreactivity is more intense closer to the center of the tumor recurrence (left picture). The border between the GB recurrence and SVZ is demarcated by a red line (left picture) and magnified on the right hand images. Co-labeling GFAP+/Nestin+ cells (yellow) are seen in this region.
Figure 3.
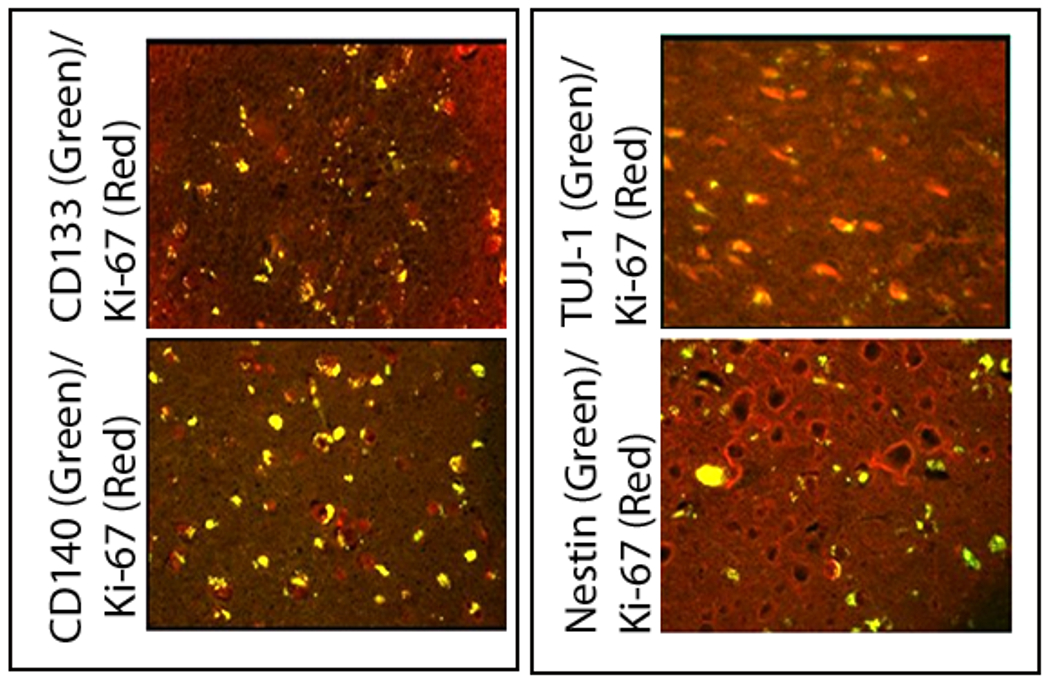
Confocal photomicrographs of left frontal GB recurrence (Case #1) showing the co-labeling of cells (yellow) with Ki-67 and CD133 (top left), CD140 (bottom left), TUJ-1 (top right) and nestin (bottom right). This demonstrates that cells labeled with several different types of progenitor markers found in and around the GB recurrence are actively dividing.
Liposomal Encapsulated Ara-C (DepoCyt) Treatment
Having failed standard therapy with evidence of bi-hemispheric spread and progression to GB, the use of intraventricular liposomal encapsulated Ara-C (DepoCyt) was discussed with the patient and informed consent obtained for its use.
The DepoCyt dosing regimen was derived from a clinical trial treating neoplastic meningitis from solid tumors [9]. Briefly, patients went through an induction phase of ITV DepoCyt, using 50mg every two weeks for one month (Cycles 1, 2). MRI, clinical response, and systemic toxicity utilizing blood chemistry analysis are performed. Patients with stable disease, not exhibiting systemic toxicity, were then treated with a three month consolidation phase of ITV DepoCyt, using 50mg every two weeks for one month, followed by 50mg ITV DepoCyt every four weeks for two months (cycles 3-6). Evaluation of the therapeutic response is again assessed by MRI, clinical examination, and blood chemistry analysis. Those without progression or toxicity were treated with further maintenance therapy using 50mg ITV DepoCyt every four weeks for three months (cycles 7-9). Patients receive dexamethasone 4 mg TID on days 1-5 of each cycle.
Patient 1 completed the induction (cycles 1, 2) and cycles 3-6. She did not have radiographic or neurological progression and received an additional 2 cycles of maintenance therapy for a total of 6 months of ITV DepoCyt. The patient tolerated the treatment well, except for headache during the first three days of treatment. No other treatment-related complications occurred. MRI demonstrating a partial response as shown in Figure 4a (II). No other treatment-related complications occurred.
The patient was restarted on DepoCyt, and tolerated induction, consolidation and maintenance phases for 10 months duration. During this period a transient 2 week period of arachnoiditis was treated with dexamethasone, and elevated ICP (highest 30 cm H2O) was successfully treated with oral acetazolamide (500mg p.o. BID) reducing ICP to below 20 cm H2O. Two cycles of ITV DepoCyt were withheld during this period while waiting for these AEs to resolve. The patient’s last MRI is shown in (Figure 4b: I, II, III) demonstrating a marked decrease in the bihemispheric tumor burden as compared to Figure 4c (I, II, III). Because of increasing ventriculomegally, further ITV DepoCyt was withheld, and a ventriculo-peritoneal shunt placed. Six weeks later the patient began to experience headaches and decrease in KPS to 70. Repeat MRI demonstrated bihemispheric tumor recurrence as seen in Figure 4c (I, II, III). The patient passed away 24 months from initial diagnosis of GB.
Case 2
History and Initial Examination
A 47 year old male presented with headaches, and new onset of seizures with a KPS of 100. MRI of the brain demonstrated diffuse right frontal edema with a 1.8 cm area of enhancement on the right adjacent to the frontal horn of the lateral ventricle.
Treatments and Pathological Analyses
The patient underwent a right frontal biopsy of the lesion. Pathology revealed anaplastic astrocytoma. Surgery was followed by a 6-week course of external-beam radiation therapy and concurrent temozolomide at 75 mg/m2. He subsequently underwent 6 cycles of adjuvant temozolomide.
Twenty-four weeks later, MR imaging showed local tumor recurrence in the right frontal lobe with extension into the corpus callosum to the contralateral side (Figure 5). The patient’s KPS was 90 and underwent biopsy of the bilateral periventricular lesions, and placement of Ommaya reservoir. Pathology results demonstrated GB in the left sided periventricular area, GFAP+ (Figure 6), with a Ki67 labeling index of 10% (Figure 5), and hypercellularity of the right frontal periventricular area, GFAP+ (Figure 6) with a Ki67 labeling index of approximately 2% (Figure 5). Both biopsy specimens demonstrated intense nestin immunoreactivity (Figure 5, 6). Both nestin immunoreactivity (green) and GFAP immunoreactivity (red) are again demonstrated in and around the GB recurrence (Figure 6). Nestin positive cells are more abundant closer to the SVZ nearest to the dorsolateral horn of the left lateral ventricle, while GFAP immunoreactivity is more intense closer to the center of the tumor recurrence (Figure 6).
Figure 5.
Preoperative MRIs demonstrating recurrent bihemispheric GB (Case# 2). Biopsy results show recurrent GB in right frontal lobe (top left) and bilateral periventricular areas (top right, and bottom left). Periventricular biopsies demonstrate a Ki-67 labeling index of 10% (top right MRI) in the left periventricular biopsy (inset), and hypercellularity of the right frontal periventricular enhancing region (top right MRI) with a Ki-67 labeling index of 2.0% (inset). Bilateral periventricular regions are nestin positive (bottom left MRI insets).
Figure 6.
Confocal photomicrographs of right frontal periventricular GB recurrence in patient #2. Nestin immunoreactivity (green) and GFAP immunoreactivity (red) are demonstrated in and around the GB recurrence. Nestin positive cells are more abundant closer to the SVZ nearest to the dorsolateral horn of the right lateral ventricle (V). Nestin+ cells are seen streaming from the ventricular surface towards the GB recurrence (left picture). Co-labeling of GFAP+/Nestin+ (yellow) cells appear in these regions.
Liposomal Encapsulated Ara-C (DepoCyt) Treatment
Having failed standard therapy with evidence of bi-hemispheric spread and progression to GB, use of intraventricular liposomal encapsulated Ara-C (DepoCyt) was discussed with the patient and informed consent obtained for its use. The patient underwent both induction and consolidation phases of ITV Depocyt (cycles 1-6) and four cycles of maintenance therapy (cycles 7-11). Treatment related toxicity was limited to headaches beginning 6-7 days after finishing the 5-day course of dexamethasone during each cycle. This was treated using a combination of intermittent Naproxen 500mg BID as needed. Further DepoCyt was withheld due to a generalized seizure several days after the 11th cycle ITV DepoCyt dose was given. The patient was hospitalized and re-loaded with Dilantin until therapeutic. An MRI was obtained at this time demonstrating diminished bilateral periventricular and right frontal tumor enhancement bilaterally (Figure 7). DepoCyt could not be restarted due to the declining clinical condition of the patient persisting after the generalized seizure. The patient was readmitted to the hospital for failure to thrive 4 weeks after the MRI shown in Figure 7b. Repeat MRI was performed demonstrating bihemispheric tumor recurrences (Figure 7c). Patient #2 died shortly thereafter The patient expired 12 months from initial diagnosis of GB.
Figure 7.
(a) Gadolinium enhanced MR images (Case 2) comparing pre- (I) and post-DepoCyt treatment (II) after completion of the induction phase (cycle 2). After one month of treatment, periventricular enhancement has significantly diminished. (b) MRI 36 weeks after starting ITV DepoCyt in patient (Case# 2). Images (I, II, III) demonstrate a marked decrease in the bihemispheric tumor burden. (c) MRI 4 weeks following termination of ITV DepoCyt in patient (Case# 2).
Discussion
Despite significant improvements in diagnostic imaging and neurosurgical techniques, the current treatment modalities for high-grade gliomas are still inadequate [10]. As such, the median survival for patients with GB is 12-18 months, even after cytoreductive surgery, radiation and chemotherapy [11]. Furthermore, the outlook for patients with these tumors has not changed significantly over the last 20 years with a 5-year survival rate of 10%, and a mortality rate of nearly 100% [12]. Recurrence is nearly inevitable and usually occurs in the form of local contiguous growth within a 2 cm margin of resection although distant periventricular failure is frequently seen [13]. The median interval from initial diagnosis to tumor recurrence, either clinical or radiographic, is 7-9 months [14–16].
The prognosis of patients with recurrent glioblastoma is poor. Either re-resection or systemic chemotherapy alone yields a median survival of 14 [14], and 24 weeks [15], respectively. Re-resection followed by systemic chemotherapy increases the median survival to approximately 36 weeks [16–20]. Results have been modest, driving a continued search for improved therapies.
Ignorance of the cellular origin of gliomas is one reason for lack of clinical advances resulting in ineffective tumor targeting and poor prediction of tumor behavior. Growing evidence exists that glial progenitor cells persisting in the adult mammalian brain, play a role in gliomagenesis [4]. As is often the case, “new concepts” are frequently well rooted in history. In fact the earliest references to the stem cell origins of gliomas date back to the 1930’s and shortly thereafter [21]. Further supporting this hypothesis are numerous laboratory investigations establishing that experimental rodent gliomas arise from the SVZ [22–24]. However, it was not until recently that these findings have been put into perspective by the demonstration of persistent proliferative glial progenitor cells in the subependymal (SEZ)/subventricular zone (SVZ), the hippocampal dentate gyrus, and subcortical white matter in the adult human [25]. Subsequently, stem-like cells were isolated from human gliomas [26], and shown to be tumorigenic after transplantation into immunodeficient mice [27].
Gliomas have been demonstrated to express the class VI intermediate filament nestin, a glial progenitor marker, as well as others also found in the SVZ of adult mammals [28]. Rat glioma models induced by in utero exposure to ethylnitrosourea, demonstrate hypertrophy and micro-tumor formation within the SVZ. Subsequent migration of these cellular clusters results in deep white matter tumor formation, further implicating glial progenitor cells within the SVZ in gliomagenesis [23]. We have previously shown that the ipsilateral SVZ in a syngeneic C6 intracranial rat glioma model is hypertrophic, and hypercellular. This region contains actively dividing nestin positive immunoreactive cells, which stream from the dorsal tip of the SVZ, and track along the ventral margin of the corpus callosum, to encompass and infiltrate deep hemispheric C6-GFP gliomas [29]. In fact roughly half of the tumor mass at the time of animal sacrifice was unrelated to the initially implanted C6-GFP tumor cells, but could be traced back to cells migrating from the hypertrophied ipsilateral SVZ. This phenomenon is highly suggestive of a role of SVZ precursor cells in gliomagenesis [29]. This work has been subsequently confirmed and furthered by Assanah et al. and Jackson et al. demonstrating glioma formation in response to PDGF signaling by glial progenitors present in the adult rodent white matter [30], and SVZ [7], respectively. Further supporting this hypothesis is a recently proposed model of SVZ organization suggesting that a population of nestin positive dividing SVZ astrocytes (“B” cells) function as glial progenitor cells, and give rise to other glia as well as to neurons via intermediate “C” and “A” cell-types [24,31].
Intraventricular administration of the chemotherapeutic anti-mitotic agent cytarabine (Ara-C), eliminates the presence of intermediate C and A cell-types and prevents division of B cells in the SVZ of adult mice [6]. Since we hypothesize that these glial precursor cells are responsible for the development, progression, and recurrence of glial tumors in humans, we have proposed the use of ITV cytarabine to suppress such activity by inhibiting the glial progenitor cells in the subependymal zone lining the ventricles. Intraventricular administration of cytarabine is not practical therapeutically due to its short half-life, but pharmacokinetic studies of DepoCyt, have demonstrated that once every 2 weeks dosing can maintain therapeutic cytarabine concentrations in the CSF with minimal side effects [8]. In one study twelve patients with neoplastic meningitis were treated with escalating doses of intrathecal liposomal cytarabine. Therapeutic intra-lumbar concentrations of free cytarabine were maintained for up to 14 days, following an intrathecal dose of 75mg [32]. Side effects such as grade II headache, fever, and nausea were reported, as well as one transient grade I cauda equina syndrome. This dosing schedule was confirmed by Chamberlain et al. [33].
Here, we report two patients with progression to GB from an anaplastic astrocytoma, SVZ gadolinium enhancement on MRI, and the presence of hypercellularity, nestin immunoreactivity, and a Ki-67 labeling index (LI) of 1-2% in the SVZ biopsy specimens (Figure 1, 5). This LI is elevated as compared to non-neoplastic brain parenchyma [34]. In comparison the primary tumor focus in these patients demonstrates similar nestin immunoreactivity, but a greater LI of ≥10%. This suggests that the SVZ may be playing a role in gliomagenesis in humans as well. Interestingly, tumor morphology and proliferative indices are different in the SVZ specimens than the hemispheric recurrences. Microscopically the SVZ component is reminiscent of a “low-grade glioma” with proliferating immature nestin and GFAP positive cells. It is possible that this “low-grade” SVZ component is the incubator for future recurrences of hemispheric GB. Using this hypothesis, we have proposed targeting SVZ progenitor cells with intraventricular liposomal encapsulated Ara-C. Both patients treated using this regimen demonstrated some response to treatment (Figure 4, and 7) potentially warranting further studies targeting the SVZ
The limitations of this study is the limited number of patients, the lack of molecular markers due to the small size of tissue obtained from the SVZ, the fact that this data was generated from a single institution making it unclear whether these findings would be confirmed in a larger number of patients across several institutions
Conclusions
Our improved understanding of SVZ progenitor cells in relation to gliomagenesis is important in defining novel therapeutic targets. Using intraventricular liposomal encapsulated Ara-C, we have shown temporary stabilization of disease in two patients with secondary GB. This potentially warrants further studies looking at targeting the SVZ as a niche allowing recurrence of gliomas..
Acknowledgement
We thank Chiang Wang, Ph.D., for assisting Dr. Bruce M. Frankel.
Funding
Completion of this project was made possible by NIH sponsored grants to Bruce M. Frankel (1R01FD003542-01).
Footnotes
Conflict of Interest:
The authors declare that they have no conflict of interest.
Ethical Approval
This research was approved by the Institutional Review Board (IRB) for Human Research in the Office of Research Integrity at the Medical University of South Carolina (MUSC). GB samples were obtained and processed using standard IRB procedure. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Subjects gave their written, informed consent to participate.
References
- 1.Nowell PC: The clonal evolution of tumor cell populations. Science 194:23–28, 1976. [DOI] [PubMed] [Google Scholar]
- 2.Sanai N, Alvarez-Buylla A, Berger MS: Neural stem cells and the origin of gliomas. N Engl J Med 353:811–822, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Glantz M, Kesari S, Recht L, Fleischhack G, Van Horn A. Understanding the origins of gliomas and developing novel therapies: cerebrospinal fluid and subventricular zone interplay. Semin Oncol. 2009. August;36(4 Suppl 2):S17–24. [DOI] [PubMed] [Google Scholar]
- 4.Berger F, Gay E, Pelletier L, Tropel P, Wion D: Development of gliomas: potential role of asymmetrical cell division of neural stem cells. Lancet Oncol 5:511–514, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Kusne Y, Sanai N. The SVZ and Its Relationship to Stem Cell Based Neuro-oncogenesis. Adv Exp Med Biol. 2015;853:23–32. [DOI] [PubMed] [Google Scholar]
- 6.Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A: Regeneration of a germinal layer in the adult mammalian brain. Proc Natl Acad Sci U S A 96:11619–11624, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jackson EL, Garcia-Verdugo JM, Gil-Perotin S, Roy M, Quinones-Hinojosa A, VandenBerg S, Alvarez-Buylla A: PDGFR alpha-positive B cells are neural stem cells in the adult SVZ that form glioma-like growths in response to increased PDGF signaling. Neuron 51:187–199, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Jaeckle KA, Batchelor T, O’Day SJ, Phuphanich S, New P, Lesser G, Cohn A, Gilbert M, Aiken R, Heros D, Rogers L, Wong E, Fulton D, Gutheil JC, Baidas S, Kennedy JM, Mason W, Moots P, Russell C, Swinnen LJ, Howell SB: An open label trial of sustained-release cytarabine (DepoCyt) for the intrathecal treatment of solid tumor neoplastic meningitis. J Neurooncol 57:231–239, 2002. [DOI] [PubMed] [Google Scholar]
- 9.Glantz MJ, Jaeckle KA, Chamberlain MC, Phuphanich S, Recht L, Swinnen LJ, Maria B, LaFollette S, Schumann GB, Cole BF, Howell SB: A randomized controlled trial comparing intrathecal sustained-release cytarabine (DepoCyt) to intrathecal methotrexate in patients with neoplastic meningitis from solid tumors. Clin Cancer Res 5:3394–3402, 1999. [PubMed] [Google Scholar]
- 10.Ryken TC, Frankel B, Julien T, Olson JJ: Surgical management of newly diagnosed glioblastoma in adults: role of cytoreductive surgery. J Neurooncol 89:271–286, 2008. [DOI] [PubMed] [Google Scholar]
- 11.Davis FG, Freels S, Grutsch J, Barlas S, Brem S: Survival rates in patients with primary malignant brain tumors stratified by patient age and tumor histological type: an analysis based on Surveillance, Epidemiology, and End Results (SEER) data, 1973–1991. J Neurosurg 88:1–10, 1998. [DOI] [PubMed] [Google Scholar]
- 12.Deen DF, Chiarodo A, Grimm EA, Fike JR, Israel MA, Kun LE, Levin VA, Marton LJ, Packer RJ, Pegg AE, et al. : Brain Tumor Working Group Report on the 9th International Conference on Brain Tumor Research and Therapy. Organ System Program, National Cancer Institute. J Neurooncol 16:243–272, 1993. [DOI] [PubMed] [Google Scholar]
- 13.Wallner KE, Galicich JH, Krol G, Arbit E, Malkin MG: Patterns of failure following treatment for glioblastoma multiforme and anaplastic astrocytoma. Int J Radiat Oncol Biol Phys 16:1405–1409, 1989. [DOI] [PubMed] [Google Scholar]
- 14.Young B, Oldfield EH, Markesbery WR, Haack D, Tibbs PA, McCombs P, Chin HW, Maruyama Y, Meacham WF: Reoperation for glioblastoma. J Neurosurg 55:917–921, 1981. [DOI] [PubMed] [Google Scholar]
- 15.Nieder C, Grosu AL, Molls M: A comparison of treatment results for recurrent malignant gliomas. Cancer Treat Rev 26:397–409, 2000. [DOI] [PubMed] [Google Scholar]
- 16.Groves MD, Puduvalli VK, Chang SM, Conrad CA, Gilbert MR, Tremont-Lukats IW, Liu TJ, Peterson P, Schiff D, Cloughesy TF, Wen PY, Greenberg H, Abrey LE, DeAngelis LM, Hess KR, Lamborn KR, Prados MD, Yung WK: A North American brain tumor consortium (NABTC 99–04) phase II trial of temozolomide plus thalidomide for recurrent glioblastoma multiforme. J Neurooncol 81:271–277, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Vredenburgh JJ, Desjardins A, Herndon JE 2nd, Marcello J, Reardon DA, Quinn JA, Rich JN, Sathornsumetee S, Gururangan S, Sampson J, Wagner M, Bailey L, Bigner DD, Friedman AH, Friedman HS: Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol 25:4722–4729, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Parney IF, Chang SM: Current chemotherapy for glioblastoma. Cancer J 9:149–156, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Brem H, Ewend MG, Piantadosi S, Greenhoot J, Burger PC, Sisti M: The safety of interstitial chemotherapy with BCNU-loaded polymer followed by radiation therapy in the treatment of newly diagnosed malignant gliomas: phase I trial. J Neurooncol 26:111–123, 1995. [DOI] [PubMed] [Google Scholar]
- 20.Choucair AK, Levin VA, Gutin PH, Davis RL, Silver P, Edwards MS, Wilson CB: Development of multiple lesions during radiation therapy and chemotherapy in patients with gliomas. J Neurosurg 65:654–658, 1986. [DOI] [PubMed] [Google Scholar]
- 21.Smyth GE, Stern K: Tumors of the thalamus: a clinico-pathological study. Brain 61:339–374, 1938. [Google Scholar]
- 22.Hopewell JW: The subependymal plate and the genesis of gliomas. J Pathol 117:101–103, 1975. [DOI] [PubMed] [Google Scholar]
- 23.Lantos PL, Cox DJ: The origin of experimental brain tumours: a sequential study. Experientia 32:1467–1468, 1976. [DOI] [PubMed] [Google Scholar]
- 24.Menn B, Garcia-Verdugo JM, Yaschine C, Gonzalez-Perez O, Rowitch D, Alvarez-Buylla A: Origin of oligodendrocytes in the subventricular zone of the adult brain. J Neurosci 26:7907–7918, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nunes MC, Roy NS, Keyoung HM, Goodman RR, McKhann G 2nd, Jiang L, Kang J, Nedergaard M, Goldman SA: Identification and isolation of multipotential neural progenitor cells from the subcortical white matter of the adult human brain. Nat Med 9:439–447, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Ignatova TN, Kukekov VG, Laywell ED, Suslov ON, Vrionis FD, Steindler DA: Human cortical glial tumors contain neural stem-like cells expressing astroglial and neuronal markers in vitro. Glia 39:193–206, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, De Vitis S, Fiocco R, Foroni C, Dimeco F, Vescovi A: Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res 64:7011–7021, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Dahlstrand J, Collins VP, Lendahl U: Expression of the class VI intermediate filament nestin in human central nervous system tumors. Cancer Res 52:5334–5341, 1992. [PubMed] [Google Scholar]
- 29.Duntsch C, Zhou Q, Weimar JD, Frankel B, Robertson JH, Pourmotabbed T: Up-regulation of neuropoiesis generating glial progenitors that infiltrate rat intracranial glioma. J Neurooncol 71:245–255, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Assanah M, Lochhead R, Ogden A, Bruce J, Goldman J, Canoll P: Glial progenitors in adult white matter are driven to form malignant gliomas by platelet-derived growth factor-expressing retroviruses. J Neurosci 26:6781–6790, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A: Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 97:703–716, 1999. [DOI] [PubMed] [Google Scholar]
- 32.Kim S, Chatelut E, Kim JC, Howell SB, Cates C, Kormanik PA, Chamberlain MC: Extended CSF cytarabine exposure following intrathecal administration of DTC 101. J Clin Oncol 11:2186–2193, 1993. [DOI] [PubMed] [Google Scholar]
- 33.Chamberlain MC, Kormanik P, Howell SB, Kim S: Pharmacokinetics of intralumbar DTC-101 for the treatment of leptomeningeal metastases. Arch Neurol 52:912–917, 1995. [DOI] [PubMed] [Google Scholar]
- 34.Colodner KJ, Montana RA, Anthony DC, Folkerth RD, De Girolami U, Feany MB: Proliferative potential of human astrocytes. J Neuropathol Exp Neurol 64:163–169, 2005. [DOI] [PubMed] [Google Scholar]


