Abstract
Efficacy of abiraterone combined with flutamide on patients with prostate cancer (PCa) and its effect on levels of miR-493-5p and miR-195-5p contained in serum were investigated. The medical records of 146 PCa patients admitted to Longhua Hospital Shanghai University of Traditional Chinese Medicine from January 2011 to December 2013 were selected. Eighty-four patients were treated with abiraterone combined with flutamide as a study group, 62 patients were treated with abiraterone alone as a control group. The curative effect, adverse reactions, quality of life and five-year overall survival (OS) of the two groups were compared. The serum prostate-specific antigen (PSA) level was measured by radioimmunoassay at 3 days (T1) before treatment, 1 month (T2), 2 months (T3), and 6 months (T4) after treatment, and the relative expression of miR-493-5p and miR-195-5p in serum were detected by qRT-PCR. The total effective rate of the study group was significantly higher than that of the control group (P<0.05). The total incidence of toxic and side effects in the study group was significantly lower than that in the control group (P<0.05). The improvement rate of quality of life in the study group was significantly higher than that in the control group (P<0.05). OS in the study group was significantly higher than that in the control group at 5 years (P<0.05). There was no significant difference in serum PSA level between the two groups at T1 (P>0.05); there was no significant difference in the relative expression of miR-493-5p and miR-195-5p between the two groups at T1 (P>0.05). In conclusion, abiraterone combined with flutamide has better curative effect and lower incidence of adverse reactions in patients with metastatic castration-resistant PCa (CRPC) than abiraterone alone, and can increase the expression levels of miR-493-5p and miR-195-5p in patient serum.
Keywords: abiraterone, flutamide, PCa, miR-493-5p, miR-195-5p
Introduction
Prostate cancer (PCa) is a common malignant tumor of genitourinary system in clinic (1). In recent years, prostate-specific antigen (PSA) screening is widely used in PCa examination, so the diagnosis rate and treatment rate of early PCa also increase (2). PCa is the second most common malignant tumor in men after lung cancer. It is estimated that more than one million new cases are diagnosed every year in the world (3), and the incidence rate is increasing (4). After the primary treatment of chemical or surgical castration for PCa patients, most cases would develop into disease state, which is the metastatic castration-resistant prostate cancer (CRPC), with median survival less than 2 years (5). For the treatment of CRPC, androgen pathway inhibitors have become the mainstream treatment method in recent years, such as flutamide, sipuleucel-T, enzalutamide, abiraterone acetate, radium-223 and cabazitaxel, which can provide survival benefits for CRPC patients (6).
Abiraterone is a selective and irreversible cytochrome P450 c17 (CYP17) inhibitor. With potency 10 to 30 times higher than ketoconazole, it is a non-selective inhibitor. CYP17 plays an important role in androgen synthesis and is a key enzyme in testosterone synthesis (7). Abiraterone acetate is a prodrug of abiraterone and a selective inhibitor of androgen biosynthesis, which effectively blocks CYP17 and androgen synthesis in adrenal gland, testis and prostate tumors (8). Flutamide is a non-steroidal oral antiandrogen that blocks the effect of testosterone. Testosterone is a natural hormone responsible for the growth and diffusion of human PCa cells. Blocking the effect of testosterone can slow down the growth and diffusion of PCa cells. Currently, it has been widely used in treating patients with PCa (9,10).
MicroRNA (miRNA) is a newly discovered class of highly conserved endogenous non-coding hairpin nucleotide transcripts with a length of approximately 19–25 bases and widely existing in eukaryotic cells (11,12). Studies have found that some miRNAs are closely related to the occurrence and development of cancer. For example, the research results of Beebe-Dimmer et al (13) show that miR-195-5p is downregulated in PCa cell lines DU145 and PC3. Overexpression of miR-195-5p significantly inhibits the migration and invasion of PCa cells, and they have proved that miR-195-5p can inhibit PCa cell movement by regulating the expression of c-Met, MMP1 and MMP9. Research results of Zhao et al (14) show that miR-493-5p is downregulated in breast cancer cells and plays an inhibitory role in invasion and tumorigenicity of breast cancer cells. Human miR-493-5p belongs to DLK1-DIO3 imprinted miRNA clusters and plays a role as a tumor inhibitor in human cancers (14). Wang et al (15) showed that miR-493-5p is a tumor suppressor gene and is downregulated in human liver cancer. Overexpression of miR-493-5p promotes apoptosis and inhibits proliferation and migration of liver cancer cells by negatively regulating VAMP expression. The research results of Wang et al (15) show that miR-493-5p is downregulated in PCa cells and acts as a tumor inhibitor in PCa cells.
At present, there are many studies on the treatment of PCa patients with single drug use of abiraterone and flutamide, but there are few on the treatment of PCa patients with abiraterone combined with flutamide. This study aimed to find more effective and safer treatment drugs for PCa patients, to prolong the survival time of patients and improve the quality of life through the efficacy and adverse reactions of abiraterone combined with flutamide in the treatment of PCa patients, as well as the effects on expression of miR-493-5p and miR-195-5p.
Patients and methods
General information
The medical records of 146 PCa patients were selected, who were admitted to Longhua Hospital Shanghai University of Traditional Chinese Medicine (Shanghai, China) from January 2011 to December 2013. Among them, 84 patients were treated with abiraterone combined with flutamide as a study group, and 62 patients were treated with abiraterone alone as a control group.
Inclusion and exclusion criteria
Inclusion criteria were as follows: Patients were confirmed as PCa by pathological examination in both groups; PSA progress was observed in both groups; both groups of patients were male, aged 18–60 years. Exclusion criteria were as follows: patients with mental illness and other serious diseases; patients allergic to the drugs used in this study; patients who used targeted therapy of enzarumine or other similar potent androgen pathways.
Patients and their families were informed and signed informed consent forms. This study was approved by the Medical Ethics Committee of Longhua Hospital Shanghai University of Traditional Chinese Medicine.
Main instruments and reagents
Human PSA kit (Cusabio); multifunctional microplate reader (model: DLK0001622, BioTek Instruments, Inc.); real-time fluorescence quantitative PCR instrument (model: 7300, Applied Biosystems; Thermo Fisher Scientific, Inc.); spectrophotometer (model: DR5000, Hach Company); high-speed refrigerated centrifuge (Eppendorf); TRIzol kit (BioTek Instruments, Inc.); reverse transcription kit (Takara); real-time fluorescence quantitative PCR kit (Beijing BioDee BioTech Corporation Ltd.); miR-493-5p, miR-195-5p and U6 small nuclear RNA (RNU6B) internal reference primers were designed and synthesized by GeneCopoeia, Inc. The detailed primer sequences are shown in Table I.
Table I.
Sequence of primers.
| Gene | Upstream primer sequence | Downstream primer sequence |
|---|---|---|
| miR-493-5p | 5′-TCCTACGGAGAGGCTCAG-3′ | 5′-TCCTCGTAGTCCAACACG-3′ |
| miR-195-5p | 5′-CGTAGCAGCACAGAAA-3′ | 5′-GTGCAGGGTCCGGGT-3′ |
| U6 | 5′-CTCGCTTCGGCAGCACA-3′ | 5′-AACGCTTCACGAATTTGCGT-3′ |
Treatment methods
Control group: patients were given 1,000 mg of abiraterone acetate orally (Xian Janssen Pharmaceutical Ltd., SFDA approval no. J20150112), once a day; study group: on the basis of the control group, the patients in the study group were given 250 mg of flutamide orally (Tasly Diyi Pharmaceutical Co., Ltd., SFDA approval no. H19990143) once a day. Altogether 28 days as a cycle, two groups of patients were treated for 4 consecutive cycles.
Observation index and therapeutic effect evaluation standard
The efficacy evaluation was divided into four parts according to the World Health Organization solid tumor evaluation standard (16): complete response (CR) is defined as complete disappearance of tumor focus and no new focus; partial response (PR) is defined as the degree of volume reduction of tumor lesions >50%; stable disease (SD) is defined as the reduction of tumor focus volume <50%; progressive disease (PD) is defined as the degree of tumor lesion volume increase above 25%. The overall response rate (ORR) = (CR + PR)/Total cases ×100%. The toxic and side effects of group A and group B during treatment were observed. The main toxic and side effects were nausea, vomiting, anemia, thrombocytopenia, myelosuppression, liver function injury and renal function injury.
According to Karnofsky (KPS) score (17), the quality of life of patients after treatment was evaluated. After treatment, KPS score was improved by >10 points, which is classified as improvement. After treatment, KPS score was decreased or increased by less than 10 points, which is classified as stability. After treatment, KPS score was decreased by >10 points, which is classified as deterioration. Quality of Life Improvement Rate = (Improvement + Stability)/Total cases ×100%.
Follow-up
The two groups of patients were followed up by telephone and visit for 5 years. The follow-up was conducted once every 3 months within 5 years, with the deadline of January 2019. The overall survival (OS) was calculated from the beginning of drug administration to the day of death or the last follow-up in both groups.
Serum standard collection
A total of 5 ml fasting venous blood of the two groups of patients was sampled and placed in EDTA-K2 anticoagulation tube three days before treatment (T1), one month after treatment (T2), two months after treatment (T3) and six months after treatment (T4). The samples were centrifuged at 1,500 × g at 4°C for 10 min, and 500 µl of upper serum was drawn and stored in EP tube for later use.
Detection of serum PSA level
The serum PSA levels of the two groups of patients were determined by radioimmunoassay at T1, T2, T3 and T4. The detection process was strictly conducted according to the PSA kit instructions.
Detection of serum miR-493-5p and miR-195-5p
At T1, T2, T3 and T4, the serum of the two groups of patients was taken for miR-493-5p and miR-195-5p detection, and the specific steps were as follows: the serum total RNA was extracted according to the instructions of the TRIzol serum extraction kit. Total RNA purity, concentration and integrity were determined by UV spectrophotometry and agarose gel electrophoresis. Total RNA (2 µl) was taken to prepare cDNA according to the instruction manual of the kit. The reverse transcription reaction conditions were as follows: 42°C for 60 min and 95°C for 5 min; the synthesized cDNA sample was stored at −20°C for later use. U6 was used as the internal reference gene, and the reaction system of the total volume (20 µl) was as follows: 1 µl of cDNA, 10 µl of PCR premix, 2 µl of upstream primer (×10), 2 µl of downstream primer (×10), and 5 µl of dd water (Rnase and Dnase free). PCR amplification cycle conditions were as follows: 90°C for 5 min, 90°C for 5 sec, 60°C for 30 sec, 72°C for 5 sec, a total of 40 cycles. Amplification data were analyzed by ABI PRISM 7500 fluorescence quantitative PCR instrument manufacturer software, and the results were expressed by 2−∆CT.
Statistical method
SPSS 21.0 (Easybio) was used for analysis, and GraphPad Prism 7 was used to visualize the data. In this study, the counting data were expressed by [n (%)], and Chi-square test was used to compare the rates. The measurement data were expressed as mean ± standard deviation (mean ± SD). t-test was used for comparison of measurement data between two groups, and one-way analysis of variance was used for the comparison of multiple groups. Kaplan-Meier method was used to draw five-year OS curves of the two groups of patients, and log-rank test for comparison. P<0.05 was considered to indicate a statistically significant difference.
Results
Comparison of general data between two groups of patients
There was no significant difference between the control group and the study group in age, smoking history, exercise, residence, ethnicity, education level, weight, diet preference, and alcohol consumption (P>0.05) (Table II).
Table II.
Comparison of general data of both groups [n (%)].
| Group | Control group (n=62) | Study group (n=84) | χ2 value | P-value |
|---|---|---|---|---|
| Age | 0.311 | 0.577 | ||
| <45 | 23 (37.10) | 35 (41.67) | ||
| ≥45 | 39 (62.90) | 49 (58.33) | ||
| Smoking history | 0.877 | 0.349 | ||
| Yes | 47 (75.81) | 69 (82.14) | ||
| No | 15 (24.19) | 15 (17.86) | ||
| Exercise | 0.835 | 0.361 | ||
| Yes | 36 (58.06) | 55 (65.48) | ||
| No | 26 (41.94) | 29 (34.52) | ||
| Residence | 1.086 | 0.297 | ||
| Urban | 40 (64.52) | 47 (55.95) | ||
| Rural | 22 (35.48) | 37 (44.05) | ||
| Ethnicity | 0.673 | 0.412 | ||
| Han | 57 (91.94) | 80 (95.24) | ||
| Minority | 5 (8.06) | 4 (4.76) | ||
| Education level | 1.331 | 0.249 | ||
| < High school | 46 (74.19) | 57 (67.86) | ||
| ≥ High school | 16 (25.81) | 27 (32.14) | ||
| Weight | 1.041 | 0.308 | ||
| <55 kg | 25 (40.32) | 27 (32.14) | ||
| ≥55 kg | 37 (59.68) | 57 (67.86) | ||
| Diet preference | 0.819 | 0.366 | ||
| Light | 33 (53.23) | 51 (60.71) | ||
| Spicy | 29 (46.77) | 33 (39.29) | ||
| Alcohol consumption | 0.199 | 0.656 | ||
| Never or seldom | 16 (25.81) | 19 (22.62) | ||
| Frequent | 46 (74.19) | 65 (77.38) |
Clinical efficacy of two groups of patients
In the control group after treatment, there were 8 cases of CR (12.91%), 15 cases of PR (24.19%), 29 cases of SD (46.77%), 10 cases of PD (16.13%), response rate (RR) was 37.10%. In the study group after treatment, there were 14 cases of CR (16.66%), 34 cases of PR (40.48%), 26 cases of SD (30.95%), 10 cases of PD (11.91%), and RR was 57.14%. RR of study group was significantly higher than that of control group (P<0.05) (Table III).
Table III.
Comparison of clinical efficacy results of two groups of patients [n (%)].
| Group | Control group (n=62) | Study group (n=84) | χ2 value | P-value |
|---|---|---|---|---|
| CR | 8 (12.90) | 14 (16.67) | – | – |
| PR | 15 (24.19) | 34 (40.48) | – | – |
| SD | 29 (46.77) | 26 (30.95) | – | – |
| PD | 10 (16.13) | 10 (11.91) | – | – |
| RR | 23 (37.10) | 48 (57.14) | 5.738 | 0.017 |
Comparison of toxic and side effects between two groups of patients
During the treatment, toxic and side effects occurred in both groups, and there was no allergic reaction. In the control group, nausea and vomiting occurred in 3 cases (4.84%), anemia in 4 cases (6.45%), thrombocytopenia in 3 cases (4.84%), myelosuppression in 5 cases (8.06%), liver function injury in 2 cases (3.23%), renal function injury in 3 cases (4.84%), and the total incidence of toxic and side effects was 32.26% (20/62). In the study group, nausea and vomiting occurred in 3 cases (3.57%), anemia in 2 cases (2.38%), thrombocytopenia in 2 cases (2.38%), myelosuppression in 4 cases (4.76%), liver function injury in 1 case (1.19%), renal function injury in 1 case (1.19%), and the total incidence of adverse reactions was 15.48% (13/84). There was no significant difference in the incidence of nausea, vomiting, anemia, thrombocytopenia, bone marrow suppression, liver function injury and renal function injury between the two groups (P>0.05), but the total incidence of adverse reactions in the study group was significantly lower than that in the control group (P<0.05) (Table IV).
Table IV.
Comparison of adverse reactions between two groups of patients [n (%)].
| Group | Control group (n=62) | Study group (n=84) | χ2 value | P-value |
|---|---|---|---|---|
| Nausea and vomiting | 3 (4.84) | 3 (3.57) | 0.145 | 0.703 |
| Anemia | 4 (6.45) | 2 (2.38) | 1.500 | 0.221 |
| Thrombocytopenia | 3 (4.84) | 2 (2.38) | 0.652 | 0.807 |
| Myelosuppression | 5 (8.06) | 4 (4.76) | 0.673 | 0.412 |
| Liver function injury | 2 (3.23) | 1 (1.19) | 0.734 | 0.392 |
| Renal function injury | 3 (4.84) | 1 (1.19) | 1.782 | 0.128 |
| Total incidence rate | 20 (32.26) | 13 (15.48) | 5.743 | 0.017 |
Comparison of quality of life between two groups of patients
The quality of life in the control group was improved in 10 cases (15.63%), stabilized in 25 cases (39.06%), deteriorated in 29 cases (45.31%), and the improvement rate of quality of life was 54.69% 30 days after discharge. In the study group, the quality of life was improved in 16 cases (19.05%), stabilized in 46 cases (54.76%), deteriorated in 22 cases (26.19%), and the improvement rate of quality of life was 73.81% 30 days after treatment. The improvement rate of quality of life in the study group was significantly higher than that in the control group (P<0.05) (Table V).
Table V.
Comparison of quality of life between two groups of patients [n (%)].
| Group | n | Improved | Stabilized | Deteriorated | Improvement rate (%) |
|---|---|---|---|---|---|
| Control group | 62 | 10 (15.63) | 25 (39.06) | 29 (45.31) | 54.69 |
| Study group | 84 | 16 (19.05) | 46 (54.76) | 22 (26.19) | 73.81 |
| χ2 value | – | – | – | – | 6.649 |
| P-value | – | – | – | – | 0.001 |
Comparison of 5-year survival rate between two groups of patients
The follow-up results showed that OS in the study group was 45.24% (38/84) at 5 years and OS in the control group was 20.97% (13/62) at 5 years. OS in the study group was significantly higher than that in the control group at 5 years (P<0.05) (Fig. 1).
Figure 1.
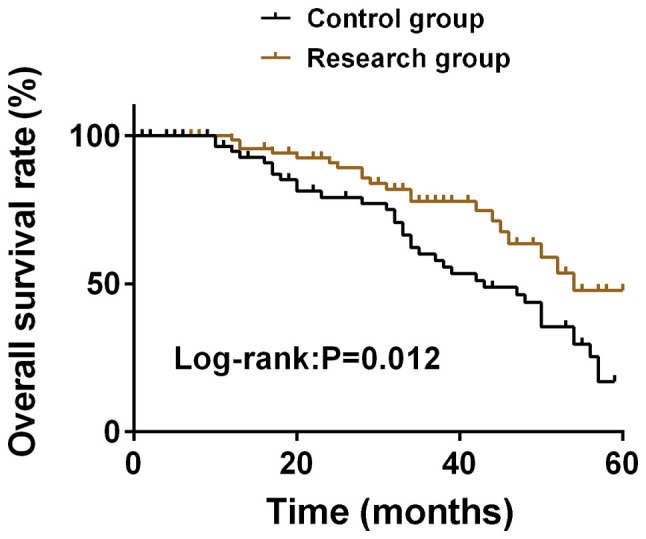
Comparison of quality of life between the two groups. The follow-up results showed that OS in the study group was significantly higher than that in the control group at 5 years (P<0.05). OS, overall survival.
Comparison of serum PSA levels of two groups of patients at different time points
There was no significant difference in serum PSA level between the two groups at T1 (P>0.05). Compared with T1, serum PSA levels in T2, T3 and T4 groups were significantly decreased (P<0.05). Compared with T2, the serum PSA level of the two groups of patients decreased significantly at T3 (P<0.05), while the serum PSA level of the study group decreased significantly at T4 (P<0.05), and the serum PSA level of the control group increased significantly (P<0.05). Compared with T3, the serum PSA levels of the two groups at T4 were significantly increased (P<0.05). At T2, T3 and T4, the serum PSA level in the study group was significantly higher than that in the control group (P<0.05). There was no significant difference in serum PSA level between the two groups at T1 (P>0.05). Compared with T1, the serum PSA levels of the two groups of patients at T2, T3 and T4 were significantly decreased (P<0.05), and the study group was significantly lower than the control group (P<0.05) (Table VI).
Table VI.
Comparison of serum PSA levels of two groups of patients at different time points (mean ± SD, ng/ml).
| Group | n | T1 | T2 | T3 | T4 | F | P-value |
|---|---|---|---|---|---|---|---|
| Control group | 62 | 63.42±22.13 | 5.82±1.87a | 4.21±1.23a,b | 6.46±1.52a–c | 418.944 | <0.001 |
| Study group | 84 | 64.14±19.24 | 4.65±1.31a | 3.44±0.87a,b | 4.13±0.93a–c | 811.626 | <0.001 |
| t value | – | 0.210 | 4.446 | 4.431 | 11.450 | – | – |
| P-value | – | 0.834 | <0.001 | <0.001 | <0.001 | – | – |
Compared with T1
P<0.05 compared with T2
P<0.05 compared with T3
P<0.05. T1, three days before treatment; T2, one month after treatment; T3, two months after treatment; T4, six months after treatment.
Comparison of relative expression of serum miR-493-5p in two groups of patients at different time points
There was no significant difference in the relative expression of serum miR-493-5p between the two groups at T1 (P>0.05). Compared with T1, the relative expression of miR-493-5p in serum of the two groups of patients at T2, T3 and T4 were significantly increased (P<0.05). Compared with T2, the relative expression levels of serum miR-493-5p at T3 and T4 were significantly increased (P<0.05). Compared with T3, the relative expression of serum miR-493-5p in the two groups at T4 was significantly increased (P<0.05). At T2, T3 and T4, the relative expression of serum miR-493-5p in the study group was significantly higher than that in the control group (P<0.05) (Table VII).
Table VII.
Comparison of relative expression levels of serum miR-493-5p in two groups of patients at different time points (mean ± SD).
| Group | n | T1 | T2 | T3 | T4 | F | P-value |
|---|---|---|---|---|---|---|---|
| Control group | 62 | 1.07±0.78 | 1.38±0.68a | 1.62±0.57a,b | 1.88±0.73a–c | 14.508 | <0.001 |
| Study group | 84 | 1.12±0.67 | 1.67±0.69a | 1.98±0.89a,b | 2.34±0.81a–c | 37.815 | <0.001 |
| t value | – | 0.416 | 2.526 | 2.789 | 3.535 | – | – |
| P-value | – | 0.678 | 0.013 | 0.006 | <0.001 | – | – |
Compared with T1
P<0.05 compared with T2
P<0.05 compared with T3
P<0.05. T1, three days before treatment; T2, one month after treatment; T3, two months after treatment; T4, six months after treatment.
Comparison of serum miR-195-5p relative expression in two groups of patients at different time points
There was no significant difference in the relative expression of serum miR-195-5p between the two groups at T1 (P>0.05). Compared with T1, the relative expression of miR-195-5p in serum at T2, T3 and T4 was significantly increased in both groups (P<0.05). Compared with T2, the relative expression of miR-195-5p in serum at T3 and T4 was significantly increased in both groups (P<0.05). Compared with T3, the relative expression of serum miR-195-5p in the two groups at T4 was significantly increased (P<0.05). At T2, T3 and T4, the relative expression of serum miR-195-5p in the study group was significantly higher than that in the control group (P<0.05) (Table VIII).
Table VIII.
Comparison of relative expression of serum miR-195-5p between two groups of patients at different time points (mean ± SD).
| Group | n | T1 | T2 | T3 | T4 | F | P-value |
|---|---|---|---|---|---|---|---|
| Control group | 62 | 0.65±0.29 | 1.12±0.35a | 1.43±0.45a,b | 1.71±0.61a–c | 65.489 | <0.001 |
| Study group | 84 | 0.64±0.34 | 1.59±0.41a | 1.97±0.54a,b | 2.48±0.65a–c | 203.549 | <0.001 |
| t value | – | 0.187 | 7.278 | 6.401 | 7.261 | – | – |
| P-value | – | 0.852 | <0.001 | <0.001 | <0.001 | – | – |
Compared with T1
P<0.05 compared with T2
P<0.05 compared with T3
P<0.05. T1, three days before treatment; T2, one month after treatment; T3, two months after treatment; T4, six months after treatment.
Diagnostic value of miR-493-5p and miR-195-5p expression levels in patients
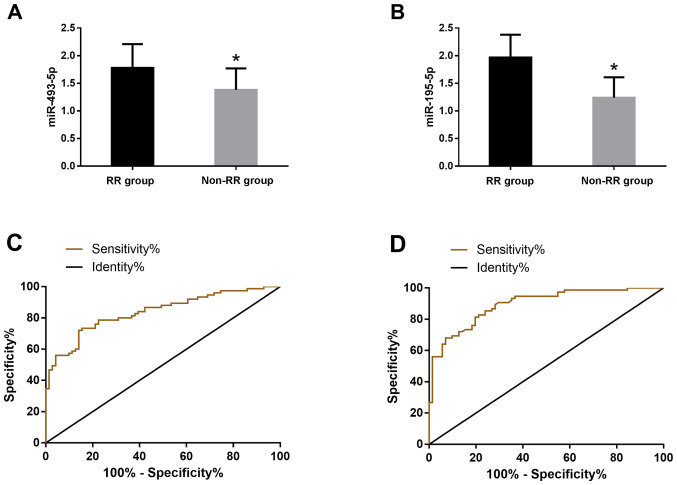
According to the clinical efficacy, the patients were divided into RR group (n=71) and non-RR group (n=75). The serum levels of miR-493-5p and miR-195-5p were detected in the two groups. The expression levels of miR-493-5p in RR group and non-RR group were 1.78±0.43 and 1.38±0.39, respectively, the difference was statistically significant (P<0.05). The expression levels of miR-195-5p in serum of RR group and non-RR group were 1.97±0.41, 1.24±0.37, the difference was statistically significant (P<0.05). The receiver operating characteristic (ROC) curve of the serum miR-493-5p and miR-195-5p expression levels in the diagnosis of patients was drawn. The AUC value of the serum miR-493-5p diagnosis was 0.829, the sensitivity was 72%, and the specificity was 85.92%; the AUC of therapeutic effect of serum miR-195-5p was 8.95, the sensitivity was 90.67%, and the specificity was 71.83% (Fig. 2).
Figure 2.
Diagnostic value of miR-493-5p and miR-195-5p expression levels in patients with therapeutic effects. (A) The expression level of miR-493-5p in serum of RR group was significantly higher than that of non-RR group (P<0.05). (B) The expression level of miR-195-5p in serum of RR group was significantly higher than that of non-RR group (P<0.05). (C) ROC curve for the diagnosis of serum miR-493-5p in patients. (D) ROC curve for the diagnosis of serum miR-195-5p in patients. ROC, receiver operating characteristic; RR group, response rate group. *P<0.05, compared with RR group.
Discussion
PCa is one of the most common adult malignant tumors. Approximately 220,000 American males are diagnosed with PCa every year. Androgen deprivation therapy (ADT) is one of the main methods for PCa treatment. Male patients with metastatic PCa almost all develop drug resistance to primary ADT and develop CRPC (18,19). In the past, treatment options for CRPC patients were limited. In recent years, new androgen pathway inhibitors are becoming the mainstream treatment methods for CRPC, such as abiraterone acetate and enzalutamide (20). At present, there is no unified administration method for treating CRPC through androgen pathway inhibitor clinically, and the curative effect and side effects by different administration methods are also different. It is of great significance for CRPC patients to explore the administration method with better curative effect and safety.
Serum PSA is widely used as a biomarker for PCa diagnosis and is also a powerful prognostic marker for PCa long-term risk (21,22). Some studies (23) show that the serum PSA level of CRPC patients has significantly decreased after using abiraterone acetate, and the early PSA change is related to the survival rate of patients using abiraterone acetate, i.e. the increase of early PSA leads to poor prognosis of early chemotherapy. The results of this study show that the serum PSA levels of the two groups of patients significantly decreased after treatment and the decrease in the study group was greater. Similar to the above results, it shows that the two administration methods in this study can effectively reduce PSA levels, and the administration method of abiraterone combined with flutamide could decrease the levels more quickly.
Abiraterone acetate is the nursing standard of CRPC, which can improve the overall survival time and progression-free survival time of CRPC patients and reduce the deterioration of the quality of life in metastatic CRPC patients. Currently, abiraterone acetate has been approved by more than 70 countries (2014) for the treatment of metastatic CRPC patients who have not received chemotherapy (24,25). Research (26) results show that abiraterone acetate also shows significant anti-tumor activity in CRPC patients after receiving docetaxel, and has good effects on safety and tolerance. Flutamide is the first nonsteroidal antiandrogen drug approved by the U.S. Food and Drug Administration (FDA) for PCa, which can block the action of androgen testosterone, prevent normal growth of PCa cells and achieve the purpose of treating PCa (27). Basch et al (28) showed that compared with placebo combined with prednisone, abiraterone combined with prednisone can prolong the response pain progression and median time of CRPC patients undergoing chemotherapy treatment, and the median time for deterioration of health-related quality of life (HRQoL) is also longer. Fizazi et al (29) also used abiraterone combined with prednisone to treat CRPC patients. The results are similar to those of Basch et al (28). The median overall survival time and radiological progression-free survival time of patients in the abiraterone group (androgen deprivation therapy and abiraterone conbined with prednisone) are significantly longer than those in the placebo group (androgen deprivation therapy and double placebo), and other indicators observed in the abiraterone group are also better than those in the placebo group, such as the time of pain progression, subsequent PCa treatment, chemotherapy initiation and prostate specific antigen progression. In recent years, it has been reported that the use of carbamide and flutamide in patients with PCa prior to the use of abiraterone does not rule out the effects of abiraterone (30). The results of this study show that RR in the study group was significantly higher than that in the control group after treatment, the total incidence of toxic and side effects in the study group was significantly lower than that in the control group, and OS in the study group was significantly higher than that in the control group for 5 years. This indicates that abiraterone combined with flutamide has better curative effect and lower incidence of adverse reactions in CRPC patients than single drug.
Wang et al (31) showed that miR-493-5p is downregulated in PCa cells, c-Met, CREB1 and EGFR are downstream target genes of miR-493-5p, and miR-493-5p inhibits cancer development through AKT/GSK-3β/ Snail signaling in prostate cancer. Cai et al (32) reported that the downregulation of miR-195-5p in PCa tissue is significantly related to high Gleason score, positive metastasis failure and biochemical recurrence, and they confirmed the tumor inhibitory effect of miR-195-5p through in vitro PCa cell invasion, migration and apoptosis experiments. Linder et al (33) also reached the same conclusion as Cai et al (32), that is, miR-195-5p can inhibit PCa tumor, overexpression of miR-195-5p can inhibit PCa cell migration, invasion and epithelial-mesenchymal transition (EMT). They also pointed out that fibroblast growth factor 2 (FGF2) is recognized as the direct target of miR-195-5p, and miR-195-5p can inhibit PCa cell metastasis by downregulating FGF2. After treatment, the relative expression of miR-493-5p and miR-195-5p in the serum of the two groups of patients in this study significantly increased. The above research shows that overexpression of miR-493-5p and miR-195-5p significantly inhibits the migration and invasion of PCa cells and acts as a tumor inhibitor in PCa cells. Therefore, we speculate that abiraterone and flutamide may inhibit the migration and invasion of PCa cells by increasing the expression levels of miR-493-5p and miR-195-5p and also their molecular mechanisms such as the antagonism of androgen receptor miR-195-5p, thus, reducing the progress of PCa and achieving the purpose of PCa treatment. At the end of the study, we also examined the diagnostic value of miR-493-5p and miR-195-5p expression levels in patients. The results showed that the AUC values of the therapeutic effects of serum miR-493-5p and miR-195-5p were respectively 0.829 and 0.895, indicating that they both have a certain diagnostic value for the efficacy of PCa.
Collectively, abiraterone combined with flutamide has better curative effect and lower incidence of adverse reactions in CRPC patients than single drug, and can increase the expression levels of miR-493-5p and miR-195-5p in patient serum.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
CY and YD conceived and designed the study, and drafted the manuscript. CY, YD, SP and XG collected, analyzed and interpreted the experimental data. XG revised the manuscript for important intellectual content. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of Longhua Hospital Shanghai University of Traditional Chinese Medicine (Shanghai, China). Signed informed consents were obtained from the patients and/or guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Afshar-Oromieh A, Holland-Letz T, Giesel FL, Kratochwil C, Mier W, Haufe S, Debus N, Eder M, Eisenhut M, Schäfer M, et al. Diagnostic performance of 68Ga-PSMA-11 (HBED-CC) PET/CT in patients with recurrent prostate cancer: Evaluation in 1007 patients. Eur J Nucl Med Mol Imaging. 2017;44:1258–1268. doi: 10.1007/s00259-017-3711-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Watson PA, Arora VK, Sawyers CL. Emerging mechanisms of resistance to androgen receptor inhibitors in prostate cancer. Nat Rev Cancer. 2015;15:701–711. doi: 10.1038/nrc4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang Y, Chen R, Sun T, Zhao L, Liu F, Ren S, Wang H, Lu X, Gao X, Xu C, et al. Efficacy and safety of combined androgen blockade with antiandrogen for advanced prostate cancer. Curr Oncol. 2019;26:e39–e47. doi: 10.3747/co.26.4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kambale PR, Haldar D, Kabi BC, Kambale KP. Study of vitamin D receptor gene polymorphism (FokI, TaqI and ApaI) among prostate cancer patients in North India. J Clin Diagn Res. 2017;11:BC05–BC08. doi: 10.7860/JCDR/2017/24290.9976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benoist GE, Hendriks RJ, Mulders PF, Gerritsen WR, Somford DM, Schalken JA, van Oort IM, Burger DM, van Erp NP. Pharmacokinetic aspects of the two novel oral drugs used for metastatic castration-resistant prostate cancer: Abiraterone acetate and enzalutamide. Clin Pharmacokinet. 2016;55:1369–1380. doi: 10.1007/s40262-016-0403-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yasui M, Uemura K, Yoneyama S, Kawahara T, Hattori Y, Teranishi JI, Inoue M, Ohta JI, Yokomizo Y, Yao M, et al. Predictors of poor response to secondary alternative antiandrogen therapy with flutamide in metastatic castration-resistant prostate cancer. Jpn J Clin Oncol. 2016;46:1042–1046. doi: 10.1093/jjco/hyw110. [DOI] [PubMed] [Google Scholar]
- 7.Reid AH, Attard G, Danila DC, Oommen NB, Olmos D, Fong PC, Molife LR, Hunt J, Messiou C, Parker C, et al. Significant and sustained antitumor activity in post-docetaxel, castration-resistant prostate cancer with the CYP17 inhibitor abiraterone acetate. J Clin Oncol. 2010;28:1489–1495. doi: 10.1200/JCO.2009.24.6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, Chi KN, Jones RJ, Goodman OB, Jr, Saad F, et al. COU-AA-301 Investigators Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iguchi T, Tamada S, Kato M, Yasuda S, Otoshi T, Hamada K, Yamasaki T, Nakatani T. Enzalutamide versus flutamide for castration-resistant prostate cancer after combined androgen blockade therapy with bicalutamide: A retrospective study. Int J Clin Oncol. 2019;24:848–856. doi: 10.1007/s10147-019-01413-1. [DOI] [PubMed] [Google Scholar]
- 10.Fukasawa S, Suzuki H, Kawaguchi K, Noguchi H, Enjo K, Tran N, Todd M, Fizazi K, Matsubara N. Efficacy and safety of abiraterone acetate plus prednisone in Japanese patients with newly diagnosed, metastatic hormone-naïve prostate cancer: A subgroup analysis of LATITUDE, a randomized, double-blind, placebo-controlled, Phase 3 study. Jpn J Clin Oncol. 2018;48:1012–1021. doi: 10.1093/jjco/hyy129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nie W, Ge HJ, Yang XQ, Sun X, Huang H, Tao X, Chen WS, Li B. LncRNA-UCA1 exerts oncogenic functions in non-small cell lung cancer by targeting miR-193a-3p. Cancer Lett. 2016;371:99–106. doi: 10.1016/j.canlet.2015.11.024. [DOI] [PubMed] [Google Scholar]
- 12.Fernandez S, Risolino M, Mandia N, Talotta F, Soini Y, Incoronato M, Condorelli G, Banfi S, Verde P. miR-340 inhibits tumor cell proliferation and induces apoptosis by targeting multiple negative regulators of p27 in non-small cell lung cancer. Oncogene. 2014;34:3240–3250. doi: 10.1038/onc.2014.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beebe-Dimmer JL, Ruterbusch JJ, Bylsma LC, Gillezeau C, Fryzek J, Schultz NM, Flanders SC, Barlev A, Heath E, Quek RGW. Patterns of bicalutamide use in prostate cancer treatment: A U.S. real-world analysis using the SEER-Medicare database. Adv Ther. 2018;35:1438–1451. doi: 10.1007/s12325-018-0738-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao L, Feng X, Song X, Zhou H, Zhao Y, Cheng L, Jia L. miR-493-5p attenuates the invasiveness and tumorigenicity in human breast cancer by targeting FUT4. Oncol Rep. 2016;36:1007–1015. doi: 10.3892/or.2016.4882. [DOI] [PubMed] [Google Scholar]
- 15.Wang G, Fang X, Han M, Wang X, Huang Q. MicroRNA-493-5p promotes apoptosis and suppresses proliferation and invasion in liver cancer cells by targeting VAMP2. Int J Mol Med. 2018;41:1740–1748. doi: 10.3892/ijmm.2018.3358. [DOI] [PubMed] [Google Scholar]
- 16.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 17.Tamada S, Iguchi T, Kato M, Asakawa J, Kita K, Yasuda S, Yamasaki T, Matsuoka Y, Yamaguchi K, Matsumura K, et al. Time to progression to castration-resistant prostate cancer after commencing combined androgen blockade for advanced hormone-sensitive prostate cancer. Oncotarget. 2018;9:36966–36974. doi: 10.18632/oncotarget.26426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teoh JY, Ng CF, Poon DM. Chemohormonal therapy for metastatic hormone-sensitive prostate cancer: An Asian perspective. Asia Pac J Clin Oncol. 2018;14(Suppl 5):5–8. doi: 10.1111/ajco.13060. [DOI] [PubMed] [Google Scholar]
- 19.Robinson D, Van Allen EM, Wu YM, Schultz N, Lonigro RJ, Mosquera JM, Montgomery B, Taplin ME, Pritchard CC, Attard G, et al. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161:1215–1228. doi: 10.1016/j.cell.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mittal RD, Mishra DK, Srivastava P, Manchanda P, Bid HK, Kapoor R. Polymorphisms in the vitamin D receptor and the androgen receptor gene associated with the risk of urolithiasis. Indian J Clin Biochem. 2010;25:119–126. doi: 10.1007/s12291-010-0023-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dong L, Zieren RC, Xue W, de Reijke TM, Pienta KJ. Metastatic prostate cancer remains incurable, why? Asian J Urol. 2019;6:26–41. doi: 10.1016/j.ajur.2018.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shariat SF, Semjonow A, Lilja H, Savage C, Vickers AJ, Bjartell A. Tumor markers in prostate cancer I: Blood-based markers. Acta Oncol. 2011;50(Suppl 1):61–75. doi: 10.3109/0284186X.2010.542174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rescigno P, Lorente D, Bianchini D, Ferraldeschi R, Kolinsky MP, Sideris S, Zafeiriou Z, Sumanasuriya S, Smith AD, Mehra N, et al. Prostate-specific antigen decline after 4 weeks of treatment with abiraterone acetate and overall survival in patients with metastatic castration-resistant prostate cancer. Eur Urol. 2016;70:724–731. doi: 10.1016/j.eururo.2016.02.055. [DOI] [PubMed] [Google Scholar]
- 24.Szmulewitz RZ, Peer CJ, Ibraheem A, Martinez E, Kozloff MF, Carthon B, Harvey RD, Fishkin P, Yong WP, Chiong E, et al. Prospective international randomized phase II study of low-dose abiraterone with food versus standard dose abiraterone in castration-resistant prostate cancer. J Clin Oncol. 2018;36:1389–1395. doi: 10.1200/JCO.2017.76.4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagai T, Naiki T, Iida K, Etani T, Ando R, Hamamoto S, Sugiyama Y, Akita H, Kubota H, Hashimoto Y, et al. Early abiraterone acetate treatment is beneficial in Japanese castration-resistant prostate cancer after failure of primary combined androgen blockade. Prostate Int. 2018;6:18–23. doi: 10.1016/j.prnil.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu L, Chen J, Liu W, Liang C, Hu H, Huang J. Targeting androgen receptor-independent pathways in therapy-resistant prostate cancer. Asian J Urol. 2019;6:91–98. doi: 10.1016/j.ajur.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ito Y, Sadar MD. Enzalutamide and blocking androgen receptor in advanced prostate cancer: Lessons learnt from the history of drug development of antiandrogens. Res Rep Urol. 2018;10:23–32. doi: 10.2147/RRU.S157116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Basch E, Autio K, Ryan CJ, Mulders P, Shore N, Kheoh T, Fizazi K, Logothetis CJ, Rathkopf D, Smith MR, et al. Abiraterone acetate plus prednisone versus prednisone alone in chemotherapy-naive men with metastatic castration-resistant prostate cancer: Patient-reported outcome results of a randomised phase 3 trial. Lancet Oncol. 2013;14:1193–1199. doi: 10.1016/S1470-2045(13)70424-8. [DOI] [PubMed] [Google Scholar]
- 29.Fizazi K, Tran N, Fein L, Matsubara N, Rodriguez-Antolin A, Alekseev BY, Özgüroğlu M, Ye D, Feyerabend S, Protheroe A, et al. LATITUDE investigators Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med. 2017;377:352–360. doi: 10.1056/NEJMoa1704174. [DOI] [PubMed] [Google Scholar]
- 30.Zhao JG, Liu JD, Shen PF, Tang X, Sun GX, Zhang XM, Chen JR, Shu KP, Shi M, Zeng H. Prior switching to a second-line nonsteroidal antiandrogen does not impact the therapeutic efficacy of abiraterone acetate in patients with metastatic castration-resistant prostate cancer: A real-world retrospective study. Asian J Androl. 2018;20:545–550. doi: 10.4103/aja.aja_58_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang S, Wang X, Li J, Meng S, Liang Z, Xu X, Zhu Y, Li S, Wu J, Xu M, et al. c-Met, CREB1 and EGFR are involved in miR-493-5p inhibition of EMT via AKT/GSK-3β/Snail signaling in prostate cancer. Oncotarget. 2017;8:82303–82313. doi: 10.18632/oncotarget.19398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cai C, Chen QB, Han ZD, Zhang YQ, He HC, Chen JH, Chen YR, Yang SB, Wu YD, Zeng YR, et al. miR-195-5 inhibits tumor progression by targeting RPS6KB1 in human prostate cancer. Clin Cancer Res. 2015;21:4922–4934. doi: 10.1158/1078-0432.CCR-15-0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Linder S, van der Poel HG, Bergman AM, Zwart W, Prekovic S. Enzalutamide therapy for advanced prostate cancer: Efficacy, resistance and beyond. Endocr Relat Cancer. 2018;26:R31–R52. doi: 10.1530/ERC-18-0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.