Abstract
In this paper, the application of biofiltration is investigated for controlled removal of gas phase chloroform through cometabolic degradation with ethanol. A trickle bed air biofilter (TBAB) operated under acidic pH 4 is subjected to aerobic biodegradation of chloroform and ethanol. The TBAB is composed of pelleted diatomaceous earth filter media inoculated with filamentous fungi species, which served as the principle biodegrading microorganism. The removal efficiencies of 5 ppmv of chloroform mixed with different ratios of ethanol as cometabolite (25, 50, 100, 150, and 200 ppmv) ranged between 69.9 and 80.9%. The removal efficiency, reaction rate kinetics, and the elimination capacity increased proportionately with an increase in the cometabolite concentration. The carbon recovery from the TBAB amounted to 69.6% of the total carbon input. It is postulated that the remaining carbon contributed to excess biomass yield within the system. Biomass control strategies such as starvation and stagnation were employed at different phases of the experiment. The chloroform removal kinetics provided a maximum reaction rate constant of 0.0018 s−1. The highest ratio of chemical oxygen demand (COD)removal/nitrogenutilization was observed at 14.5. This study provides significant evidence that the biodegradation of a highly chlorinated methane can be favored by cometabolism in a fungi-based TBAB.
Introduction
Trichloromethane (CAS No. 67–66-3), commonly known as chloroform, is a colorless, heavy volatile liquid with an ether-like odor (USNIH 2016). Chloroform is widely used for laboratory purposes, the manufacture of refrigerants, pharmaceuticals, and household cleaning products, and is also formed as a byproduct of chlorination disinfection in drinking water, wastewater, and swimming pools. Sources of chloroform emissions include publicly owned treatment works (POTWs), cooling towers, pulp and paper mills, hazardous waste sites, and sanitary landfills (CARB 1990). Chloroform persists as a fairly stable, non-reactive compound in the atmosphere for about 0.5 years causing long-range air pollution (Khalil and Rasmussen 1999). The U.S. EPA has designated it as one of the 189 hazardous air pollutants under the Clean Air Act and also as a disinfection byproduct with a maximum contaminant level of 70 ppb under the Safe Drinking Water Act (Hua and Reckhow 2007; USEPA 2016). Since chlorination is a universal form of disinfection, the occurrence of chloroform as an intermediary byproduct in treated water is a common phenomenon. A national groundwater supply survey stated that 45% of the sample sites had detectable levels of chloroform, with the minimum and maximum concentrations of 1.5 and 300 ppb, respectively (Westrick et al. 1984). A drinking water study performed in 53 water treatment facilities in nine Canadian provinces showed chloroform levels reaching a maximum of 336 ppb, for 214 samples measured with >0.2 ppb of chloroform detected in all the samples (Williams et al. 1995). Another study performed in 2001 in Mumbai, India, showed that the levels of chloroform ranged from 29.1 to 231.26 ppb in treated drinking water (Thacker et al. 2002).
Chloroform exposure occurs through various ancillary routes such as from chlorinated tap water used for cooking, showering, and domestic cleaning. Inhalation exposure is reported to be an average concentration ranging between 2 and 2200 μg/day in rural, urban, and source-dominated areas (ATSDR 1997). The implications of chloroform ingestion into the human system have been researched over the years. The International Agency for Research on Cancer (IARC) has determined that chloroform is possibly carcinogenic to humans (2B) (Komulainen 2004). Some studies reveal that increased risk of bladder and colorectal cancer is associated with chronic exposure to chloroform (Morris et al. 1992). Treatment techniques that are currently employed for the removal of volatile organic compounds (VOCs) include carbon adsorption, and catalytic and thermal oxidation (FRTR 2002). These technologies are often not economical to treat dilute gas streams and energy-intensive when treating moisture-laden emissions, and often form secondary pollutants that may require hazardous waste handling procedures (FRTR 2002).
Biofiltration is an air pollution control technology traditionally used in the UK and Japan for the treatment of odorous compounds, hydrocarbon, and vapor emissions from remediation systems. Biofiltration is a low-cost destructive mechanism in which vapor-phase organic contaminants are passed through a bed of porous media and sorbed to the media surface where they are degraded by microorganisms (Smith et al. 1998). Biofilters rely on the microbial catabolic reactions for the removal of contaminants (Jang and Jang 2000). They are well suited for dilute waste streams of hydrocarbons that upon biodegradation are reduced to carbon dioxide, water, and biomass (Devinny et al. 1998). They are reported to be efficient treatment mechanisms for alcohols, ethers, aldehydes, ketones, and common monocyclic aromatics, although some compounds like chlorinated hydrocarbons, polyaromatic hydrocarbons, however, highly halogenated hydrocarbons show moderate to slow biodegradation rates (Kumar et al. 2011). In halogenated organics, the nature of the halogen bond and the halogen itself can significantly affect the biofiltration process (Leson and Winer 1991). In order to overcome this, halogenated organic compounds often require the presence of an easily degradable substrate that can increase their biodegradability by co-metabolism (Leson and Winer 1991). Higher chlorinated methanes, such as chloroform and carbon tetrachloride, are only known to biodegrade through fortuitous cometabolism as they are not favored as sole sources of carbon and energy by the microorganisms (Field and Sierra-Alvarez 2004). Frascari et al. (2008) studied the cometabolic biodegradation of chloroform along with other chlorinated aliphatic hydrocarbons using propane-grown Rhodococcus sp. PB1 under aerobic conditions (Frascari et al. 2008). The study concluded that propane exerted significant inhibition on the biodegradation of chloroform. The same authors tested the aerobic degradation of chloroform using butane as the primary substrate and Rhodococcus aetherovorans strain, BCP1, as the biodegrading microorganism. They analyzed chloroform at the levels of 0–75.5 mg/l and obtained a maximum specific degradation rate of 22 μmol/(mgprotein. day) (Frascari et al. 2006). Another study performed by Wahman et al. (2006) used nitrifying bacteria to cometabolically biodegrade trihalomethanes in drinking water containing ammonia and reported that chloroform showed the lowest reaction rate among all four trihalomethanes (Wahman et al. 2006). Bagley et al. (2000) reported the anaerobic biodegradation of tetrachloroethane, carbon tetrachloride, and chloroform in the presence of propionic and acetic acid (Bagley et al. 2000). The aforementioned studies have invariably analyzed chloroform in its aqueous phase, while the focus of our study is to understand the cometabolic biodegradation of stripped chloroform in the gas phase.
In this study, ethanol is used as a cometabolite in combination with chloroform since it is a strong polar solvent and a simple straight chain alcohol. Both compounds are often emitted in a mixture by pharmaceutical industries and wastewater sources (Balasubramanian et al. 2011; Cecen and Aktas 2011). Balasubramanian et al. (2011) studied the aerobic biodegradation rates of different organic solvents like methanol, ethanol, isopropanol, acetone, acetonitrile, and toluene in individual batch systems. Ethanol was degraded in the least amount of time and also formed the maximum microbial growth rate of 0.0415/h. According to Hernandez-Perez et al. (2001), the addition of ethanol as the primary substrate served as the sole carbon source for Gordonia terrae to biodegrade methyl t-butyl ether and t-amyl methyl ether.
Additionally, the hydrophobic nature of chloroform slows down the mass transfer of the contaminant into the liquid phase within the filter bed affecting the reaction kinetics. Kennes and Veiga (2004) and Vergara‐Fernández et al. (2008) proposed that this obstacle can be overcome by using filamentous fungi as the biodegrading microorganism. So far, many aromatic hydrocarbons have been subjected to biofiltration with filamentous fungi (García‐Peña et al. 2001; Zehraoui et al. 2013; Prado et al. 2002; Chheda and Sorial 2016; Hassan and Sorial 2010a). This paper extends its application for the treatment of a chlorinated aliphatic compound. Filamentous fungi are known to use hydrocarbons as their growth substrates and can be readily isolated from the soil (Hardison et al. 1997). Fungi have several advantages for the treatment of hydrophobic VOCs in trickle bed air biofilters (TBABs) including the ability to degrade a large number of VOCs and the resistance to low humidity favored by the presence of cysteine-rich proteins called hydrophobins in the hyphal surface (Vergara‐Fernández et al. 2008; Chheda and Sorial 2016). Filamentous fungi are also capable of colonizing the void space within the growth media with their aerial hyphae in order to increase the availability of nutrients (Vergara‐Fernández et al. 2008). Vergara‐Fernández et al. (2008) devised a mathematical model that linearly correlates the elimination capacity for n-hexane to the specific surface area of transport (SSAT) formed by the hyphal elongation of filamentous fungi. Due to the typical recalcitrance of chlorinated organics, the acidification of the filter bed is also an issue in the treatment of such compounds in a biofilter (Leson and Winer 1991). Fungi are metabolically active over a wide pH range of 2 to 7 and are tolerant to pH fluctuations unlike bacteria which requires neutral pH for sustenance (Kennes and Veiga 2004). Up to the present, biofiltration of chloroform has been performed only under neutral conditions. In one study, a TBAB running at neutral pH was used to treat a combination of VOCs including chloroform, from waste air in a wastewater treatment plant. No removal of chloroform was observed at feed concentrations ranging from 16 to 102 ppb while maintaining an empty bed residence time (EBRT) of 24 s (Cox et al. 2002). A similar study involved the use of a pilot-scale TBAB for the removal of hydrogen sulfide along with a combination of VOCs containing chloroform. The TBAB was run at an EBRT of 24–52 s under neutral conditions and showed low to nil removal for treating 50–76 ppbv of chloroform (Converse et al. 2003).
The current study will investigate the removal of chloroform under acidic pH conditions in order to facilitate the growth of fungal colonies. Chloroform at inlet concentration of 5 ppmv is subjected to cometabolic biofiltration with ethanol under acidic conditions in a TBAB using filamentous fungi. Five parts per million by volume (ppmv) of gas phase chloroform was equivalent to 96.5 ppb in the liquid phase, which is more than the MCL of chloroform in drinking water. It also imitates the environmentally relevant concentrations of chloroform in wastewater discharges, industrial effluents, and source-dominated areas (Jolley et al. 1990; ATSDR 1998). Previous research in our laboratory has proven that a TBAB can effectively treat both hydrophobic and hydrophilic VOCs such as methyl ethyl ketone, methyl isobutyl ketone, benzene, and n-hexane, and achieve stable removal performance for a wide range of feed concentrations (Cai et al. 2004, 2005; Hassan and Sorial 2010c; Zehraoui et al. 2012). The objectives of this paper are to (1) investigate the removal performance of chloroform under different cometabolite loading rates, (2) study the removal kinetics of chloroform and identify the reaction rates at different VOC loading rates, (3) draw a carbon mass balance for the TBAB, and (4) determine the optimum chemical oxygen demand (COD)removal/nitrogenutilization ratio to understand the growth of biomass.
Materials and Methods
Chemicals
In this study, two volatile organic compounds namely chloroform (CAS: 67–66-3) with 99.8% purity obtained from Fisher Scientific (Pittsburgh, PA, USA) and ethyl alcohol (CAS: 64–17-5) with 99.5% purity obtained from Sigma Aldrich (St. Louis, MO, USA) were used. Chloroform is highly hydrophobic with a Henry’s law constant, K H of 3.5 × 10−3 atm.m3/mol, and the K H value of a hydrophilic ethanol is known to be 5.1 × 10−6 atm.m3/mol at 25 °C (Chen et al. 2012; Butler et al. 1935).
Trickle Bed Air Biofilter
The TBAB was made up of seven cylindrical glass sections with an internal diameter of 7.6 cm and a total length of 130 cm. It was packed with synthetic biological support media (Celite® 6-mm R-635 Bio-Catalyst Carrier; Celite Corp., Lompoc, CA, USA) comprising of pelleted diatomaceous earth covering a depth of 60 cm. Figure 1 presents a schematic of the TBAB. A constant temperature of 35 °C was maintained inside the TBAB. The temperature was noted to be higher than that reported in previous publications due to the oxidation of ethanol during which micro-organisms convert chemical energy to heat as their primary energy source (Devinny et al. 1998). To maintain satisfactory conditions of moisture and nutrients for the microorganisms’ activity, the buffered nutrient solution was delivered intermittently into the TBAB through a spray nozzle. The nutrients were supplied at an acidic pH of 4 by the addition of sodium formate buffer to encourage the growth of fungi colonies. The buffered solution contains all necessary micronutrients and vitamins essential for biomass growth, as described by Sorial et al. (1995).
Figure 1.
Schematic of the trickle bed air biofilter
Compressed air was supplied as the carrier gas at the flow rate of 0.50 sL/min with a corresponding empty bed residence time (EBRT) of 5.44 min. Liquid chloroform and ethanol were injected into the air stream via syringe pumps and vaporized. The vapors were homogenously mixed inside a mixing chamber and then fed to the TBAB as shown in Fig. 1. The nutrient solution was supplied at the rate of 1.5 L/day. The TBAB was continuously operated in a co-current gas and liquid downward flow mode to acclimatize and enhance the growth of biomass. A combination of two biomass control technologies namely starvation and stagnation was used through the length of the experimental phase. Both non-use periods were observed during two consecutive days per week. During the starvation period, the TBAB only received the nutrients, devoid of any supply of VOCs and air. Under stagnation, the TBAB did not get any nutrients, VOCs, or air (Hassan and Sorial 2009).
Analytical Methods
Gas Sampling
Gas phase samples were taken from seven equidistant ports through the length of the TBAB. The samples were manually drawn using gas-tight syringes through low-bleed and high-puncture-tolerance silicone gas chromatograph (GC) septa installed in the sampling ports. Samples for chloroform and ethanol were immediately analyzed using GC–HP 6890 Series, Column: HP 608, 30 m × 530 μm film thickness, part no. Agilent 19095S – 023. The GC was equipped with a flame ionization detector (FID). The GC oven was programmed to an isothermal setting of 60 °C for 2 min and then ramped to 90 °C @ 10 °C/min. The carrier gas (He) flow rate was set at 3.5 mL/min. The FID was used with N2 make-up gas at a flow rate of 30 mL/min, a fuel gas flow (H2) of 40 mL/min, and airflow of 400 mL/min. Retention time for chloroform was 3.3 min and ethanol was 2.5 min under the above conditions. Samples of CO2 were also taken manually from each sampling port using GC Model No: Agilent 19095P equipped with CarbPLOT capillary column with dimensions 30 m × 530 μm × 0.83 μm, and thermal conductivity detector (TCD). The GC oven was programmed at 60 °C for 1 min and ramped to 115 °C at 25 °C/min. The TCD was used with helium make-up gas at a flow rate of 5 mL/min.
Liquid Sampling
Liquid phase data included the measurement of the influent and effluent concentrations of total carbon (TC), inorganic carbon (IC), nitrate, chloride, and volatile suspended solids (VSS). TC and IC contents of the aqueous samples were determined using a Shimadzu TOC-L total organic carbon analyzer (Shimadzu Corp., Tokyo, Japan). Nitrate and chloride were analyzed using an ion chromatograph fitted with an anion exchange column (Dionex Corp., Sunnyvale, CA, USA). VSS analysis was carried out according to Standard Methods 2540G (Clescerl et al. 1999).
Results and Discussion
The length of the experiment spanned to approximately 1 year. The tests consisted of five phases of operation where each phase constituted a different ethanol concentration in the feed mixture as presented in Table 1. Chloroform was analyzed at a fixed concentration of 5 ppmv (0.27 g/(m3.h)) mixed with ethanol at 25 ppmv (0.57 g/(m3.h)), 50 ppmv (1.15 g/(m3.h)), 100 ppmv (2.30 g/(m3.h)), 150 ppmv (3.45 g/(m3.h)), and 200 ppmv (4.59 g/(m3.h)) forming different feed ratios of 1:5, 1:10, 1:20, 1:30, and 1:40, respectively. The TBAB was seeded with a filamentous fungal consortium that was previously utilized in a study published by Hassan and Sorial (2010c). A SEM analysis on a fungal sample collected from the chloroform degrading TBAB produced a surface imagery of highly filamentous structures as shown in Fig. 2a, b.
Table 1.
Operating conditions and performance of the TBAB under continuous loading conditions degrading chloroform and ethanol at pH 4
| Experimental conditions and removal performance | |||||
| Phases of operation | I | II | III | IV | V |
| Influent chloroform concentration, ppmv | 5 | 5 | 5 | 5 | 5 |
| Influent chloroform loading rate, g/m3.h | 0.27 | 0.27 | 0.27 | 0.27 | 0.27 |
| Influent ethanol concentration, ppmv | 25 | 50 | 100 | 150 | 200 |
| Influent ethanol loading rate, g/m3.h | 0.57 | 1.15 | 2.30 | 3.45 | 4.59 |
| Days of operation | 0–30 | 31–59 | 60–181 | 182–211 | 212–244 |
| Average chloroform removal efficiency, % | 69.9 | 71.6 | 75.1 | 78.4 | 80.9 |
| Standard deviation,% | 9.1 | 5.3 | 8.6 | 4.4 | 4.4 |
| Average chloroform elimination capacity, g/m3.h | 0.224 | 0.225 | 0.226 | 0.235 | 0.238 |
| Average ethanol removal efficiency, % | 99.9 | 99.9 | 99.4 | 99.8 | 98.6 |
| Standard deviation,% | 0.0 | 0.0 | 2.2 | 0.5 | 3.7 |
Figure 2.
a Surface imagery of filamentous fungi produced by SEM analysis under 2-μm magnification.
b. Surface imagery of filamentous fungi produced by SEM analysis under 100-μm magnification.
Figure 3 represents the daily performance of the TBAB with respect to influent and effluent concentrations of chloroform in addition to a statistical summary of its removal efficiency at different ethanol loading rates. The efficiency is represented as a box plot which plots the data points in the form of a box representing statistical values. The boundary of the box closest to zero represents the 25th percentile; the median is marked by the line within the box plot, and the boundary of the box that is farthest from zero represents the 75th percentile. The 90th and 10th percentiles are shown by the error bars above and below the boundaries, respectively.
Figure 3.
Performance of the TBAB with sequential time for the removal of chloroform at pH 4
TBAB Performance
The TBAB was set at phase I conditions maintaining a constant feed concentration of 5 ppmv of chloroform and 25 ppmv of ethanol providing a VOC mixing ratio of 1:5. Data analysis was performed for 30 consecutive days. With a chloroform and ethanol loading rate of 0.27 and 0.57 g/(m3.h), respectively, the removal efficiency of chloroform was achieved at 69.9% with a standard deviation of 9.1%. On day 31, phase II conditions were observed when the loading rate of ethanol was increased to 1.15 g/(m3.h), and the removal efficiency of chloroform was 71.6% with a standard deviation of 5.3%. Complete removal of ethanol at 99.9% efficiency was achieved for both phases I and II. Due to ethanol’s hydrophilic nature, its dissolved form is more dominant in the water phase; therefore, with excess moisture at the top of the TBAB, it quickly transitioned from the air to the water phase resulting in its complete removal. On day 60, the loading rate of ethanol was increased to 2.30 g/(m3.h) according to phase III conditions while maintaining the same loading rate for chloroform. The removal efficiency of chloroform showed a corresponding increase from 70 to 75.1% with a standard deviation of 8.6%. The average removal efficiency of ethanol was observed at 99.4% with a standard deviation of 2.2%. This phase was continued for the next 121 days to enable cell synthesis and proliferation of biomass through the length of the bed by maintaining the same operating conditions. The long duration of this phase significantly supported the visible growth of fungal colonies on the pellets. The microbial ecosystem transitioned from a low-density inoculum to a thick, well-acclimated biofilm. On day 182, the TBAB was subjected to the ethanol loading rate of 3.45 g/(m3.h) under phase IV conditions. This phase showed a chloroform removal efficiency of 78.4% with a standard deviation of 4.4% and ethanol removal efficiency of 99.8% with a standard deviation of 0.5%. During the phase change from III to IV, the biomass control strategy was switched from starvation to stagnation to control high-pressure drops across the system and to control excessive biomass growth around the nutrient spray nozzle. This strategy also minimized the periodic need for backwashing the filter bed that is usually performed to avoid short-circuiting within the biofilter. Backwashing is one of the three biomass control strategies typically used throughout the life cycle of a TBAB. It involves flushing the media bed with 18 L of buffered nutrient solution, inducing medium fluidization at approximately 50% bed expansion when the system is offline. Following this, the recirculation of the nutrients will be shut down, and another 18 L of the nutrients will be supplied for a final rinse. More details on backwashing duration and frequency can be found in Hassan and Sorial (2009). Up until phase III, backwashing was performed during two different times in order to remove the excess biomass buildup surrounding the nutrient feed nozzle. After subjecting the biofilter weekly to stagnation mode, no such formations were observed around the spray nozzle eliminating the occasional need to backwash the biofilter. On day 212, the final phase V was set where the ethanol loading rate was increased to 4.59 g/(m3.h) during which the removal efficiency of chloroform peaked at 80.9% with a standard deviation of 4.4% and ethanol removal was observed at 98.6% with a 3.7% standard deviation. The results obtained represent an improvement in the performance of the TBAB with a corresponding increase in the cometabolite concentration. So far, chloroform has been studied only under aerobic co-oxidation with methane- and butane-oxidizing and nitrifying bacterium (Field and Sierra-Alvarez 2004). In this paper, chloroform displayed significant biodegradation rates when using ethanol as a co-substrate in a fungi-based system. Ethanol served as an electron donor and a carbon source to support biomass growth. It can be assumed that the transfer of electrons from ethanol to an electron acceptor such as O2 released energy to support cell synthesis (Field and Sierra-Alvarez 2004). Chloroform displayed fortuitous cometabolic degradation during this process and may not be directly linked to the growth of fungi. In other words, the growth of the microbial population solely depended on the addition of the electron donating substrate which validates the increased removal efficiencies obtained with increasing ethanol concentrations. The elimination capacity of chloroform is represented in Fig. 4 in the form of a box plot on the different total loading rates studied. This represents the volume of the contaminant biodegraded per cubic meter (m3) of the filtering media per unit time. This graph demonstrates more reliability of the removal performance data collected considering the long observational period of study.
Figure 4.
Elimination capacity of chloroform vs. total VOC loading rates
Reaction Rate Kinetics
Gas phase samples were drawn from each port of the TBAB 1 day following every non-use period to evaluate the reaction rate kinetics of chloroform corresponding to the total VOC loading rates. The samples collected immediately after stagnation or starvation promised uniformity of the biomass through the length of the TBAB. The samples of gas phase chloroform and ethanol were collected from ports installed at a distance of 7.60, 23, 38, 53, and 60 cm from the top of the TBAB media. The TBAB is assumed to function as a plug flow reactor, and the removal kinetics was based on the pseudo first-order reaction as a function of the depth of the TBAB. Natural logarithm of the ratio of residual chloroform concentration at each port to the inlet chloroform concentration (ln(C/C0)) is plotted against the independent variable, time (seconds). The data were fit to a linear model, and the slopes of the regression represented the reaction rate constants, k in (seconds−1).
Figure 5 represents the reaction rate constants for the five phases with respect to time in seconds. The rate constants obtained for all the five phases of operation in sequential order were 0.0011, 0.0013, 0.0015, 0.0016, and 0.0018 s−1. The reaction rate constants showed a consistent increase with the cometabolite loading rate much similar to the removal profile. This increase can further support the theory that increasing ethanol-loading rates favored the growth of microbial population resulting in an increase in the biocatalyst, and thus improving the rates of biodegradation. This trend was consistent with the removal kinetics observed for n-hexane and methanol by Zehraoui et al. (2012). The highest reaction rate constant achieved is less than the values for n-hexane (0.03 s−1), benzene (0.0189 s−1), and the mixture of n-hexane and methanol (0.0144 s−1) studied in similar fungi-based TBABs operated under pH 4 (Zehraoui et al. 2014; Hassan and Sorial 2010a, b). The low rate constant values could be attributed to the slow reaction rates of heavily chlorinated methanes such as chloroform (Haag and Yao 1992). On the other hand, it was not possible to evaluate the reaction rate constant for ethanol since over 98% of ethanol was removed at the top of the TBAB.
Figure 5.
Reaction rate constants for chloroform vs. total VOC loading rates
Carbon Mass Balance
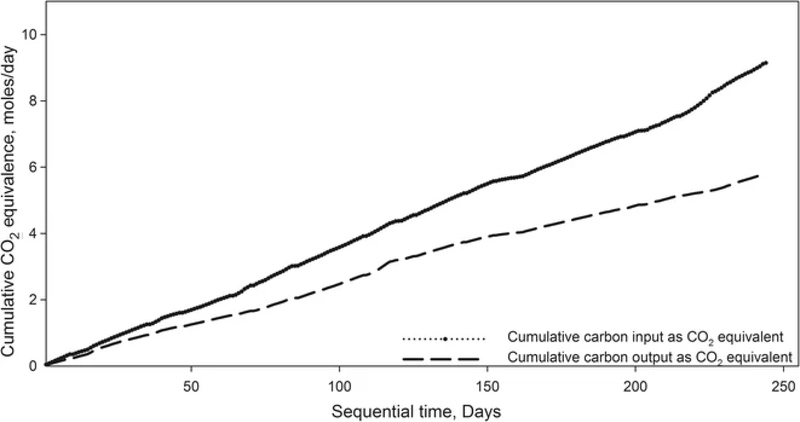
Figure 6 represents the cumulative CO2 equivalent of chloroform, ethanol, and the nutrients entering and leaving the TBAB. The input carbon was the summation of the carbon from the VOCs and TC from the nutrients in the aqueous phase. The carbon exiting the TBAB was the summation of the carbon present in the residual VOC concentration in the gas phase, volatile suspended solids in the TBAB effluent (VSS), effluent aqueous carbon (TC), and effluent gaseous CO2. The CO2 equivalence of all the carbon components was theoretically calculated in moles/day and a cumulative input, and output CO2 equivalence of carbon was plotted with respect to sequential time. The carbon recovery was obtained as 69.6%. The recovery percent obtained was lower than those presented in previous publications on n-hexane and toluene (Sorial et al. 1995; Hassan and Sorial 2010b). This is because chloroform and ethanol are not as carbon-rich as the former and in addition, they were supplied at a relatively lower flow rate of 0.5 sL/m to avoid intoxication of the microorganisms. The carbon loss of 30.3% between the input and output carbon is assumed to be utilized for biomass growth within the TBAB. This hypothesis is justified by comparing the loss of carbon to the amount of biomass accumulated within the filter bed. The biomass production was computed by representing filamentous fungi with the molecular formula of C9H15O5N. It was assumed that the biodegradation of chloroform and ethanol occurred independently. The daily nitrogen consumption divided by the mass percent of nitrogen in the biomass (N/C9H15O5N), provided the daily biomass production rate. A t test was performed to compare the results of the carbon loss and the biomass produced. The test generated a p value <0.05 indicating that the difference between the carbon retained and the biomass produced was statistically significant, and confirming that the loss of carbon within the TBAB was utilized for biomass yield.
Figure 6.
Cumulative carbon input/output as CO2 equivalence for the TBAB
To investigate the formation of chloroform oxidation byproducts, a study was performed to estimate the percentage fraction of inorganic and organic carbon from the total carbon recovered from the biofilter. This was combined with a mass balance analysis of chloride in the biofilter. The influent carbon entailed the carbon from the VOCs and TC measured in the nutrients in mg/day. The effluent organic carbon (OC) was a summation of the carbon from effluent VOCs, volatile suspended solids (VSS), and total organic carbon (TOC) in the biofilter effluent in mg/day. The IC was a summation of carbon fraction of carbon dioxide generated within the biofilter, and inorganic carbon in the biofilter effluent in mg/day. The IC fraction of the total output carbon was 89.84% while the OC fraction contributed the remaining 10.15%. Meanwhile, the average difference between the chloride in the influent and effluent gas phases was 11.64 mg/day, and the difference between the effluent and the influent liquid chloride concentration was 17.34 mg/day. It can be assumed that the chloride lost in the gas phase appeared in the liquid phase at a recovery rate of 67.12%. Thus, the absence of additional chromatographic peaks, the recovery of chloride, and the low concentrations of organic carbon fraction in the liquid phase demonstrate that chloroform was mineralized to simple inorganic compounds and that no VOC byproducts were formed during the biofiltration of chloroform.
Nitrogen Utilization and COD Reduction
Microorganisms uptake readily available inorganic nitrogen sources such as NH4+ and NO3 − which is very essential for their growth and development (Moe et al. 2013). In this study, nitrates were the only source of nitrogen supplied with the nutrients. Daily analyses of influent and effluent concentrations of NO3–N were performed. The net nitrogen utilization was computed from the NO3–N in the TBAB nutrients and the effluent liquid (Zehraoui et al. 2012).
The chemical oxidation demand (COD) for ethanol and chloroform degradation is illustrated using the following reactions.
| (1) |
| (2) |
Equation 1 was used to determine the mass of COD consumed to the mass of ethanol supplied. The ratio of gCOD/gVOC oxidized was 2.09. This value was used to determine the COD consumed from the influent and effluent ethanol in the TBAB. Similarly, Eq. 2 represents the oxidation of chloroform to its end products, forming a gCOD/gVOC ratio of 0.13. This ratio was used to determine the COD consumed from the influent and effluent chloroform. The net chemical oxygen demand was calculated as the difference between COD of the feed and the COD of the effluent gas and liquid streams (Zehraoui et al. 2012). Figure 7 shows dimensionless CODremoval/Nutilization ratios plotted against the total loading rates of ethanol and chloroform in box plots. Phase I produced a CODremoval/Nutilization ratio of 3 for a total VOCs loading rate of 0.84 g/(m3.h) and increased to 4.5 for a total loading rate of 1.42 g/(m3.h) in phase II. Following this, phase III was run with a total loading rate of 2.57 g/(m3.h) for 120 consecutive days with a view to enhance the biomass yield by maintaining a constant growth environment. The CODremoval/Nutilization ratio at phase III increased to 14.5. The ratios showed apparent dependency on the loading rate for phases I, II, and III. The TBAB consumed more COD per mole of nitrogen utilized for each consecutive phase. The active COD consumption could be related to the relatively new support media that is highly porous and at its maximum absorption potential. Chloroform is highly hydrophobic whose major reservoir is the organic material on the TBAB media which became a dense biofilm over time with increasing substrate loading. As a result, to avoid pressure drop with the TBAB, the biomass control strategy was switched from starvation to stagnation following phase III. Phase IV was operated at a total VOC loading rate of 3.72 g/(m3.h), and the CODremoval/Nutilization ratio drastically dropped to 4. It further reduced to 3.5 at phase V operated with the highest VOC loading rate of 4.86 g/(m3.h). The decrease in the COD consumption could be correlated with the change in the biomass control technique that led to the sudden withdrawal of nutrients 2 days a week. Nitrogen utilization increased drastically following the non-use periods during phases IV and V. Another reason could be the reduced absorption capacity of the media over time resulting in reduced COD consumption. Microbial population fluctuates over time for reasons difficult to determine, although these results indicate that microbial viability reached stability at phase III giving an optimum CODremoval/Nutilization ratio of 14.5.
Figure 7.
CODremoval/Nutilization vs. total VOC loading rates for the TBAB
Conclusion
This study investigated the effect of fungi and cometabolism on the performance of the TBAB for the stable removal of chloroform. Fungal strains grown on diatomaceous earth pellets proved to be an effective medium for the removal of a highly persistent compound through cometabolism. Under pH 4, the filamentous fungi medium was able to effectively biodegrade 5 ppmv of chloroform up to a removal efficiency of 81% when mixed with 200 ppmv of ethanol. The biomass control strategies were effectively utilized to control excess biomass growth and prevent pressure drop along the biofilter. The elimination capacity increased linearly from 0.22 to 0.24 g/(m3.h) with increasing ethanol concentration. The reaction rate kinetics ranged from 0.0011 to 0.0018 s−1 from phase I to phase V consistent with the removal performance of the TBAB. This demonstrated that the ethanol served as an excellent carbon and energy source for the fungi species and also that the TBAB media adapted well to changing VOC concentrations (Devinny et al. 1998). The carbon mass balance indicated that most of the input carbon was recovered in the form of inorganic carbon, suggesting that no VOC oxidation byproducts were formed during chloroform degradation. The CODremoval/Nutilization analysis provided an optimum ratio, which can be used to improvise the performance of the TBAB in the future. The results of this study prove that a trickle bed air biofilter is an effective technique to treat gas phase chloroform through cometabolism under suitable environmental conditions.
Footnotes
Disclaimer
The views expressed in this article are those of the authors and do not reflect the official policy or position of the Unites State Environmental Protection Agency. Mention of trade names, products, or services does not convey official EPA approval, endorsement, or recommendation. This manuscript has been subjected to the agency’s review and has been approved for publication.
References
- ATSDR (1997). Toxicological profile for chloroform. http://www.atsdr.cdc.gov/toxprofiles/tp6-c5.pdf. Accessed 17 August 2016.
- ATSDR (1998). Chloroform: potential for human exposure. http://www.atsdr.cdc.gov/toxprofiles/tp6-c5.pdf. Accessed 11 October 2014.
- Bagley DM, Lalonde M, Kaseros V, Stasiuk KE, & Sleep BE (2000). Acclimation of anaerobic systems to biodegrade tetrachloroethene in the presence of carbon tetrachloride and chloroform. Water Research, 34(1), 171–178. [Google Scholar]
- Balasubramanian P, Philip L, & Bhallamudi SM (2011). Biodegradation of chlorinated and non-chlorinated VOCs from pharmaceutical industries. Applied Biochemistry and Biotechnology, 163(4), 497–518. [DOI] [PubMed] [Google Scholar]
- Butler J, Ramchandani C, & Thomson D (1935). 58. The solubility of non-electrolytes. Part I. The free energy of hydration of some aliphatic alcohols. Journal of the Chemical Society (Resumed), 280–285. [Google Scholar]
- Cai Z, Kim D, & Sorial GA (2004). Evaluation of trickle-bed air biofilter performance for MEK removal. Journal of Hazardous Materials, 114(1), 153–158. [DOI] [PubMed] [Google Scholar]
- Cai Z, Kim D, & Sorial GA (2005). Removal of methyl isobutyl ketone from contaminated air by trickle-bed air biofilter. Journal of Environmental Engineering, 131(9), 1322–1329. [Google Scholar]
- CARB (1990). Chloroform as a toxic air contaminant. http://www.arb.ca.gov/toxics/id/summary/chloroform_A.pdf. Accessed 17 August 2016.
- Cecen F, & Aktas Ö (2011). Activated carbon for water and wastewater treatment: integration of adsorption and biological treatment: Wiley. [Google Scholar]
- Chen F, Freedman DL, Falta RW, & Murdoch LC (2012). Henry’s law constants of chlorinated solvents at elevated temperatures. Chemosphere, 86(2), 156–165. [DOI] [PubMed] [Google Scholar]
- Chheda D, & Sorial GA (2016). Effect of a ternary mixture of volatile organic compounds on degradation of TCE in biotrickling filter systems. Water, Air, & Soil Pollution, 227(7), 1–11. [Google Scholar]
- Clescerl LS, Greenberg AE, & Eaton AD (1999). Standard methods for examination of water and wastewater.
- Converse B, Schroeder E, Iranpour R, Cox H, & Deshusses M (2003). Odor and volatile organic compound removal from wastewater treatment plant headworks ventilation air using a biofilter. Water Environment Research, 75(5), 444–454. [DOI] [PubMed] [Google Scholar]
- Cox H, Deshusses M, Converse B, Schroeder E, & Iranpour R (2002). Odor and volatile organic compound treatment by biotrickling filters: pilot-scale studies at hyperion treatment plant. Water Environment Research, 74(6), 557–563. [DOI] [PubMed] [Google Scholar]
- Devinny JS, Deshusses MA, & Webster TS (1998). Biofiltration for air pollution control: CRC press. [Google Scholar]
- Field J, & Sierra-Alvarez R (2004). Biodegradability of chlorinated solvents and related chlorinated aliphatic compounds. Reviews in Environmental Science and Bio/Technology, 3(3), 185–254. [Google Scholar]
- Frascari D, Pinelli D, Nocentini M, Fedi S, Pii Y, & Zannoni D (2006). Chloroform degradation by butane-grown cells of Rhodococcus aetherovorans BCP1. Applied Microbiology and Biotechnology, 73(2), 421–428. [DOI] [PubMed] [Google Scholar]
- Frascari D, Pinelli D, Nocentini M, Baleani E, Cappelletti M, & Fedi S (2008). A kinetic study of chlorinated solvent cometabolic biodegradation by propane-grown Rhodococcus sp. PB1. Biochemical Engineering Journal, 42(2), 139–147. [Google Scholar]
- FRTR (2002). Remediation technologies screening matrix and reference guide, version 4.0. https://frtr.gov/matrix2/section1/toc.html. Accessed 17 August 2016.
- García‐Peña EI, Hernández S, Favela‐Torres E, Auria R, & Revah S (2001). Toluene biofiltration by the fungus Scedosporium apiospermum TB1. Biotechnology and Bioengineering, 76(1), 61–69. [DOI] [PubMed] [Google Scholar]
- Haag WR, & Yao CD (1992). Rate constants for reaction of hydroxyl radicals with several drinking water contaminants. Environmental Science & Technology, 26(5), 1005–1013. [Google Scholar]
- Hardison LK, Curry SS, Ciuffetti LM, & Hyman MR (1997). Metabolism of diethyl ether and cometabolism of methyl tert-butyl ether by a filamentous fungus, a Graphium sp. Applied and Environmental Microbiology, 63(8), 3059–3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan AA, & Sorial G (2009). Biological treatment of benzene in a controlled trickle bed air biofilter. Chemosphere, 75(10), 1315–1321. [DOI] [PubMed] [Google Scholar]
- Hassan AA, & Sorial GA (2010a). Biofiltration of n‐hexane in the presence of benzene vapors. Journal of Chemical Technology and Biotechnology, 85(3), 371–377. [Google Scholar]
- Hassan AA, & Sorial GA (2010b). A comparative study for destruction of n-hexane in trickle bed air biofilters. Chemical Engineering Journal, 162(1), 227–233. [Google Scholar]
- Hassan AA, & Sorial GA (2010c). Removal of benzene under acidic conditions in a controlled trickle bed air biofilter. Journal of Hazardous Materials, 184(1), 345–349. [DOI] [PubMed] [Google Scholar]
- Hernandez-Perez G, Fayolle F, & Vandecasteele J-P (2001). Biodegradation of ethyl t-butyl ether (ETBE), methyl t-butyl ether (MTBE) and t-amyl methyl ether (TAME) by Gordonia terrae. Applied Microbiology and Biotechnology, 55(1), 117–121. [DOI] [PubMed] [Google Scholar]
- Hua G, & Reckhow DA (2007). Comparison of disinfection byproduct formation from chlorine and alternative disinfectants. Water Research, 41(8), 1667–1678. [DOI] [PubMed] [Google Scholar]
- Jang SR, & Jang BW (2000). Thresholds for mathematical models of microbial interaction. In Computer-Based Medical Systems, 2000. CBMS 2000. Proceedings. 13th IEEE Symposium on, (pp. 51–56): IEEE. [Google Scholar]
- Jolley RL, Condie LW, Johnson JD, Katz S, Minear RA, Mattice JS, et al. (1990). Water chlorination: chemistry, environmental impact and health effects. In Conference on Water Chlorination: Environmental Impact and Health Effects, 6,: Lewis publishers [Google Scholar]
- Kennes C, & Veiga MC (2004). Fungal biocatalysts in the biofiltration of VOC-polluted air. Journal of Biotechnology, 113(1), 305–319. [DOI] [PubMed] [Google Scholar]
- Khalil M, & Rasmussen R (1999). Atmospheric chloroform. Atmospheric Environment, 33(7), 1151–1158. [Google Scholar]
- Komulainen H (2004). Experimental cancer studies of chlorinated by-products. Toxicology, 198(1), 239–248. [DOI] [PubMed] [Google Scholar]
- Kumar TP, Rahul M, & Chandrajit B (2011). Biofiltration of volatile organic compounds (VOCs)—an overview. Res J Chem Sci, 2231, 606X. [Google Scholar]
- Leson G, & Winer AM (1991). Biofiltration: an innovative air pollution control technology for VOC emissions. Journal of the Air & Waste Management Association, 41(8), 1045–1054. [DOI] [PubMed] [Google Scholar]
- Moe WM, Hu W, Key TA, & Bowman KS (2013). Removal of the sesquiterpene β-caryophyllene from air via biofiltration: performance assessment and microbial community structure. Biodegradation, 24(5), 685–698. [DOI] [PubMed] [Google Scholar]
- Morris RD, Audet A-M, Angelillo IF, Chalmers TC, & Mosteller F (1992). Chlorination, chlorination by-products, and cancer: a meta-analysis. American Journal of Public Health, 82(7), 955–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado Ó, Mendoza J, Veiga M, & Kennes C (2002). Optimization of nutrient supply in a downflow gas-phase biofilter packed with an inert carrier. Applied Microbiology and Biotechnology, 59(4–5), 567–573. [DOI] [PubMed] [Google Scholar]
- Smith FL, Sorial GA, Suidan MT, Pandit A, Biswas P, & Brenner RC (1998). Evaluation of trickle bed air biofilter performance as a function of inlet VOC concentration and loading, and biomass control. Journal of the Air & Waste Management Association, 48(7), 627–636. [DOI] [PubMed] [Google Scholar]
- Sorial GA, Smith FL, Suidan MT, Biswas P, & Brenner RC (1995). Evaluation of trickle bed biofilter media for toluene removal. Journal of the Air & Waste Management Association, 45(10), 801–810. [Google Scholar]
- Thacker NP, Kaur P, & Rudra A (2002). Trihalomethane formation potential and concentration changes during water treatment at Mumbai (India). Environmental Monitoring and Assessment, 73(3), 253–262. [DOI] [PubMed] [Google Scholar]
- USEPA (2016). Original list of hazardous air pollutants. https://www3.epa.gov/airtoxics/188polls.html. Accessed 17 August 2016.
- USNIH (2016). Chloroform (Code C29815). https://ncit.nci.nih.gov/ncitbrowser/ConceptReport.jsp?dictionary=NCI_Thesaurus&version=16.02d&ns=NCI_Thesaurus&code=C29815. Accessed 17 August 2016.
- Vergara‐Fernández A, Hernández S, & Revah S (2008). Phenomenological model of fungal biofilters for the abatement of hydrophobic VOCs. Biotechnology and Bioengineering, 101(6), 1182–1192. [DOI] [PubMed] [Google Scholar]
- Wahman DG, Henry AE, Katz LE, & Speitel GE (2006). Cometabolism of trihalomethanes by mixed culture nitrifiers. Water Research, 40(18), 3349–3358. [DOI] [PubMed] [Google Scholar]
- Westrick JJ, Mello JW, & Thomas RF (1984). The groundwater supply survey. Journal (American Water Works Association), 52–59. [Google Scholar]
- Williams DT, LeBel GL, & Benoit FM (1995). A national survey of chlorinated disinfection by-products in Canadian drinking water (E. H. D. Health Canada, Trans.). Ottawa, Ontario, Canada. [Google Scholar]
- Zehraoui A, Hassan AA, & Sorial GA (2012). Effect of methanol on the biofiltration of n-hexane. Journal of Hazardous Materials, 219, 176–182. [DOI] [PubMed] [Google Scholar]
- Zehraoui A, Hassan AA, & Sorial GA (2013). Biological treatment of n-hexane and methanol in trickle bed air biofilters under acidic conditions. Biochemical Engineering Journal, 77, 129–135. [Google Scholar]
- Zehraoui A, Kapoor V, Wendell D, & Sorial GA (2014). Impact of alternate use of methanol on n-hexane biofiltration and microbial community structure diversity. Biochemical Engineering Journal, 85, 110–118. [Google Scholar]