Abstract
Trends in greener and sustainable process development during the past 25 years are abridged involving the use of alternate energy inputs (mechanochemistry, ultrasound- or microwave irradiation), photochemistry, and greener reaction media as applied to synthesis of organics and nanomaterials. In the organic synthesis arena, examples comprise assembly of heterocyclic compounds, coupling and a variety of other name reactions catalyzed by basic water or recyclable magnetic nanocatalysts. Generation of nanoparticles benefits from the biomimetic approaches where vitamins, sugars, and plant polyphenols, including agricultural waste residues, can serve as reducing and capping agents. Metal nanocatalysts (Pd, Au, Ag, Ni, Ru, Ce, Cu, etc.) immobilized on biodegradable supports such as cellulose and chitosan, or on recyclable magnetic ferrites via ligands, namely dopamine or glutathione, are receiving special attention. These strategic approaches attempt to address most of the Green Chemistry Principles while producing functional chemicals with utmost level of waste minimization.
Keywords: Green chemistry, Magnetic nanocatalysts, Nanomaterials, Organic synthesis, Sustainable processes Synopsis
Abstract Figure
Greener trends in organic synthesis and assembly of nanomaterials are highlighted, including their sustainable applications.
Continual improvements in chemical synthesis and transformation during the last two centuries have contributed vastly to chemical processing efficiencies by utilizing cost-effective synthetic precursors and catalysts and have obliquely contributed to some aspects of greenness. The evolving central theme of green chemistry with its definition and associated 12 Principles were coined only during the last 25 years, yet we learn from history that a large number of farmers suddenly lost their livelihood when a Noble-winning efficient synthesis of indigo blue dye was realized in the laboratory; these advances could be traced back to 1865 when the German chemist, Adolf von Baeyer, began working on the synthesis of indigo.(1) In contrast, fossil-derived, nonrenewable chemical entities are not considered sustainable today, and their substitution with biomass-derived chemicals is the order of the day. However, a holistic lifecycle analysis of the process probably would favor age-old Baeyer’s solvent-free synthesis of the blue dye that spares precious land for growing much-needed foodstuff; recent biofuel story teaches us that renewability need not be confused with sustainability. The modern green chemistry movement(2, 3) initiated in 1998, strives to meet the scientific challenges of protecting human health and the environment and has drawn attention and relevance demonstrated by the 2005 Noble award to catalysis.(4)
The diverse field of chemistry requires various greener pathways in our quest toward attaining sustainability. Strides in green chemistry have led to development of cleaner processes as alternatives to traditional chemical syntheses and transformations via newer concepts, such as step- and atom-economy(5) and E-factor.(6) Greener features in molecular design now regularly include the use of biorenewable resources in benign reaction media and recyclable magnetic nanocatalysts in atom-economic syntheses as thrust areas; minimum formation of byproducts or waste generation with efficient separation is routinely emphasized. The concept of the use of greener solvents, namely water, polyethylene glycol (PEG), molten organic salts, and supercritical CO2, is gaining traction to replace traditional organic solvents. Several newer strategies have appeared, such as reactions under solvent-free (dry media) conditions,(7) mechanochemical mixing,(8) and the use of solid-supported reagents. The alternate heating and activation methods utilize microwave- (MW),(11, 12) and ultrasonic irradiation(14, 15) for rapid syntheses; these techniques overcome some of the problems associated with excessive or wasteful heating. Ball-mill processing, which can be easily scaled up, finds its utility often in the paint industry, materials science, and for environmental remediation.
Alternative energy input systems, such as mechanochemical grinding, photocatalysis, and MW- and ultrasonic irradiation have been incessantly investigated and adapted in conjunction with emerging continuous flow processes.(7–15) MW heating has emerged as an important energy source to accomplish swift chemical transformations in organic(16–18) and, lately, in nanomaterial syntheses.(19–22) Chemical processes in the syntheses of pharmaceuticals, fine chemicals, and polymers that use alternative energy input in combination with nanocatalysts shorten the reaction time and eliminate or minimize the formation of side products.(23) Recent progress in these thematic areas encompassing greener reaction media, such as PEG and water, and in combination with photoactivation, MW and ultrasonic irradiation, and/or mixing under solvent-free conditions,(24) ensued numerous sustainable alternatives.
The burgeoning field of nanomaterials, and the preparation of nanocatalysts in particular, has benefitted immensely from the technique of MW heating as material properties are largely a reflection of their size and shape; nanoparticles of uniformly small size are reproducibly accessible using MW irradiation,(19–21) especially under continues flow conditions.(22) This is because the growth of the materials at nanoscale is chiefly dependent on the thermodynamic and kinetic barriers in the reaction as defined by the reaction path. Reuse and recyclability features are of overriding importance when using precious and rare nanocatalysts; mounting them in very small amounts on magnetic supports provides for reclaim and recycling of the catalysts.(20, 23, 25, 26) Numerous “greener” catalytic applications have been realized through relatively inexpensive, earth-abundant, iron-based, and magnetically recyclable nanocatalysts for routine oxidation, reduction, and condensation reactions;(23, 25–27) their regular deployment has made a significant impact on sustainable synthesis in recent years.
Mechanochemistry
he milling of solid substances produces a series of complex changes. The input of external energy rupturing the order of the crystalline structure, generating cracks and creating newer surfaces. The deformation and melting of solids occurs when edges collide—the solids form hot points where the molecules can reach very high vibrational agitation culminating in bond breakage. High-energy vibrators or high impact stainless steel ball mills are deployed for prolonged high-energy milling, e.g., in amorphization of hard crystalline solids or mechanical alloying. Use of mortar and pestle, high-speed ball-milling (HSBM), and high intensity pulverization are some of the modes commonly used in mechanochemistry.
Neat chemical entities (solid–solid or solid–liquid) reacting together in the absence of a reaction media has received plenty of attention in the past decade(28) with delineation of mechanistic details;(29) some representative solventless examples appended below highlight their prowess.
The regio- and enantioselective reductions of chiral diketone have been accomplished using mortar and pestle in the absence of solvent (Scheme 1).(30)
Scheme 1.

Regio- and Enantioselective NaBH4 Reduction of Chiral Diketone under Solvent-Free Conditions
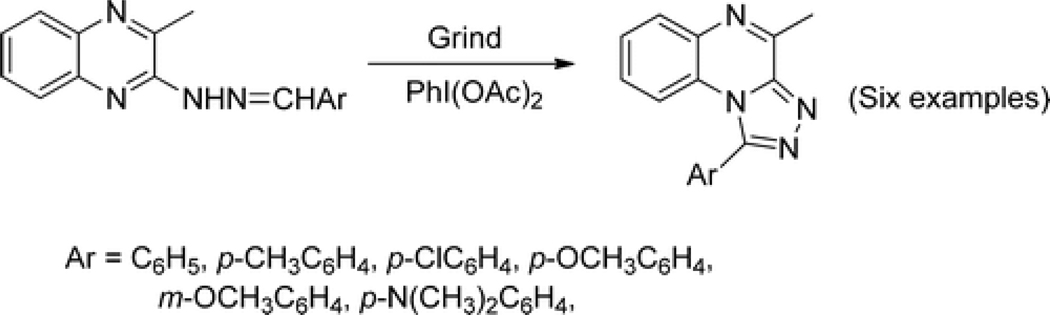
A rapid transformation of hydrazones to quinoxaline derivatives can be accomplished even in a basic laboratory using a nonmetallic hypervalent oxidant, iodobenzene diacetate, PhI(OAc)2, and a pestle and mortar (Scheme 2).(10)
Scheme 2.
Synthesis of 1-Aryl-4-methyl-1,2,4-triazolo[4,3-a]quinoxalines from Hydrazones
Similarly, hypervalent iodine reagents have been used for the synthesis of β-keto sulfones from ketones(31) and α-tosyloxy β-keto sulfones under similar solventless conditions.(32) This simple and greener technique does help in assembly of supramolecular complex in solid state where solubilization of substrates is challenging, as exemplified in the case of fullerene C60.(33)
Mechanochemistry accomplishes various named reactions such as Reformatsky,(34) Luche,(34) Biginelli,(35) and Morita–Baylis–Hillman reactions(36) mostly under solvent-free HSBM conditions; Sonogashira reaction is achievable using Pd(II) catalysts in the absence of additional ligands and copper.(37) Interestingly, the inside lining of reacting vessels with various elements such as copper do serve as catalyst in such ball-milling operations.(38) Friscic et al. conducted the real-time study of mechanochemical transformations in a ball mill by means of in situ diffraction of high-energy synchrotron X-rays.(39) The impactful field of polymer mechanochemistry has witnessed fast growth during the past decade, driven mainly by the development of mechanophores which are essentially force-activated functional groups. These multidisciplinary methodologies engaging various engineering fields, computational-assisted design, and modeling have enabled the development of polymer mechanochemistry applications.(40)
Ultrasound in Chemistry
Ultrasound is an integral part of the sonic spectrum ranging from about 20 kHz to 10 MHz and can be segmented into three regions: low frequency, high-power ultrasound (20–100 kHz); high frequency, medium-power ultrasound (100 kHz–1 MHz); and high frequency, low-power ultrasound (1–10 MHz); 20 kHz to ∼1 MHz range is typically utilized in sonochemistry whereas frequencies above 1 MHz are deployed for diagnostic and medical applications. Ultrasonic irradiation is used to substitute for the conventional energy resources in synthesis,(41) and can be essentially categorized into two main classes, namely heterogeneous and homogeneous sonochemistry. Improved mass transfer, reduction in particle sizes among nanoparticles, and the surface cleaning via the mechanical effects of cavitation are some of the hallmarks of heterogeneous sonochemistry. In liquid–solid systems, cavity collapse close to solid periphery generates high-speed liquid jets that impact the solid surface with incredible force culminating in newer exposed, vastly reactive surfaces. Homogeneous sonochemistry, in contrast, advances via radical-ion intermediaries wherein volatile molecules form bubbles that serve as microreactors, and localized high pressure and temperature are attained during cavitation that can break the chemical bonds.
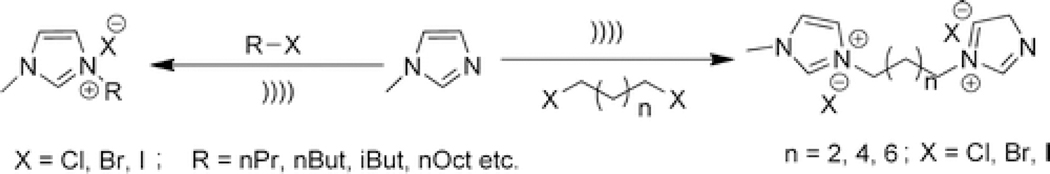
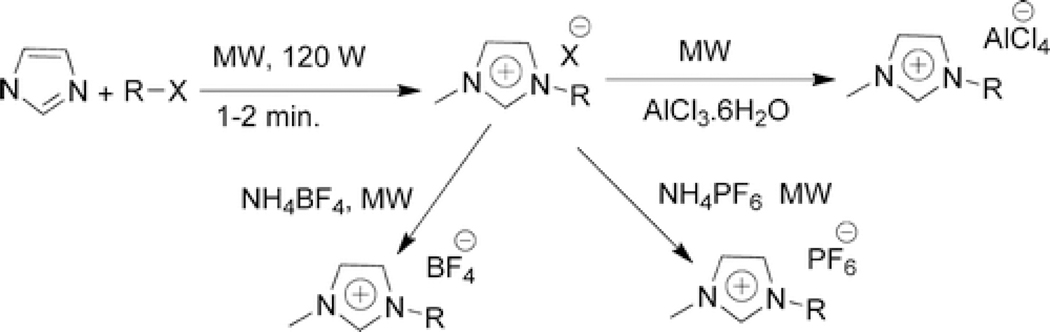
Synthesis of Ionic Liquid under Solvent-Free Conditions
Ionic liquids (IL) comprising imidazolium salts, among a sizable variety of organic salts, have been extensively explored as a reaction medium for a variety of reactions in view of their unique properties; especially, their lack of measurable vapor pressure renders them a good substitute for volatile organic solvents. Normally, their preparation entails the use of a large excess of alkyl halides in organic solvents until Varma et al. developed an expeditious method by simply exposing neat reactants to ultrasonic irradiation (Scheme 3).(14)
Scheme 3.
Synthesis of Ionic Liquids (IL) under Ultrasonic Irradiation
Coupling Reactions
Several beneficial effects of ultrasound have been discerned for popular coupling reactions namely Suzuki,(42) Sonogashira,(43) and Ullmann in terms of selectivity and speeding up of reactions. Heck reaction executed with numerous aryl iodides and alkenes in IL under ultrasound irradiation provides exclusively the E-products in shorter duration and precludes the use of conventional polar solvents, e.g., DMF under reflux conditions (Scheme 4).(44)
Scheme 4.
Heck Reaction in IL Facilitated by Ultrasound Irradiation
Ultrasound Can Substitute for Phase Transfer Catalysts (PTC)
Standard permanganate oxidation of olefins can be expedited by ultrasonic irradiation as the cleavage of the rate limiting intermediate, dioxo species, is expedited for the conversion to products, 1,2-cis diols.(45)
Strong agitation is essential for speeding up the reaction between two or more immiscible components often in the presence of a phase transfer catalyst (PTC); transfer of the active species from one phase to the other ensues. Ultrasonic irradiation generates exceptionally fine emulsion of immiscible liquids, promotes surface activation (in case of liquid/solid combination), or assists in mass transfer thus replacing PTC entirely or enhancing PTC-catalyzed heterogeneous reactions.(46)
Ultrasound “Switching” of Reaction Pathway
A “sonochemical switching” reaction has been described by Ando and Kimura(47) wherein a suspension of benzyl bromide and alumina-supported potassium cyanide are admixed in toluene (Scheme 5). Under conventional mixing, the reaction provided diphenylmethane as a product via a typical Friedel–Crafts reaction between the bromo compound and the solvent, catalyzed by surface Lewis acid sites of the solid phase reagent. In contrast, the same constituents upon exposure to sonication produced exclusively the substitution product, benzyl cyanide; the rationale being that cavitation produces a structural change to the catalytic sites of the solid support, possibly by masking them via cavitationally induced cyanide absorption.
Scheme 5.
Reaction of Benzyl Bromide with Alumina-Supported Potassium Cyanide
Alternate Energy Input: Microwave Heating in Chemical Synthesis
Microwave (MW) heating technique is now accepted globally as an energy source to accomplish expeditious chemical conversions, instead of a prolonged reaction time of hours or days.(7, 8, 11–13, 16–18) Regular heating drives the reaction through the conduction of blackbody radiation where the reaction container acts as a mediator to transfer the energy from the oil bath or electrical element to the reaction media and finally to the molecules undergoing the reaction. This uneven heating is especially detrimental for the synthesis of nanomaterials where uniform nucleation and growth rates are crucial to the quality of ensuing materials. Such inhomogeneous heating problems, typically encountered in conventional techniques, are suitably dealt with via MW heating as it offers enhanced reaction kinetics, and rates providing relatively clean reaction products in higher yields.(19–22) The practice of MW heating has been applied to a broad range of chemical entities such as fine chemicals, pharmaceutical precursors, polymers, and even to biological and enzymatic procedures.
MW-Assisted Synthesis of Organics under Solvent-Free Conditions
Our laboratory, in early 1990s, initiated exploratory MW-accelerated reactions in open glass vessels without using solvents and utilizing an unmodified household MW oven operating at 2450 MHz; commercial laboratory-grade MW systems were not available at that time. Quite often, the procedure entailed simply mixing neat reactants with the catalyst (mineral oxides or their “doped” equivalents were commonly used). A wide variety of industrially significant compounds and valuable intermediates, such as imines, nitroalkenes, enamines, enones, oxidized sulfur species (sulfoxides and sulfones), and nitrogen and oxygen-heterocyclic compounds, could be prepared in minutes;(7, 8) representative compounds, such as 2-aroylbenzofurans, tetrahydroquinolones, and thiazoles, could be synthesized via the usage of in situ generated intermediates in a single-pot operation.(48) The approach was subsequently broadened to solventless parallel synthesis involving multicomponent reactions and was validated for the preparation of imidazo[1,2-a]annulated pyridines, pyrazines, pyrimidines (Ugi reaction),(49) and a library of dihydropyrimidine-2(1H)-ones (Biginelli reaction).(50)
A simple and expeditious condensation of hydrazines/hydrazides and diamines with 1,3-diketones/β-ketoester leads to ready formation of pyrazoles and diazepines in high yields without any solvent or catalyst under MW irradiation (Scheme 6).(51)
Scheme 6.
Solvent-Free and Catalyst-Free Synthesis of Pyrazoles and Diazepines
This is evocative of our initial discovery, which revealed that the reaction can proceed effortlessly, even between solid reactants,(52) and essentially gets completed below the melting points of the two reactants, likely via the formation of a eutectic (Scheme 7);(53) the reaction could be followed by visual inspection when a melt or globule formation is noticed.
Scheme 7.
Formation of Hydrazones under Solvent-Free and Catalyst-Free Conditions
The first solvent-free preparation of ionic liquid, 1,3-dialkylimidazolium halides, via MW irradiation (Scheme 8) was accomplished by Varma et al. thus reducing the reaction time from several hours to few minutes;(54) it did not make sense to use an organic solvent to make another solvent which would not be an overall greener process. However, indium and gallium-bearing ionic liquids, [Rmim][InCl4](55) and [bmim][GaCl4],(56) prepared similarly using the solvent-free MW protocol, could be deployed as catalysts rather than solvent; notable among them is the reaction that consumes greenhouse contributing gas, CO2, via reaction with epoxides, to generate useful cyclic carbonates.(57)
Scheme 8.
MW-Assisted Solvent-Free Synthesis of Ionic Liquids
Assembly of N-Heterocycles in Water Using Microwaves
Nitrogen-heterocycles, a significant class of valuable compounds, constitute an essential component of several pharmaceuticals, alkaloids, natural products, antibiotics, vitamins, and hormones. With the advent of newer laboratory MW equipment equipped with proper controls, greener options have now become widely available that enables chemical reactions to be performed in benign media such as water and PEG. A general approach to assortment of N-heterocycles, namely azetidines, pyrrolidines, piperidines, azepanes, N-substituted 2,3-dihydro-1H-isoindoles, pyrazolidines, 4,5-dihydropyrazoles, and 1,2-dihydrophthalazines, has been developed by Varma et al.(60, 61) The reactions proceed in a basic aqueous carbonate medium via double N-alkylation of primary amines and hydrazine derivatives (Scheme 9) with easily accessible dihalides (or ditosylates) under MW irradiation; expeditious entry to significant building blocks in pharmaceuticals is thus facilitated.(60–62)
Scheme 9.
Synthesis of N-Heterocycles in Carbonated Water Using MW Irradiation
The formation of two C–N bonds occurs via this expedited MW-assisted method in a simple SN2-like sequential heterocyclization that exploits the use of readily available entities such as amines, hydrazines, alkyl dihalides or ditosylates. Abiding by the Green Chemistry Principles, the mild reaction protocol avoids the functional group protection/deprotection sequences, multistep reactions, and eliminates the use of expensive phase transfer- and transition metal catalysts. The absorption of microwaves by ensuing polar intermediates in a multiphase system circumvents the use of a phase transfer catalyst; similar acceleration has been observed earlier for ultrasonic irradiation as well.(46)
Such scenarios have been exploited subsequently for numerous other reactions wherein the polar transition state of the reaction has proven ideal for coupling to microwaves because of the dielectric polarization nature of the MW energy transmission. An additional salient feature is that the phase separation of the desired product occurs in aqueous media, thus facilitating the product purification via simple decantation or filtration rather than the elaborate and wasteful extraction, distillation, or column chromatography, which lessens the use of volatile organic solvents.(61, 62)
PEG is another nontoxic and nonvolatile solvent that can be used as a medium for various organic transformations.(63) In our laboratory, we found that palladium chloride catalyst and potassium fluoride base could be recycled during MW-assisted Suzuki cross coupling of aryl halides with aryl boronic acids in PEG;(64) self-coupling of boronic acids was not observed.
The general limitation for larger scale MW-assisted reactions is due to the depth of penetration of microwaves, which has been addressed by switching a traditional batch operation to a continuous flow mode. The “microwave-to-flow” experiments with appropriately equipped fluidic flow devices can generate substantial amount of product in a continuous fashion.(65)
Flow Chemistry
To meet the challenges encountered in Green Chemistry implementation, versatile flow chemistry tools are being introduced. This process intensification option has demonstrated its success by offering enhanced mass/heat transfer prospects, waste minimization, use of less solvent, restraining the usage of hazardous, and reactive compounds with round the clock unmanned operations. This robotic approach has been successfully demonstrated by Ley et al., where reagent and scavenger cartridges are routinely deployed in monotonous or repetitive processes to accomplish the multistep assembly of complicated natural products and pharmaceutical entities.(66) The strategy has been adapted for the synthesis of active pharmaceutical precursors(67) where even hazardous materials can be used in a controlled manner.(68)
Photochemistry
Photochemically driven chemical reactions are valuable in synthetic chemistry domain.(69) They generally proceed via the excited-state route and are capable of generating products that may not be obtainable by standard thermal reactions. However, successful examples of catalysis via photochemistry are limited as it is arduous to foresee and restrain the ensuing results, especially in homogeneous solutions; the reactive components behave in an utterly disordered manner.
Photochemical Transformations: Challenges in Selectivity
Photocatalysis has application potential in numerous disciplines, namely source of greener energy, medicine, chemical synthesis, and environmental technology. However, photocatalysis has customarily been considered as an indiscriminative process (particularly in water), because these reactions are normally regulated via a free radical mechanism (•OH, O2–•, HO2•). Consequently, for a long time, the research on selective photocatalysis has been largely neglected.
The enhancement of the selectivity in photocatalytic processes, a huge challenge, can be addressed via many strategies that can chiefly be divided into two groups, namely the modification of photocatalysts and the change of external operating conditions. Because the ensuing •OH radical is usually responsible for the nonselectivity, various modifications of photocatalysts are customized to avoid the involvement of •OH via band gap modification, selective growth of crystal facets, and the surface treatment.
Among several investigations,(70) oxidation reactions have been most studied and generally center on the oxidation of hydroxyl groups to carbonyls. Some photocatalysts, e.g., graphitic carbon nitrides (C3N4), are endowed with good selectivity due to their narrow band gap, which is unsuitable to generate •OH. For others with wide band gap, modifications are essential to obtain high selectivity, which can be achieved by using dopants, forming special crystal facets, and phase. However, the improved selectivity is attained at the expense of less conversion. The experimental conditions can be used to improve the selectivity of photocatalysis by changing the solvent and pH value.
For reduction reactions, modifying the surfaces, exposing particular phase and crystal facets, as well as changing external conditions are strategies often used for enhancing the selectivity. In recent years, it has become clear that the burgeoning interest in photocatalytic CO2 conversion into organic fuels is due to environmental concerns. CO2, being an inert entity, has thermodynamic limitations for its conversion to other compounds; several products namely CO, CH4, CH3OH, HCOOH, etc. are obtainable. Future investigation regarding the conversion of CO2 to high value product, especially methanol, should receive more attention in the context of “Methanol Economy”.
The selective photocatalytic oxidation of organic molecules via the oxygen-mediated method has been accomplished using the most commonly used oxide, titanium oxide (TiO2).(71) The oxidation entails an oxygen transfer pathway for the interfacial reactions on TiO2 that may encourage the development of highly selective oxygen-mediated oxidation of organics driven by solar energy to meet the energy and environmental challenges of the future.
The field of selective photocatalysis is emerging rapidly with several greener applications such as selective CO2 transformation to fuels, novel selective catalytic reactions, and selective elimination or degradative oxidation of diluted pollutants. The selective CO2 conversion to fuels is especially attractive from an environmental and renewal of energy viewpoint. Importantly, these investigations are very appealing because they provide a sustainable process to utilize abundant solar energy as the global damaging influence of fossil fuel consumption is clearly apparent. The photocatalytic transformations are, in general, supportive to relieve the energy stress, which is one of the most crucial issues of the century.
Greener Pathways to Nanomaterials and Their Sustainable Applications
Nanoparticles are extremely tiny building units used to create novel consumer materials in the emergent nanotechnology field; they are identified and introduced in the market at a brisk pace, and their generation represents a major enterprise in nanobased material science.(72) Their impact on health and the environment, and the risks associated in their generation and handling is a major concern.(73) The general bottom-up approach for the production of nanoparticles often entails the use of hazardous reducing agents, typically hydrides or hydrazines and a capping agent, namely polyvinylpyrrolidone (PVP), in addition to volatile organic solvents. Consequently, the development of eco-friendly methods for the production of these nanomaterials is imperative.(74, 75) To circumvent the aggregation problems in nanoparticles that reduce the specific surface area and interfacial free energy and hence diminishing their desired reactivity profile, stability needs are paramount which require added use of surfactants or polyelectrolytes thus contributing to additional waste generation.
Greener Synthetic Approaches to Nanoparticles by Mimicking Nature
In view of the aforementioned arguments, it is essential for “greener” synthesis of nanoparticles to seek nontoxic, nonimmunogenic and hydrophilic stabilizing agents,(76) an eco-friendly reducing agent, and a safe capping entity for stabilizing the ensuing nanoparticles.(77) Our own risk-reduction approach to prepare nanomaterials via several pathways has been largely driven by lessons from Nature; emphasis has been to use benign and safer reagents chiefly in the matrix in which they are to be used, thus avoiding their undue manipulation or handling.(77) As an example, the sustainable preparation of nanoparticles has been demonstrated using natural antioxidants found in wine waste or tea polyphenols,(76, 77) which serve both as reducing and capping agents, thus generating them in bulk quantities with application in catalysis arena.(78) One can envision the preparation of nanoparticles in the total absence of reducing or capping agents using benign natural materials that perform efficient redox chemistry in living systems such as sugars,(79) vitamin B1 (Figure 1),(80) vitamin B2 (riboflavin),(81) vitamin C (ascorbic acid),(82) coffee- and tea-extracts,(83) beet juice,(84, 85) and mammoth amount of waste next to any winery, namely grape pomace.(86) The synthesis of stable nanostructured composites would simply entail the use of biorenewable plant extracts and polyphenolics from varied origins;(87) biodegradable carboxymethyl cellulose (CMC),(88) sugars,(79) agricultural products (beet),(84, 85) and residual winery waste (grape pomace).(86)
Figure 1.

SEM images for Pd nanoparticles generated using vitamin B1 (Reproduced with permission from the Royal Society of Chemistry).(80)
Similarly, glutathione,(89) a ubiquitous tripeptide and an essential component of all cells, affords a straightforward method to bulk amounts of varied-shaped metal nanoparticles in water, avoiding the necessity of sizable amounts of insoluble templates.
Microwave-Assisted Synthesis of Nanoparticles
In-core MW heating has been used as a favorable technique for the synthesis of metal nanoparticles;(19–21) single crystalline polygonal sheets, plates, rods, wires, tubes, dendrites, etc. (Figure 2) are readily obtainable.(90) Nanoparticles with relatively smaller sizes and narrower size distributions are more often conveniently attained using a MW-assisted route than those made in a typical oil-bath; additional greener attributes comprise shorter reaction times, reduced energy consumption, and better product yields.
Figure 2.
Shape-selective synthesis of dendritic structures via aqueous MW hydrolysis of cyano salts without any reducing or capping reagent.(90)
MW heating enables the shape-controlled bulk synthesis of Ag and Fe nanorods in PEG(91) and efficient cross-linking of poly(vinyl alcohol) (PVA) with metallic and bimetallic systems;(92) single-wall carbon nanotubes (SWNT); multiwall carbon nanotubes (MWNT), and C-60;(93) alignment of carbon nanotubes in CMC is facilitated via MW-assisted method.(94)
Biorenewable and naturally abundant polymers, namely chitosan(95) and cellulose (Figure 3),(88) can be used to produce nanocomposites that can find interesting catalytic applications.
Figure 3.
Biodegradable and thermally stable nanocomposites from carboxymethyl cellulose and metals (reproduced by permission from Royal Society of Chemistry).(108)
Abundant, inexpensive, and nontoxic byproduct from biofuel processing, glycerol, could find noteworthy uses as a solvent, as a reagent in transfer hydrogenation reactions, and to produce nanomaterials (Figure 4).(96)
Figure 4.
MW-assisted synthesis of nanometals (Au, Pt, Pd) using glycerol (Reproduced from with permission from the American Chemical Society).(96)
Sustainable Catalysis with Nanoparticle-Adorned Magnetic Supports
Recently, consumer products bearing metal nanoparticles have seen a massive surge, thus increasing the possibility of human or ecosystem exposure(72) due to their release into the environment.(73) Silver nanoparticles (AgNPs), because of their antibacterial properties, are becoming ubiquitous in a range of products such as clothing, dishwashers, detergents, water treatment filters, medical appliances, and food packaging materials. Consequently, there is a pressing demand to develop tools that can help characterize and measure these materials in complex matrices and at low concentrations. Not aware of any other means to retrieve nanoparticles after their discharge, we embarked on the generation of iron-based magnetic nanoparticles (MNPs) that can be recovered using an external magnet.(97) A simple and reproducible technique for the separation/preconcentration of trace amounts of silver nanoparticles has been developed which can enable their quantitation using inductively coupled plasma mass spectrometry (ICP-MS). The modified magnetic particles could successfully capture trace amounts of silver nanoparticles (∼2 ppb) and concentrate (up to 250 times) the particles for analysis with ICP-MS.(98)
The intrinsic instability of MNPs has been addressed via functionalization, and coating them with polymers, surfactants, carbon and silica species;(99) benign ligands, such as glutathione or dopamine, can be affixed on MNPs, which can be used as heterogeneous catalysts for a wide variety of organic transformations and syntheses.(25–27, 100–102) The heterogenization of the catalyst in the form of MNPs facilitates their efficient recovery and the subsequent reuse of precious nanocatalysts comprise some of the salient features of these sustainable process developments(25–27, 100–102) that are applicable to a variety of reduction, oxidation, coupling,(103) and condensation reactions.(100–102) Because of the nm-range size of MNPs, most of the catalysts surface is accessible for reaction as it provides quasi homogeneous media for the catalysts that serves as a bridge between heterogeneous and homogeneous catalysis, thus capturing the good attributes of both systems (Figure 5).
Figure 5.
Nanocatalysts serve as a quasi-homogeneous phase bridging heterogeneous and homogeneous catalysis (reproduced by permission from Royal Society of Chemistry).(25)
MNPs, integral part of sustainable process development, can be subdivided based on the type of magnetic core, which may comprise either reduced species or oxides, e.g., iron oxide NPs (Fe2O3 and Fe3O4), with proven utility in a variety of oxidative and coupling reactions. Bare iron oxides, Fe3O4 and Fe2O3, are active catalysts for the coupling of aldehyde, alkyne, and amine (A3 coupling) and afford an easy route to propargylamines.(104)
The postsynthetic functionalization of nanoferrites with dopamine and adorning them with metal particles (Scheme 10) provides numerous possibilities to use such nanocatalysts; hydration of benzonitrile to benzamide occurs with ruthenium hydroxide on magnetic nanoferrites wherein the complete procedure is performed exclusively in aqueous medium without any organic solvents.(105)
Scheme 10.
Hydration of Nitriles Catalyzed by Nanoferrite–[Ru(OH)]xa
aReproduced by permission from Royal Society of Chemistry.(108)
Similarly, organocatalytic reactions can be performed by anchoring the ubiquitous glutathione (GT) on magnetic nanoferrites via their thiol group(106) as the ensuing catalyst (nano-FGT) can be used for the synthesis of a wide variety of alkyl, aryl, and heterocyclic amines (Scheme 11); facile transformation of functionalized amines to the corresponding pyrroles occurs selectively while tolerating some sensitive functional groups,(107) thus saving on solvent usage and circumventing the tedious conventional chromatographic separations.
Scheme 11.
Nano-FGT-Catalyzed Paal–Knorr Reactionsa
aReproduced by permission from Royal Society of Chemistry.(106)
Magnetically recoverable organocatalysts enable the synthesis of heterocyclic compounds in aqueous medium, thereby avoiding the use of volatile organic solvents at all stages of the reactions.(23, 106–108)
Magnetic nano-FGT-Cu catalyst could accomplish one-pot azide alkyne cycloaddition (AAC) reactions via in situ generation of azides followed by cycloaddition in aqueous media (Scheme 12).(109)
Scheme 12.
Nano-FGT-Cu Catalyzed 1,3-Dipolar Cycloadditions Reactiona
aReproduced by permission from Royal Society of Chemistry.(108)
In an analogous manner, a bimetallic nano-FeDOPACu catalyst (Figure 6) could perform the C–S coupling of aryl halides with thiophenols under MW irradiation conditions to afford diaryl sulfides (Scheme 13)(110).
Figure 6.
Nano-Fe3O4-DOPA-Cu catalyst (nano-FeDOPACu) (reproduced by permission from Royal Society of Chemistry).(108)
Scheme 13.
Cross Coupling of Aryl Halides with Thiophenols Using Nano-FeDOPACua
aReproduced by permission from Royal Society of Chemistry.(108)
Interestingly, the activity of bimetallic Fe–Cu catalyst gets modified as the anchoring ligand for the immobilization of Cu nanoparticles on the magnetic nanoferrite (Fe3O4) surface is changed; whereas the catalyst bearing a GT ligand displays activity for Huisgen cycloaddition.(109) In contrast, dopamine works for C–S coupling and is completely inactive for the Huisgen cycloaddition reactions.(111)
Living cells and enzymes are used as biocatalysts for the highly enantioselective synthesis of chiral molecules with minimal environmental hazards although success in this domain lies in finding an active biocatalyst. Alternatively, the combination of ligands admixed with a suitable transition metal or chiral metal complex that induces chirality via catalytic asymmetric synthesis, is frequently used. Often, precious metal and important organic ligands or asymmetric organocatalysts are lost that not only add to the cost but also generates hazardous waste that directly impacts human health and the environment; fortunately, interest in chiral iron catalysts is on rise.(112) Surface modification of MNPs with chiral scaffolds for asymmetric catalytic applications is an elegant way of providing a special pseudohomogeneous phase which could be separated using an external magnet.
Carbon-coated magnetic nanoparticles not only provide a protective shell but also confers on them superior chemical and thermal stabilities. The reactivity of the catalyst immobilized over such carbon-coated nanoparticles using π–π stacking interaction of pyrene has been truly striking (Figure 7); activity was maintained even after the 16th cycle when hydroxycarbonylation of aryl halides was carried out in water. The mechanistic study has disclosed that the active catalytic species becomes free in solution during agitation of the reaction mixture at elevated temperatures. After the reaction is complete, it returns back to bind with the magnetic beads, which could be easily recovered using an external magnet.(113)
Figure 7.
Carbon-coated nanoparticles and their utility via stacking interaction.
MNPs are getting distinctive attention in the emerging area of flow chemistry. The twin function of agitation and confinement of the nanoparticle-bound catalyst can be realized in a reactor via a rotating magnetic field, which avoids the impending problems of clogging membranes or filters that are well-known barriers for immobilized catalysts. The concept has been well demonstrated in a close circuit reactor for the asymmetric benzoylation of racemic 1,2-diols using a copper(II)-azabis(oxazoline) catalyst, which had been covalently linked to carbon-coated cobalt nanoparticles;(114) magnetic field induced flow mixing is beginning to make critical impact for handling of slurries and precipitates in newer small footprint flow reactors.(115)
Biocompatibility of Nanoparticles
The fate, transport, and toxicity of engineered metal nanoparticles (ENPs) have been extensively studied in recent years,(116) whereas the formation and ecological effects of natural nanoparticles (NNPs) have been barely examined.(117) NNPs are frequently present in all spheres of the Earth, regardless of human activities, and their existence as Ag, Au, Fe, Mn, pyrite (FeS2), Ag2S, CuS, CdS, and ZnS nanoparticles is dictated mostly by environmental conditions, namely temperature, pH, oxic/anoxic, light, and concentration and characteristics of natural organic matter (NOM). Organic-matter-coated natural metal nanoparticles display less toxicity than ENPs that are coated by polymers and/or surfactants, which is also corroborated by the fact that Ag nanoparticles generated by tea or epicatechin were nontoxic even at high concentrations (100 μg/mL);(118) in most cases, these nanoparticles created a prolific response, which is most likely a result of the presence of antioxidants on the surface of the nanoparticles. The synthesis of silver nanoparticles using plant extracts and biodegradable polymers has been well documented(119) and such coated silver nanoparticles have been examined in terms of alteration of the membrane permeability of barrier (intestinal, brain endothelial) cells.(120) The ideal objective should be to attain the functional properties of nanomaterials while minimizing inherent threat, thus circumventing risk to the environment and health at various stages of the life cycle.(121) In this context, learning from Nature and pursuing the bioinspired synthetic strategies will aid our understanding of the vital interactions and mechanisms to design the next generation of biocompatible nanomaterials for varied applications.(122)
Conclusion
The diversity of our chemical universe provides room for several stimulating philosophies and disciplines to advance the knowledge base, which may form the foundation to orchestrate newer design trends ranging from cradle to cradle to biomimicry. Debating and analyzing green and sustainable chemistry endeavors,(123) and associated misunderstanding of the thematic discussions,(124) will help us to gain better insight in to the holistic view. Computational toxicological advancements armed with predictive methods may enable creators of new chemical entities to integrate both functional and safety considerations at early stages of development, well before embarking on the synthesis of desired molecules.(125) Such practices can foresee and predict other pursued properties in early assessments and will be of utmost interest not only for the identifying “lead” compounds with a chosen property but at the same time eliminating molecules that present unsafe propositions that surpass their advantages. Design of novel nanocatalysts should accommodate and preferably preserve the functionality present in the renewable biopolymers during the depolymerization, especially for the nonedible portions both from seafood- or forestry waste; use of earth-abundant materials (CO2 and base metals), and energy efficient (solar or visible light) and atom-economic processes will comprise the future sustainable chemical enterprise. The possibilities for innovation in Green and Sustainable Chemistry are limitless and they will provide unceasing opportunities for future explorations.
Acknowledgment
I thank my collaborators and colleagues, past and present, whose names appear in the reference section for their immense contribution to our efforts in Green and Sustainable Chemistry over the years. The views expressed in this article are those of the author and do not necessarily represent the views or policies of the U.S. Environmental Protection Agency. Any mention of trade names or commercial products does not constitute endorsement or recommendation for use.
Footnotes
The author declares no competing financial interest.
References
- 1.Baeyer A (1883) ″Ueber die Verbindungen der Indigogruppe″ [On the compounds of the indigo group] Ber. Dtsch. Chem. Ges. 1883, 16 (16) 2188–2204DOI: 10.1002/cber.188301602130 [DOI] [Google Scholar]
- 2.Anastas PT; Warner JC Green Chemistry Theory and Practice; Oxford University Press: New York, 1998. [Google Scholar]
- 3.Nasir Baig RB; Varma RS Solvent-free synthesis In An Introduction to Green Chemistry Methods; Luque R; Colmenares JC, Eds.; Future Science: London, U. K., 2013; http://www.futuremedicine.com/doi/book/10.4155/9781909453104. [Google Scholar]
- 4. http://www.nobelprize.org/nobel_prizes/chemistry/laureates/2005/press.html.
- 5.Trost BM The atom economy--a search for synthetic efficiency Science 1991, 254, 1471–1477DOI: 10.1126/science.1962206 [DOI] [PubMed] [Google Scholar]
- 6.Sheldon RA Green chemistry and resource efficiency: towards a green economy Green Chem. 2016, 18, 3180–3183and references cited therein.DOI: 10.1039/C6GC90040B [DOI] [Google Scholar]
- 7.Varma RS Clay and Clay-supported Reagents in Organic Synthesis Tetrahedron 2002, 58, 1235–1255DOI: 10.1016/S0040-4020(01)01216-9 [DOI] [Google Scholar]
- 8.Varma RS Solvent-free organic syntheses using supported reagents and microwave irradiation Green Chem. 1999, 1, 43–55DOI: 10.1039/a808223e [DOI] [Google Scholar]
- 9.Mack J; Fulmer D; Stofel S; Santos N The first solvent-free method for the reduction of esters Green Chem. 2007, 9, 1041–1043DOI: 10.1039/b706167f [DOI] [Google Scholar]
- 10.Kumar D; Chandra Sekhar KVG; Dhillon H; Rao VS; Varma RS An expeditious synthesis of 1-aryl-4-methyl-1,2,4-triazolo[4,3a]quinoxalines under solvent-free conditions using iodobenzene diacetate Green Chem. 2004, 6, 156– 157DOI: 10.1039/b315031c [DOI] [Google Scholar]
- 11.Nasir Baig RB; Varma RS Alternate energy input: mechanochemical, microwave and ultrasound-assisted organic synthesis Chem. Soc. Rev. 2012, 41, 1559–1584DOI: 10.1039/C1CS15204A [DOI] [PubMed] [Google Scholar]
- 12.Gawande MB; Shelke SS; Zboril R; Varma RS Microwave-assisted chemistry: synthetic applications for rapid assembly of nanomaterials and organics Acc. Chem. Res. 2014, 47, 1338–1348DOI: 10.1021/ar400309b [DOI] [PubMed] [Google Scholar]
- 13.Varma RS Chemical activation by mechanochemical mixing, microwave, and ultrasonic irradiation Green Chem. 2008, 10, 1129–1130DOI: 10.1039/b817559b [DOI] [Google Scholar]
- 14.Namboodiri VV; Varma RS Solvent-free sonochemical preparation of ionic liquids Org. Lett. 2002, 4, 3161–3163DOI: 10.1021/ol026608p [DOI] [PubMed] [Google Scholar]
- 15.Luche JL Synthetic Organic Chemistry; Plenum Press: New York, 1998. [Google Scholar]
- 16.Varma RS In Microwave Technology: Applications in Chemical Synthesis; Kik-Othmer On-line Encyclopedia of Chemical Technology, 6th ed.; John Wiley & Sons, Inc.: 2013; pp 1–68; DOI: DOI: 10.1002/0471238961.1309031822011813.a01.pub2. [DOI] [Google Scholar]
- 17.Strauss CR; Varma RS Microwaves in green and sustainable chemistry In Microwave Methods in Organic Synthesis; Larhed M; Olofsson K, Eds.; Springer-Verlag: Heidelberg, 2006; 266, pp 199–231. [Google Scholar]
- 18.Bruckmann A; Krebs A; Bolm C Organocatalytic reactions: effects of ball milling, microwave and ultrasound irradiation Green Chem. 2008, 10, 1131–1141DOI: 10.1039/b812536h [DOI] [Google Scholar]
- 19.Nadagouda MN; Speth T; Varma RS Microwave-assisted green synthesis of silver nanostructures Acc. Chem. Res. 2011, 44, 469–478DOI: 10.1021/ar1001457 [DOI] [PubMed] [Google Scholar]
- 20.Polshettiwar V; Nadagouda MN; Varma RS Microwave-assisted chemistry: A rapid and sustainable route to synthesis of organics and nanomaterials Aust. J. Chem. 2009, 62, 16–26DOI: 10.1071/CH08404 [DOI] [Google Scholar]
- 21.Baghbanzadeh M; Škapin SD; Orel ZC; Kappe CO A critical assessment of the specific role of microwave irradiation in the synthesis of ZnO micro- and nanostructured materials Chem. - Eur. J. 2012, 18, 5724–5731DOI: 10.1002/chem.201103548 [DOI] [PubMed] [Google Scholar]
- 22.Harada M; Cong C Microwave-assisted polyol synthesis of polymer-protected monometallic nanoparticles prepared in batch and continuous-flow processing Ind. Eng. Chem. Res. 2016, 55, 5634–5643DOI: 10.1021/acs.iecr.6b00991 [DOI] [Google Scholar]
- 23.Polshettiwar V; Varma RS Green chemistry by nano-catalysis Green Chem. 2010, 12, 743–754DOI: 10.1039/b921171c [DOI] [Google Scholar]
- 24.Gawande MB; Bonifácio VDB; Luque R; Branco PS; Varma RS Benign by design: catalyst-free in-water, on-water, green chemical methodologies in organic synthesis Chem. Soc. Rev. 2013, 42, 5522–5551DOI: 10.1039/c3cs60025d [DOI] [PubMed] [Google Scholar]
- 25.Baig RBN; Varma RS Magnetically retrievable catalysts for organic synthesis Chem. Commun. 2013, 49, 752– 770DOI: 10.1039/C2CC35663E [DOI] [PubMed] [Google Scholar]
- 26.Gawande MB; Rathi AK; Branco PS; Varma RS Sustainable utility of magnetically recyclable nano-catalysts in water: applications in organic synthesis Appl. Sci. 2013, 3, 656–674DOI: 10.3390/app3040656 [DOI] [Google Scholar]
- 27.Varma RS Greener routes to organics and nanomaterials: sustainable applications of nano-catalysts Pure Appl. Chem. 2013, 85, 1703–1710DOI: 10.1351/PAC-CON-13-01-15 [DOI] [Google Scholar]
- 28.Tanaka K Solvent-free Organic Synthesis; Wiley-VCH: Weinheim, 2003. [Google Scholar]
- 29.Rothenberg G; Downie AP; Raston CL; Scott JL Understanding solid/solid organic reactions J. Am. Chem. Soc. 2001, 123, 8701–8708DOI: 10.1021/ja0034388 [DOI] [PubMed] [Google Scholar]
- 30.Toda F; Kiyoshige K; Yagi M NaBH4 Reduction of ketones in the solid state Angew. Chem., Int. Ed. Engl. 1989, 28, 320–321DOI: 10.1002/anie.198903201 [DOI] [Google Scholar]
- 31.Kumar D; Sundaree S; Rao VS; Varma RS A facile one-pot synthesis of β-keto sulfones from ketones under solvent-free conditions Tetrahedron Lett. 2006, 47, 4197–4199DOI: 10.1016/j.tetlet.2006.04.076 [DOI] [Google Scholar]
- 32.Kumar D; Sundaree S; Patel G; Rao VS; Varma RS Solvent-free facile synthesis of novel α-tosyloxy β-keto sulfones using [hydroxy(tosyloxy)iodo]benzene Tetrahedron Lett. 2006, 47, 8239–8241DOI: 10.1016/j.tetlet.2006.09.107 [DOI] [Google Scholar]
- 33.Constabel F; Geckeler KE Solvent-free self-assembly of C60 and cucurbit[7]uril using high-speed vibration milling Tetrahedron Lett. 2004, 45, 2071–2073DOI: 10.1016/j.tetlet.2004.01.071 [DOI] [Google Scholar]
- 34.Tanaka K; Kishigami S; Toda F Reformatsky and Luche reaction in the absence of solvent J. Org. Chem. 1991, 56, 4333–4334DOI: 10.1021/jo00013a055 [DOI] [Google Scholar]
- 35.Bose AK; Pednekar S; Ganguly SN; Chakraborty G; Manhas MS A simplified green chemistry approach to the Biginelli reaction using ‘Grindstone Chemistry’ Tetrahedron Lett. 2004, 45, 8351–8353DOI: 10.1016/j.tetlet.2004.09.064 [DOI] [Google Scholar]
- 36.Mack J; Shumba M Rate enhancement of the Morita–Baylis–Hillman reaction through mechanochemistry Green Chem. 2007, 9, 328–330DOI: 10.1039/B612983H [DOI] [Google Scholar]
- 37.Thorwirth R; Stolle A; Ondruschka B Fast copper-, ligand- and solvent-free Sonogashira coupling in a ball mill Green Chem. 2010, 12, 985–991DOI: 10.1039/c000674b [DOI] [Google Scholar]
- 38.Cook TL; Walker JA; Mack J Scratching the catalytic surface of mechanochemistry: a multi-component CuAAC reaction using a copper reaction vial Green Chem. 2013, 15, 617–619DOI: 10.1039/c3gc36720g [DOI] [Google Scholar]
- 39.Friscic T; Halasz I; Beldon PJ; Belenguer AM; Adams F; Kimber SAJ; Honkimäki V; Dinnebier RE Real-time and in situ monitoring of mechanochemical milling reactions Nat. Chem. 2013, 5, 66–73DOI: 10.1038/nchem.1505 [DOI] [PubMed] [Google Scholar]
- 40.Larsen MB; Boydston AJ Investigations in fundamental and applied polymer mechanochemistry Macromol. Chem. Phys. 2016, 217, 354–364DOI: 10.1002/macp.201500292 [DOI] [Google Scholar]
- 41.Mason TJ; Lorimer JP Applied Sonochemisrty, The uses of ultrasound in chemistry and processing; Wiley-VCH Verlag GmbH: Weinheim, 2002. [Google Scholar]
- 42.Rajagopal R; Jarikote VD; Srinivasan KV Ultrasound promoted Suzuki cross-coupling reactions in ionic liquid at ambient conditions Chem. Commun. 2002, 616–617DOI: 10.1039/b111271f [DOI] [PubMed] [Google Scholar]
- 43.Gholap AR; Venkatesan K; Pasricha R; Daniel T; Lahoti RJ; Srinivasan KV Copper- and ligand-free Sonogashira reaction catalyzed by Pd(0) nanoparticles at ambient conditions under ultrasound irradiation J. Org. Chem. 2005, 70, 4869–4872DOI: 10.1021/jo0503815 [DOI] [PubMed] [Google Scholar]
- 44.Deshmukh RR; Rajagopal R; Srinivasan KV Ultrasound promoted C–C bond formation: Heck reaction at ambient conditions in room temperature ionic liquids Chem. Commun. 2001, 1544–1545DOI: 10.1039/b104532f [DOI] [PubMed] [Google Scholar]
- 45.Varma RS; Naicker KP Ultrasound accelerated permanganate oxidation: an improved procedure for the synthesis of 1,2-cis diols from olefins Tetrahedron Lett. 1998, 39, 7463–66DOI: 10.1016/S0040-4039(98)01671-2 [DOI] [Google Scholar]
- 46.Varma RS; Naicker KP; Kumar D Can Ultrasound substitute for a phase-transfer catalyst? triphase catalysis and sonochemical acceleration in nucleophilic substitution of alkyl halides and α-tosyloxyketones: synthesis of alkyl azides and α-azidoketones J. Mol. Catal. A: Chem. 1999, 149, 153–160DOI: 10.1016/S1381-1169(99)00168-5 [DOI] [Google Scholar]
- 47.Ando T; Kimura T Ultrasonic organic synthesis involving nonmetal solids In Advances in Sonochemistry; Mason TJ, Ed.; JAI Press: London, 1991; 2, p 211. [Google Scholar]
- 48.Varma RS; Dahiya R An Expeditious and solvent-free synthesis of 2-amino substituted isoflav-3-enes using microwaves J. Org. Chem. 1998, 63, 8038–8041DOI: 10.1021/jo980985r [DOI] [Google Scholar]
- 49.Varma RS; Kumar D Microwave-accelerated three-component condensation reaction on clay: solvent-free synthesis of imidazo[1,2-a] annulated pyridines, pyrazines and pyrimidines Tetrahedron Lett. 1999, 40, 7665–7669DOI: 10.1016/S0040-4039(99)01585-3 [DOI] [Google Scholar]
- 50.Kappe CO; Kumar D; Varma RS Microwave-assisted high-speed parallel synthesis of 4-aryl-dihydro-pyrimidin-2(1h)-ones using a solventless Biginelli condensation protocol Synthesis 1999, 1999, 1799–1803DOI: 10.1055/s-1999-3592 [DOI] [Google Scholar]
- 51.Vaddula BR; Varma RS; Leazer J Mixing with microwaves: a simple catalyst-free and solvent-free synthesis of pyrazoles and diazepines Tetrahedron Lett. 2013, 54, 1538–1541DOI: 10.1016/j.tetlet.2013.01.029 [DOI] [Google Scholar]
- 52.Ješelnik M; Varma RS; Polanc S; Kočevar M Solid-state synthesis of heterocyclic hydrazones using microwaves under catalyst-free conditions Green Chem. 2002, 4, 35–38DOI: 10.1039/b108029f [DOI] [PubMed] [Google Scholar]
- 53.Ješelnik M; Varma RS; Polanc S; Kočevar M Catalyst-free reactions under solvent-free conditions: microwave-assisted synthesis of heterocyclic hydrazones below the melting points of neat reactants Chem. Commun. 2001, 1716–1717DOI: 10.1039/b104508n [DOI] [PubMed] [Google Scholar]
- 54.Varma RS; Namboodiri VV An expeditious solvent-free route to ionic liquids using microwaves Chem. Commun. 2001, 643–644DOI: 10.1039/b101375k [DOI] [Google Scholar]
- 55.Kim YJ; Varma RS Microwave-assisted preparation of imidazolium-based tetrachloroindate and their application in the solvent-free tetrahydropyranylation of aromatic alcohols Tetrahedron Lett. 2005, 46, 1467–1469DOI: 10.1016/j.tetlet.2005.01.025 [DOI] [Google Scholar]
- 56.Kim YJ; Varma RS Microwave-assisted preparation of 1-butyl-3-methylimidazolium tetrachlorogallate and its catalytic use in acetal formation under mild conditions Tetrahedron Lett. 2005, 46, 7447–7449DOI: 10.1016/j.tetlet.2005.08.059 [DOI] [Google Scholar]
- 57.Kim YJ; Varma RS Tetrahaloindate(III)-based ionic liquids in the coupling reaction of carbon dioxide and epoxides to generate cyclic carbonates: H-bonding and mechanistic studies J. Org. Chem. 2005, 70, 7882–7891DOI: 10.1021/jo050699x [DOI] [PubMed] [Google Scholar]
- 58.Gawande MB; Bonifácio VDB; Luque R; Branco P; Varma RS Solvent-free and catalyst-free chemistry–A benign pathway to sustainability ChemSusChem 2014, 7, 24–44DOI: 10.1002/cssc.201300485 [DOI] [PubMed] [Google Scholar]
- 59.Varma RS; Nasir Baig RB In Microwaves in Organic Synthesis, 3rd ed.; de la Hoz A; Loupy A, Eds.;Wiley-VCH: Weinheim, 2012; Chapter 10, pp 431–487. [Google Scholar]
- 60.Ju Y; Varma RS An efficient and simple aqueous N-heterocyclization of aniline derivatives: microwave-assisted synthesis of N-aryl azacycloalkanes Org. Lett. 2005, 7, 2409–2411DOI: 10.1021/ol050683t [DOI] [PubMed] [Google Scholar]
- 61.Ju Y; Varma RS Aqueous N-heterocyclization of primary amines and hydrazines with dihalides: microwave-assisted syntheses of N-azacycloalkanes, isoindole, pyrazole, pyrazolidine and phthalazine derivatives J. Org. Chem. 2006, 71, 135–141DOI: 10.1021/jo051878h [DOI] [PubMed] [Google Scholar]
- 62.Nasir Baig RB; Varma RS Nanocatalysis in water in Metal-catalyzed Reactions in Water; Dixneuf P; Cadierno V, Eds.; Wiley-VCH GmbH & Co; KGaA: Weinheim, 2013; Chapter 9, pp 337–394. [Google Scholar]
- 63.Chen J; Spear SK; Huddleston JG; Rogers RD Polyethylene glycol and solutions of polyethylene glycol as green reaction media Green Chem. 2005, 7, 64–82DOI: 10.1039/b413546f [DOI] [Google Scholar]
- 64.Namboodiri VV; Varma RS Microwave-accelerated Suzuki cross-coupling reaction in polyethylene glycol (PEG) Green Chem. 2001, 3, 146–148DOI: 10.1039/b102337n [DOI] [Google Scholar]
- 65.Glasnov TN; Kappe CO The microwave-to-flow paradigm: translating high-temperature batch microwave chemistry to scalable continuous flow processes Chem. - Eur. J. 2011, 17, 11956–11968DOI: 10.1002/chem.201102065 [DOI] [PubMed] [Google Scholar]
- 66.Ley SV On being green: can flow chemistry help? Chem. Rec. 2012, 12, 378–390DOI: 10.1002/tcr.201100041 [DOI] [PubMed] [Google Scholar]
- 67.Gutmann B; Cantillo D; Kappe CO Use of continuous flow technology to harness hazardous chemistries and process conditions–a tool for the manufacturing of active pharmaceutical ingredients Angew. Chem., Int. Ed. 2015, 54, 6688–6729DOI: 10.1002/anie.201409318 [DOI] [PubMed] [Google Scholar]
- 68.Pieber B; Kappe CO Generation and synthetic application of trifluoromethyl diazomethane utilizing continuous flow technologies Org. Lett. 2016, 18, 1076–1079DOI: 10.1021/acs.orglett.6b00194 [DOI] [PubMed] [Google Scholar]
- 69.Svoboda J; Konig B Templated photochemistry: toward catalysts enhancing the efficiency and selectivity of photoreactions in homogeneous solutions Chem. Rev. 2006, 106, 5413–5430DOI: 10.1021/cr050568w [DOI] [PubMed] [Google Scholar]
- 70.Heterogeneous Photocatalysis-from Fundamentals to Green Applications; Colmenares JC; Xu Y-J, Eds.; Springer-Verlag: Berlin, Heidelberg, 2016. [Google Scholar]
- 71.Lang X; Ma W; Chen C; Ji H; Zhao J Selective aerobic oxidation mediated by TiO2 photocatalysis Acc. Chem. Res. 2014, 47, 355–363DOI: 10.1021/ar4001108 [DOI] [PubMed] [Google Scholar]
- 72.Nadagouda MN; Varma RS In Nanomaterials: Risks and Benefits; Linkov I; Steevens J, Eds.; Springer: Dordrecht, 2009; pp 209–218. [Google Scholar]
- 73.Dwivedi AD; Dubey SP; Sillanpää M; Kwon Y–N; Lee C; Varma RS Fate of engineered nanoparticles: implications in the environment Coord. Chem. Rev. 2015, 287, 64–78DOI: 10.1016/j.ccr.2014.12.014 [DOI] [Google Scholar]
- 74.Virkutyte J; Varma RS Green Synthesis of Nanomaterials; Environmental Aspects in “Sustainable Nanotechnology and the Environment: Advances and Achievements; Shamim N, Ed.; ACS Symposium Series, Vol. 1124; American Chemical Society: Washington, DC, 2013; Chapter 2, pp 11–39. [Google Scholar]
- 75.Sustainable Preparation of Metal Nanoparticles-Methods and Applications; Luque R; Varma RS, Eds.; RSC Green Chemistry # 19; Royal Society Chemistry: Thomas Graham House, Cambridge, United Kingdom, 2013. [Google Scholar]
- 76.Virkutyte J; Varma RS Green synthesis of metal nanoparticles: biodegradable polymers and enzymes in stabilization and surface functionalization Chem. Sci. 2011, 2, 837–846DOI: 10.1039/C0SC00338G [DOI] [Google Scholar]
- 77.Varma RS Greener approach to nanomaterials and their sustainable applications Curr. Opin. Chem. Eng. 2012, 1, 123–128DOI: 10.1016/j.coche.2011.12.002 [DOI] [Google Scholar]
- 78.Nadagouda MN; Hoag GE; Collins JB; Varma RS Green synthesis of Au nanostructures at room temperature using biodegradable plant surfactants Cryst. Growth Des. 2009, 9, 4979–4983DOI: 10.1021/cg9007685 [DOI] [Google Scholar]
- 79.Mallikarjuna MN; Varma RS Microwave-assisted shape-controlled bulk synthesis of noble nanocrystals and their catalytic properties Cryst. Growth Des. 2007, 7, 686–690DOI: 10.1021/cg060506e [DOI] [Google Scholar]
- 80.Nadagouda MN; Polshettiwar V; Varma RS Self-Assembly of palladium nanoparticles: synthesis of nanobelts, nanoplates and nanotrees using vitamin B1 and their application in carbon-carbon coupling reactions J. Mater. Chem. 2009, 19, 2026–2031DOI: 10.1039/b817112b, [DOI] [Google Scholar]
- 81.Nadagouda MN; Varma RS Green and controlled synthesis of gold and platinum nanomaterials using vitamin B2: density-assisted self-assembly of nanospheres, wires and rods Green Chem. 2006, 8, 516–518DOI: 10.1039/b601271j [DOI] [Google Scholar]
- 82.Nadagouda MN; Varma RS A greener synthesis of core (Fe, Cu)-shell (Au, Pt, Pd and Ag) nanocrystals using aqueous vitamin C Cryst. Growth Des. 2007, 7, 2582–2587DOI: 10.1021/cg070554e [DOI] [Google Scholar]
- 83.Nadagouda MN; Varma RS Green synthesis of silver and palladium nanoparticles at room temperature using coffee and tea extract Green Chem. 2008, 10, 859–862DOI: 10.1039/b804703k [DOI] [Google Scholar]
- 84.Kou J; Varma RS Beet juice-induced green fabrication of plasmonic AgCl/Ag nanoparticles ChemSusChem 2012, 5, 2435–2441DOI: 10.1002/cssc.201200477 [DOI] [PubMed] [Google Scholar]
- 85.Kou J; Varma RS Beet juice utilization: expeditious green synthesis of nobel metal nanoparticles (Ag, Au, Pt, and Pd) using microwaves RSC Adv. 2012, 2, 10283–10290DOI: 10.1039/c2ra21908e [DOI] [Google Scholar]
- 86.Baruwati B; Varma RS High value products from waste: grape pomace extract - a three-in-one package for the synthesis of metal nanoparticles ChemSusChem 2009, 2, 1041–1044DOI: 10.1002/cssc.200900220 [DOI] [PubMed] [Google Scholar]
- 87.Nadagouda N; Iyanna N; Lalley J; Han C; Dionysiou DD; Varma RS Synthesis of silver and gold nanoparticles using antioxidants from blackberry, blueberry, pomegranate and turmeric extracts ACS Sustainable Chem. Eng. 2014, 2, 1717–1723DOI: 10.1021/sc500237k [DOI] [Google Scholar]
- 88.Nadagouda MN; Varma RS Synthesis of thermally stable carboxymethyl cellulose/metal biodegradable nanocomposite films for potential biological applications Biomacromolecules 2007, 8, 2762–2767DOI: 10.1021/bm700446p [DOI] [PubMed] [Google Scholar]
- 89.Baruwati B; Polshettiwar V; Varma RS Glutathione promoted expeditious green synthesis of silver nanoparticles in water using microwaves Green Chem. 2009, 11, 926–930DOI: 10.1039/b902184a [DOI] [Google Scholar]
- 90.Polshettiwar V; Baruwati B; Varma RS Self-assembly of metal oxides into three-dimensional nanostructures: synthesis and application in catalysis ACS Nano 2009, 3, 728–736DOI: 10.1021/nn800903p [DOI] [PubMed] [Google Scholar]
- 91.Nadagouda MN; Varma RS Microwave-assisted shape controlled bulk synthesis of Ag and Fe nanorods in poly (ethylene glycol) solutions Cryst. Growth Des. 2008, 8, 291–295DOI: 10.1021/cg070473i [DOI] [Google Scholar]
- 92.Nadagouda MN; Varma RS Preparation of novel metallic and bimetallic cross-linked poly (vinyl alcohol) nanocomposites under microwave irradiation Macromol. Rapid Commun. 2007, 28, 465–472DOI: 10.1002/marc.200600735 [DOI] [Google Scholar]
- 93.Nadagouda MN; Varma RS Microwave-assisted synthesis of cross-linked poly (vinyl alcohol) nanocomposites comprising single-wall carbon nanotubes (SWNT), Multi-wall carbon nanotubes (MWNT) and Buckminsterfullerene (C-60) Macromol. Rapid Commun. 2007, 28, 842–847DOI: 10.1002/marc.200600878 [DOI] [Google Scholar]
- 94.Nadagouda MN; Varma RS Noble metal decoration and alignment of carbon nanotubes in carboxymethyl cellulose Macromol. Rapid Commun. 2008, 29, 155–159DOI: 10.1002/marc.200700616 [DOI] [Google Scholar]
- 95.Nasir Baig RB; Nadagouda MN; Varma RS Ruthenium on chitosan: a recyclable heterogeneous catalyst for aqueous hydration of nitriles to amides Green Chem. 2014, 16, 2122–2127DOI: 10.1039/c3gc42004c [DOI] [Google Scholar]
- 96.Kou J; Bennett-Stamper C; Varma RS Green synthesis of noble nanometals (Au, Pt, Pd) using glycerol under microwave irradiation conditions ACS Sustainable Chem. Eng. 2013, 1, 810–816DOI: 10.1021/sc400007p [DOI] [Google Scholar]
- 97.Baruwati B; Nadagouda MN; Varma RS Bulk synthesis of monodisperse ferrite nanoparticles at water-organic interfaces under conventional and microwave hydrothermal treatment and their surface functionalization J. Phys. Chem. C 2008, 112, 18399–18404DOI: 10.1021/jp807245g [DOI] [Google Scholar]
- 98.Mwilu SK; Siska E; Nasir Baig RB; Varma RS; Heithmar E; Rogers KR Separation and measurement of silver nanoparticles and silver ions using magnetic particles Sci. Total Environ. 2014, 472, 316–323DOI: 10.1016/j.scitotenv.2013.10.077 [DOI] [PubMed] [Google Scholar]
- 99.Nasir Baig RB; Varma RS Magnetic silica supported ruthenium nanoparticles: an efficient catalyst for transfer hydrogenation of carbonyl compounds ACS Sustainable Chem. Eng. 2013, 1, 805–809DOI: 10.1021/sc400032k [DOI] [Google Scholar]
- 100.Nasir Baig RB; Nadagouda MN; Varma RS Magnetic retrievable catalysts for asymmetric synthesis Coord. Chem. Rev. 2015, 287, 137–156DOI: 10.1016/j.ccr.2014.12.017 [DOI] [Google Scholar]
- 101.Gawande MB; Branco PS; Varma RS Nano-magnetite (Fe3O4) as a support for recyclable catalysts in the development of sustainable methodologies Chem. Soc. Rev. 2013, 42, 3371–3393DOI: 10.1039/c3cs35480f [DOI] [PubMed] [Google Scholar]
- 102.Nasir Baig RB; Varma RS Organic synthesis via magnetic attraction: benign and sustainable protocols using magnetic nanoferrites Green Chem. 2013, 15, 398–417DOI: 10.1039/C2GC36455G [DOI] [Google Scholar]
- 103.Sharma RK; Dutta S; Sharma S; Zboril R; Varma RS; Gawande MB Fe3O4 (Iron oxide)-supported nanocatalysts: synthesis, characterization and applications in coupling reactions Green Chem. 2016, 18, 3184–3209DOI: 10.1039/C6GC00864J [DOI] [Google Scholar]
- 104.Zeng TQ; Chen WW; Cirtiu CM; Moores A; Song GH; Li CJ Fe3O4 nanoparticles: a robust and magnetically recoverable catalyst for three-component coupling of aldehyde, alkyne and amine Green Chem. 2010, 12, 570–573DOI: 10.1039/b920000b [DOI] [Google Scholar]
- 105.Polshettiwar V; Varma RS Nanoparticle-supported and magnetically recoverable ruthenium hydroxide catalyst: efficient hydration of nitriles to amides in aqueous medium Chem. - Eur. J. 2009, 15, 1582–1586DOI: 10.1002/chem.200802264 [DOI] [PubMed] [Google Scholar]
- 106.Polshettiwar V; Baruwati B; Varma RS Magnetic nanoparticle-supported glutathione: A conceptually sustainable organocatalyst Chem. Commun. 2009, 1837–1839DOI: 10.1039/b900784a [DOI] [PubMed] [Google Scholar]
- 107.Polshettiwar V; Varma RS Nano-organocatalyst: magnetically retrievable ferrite-anchored glutathione for microwave-assisted Paal-Knorr reaction, aza-Michael addition, and pyrazole synthesis Tetrahedron 2010, 66, 1091–1097DOI: 10.1016/j.tet.2009.11.015 [DOI] [Google Scholar]
- 108.Varma RS Journey on greener pathways: from the use of alternate energy inputs and benign reaction media to sustainable applications of nano-catalysts in synthesis and environmental remediation Green Chem. 2014, 16, 2027–2047DOI: 10.1039/c3gc42640h [DOI] [Google Scholar]
- 109.Nasir Baig RB; Varma RS A highly active magnetically recoverable nano ferrite-glutathione-copper (nano-FGT-Cu) catalyst for Huisgen 1,3-dipolar cycloadditions Green Chem. 2012, 14, 625–632DOI: 10.1039/c2gc16301b [DOI] [Google Scholar]
- 110.Nasir Baig RB; Varma RS A highly active and magnetically retrievable nanoferrite-DOPA-copper catalyst for the coupling of thiophenols with aryl halides Chem. Commun. 2012, 48, 2582–2584DOI: 10.1039/c2cc17283f [DOI] [PubMed] [Google Scholar]
- 111.Nasir Baig RB; Varma RS Copper modified magnetic bimetallic nano-catalysts: ligand regulated catalytic activity Curr. Org. Chem. 2013, 17, 2227–2237DOI: 10.2174/13852728113179990045 [DOI] [Google Scholar]
- 112.Gopalaiah K Chiral iron catalysts for asymmetric synthesis Chem. Rev. 2013, 113, 3248–3296DOI: 10.1021/cr300236r [DOI] [PubMed] [Google Scholar]
- 113.Wittmann S; Schatz A; Grass RN; Stark WJ; Reiser O A recyclable nanoparticle-supported palladium catalyst for the hydroxycarbonylation of aryl halides in water Angew. Chem., Int. Ed. 2010, 49, 1867–1870DOI: 10.1002/anie.200906166 [DOI] [PubMed] [Google Scholar]
- 114.Schatz A; Grass RN; Kainz Q; Stark WJ; Reiser O Cu(II)–azabis(oxazoline) complexes immobilized on magnetic Co/C nanoparticles: kinetic resolution of 1,2-diphenylethane-1,2-diol under batch and continuous-flow conditions Chem. Mater. 2010, 22, 305–310DOI: 10.1021/cm9019099 [DOI] [Google Scholar]
- 115.Koos P; Browne DL; Ley SV Continuous stream processing: a prototype magnetic field induced flow mixer Green Process. Synth. 2012, 1, 11–18DOI: 10.1515/greenps-2011-0501 [DOI] [Google Scholar]
- 116.Krug HF Nanosafety research—are we on the right track? Angew. Chem., Int. Ed. 2014, 53, 12304–12319DOI: 10.1002/anie.201403367 [DOI] [PubMed] [Google Scholar]
- 117.Sharma VK; Filip J; Zboril R; Varma RS Natural inorganic nanoparticles– formation, fate, and toxicity in the environment Chem. Soc. Rev. 2015, 44, 8410–8423DOI: 10.1039/C5CS00236B [DOI] [PubMed] [Google Scholar]
- 118.Moulton MC; Braydich-Stolle LK; Nadagouda MN; Kunzelman S; Hussain SM; Varma RS Synthesis, characterization and biocompatibility of “green” synthesized silver nanoparticles using tea polyphenols Nanoscale 2010, 2, 763–770DOI: 10.1039/c0nr00046a [DOI] [PubMed] [Google Scholar]
- 119.Hebbalalu D; Lalley J; Nadagouda MN; Varma RS Greener techniques for the synthesis of silver nanoparticles using plant extracts, enzymes, bacteria, biodegradable polymers and microwaves ACS Sustainable Chem. Eng. 2013, 1, 703–712DOI: 10.1021/sc4000362 [DOI] [Google Scholar]
- 120.Baruwati B; Simmons S; Varma RS; Veronesi B Green” synthesized and coated nanosilver alters the membrane permeability of barriers (intestinal, brain endothelial) cells and stimulates oxidative stress pathways in neurons ACS Sustainable Chem. Eng. 2013, 1, 753–759DOI: 10.1021/sc400024a [DOI] [Google Scholar]
- 121.Gilbertson LM; Zimmerman JB; Plata DL; Hutchison JE; Anastas PT Designing nanomaterials to maximize performance and minimize undesirable implications guided by the Principles of Green Chemistry Chem. Soc. Rev. 2015, 44, 5758–5777DOI: 10.1039/C4CS00445K [DOI] [PubMed] [Google Scholar]
- 122.Huang J; Lin L; Sun D; Chen H; Yang D; Li Q Bio-inspired synthesis of metal nanomaterials and applications Chem. Soc. Rev. 2015, 44, 6330–6374DOI: 10.1039/C5CS00133A [DOI] [PubMed] [Google Scholar]
- 123.Winterton N Green chemistry: deliverance or distraction? Clean Technol. Environ. Policy 2016, 18, 991–1001DOI: 10.1007/s10098-016-1118-y [DOI] [Google Scholar]
- 124.Clark J The 12 misunderstandings of green chemistry. Env. Sci. Eng. Mag., 2012, 6–8. [Google Scholar]
- 125.Nieto-Draghi C; Fayet G; Creton B; Rozanska X; Rotureau P; de Hemptinne J-C; Ungerer P; Rousseau B; Adamo C A general guidebook for the theoretical prediction of physicochemical properties of chemicals for regulatory purposes Chem. Rev. 2015, 115, 13093–13164DOI: 10.1021/acs.chemrev.5b00215 [DOI] [PubMed] [Google Scholar]