Abstract
Parasitic infections are increasingly recognized as influential forces in the migratory behaviors of hosts ranging from butterflies to whales. In aquatic zooplankton, diel vertical migrations (DVMs) are among the most recurrent behaviors with implications for predator-prey interactions, nutrient cycling, and energy flow, yet how parasitism affects such migrations remains an open question. Here, we tested the effects of sporangia cluster disease (SCD) on DVM of the large-bodied Daphnia pulicaria, which is often considered a key component of lake food webs. By collecting depth-specific zooplankton samples across diel cycles, between years, and among lakes, we show that infection is associated with strong inhibition of host DVM; while all Daphnia tended to occur deeper during the day, uninfected Daphnia and especially gravid individuals migrated to shallower waters at night. In contrast, infected hosts—which could comprise 40% of the population—were more likely to remain deep regardless of time of day. Among infected hosts, the intensity of SCD (sporangia count per host) predicted the degree of DVM inhibition. These observations—coupled with lab-based assays showing that infected hosts exhibited fewer swimming movements and persisted at lower depths than uninfected conspecifics—suggest that parasite-induced inhibition of DVM is a “sickness behavior” resulting from increasing morbidity and energy depletion as the infection intensifies toward host death. Considering the importance of large-bodied Daphnia as regulators of water clarity and prey for fishes, parasite-induced alterations of host migratory behavior have broad potential to affect the redistribution of energy and nutrients within lake ecosystems.
How parasites affect host behavior is among the most fascinating yet challenging questions in biology (e.g., Lefevre et al. 2009). Alongside their more conspicuous effects on host growth, survival, and reproduction, parasites across a wide range of taxa can induce behavioral changes in their hosts ranging from subtle to bizarre (see reviews by Zimmer 2000; Moore 2002). For instance, the fungal pathogen (Ophiocordyceps unilateralis) causes infected ants to climb into the understory vegetation in tropical forests, firmly affix their mandibles to a leaf, and subsequently starve to death, thereby facilitating the spread of fungal spores via aerial transmission from the host’s cadaver (e.g., Andersen et al. 2009). Host behavioral changes associated with infection broadly fall into one of three categories (Poulin 2010). Hosts can change their behaviors to prevent initial exposure to infection (parasite avoidance), including alterations in activity, habitat use, mate selection, or foraging (Behringer et al. 2006; Daly and Johnson 2011; Fritzsche and Allan 2012). Second, as in the ant-fungus example, induced changes in behavior can adaptively benefit the parasite by increasing the probability of infection persistence or subsequent transmission to new hosts (parasite manipulation) (Bethel and Holmes 1973; Lafferty and Morris 1996; Moore 2002). Finally, behavioral changes may be the consequence of the host’s response to infection, resulting either as a direct byproduct of the infection (e.g., acute morbidity) or indirectly as hosts reallocate energy toward controlling its spread (sickness behaviors) (Adelman et al. 2009; Hawley and Altizer 2011). For instance, movement of ectothermic hosts into colder or warmer environments (i.e., behavioral fever) can inhibit pathogen replication (Lefcort and Blaustein 1995; Boltana et al. 2013), whereas isolation from a social group may prevent transmission to other hosts including kin (Kiesecker et al. 1999; Shakhar and Shakhar 2015).
Growing evidence highlights the importance of parasitism in the ecology and evolution of zooplankton (Yan and Larsson 1988; Ebert 2000; Ebert et al. 2001; Decaestecker et al. 2002; Duffy et al. 2009; Caceres et al. 2014). Species of Daphnia frequently become infected by bacterial, fungal, and protozoan microparasites that can reduce growth, inhibit reproduction, alter nutrient chemistry, and lead to population crashes (Green 1974; Brambilla 1983; Ebert 2000; Bittner et al. 2002; Hall et al. 2011). Considerably less is known about how infections affect zooplankton behavior, however. In lakes, one prominent behavior is diel vertical migration (DVM), in which zooplankton retreat to deeper waters during the day but move toward the surface at night (Lampert 1989; Hays 2003). While many factors influence this pattern, it often represents a balance between avoiding visual predators during the day while taking advantage of greater food availability and warmer temperatures at night (e.g., Ringelberg 2010). The role of parasites on DVM has rarely been studied (Fels et al. 2004). Decaestecker et al. (2002) showed that Daphnia DVM is influenced by parasite avoidance; because many infections come from the sediment (i.e., the parasite “spore bank”), the vertical position of Daphnia is the product of a tradeoff between limiting fish predation (by staying deep) and avoiding parasite exposure (but not too deep) (De Meester 1993). Similarly, both field and laboratory experiments have demonstrated that acanthocephalan parasites cause amphipod hosts to become more active and spend more time in illuminated environments, thus increasing their vulnerability to predation by fish that are necessary hosts for parasite reproduction (Bethel and Holmes 1973; Kennedy et al. 1978; McCahon et al. 1991).
Polycaryum laeve is a chytridiomycete parasite that causes reduced growth, reproductive castration, and increased mortality in large-bodied Daphnia, a condition referred to as “sporangia cluster disease” or SCD (Johnson et al. 2006a,b; Johnson et al. 2009). The parasite is an “obligate killer” (Ebert 2005), meaning that maturation of sporangia and subsequent release of motile zoospores occurs only after host death. Sporangia, which occur throughout the hemocoel of infected hosts, dramatically increase the conspicuousness of Daphnia and their resultant vulnerability to fish predators, both in mecoscosm experiments and natural lakes (Johnson et al. 2006b). Although P. laeve has been observed infrequently, with isolated reports from Germany and Greenland (Stempell 1903; Green 1974), surveys of lakes in northern Wisconsin indicated that the parasite was regionally widespread, occurring in 13 of 58 lakes and at prevalences of up to 85% (Johnson et al. 2006b). This infection is well-suited to address questions about parasite-induced changes in host distribution and vertical migration behavior owing to its high prevalence in many natural host populations, the ease with which the presence and intensity of infection can be scored, and the likely importance of lake mixing for transmission (Johnson et al. 2009).
Here, we combined field surveys with laboratory assays to investigate the influence of P. laeve infection on vertical migration by Daphnia pulicaria. Using samples collected across depths in the water column, over multiple diel cycles (night vs. day), and between years, we tested whether infected and uninfected Daphnia exhibited differential DVM behavior and the degree to which such differences could be explained by infection intensity, fecundity, and host body size. We coupled our field observations with laboratory-based assays of Daphnia activity and vertical position in isothermic chambers to evaluate whether infection-mediated behaviors stemmed from host morbidity or adaptive strategies by the host or parasite. For instance, if infection affects hosts’ photoresponse, Daphnia with SCD might show no DVM behavior or even a reversed DVM (Chae and Nishida 1995), whereas if the effects of infection are primarily associated with energy depletion and host morbidity, infected hosts may have difficulty migrating into shallower waters regardless of time of day. Alternatively, if shifts in the vertical distribution of infected Daphnia are primarily the outcome of their greater vulnerability to visual predators (such as planktivorous fishes), we expected the pattern to be strongest during the day and less pronounced or absent in night samples and laboratory trials. Given the importance of large-bodied Daphnia in lake food webs as well as the influence of their vertical movements on processes such as nutrient cycling (Brooks and Dodson 1965; Carpenter et al. 1987; Haupt et al. 2010), parasite-mediated changes in DVM offer a tractable system in which to investigate links among infection, host behavior, and ecological responses.
Materials
Field sampling
In the summers of 2003 and 2004, we collected depth-specific zooplankton samples from Allequash Lake in Vilas County, Wisconsin (latitude: 46.036939, longitude: −89.628844). Allequash is a 168 ha lake that is regularly sampled by the North Temperate Lakes Long-Term Ecological Research (LTER) program based out of the University of Wisconsin, Madison (https://lter.limnology.wisc.edu). Based on previous observations of SCD at high prevalence in 2002 (Johnson et al. 2006a), we selected this lake for a targeted study of infection and DVM. Allequash was visited twice on each sampling date: once during the daytime (1000–1400 h) and again at night (2100–2400 h). On each visit, we used a 45 L Schindler-Patalas trap to collect depth-specific water samples (0–2 m, 2–4 m, and 5–7 m from the deepest portion of the lake) and immediately preserved the contents in 80% ethanol for subsequent examination. In total, we collected paired day–night samples three times in 2003 (17 June, 23 June, and 05 July) and seven times in 2004 (16 May, 31 May, 15 June, 21 June, 28 June, 13 July, and 26 July). We complemented these collections with an examination of depth-specific zooplankton samples from Devil’s Lake (Sauk County, Wisconsin, 43.411891, −89.745120) collected between April and November in 1987 (18 samples). A previous study documented the occurrence of SCD in Devil’s Lake at variable prevalence (< 1–34%) between 1982 and 2003 (Johnson et al. 2009). These samples were collected over 3-m depth intervals (0–3 m, 3–6 m, 6–9 m, 9–12 m, and 12–14 m) using an 80 μm Wisconsin net with a self-closing feature (Lathrop et al. 1998; Johnson et al. 2009). Although no night samples were collected, Devil’s Lake nonetheless offers an interesting contrast with Allequash owing to its greater depth (14 m), extensive history of trout stocking, and geographic separation (Lillie and Mason 1986).
Sample processing
For each sample, we used a stereo dissecting microscope to quantify the number of infected D. pulicaria, the number of females with eggs or ephippia, and the total number of adults (> 1.2 mm in body length). Because examination was of preserved rather than living Daphnia, it is possible that recorded values of fecundity represent underestimates; however, few unassociated eggs or embryos were found loose within the samples. Among randomly selected subsets of uninfected and infected hosts (∼ 30–50 of each), we measured body length using a calibrated ocular micrometer, the number of eggs or embryos per gravid female, and the intensity of infection. Infection intensity is scored visually and ranges from 1 (light infection with few sporangia, mostly concentrated around the heart and head) to 3 (heavy infection with sporangia throughout much of the hemocoel) (Johnson et al. 2006a). We excluded neonates (< 1.2 mm in body length) and male Daphnia because they are less commonly infected.
Laboratory assay of swimming movements
To examine the relationship between P. laeve infection status (infected vs. uninfected) and the swimming behavior of D. pulicaria, we collected zooplankton from Allequash Lake and acclimated them to laboratory conditions over 12 h. Under a stereo dissecting microscope, we isolated 20 infected and 20 uninfected adult female D. pulicaria, measured their body length, and transferred them to observation chambers (200 × 25 mm) filled with 70 mL of filtered (0.1 μm) lake water. Only one Daphnia was included per chamber. Chambers were divided into seven, evenly-spaced sections to facilitate scoring the vertical position (i.e., distance from the bottom) of each Daphnia, which we recorded hourly over 8 h under low-light conditions at 20°C. At 1 h, 5 h, and 8 h, we also visually recorded the number of swimming movements by the second antennae on each host over a 30 s period. The order of observations was randomized among chambers and between observation periods.
Data analysis
For the lake data, we used generalized linear mixed effects models (GLMM) with the appropriate distributions to evaluate how infected and uninfected Daphnia differed in body size (Gaussian response) and fecundity (binomial response, fecund vs. not-fecund) while incorporating sampling date as a random intercept term. We tested for overdispersion by calculating the sum of squared Pearson residuals and comparing it to the residual degrees of freedom (Venables and Ripley 2002). To test how infection and other host traits affected DVM behavior, we used cumulative link mixed models (CLMMs; R package ordinal, Christensen 2015) with host infection status (yes or no), fecundity (yes or no), time of day (day or night), and interactions (infection * time and fecundity * time) to predict depth within the lake. We did not include an interaction between infection and fecundity given that infected hosts were almost always castrated, but we did compare the Akaike Information Criterion (AIC) value of models with and without infection or fecundity to assess whether the effects of infection might simply be mediated by their lack of eggs. Sampling date was included as a random effect to account for seasonal variation in infection and host abundance. We were specifically interested in a main effect of time of day (day vs. night), which would indicate changes in vertical position with time, and its interaction with infection or fecundity, which would indicate differential DVM behavior between groups. For samples from Devil’s Lake, we performed the same analysis only without a covariate for time of day. For the subset of measured individuals, we also included body size, number of eggs (if fecund), and infection intensity (if infected) as additional predictors. Beyond the effects of infection status as a dichotomous variable, we also restricted the data to infected hosts only and assess how infection intensity (which scaled between 1 and 3) influenced vertical position among infected individuals.
We used a similar approach to analyze data from the activity assays. Using vertical position within observation chambers as an ordinal response, we evaluated the influence of infection status, body size, and light while accounting for observation period and host individual as random effects. We modeled antennae movements over 30 s as a Poisson-distributed response with a log-link. Any individuals that died during the observation period or became immobilized in the surface tension were excluded from those time points.
Results
Over 2 yr, both the abundance of large-bodied D. pulicaria and the prevalence of infection by P. laeve varied considerably (Fig. 1). In Allequash, Daphnia abundance tended to decrease over the summer, dropping abruptly in late June or early July; in 2003, Daphnia L−1 decreased from 8.5 on 17 June to 1.45 on 05 July, whereas in 2004 abundance decreased from 11.01 L−1 on 16 May to 0.06 L−1 on 28 June, with < 0.02 individuals L−1 on 13 July and 0 individuals L−1 on 26 July (Fig. 1A). Infection patterns—while variable among sampling dates and depths—were broadly similar between years, with an average prevalence ± 1 SE of 23.6% ± 0.019 in 2003 and 25.3% ± 0.038 in 2004. In Devil’s Lake, infection prevalence peaked on 10 June at 19.5% and dropped to 0% for all sampling dates after 19 August (Fig. 1B). On average, infected hosts were 6.4% larger than uninfected hosts (linear mixed effects [LME], infection coefficient = 0.167 ± 0.016, t = 10.18, p < 0.0001) and rarely supported eggs or ephippia, consistent with the castrating effects of this parasite. Among 1979 infected D. pulicaria examined, four had ephippia and seven supported eggs or embyros (∼ 0.55%, GLMM, infection coefficient = −4.69 ± 0.347, z = −13.51, p < 0.0001).
Figure 1.
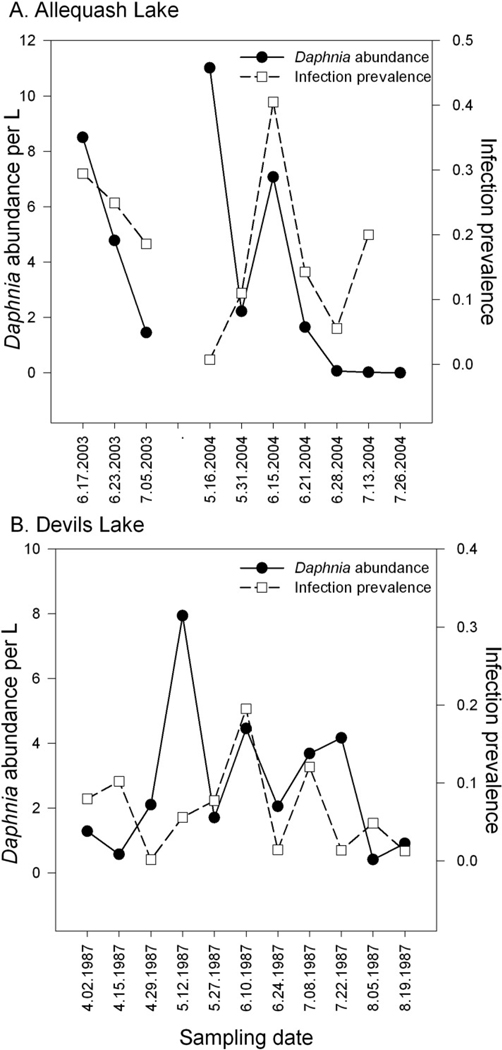
Patterns of P. laeve infection prevalence and D. pulicaria host abundance from (A) Allequash Lake in 2003–2004 and (B) Devil’s Lake in 1987. Presented is the number of mature D. pulicaria (> 1.2 mm) per L as well as the overall proportion infected hosts as a function of each sampling date. For Allequash Lake, each date represents the combination of day- and night-time samples collected within 24 h (infection prevalence is not presented for 7.26.2004 because no mature D. pulicaria were detected). For Devil’s Lake, all samples were collected during daylight hours.
Infected hosts also exhibited stark differences in vertical position within the water column relative to uninfected individuals (Figs. 2, 3). Consistent with classical DVM behavior, the depth at which Daphnia occurred in Allequash Lake shifted with time of day, such that a greater proportion of individuals were found at shallower depths during night collections relative to daytime samples (CLMM, night coefficient = −0.284 ± 0.071, z = −4.02, p < 0.00005). However, we also detected main effects of infection status and fecundity as well as their interactions with time in determining host position. Among daytime samples, both infection status and fecundity positively influenced Daphnia depth (CLMMs, infection coefficient = 0.612 ± 0.172, z = 3.56, p < 0.001; gravid coefficient = 0.468 ± 0.091, z = 5.15, p < 0.00001). At night, however, infection status continued to positively affect depth whereas fecundity had a negative effect (CLMMs, infection coefficient = 0.476 ± 0.059, z = 8.054, p < 0.00001; gravid coefficient = −0.484 ± 0.046, z = −10.34, p < 0.000001). Thus, infected hosts occurred at greater depths both at night and during the day (Fig. 2), while Daphnia with eggs or ephippia were more likely to migrate to shallower waters at night. Results were similar among the random subset of individuals for which carapace length was measured, but with only a marginal influence of host size (CLMM, scaled [size] = 0.123 ± 0.069, z = 1.78, p = 0.08). In part, this was likely due to the correlations between size and both infection and fecundity; because infected or fecund hosts were larger than uninfected D. pulicaria without eggs, body size explained little additional variance in vertical position. Among infected hosts, more severely infected individuals were also more likely to be found deeper in the lake (CLMM, scaled [infection intensity] = 0.446 ± 0.074, z = 6.04, p < 0.00001; night coefficient = −1.999 ± 0.225, z = −8.901, p < 0.00001), without any additional influence of body size (scaled [size] = 0.049 ± 0.077, z = 0.65, p = 0.52).
Figure 2.
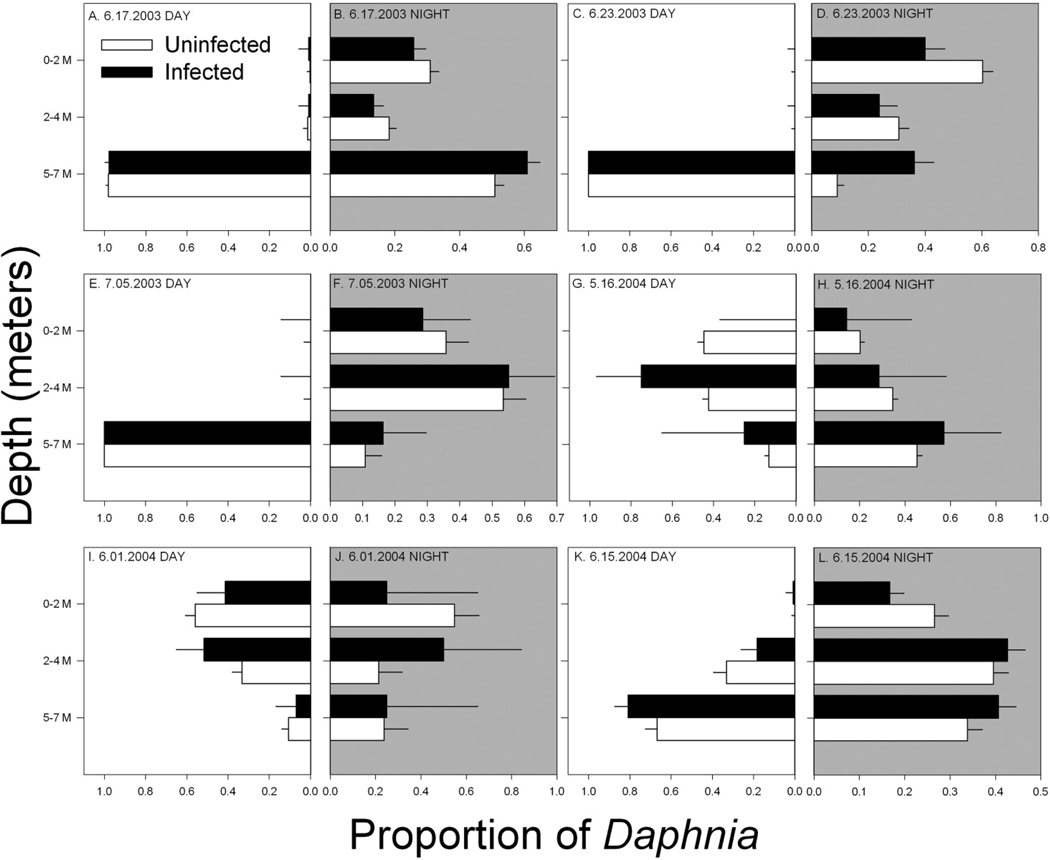
Vertical distribution of infected and uninfected D. pulicaria in Allequash Lake. For each sampling date and time (day vs. night), values represent the proportion of infected and uninfected hosts found in each depth (i.e., of the total number of (un)infected hosts collected on a given date, the fraction that occurred at a particular depth). Shaded plots represent samples collected at night. Error bars are the 95% upper confidence interval for a proportion.
Figure 3.
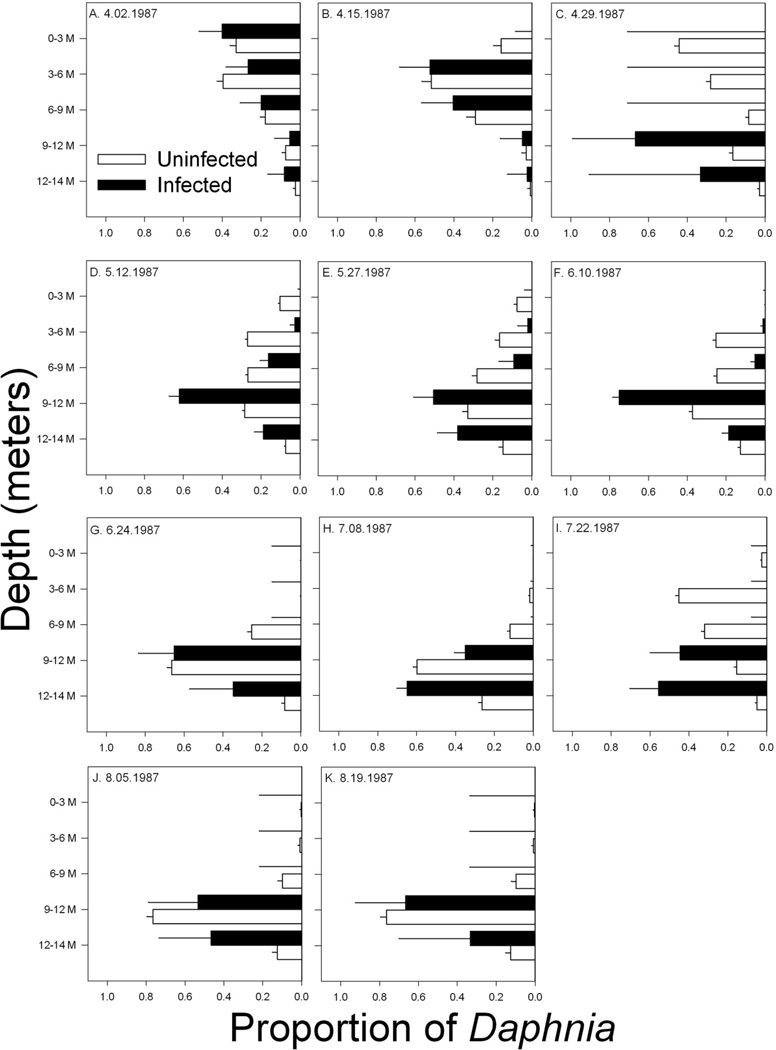
Vertical distribution of infected and uninfected D. pulicaria in Devil’s Lake. For each sampling date, values represent the proportion of infected and uninfected hosts found in each depth (i.e., of the total number of (un)infected hosts collected on a given date, the fraction that occurred at a particular depth). Samples were collected only during the daytime. Error bars are the 95% upper confidence interval for a proportion. No infection was detected in samples processed after August.
Based on the daytime zooplankton samples collected from Devil’s Lake in 1987, infected D. pulicaria were consistently overrepresented deeper in the water column (CLMM, infection coefficient = 1.297 ± 0.032, z = 40.0, p < 0.00001). Similarly, gravid females (almost none of which were infected) were also associated with lower depths (gravid coefficient = 0.543 ± 0.021, z = 25.3, p < 0.00001). For models of Devil’s Lake, we used the complementary log-log (cloglog) link, which had a lower AIC than models with the logistic link likely because infection prevalence was low in some samples. Within the subset of hosts on which carapace size measured, body size, infection status, and fecundity status all positively predicted Daphnia depth (CLMM, gravid coefficient = 0.294 ± 0.119, z = 2.46, p = 0.014; infection coefficient = 1.039 ± 0.091, z = 11.38, p < 0.00001; scaled [size] coefficient = 0.104 ± 0.045, z = 2.33, p = 0.02).
In the laboratory assays of swimming movements, infection status was similarly associated with a lower vertical position in the observation chambers (Fig. 4A). Over the course of 8 h, the vertical position of uninfected and infected hosts averaged 1.43 ± 0.08 and 0.85 ± 0.09, respectively (range of 0–6 above the bottom). Accounting for host individual and observation period as random effects, infection status had a strongly negative effect on position (CLMM, infection coefficient = −1.390 ± 0.345, z = −4.02, p < 0.0001) with a nonsignificant effect of host body size (size coefficient = 1.728 ± 0.764, z = 0.95, p = 0.34). Infection status also had a sharply negative influence on host swimming movements with no additional effect of host size (GLMM, infection coefficient = −0.306 ± 0.083, z = −3.81, p < 0.001; size coefficient = 0.254 ± 0.158, z = 1.61, p = 0.11) (Fig. 4B). Averaged across time periods and individuals, infected hosts exhibited 45.23 ± 2.82 movements per 30 s relative to 62.54 ± 1.62 for uninfected individuals. There was no evidence of overdispersion in the model.
Figure 4.
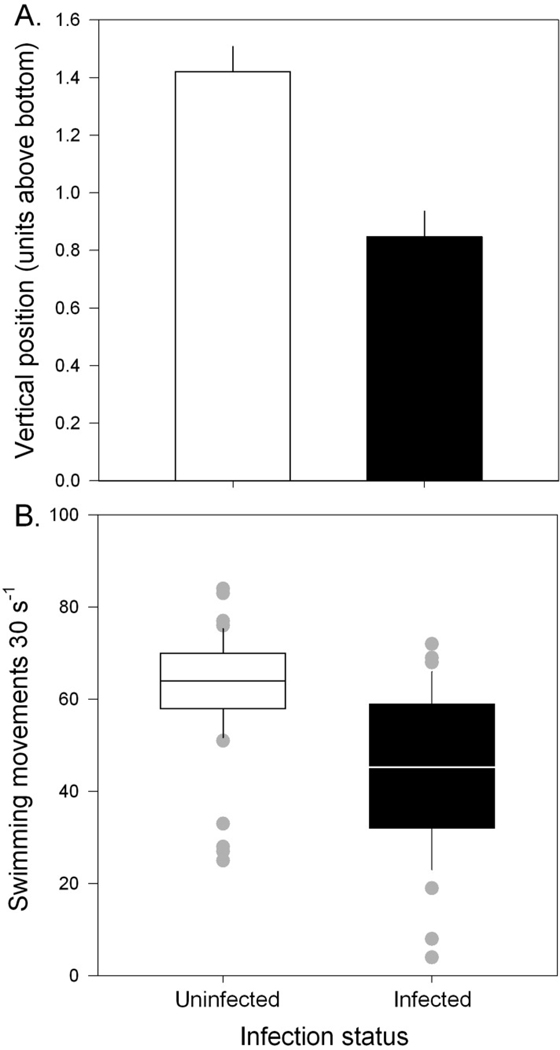
Laboratory assays to assess (A) host swimming movements per 30 s and (B) vertical position within observation chambers as a function of infection. Vertical position is measured as distance from the bottom of an observation chamber (range of 0 through 6). Error bars represent + 1 SE.
Discussion
Parasites can have striking effects on host behaviors and even alter extended migratory movements (e.g., Altizer et al. 2011), yet such changes are challenging to study owing to their sublethal nature, the difficulties in quantifying infection among living hosts, and broad intrinsic variation in behavior stemming from genetic and environmental signals (e.g., Moore 2002; Poulin 2010). Our results provide evidence that chytrid fungal infection alters D. pulicaria DVM behavior, which is among the most prominent and well-studied zooplankton responses in natural systems. Based on depth-specific samples collected over 2 yr from Allequash Lake, Daphnia with SCD exhibited sharply inhibited DVM relative to uninfected hosts; while all Daphnia were more likely to occupy deeper strata during the day, particularly as the season progressed, uninfected hosts and especially gravid females were much more likely to move to shallow waters at night. By contrast, infected Daphnia often remained deeper in the water column, such that infection prevalence at night increased with depth (Fig. 2). This result was not explained by alternative factors, such as host body size, fecundity status, or sampling date, which were also included in models. Further supporting this pattern, daytime collections of D. pulicaria from Devil’s Lake also revealed that infected Daphnia consistently occurred deeper than uninfected conspecifics despite the weaker influence of planktivory (Fig. 3).
Multiple mechanistic processes may underlie the observed shifts in DVM associated with SCD. For instance, if the strength of DVM behavior covaries genetically with susceptibility (or exposure) to infection, patterns of infection prevalence will correlate with water column depth—a hypothesis that requires experimental infection studies and genetic analyses (P. laeve has not been successfully transmitted under laboratory conditions). Decaestecker et al. (2002) showed that more negatively phototactic Daphnia clones with stronger DVM behavior experienced a lower risk from visual predators but a greater exposure to infectious parasites from the sediment (see also De Meester 1993). Moreover, visual predators will often preferentially consume infected Daphnia, in part owing to the increased conspicuousness association with many infections (e.g., Duffy et al. 2005; Johnson et al. 2006b). In mesocosm and field studies with SCD, fish predators were 2–5× more likely to eat infected Daphnia, particularly when water clarity was high (Johnson et al. 2006b). Selective predation could thus help explain the relative rarity of infected D. pulicaria in shallower waters from Devil’s Lake, for which samples were collected during daylight hours. For Allequash Lake, however, the disparity was more manifest at night when visual predation risk is expected to be low, and infected hosts also occupied lower (deeper) positions in laboratory trials relative to uninfected conspecifics, suggesting that predators alone are unlikely to drive the observed shift in DVM.
The inhibition of DVM behavior associated with P. laeve infection likely also stems from the parasite’s effects on host morbidity and energy availability. Previous work indicates that infected D. pulicaria exhibit lower growth, cessation of reproduction, and slower heart rates relative to healthy hosts (Johnson et al. 2006a; Forshay et al. 2008; Peñalva-Arana et al. 2011). Forshay et al. (2008) found that infected individuals had lower mass, less nitrogen and phosphorus per g of tissue, and reduced fatty acid concentrations compared with similarly-sized uninfected Daphnia. Thus, the relative rarity of infected hosts higher in the water column, regardless of the time of day, was likely driven—at least in part—from depleted energy reserves and an impaired ability to swim upward to shallower waters. Infected D. pulicaria rarely recover, and progressive replication of sporangia eventually fills much of the hemocoel before causing host death, which is required for the pathogen to release zoospores (Johnson et al. 2006a). Correspondingly, even after accounting for the difference between infected and uninfected hosts, the intensity of infection among infected Daphnia was a strong, positive predictor of their depth, such that heavily infected individuals—which are closer to parasite-induced mortality—were more likely to occur in deeper depths than individuals with low-intensity infections. We observed a similar over-representation of infected D. pulicaria at lower vertical positions within controlled laboratory assays, which lacked predators. Infected Daphnia exhibited 17.7% fewer swimming movements per 30 s, helping to explain their consistently lower position within observation chambers over the observation period.
Previous research on parasitism and DVM has reported altered or reversed vertical movement patterns in response to infection. In a laboratory study of diatoms (Akashiwo sanguinea) infected with a parasitic dinoflagellate, Park et al. (2002) found that late-stage infections reduced host swimming speed and inhibited phototaxis. Infected diatoms exhibited a much weaker or reversed vertical migratory pattern, which has the potential to alter the magnitude and spatial position of host blooms in natural ecosystems. In one of the few experimental studies of Daphnia DVM and parasites, Fels et al. (2004) evaluated how five microparasites affected the vertical position of the pond-dwelling daphnid, Daphnia magna. While there were no differences in position at night, infected individuals were often lower in the water column during the day. The authors suggested this change stemmed from increased fish avoidance by infected hosts, many of which are more conspicuous owing to infection (Willey et al. 1990; Willey and Willey 1993; Johnson et al. 2006b). In a study of Calanus copepods off the coast of Norway, by contrast, Torgersen et al. (2002) found that infection by the fungal pathogen Ichthyophonus was restricted almost exclusively to copepod hosts in the uppermost portion of the water column, which the authors hypothesized might increase predation by fishes and thereby enhance transmission. Similarly, altered behavioral patterns have been reported for acanthocephalans that depend on predation of the intermediate hosts for transmission (Bethel and Holmes 1973; McCahon et al. 1991).
Ecological studies of natural enemies have increasingly suggested that organisms experience risk tradeoffs between the threats of infection and predation (Rohr et al. 2009; Fritzsche and Allan 2012). In this proverbial “rock and a hard place” scenario, physiological or behavioral shifts by hosts/prey to mitigate one enemy may exacerbate the danger posed the other. Greater vertical migration by Daphnia to avoid fish predation can amplify host exposure to parasite “spore banks” from the sediment (Thiemann and Wassersug 2000; Decaestecker et al. 2002; Szuroczki and Richardson 2012). In a shallow lake such as Allequash, which has high planktivory as young-of-the-year yellow perch become large enough to consume D. pulicaria, this tradeoff of threats could become pronounced. In deeper lakes such as Devil’s, for instance, which tended to have less infection, differences in the vertical position of infected and uninfected Daphnia were evident even during the day. However, as stratification intensifies later in the season and the hypolimnion becomes more hypoxic, transmission is expected to weaken as hosts spend less time close to the sediment. A long-term (15 yr) analysis of SCD epizootics in Devil’s Lake identified stratification strength as among the most important drivers of seasonal infection peaks, which often exhibited local maxima corresponding with spring and fall mixing events with comparatively little infection in late summer (Johnson et al. 2009).
Recent investigations focused at the nexus of disease, host behavior, and animal migratory movements have illustrated the diversity of mechanisms through which these topics interact (e.g., Altizer et al. 2011). In some cases, the mass movement of hosts during migrations can redistribute pathogens over long distances, whereas in others migratory movements allow hosts to “escape” infections that accumulate in residential populations (Poulin et al. 2012; Satterfield et al. 2015). If infections cause significant host morbidity or mortality, disease can also “cull” the number of hosts able to complete a migration (Bartel et al. 2011). Although less research has explored the effects of disease on fine-scale migratory behaviors (e.g., between habitats), parasite-induced changes in zooplankton vertical migration could have significant implications for parasite transmission or ecological processes in aquatic ecosystems. For instance, while models of transmission typically focus on overall host density, reduced migrations by infected Daphnia could inhibit infection by creating greater spatial separation from susceptible hosts, independent of changes in density. Alternatively, they could enhance transmission by limiting the selective removal of infected hosts by fish predators [although our own observations indicate that P. laeve sporangia remain viable even following digestion (Johnson et al. 2006b), see also Duffy 2009]. Finally, because diel vertical movements by Daphnia affect the translocation of organic matter, essential nutrients, and energy between habitats (Vanni et al. 2002; Haupt et al. 2009, 2010), parasite-induced reduction in DVM have the potential to also reduce the mixing of materials within lakes, inhibit the capacity of grazers to regulate algal blooms, and decrease the availability of zooplankton for consumption by predators, including young-of-the-year fishes (Brooks and Dodson 1965; Carpenter et al. 1987). Altered patterns of vertical migration could thus shorten food chain length and shunt energetic resources toward the benthos through the greater downward movement of moribund and dying hosts, as also documented for viral-induced mortality of marine bacteria and the resulting acceleration in oceanic carbon cycling (Middelboe et al. 2003). Considering the often-high prevalence of SCD in these lakes, which was ∼ 20% overall and ranged as high as 53%, observed differences in migratory behavior affect a substantial component of pelagic food web biomass, underscoring the potential of disease epizootics to have large-scale effects on within-lake cycling of energy and nutrients (Preston et al. 2016).
Supplementary Material
Acknowledgments
This project benefited enormously from the resources and logistical support provided by Stephen Carpenter and the North Temperate Lakes Long-Term Ecological Research project, including direct help from Tim Kratz, Pam Montz, and the Trout Lake Field Station staff. For assistance with collecting samples, we thank Eric Preu for his tireless support and commitment. We also thank Julia Logeman, James Ellwein, and Melanie Stock for their efforts to help process collected samples and Richard Lathrop for his generosity in sharing collected samples from Devil’s Lake. Stanley Dodson, Michael Sierszen, and two anonymous reviewers offered conceptual and editorial feedback helpful in shaping the project. This research was funded, in part, through grants from the National Science Foundation (Graduate Research Fellowship Program to PTJJ and the Long-Term Ecological Research [LTER] programs for North Temperate Lakes [DEB 1440297] and Niwot Ridge [DEB 1027341]), a fellowship from the Birge and Juday families, and support from the David and Lucile Packard Foundation. Although an EPA employee contributed to this article, the research presented was not performed or funded by and was not subject to EPA’s quality system requirements. Consequently, the views, interpretations, and conclusions expressed in the article are solely those of the authors and do not necessarily reflect or represent EPA’s views or policies.
Footnotes
Conflict of Interest
None declared.
References
- Adelman JS, Wikelski MC, and Hau M. 2009. Sickness behavior and fever vary among free-living sparrows along a life history gradient. Integr. Comp. Biol. 49: E1–E190. doi: 10.1093/icb/icp002 [DOI] [Google Scholar]
- Altizer S, Bartel R, and Han BA. 2011. Animal migration and infectious disease risk. Science 331: 296–302. doi: 10.1126/science.1194694 [DOI] [PubMed] [Google Scholar]
- Andersen SB, Gerritsma S, Yusah KM, Mayntz D, Hywel-Jones NL, Billen J, Boomsma JJ, and Hughes DP. 2009. The life of a dead ant: The expression of an adaptive extended phenotype. Am. Nat. 174: 424–433. doi: 10.1086/603640 [DOI] [PubMed] [Google Scholar]
- Bartel RA, Oberhauser KS, de Roode JC, and Altizer SM. 2011. Monarch butterfly migration and parasite transmission in eastern North America. Ecology 92: 342–351. doi: 10.1890/10-0489.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behringer DC, Butler MJ, and Shields JD. 2006. Avoidance of disease by social lobsters. Nature 441: 421. doi: 10.1038/441421a [DOI] [PubMed] [Google Scholar]
- Bethel WM, and Holmes JC. 1973. Altered evasive behavior and responses to light in amphipods harboring acanthocephalan cystacanths. J. Parasitol. 59: 945–956. doi: 10.2307/3278623 [DOI] [PubMed] [Google Scholar]
- Bittner K, Rothhaupt KO, and Ebert D. 2002. Ecological interactions of the microparasite Caullerya mesnili and its host Daphnia galeata. Limnol. Oceanogr. 47: 300–305. doi: 10.4319/lo.2002.47.1.0300 [DOI] [Google Scholar]
- Boltana S, and others. 2013. Behavioural fever is a synergic signal amplifying the innate immune response. P. R. Soc. B Biol. Sci. 280: 20131381. doi: 10.1098/rspb.2013.1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambilla DJ 1983. Microsporidiosis in a Daphnia pulex population. Hydrobiologia 99: 175–188. doi: 10.1007/BF00008769 [DOI] [Google Scholar]
- Brooks JL, and Dodson SI. 1965. Predation body size and composition of plankton. Science 150: 28–35. doi: 10.1126/science.150.3692.28 [DOI] [PubMed] [Google Scholar]
- Caceres CE, Tessier AJ, Duffy MA, and Hall SR. 2014. Disease in freshwater zooplankton: What have we learned and where are we going? J. Plankton Res. 36: 326–333. doi: 10.1093/plankt/fbt136 [DOI] [Google Scholar]
- Carpenter SR, and others. 1987. Regulation of lake primary productivity by food web structure. Ecology 68: 1863–1876. doi: 10.2307/1939878 [DOI] [PubMed] [Google Scholar]
- Chae J, and Nishida S. 1995. Vertical distribution and diel migration in the iridescent copepods of the family Sapphirinidae: a unique example of reverse migration?. Marine Ecology Progress Series 119: 111–124. [Google Scholar]
- Christensen RHB 2015. ordinal: Regression models for ordinal data. R package version 2015.6–28. Available from http://www.cran.r-project.org/package=ordinal. Accessed on August 24, 2017. [Google Scholar]
- Daly EW, and Johnson PTJ. 2011. Beyond immunity: Quantifying the effects of host anti-parasite behavior on parasite transmission. Oecologia 165: 1043–1050. doi: 10.1007/s00442-010-1778-y [DOI] [PubMed] [Google Scholar]
- De Meester L 1993. Genotype, fish-mediated chemicals, and phototactic behavior in Daphnia magna. Ecology 74: 1467–1474. doi: 10.2307/1940075 [DOI] [Google Scholar]
- Decaestecker E, De Meester L, and Ebert D. 2002. In deep trouble: Habitat selection constrained by multiple enemies in zooplankton. Proc. Natl. Acad. Sci. USA. 99: 5481–5485. doi: 10.1073/pnas.082543099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy MA, Hall SR, Tessier AJ, and Huebner M, 2005. Selective predators and their parasitized prey: Are epidemics in zooplankton under top-down control?. Limnol. Oceanogr. 50: 412–420. [Google Scholar]
- Duffy MA 2009. Staying alive: The post-consumption fate of parasite spores and its implications for disease dynamics. Limnol. Oceanogr. 54: 770–773. doi: 10.4319/lo.2009.54.3.0770 [DOI] [Google Scholar]
- Duffy MA, Hall SR, Caceres CE, and Ives AR. 2009. Rapid evolution, seasonality, and the termination of parasite epidemics. Ecology 90: 1441–1448. doi: 10.1890/08-1130.1 [DOI] [PubMed] [Google Scholar]
- Ebert D 2000. Experimental evidence for rapid parasite adaptation and its consequences for the evolution of virulence. Evolutionary Biology of Host-Parasite Relationships: Theory Meets Reality 32: 163–184. [Google Scholar]
- Ebert D 2005. Ecology, epidemiology, and evolution of parasitism in Daphnia National Library of Medicine; (US: ), National Center for Biotechnology Information. [Google Scholar]
- Ebert D, Hottinger JW, and Pajunen VI. 2001. Temporal and spatial dynamics of parasite richness in a Daphnia metapopulation. Ecology 82: 3417–3434. doi: 10.2307/2680162 [DOI] [Google Scholar]
- Fels D, Lee VA, and Ebert D. 2004. The impact of microparasites on the vertical distribution of Daphnia magna. Arch. Hydrobiol. 161: 65–80. doi: 10.1127/0003-9136/2004/0161-0065 [DOI] [Google Scholar]
- Forshay KJ, Johnson PTJ, Stock M, Penalva C, and Dodson SI. 2008. Festering food: Chytridiomycete pathogen reduces quality of Daphnia host as a food resource. Ecology 89: 2692–2699. doi: 10.1890/07-1984.1 [DOI] [PubMed] [Google Scholar]
- Fritzsche A, and Allan BF. 2012. The ecology of fear: Host foraging behavior varies with the spatio-temporal abundance of a dominant ectoparasite. Ecohealth 9: 70–74. doi: 10.1007/s10393-012-0744-z [DOI] [PubMed] [Google Scholar]
- Green J 1974. Parasites and epibionts of Cladocera. Trans. R. Soc. Lond. 32: 417–515. doi: 10.1111/j.1096-3642.1974.tb00031.x [DOI] [Google Scholar]
- Hall SR, Becker CR, Duffy MA, and Caceres CE. 2011. Epidemic size determines population-level effects of fungal parasites on Daphnia hosts. Oecologia 166: 833–842. doi: 10.1007/s00442-011-1905-4 [DOI] [PubMed] [Google Scholar]
- Haupt F, Stockenreiter M, Baumgartner M, Boersma M, and Stibor H. 2009. Daphnia diel vertical migration: Implications beyond zooplankton. J. Plankton Res. 31: 515–524. doi: 10.1093/plankt/fbp003 [DOI] [Google Scholar]
- Haupt F, Stockenreiter M, Reichwaldt ES, Baumgartner M, Lampert W, Boersma M, and Stibor H. 2010. Upward phosphorus transport by Daphnia diel vertical migration. Limnol. Oceanogr. 55: 529–534. doi: 10.4319/lo.2009.55.2.0529 [DOI] [Google Scholar]
- Hawley DM, and Altizer SM. 2011. Disease ecology meets ecological immunology: Understanding the links between organismal immunity and infection dynamics in natural populations. Funct. Ecol. 25: 48–60. doi: 10.1111/j.1365-2435.2010.01753.x [DOI] [Google Scholar]
- Hays GC 2003. A review of the adaptive significance and ecosystem consequences of zooplankton diel vertical migrations. Hydrobiologia 503: 163–170. doi: 10.1023/B:HYDR.0000008476.23617.b0 [DOI] [Google Scholar]
- Johnson PTJ, Longcore JE, Stanton DE, Carnegie RB, Shields JD, and Preu ER. 2006a. Chytrid infections of Daphnia pulicaria: Development, ecology, pathology and phylogeny of Polycaryum laeve. Freshw. Biol. 51: 634–648. doi: 10.1111/j.1365-2427.2006.01517.x [DOI] [Google Scholar]
- Johnson PTJ, Stanton DE, Preu ER, Forshay KJ, and Carpenter SR. 2006b. Dining on disease: How interactions between infection and environment affect predation risk. Ecology 87: 1973–1980. doi: 10.1890/0012-9658(2006)87[1973:DODHIB]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Johnson PTJ, Ives AR, Lathrop RC, and Carpenter SR. 2009. Long-term disease dynamics in lakes: Causes and consequences of chytrid infections in Daphnia populations. Ecology 90: 132–144. doi: 10.1890/07-2071.1 [DOI] [PubMed] [Google Scholar]
- Kennedy CR, Broughton PF, and Hine PM. 1978. Status of brown and rainbow trout, Salmo trutta and S. gairdneri as hosts of acanthocephalan, Pomphorhynchus laevis. J. Fish Biol. 13: 265–275. doi: 10.1111/j.1095-8649.1978.tb03434.x [DOI] [Google Scholar]
- Kiesecker JM, Skelly DK, Beard KH, and Preisser E. 1999. Behavioral reduction of infection risk. Proc. Natl. Acad. Sci. USA. 96: 9165–9168. doi: 10.1073/pnas.96.16.9165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafferty KD, and Morris AK. 1996. Altered behavior of parasitized killifish increases susceptibility to predation by bird final hosts. Ecology 77: 1390–1397. doi: 10.2307/2265536 [DOI] [Google Scholar]
- Lampert W 1989. The adaptive significance of diel vertical migration of zooplankton. Funct. Ecol. 3: 21–27. doi: 10.2307/2389671 [DOI] [Google Scholar]
- Lathrop RC, Carpenter SR, Stow CA, Soranno PA, and Panuska JC. 1998. Phosphorus loading reductions needed to control blue-green algal blooms in Lake Mendota. Can. J. Fish. Aquat. Sci. 55: 1169–1178. doi: 10.1139/cjfas-55-5-1169 [DOI] [Google Scholar]
- Lefcort H, and Blaustein AR. 1995. Disease, predator avoidance, and vulnerability to predation in tadpoles. Oikos 74: 469–474. doi: 10.2307/3545992 [DOI] [Google Scholar]
- Lefevre T, Adamo SA, Biron DG, Misse D, Hughes D, and Thomas F. 2009. Invasion of the body snatchers: The diversity and evolution of manipulative strategies in host-parasite interactions. Adv. Parasitol. 68: 45–83. doi: 10.1016/S0065-308X(08)00603-9 [DOI] [PubMed] [Google Scholar]
- Lillie RA, and Mason JW. 1986. Historical changes in water quality and biota of Devils Lake, Sauk County, 1866–1985. Trans. Wis. Acad. Sci. Arts Lett. 74: 81–104. [Google Scholar]
- McCahon CP, Maund SJ, and Poulton MJ. 1991. The effect of the acanthocephalan parasite (Pomphorhynchus laevis) on the drift of Its intermediate host (Gammarus pulex). Freshw. Biol. 25: 507–513. doi: 10.1111/j.1365-2427.1991.tb01393.x [DOI] [Google Scholar]
- Middelboe M, Riemann L, Steward GF, Hansen V, and Nybroe O. 2003. Virus-induced transfer of organic carbon between marine bacteria in a model community. Aquat. Microb. Ecol. 33: 1–10. doi: 10.3354/ame033001 [DOI] [Google Scholar]
- Moore J 2002. Parasites and the behavior of animals. Oxford Univ. Press. [Google Scholar]
- Park MG, Cooney SK, Kim JS, and Coats DW. 2002. Effects of parasitism on diel vertical migration, phototaxis/geotaxis, and swimming speed of the bloom-forming dinoflagellate Akashiwo sanguinea. Aquat. Microb. Ecol. 29: 11–18. doi: 10.3354/ame029011 [DOI] [Google Scholar]
- Peñalva-Arana DC, Forshay K, Johnson PTJ, Strickler JR, and Dodson SI. 2011. Chytrid infection reduces thoracic beat and heart rate of Daphnia pulicaria. Hydrobiologia 668: 147–154. [Google Scholar]
- Poulin R 2010. Parasite manipulation of host behavior: An update and frequently asked questions. Adv. Study Behav. 41: 151–186. doi: 10.1016/S0065-3454(10)41005-0 [DOI] [Google Scholar]
- Poulin R, Closs GP, Lill AWT, Hicks AS, Herrmann KK, and Kelly DW. 2012. Migration as an escape from parasitism in New Zealand galaxiid fishes. Oecologia 169: 955–963. doi: 10.1007/s00442-012-2251-x [DOI] [PubMed] [Google Scholar]
- Preston DL, Mischler JA, Townsend AR, and Johnson PTJ. 2016. Disease ecology meets ecosystem science. Ecosystems 19: 737–748. doi: 10.1007/s10021-016-9965-2 [DOI] [Google Scholar]
- Ringelberg J 2010. Diel vertical migration of zooplankton in lakes and oceans: Causal explanations and adaptive significances, p. 1–356. In Ringelberg J [ed.] Diel vertical migration of zooplankton in lakes and oceans: Causal explanations and adaptive significances. London NY: Springer Science & Business Media. [Google Scholar]
- Rohr JR, Swan A, Raffel TR, and Hudson PJ. 2009. Parasites, info-disruption, and the ecology of fear. Oecologia 159: 447–454. doi: 10.1007/s00442-008-1208-6 [DOI] [PubMed] [Google Scholar]
- Satterfield DA, Maerz JC, and Altizer S. 2015. Loss of migratory behaviour increases infection risk for a butterfly host. Proc. R. Soc. B Biol. Sci. 282: 20141734. doi: 10.1098/rspb.2014.1734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakhar K, and Shakhar G. 2015. Why do we feel sick when infected: Can altruism play a role? PLoS Biol. 13: e1002276. doi: 10.1371/journal.pbio.1002276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stempell W 1903. Beiträge zur Kenntnis der Gattung Polycaryum. Arch. Protistenkunde 2: 349–363. [Google Scholar]
- Szuroczki D, and Richardson JML. 2012. The behavioral response of larval amphibians (Ranidae) to threats from predators and parasites. PLoS One 7: e495492. doi: 10.1371/journal.pone.0049592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiemann GW, and Wassersug RJ. 2000. Biased distribution of trematode metacercariae in the nephric system of Rana tadpoles. J. Zool. 252: 534–538. doi: 10.1111/j.1469-7998.2000.tb01236.x [DOI] [Google Scholar]
- Torgersen T, Karlsbakk E, and Kaartvedt S. 2002. Deviating vertical distribution and increased conspicuousness of parasitized Calanus. Limnol. Oceanogr. 47: 1187–1191. doi: 10.4319/lo.2002.47.4.1187 [DOI] [Google Scholar]
- Vanni MJ, Flecker AS, Hood JM, and Headworth JL. 2002. Stoichiometry of nutrient recycling by vertebrates in a tropical stream: Linking species identity and ecosystem processes. Ecol. Lett. 5: 285–293. doi: 10.1046/j.1461-0248.2002.00314.x [DOI] [Google Scholar]
- Venables W, and Ripley B. 2002. Modern applied statistics with S, 4th ed Springer. [Google Scholar]
- Willey RL, Cantrell PA, and Threlkeld ST. 1990. Epibiotic euglenoid flagellates increase the susceptibility of some zooplankton to fish predation. Limnol. Oceanogr. 35: 952–959. doi: 10.4319/lo.1990.35.4.0952 [DOI] [Google Scholar]
- Willey RL, and Willey RB. 1993. Planktivore effects on zooplankton epibiont communities - epibiont pigmentation effects. Limnol. Oceanogr. 38: 1818–1822. doi: 10.4319/lo.1993.38.8.1818 [DOI] [Google Scholar]
- Yan ND, and Larsson JIR. 1988. Prevalence and inferred effects of microsporidia of Holopedium gibberum (Crustacea, Cladocera) in a Canadian shield lake. J. Plankton Res. 10: 875–886. doi: 10.1093/plankt/10.5.875 [DOI] [Google Scholar]
- Zimmer C 2000. Parasite rex: Inside the bizarre world of nature’s most dangerous creatures. Simon & Schuster. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


