To the Editor:
Prurigo nodularis (PN) is an extremely pruritic, inflammatory skin disease associated with multiple underlying comorbidities.1 Case reports have noted an association between PN and malignancies, including lymphoma2,3 and solid organ tumors.4 The goal of this cross-sectional study was to evaluate an association between PN and a variety of malignancies in a diverse patient population.
Institutional review board approval was waived for this study because only anonymous aggregate-level data were used. The study population consisted of 695 patients aged 40–69 years who presented to the Johns Hopkins Health System during 2013–2017 with a PN diagnosis. They were compared with 2,446,880 control patients also aged 40–69 years (Table I) because this age range is the peak for PN occurrence.1 Patients with a diagnosed malignancy were identified within the PN and control groups, and odds ratios (ORs) were calculated. P values were calculated by using χ2 statistics with 1 degree of freedom. A Bonferroni correction was applied (alpha = 0.002). For malignancies of the genital tracts, only patients of the relevant sex were included.
Table I.
Demographic characteristics of the PN group and comparison group without PN
| Characteristic | PN group, n = 695, % | Control group, n = 2,454,685, % |
|---|---|---|
| Sex | ||
| Male | 48.35 | 45.42 |
| Female | 51.65 | 54.58 |
| Race | ||
| White, Caucasian | 41.44 | 60.98 |
| Black, African American | 51.37 | 22.13 |
| Asian | 2.59 | 3.81 |
| American Indian or Alaskan native | 0.14 | 0.21 |
| Native Hawaiian, other | 0.14 | 0.05 |
| Pacific Islander | ||
| Other | 4.32 | 6.31 |
| Unknown | 1.15 | 8.13 |
| Declined to answer | 0.14 | 0.21 |
| Age, y | ||
| 40–49 | 25.61 | 32.85 |
| 50–59 | 44.93 | 36.34 |
| 60–69 | 29.46 | 30.80 |
In total, 695 patients aged 40–69 years who presented to Johns Hopkins Health System during April 2013-December 2017 with a visit diagnosis, billing diagnosis, or active problem list entry of PN were identified. The comparison population was 2,446,880 patients aged 40–69 years without a diagnosis of PN.
PN, Prurigo nodularis.
Of the 695 patients with PN, 124 had a concomitant diagnosis of malignancy during the study period (Fig 1, A). Patients with PN were >4 times more likely than controls to have a malignancy diagnosis (OR 4.54, 95% confidence interval [CI] 3.74–5.52). PN was significantly associated with cancers of the skin (OR 10.94, 95% CI 8.01–14.96), hematopoietic system (OR 5.41, 95% CI 3.39–8.64), and solid organs (OR 3.44, 95% CI 2.34–5.06) (Fig 1, B). Among the hematologic malignancies, PN was most strongly associated with primary cutaneous lymphoma (OR 24.82, 95% CI 7.96–77.46), multiple myeloma (OR 6.55, 95% CI 2.45–17.51), and non-Hodgkin lymphoma (OR 5.66, 95% CI 2.82–11.38). Among solid organ malignancies, PN was most strongly associated with cancers of the female genital organs (OR 11.00, 95% CI 4.90–24.68), gastrointestinal tract (OR 5.08, 95% CI 2.11–12.26), and lung (OR 4.63, 95% CI 1.92–11.16).
Fig 1.
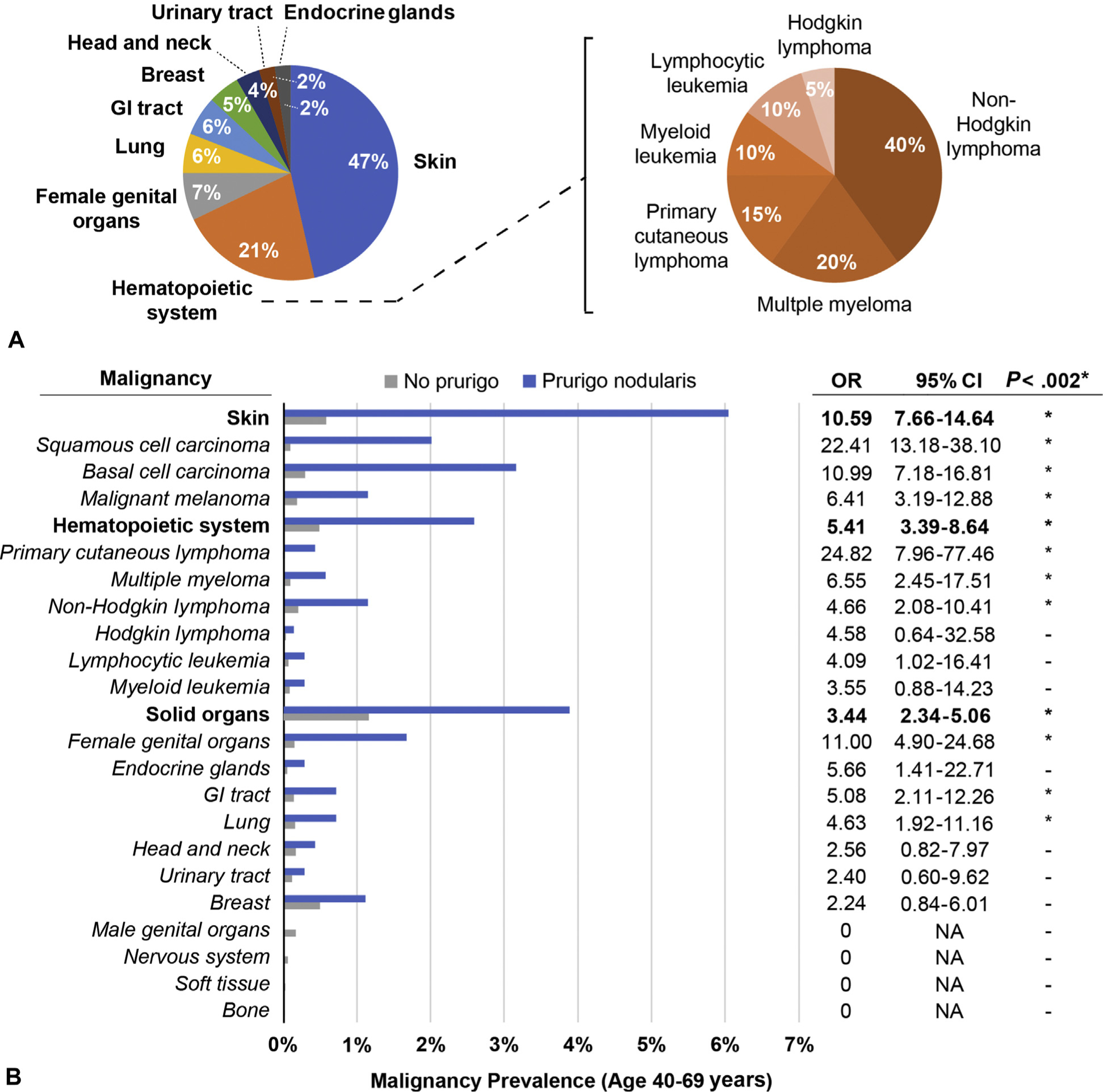
Malignancies in prurigo nodularis (PN) patients. A, Distribution of 124 malignancies diagnosed in PN patients during the 5-year study period, by anatomic origin. Hematopoietic system malignancies are further subdivided by disease entity. B, Prevalence of different malignancy categories in PN patients (blue) and patients without PN (gray) in the Johns Hopkins Health System population. ORs and 95% CIs for each malignancy type are tabulated on the right. Row section heads are indicated in bold *P < .002 (χ2 test). †Noncutaneous. CI, Confidence interval; GI, gastrointestinal; NA, not applicable; OR, odds ratio; PN, prurigo nodularis.
There are several limitations to this study. There might be a selection bias for the detection of dermatologic malignancies because PN patients are more likely to be under the care of dermatologists. Also, we cannot draw conclusions about the causal relationship between PN and malignancy from these data. However, case reports have shown that PN can improve with cancer treatment,2–4 suggesting that PN might be downstream. In the case of hematologic malignancies, PN might be the result of inflammatory infiltration of the skin, leading to pruritus and excoriation. The mechanisms by which other malignancies predispose patients to PN remain unclear but might be related to systemic inflammatory states.
We conclude that PN might be associated with a wide variety of malignancies. The time course of the association and the risk-to-benefit ratio of cancer screening in this patient population requires further investigation, but cohort studies of generalized pruritus have suggested that the risk of cancer diagnosis is highest in the first 3 months after onset.5 At minimum, clinicians caring for PN patients should be vigilant for signs or symptoms suggestive of malignancy and ensure that these patients receive all recommended age-appropriate cancer screening.
Funding sources:
None.
Conflicts of interest: Dr Miller has received grant support from AstraZeneca, MedImmune (a subsidiary of AstraZeneca), Pfizer, Boerhinger Ingelheim, Regeneron Pharmaceuticals, Moderna Therapeutics; is a shareholder of Noveome Biotherapeutics; and is on the scientific advisory board of Integrated Biotherapeutics, which are all unrelated to the work reported in this article. Dr Kwatra is an advisory board member for Menlo and Trevi Therapeutics. Ms Larson, Ms Tang, Dr Stander, and Dr Kang have no conflicts of interest to disclose.Reprints not available from the authors.
REFERENCES
- 1.Boozalis E, Tang O, Patel S, et al. Ethnic differences and comorbidities of 909 prurigo nodularis patients. J Am Acad Dermatol. 2018. pii: S0190(18):30655–30658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schweda K, Hainz M, Loquai C, Grabbe S, Saloga J, Tuettenberg A. Prurigo nodularis as index symptom of (non-Hodgkin) lymphoma: ultrasound as a helpful diagnostic tool in dermatological disorders of unknown origin. Int J Dermatol. 2015;54(4):462–464. [DOI] [PubMed] [Google Scholar]
- 3.Rubenstein M, Duvic M. Cutaneous manifestations of Hodgkin’s disease. Int J Dermatol. 2006;45(3):251–256. [DOI] [PubMed] [Google Scholar]
- 4.Funaki M, Ohno T, Dekio S, et al. Prurigo nodularis associated with advanced gastric cancer: report of a case. J Dermatol. 1996;23(10):703–707. [DOI] [PubMed] [Google Scholar]
- 5.Johannesdottir SA, Farkas DK, Vinding GR, et al. Cancer incidence among patients with a hospital diagnosis of pruritus: a nation-wide Danish cohort study. Br J Dermatol. 2014;171(4):839–846. [DOI] [PubMed] [Google Scholar]


