Abstract
Tidal freshwater marshes can protect downstream ecosystems from eutrophication by intercepting excess nutrient loads, but recent studies in salt marshes suggest nutrient loading compromises their structural and functional integrity. Here, we present data on changes in plant biomass, microbial biomass and activity, and soil chemistry from plots in a tidal freshwater marsh on the Altamaha River (GA) fertilized for 10 yr with nitrogen (+N), phosphorus (+P), or nitrogen and phosphorus (+NP). Nitrogen alone doubled aboveground biomass and enhanced microbial activity, specifically rates of potential nitrification, denitrification, and methane production measured in laboratory incubations. Phosphorus alone increased soil P and doubled microbial biomass but did not affect microbial processes. Nitrogen or P alone decreased belowground biomass and soil carbon (C) whereas +NP increased aboveground biomass, microbial biomass and N cycling, and N, P, and C assimilation and burial more than either nutrient alone. Our findings suggest differential nutrient limitation of tidal freshwater macrophytes by N and microbes by P, similar to what has been observed in salt marshes. Macrophytes outcompete microbes for P in response to long-term N and P additions, leading to increased soil C storage through increased inputs of belowground biomass relative to N and P added singly. The susceptibility of tidal freshwater marshes to long-term nutrient enrichment and, hence their ability to mitigate eutrophication will depend on the quantity and relative proportion of N vs. P entering estuaries and tidal wetlands.
Tidal freshwater marshes occur at the intersection of riverine freshwater and marine tides. They exist in high-energy, high-exchange environments, but do not experience the ionic or sulfide-induced changes in biogeochemistry associated with seawater. They tend to have higher species diversity, higher primary productivity, and lower decomposition rates, leading to higher rates of carbon (C) burial than their brackish and saline counterparts (Odum 1988; Hopkinson 1992; Craft 2007; Loomis and Craft 2010; Wieski et al. 2010). Tidal freshwater marshes also tend to have higher rates of nitrogen (N) removal via both denitrification and N burial (Craft 2007; Craft et al. 2009), and higher rates of phosphorus (P) removal via sorption and burial than more saline tidal marshes (Sundareshwar and Morris 1999; Craft 2007; Loomis and Craft 2010). Despite the high levels of ecosystem services provided by tidal freshwater marshes, they are less well studied than brackish and saline marshes (but see Barendregt et al. 2009), especially with regard to anthropogenic disturbances such as nutrient loading (Shifflet and Schubauer-Berigan 2019).
Human activities have accelerated the riverine export of dissolved reactive N and P to the coastal zone. The resulting widespread hypoxia, harmful algal blooms, and habitat loss negatively impact economically valuable natural resources, illustrated by the repeated closures of valuable fin- and shell-fisheries, popular swimming beaches, and other tourist destinations (Smith et al. 2006; Verhoeven et al. 2006; Selman et al. 2008; Rabalais et al. 2009). While tidal freshwater marshes are poised to intercept these excess nutrients as they enter estuaries, studies in salt marshes reveal that the consequences of these nutrient removal processes include increased organic matter decomposition and reduced biomass allocation to roots and rhizomes (Turner et al. 2009; Deegan et al. 2012). There is growing concern that increased nutrients may compromise the ability of coastal wetlands to accumulate the organic matter needed to maintain soil strength and elevation in the face of erosion and rising sea levels (Morris et al. 2013).
Belowground biomass contributes to the physical strength of marsh soils (Deegan et al. 2012) and is the primary contributor to soil organic matter (SOM) (Craft 2007; Neubauer 2008). While increased fertilization enhances aboveground productivity in both tidal salt (Sundareshwar et al. 2003; Crain 2007; Deegan et al. 2007, 2012; Langley et al. 2013) and fresh marshes (Crain 2007; Frost et al. 2009; Ket et al. 2011), there is considerable debate on how increased nutrients affect belowground biomass (Shifflet and Schubauer-Berigan 2019). Some studies report that increased nutrient loads increase belowground biomass (Haines 1979; Buresh et al. 1980). However, many others report decreases in belowground biomass (Morris and Bradley 1999; Darby and Turner 2008a,b; Ket et al. 2011; Deegan et al. 2012; Graham and Mendelssohn 2014; Valiela 2015). Changes in belowground biomass in particular can impact microbial metabolism as the delivery of C and oxygen to the soil profile are positively related to root biomass (Mueller et al. 2016).
Fertilization can also directly affect microbial metabolism. Because nitrate () reduction, or denitrification, is the most energetically efficient anaerobic microbial mineralization pathway, increased availability can accelerate organic matter mineralization in tidal freshwater marshes (Caffrey et al. 2007; Dodla et al. 2009; Deegan et al. 2012). Increased nutrient loads can also alleviate stoichiometric constraints on microbial growth and activity by enhancing nutrient availability and the quality (C : N ratio) of organic matter (Paludan and Blicher-Mathiesen 1996; Morris and Bradley 1999; Caffrey et al. 2007; Deegan et al. 2007; Koop-Jakobsen and Giblin 2010). Several saltmarsh studies have reported that marsh eutrophication decreases SOM, soil strength, or soil volume (Turner et al. 2009; Kearney et al. 2011; Deegan et al. 2012), while others report no effect (Anisfeld and Hill 2011) and yet others report positive effects of nutrients on soil accumulation (Morris et al. 2002). The applicability of saltmarsh studies to tidal freshwater marshes is limited because tidal freshwater marshes are dominated by markedly different biogeochemical processes, have more heterogeneous and productive macrophyte communities compared to saltmarshes, and tend to rely more on organic matter to maintain elevation (Odum 1988; Sundareshwar and Morris 1999; Neubauer 2008; Koop-Jakobsen and Giblin 2009).
Nutrient enrichment can alter C, N, and P cycling rates and the efficiency of tidal freshwater marshes as C and nutrient sinks. Much like saltmarshes, the large stocks of SOM in freshwater marshes are vulnerable to enhanced mineralization via denitrification (Koop-Jakobsen and Giblin 2010; Deegan et al. 2012). Unlike saltmarshes, tidal freshwater marshes emit large amounts of methane (CH4), a greenhouse gas with a global warming potential 25–45 times that of CO2 (Poffenbarger et al. 2011; Neubauer and Megonigal 2015). While increased denitrification may increase CO2 emissions, these emissions may be offset by reduced CH4 production as methanogens are outcompeted for C substrates by denitrifiers (Dodla et al. 2009; Helton et al. 2014).
Although marshes have been shown to have a high capacity to remove and retain excess N through biological processes like denitrification and organic matter burial (Valiela et al. 1973; Chalmers 1979; Gribsholt et al. 2005, 2006; Deegan et al. 2012), P removal capacity tends to be strongly related to the physicochemical properties of soils (Bridgham et al. 2001). Concentrations of extractable iron and aluminum dictate the number of sites available for P sorption, and P sorption sites can become saturated over time (Richardson 1985; Reddy and DeLaune 2008). Enhanced macrophyte productivity under high nutrient loads can enhance P burial by increasing inorganic sediment trapping (Morris et al. 2002, 2013) or increasing P burial as organic matter (Craft and Richardson 1993a,b).
Eutrophication induces changes in plant and microbial communities and the complex interaction between these communities mediates ecosystem responses to disturbance (Wardle et al. 2004; Sutton-Grier and Megonigal 2011; Bardgett et al. 2013). There is evidence that fertilization has both direct impacts on nutrient cycling, dictated by direct biotic responses to increased nutrients, and indirect impacts on nutrient cycling, in which microbial processes are mediated by changes in plant biomass and allocation (Neubauer et al. 2013). Despite the potential for changes in root biomass due to nutrient enrichment (Darby and Turner 2008a,b; Ket et al. 2011; Deegan et al. 2012; Graham and Mendelssohn 2014) and the importance of rhizosphere processes in the regulation of elemental cycling in wetlands (Van Der Nat and Middelburg 1998; Lu et al. 2004; Nikolausz et al. 2008; Lamers et al. 2012), the link between belowground macrophyte productivity and nutrient enrichment has often been overlooked (Koop-Jakobsen and Giblin 2010). Furthermore, it has been suggested that plant (N limitation) and microbial communities (P limitation) may be limited by different nutrients (Sundareshwar et al. 2003). The majority of nutrient enrichment manipulations that examine both macrophyte and soil response either add mixed N-P, N-P-K (potassium), highnutrient river water, or sewage sludge (Valiela et al. 1973; Chalmers 1979; Caffrey et al. 2007; Deegan et al. 2007, 2012; Swarzenski et al. 2008; Koop-Jakobsen and Giblin 2010; Graham and Mendelssohn 2014; Valiela 2015), making it difficult to tease apart the effects of different nutrients and how the stoichiometry of nutrient additions affect ecosystem responses.
We present results from a fully factorial 10-yr fertilization experiment to assess the response of a tidal freshwater marsh to the long-term effects of elevated loads of N, P, or N and P in combination. Long-term experiments often reveal ecosystem responses that are not obvious after only a few years of treatment (Deegan et al. 2012; Neubauer et al. 2013); for instance, Neubauer et al. (2013) showed that wetland soil carbon mineralization increased after short-term exposure (2 d) to increased salinity but decreased after long-term (3.5 yr) exposure, and that the difference was mediated by changes in plant productivity and soil quality over the duration of their experiment.
Our study continues the work of Frost et al. (2009) and Ket et al. (2011) who investigated aboveground productivity at the site, finding that N and N + P in combination increase aboveground biomass (Frost et al. 2009; Ket et al. 2011). The goals of our study are to build on the measurements of Frost et al. (2009) and Ket et al. (2011), who did not measure microbial or soil processes, by (1) simultaneously assessing nutrient limitation (N, P) of macrophytes vs. microorganisms and (2) by exploring relationships between inorganic nutrient availability, macrophyte productivity, microbial activity, and C, N, and P pools. Macrophyte productivity in this tidal marsh is strongly N-limited (Frost et al. 2009; Ket et al. 2011). However, we hypothesize that microbial biomass will be P-limited because microbes have a higher P demand (lower N : P ratio) than macrophytes (Sundareshwar and Morris 1999; Sterner and Elser 2000). We hypothesize that P fertilization will increase P burial through both abiotic (mineral sorption and precipitation) and biotic (increased uptake and burial of plant biomass) processes, and N fertilization will increase denitrification and N storage in soil. Finally, we expect that the effects of N will be magnified when added in combination with P.
Methods
Site description
Sixteen experimental plots were established in a tidal freshwater marsh on Carr’s Island (31.334364°, −81.475900°) on the Altamaha River (Georgia, U.S.A.) in 2004 (Fig. 1a). The site is classified as fresh with average salinities < 0.5 psu (but occasionally reach up to 2 psu) (Wieski et al. 2010) and ~ 1.7 m tide range. Soils at the site are classified as Swamp—fine, mixed, acid, thermic Typic Fluvaquents (National Resource Conservation Service 2014; http://websoilsurvey.sc.egov.usda.gov) and contain ~ 20% organic matter (Loomis and Craft 2010). The site is dominated by giant cutgrass (Zizaniopsis miliacea [Michx.] Döll & Asch) with less than 3% cover of pickerelweed (Pontederia cordata L.), bull tongue arrow-head (Sagittaria lancifolia L.) and several knotweed (Polygonum) species. To account for gradients in inundation associated with distance from channel and elevation, we used a complete randomized block design. The 4-m2 plots are arrayed in a 4 row by 4 column grid with rows (blocks) oriented parallel to Hammersmith Creek (Fig. 1b). We measured the elevation of the four corners of each treatment plot in March 2015 using a Trimble R6 RTK GPS receiver (NAVD88 GEOID12) with a postsurvey accuracy observed root-square mean error of 0.0037 m. We used elevation data to calculate the cumulative difference in percent time flooded over the 10-yr study period using 30-min water level data from a nearby water level recording station (Di Iorio 2016). The flooding duration was 36%, 35%, 33%, and 31% of the total study period (2004–2014) for rows in order from lowest elevation row to highest elevation row. The lowest block had an average flooding depth 34 cm (± 18 cm) above the soil surface and a maximum depth of ~ 120 cm.
Fig. 1.
Site map showing (a) the location of the study site on the Altamaha River in relation to the Georgia coast and Sapelo Island and (b) the layout of treatment plots along Hammersmith Creek (N = nitrogen and P = phosphorus) with mean block elevations in meters. Elevations (relative to NAVD88) with the same letter are not significantly different (p > 0.05, Einot-Gabriel-Welsch Multiple Range Test).
Treatments (nitrogen-+N, phosphorus-+P, +NP, and control) were randomly assigned within rows (blocks) providing four replicates of each treatment type. Slow-release fertilizer pellets were hand-broadcast in plots twice a year in March and May/June throughout the 10-yr period (2004–2014). Nitrogen is added as polymer coated urea (CO(NH2)2 (Polyon, Pursell Technologies, Sylacauga, AL, U.S.A.) at a rate of 50 g N m−2 yr−1, and P is added as triple superphosphate (45% P2O5) at a rate of 10 g P m−2 yr−1. Control plots receive no amendments. Pellets of polymer coated urea are 2–5 mm diameter that release urea over a 10–14 week period (Simplot Professional Products, Latrop, CA). Pellets of triple superphosphate are 2.7 mm diameter and dissolve over 6–8 weeks. Both types of pellets persisted on the soil surface for 3–4 months or longer based on follow-up visits to the site (C. Craft pers. obs.). The fact that we observed a significant plant response to N (increased biomass) after 2 yr (Frost et al. 2009) and to N (increased biomass, leaf N) and P (increased leaf P) after 5 yr (Ket et al. 2011) while, at the same time, not detecting any increased biomass, pore water, or tissue nutrient concentrations in control plots downstream or down slope of treatment plots over the 10-yr experimental period (Frost et al. 2009; Ket et al. 2011; this study) indicates that N and P additions generally were retained in the plots without leakage among plots.
Biomass and soil sampling
Aboveground biomass was sampled nondestructively in August yearly (2004–2010) and every other year thereafter by measuring the height and number of leaves in a 0.25-m2 subplot within each treatment plot and converting these heights into biomass using an allometric equation as previously described in Frost et al. (2009) and Ket et al. (2011). For belowground biomass, we collected one 18-cm diameter by 60-cm deep core from each plot in 2014. Following Ket et al. (2011), cores were separated into 10-cm increments, washed through a 2 mm sieve and the material remaining in the sieve was sorted to separate live rhizomes from remaining macro-organic matter (MOM) using the methods of Schubauer and Hopkinson (1984).
Leaf C, N, and P were measured annually by harvesting five random Z. miliacea leaves from each plot. Oven-dried (50°C) leaves were ground using a Wiley Mill (40-mesh, Thomas Scientific, Swedesboro, NJ, U.S.A.). Total C and N were analyzed by combustion on a Perkin-Elmer 2400 CHN elemental analyzer (PerkinElmer, Waltham, MA; method detection limit [MDL] = 0.001 mg C and N). Total P was analyzed colorimetrically by hand using the molybdate blue method with pH adjustment following HNO3-HClO4 digestion of oven-dried samples (Sommers and Nelson 1972; MDL = 0.05 mg P). Recovery of N (NIST 1515 Apple Leaves) was 96% and P (NIST 1575a Pine Needles) was 92%.
In August 2012 and 2013, two randomly selected cores (8.5-cm diameter by 20-cm depth) were collected within each 4-m2 plot. A depth-integrated ~ 40 mL subsample was transferred to a plastic screw-top container for extracellular enzyme activity (EEA) analysis (2013 only) and the remainder of the cores were transferred to glass jars and topped with site water to maintain anaerobic conditions. We chose to depth-integrate all samples to represent activity in the surface soil and in the soil layers where the majority of live root biomass occurs, although we acknowledge this approach does not capture depth-driven variations in reduction–oxidation potential, solute concentration, and other factors. Five liters of river water was collected in a plastic bladder for use in soil incubations. In 2014, we conducted additional EEA sampling directly adjacent to the root biomass cores. Soil and water samples were stored on ice and transported to the lab at the University of Georgia Marine Institute where water was filtered through 0.22 μm nitrocellulose filters (Sigma-Aldrich) and EEA samples were frozen at −80°C. Water (< 1 week holding time) and soils (< 2 week holding time before extracting) were held at 4°C and EEA samples at −80°C until analysis at Indiana University.
Pore water and soil nutrients
We measured concentrations of dissolved inorganic N (, ) and dissolved inorganic P () and dissolved organic C, N, and P (DOC, DON, DOP) by centrifuging 40mL of homogenized soil and analyzing the supernatant. Samples were analyzed using a Lachat QuickChem 8500 Flow Injection Analysis system (Hach Company, Loveland, CO, U.S.A.) using QuickChem methods 10–107-06–1-J for (indophenol blue complex, MDL = 10 μgNL−1), 10–107-04–1-A for (cadmium reduction/EDTA red complex, MDL = 2.5 μgNL−1), and 10–115-01–1-Q for (molybdate blue complex, MDL = 5 μg PL−1). Total dissolved N and P were converted to and via an acidic persulfate digestion (Langner and Hendrix 1982) of diluted pore water (1:9) and subsequently quantified using the methods described above using a diluted persulfate carrier. DON/DOP concentrations were calculated as the difference between total dissolved and dissolved inorganic N/P. DOC was analyzed via high-temperature oxidation using a Shimadzu TOC-VCPN analyzer (Columbia, MD, U.S.A.; MDL = 150 μgCL−1). For all analyses, known standards and ultrapure deionized water blanks were run every 10 samples to ensure accuracy and correct for instrumental drift.
Bicarbonate-extractable , a measure of readily available soil-bound , was determined by extracting 25 g of field-moist soil with 30mL of 0.5 mol L−1 NaHCO3 (Murphy and Riley 1962) for 16 h followed by centrifuging and analysis of the supernatant as described for above after acidifying to remove CO2 bubbles. Exchangeable inorganic N ( and ) was determined by extracting 25 g of field-moist soil with 30mL of 2 mol L−1 KCl for 1 h (Cataldo et al. 1974) followed by centrifuging and analysis of the supernatant as described above for and using a KCl carrier. Phosphorus sorption capacity was determined using a single-point isotherm (phosphorus sorption index [PSI]; Bache and Williams 1971). Twenty-five milliliters of 130mg -P L−1 solution was shaken for 18 h with 5 g of field-moist soil. The concentration of P in solution after the incubation, Ct (mg P L−1), was determined as described above for . PSI is calculated as:
where S is the amount of P sorbed (mg P dry 100 g soil−1). We also calculated the quantity/intensity (Q/I) relationship described by Reddy and DeLaune (2008) as the ratio between the amount of P adsorbed on the soil surface to the concentration of P in soil pore water.
A homogenized subsample of soil was dried at 60°C to determine soil moisture content, then the subsample was ground and sieved (2 mm) for bulk analysis of C, N, and P as described for biomass samples. Recovery of C and N (in-house estuarine sediment; 6.106% N, 0.365% P) was 100% and 94%, respectively, and for P (NIST Estuarine Sediment) was 93%.
Microbial C, N, and P
Microbial C, N, and P were determined via a modified chloroform-extraction method (Hedley and Stewart 1982; Brookes et al. 1985; Vance et al. 1987). For C and N, 10 g of field-moist soil was fumigated for 24 h with chloroform (CHCl3). The fumigated soils and a second set of unfumigated soils were extracted with 30 mL 0.5 mol L−1 K2SO4 for 1 h. After centrifuging, the supernatant was analyzed for DOC and DON as described above. For P, fumigation was followed by extraction with 0.5 mol L−1 NaHCO3 and DOP was determined as described above. Extracted (Ex; where x = C, N, or P) C, N, or P were then calculated as the difference between C, N, or P concentrations in fumigated and unfumigated soils. Biomass (Bx) C, N, or P was calculated by dividing by a recovery constant (kx) to correct for the fact that only a fraction of the total element liberated from microbial biomass is fully mineralized during the extraction (kC = 0.38, Vance et al. 1987; kN = 0.54, Brookes et al. 1985; kP = 0.37, Hedley and Stewart 1982).
Nitrification, denitrification, and greenhouse gas production incubations
Incubations for nitrification potential followed the methods of Bernhard et al. (2007). Five grams of homogenized field-moist soil were placed in quadruplicate 50-mL centrifuge tubes with 30 mL of filtered site water amended with NH4Cl (300 μmol L−1 NH4-N) and a 1:1 mix of K2HPO4 and KH2PO4 (60 μmol L−1 PO4-P). Tubes were agitated on a shaker table and one tube from each soil was sacrificed at 12, 24, 48, and 72 h, centrifuged and the supernatant was filtered through a 0.22-μm nitrocellulose filter (Sigma-Aldrich). Samples were frozen until analysis for as described above. Nitrification potential was then calculated as the slope of the regression of increase per dry gram soil over time (r2 ≥ 0.90, ≥ 3 points per regression). In 2012 and 2013, we assessed potential denitrification enzyme activity (DEA) using the chloramphenicol-amended acetylene inhibition method (Wall et al. 2005). Approximately 25 g of field-moist soil was homogenized with 50mL of a solution of filtered site water amended with 0.2 mmol L−1 chloramphenicol to inhibit de novo enzyme synthesis (Smith and Tiedje 1979) in Wheaton glass bottles fitted with gray butyl septa. The headspace was flushed with ultra-high purity N2 gas for 8 min prior to the incubation and acetylene (C2H2) gas was injected to achieve a final concentration of 10% C2H2 in the incubation headspace to block the conversion of N2O to N2. At 15, 30, 60, 120, and 240 min, bottles were vigorously agitated to equilibrate gas between the dissolved and gaseous phase and 5-mL gas samples were taken from the bottles using a gas-tight syringe. Samples were injected into N2-flushed and evacuated 2-mL Wheaton glass vials fitted with aluminum crimp-tops and gray butyl rubber septa for storage. Headspace was replaced after each sample with N2 gas containing 10% C2H2. Bottle incubations were performed in the laboratory to determine the potential rates of anaerobic production of N2O, CO2, and CH4 from soil slurries. In 2012 and 2013, 25 g of homogenized field-moist soil was mixed with 30 mL of filtered site water in 125 mL Wheaton glass bottles fitted with gray butyl septa. The headspace of the bottles was flushed with N2 gas prior to the incubation. At 15, 30, 60, and 120 min, 5-mL gas samples were taken from the bottles using a gas-tight syringe and stored in glass vials as described for denitrification potential incubations above. Five millilitersN2 gas was injected into each bottle after sampling to maintain constant pressure through the incubation.
Gas samples were analyzed within 1 month of collection using a Varian 450-Gas Chromatograph (GC) (Varian/Agilent Technologies, Palo Alto/Santa Clara, CA, U.S.A.) equipped with a thermal conductivity detector for CO2 (Helium [He] carrier; method detection limit [MDL] = 150.3 ppm), flame ionization detector for CH4 (He carrier; MDL = 0.37 ppm), and 63Ni electron capture detector for N2O (N2; MDL = 0.14 ppm). The Gas Chromatograph (GC) was calibrated daily with 4–5 certified Scott™ standards for each gas (AirLiquid, Troy, MI). Certified standard mixtures and blanks were run every 15 samples to ensure accuracy and correct for instrumental drift. Analysis of stored standards showed there was no effect of the holding time on gas concentrations.
Gas concentrations were calculated for the dissolved phase using the appropriate Bunsen coefficient (Murray and Riley 1971; Wiesenburg and Guinasso 1979; Weiss and Price 1980) and summed with the headspace gas phase. The gas concentrations were corrected for dilution from sampling and headspace replacement and expressed on a per dry gram soil basis and then regressed against sampling time. Production rates were calculated using the slopes of the regression for the linear portion of the curve (≥ 3 points, general time 15–120 min). We did not include gas concentrations below the MDL in regressions nor did we utilize regressions with r2 < 0.95. Denitrification was calculated as N2 production by first subtracting the N2O production from incubations without C2H2 (the incubations for CH4 and CO2 previously described in the “Nitrification, denitrification, and greenhouse gas production incubations” section) from the C2H2 incubations to correct for ambient N2O production and then assuming that 100% of the remaining N2O would be converted to N2 in the absence of C2H2.
Extracellular enzyme activity
We assessed extracellular enzyme activity (EEA) from 2013 and 2014 soil collections (0–20 cm) for eight enzymes associated with C, N, and P acquisition (Table 1) using fluorometric assays following modified protocols from Bell et al. (2013). We chose a depth-integrated sampling approach to compare our enzyme activities with comparable measurements of belowground biomass (0–20 cm) where most roots are located and where competition between macrophytes and microorganisms for nutrients is most likely to occur. Neubauer et al. (2013) found no difference in the activity of C-acquiring enzymes measured at three depths (0–3, 8–13, and 23–28 cm) in a tidal freshwater marsh in South Carolina, suggesting that our integrated (0–20 cm) sampling is appropriate for measuring EEA in our treatment plots. Soil slurries were prepared from the −80°C preserved samples using 10 g of soil and 91 mL of 50 mmol L−1 acetate buffer adjusted to the pH (5.6) of the field-moist soils. Freezing is common (Keeler et al. 2009) and previous work (DeForest 2009) found no consistent shift in EEA associated with freezing samples. For example, DeForest (2009) found no difference in activities of β-glucosidase, β-xylosidase, N-acetylglucosaminidase, or phosphatase of soils stored at −20°C vs. 4°C when compared to fresh soils. Furthermore, our measured values (in nmol g OM−1 h−1) are comparable to soil EEA measured in unamended and fertilized herbaceous and forest plot experiments (Keeler et al. 2009), suggesting that freezing did not materially alter EEA in our experiment. Organic matter was determined for a subsample of the slurry via loss on ignition at 550°C to correct for quenching (i.e., decreased florescence intensity caused by organic matter).
Table 1.
Enzymes for which activities were assessed in this study and their abbreviation, elemental cycling with which they are associated, model substrate, and their related hydrolytic functions.
| Enzyme | Element | Model substrate | Hydrolysis reaction |
|---|---|---|---|
| β-D-glucosidase (BG) | C | 4-MUB β-D-glucopyranoside | Cellulose → glucose |
| 1,4-β-cellobiosidase (CBH) | C | 4-MUB β-D-cellobioside | Cellulose → disaccharide |
| β-Xyloxidase (BX) | C | 4-MUB-β-D-xylopyranoside | Hemicellulose hydrolysis |
| β-1,4-N-acetylglucosaminidase (NAG) | C | 4-MUB N-acetyl-β-D-glucosaminide | Chitin hydrolysis |
| Leucine-aminopeptidase (LAP) | N | L-Leucine-7-amido-4-AMC | Polypeptides → leucine |
| Arginine-aminopeptidase (AAP) | N | L-Arginine-7-amido-4-AMC | Polypeptides → arginine |
| Alkaline phosphatase (PHOS) | P | 4-MUB phosphate | Phosph-monoesters → phosphate |
Eight hundred microliters of slurry was pipetted in triplicate into black, deep 96-well plates, incubated at room temperature for 3 h with 250 μL of 300 μmol L−1 model substrate (Table 1; Sigma-Aldrich), and agitated periodically. This substrate concentration was determined to be nonlimiting over the course of a 3–4 h incubation in previous assays. For each substrate, three deionized water blanks were also incubated. Standard quench curves (0–100 μmol L−1) for individual soils were prepared in separate plates for both 7-amido-4-methylcoumarin hydrochloride (AMC) and 4-methylmubelliferyl (MUB). This step is necessary to account for differences in fluorescent quenching between soils. Following the incubation period, 250-μL samples of the supernatant were pipetted into a black, flat-bottomed 96-well plate to eliminate backscatter and fluorescence was quantified at 360 nm excitation and 460 nm emission wavelengths on a Synergy 2 plate reader (Bioteck, Winooski, VT, U.S.A.). Enzyme activity was calculated by regressing fluorescent intensity against concentration for MUB or AMC standard quench curves for each soil and then calculating the change in MUB or AMC concentration per gram organic matter over incubation time. We used negative assay controls (buffer plus substrate solution) to monitor substrate inconsistencies/changes over time. To simplify our assessment of the potential patterns in soil C, N, and P cycling, we summed extracellular enzyme activities (EEAs) associated with each element (Table 1).
Statistical analyses
All statistics were performed using SAS 9.4 (2012, SAS Institute, Cary, NC). Prior to statistical tests, we verified assumptions of normality and homoscedasticity. To meet these assumptions, data were log-transformed (all nutrient and belowground biomass data) or square-root-transformed (EEA, microbial process rates, belowground biomass). For presentation, means and standard errors (SEs) were back-transformed. We used a three-way ANOVA to examine the effect of time, block, and fertilization on aboveground biomass and aboveground C, N, and P for the years 2004–2014. For soil nutrients and microbial process rates, we compared the arithmetic means of the duplicate cores for each treatment plot for each year using a two-way ANOVA for the effect of year and treatment. With the exception of EEA, there was no significant effect of year and no significant interaction between year and treatment; therefore, we averaged values for each plot from both years to simplify further analysis.
RTK GPS surveys showed that treatment blocks were not only significantly different in their distance from channel but also in their elevation (p < 0.001; Fig. 1b); however, average elevation did not vary between treatments (p = 0.6). Because elevation and distance from channel are known to have significant effects on both biotic and abiotic processes, averaged data were then analyzed using an ANOVA for a randomized block design which tests for the effect of differences among fertilization treatments while adjusting for difference between blocks. Treatment means were separated post-ANOVA using the Ryan-Einot-Welsch Multiple Range Test. All tests were conducted at α = 0.05 level. Additionally, we explored the interaction between macrophyte productivity, microbial process rates, and nutrient chemistry using Spearman’s rank-order correlation (ρ) for untransformed data.
Results
Macrophyte biomass
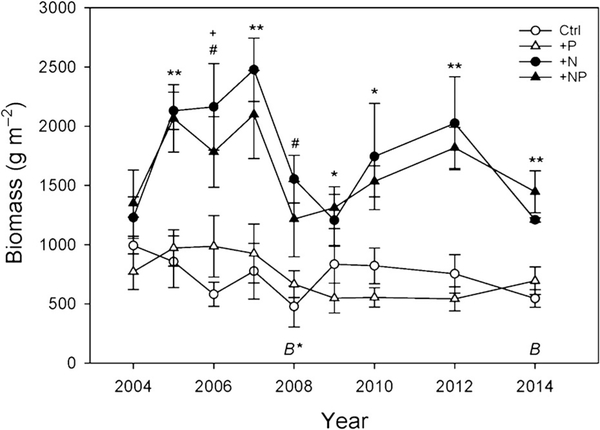
Aboveground biomass was 2–3 times greater in +N and +NP plots than in +P or control plots (p < 0.0001) in all years except 2004 (year 1 of the study; Fig. 2). Although there were differences in biomass between years (p < 0.001), there was no significant interaction between treatment and year (p = 0.07). This N limitation was consistent despite a wide range of hydrologic and climatic conditions throughout the 10-yr study period (Li et al. 2018). Belowground, differences in biomass were only apparent in the top 10 cm of soil where MOM was 20–24% higher and rhizome biomass was 2–3 times higher in control and +NP plots than in +N and +P plots (p = 0.003 and 0.002; Table 2). Belowground (MOM + Rhizome) to aboveground biomass ratios ranged from 5.2 to 6.7 and were highest in +NP plots, followed by control plots and lowest in +N and +P plots (p = 0.004; Table 2).
Fig. 2.
Annual aboveground biomass (g m−2) of cutgrass (Zizaniopsis millacea) for 2004–2014. Bars = SE. ** = +N and +NP are significantly different than +P and Ctrl. * = +N and +NP significantly different than +P. # = +N significantly different than P and Ctrl. + = +NP significantly different than Ctrl. For all tests, α = 0.05 (Einot-Gabriel-Welsch Multiple Range Test). B* signifies belowground biomass sampling from Ket et al. 2011 and B signifies belowground biomass sampling for this study.
Table 2.
Belowground MOM and rhizome biomass (g m−2) for 0–10 cm and 10–20 cm depth increments and belowground (0–20 cm MOM + rhizome) to aboveground ratio (g:g) for 2014. Treatment means (± SE) within the same column followed by the same letter are not significantly different (p > 0.05;Ryan-Einot-Gabriel-Welsch Multiple Range Test). There were no significant differences between treatments for MOM or rhizome biomass at the 10–20 cm depth increment.
| MOM (g m−2) |
Rhizome (g m −2) |
||||
|---|---|---|---|---|---|
| Treatment | 0–10 cm | 10–20 cm | 0–10 cm | 10–20 cm | Below: Above (g:g) |
| Control | 1894.8 ± 42.8a | 1576.0 ± 67.2 | 578.9 ± 36.0a | 56.6 ± 5.9 | 6.2 ± 0.1a,b |
| +P | 1582.2 ± 137.1b | 1660.5 ± 29.6 | 190.9 ± 62.0b | None | 5.2 ± 0.2c |
| +N | 1538.7 ± 100.1b | 1688.5 ± 197 | 303.0 ± 62.1b | 41.69 | 5.5 ± 0.4b,c |
| +NP | 1903.3 ± 81.7a | 1645.16 ± 59.1 | 732.2 ± 56.7a | 100.9 ± 33.9 | 6.7 ± 0.2a |
Nitrogen in aboveground biomass was 20% higher in +N and +NP plots than control or +P plots (p < 0.0001; Table 3). Phosphorus content was highest in +P plots and +NP plots and lowest in +N plots (p < 0.0001). Nitrogen fertilization decreased the C : N ratio of aboveground biomass from 51 in control and +P plots to 45 in +N and +NP plots (p < 0.0001). Aboveground biomass N : P was lowest in the +P plots (19), and increased in the control and +NP plots, with +N plots having the highest N : P (30) (p < 0.0001).
Table 3.
Biomass C, N, and P for aboveground plant (mg dry g−1) and microbial (μg dry g soil−1) biomass and C:N and N:P ratios (mol : mol). Treatment means (± SE) within the same column followed by the same letter are not significantly different (p > 0.05; Ryan-Einot-Gabriel-Welsch Multiple Range Test). There was no significant difference between treatments for aboveground biomass C.
| Treatment | C | N | P | C:N | N:P |
|---|---|---|---|---|---|
| Aboveground (mg dry g−1)† | |||||
| Control | 434 ± 5 | 10.4 ± 1.1b | 1.18 ± 0.16b | 51 ± 5a | 20.9 ± 3.6c |
| +P | 437 ± 16 | 10.3 ± 1.1b | 1.27 ± 0.16a | 52 ± 6a | 18.8 ± 2.8d |
| +N | 441 ± 7 | 12.7 ± 1.9a | 1.06 ± 0.23c | 45 ± 8b | 29.6 ± 6.1a |
| +NP | 438 ± 18 | 12.5 ± 1.9a | 1.24 ± 0.20a,b | 45 ± 8b | 23.7 ± 4.9b |
| Microbial (μg dry g soil−1)* | |||||
| Control | 1207 ± 88b | 68 ± 20b | 23.5 ± 4.1b | 21.2 ± 1.2a | 8 ± 1.6 |
| +P | 1681 ± 118a,b | 117 ± 17b | 50.8 ± 7.8a,b | 17.6 ± 2.7a,b | 6.7 ± 0.5 |
| +N | 1145 ± 33b | 147 ± 12.8a,b | 27 ± 3.4b | 9.2 ± 0.7c | 11.7 ± 2.0 |
| +NP | 2353 ± 1243a | 255 ± 54a | 85.4 ± 14.2a | 11.4 ± 1.3b,c | 12.5 ± 3.4 |
Pooled data from 2 yr (2012, 2013); two-way ANOVA (block × treatment).
2004–2014 data; multivariate ANOVA (block × treatment × year).
C, N, and P pools
Pore-water was 5–6 times higher in +N and +NP plots (p < 0.001) and exchangeable was 2–3 times higher in +N and +NP plots than in control or +P plots (Table 4). Pore-water and exchangeable were also higher in +NP and +N plots than in +P or control plots. Pore-water and exchangeable were 2–3 times higher in +P and +NP plots than +N or control plots. PSI ranged from 117.2 to 140.2 and did not differ significantly between treatments. Q/I ranged from 0.21 to 0.33 and also did not differ significantly between treatments (data not shown). There were no significant differences between treatments for DOC, DON, or DOP (Table 4).
Table 4.
Concentrations of dissolved inorganic nutrients (, , and ) from soil pore water, soil KCl-exchangeable and , -exchangeable , and DOC, DON, and DOP from soil in the top 20 cm of plots (pooled 2013 and 2014 values). Treatment means (± SE) within the same column followed by the same letter are not significantly different (p >0.05; Ryan-Einot-Gabriel-Welsch Multiple Range Test).
| Pore water (μg L−1) |
Exchangeable (μg dry g−1) |
Dissolved organic |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment | -N | -N | -P | -N | -N | -P | C | N | P |
| Control | 2.8 ± 0.6b | 0.84 ± 0.1b | 8.0 ± 1.4b | 46.0 ± 4.5b | nd | 1.4 ± 0.2b | 24.0 ± 7.6 | 11.2 ± 8.5 | 3.0 ± 0.6 |
| +P | 3.2 ± 0.8b | 0.95 ± 0.2a,b | 17.8 ± 2.1a | 58.2 ± 8.3b | nd | 4.9 ± 1.3a | 24.3 ± 5.6 | 12.2 ± 7.4 | 3.5 ± 0.6 |
| +N | 19.2 ± 1.3a | 1.3 ± 0.3a,b | 8.0 ± 0.2b | 154.6 ± 28.4a | 2.5 ± 1.7 | 2.2 ± 0.3b | 44.3 ± 21.3 | 23.7 ± 16.1 | 3.1 ± 0.9 |
| +NP | 21.0 ± 1.5a | 1.2 ± 0.2a | 26.2 ± 3.6a | 104.3 ± 32.7a,b | 2.3 ± 2.0 | 5.6 ± 0.7a | 27.7 ± 8.6 | 16.5 ± 16.7 | 4.4 ± 0.9 |
Total soil C was lower in the +N plots (12.9% ± 1.4%) and marginally lower in the +P plots (13.4% ± 2.2%) than in the control (15.5% ± 1.5%) and +NP plots (15.4% ± 1.8%) (Fig. 3a). Total N was significantly higher in the +NP plots (1.23% ± 0.1%) and marginally higher in the +N plot (1.0% + 0.2%) than in other treatments (0.91% ± 0.1% to 0.92% ± 0.1%) (p = 0.0007; Fig. 3b). Total P was highest in the +NP plots (1.5 ± 0.3 mg dry g−1) and marginally higher in +P plots (1.2 ± 0.2 mg dry g−1) than in +N or control plots (Fig. 3c).
Fig. 3.
Total (a) C (%), (b) N (%), and (c) P (μg P dry g−1) in the top 20 cm of soil in each treatment. Bars = SE. Treatment means with the same letter are not significantly different (p > 0.05, Einot-Gabriel-Welsch Multiple Range Test).
Microbial activity
Microbial biomass C was ~ 200% higher in +NP plots and ~ 40% higher in +P plots than in control or +N plots, although the difference was only statistically significant for the +NP plots (Table 3). Microbial biomass N was 2–3 times higher in +NP plots and ~ 50% higher in +N plots compared to +P and control plots. Microbial biomass P was three times higher in +NP plots and two times higher in +P plots than in +N or control plots. Microbial biomass C : N ratios were highest in the control plots and were reduced by half in the +N and +NP plots, while microbial biomass N : P ratios were not significantly different between treatments (Table 3).
Laboratory nitrification potential was an order of magnitude higher in +N and +NP soils (1018 ± 225 nmol -N dry g−1 d−1 and 1573 ± 362 nmol -N dry g−1 d−1, respectively) than in control or +P soils (193 ± 49 nmol -N dry g−1 d−1 and 105 ± 63 nmol -N dry g−1 d−1, respectively) (Fig. 4a). Potential DEA was significantly higher in the +NP plot (135 ± 6270 nmol N dry g−1 d−1) than in control or +P plots (265 ± 124 nmol N dry g−1 d−1 and 420 ± 51 nmol N dry g−1 d−1, respectively) and was also higher in the +N plot (784 ± 201 nmol N dry g−1 d−1) than control or +P plots (Fig. 4b). Rates of ambient N2O production (from incubations without acetylene) were small compared to gaseous N production via denitrification (0.6–24.1 nmol N dry g−1 d−1) and we observed no significant difference between treatments (data not shown). Nitrification potential was positively correlated with pore-water concentration (ρ = 0.971, p < 0.0001), and nitrification potential and potential DEA were also positively correlated (ρ = 0.565, p = 0.003). Microbial CO2 production potential did not differ between treatments (Fig. 4c). Laboratory CH4 production rates were significantly higher in +N soils (3.4 ± 0.5 μgCH4 dry g−1 d−1) than in control, +P or +NP soils (all ≤ 1.1 μg CH4 dry g−1 d−1) (Fig. 4d).
Fig. 4.
Potential rates of (a) nitrification (nmol -N dry g−1 d−1), (b) denitrification (nmol N dry g−1 d−1), (c) CO2 production (μg CO2 dry g−1 min−1), and (d) CH4 production (μg CH4 dry g−1 min−1) determined in laboratory incubations of the top 20cm of soil for each treatment. Bars = SE. Treatment means with the same letter are not significantly different (p > 0.05, Einot-Gabriel-Welsch Multiple Range Test). There was no significant difference in CO2 production between treatments (p > 0.05).
Although the magnitude of EEA varied between sampling years, patterns between treatments were generally similar. Carbon-acquiring EEA was highest in the +NP plots, followed by control plots with the +N and +P plots having the lowest activity (p < 0.01 for both years; Fig. 5a). Carbon-acquiring EEA was positively related to DOC, DON, live rhizome, and total belowground biomass (Table 5). N-acquiring EEA was significantly higher in +P plots (p < 0.01 for both years; Fig. 5b) and was positively correlated with all measured forms of P (Table 5). P-acquiring EEA was highest in +NP plots, followed by +N plots and was lowest in +P plots (p < 0.001 for both years; Fig. 5c). P-acquiring EEA was positively correlated with pore-water , DON, and DOC (Table 5). Across years and treatments, C- and P-acquiring EEA were an order of magnitude higher (250–1400 nmol g OM−1 h−1) than N-acquiring EEA (55–395 nmol g OM−1 h−1). Total EEA was highest in +NP plots and lowest in +P plots in both 2013 and 2014 (p = 0.0008 and p = 0.0024, respectively) and it was not significantly correlated with any individual environmental variable. With the exception of potential nitrification, which was correlated with P acquiring enzymes (r = 0.82) and total EEA (r = 0.54), there were no correlations between EEA and laboratory incubations of microbial processes.
Fig. 5.
Activity (nmol g OM−1 h−1) of extracellular enzymes associated with (a) C-acquisition (sum of BG, CBH, BX, and NAG), (b) N-acquisition (sum LAP and AAP), (c) P-acquisition (PHOS), and (d) total EEA (sum of C-, N-, and P-acquiring EEA) in 2013 (gray) and 2014 (black) determined using laboratory incubations for the top 20 cm of soil for each treatment. Bars = SE. Treatment means for the same enzyme in the same year with the same letter are not significantly different (p > 0.05, Einot-Gabriel-Welsch Multiple Range Test).
Table 5.
Spearman correlation coefficient (ρ, n = 16) for interactions between C-, N-, and P-acquiring and total extracellular enzyme activities and select soil properties. Significance level is indicated as *α < 0.05, **α < 0.01, ***α < 0.001, ****α < 0.0001. Missing values indicate no significant interaction (p > 0.05).
| Dissolved inorganic† |
Dissolved organic† |
Soil total† |
Belowground biomass‡ |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Enzyme | DOC | DON | DOP | C | N | P | Rhizome | Total | ||
| C | 0.7*** | 0.7** | 0.7** | 0.6* | ||||||
| N | 0.7** | 0.6* | 0.7** | |||||||
| P | 0.8 **** | 0.6* | 0.9*** | |||||||
2013 soil and EEA data.
2014 root and EEA data.
Discussion
Effects of fertilization on macrophyte productivity and belowground biomass
This study provides evidence of strong and consistent long-term N limitation of plant productivity in a tidal freshwater marsh (Frost et al. 2009; Ket et al. 2011). Aboveground biomass was 2–3 times greater and leaf N content was 20% greater in +N and +NP plots than in control or +P plots over the duration of the 10 yr experiment, consistent with Ket et al. (2011)’s findings from the first 4 yr. Furthermore, leaf N : P ratios (mol : mol) were < 31–35 in all treatments, the threshold between macrophyte N vs. P limitation, which is strongly suggestive of N limitation (Koerselman and Mueleman 1996; Reich and Orelsyn 2004). These findings are also consistent with decades of research in salt marsh systems (see Chalmers 1979 and Morris et al. 2013 for extensive documentation). Several short-term (3–4 yr) multifactorial fertilization experiments in oligohaline marshes of Louisiana and Maine support the idea that N (Graham and Mendelssohn 2010) or N + P (Crain 2007) limit aboveground macrophyte productivity. Our results, however, are in contrast to the only other multiyear (3 yr) factorial wetland fertilization study (to our knowledge) which concluded that aboveground macrophyte productivity in oligohaline marshes in Maine was colimited by N and P (Crain 2007) possibly induced by low biologically available P (Paludan and Morris 1999). In the Maine experiment, colimitation by N and P was thought to be induced by low available P (Paludan and Morris 1999). Ecosystem-wide P limitation is often predicted for freshwater systems due to (1) lower biological availability of P due to complexation with iron and (2) higher rates of biological N-fixation in tidal freshwater marshes. However, we are among the first to show conclusive N limitation of aboveground macrophyte productivity in tidal freshwater marshes.
We found that, compared to control plots, both rhizome and MOM biomass were lower in the +N and +P plots relative to the control and +NP plots in the top 10 cm where > 70% of Z. miliacea live root biomass tends to occur (Li et al. 2018). Although increased nutrients have been identified as a driver of decreased belowground biomass (Valiela and Teal 1974; Valiela et al. 1976; Darby and Turner 2008a,b; Ket et al. 2011; Deegan et al. 2012; Graham and Mendelssohn 2014; Valiela 2015), leading to concerns of marsh soil collapse, only a few studies have examined the response of belowground biomass to individual nutrients. For example, Graham and Mendelssohn (2016) reported that long-term (7 yr) additions of N reduced live root biomass of a S. lancifolia marsh, similar to our findings, but there was no effect of P. In contrast, Darby and Turner (2008a) found that P alone or in combination with N or Fe reduced root and rhizome biomass of salt marsh vegetation, which they attributed to decreased competition for P with the microbial community. Cleland et al. (2019) found that, in a globally distributed fertilization experiment of terrestrial grasslands, N and P applied singly reduced carbon allocated to roots and root biomass (0–10 cm), where most roots are located.
Reduced MOM biomass in +N and +P plots relative to control and +NP plots (Table 2) may be explained by the optimal allocation hypothesis (Gleeson and Tilman 1992). The theory predicts that when plant growth is limited by belowground resources such as nutrients, carbon allocation to roots should increase (Gleeson and Tilman 1992; Cleland et al. 2019) but when the limitation is removed, in this case by fertilization, C allocation to belowground structures is reduced. This is especially true for N since reduced root foraging allows more N to be allocated aboveground where it is a component of the chlorophyll molecule and photosynthesis (Sterner and Elser 2000). Reduced MOM and rhizomes in the +P plots also may be linked to reduced foraging for P. Phosphorus is a component of energy transfer (adenosine triphosphate, reduced nicotinamide) in cells (C adenine dinucleotide phosphate—NADPH) (Aerts and Chapin 2000) and P additions increased leaf P relative to control and +N treatments (Table 3).
Increased competition for P with microbes after 10 yr of fertilization may explain the increase in MOM and rhizome biomass in the +NP plots. Microbial biomass C was significantly greater in the +NP treatment than in the control and +N treatments (Table 3). Results from this study clearly support the idea that the microbial community is P-limited (Table 3, Fig. 5) whereas the plant community is N-limited (Fig. 2, Table 3) so increased competition for P with a resultant increase in root and rhizome biomass in the +NP plots relative to the +N and +P plots after 10 yr of fertilization is reasonable (see “Effects of fertilization on microbial activity” section below for more detailed explanation).
Effects of fertilization on microbial activity
Our long-term field experiment indicates P limitation of the microbial community. Microbial biomass C is higher with +P and +NP fertilization, but not +N alone. Cleveland and Liptzin (2007) suggest that microbial N:P > 6.9 (± 0.4) are indicative of P limitation, and our results indicate that microbial N : P is ≥ 8 for all but the +P plots. EEA data also support P limitation (Fig. 5). Furthermore, rates of P-acquiring EEA are elevated roughly three times relative to N-acquiring EEA regardless of treatment and are especially high in +N and +NP treated plots (Fig. 5). The elevated microbial N : P ratios (Table 3) and increased P-acquiring EEA observed in +NP plots indicates that combined fertilization with N and P at 15:1 (mol : mol), fertilizer ratio is inducing competition for P between plants and microbes and that plants appear to be “winning” this competition. In contrast, differential nutrient limitations between macrophytes (N-limited) and microbes (P-limited) could reduce competition between plants and microbes when individual nutrients (N or P) are added (Sterner and Elser 2000; Sundareshwar et al. 2003), resulting in reduced investment in belowground biomass for nutrient foraging or storage as seen in the +N and +P plots (Table 2).
We observed increased N-acquiring enzymes in response to P additions, increased P-acquiring enzymes in response to N additions, and increased C-acquiring enzymes with +NP (Fig. 5). Other studies report similar response of EEA to nutrient loading. For example, Jackson and Vallaire (2009) found that N additions increased P-acquiring enzymes in freshwater wetland soils of Louisiana. Phosphorus additions, however, had no effect on C-, N-, or P-acquiring enzymes. In the Everglades, Wright and Reddy (2001) and Penton and Newman (2007) reported that P enrichment increased N- and C-acquiring enzymes and reduced the activity of P-acquiring enzymes. In contrast, Min et al. (2011) observed no change in C-, N-, or P-acquiring enzymes in response to additions of NH4NO3 in a freshwater wetland in Korea.
Our EEA measurements are comparable to other studies in tidal freshwater marshes. For example, Morrissey et al. (2014a) reported activities of 270–400 nmol g OM−1 h−1 for C-acquiring enzymes (sum of BG, CBH, and BX), somewhat lower than measured values in our control plots (Fig. 5a), and 220–800 nmol g OM−1 h−1 for P-acquiring enzymes, comparable to our control plots (Fig. 5c). Neubauer et al. (2013) measured C-acquiring EAA of 1750 nmol gdw−1 h−1 in a tidal freshwater marsh in South Carolina that, corrected for organic matter content (approximately 60%) was 2920 nmol g OM−1 h−1, was higher than measurements in our control plots (Fig. 5a). Our samples were frozen at −80°C whereas Morrissey et al. and Neubauer et al. used fresh or refrigerated samples. Thus, it appears that freezing did not have a measureable effect on EEA as has been shown by other studies (DeForest 2009; Keeler et al. 2009).
It is hypothesized that competition between macrophytes and microorganisms for nutrients involves a temporal niche differentiation reflecting their different generation times (Kuzyakov and Xu 2013). In the short-term, microbes are more efficient at assimilating nutrients than macrophytes. However, because of the unidirectional flow of nutrients from soil to roots, macrophytes are the winners for nutrients in the long run (Kuzyakov and Xu 2013).
Laboratory measured potential nitrifier and denitrifier activity all increased with N enrichment added as urea. High potential nitrification rates combined with low ambient concentrations in field soils and the strong correlation between concentration and potential DEA suggest that N fertilization enhances coupled nitrification-denitrification, and this may be a significant pathway for the removal of excess N. Long-term N fertilization has been shown to enhance in situ coupled nitrification-denitrification (White and Howes 1994; Hamersley and Howes 2005; Koop-Jakobsen and Giblin 2010) and DEA in laboratory incubations (Deegan et al. 2012) and the number of genes that encode the enzymes for denitrification (nirK and nirS; Chen et al. 2010). Fertilization studies with durations < 1 yr tend not to show this response (Caffrey et al. 2007), highlighting the importance of long-term studies.
Effect of fertilization on C storage and mineralization
We observed that fertilization with either N or P decreases C storage in wetlands by ~ 15% (Fig. 3). Some studies document a decrease (Mack et al. 2004), an increase (Fornara et al. 2013; Wigand et al. 2015), or no effect (Lu et al. 2011) of long-term N or multifertilizer additions on soil C storage. Wigand et al. (2015) reported that long-term (12 yr) additions of N increased SOM whereas there was no effect of P or N + P additions in a minerogenic salt marsh. Lu et al. (2011), in a meta-analysis of 257 studies, found no effects of N additions on C soil storage in grasslands or forests that included wetland studies. In our experiment, most of the change in soil C storage was due to reduced belowground biomass and total organic C in the top 30 cm in +N and +P only plots (soil + root C = 6.6–6.7 kg C m−2) compared to control and +NP plots (7.5–7.6 kg C m−2). Our findings suggest that macrophytes are the dominant control on changes in C accumulation in the soils at our site through the deposition of root material into the soil profile as seen by greater rhizomes and MOM in +NP plots and fewer rhizomes and MOM in +N and +P plots (Table 2). Decomposition does not appear to drive changes in soil C pools which is supported by our observations of no difference in laboratory microbial CO2 production potential (Fig. 4) and lower C-acquiring EEA in the +P and +N plots where soil C loss occurred.
Many studies show an increased potential for biogenic production of CO2 (Morris and Bradley 1999; Zheng et al. 2007; Min et al. 2011; Deegan et al. 2012), CH4 (Zheng et al. 2007), or N2O (Smith et al. 1982; Paludan and Blicher-Mathiesen 1996; Helton et al. 2014) from fertilized wetland soils that could increase the net radiative forcing of tidal wetlands and potentially shift them from a C sink to a C source. However, we saw no change in lab-incubated CO2 or N2O production, but saw increases in lab-incubated CH4 from +N plots.
Our results suggest that N eutrophication could enhance CH4 emissions through a combination of increased CH4 production and reduced CH4 oxidation. Irvine et al. (2012) showed that N fertilization enhanced CH4 emissions from both field and laboratory incubations of saltmarsh sediments by enhancing CH4 production. Liu and Greaver (2009) showed in a meta-analysis of 109 studies that CH4 oxidation was reduced by ~ 40% on average by N fertilization ranging from 1 to 56 g N m−2 yr−1.
Fate of added nutrients
After 10 yr, fertilization increased levels of pore water, exchangeable, and total soil nutrients. Though inorganic nutrients were elevated in fertilized plots, across all treatments inorganic species were < 0.01% of the annual amount of fertilizer added, indicating that the fertilizer is rapidly assimilated into biomass and/or exported from the system. We calculated the recovery of excess fertilizer N and P as the difference in ecosystem pools (dissolved, soil, plant, and microbial) between control and fertilized treatments. Between 1% and 18% of the 500 g N m−2 added over the 10 yr was recovered in the biomass and soil from +N and +NP plots. Of the added N retained in the plots, 30% was stored in aboveground biomass with 70% stored in soils, respectively (Table 6). Phosphorus recovery in biomass and soil of the +NP plots (20% of the 100 g P m−2 added) was nearly double that recovered in the +P-only plots (11%), likely because total biomass is 2–3 times greater in +NP than +P plots (Table 6). Of the added P retained in the plots, 94–97% was stored in the soil.
Table 6.
Nitrogen and phosphorus storage in macrophyte aboveground biomass and soil (0–20 cm) and amount returned via turnover of aboveground biomass in control N, P, and N + P fertilized plots in 2014. Numbers in parentheses represent the net increase in storage/turnover in response to N, P, and N + P relative to the control plots.
| Macrophyte | Soil (g m−2) | Total | Returned via turnover* (g m−2 yr−1) | |
|---|---|---|---|---|
| Nitrogen | ||||
| Control | 5.6 | 218 | 224 | 11.8 |
| Phosphorus | 7.2 | 221 | 228 | 15.1 |
| Nitrogen | 15.3 | 240 | 255 | 32.1 |
| (31) (1%)† | (20.3) (41%)‡ | |||
| N + P | 18.1 | 296 | 314 | 38.0 |
| (90) (18%) | (26.2) (52%) | |||
| Phosphorus | ||||
| Control | 0.64 | 19.0 | 19.6 | 1.34 |
| Phosphorus | 0.88 | 29.8 | 30.7 | 1.85 |
| (11.1) (11%) | (0.51) (5%) | |||
| Nitrogen | 1.28 | 21.1 | 22.4 | 2.69 |
| N + P | 1.79 | 37.4 | 39.2 | 3.76 |
| (19.6) (20%) | (2.42) (24%) | |||
Turnover rate = 2.1 based on aboveground productivity of 1100 g m−2 yr−1 (Li et al. 2018) and aboveground biomass (2014 control treatment of 546 g m−2) in Fig. 2.
Percent of N and P retained in the fertilized plots from 10 yr of N (500 g m−2) and P (100 g m−2) additions minus N and P pools in the control treatment.
Percent of N and P returned to the soil from the 2014 addition of N (50 g m−2) and P (10 g m−2) minus turnover of N and P from the control treatment.
The low measured retention of fertilizer N in the plots may be due in part to turnover of macrophyte production that was not accounted for in the calculation of pools, as well as stimulation of denitrification (Fig. 4b). Li et al. (2018) estimated aboveground productivity of unfertilized Zizaniopsis in the Altamaha River to be 1100 g m−2 yr−1. Using our aboveground biomass measurements in the control plots of 546 g m−2 yr−1 in 2014, we calculated the turnover rate (productivity/biomass) to be 2.1. Assuming the turnover rate is similar for N fertilized plots, this amounts to an additional 20.3–26.2 g N m−2 yr−1 added to the soil. This represents 41–52% of the N added in 1 yr (2014) (Table 6).
Stimulation of microbial metabolism of N, specifically N removal via the nitrification-denitrification pathway in which N is converted to atmospheric N, also may represent some of the unaccounted for N. Potential nitrification and denitrification rates measured in laboratory incubations were stimulated by N additions. In salt marshes, White and Howes (1994) estimated 54–77% of the loss of a tracer was due to coupled nitrification-denitrification. Rates of nitrification and denitrification are higher in freshwater marshes than saline marshes (Rysgaard et al. 1999; Craft et al. 2009), so it is likely that a considerable portion of added N was removed via nitrification-denitrification.
If we consider P returned to the soil via turnover, we can account for 5–24% of the 10 g P m−2 added each year which is comparable to the P stored in macrophyte and soil pools (Table 6). Our estimates of N and P turnover may underestimate long-term (10 yr) fertilizer retention since we did not measure turnover of belowground biomass. Belowground productivity of Zizaniopsis marshes of the Altamaha River is double (2231 g m−2 yr−1) that of aboveground productivity (1100 g m−2 yr−1) (Li et al. 2018). However, turnover of belowground biomass is much less (0.28 per year) than aboveground biomass (2.1 per year). Belowground biomass of Zizaniopsis also contains less N (0.73%) and P (0.66 mg g−1) (Solohin et al. unpubl.) than aboveground biomass. So, while some N and P is returned by turnover of belowground biomass, it is likely considerably less than N and P returned by turnover of aboveground biomass.
Most soil P in our fertilized and unfertilized plots likely is associated with the organic fraction. Soil pH of tidal freshwater marshes of the Altamaha River average about 5.5 (Widney et al. 2019) suggesting that most mineral P is associated with Fe and Al rather than Ca (Brady and Weil 2017). Iron-bound and Al-P concentrations of 20–100 μg g−1 soil and 5–25 μg g−1 soil (0–20 cm), respectively, were measured in a nearby Zizaniopsis-dominated tidal freshwater marsh with highest concentrations in surface (0–5 cm) soil (C. Craft unpubl. data). We estimate that soil Fe-P pools in the top 20 cm are 0.84–1.26 g Fe-P m−2, substantially less than the 19 g P m−2 measured in control plots of this experiment. These findings suggest that some of the added P is stored in association with Fe. However, P taken up by macrophytes and then deposited as organic matter (i.e., organic P) likely is more important. Organic P typically represents the largest P fraction in soil (Brady and Weil 2017). Macrophyte (Table 3) and soil P concentrations (Fig. 3) were highest in +P and +NP plots. Thus, it is likely that deposition of P enriched macrophyte biomass in these plots is incorporated into the soil as organic P (see paragraph below), contributing to long-term retention of P.
We also do not account for N and P retention via soil accretion in fertilized (and unfertilized) plots. Short-term (4 yr) and long-term (50+ years) soil accretion in Zizaniopsis marshes of the Altamaha River are 4.3 mm yr−1 and 4.05 mm yr−1, respectively (Craft 2007; Solohin et al. unpubl.), returning 8.0 g N m−2 yr−1 and 0.63 P m−2 yr−1 in unfertilized plots (Craft 2007). Craft and Richardson (1993a,b) reported that nutrient enrichment increased soil accretion by a factor of 2–3 relative to unenriched freshwater marshes of the Florida Everglades. Though we did not measure soil accretion in our fertilizer experiment, a doubling or tripling of soil accretion would increase N retention by 8–16 g m−2 yr−1 and P retention by 0.6–1.3 g m−2 yr−1 or 16–32% and 12–26% of the N and P added annually. These values are comparable to N (20–26 g m−2 yr−1) and P (0.5–2.4 g m−2 yr−1) returned via turnover in fertilized plots (Table 6).
Caveats
Limitations of our experimental design and measurement protocols include (1) potential loss of fertilizer by tidal action, (2) difficulties in scaling laboratory incubations of potential nitrification and denitrification and C fluxes to the field, and (3) linking EEA to microbial processes (i.e., laboratory incubations). Undoubtedly, there is some loss of fertilizer from the treatments from tidal inundation. However, the use of large pellets and slow release fertilizer (polymer coasted urea, pelletized triple superphosphate) reduced their loss as seen by the relatively high N (41–54%) and P (5–24%) retention based on annual biomass turnover (Table 6). Furthermore, our calculations may underestimate retention since we did not account for soil accretion (4–4.3 mm yr−1) or losses from denitrification.
Second, while it is challenging to scale laboratory incubations, nitrification, denitrification, and greenhouse gases to the field, this was not the goal of our experiment. Rather, we wanted to understand how long-term fertilization affects various macrophyte and microbial processes and to determine whether and to what extent differential (N vs. P) nutrient limitation occurs, how macrophytes and microbes compete for these resources, and who wins out over the long term. Our measurements of macrophyte and microbial communities clearly show a response to N and P additions that varies depending on whether N and P are added singly or in combination.
Finally, linking measurements of EEA to microbial processes is difficult as pointed out by Morrissey et al. (2014b). In our experiment and in Morrissey et al. who sampled a tidal freshwater marsh in Virginia, there was no correlation between CO2 production and C-acquiring enzymes (BG, CBH, BX). Morrissey et al. attributed the lack of a relationship to the high functional redundancy of microorganisms capable of producing CO2 and limited number (3) of enzymes measured. Morrissey et al. did observed a significant correlation between CH4 production and C- and N- acquiring enzymes whereas we did not observed such associations in our experiment. Regardless, our EEA and laboratory incubations (nitrification, denitrification, CH4 production) clearly show a microbial response to nutrient additions that depends on whether N and P are added singly or in combination.
Conclusions
Our results suggest that nutrient additions to a tidal freshwater marsh increase N removal via increased productivity, denitrification, and burial in soil and increased P retention in soil. However, these ecosystems respond differently to long-term increases in N and P alone or in combination, leading to differences in belowground C inputs and soil C storage. When N and P are applied singly, macrophytes reduce allocation of carbon to belowground biomass with a resultant decrease in soil C. Application of N and P together induce competition between macrophytes and microbes that is linked to differential nutrient limitation, N limitation of macrophytes vs. P limitation of microbes. Under these conditions, macrophytes increase above- and belowground biomass to outcompete microbes for P, and increase soil C storage as compared to when N and P are applied singly. Changes in soil C in our study were associated with changes in root biomass, not microbial activity, suggesting that macrophytes are the dominant control on changes in soil C accumulation. Long-term susceptibility of tidal freshwater marshes and their ability to mitigate eutrophication will depend on the quantity and relative proportion of anthropogenic (and natural) N vs. P inputs.
Acknowledgments
We thank Laura Trice, whose 2011 National Science Foundation (NSF) Research Experience for Undergraduates (REU) project inspired the present study and Anya Hopple who was instrumental in the early phases of the study. We thank Sean Graham, Josh Frost, Wes Ket, and countless other Indiana University Wetlands Laboratory students who have measured and maintained the fertilization study since 2004. A special thanks to Sarah Widney and three anonymous reviewers who made substantive improvements to the manuscript. This research was supported by funding from the National Science Foundation to C.B.C. through the NSF Long Term Ecological Research (LTER) program (Georgia Coastal Ecosystems LTER, OCE-9982133, OCE-0620959, and OCE-1237140) and to E.R.H. through the NSF GRFP (2011117001) and NSF Division of Environmental Biology (DEB) Doctoral Dissertation Improvement Grant (DDIG) program (DEB-1401070). This is contribution 1066 of the University of Georgia Marine Institute.
Footnotes
Conflict of Interest
None declared.
References
- Aerts R, and Chapin III FS 2000. The mineral nutrition of wild plants revisited: A re-evaluation of processes and patterns. Adv. Ecol. Res 30: 1–67. doi: 10.1016/S0065-2504(08)60016-1 [DOI] [Google Scholar]
- Anisfeld SC, and Hill TD 2011. Fertilization effects on elevation change and belowground carbon balance in a Long Island Sound tidal marsh. Estuaries Coast. 35: 201–211. doi: 10.1007/s12237-011-9440-4 [DOI] [Google Scholar]
- Bache B, and Williams E 1971. A phosphate sorption index for soils. J. Soil Sci 22: 289–301. doi: 10.1111/j.1365-2389.1971.tb01617.x [DOI] [Google Scholar]
- Bardgett RD, Manning P, Morriën E, and De Vries FT 2013. Hierarchical responses of plant-soil interactions to climate change: Consequences for the global carbon cycle. J. Ecol 101: 334–343. doi: 10.1111/1365-2745.12043 [DOI] [Google Scholar]
- Barendregt A, Whigham D, and Baldwin A [eds.]. 2009. Tidal freshwater wetlands. Backhuys. [Google Scholar]
- Bell CW, Fricks BE, Rocca JD, Steinweg JM, McMahon SK, and Wallenstein MD 2013. High-throughput fluorometric measurement of potential soil extracellular enzyme activities. J. Vis. Exp 81: e50961. doi: 10.3791/50961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhard AE, Tucker J, Giblin AE, and Stahl DA 2007. Functionally distinct communities of ammonia-oxidizing bacteria along an estuarine salinity gradient. Environ. Microbiol 9: 1439–1447. doi: 10.1111/j.1462-2920.2007.01260.x [DOI] [PubMed] [Google Scholar]
- Brady NC, and Weil RR 2017. The nature and properties of soils, 15th ed. Prentice Hall. [Google Scholar]
- Bridgham SD, Johnston CA, Schubauer-Berigan JP, and Weishampel P 2001. Phosphorus sorption dynamics in soils and coupling with surface and pore water in riverine wetlands. Soil Sci. Soc. Am. J 65: 577–588. doi: 10.2136/sssaj2001.652577x [DOI] [Google Scholar]
- Brookes PC, Landman A, Pruden G, and Jenkinson DS 1985. Chloroform fumigation and the release of soil nitrogen: A rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol. Biochem 17: 837–842. doi: 10.1016/0038-0717(85)90144-0 [DOI] [Google Scholar]
- Buresh RJ, DeLaune RD, and Patrick WH 1980. Nitrogen and phosphorus distribution and utilization by Spartina alterniflora in a Louisiana gulf coast marsh. Estuaries 3: 111–121. doi: 10.2307/1351555 [DOI] [Google Scholar]
- Caffrey JM, Murrell MC, Wigand C, and McKinney R 2007. Effect of nutrient loading on biogeochemical and microbial processes in a New England salt marsh. Biogeochemistry 82: 251–264. doi: 10.1007/s10533-007-9068-4 [DOI] [Google Scholar]
- Cataldo AE, Schrader LE, and Youngs VL 1974. Analysis by digestion and colorimetric assay of total nitrogen in plant tissues high in nitrate. Crop Sci. 14: 854–856. doi: 10.2135/cropsci1974.0011183X001400060024x [DOI] [Google Scholar]
- Chalmers AG 1979. The effects of fertilization on nitrogen distribution in a Spartina alterniflora salt marsh. Estuar. Coast. Mar. Sci 8: 327–337. doi: 10.1016/0302-3524(79)90050-1 [DOI] [Google Scholar]
- Chen Z, Luo X, Hu R, Wu M, Wu J, and Wei W 2010. Impact of long-term fertilization on the composition of denitrifier communities based on nitrite reductase analyses in a paddy soil. Microb. Ecol 60: 850–861. doi: 10.1007/s00248-010-9700-z [DOI] [PubMed] [Google Scholar]
- Cleland EE, and others. 2019. Belowground biomass response to nutrient enrichment depends on light limitation across globally distributed grasslands. Ecosystems. 22: 1466–1477. doi: 10.1007/s10021-0919-00350-4 [DOI] [Google Scholar]
- Cleveland CC, and Liptzin D 2007. C:N:P stoichiometry in soil: Is there a “Redfield ratio” for the microbial biomass? Biogeochemistry 85: 235–252. doi: 10.1007/s10533-007-9132-0 [DOI] [Google Scholar]
- Craft C 2007. Freshwater input structures soil properties, vertical accretion, and nutrient accumulation of Georgia and U.S tidal marshes. Limnol. Oceanogr 52: 1220–1230. doi: 10.4319/lo.2007.52.3.1220 [DOI] [Google Scholar]
- Craft C, Clough J, Ehman J, Joye S, Park R, Pennings S, Guo H, and Machmuller M 2009. Forecasting the effects of accelerated sea-level rise on tidal marsh ecosystem services. Front. Ecol. Environ 7: 73–78. doi: 10.1890/070219 [DOI] [Google Scholar]
- Craft CB, and Richardson CJ 1993a. Peat accretion and N, P, and organic C accumulation in nutrient-enriched and unenriched everglades peatlands. Ecol. Appl 3: 446–458. doi: 10.2307/1941914 [DOI] [PubMed] [Google Scholar]
- Craft CB, and Richardson CJ 1993b. Peat accretion and phosphorus accumulation along a eutrophication gradient in the northern Everglades. Biogeochemistry 22: 133–156. doi: 10.1007/BF00002708 [DOI] [Google Scholar]
- Crain CM 2007. Shifting nutrient limitation and eutrophication effects in marsh vegetation across estuarine salinity gradients. Estuaries Coast. 30: 26–34. doi: 10.1007/BF02782964 [DOI] [Google Scholar]
- Darby FA, and Turner RE 2008a. Below- and aboveground biomass of Spartina alterniflora: Response to nutrient addition in a Louisiana salt marsh. Estuaries Coast. 31: 326–334. doi: 10.1007/s12237-008-9037-8 [DOI] [Google Scholar]
- Darby FA, and Turner RE 2008b. Effects of eutrophication on salt marsh root and rhizome biomass accumulation. Mar. Ecol. Prog. Ser 363: 63–70. doi: 10.3354/meps07423 [DOI] [Google Scholar]
- Deegan LA, and others. 2007. Susceptibility of salt marshes to nutrient enrichment and predator removal. Ecol. Appl 17: 42–63. doi: 10.1890/06-0452.1 [DOI] [Google Scholar]
- Deegan LA, Johnson DS, Warren RS, Peterson BJ, Fleeger JW, Fagherazzi S, and Wollheim WM 2012. Coastal eutrophication as a driver of salt marsh loss. Nature 490: 388–392. doi: 10.1038/nature11533 [DOI] [PubMed] [Google Scholar]
- DeForest JL 2009. The influence of time, storage temperature, and substrate age on potential soil enzyme activity in acidic forest soils using MUB-linked substrates and L-DOPA. Soil Biol. Biochem 46: 1180–1186. doi: 10.1016/j.soilbio.2009.02.029 [DOI] [Google Scholar]
- Di Iorio D 2016. Long-term hydrographic mooring data from the Georgia Coastal Ecosystems LTER Salinity Monitoring Program - primary 30 minute observational data Georgia Coastal Ecosystems LTER Project. Univ. of Georgia, Long Term Ecological Research Network. doi: 10.6073/pasta/b6eaef49fa1ad5d4e1f3ce2e15b78152 [DOI] [Google Scholar]
- Dodla SK, Wang JJ, DeLaune RD, and Breitenbeck G 2009. Carbon gas production under different electron acceptors in a freshwater marsh soil. Chemosphere 76: 517–522. doi: 10.1016/j.chemosphere.2009.03.013 [DOI] [PubMed] [Google Scholar]
- Fornara DA, Banin L, and Crawley MJ 2013. Multinutrient versus nitrogen-only effects on carbon sequestration in grassland soils. Glob. Chang. Biol 19: 3848–3857. doi: 10.1111/gcb.12323 [DOI] [PubMed] [Google Scholar]
- Frost JW, Schleicher T, and Craft C 2009. Effects of nitrogen and phosphorus additions on primary production and invertebrate densities in a Georgia (USA) tidal freshwater marsh. Wetlands 29: 196–203. doi: 10.1672/07-79.1 [DOI] [Google Scholar]
- Gleeson SK, and Tilman D 1992. Plant allocation and the multiple limitation hypothesis. Am. Nat 139: 1322–1343. [Google Scholar]
- Graham SA, and Mendelssohn IA 2010. Multiple levels of nitrogen applied to an oligohaline marsh identify a plant community response sequence to eutrophication. Mar. Ecol. Prog. Ser 417: 73–82. doi: 10.3354/meps08808 [DOI] [Google Scholar]
- Graham SA, and Mendelssohn IA 2014. Coastal wetland stability maintained through counterbalancing accretionary responses to chronic nutrient enrichment. Ecology 95: 3271–3283. doi: 10.1890/14-0196.1 [DOI] [Google Scholar]
- Graham SA, and Mendelssohn IA 2016. Contrasting effects of nutrient enrichment on belowground biomass in coastal wetlands. J. Ecol 104: 249–260. doi: 10.1111/1365-2745.12498 [DOI] [Google Scholar]
- Gribsholt B, and others. 2005. Nitrogen processing in a tidal freshwater marsh: A whole-ecosystem 15N labeling study. Limnol. Oceanogr 50: 1945–1959. doi: 10.4319/lo.2005.50.6.1945 [DOI] [Google Scholar]
- Gribsholt B, Struyf E, Tramper A, De Brabandere L, and Brion N 2006. Nitrogen assimilation and short term retention in a nutrient-rich tidal freshwater marsh: A whole ecosystem 15N enrichment study. Biogeosci. Discuss 3: 1081–1119. doi: 10.5194/bg-4-11-2007 [DOI] [Google Scholar]
- Haines EB 1979. Growth dynamics of cordgrass, Spartina alterniflora loisel., on control and sewage sludge fertilized plots in a Georgia salt marsh. Estuaries 2: 50–53. doi: 10.2307/1352040 [DOI] [Google Scholar]
- Hamersley MR, and Howes BL 2005. Coupled nitrification-denitrification measured in situ in a Spartina alterniflora marsh with a 15NH4+ tracer. Mar. Ecol. Prog. Ser 299: 123–135. doi: 10.3354/meps299123 [DOI] [Google Scholar]
- Hedley MJ, and Stewart JWB 1982. Method to measure microbial phosphate in soils. Soil Biol. Biochem 14: 377–385. doi: 10.1016/0038-0717(82)90009-8 [DOI] [Google Scholar]
- Helton AM, Bernhardt ES, and Fedders A 2014. Biogeochemical regime shifts in coastal landscapes: The contrasting effects of saltwater incursion and agricultural pollution on greenhouse gas emissions from a freshwater wetland. Biogeochemistry 120: 133–147. doi: 10.1007/s10533-014-9986-x [DOI] [Google Scholar]
- Hopkinson CS 1992. A comparison of ecosystem dynamics in freshwater wetlands. Estuaries 15: 549–562. doi: 10.2307/1352397 [DOI] [Google Scholar]
- Irvine IC, Vivanco L, Bentley PN, and Martiny JBH 2012. The effect of nitrogen enrichment on C1-cycling microorganisms and methane flux in salt marsh sediments. Front. Microbiol 3: 90. doi: 10.3389/fmicb.2012.00090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson CR, and Vallaire SC 2009. Effects of salinity and nutrients on microbial assemblages in Louisiana wetland sediments. Wetlands 29: 277–287. doi: 10.1672/08-86.1 [DOI] [Google Scholar]
- Kearney MS, Riter JCA, and Turner RE 2011. Freshwater river diversions for marsh restoration in Louisiana: Twenty-six years of changing vegetative cover and marsh area. Geophys. Res. Lett 38: L16405. doi: 10.1029/2011GL047847 [DOI] [Google Scholar]
- Keeler BL, Hobbie SE, and Kellogg LE 2009. Effects of long-term nitrogen addition on microbial enzyme activity in eight forested and grassland sites: Implications for litter and soil organic matter decomposition. Ecosystems 12: 1–15. doi: 10.1007/s10021-008-9199-z [DOI] [Google Scholar]
- Ket WA, Schubauer-Berigan JP, and Craft CB 2011. Effects of five years of nitrogen and phosphorus additions on a Zizaniopsis miliacea tidal freshwater marsh. Aquat. Bot 95: 17–23. doi: 10.1016/j.aquabot.2011.03.003 [DOI] [Google Scholar]
- Koerselman W, and Meuleman AFM 1996. The vegetation N:P ratio: A new tool to detect the nature of nutrient limitation. J. Appl. Ecol 33: 1441–1450. doi: 10.2307/2404783 [DOI] [Google Scholar]
- Koop-Jakobsen K, and Giblin AE 2009. Anammox in tidal marsh sediments: The role of salinity, nitrogen loading, and marsh vegetation. Estuaries Coast. 32: 238–245. doi: 10.1007/s12237-008-9131-y [DOI] [Google Scholar]
- Koop-Jakobsen K, and Giblin AE 2010. The effect of increased nitrate loading on nitrate reduction via denitrification and DNRA in salt marsh sediments. Limnol. Oceanogr 55: 789–802. doi: 10.4319/lo.2010.55.2.0789 [DOI] [Google Scholar]
- Kuzyakov Y, and Xu X 2013. Competition between roots and microorganisms for nitrogen: Mechanisms and relevance. New Phytol. 198: 656–669. doi: 10.1111/nph.12235 [DOI] [PubMed] [Google Scholar]
- Lamers LPM, and others. 2012. Microbial transformations of nitrogen, sulfur, and iron dictate vegetation composition in wetlands: A review. Front. Microbiol 3: 1–12. doi: 10.3389/fmicb.2012.00156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley JA, Mozdzer TJ, Shepard KA, Hagerty SB, and Megonigal PJ 2013. Tidal marsh plant responses to elevated CO2, nitrogen fertilization, and sea level rise. Glob. Chang. Biol 19: 1495–1503. doi: 10.1111/gcb.12147 [DOI] [PubMed] [Google Scholar]
- Langner CL, and Hendrix PF 1982. Evaluation of a persulfate digestion method for particulate nitrogen and phosphorus. Water Res. 16: 1451–1454. doi: 10.1016/0043-1354(82)90243-3 [DOI] [Google Scholar]
- Li S, Hopkinson CS, Schubauer-Berigan JP, and Pennings SC 2018. Climate drivers of Zizaniopsis miliacea biomass in a Georgia, U.S.A. tidal fresh marsh. Limnol. Oceanogr 63: 2266–2276. doi: 10.1002/lno.10937 [DOI] [Google Scholar]
- Liu L, and Greaver T 2009. A review of nitrogen enrichment effects on three biogenic GHGs: The CO2 sink may be largely offset by stimulated N2O and CH4 emissions. Ecol. Lett 10: 1103–1107. doi: 10.1111/j.1461-0248.2009.01351.x [DOI] [PubMed] [Google Scholar]
- Loomis MJ, and Craft CB 2010. Carbon sequestration and nutrient (nitrogen, phosphorus) accumulation in riverdominated tidal marshes, Georgia, USA. Soil Sci. Soc. Am. J 74: 1028–1036. doi: 10.2136/sssaj2009.0171 [DOI] [Google Scholar]
- Lu M, Zhou X, Luo Y, Yang Y, Fang C, Chen J, and Li B 2011. Minor stimulation of soil carbon by nitrogen addition: A meta-analysis. Agric. Ecosyst. Environ 140: 234–244. doi: 10.1016/j.agee.2010.12.010 [DOI] [Google Scholar]
- Lu Y, Murase J, Watanabe A, Sugimoto A, and Kimura M 2004. Linking microbial community dynamics to rhizosphere carbon flow in a wetland rice soil. FEMS Microbiol. Ecol 48: 179–186. doi: 10.1016/j.femsec.2004.01.004 [DOI] [PubMed] [Google Scholar]
- Mack MC, Schurr EAG, Syndonia Bret-Harte M, Shaver GR, and Chapin III FS 2004. Ecosystem carbon storage in arctic tundra reduced by long-term nutrient fertilization. Nature 431: 440–443. doi: 10.1038/nature02887 [DOI] [PubMed] [Google Scholar]
- Min K, Kang H, and Lee D 2011. Effects of ammonium and nitrate additions on carbon mineralization in wetland soils. Soil Biol. Biochem 43: 2461–2469. doi: 10.1016/j.soilbio.2011.08.019 [DOI] [Google Scholar]
- Morris J, and Bradley P 1999. Effects of nutrient loading on the carbon balance of coastal wetland sediments. Limnol. Oceanogr 44: 699–702. doi: 10.4319/lo.1999.44.3.0699 [DOI] [Google Scholar]
- Morris JT, Sundareshwar PV, Nietch CT, Kjerve B, and Cahoon DR 2002. Responses of coastal wetlands to rising sea level. Ecology 83: 2869–2877. doi: 10.1890/0012-9658(2002)083[2869:ROCWTR]2.0.CO;2 [DOI] [Google Scholar]
- Morris JT, Shaffer GP, and Nyman JA 2013. Brinson review: Perspectives on the influence of nutrients on the sustainability of coastal wetlands. Wetlands 33: 975–988. doi: 10.1007/s13157-013-0480-3 [DOI] [Google Scholar]
- Morrissey EM, Gillespie JM, Morina JC, and Franklin RB 2014a. Salinity affects microbial activity and soil organic matter content in tidal wetlands. Glob. Chang. Biol 20: 1351–1362. doi: 10.1111/gcb.12431 [DOI] [PubMed] [Google Scholar]
- Morrissey EM, Berrier DJ, Neubauer SC, and Franklin RB 2014b. Using microbial communities and extracellular enzymes to link organic matter characteristics to greenhouse gas production in a tidal freshwater marsh. Biogeochemistry 117: 473–490. doi: 10.1007/s10533-013-9894-5 [DOI] [Google Scholar]
- Mueller KE, Blumenthal DM, Pendall E, Carrillo Y, Dijkstra FA, Williams DG, Follett RF, and Morgan JA 2016. Impacts of warming and elevated CO2 on a semi-arid grassland are non-additive, shift with precipitation, and reverse over time. Ecol. Lett 19: 956–966. doi: 10.1111/ele.12634 [DOI] [PubMed] [Google Scholar]
- Murphy J, and Riley JP 1962. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 27: 31–36. doi: 10.1016/S0003-2670(00)88444-5 [DOI] [Google Scholar]
- Murray CN, and Riley JP 1971. The solubility of gases in distilled water and sea water—IV. Carbon dioxide. Deep-Sea Res. Oceanogr. Abstr 18: 533–541. doi: 10.1016/0011-7471(71)90077-5 [DOI] [Google Scholar]
- Neubauer SC 2008. Contributions of mineral and organic components to tidal freshwater marsh accretion. Estuar. Coast. Shelf Sci 78: 78–88. doi: 10.1016/j.ecss.2007.11.011 [DOI] [Google Scholar]
- Neubauer SC, Franklin RB, and Berrier DJ 2013. Saltwater intrusion into tidal freshwater marshes alters the biogeochemical processing of organic carbon. Biogeosciences 10: 8171–8183. doi: 10.5194/bg-10-8171-2013 [DOI] [Google Scholar]
- Neubauer SC, and Megonigal JP 2015. Moving beyond global warming potentials to quantify the climatic role of ecosystems. Ecosystems 18: 1000–1013. doi: 10.1007/s10021-015-9879-4 [DOI] [Google Scholar]
- Nikolausz M, Kappelmeyer U, Székely A, Ruznyak A, Marialigeti K, and Kastner M 2008. Diurnal redox fluctuation and microbial activity in the rhizosphere of wetland plants. Eur. J. Soil Biol 44: 324–333. doi: 10.1016/j.ejsobi.2008.01.003 [DOI] [Google Scholar]
- Odum W 1988. Comparative ecology of tidal freshwater and salt marshes. Annu. Rev. Ecol. Syst 19: 147–176. doi: 10.1146/annurev.es.19.110188.001051 [DOI] [Google Scholar]
- Paludan C, and Blicher-Mathiesen G 1996. Losses of inorganic carbon and nitrous oxide from a temperate freshwater wetland in relation to nitrate loading. Biogeochemistry 35: 305–326. doi: 10.1007/BF02179957 [DOI] [Google Scholar]
- Paludan C, and Morris JT 1999. Distribution and speciation of phosphorus along a salinity gradient in intertidal marsh sediments. Biogeochemistry 45: 197–221. doi: 10.1023/A:1006136621465 [DOI] [Google Scholar]
- Penton CR, and Newman S 2007. Enzyme activity responses to nutrient loading in subtropical wetlands. Biogeochemistry 84: 83–98. doi: 10.1007/s10533-007-9106-2 [DOI] [Google Scholar]
- Poffenbarger HJ, Needelman BA, and Megonigal JP 2011. Salinity influence on methane emissions from tidal marshes. Wetlands 31: 831–842. doi: 10.1007/s13157-011-0197-0 [DOI] [Google Scholar]
- Rabalais NN, Turner RE, Díaz RJ, and Justić E 2009. Global change and eutrophication of coastal waters. ICES J. Mar. Sci 66: 1528–1537. doi: 10.1093/icesjms/fsp047 [DOI] [Google Scholar]
- Reddy KR, and DeLaune RD 2008. Biogeochemistry of wetlands: Science and applications. CRC Press. [Google Scholar]
- Reich PB, and Orelsyn J 2004. Global patterns of plant leaf N and P in relation to temperature and latitude. Proc. Natl. Acad. Sci. USA 101: 11001–11006. doi: 10.1073/pnas.0403588101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson CJ 1985. Mechanisms controlling phosphorus retention capacity in freshwater wetlands. Science 228: 1424–1427. doi: 10.1126/science.228.4706.1424 [DOI] [PubMed] [Google Scholar]
- Rysgaard S, Thastum P, Dalsgaard T, Christensen PB, and Sloth NP 1999. Effects of salinity on NH4+ adsorption capacity, nitrification, and denitrification in Danish estuarine sediments. Estuaries 22: 21–30. doi: 10.2307/1352923 [DOI] [Google Scholar]
- Schubauer JP, and Hopkinson CS 1984. Above- and belowground emergent macrophyte production and turnover in a coastal marsh ecosystem, Georgia. Limnol. Oceanogr 29: 1052–1065. doi: 10.4319/lo.1984.29.5.1052 [DOI] [Google Scholar]
- Selman M, Greenhalgh S, Diaz R, and Sugg Z 2008. Eutrophication and hypoxia in coastal areas: A global assessment of the state of knowledge, p. 1–6. World Resources Institute Policy Note, No. 1. [Google Scholar]
- Shifflet SD, and Schubauer-Berigan JP 2019. Assessing the risk of utilizing tidal coastal wetlands for wastewater management. J. Environ. Manage 236: 269–279. doi: 10.1016/j.jenvman.2018.12.082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CJ, Brandon M, and Patrick WH 1982. Nitrous oxide emission following urea-N fertilization of wetland rice. J. Soil Sci. Plant Nutr 28: 161–171. doi: 10.1080/00380768.1982.10432433 [DOI] [Google Scholar]
- Smith MS, and Tiedje JM 1979. Phases of denitrification following oxygen depletion in soil. Soil Biol. Biochem. 11: 261–267. doi: 10.1016/0038-0717(79)90071-3 [DOI] [Google Scholar]
- Smith VH, Joye SB, and Howarth RW 2006. Eutrophication of freshwater and marine ecosystems. Limnol. Oceanogr. 51: 351–355. doi: 10.4319/lo.2006.51.1_part_2.0351 [DOI] [Google Scholar]
- Sommers LE, and Nelson DW 1972. Determination of total phosphorus in soils: A rapid perchloric acid digestion procedure. Soil Sci. Soc. Am. J 36: 902–904. doi: 10.2136/sssaj1972.03615995003600060020x [DOI] [Google Scholar]
- Sterner RW, and Elser JJ 2000. Ecological stoichiometry. Princton Univ. Press. [Google Scholar]
- Sundareshwar PV, and Morris JT 1999. Phosphorus sorption characteristics of intertidal marsh sediments along an estuarine salinity gradient. Limnol. Oceanogr 44: 1693–1701. doi: 10.4319/lo.1999.44.7.1693 [DOI] [Google Scholar]
- Sundareshwar PV, Morris JT, Koepfler EK, and Fornwalt B 2003. Phosphorus limitation of coastal ecosystem processes. Science 299: 563–565. doi: 10.1126/science.1079100 [DOI] [PubMed] [Google Scholar]
- Sutton-Grier AE, and Megonigal JP 2011. Plant species traits regulate methane production in freshwater wetland soils. Soil Biol. Biochem 43: 413–420. doi: 10.1016/j.soilbio.2010.11.009 [DOI] [Google Scholar]
- Swarzenski CM, Doyle TW, Fry B, and Hargis TG 2008. Biogeochemical response of organic-rich freshwater marshes in the Louisiana delta plain to chronic river water influx. Biogeochemistry 90: 49–63. doi: 10.1007/s10533-008-9230-7 [DOI] [Google Scholar]
- Turner RE, Howes BL, Teal JM, Milan CS, Swenson EM, and Toner DDG 2009. Salt marshes and eutrophication: An unsustainable outcome. Limnol. Oceanogr 54: 1634–1642. doi: 10.4319/lo.2009.54.5.1634 [DOI] [Google Scholar]
- Valiela I 2015. The Great Sippewissett salt marsh plots – some history, highlights and contrails from a long-term study. Estuaries Coast. 38: 1099–1120. doi: 10.1007/s12237-015-9976-9 [DOI] [Google Scholar]
- Valiela I, Teal JM, and Sass W 1973. Nutrient retention in salt marsh plots experimentally fertilized with sewage sludge. Estuar. Coast. Mar. Sci 1: 261–269. doi: 10.1016/0302-3524(73)90039-X [DOI] [Google Scholar]
- Valiela I, and Teal JM 1974. Nutrient limitation in salt marsh vegetation, p. 547–563. In Reimold RJ and Queen WH [eds.], Ecology of halophytes Academic Press. [Google Scholar]
- Valiela I, Teal JM, and Persson NY 1976. Production and dynamics of experimentally enriched salt marsh vegetation. Limnol. Oceanogr 21: 245–252. doi: 10.4319/lo.1976.21.2.0245 [DOI] [Google Scholar]
- Van Der Nat FJWA, and Middelburg JJ 1998. Seasonal variation in methane oxidation by the rhizosphere of Phragmites australis and Scirpus lacustris. Aquat. Bot 61: 95–110. doi: 10.1016/S0304-3770(98)00072-2 [DOI] [Google Scholar]
- Vance ED, Brooks PC, and Jenkinson DS 1987. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 19: 703–707. doi: 10.1016/0038-0717(87)90052-6 [DOI] [Google Scholar]
- Verhoeven JT, Arheimer B, Yin C, and Hefting MM 2006. Regional and global concerns over wetlands and water quality. Trends Ecol. Evol 21: 96–103. doi: 10.1016/j.tree.2005.11.015 [DOI] [PubMed] [Google Scholar]
- Wall LG, Tank JL, Royer TV, and Bernot MJ 2005. Spatial and temporal variability in sediment denitrification within an agriculturally influenced reservoir. Biogeochemistry 76: 85–111. doi: 10.1007/s10533-005-2199-6 [DOI] [Google Scholar]
- Wardle DA, Bardgett RD, Klironomos JN, Setälä H, van der Putten WH, and Wall DH 2004. Ecological linkages between aboveground and belowground biota. Science 304: 1629–1633. doi: 10.1126/science.1094875 [DOI] [PubMed] [Google Scholar]
- Weiss RF, and Price A 1980. Nitrous oxide solubility in water and seawater. Mar. Chem 8: 347–359. doi: 10.1016/0304-4203(80)90024-9 [DOI] [Google Scholar]
- White DS, and Howes BL 1994. Long-term 15N-nitrogen retention in the vegetated sediments of a New England salt marsh. Limnol. Oceanogr 39: 1878–1892. doi: 10.4319/lo.1994.39.8.1878 [DOI] [Google Scholar]
- Widney S, Smith D, Herbert E, Schubauer-berigan J, Li F, Pennings SC, and Craft C 2019. Chronic but not acute saltwater intrusion leads to large release of inorganic N in a tidal freshwater marsh. Sci. Total Environ 695: 1–111. doi: 10.1016/j.scitotenv.2019.133779 [DOI] [PubMed] [Google Scholar]
- Wiesenburg DA, and Guinasso NL 1979. Equilibrium solubilities of methane, carbon monoxide, and hydrogen in water and sea water. J. Chem. Eng. Data 24: 356–360. doi: 10.1021/je60083a006 [DOI] [Google Scholar]
- Wieski K, Guo H, Craft CB, and Pennings SC 2010. Ecosystem functions of tidal fresh, brackish, and salt marshes on the Georgia coast. Estuaries Coast. 33: 161–169. doi: 10.1007/s12237-009-9230-4 [DOI] [Google Scholar]
- Wigand C, Davey E, Johnson R, Sundberg K, Morris J, Kenny P, Smith E, and Holt M 2015. Nutrient effects on belowground organic matter in a minerogenic salt marsh, North Inlet, SC. Estuaries Coast. 38: 1838–1853. doi: 10.1007/s12237-014-9937-8 [DOI] [Google Scholar]
- Wright AL, and Reddy KR 2001. Phosphorus loading effects on extracellular enzyme activity in Everglades wetland soils. Soil Sci. Soc. Am. J 65: 588–595. doi: 10.2136/sssaj2001.652588x [DOI] [Google Scholar]
- Zheng J, Zhang X, Li L, Zhang P, and Pan G 2007. Effect of long-term fertilization on C mineralization and production of CH4 and CO2 under anaerobic incubation from bulk samples and particle size fractions of a typical paddy soil. Agric. Ecosyst. Environ 120: 129–138. doi: 10.1016/j.agee.2006.07.008 [DOI] [Google Scholar]