Presentation of Case
Dr. Daniel A. Zlotoff: A 44-year-old woman was admitted to this hospital because of shortness of breath and chest pain.
Eight days before admission — and 3 days after her husband had begun to have fatigue, a nonproductive cough, and a fever — the patient started to have chills, a sore throat, a nonproductive cough, and myalgias. After 2 days of progressive symptoms, she contacted her primary care physician. A telemedicine visit was arranged as part of a local public health strategy to reduce the spread of coronavirus disease 2019 (Covid-19), the disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), during the pandemic. The patient reported that she had rib soreness with coughing, as well as a temperature of 35.3°C. Infection with SARS-CoV-2 was suspected. Rest, isolation measures to reduce viral transmission, and increased oral intake of fluid were recommended, along with acetaminophen and dextromethorphan–guaifenesin as needed. Three days later, she had diarrhea and back pain, but the coughing had become less frequent. During a follow-up telemedicine visit, the primary care physician recommended that the patient take acetaminophen as needed for back pain and counseled her to seek in-person medical evaluation if symptoms worsened.
Three days later, the patient started to have chest pain that was different from the rib soreness with coughing; the chest pain was present at rest and was accompanied by new dyspnea. She called emergency medical services. On the initial evaluation, the heart rate was 116 beats per minute, the systolic blood pressure 110 mm Hg, the respiratory rate 20 breaths per minute, and the oxygen saturation 99% while she was breathing ambient air. The patient was brought by ambulance to the emergency department of this hospital.
On arrival, the patient reported feeling weak, light-headed, and feverish, with chills. She reported that the chest discomfort felt like pressure, was primarily located in the anterior chest, and was mild in intensity but worsened with deep inspiration or coughing. She also reported nausea and a few episodes of nonbloody diarrhea. She had received an influenza vaccine 6 months earlier.
The patient’s medical history was notable for gastroesophageal reflux disease with histologic evidence of intestinal metaplasia of the esophagus, chronic abdominal bloating and constipation, mild obstructive sleep apnea, subclinical hyperthyroidism, uterine cysts, and depression. Medications included trazodone and acetaminophen as needed. There were no known drug allergies.
The patient was born in Central America and had immigrated to the United States approximately 20 years earlier. She lived in New England with her husband and teenage children. She worked as a custodian but had no known exposure to dust, allergens, or solvents. She did not smoke tobacco, drink alcohol, or use illicit drugs. Her mother had had hypertension and a myocardial infarction; her father had died of genitourinary cancer, and multiple paternal relatives had a history of cancer.
On examination, the temperature was 36.4°C, the heart rate 103 beats per minute, the blood pressure 79/51 mm Hg, the respiratory rate 30 breaths per minute, and the oxygen saturation 99% while the patient was breathing ambient air. The body-mass index (the weight in kilograms divided by the square of the height in meters) was 23.7. The patient appeared pale and slightly diaphoretic. She was able to speak in full sentences but appeared lethargic. The lungs were clear on auscultation. The heart was tachycardic, with normal first and second heart sounds (S1 and S2) and no gallops (S3 or S4). There was mild, diffuse abdominal tenderness on palpation. The legs were cool to the touch. The remainder of the examination was normal. Lactated Ringer’s solution was administered intravenously.
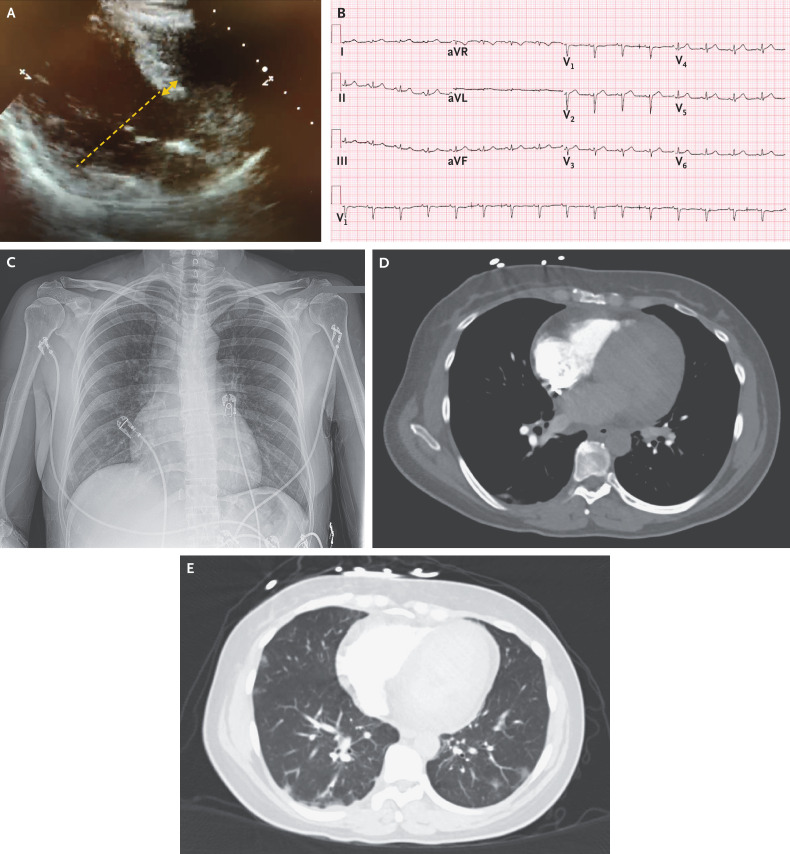
Dr. Judy Hung: Point-of-care cardiac ultrasonography revealed severely depressed left ventricular function. The left ventricular wall thickness was normal; the left ventricular cavity was mildly dilated (Figure 1A; also see Video 1, available with the full text of this article at NEJM.org). A trace pericardial effusion was visible.
Figure 1. Studies Obtained on Presentation.
A point-of-care cardiac ultrasonographic image (Panel A) shows that the left ventricle is mildly dilated, with a left ventricular end-diastolic dimension of 55 mm (dashed line), which suggests that the cardiac dysfunction is acute. The left ventricular wall thickness is normal, with an interventricular septal wall thickness of 9 mm (double arrow). An electrocardiogram (Panel B) shows sinus rhythm at 96 beats per minute, low QRS voltage, and submillimeter ST-segment elevation in leads I and aVL. A chest radiograph (Panel C) shows clear lungs, without evidence of focal airspace consolidation or pulmonary edema. Computed tomographic angiographic images of the chest obtained in the pulmonary angiographic phase (Panels D and E) show excellent opacification of the right side of the heart and pulmonary arteries but poor opacification of the left side of the heart and aorta, which suggests cardiac dysfunction (Panel D). Scattered, predominantly peripheral ground-glass opacities in the lingula, the right middle lobe, and both lower lobes are present (Panel E), as is a trace pleural effusion. Although the pulmonary findings are nonspecific, they are suggestive of inflammation or infection that, in this clinical context, is likely to be due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Dr. Zlotoff: On blood testing, human chorionic gonadotropin was undetectable. Blood levels of magnesium, bilirubin, alkaline phosphatase, and free thyroxine were normal, as were the prothrombin time, international normalized ratio, and partial-thromboplastin time; other test results are shown in Table 1. A nasopharyngeal swab was obtained for testing, and blood was obtained for culture. Intravenous vancomycin and cefepime were administered.
Table 1. Laboratory Data.*.
| Variable | Reference Range, Adults† | On Arrival, This Hospital | 12 Hr after Arrival, ICU | During ECMO, Hospital Day 2 |
|---|---|---|---|---|
| Days after symptom onset | 8 | 9 | 9 | |
| Hemoglobin (g/dl) | 12.0–16.0 | 13.4 | 13.4 | 9.6 |
| Hematocrit (%) | 36.0–46.0 | 40.3 | 39.7 | 28.2 |
| White-cell count (per μl) | 4500–11,000 | 7240 | 13,270 | 9330 |
| Differential count (%) | ||||
| Neutrophils | 40–70 | 67.7 | 76.5 | |
| Lymphocytes | 22–44 | 23.9 | 16.2 | |
| Monocytes | 4–11 | 8.0 | 6.8 | |
| Eosinophils | 0–8 | 0.0 | 0.0 | |
| Platelet count (per μl) | 150,000–400,000 | 119,000 | 124,000 | 85,000 |
| Sodium (mmol/liter) | 135–145 | 139 | 143 | 148 |
| Potassium (mmol/liter) | 3.4–5.0 | 3.9 | 4.9 | 4.1 |
| Chloride (mmol/liter) | 98–108 | 100 | 103 | 111 |
| Carbon dioxide (mmol/liter) | 23–32 | 22 | 19 | 26 |
| Urea nitrogen (mg/dl) | 8–25 | 14 | 25 | 14 |
| Creatinine (mg/dl) | 0.60–1.50 | 0.82 | 1.19 | 0.73 |
| Glucose (mg/dl) | 70–110 | 160 | 235 | 139 |
| Lactate (mmol/liter) | 0.5–2.0 | 5.5 | 10.1 | 4.1 |
| Calcium (mg/dl) | 8.5–10.5 | 8.4 | 7.8 | 8.5 |
| Ionized calcium (mmol/liter) | 1.14–1.30 | 1.04 | 1.10 | |
| Phosphorus (mg/dl) | 2.6–4.5 | 3.6 | 2.0 | 1.9 |
| Alanine aminotransferase (IU/liter) | 7–33 | 242 | 225 | |
| Aspartate aminotransferase (IU/liter) | 9–32 | 186 | 178 | |
| Albumin (g/dl) | 3.3–5.0 | 3.7 | 3.0 | |
| Creatine kinase (IU/liter) | 40–150 | 292 | 501 | |
| N-terminal pro–B-type natriuretic peptide (pg/ml) | 0–450 | 6381 | ||
| High-sensitivity troponin T (ng/liter) | 0–9 | 375 | 1810 | 1282 |
| d-dimer (ng/ml) | <500 | 4903 | 3489 | |
| Lactate dehydrogenase (IU/liter) | 110–210 | 297 | 346 | |
| Mixed venous oxygen saturation (%) | 65.0–75.0 | 46.2 | ||
| Erythrocyte sedimentation rate (mm/hr) | 0–20 | 2 | ||
| C-reactive protein (mg/liter) | <0.7 | 4.3 | ||
| Interleukin-6 (pg/ml) | 0–15.5 | 18.0 | ||
| Arterial blood gases | ||||
| Fraction of inspired oxygen | 0.60 | 0.40 | ||
| pH | 7.35–7.45 | 7.40 | 7.38 | |
| Partial pressure of carbon dioxide (mm Hg) | 35–42 | 31 | 47 | |
| Partial pressure of oxygen (mm Hg) | 80–100 | 220 | 229 |
To convert the values for urea nitrogen to millimoles per liter, multiply by 0.357. To convert the values for creatinine to micromoles per liter, multiply by 88.4. To convert the values for glucose to millimoles per liter, multiply by 0.05551. To convert the values for lactate to milligrams per deciliter, divide by 0.1110. ECMO denotes extracorporeal membrane oxygenation, and ICU intensive care unit.
Reference values are affected by many variables, including the patient population and the laboratory methods used. The ranges used at the Massachusetts General Hospital are for adults who are not pregnant and do not have medical conditions that could affect the results. They may therefore not be appropriate for all patients.
Electrocardiography (ECG) showed sinus rhythm at 96 beats per minute, submillimeter ST-segment elevation in leads I and aVL, and low QRS voltage (Figure 1B). Results of chest radiography were normal (Figure 1C).
One hour after the administration of intravenous fluids and antibiotic agents, hypotension persisted and treatment with intravenous norepinephrine was initiated. The patient had a brief episode of unresponsiveness in the context of worsened hypotension, and intravenous dobutamine was added. Repeat bedside cardiac ultrasonography revealed severe left ventricular dysfunction, a small anterior pericardial effusion, and a dilated inferior vena cava without respirophasic variation; the left ventricular wall thickness was normal.
Dr. Andrey Rupasov: Computed tomographic (CT) angiography of the chest, performed after the administration of intravenous contrast material, revealed no evidence of pulmonary embolism. Images of the heart and great vessels showed excellent opacification of the right side of the heart and pulmonary arteries but poor opacification of the left side of the heart and aorta due to prolongation of the pulmonary circulation time, which is suggestive of cardiac dysfunction (Figure 1D). Radiographically significant coronary-artery calcification was absent. Images of the lungs showed a few scattered, predominantly peripheral ground-glass opacities in the lingula, the right middle lobe, and both lower lobes (Figure 1E), as well as a trace pleural effusion. Although the pulmonary findings are nonspecific, they are suggestive of inflammation or infection that, in this clinical context, is likely to be due to SARS-CoV-2. Because the patient had abdominal tenderness and hypotension, a CT scan of the abdomen and pelvis was also obtained; the images showed a small amount of ascites and periportal edema, findings that are indicative of elevated central venous pressure.
Dr. Zlotoff: Three hours after the initial presentation, the patient was admitted to the cardiac intensive care unit (ICU). She was lethargic and reported nausea. The temperature was 36.4°C, the heart rate 121 beats per minute, the blood pressure 99/79 mm Hg while she was receiving norepinephrine and dobutamine, the respiratory rate 24 breaths per minute, and the oxygen saturation 98% while she was breathing ambient air. The arms and legs were cool.
Specialists in heart failure, critical care, and cardiac surgery were consulted. During the next 4 hours, catheters were placed in the radial artery and in the pulmonary artery through the right internal jugular vein. Arterial blood gas measurements, obtained while the patient was breathing ambient air, were notable for a pH of 7.45, a partial pressure of carbon dioxide of 20 mm Hg, and a partial pressure of oxygen of 103 mm Hg. The central venous pressure was 21 mm Hg, the pulmonary arterial pressure 37/19 mm Hg, the pulmonary capillary wedge pressure 25 mm Hg, and the thermodilution cardiac output 2.2 liters per minute. The dose of dobutamine was increased. Testing of the nasopharyngeal swab was negative for influenza A and B virus and respiratory syncytial virus DNA but was positive for SARS-CoV-2 RNA.
During the next 3 hours, the patient remained lethargic, with light-headedness, nausea, and one episode of nonbloody emesis. The temperature increased to 37.9°C, the heart rate to 140 beats per minute, and the respiratory rate to 31 breaths per minute; the blood pressure was labile, with intermittent episodes of a systolic blood pressure lower than 70 mm Hg despite treatment with norepinephrine and dobutamine. The trachea was intubated and mechanical ventilation was initiated to reduce cardiopulmonary metabolic demands. Intravenous vasopressin and milrinone were added. The heart rate remained higher than 140 beats per minute, the systolic blood pressure remained lower than 70 mm Hg, and the thermodilution cardiac output was 1.7 liters per minute. Oliguria developed. Additional laboratory studies were obtained (Table 1). Urgent management decisions were made.
Differential Diagnosis
Dr. Christopher Newton-Cheh: This patient with SARS-CoV-2 infection had severe, presumably reversible cardiovascular dysfunction in the absence of clinically significant pulmonary manifestations of infection. Several published case reports suggest that her presentation, although uncommon, is not unique.1-9
The basis of myocardial dysfunction in patients with Covid-19 has not been elucidated, but several pathobiologic mechanisms have been suggested, including stress cardiomyopathy, macrovascular or microvascular supply–demand mismatch, cytokine storm, and myocarditis with or without pericarditis. It seems likely that each of these mechanisms would be found in some cases of Covid-19–associated myocardial dysfunction, but none would be a single unifying cause to explain all cases. I will consider each of these mechanisms as a potential cause of this patient’s myocardial dysfunction, in light of our prepandemic understanding of these syndromes and our evolving understanding of SARS-CoV-2, other coronaviruses, and the heart.
Stress Cardiomyopathy
Stress cardiomyopathy is a syndrome characterized by chest discomfort, ST-segment abnormalities on ECG, elevated troponin levels, and myocardial dysfunction in a noncoronary distribution on echocardiography.10 It classically occurs in older women and typically follows emotional or physical stress. The underlying mechanisms are not completely understood, but given the phenotypic overlap of this syndrome with the cardiomyopathy that can follow intracranial hemorrhage or electroconvulsive therapy — in particular, the frequent development of T-wave inversion and QT-interval prolongation — a neurocardiogenic mechanism has been suggested.11 This patient had precipitous cardiovascular collapse, a mildly elevated troponin level, and subtle ST-segment elevation, features that are compatible with this syndrome. However, she was relatively young, did not have a clear stressor other than mild Covid-19 symptoms, and did not have apical ballooning (the takotsubo pattern, which is seen in three fourths of cases). These factors make stress cardiomyopathy an unlikely diagnosis in this case.
Macrovascular or Microvascular Supply–Demand Mismatch
Elevated troponin levels are common among hospitalized patients with Covid-19 and are associated with an increased risk of arrhythmias, hypoxemic respiratory failure, and death.12-15 Stress and infection can provoke plaque rupture in the epicardial coronary arteries that results in myocardial infarction. However, this young woman did not have any known cardiovascular risk factors or coronary calcification, and she had global myocardial dysfunction rather than regional myocardial dysfunction, which would be accompanied by a much greater elevation in the troponin level than was seen in this case.
In patients with Covid-19, elevated d-dimer levels have been associated with severe illness, often with multisystemic dysfunction. This association has led to the hypothesis that microvascular thrombosis from a thromboinflammatory state may be widespread among these patients.12 Disseminated intravascular coagulation seems unlikely in this patient, given the modest thrombocytopenia and minimal evidence of a consumptive coagulopathy; the elevation of the activated partial-thromboplastin time that occurs in some cases has been suggested to result from a lupus anticoagulant.16,17 Microthrombi in the lung, kidney, and liver have been reported in some series, but microthrombi in the heart have not been described in reports regarding patients with severe myocardial dysfunction2,5 or in reports from postmortem examinations of patients with Covid-19, including some who died from cardiovascular causes and some who died from noncardiovascular causes.18-20 Microthrombi that are widespread enough to cause the degree of myocardial dysfunction seen in this patient would probably cause a much higher and more sustained elevation of the cardiac troponin T level than was seen in this case. However, endotheliitis, which has been reported in multiple tissues,21,22 may result from direct viral entry or cytokine-mediated effects on the endothelium that potentially alter its permeability and lead to myocardial edema.
Cytokine Storm
A precipitous rise in inflammatory cytokines has been observed in patients who receive chimeric antigen receptor (CAR) T-cell therapy and probably plays some role in the multisystemic dysfunction that can occur in patients with severe Covid-19, which is marked by very high levels of C-reactive protein, interleukin-6, and ferritin.23 Cytokine storm from CAR T-cell therapy has been associated with myocardial dysfunction and arrhythmias.24 In patients with Covid-19, a course that progresses from mild-to-moderate illness to acute respiratory distress syndrome followed by myocardial dysfunction is commonly accompanied by an elevation in ferritin and interleukin-6 levels.12 Trials of interleukin-6 and other cytokine antagonists are ongoing. In this case, the levels of C-reactive protein and interleukin-6 were minimally elevated and pulmonary involvement was not prominent at the time of severe myocardial dysfunction. These findings led us to initially rule out a role for systemic inflammation. Whether locally produced inflammatory mediators in the heart could explain this patient’s myocardial dysfunction in the absence of clinically significant pulmonary involvement at the outset remains unknown.
Myocarditis with or without Pericarditis
Many reports are emerging in the Covid-19 literature that suggest that myocardial dysfunction may reflect myocarditis with or without pericardial involvement.1-4,6,7 In theory, the presence of angiotensin-converting–enzyme 2 (ACE2) receptors on cardiomyocytes could be a mechanism by which SARS-CoV-2 causes lymphocytic myocarditis.
This patient had pleuritic chest pain, a pericardial effusion, low QRS voltage with subtle ST-segment elevation on ECG, and a rapid rise in the cardiac troponin level, findings that are all of a precipitous nature and are clinically suggestive of fulminant myopericarditis. Identification of increased ventricular wall thickness would also correlate with myocardial edema and myocarditis. The two echocardiographic studies obtained early after presentation showed normal wall thickness, but subsequent echocardiography revealed an increase in left ventricular wall thickness. Since the lung is an important source of systemic inflammation in severe Covid-19, the relatively low initial levels of inflammatory markers led us to rule out cytokine storm, but it is possible that the biomarker profile was measured at an early stage in the clinical course, especially given that acute respiratory distress syndrome had not developed.
However, because there is a lack of convincing histologic evidence associated with lymphocytic myocarditis in cases reported to date,25 a less specific term such as “acute inflammatory cardiomyopathy” may be more appropriate.2,5,15,22 Most of the clinical findings in this case could be explained solely by myocardial edema, potentially on the basis of diffuse endothelial permeability, without an accompanying inflammatory-cell infiltrate or direct viral entry in cardiomyocytes. MRI has largely replaced routine endomyocardial biopsy in the evaluation of suspected lymphocytic myocarditis.25 However, T2-weighted signal and late gadolinium enhancement result from myocardial edema that cannot be distinguished from edema from nonmyocardial causes such as primary endothelial injury. Owing to concern about infection control and the patient’s critical illness, MRI was not performed.
Clinical Diagnosis
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, with isolated acute cardiomyopathy and refractory cardiogenic shock.
Discussion of Management
Dr. Masaki Funamoto: If acute cardiogenic shock is not reversed, it is associated with a high mortality, with death resulting from the progressive development of end-organ dysfunction. In this case, we followed our institutional practice to implement a multidisciplinary shock-team approach that involves rapid consultation with cardiac intensivists, cardiac surgeons, and cardiologists. This approach, which calls for consideration of the full range of options available for mechanical and pharmacologic support, has led to improved outcomes in patients with cardiogenic shock.26
Extracorporeal Membrane Oxygenation
Dr. Jerome C. Crowley: The appropriate option for mechanical support depends on many factors, including the patient’s size and requirements for cardiac output, the left ventricular size, whether the patient has a need for pulmonary support, the degree of aortic-valve insufficiency, and technical factors. Because this patient had severe biventricular dysfunction, we were concerned that insertion of a percutaneous device would not provide sufficient cardiac output and that the device would be too large to be accommodated in the nondilated left ventricle. We therefore considered extracorporeal membrane oxygenation (ECMO).
A consideration during the Covid-19 pandemic is the use of limited resources, including ICU beds, ECMO circuits, and personnel. Because this patient was young and did not have functional impairment or coexisting conditions at baseline, she was potentially eligible to be a candidate for a durable ventricular assist device or transplantation if the myocardial dysfunction did not improve, and thus, she was a candidate for short-term ECMO support. At the bedside, we obtained femoral arterial and venous access to facilitate emergency peripheral venoarterial ECMO, which was initiated at a flow rate of 4.1 liters per minute.
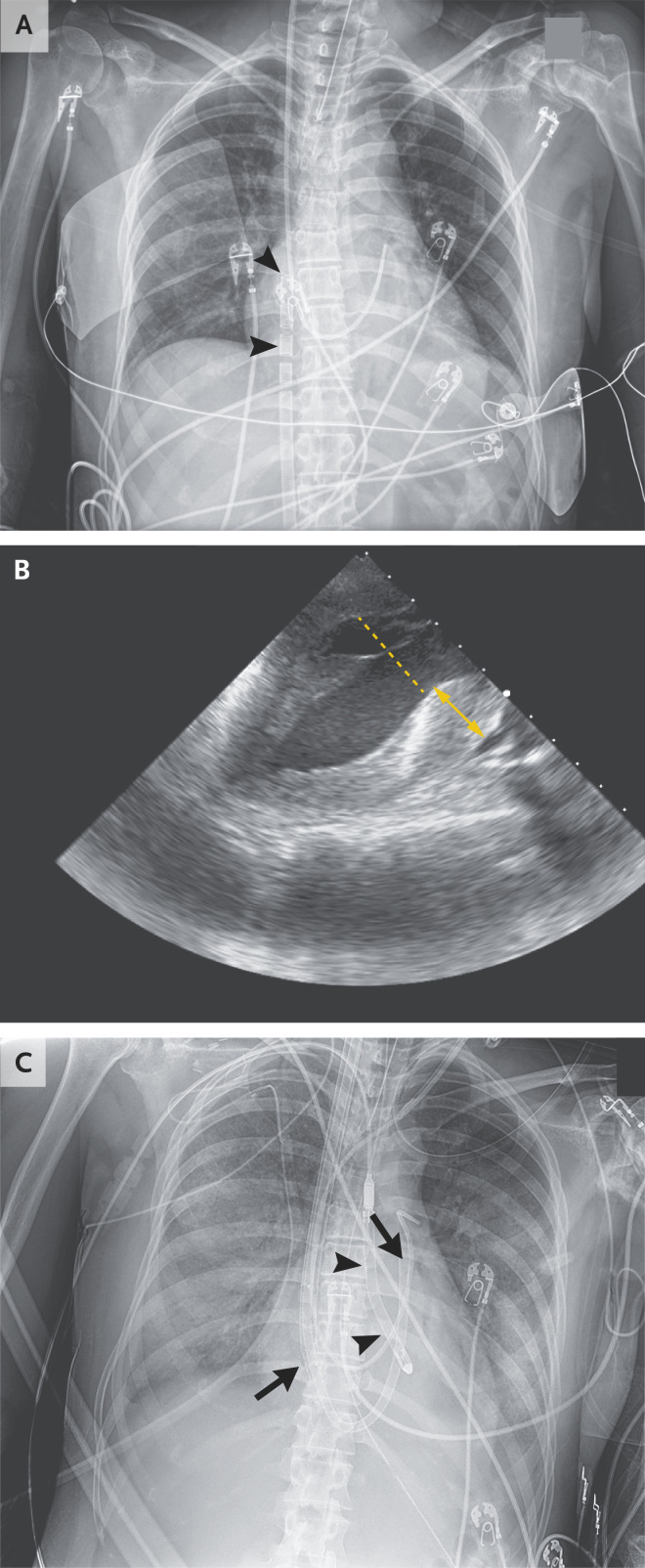
Dr. Rupasov: Portable chest radiography confirmed satisfactory positioning of the endotracheal tube, the pulmonary-artery catheter, and the venous ECMO drainage cannula (Figure 2A).
Figure 2. Studies Obtained after Initiation of Mechanical Circulatory Support.
A chest radiograph obtained after venoarterial extracorporeal membrane oxygenation (ECMO) cannulation (Panel A) shows satisfactory positioning of the venous ECMO cannula (arrowheads) in the right atrium, as well as satisfactory positioning of the endotracheal tube and the pulmonary-artery catheter. A transesophageal echocardiogram obtained during ECMO (Panel B) shows decompression of the left ventricular cavity, with a decreased left ventricular dimension (dashed line). However, wall thickness in both the left ventricle (double arrow) and the right ventricle is increased, as compared with ultrasonographic findings from the previous day, which suggests myocardial edema. A portable chest radiograph obtained after ECMO decannulation and placement of right and left ventricular assist devices (RVAD and LVAD, respectively) (Panel C) shows satisfactory positioning of the RVAD (arrows; the RVAD tip is in the pulmonary trunk, and the catheter is in the left main pulmonary artery) and of the transaortic LVAD (arrowheads). The radiograph also shows small bilateral pleural effusions, as well as development of diffuse bilateral airspace opacities, a finding consistent with alveolar pulmonary edema.
Dr. Crowley: In the hours after venoarterial ECMO cannulation, the heart rate decreased to 80 to 90 beats per minute, with a mean arterial pressure of approximately 70 mm Hg. Urine output normalized. Laboratory test results are shown in Table 1.
Dr. Hung: Transthoracic echocardiography that was performed after ECMO cannulation showed severe biventricular dysfunction. The left ventricular septal and posterior wall thicknesses were approximately 11 mm. The aortic valve did not open.
Dr. Funamoto: Although systemic perfusion was well supported, we were concerned that the left ventricle was not ejecting despite inotropic support, as evidenced by the findings on echocardiography and the lack of a difference between the systolic and diastolic blood pressure measurements (i.e., lack of pulsatility). A lack of left ventricular ejection results from pump failure and the increased afterload exerted on the heart by retrograde blood flow into the aorta during ECMO, and it can result in pulmonary edema. Since Covid-19–associated cardiomyopathy is a new entity, we could not predict a timeline for myocardial recovery, but we thought that mechanical circulatory support might be needed for many days.
Strategies to Unload the Left Ventricle and Augment Cardiac Function
Dr. Crowley: We devised a percutaneous strategy that could unload the left ventricle, independently provide right ventricular support if needed, and be configured to provide oxygenation. We sought a strategy that would be amenable to bedside procedures in order to minimize the need for surgical interventions and transportation to operating rooms and thus decrease the risk of viral transmission to health care workers or other patients. A microaxial left ventricular assist device (LVAD) cannula was placed through cutdown of the right axillary artery, and a percutaneous right ventricular assist device (RVAD) cannula was placed in the right internal jugular vein; thereafter, the venoarterial ECMO cannulas were removed. During the procedure, copious frothy endotracheal secretions were noted; this finding was consistent with pulmonary edema and was probably the result of hours of venoarterial ECMO support without left ventricular ejection. An oxygenator was therefore added to the RVAD circuit to support gas exchange.
Dr. Hung: Intraprocedural transesophageal echocardiography showed severe biventricular dysfunction. The left ventricular cavity size was markedly reduced with the use of an LVAD. However, wall thickness in both the left and right ventricles was increased (Figure 2B and Video 2), as compared with ultrasonographic findings from the previous day, which indicates possible myocardial edema.
Dr. Rupasov: After ECMO decannulation, portable chest radiography confirmed satisfactory positioning of the RVAD and the transaortic LVAD. Radiography also revealed small bilateral pleural effusions, as well as development of diffuse bilateral airspace opacities, a finding consistent with alveolar pulmonary edema (Figure 2C).
Follow-up
Dr. Newton-Cheh: In the absence of clear data from randomized, controlled trials of antiviral or antiinflammatory therapies for the treatment of Covid-19 at the time, we provided general supportive care for this patient. Because we initially considered the possibility of fulminant viral myocarditis, we administered intravenous immune globulin (IVIG) for 5 days, starting on hospital day 3, on the basis of historical and contemporary data from nonrandomized studies of its use in patients with proven or suspected myocarditis.25
Dr. Funamoto: The oxygenator was removed from the RVAD circuit after 4 days. The patient’s biventricular ejection was improved, with increased pulsatility on both the systemic and the pulmonary arterial pressure tracings. Bedside echocardiography showed evidence of improved left ventricular function. The flows of the ventricular assist devices were tapered, and on hospital day 7, after 6 days of mechanical support, the RVAD and LVAD were removed. Intraoperative transesophageal echocardiography showed evidence of improvement in biventricular function.
Dr. Crowley: After removal of the ventricular assist devices, inotropes were tapered. The patient’s clinical course was notable for vasodilatation, with the thermodilution cardiac output exceeding 11 liters per minute. There was no clinically significant leukocytosis or fever, and investigations for other infectious causes of vasodilatation were negative, except for the identification of a few pseudomonas and serratia species in cultures of tracheal aspirate.
Dr. Newton-Cheh: The evolution of this patient’s course is notable for a rise in inflammatory markers, with levels of acute-phase reactants peaking on hospital days 2 to 5 (days 10 to 13 after the onset of symptoms) and the C-reactive protein level peaking a bit later, at 127.1 mg per liter on hospital day 4. Of note, the high-sensitivity troponin T level peaked within 24 hours after presentation and then slowly fell, with the decrease occurring earlier than the decrease in inflammatory markers and before the administration of IVIG (Table 2). The patient’s clinical state transitioned from primary cardiogenic shock on hospital days 1 to 5 (days 9 to 13 after the onset of symptoms) to vasodilatation on hospital days 7 to 10 (days 15 to 18 after the onset of symptoms) that was very well compensated and did not lead to the use of vasoactive agents.
Table 2. Inflammatory Markers.
| Variable | Reference Range | Peak Value | Hospital Day on Which Peak Value Was Obtained |
|---|---|---|---|
| C-reactive protein (mg/liter) | <0.7 | 127.1 | 4 |
| Interleukin-6 (pg/ml) | 0–15.5 | 53.3 | 4 |
| d-dimer (ng/ml) | <500 | 8691 | 13 |
| Ferritin (ng/ml) | 10–200 | 2564 | 2 |
Iatrogenic factors may have modulated the natural course of this patient’s illness. The use of ECMO can be associated with a systemic inflammatory response.27 The administration of empirical IVIG may directly modulate inflammatory pathways. In addition, the effects of congesting the lungs when there is inadequate “venting” of the left ventricle on peripheral venoarterial ECMO are uncertain.
Of note, the disease presentation and course observed in this patient are similar to recent descriptions of multisystem inflammatory syndrome in children.8,9
Dr. Crowley: This patient completed an empirical 7-day course of intravenous vancomycin and cefepime and was extubated on hospital day 11. She was transferred out of the ICU on hospital day 15.
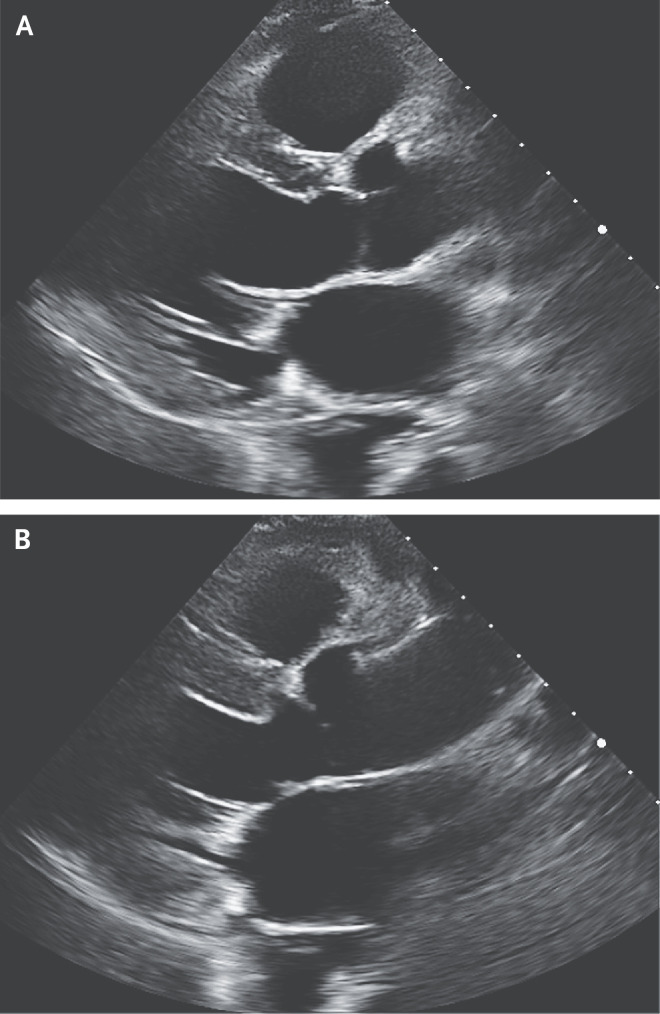
Dr. Hung: Repeat transthoracic echocardiography was performed on hospital day 16 (day 24 after the onset of symptoms) (Figure 3 and Video 3). The patient had a left ventricular ejection fraction of 47%, an interventricular septal wall thickness of 11 mm, and a left ventricular posterior wall thickness of 9 mm.
Figure 3. Follow-up Echocardiogram.
Repeat transthoracic echocardiography was performed on hospital day 16. There is an interventricular septal wall thickness of 11 mm and a left ventricular posterior wall thickness of 9 mm. Biventricular function is mildly decreased, and the left ventricle is no longer dilated, with a left ventricular end-diastolic dimension of 50 mm (Panel A) and a left ventricular end-systolic dimension of 38 mm (Panel B).
Dr. Zlotoff: Therapy with a β-adrenergic receptor antagonist and an ACE inhibitor was started. No diuretic agent was required. The patient reported feeling well, with no shortness of breath, cough, or chest pain. She had marked deconditioning and impaired balance during physical therapy and was therefore discharged to a rehabilitation facility; 1 week later, she was discharged home. The patient was advised to avoid high-intensity exercise for at least 3 months. Two weeks after discharge, at a follow-up telemedicine visit, she reported no ongoing symptoms. She will undergo repeat echocardiography 3 months after discharge.
Final Diagnosis
SARS-CoV-2 infection complicated by severe acute inflammatory cardiomyopathy and cardiogenic shock.
Acknowledgments
We thank David D’Alessandro, Roby Bhattacharyya, James Stone, Matt Frigault, Tomas Neilan, and Walter Dzik for thoughtful discussions of this case.
Disclosure Forms
This article was published on July 15, 2020, at NEJM.org.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Zeng J-H, Liu Y-X, Yuan J, et al. First case of COVID-19 complicated with fulminant myocarditis: a case report and insights. Infection 2020. April 10 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sala S, Peretto G, Gramegna M, et al. Acute myocarditis presenting as a reverse Tako-Tsubo syndrome in a patient with SARS-CoV-2 respiratory infection. Eur Heart J 2020;41:1861-1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inciardi RM, Lupi L, Zaccone G, et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020. March 27 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fried JA, Ramasubbu K, Bhatt R, et al. The variety of cardiovascular presentations of COVID-19. Circulation 2020;141:1930-1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tavazzi G, Pellegrini C, Maurelli M, et al. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur J Heart Fail 2020;22:911-915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu H, Ma F, Wei X, Fang Y. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. Eur Heart J 2020. March 16 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim I-C, Kim JY, Kim HA, Han S. COVID-19-related myocarditis in a 21-year-old female patient. Eur Heart J 2020;41:1859-1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feldstein LR, Rose EB, Horwitz SM, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dufort EM, Koumans EH, Chow EJ, et al. Multisystem inflammatory syndrome in children in New York state. N Engl J Med. 10.1056/NEJMoa2021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Case Records of the Massachusetts General Hospital (Case 8-2018). N Engl J Med 2018;378:1043-1053.29539275 [Google Scholar]
- 11.Medina de Chazal H, Del Buono MG, Keyser-Marcus L, et al. Stress cardiomyopathy diagnosis and treatment: JACC state-of-the-art review. J Am Coll Cardiol 2018;72:1955-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020. March 27 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lala A, Johnson KW, Russak AJ, et al. Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 infection. April 27, 2020. (https://www.medrxiv.org/content/10.1101/2020.04.20.20072702v2). preprint. [DOI] [PMC free article] [PubMed]
- 15.Case Records of the Massachusetts General Hospital (Case 18-2020). N Engl J Med 2020;382:2354-2364.32521138 [Google Scholar]
- 16.Zhang Y, Xiao M, Zhang S, et al. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med 2020;383(17):e38-e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bowles L, Platton S, Yartey N, et al. Lupus anticoagulant and abnormal coagulation tests in patients with Covid-19. N Engl J Med. DOI: 10.1056/NEJMc2013656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barton LM, Duval EJ, Stroberg E, Ghosh S, Mukhopadhyay S. COVID-19 autopsies, Oklahoma, USA. Am J Clin Pathol 2020;153:725-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020;8:420-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bradley BT, Maioli H, Johnston R, et al. Histopathology and ultrastructural findings of fatal COVID-19 infections. April 21, 2020. (https://www.medrxiv.org/content/10.1101/2020.04.17.20058545v1). preprint. [DOI] [PMC free article] [PubMed]
- 21.Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res 2020;220:1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020;395:1417-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moore JB, June CH. Cytokine release syndrome in severe COVID-19. Science 2020;368:473-474. [DOI] [PubMed] [Google Scholar]
- 24.Alvi RM, Frigault MJ, Fradley MG, et al. Cardiovascular events among adults treated with chimeric antigen receptor T-cells (CAR-T). J Am Coll Cardiol 2019;74:3099-3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caforio ALP, Pankuweit S, Arbustini E, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2013;34:2636-2648. [DOI] [PubMed] [Google Scholar]
- 26.Tehrani BN, Truesdell AG, Sherwood MW, et al. Standardized team-based care for cardiogenic shock. J Am Coll Cardiol 2019;73:1659-1669. [DOI] [PubMed] [Google Scholar]
- 27.Millar JE, Fanning JP, McDonald CI, McAuley DF, Fraser JF. The inflammatory response to extracorporeal membrane oxygenation (ECMO): a review of the pathophysiology. Crit Care 2016;20:387-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.