Abstract
Background
Although mother-to-child human immunodeficiency virus (HIV) transmission has dramatically decreased with maternal antiretroviral therapy, breast milk transmission accounts for most of the 180 000 new infant HIV infections annually. Broadly neutralizing antibodies (bNAb) may further reduce transmission.
Methods
A Phase 1 safety and pharmacokinetic study was conducted: a single subcutaneous (SC) dose of 20 or 40 mg/kg (Dose Groups 1 and 2, respectively) of the bNAb VRC01 was administered to HIV-exposed infants soon after birth. Breastfeeding infants (Dose Group 3) received 40 mg/kg SC VRC01 after birth and then 20 mg/kg/dose SC monthly. All infants received appropriate antiretroviral prophylaxis.
Results
Forty infants were enrolled (21 in the United States, 19 in Africa). Subcutaneous VRC01 was safe and well tolerated with only mild-to-moderate local reactions, primarily erythema, which rapidly resolved. For multiple-dose infants, local reactions decreased with subsequent injections. VRC01 was rapidly absorbed after administration, with peak concentrations 1–6 days postdose. The 40 mg/kg dose resulted in 13 of 14 infants achieving the serum 50 micrograms (mcg)/mL target at day 28. Dose Group 3 infants maintained concentrations greater than 50 mcg/mL throughout breastfeeding.
Conclusions
Subcutaneous VRC01 as single or multiple doses is safe and well tolerated in very young infants and is suitable for further study to prevent HIV transmission in infants.
Keywords: broadly neutralizing antibodies, HIV-1, mother-to-child transmission of HIV, neonates, VRC01
Safety and pharmacokinetics of VRC01, a broadly neutralizing monoclonal antibody, was evaluated in HIV-exposed neonates. VRC01 was well tolerated with frequent mild local reactions. Birth dose of 40 mg/kg and monthly dose of 20 mg/kg achieved target trough serum levels.
(See the Editorial Commentary by Gaebler and Caskey, on pages 525–7.)
Antiretroviral therapy (ART) for pregnant and breastfeeding women with human immunodeficiency virus (HIV)-infection, along with antiretroviral (ARV) prophylaxis for their infants, has resulted in dramatic decreases in HIV transmission worldwide [1]; however, an estimated 180 000 infant infections occurred in 2017 (World Health Organization Data and Statistics, https://www.who.int/hiv/data/en/). Transmission continues because of late diagnosis of maternal infection, incomplete adherence to ART, infection with ART-resistant virus, and breastmilk transmission, especially among women who acquire HIV while breastfeeding [2]. To eliminate perinatal HIV transmission, additional strategies are needed. Advances in the identification of potent and broadly neutralizing monoclonal antibodies (bNAb) make it possible to consider a birth dose of bNAbs with continued administration during breastfeeding as a potential option to further reduce vertical HIV transmission.
Anti-HIV bNAbs [3, 4] vary in breadth (proportion of viruses neutralized), potency (concentration to neutralize virus), antiself properties (antibody binding to human tissue), and pharmacokinetics (PKs). Ideally, bNAbs to prevent HIV transmission should be manufacturable at large scale, formulated at high concentrations, stable under routine storage conditions, easy to administer, safe, and well tolerated. They also need high potency and breadth and limited or no self-reactivity. VRC01, a bNAb specific for the HIV-1 CD4 binding site [5, 6], meets many of these criteria. VRC01 was derived from an HIV-infected subject who remained clinically asymptomatic for >10 years [5]. VRC01 has been shown to block transmission of HIV in animal models [7], is well tolerated when given intravenously (IV) or subcutaneously (SC) to adults [8, 9], has antiviral activity in adults with HIV [10], and does not react with human tissue [11]. In initial studies of breadth and potency of VRC01, the antibody was shown to neutralize 94% of viruses in a 3-clade panel of tier-2 viruses at levels ≤50 micrograms (mcg)/mL. Infection in infants is typically due to a single founder virus; thus, VRC01 may have sufficient breadth and potency to prevent HIV-infection in HIV-exposed infants at risk of intrapartum or postnatal transmission. In circumstances when maternal ART adherence is poor, resistance is present, or there are other complications, bNAb may provide an additional measure to further reduce vertical HIV transmission. The present study evaluated safety, tolerability, and PKs of VRC01 in HIV-exposed infants at increased risk of HIV infection.
METHODS
Study Participants
P1112 was an open-label, dose-escalating, Phase 1, multicenter study of VRC01 (VRC-HIVMAB-060-00-AB) conducted in the United States, Zimbabwe, and South Africa (ClinicalTrials.gov NCT02256631). The target sample size was 39; 13 per dose group. Eligible infants were born to women with HIV, ≥36 weeks gestation, and ≥2 kg. Nonbreastfeeding infants enrolled into the single-dose cohorts (Dose Groups 1 and 2) were at increased risk of HIV infection, defined as follows: maternal ART for <30 days before delivery; missing all ART for ≥7 days immediately before delivery; HIV ribonucleic acid (RNA) >1000 copies/mL at last measurement within 30 days of delivery; rupture of membranes >12 hours; or 3 drug class-resistant virus. Criteria were expanded in May 2016 to include mothers who began or reinitiated ART after an interruption of >14 days during the third trimester; HIV RNA above the limit of detection at last measurement before delivery; or 2 drug class-resistant viruses. Breastfed infants were enrolled into Dose Group 3 and did not require any additional high-risk criteria.
Dose Group Management
Infants in Dose Groups 1 and 2 received a single SC 20 mg/kg or 40 mg/kg VRC01 dose, respectively, administered <72 hours after birth. Infants in Dose Group 3 received a single SC 40 mg/kg dose within 5 days of birth, followed by monthly 20 mg/kg SC doses for at least 6 months, and continued for the duration of breastfeeding to a maximum of 72 weeks. Because this group had ongoing risk due to breastfeeding, enrollment was allowed up to 120 hours of life; however, sites were encouraged to administer VRC01 as early as possible. All infants received prophylactic ARV per local standard of care.
Dose Groups 2 and 3 opened sequentially, after accrual in the previous group was completed, 6 infants reached day 28, and all infants passed safety criteria. Safety stopping rules were predefined as (1) any infant death or life-threatening adverse event (AE) or any Grade 4 event probably or definitely attributable to VRC01 or (2) ≥2 of the first 6 infants immunized in the previous dose group having a Grade ≥3 AE at least possibly related to VRC01 (excluding neutropenia or anemia). If any of these criteria were met, then a safety pause would occur and a Safety Monitoring Committee would review. Safety stopping rule criteria were not met.
VRC01 was administered by slow SC push in the thigh using a BD Safety-Lok infusion set, a 25 G × 0.75-inch needle, 7 inches of tubing, and a luer-lock adapter. Study drug was administered as 1 or 2 injections, as determined by site investigators. Infants were observed for at least 4 hours after the first injection and, in Dose Group 3, at least 1 hour after subsequent doses. Follow-up continued for 48 weeks for Dose Groups 1 and 2 and 96 weeks for Dose Group 3. Details of safety assessments are presented in Supplemental Table 1. Toxicities were graded according to Division of AIDS Table for Grading Severity of Maternal and Pediatric Adverse Events, Version 1.0, December 2004, Clarification August 2009, and were assessed by site investigators and the protocol team as either definitely, probably, possibly, probably not, or not related to VRC01. Assessment included local reactions (pain, swelling, redness, or bruising at the injection site), systemic reactions (potentially vaccine-associated AEs such as irritability, fever, or lethargy), and other AEs (any abnormality not fitting into one of the above categories, regardless of association with immunization). Pain at the injection site was graded using a study-specific scale: Grade 1 (mild) = mild reaction to light touch with no or minimal limitation of limb movement; Grade 2 (moderate) = persistent crying with no or light touch, or significant limitation of movement of extremity; Grade 3 (severe) = persistent crying >1 hour or interfering with infant’s ability to sleep or eat; Grade 4 = inconsolable crying >2 hours.
Study Drug
VRC01 is a recombinant human immunoglobulin (Ig)G1 produced in a Chinese Hamster Ovary cell line in accordance with current Good Manufacturing Practice regulations. VRC01 was manufactured at the National Institute of Allergy and Infectious Diseases (NIAID) Vaccine Research Center Pilot Plant operated by Leidos Biomedical Research, Inc. (Frederick, MD) as a 100-mg/mL solution in single-dose vials. VRC01 was stored at site pharmacies in a nonfrost-free freezer with a temperature range of −45°C to −10°C (−49°F to 14°F).
Regulatory
The study was approved by the Institutional Review Board at each site. Each woman provided written informed consent for her own and her infant’s enrollment. Only the infants received VRC01. Human experimentation guidelines of the US Department of Health and Human Services and those of the authors’ institutions were followed.
Laboratory
Hematology, chemistries, and HIV testing were performed at clinical laboratories. VRC01 quantification in plasma was performed with a Beckman Biomek-based automation platform. The anti-idiotype 5C9 monoclonal antibody was added to Immulon-4HXB microtiter overnight before blocking. Three-fold dilutions, from 1:100 to 1:218 700, were analyzed in duplicate. Antihuman IgG1 conjugated with horseradish peroxidase and 3,5’,5,5’-tetra-methylbenzidine (TMB) substrate was used to develop the reaction. Sulfuric acid was then added to halt color development. Plates were then read within 30 minutes at 450 nm with a Molecular Devices Paradigm plate reader. Sample concentrations were quantified using a linear regression of a standard curve of VRC01 covering a range between 5 and 125 ng/mL.
Analysis
Descriptive summaries of baseline characteristics and AEs are presented by Dose Group. No formal statistical analyses to compare the groups were performed.
Pharmacokinetic Evaluation
Plasma VRC01 concentrations were analyzed by noncompartmental methods using the computer program PkPlus, version 2.0 (Simulations Plus, Lancaster, CA). Maximum concentration (Cmax), time of maximum concentration (Tmax), and concentration at day 28 (C28D) were taken directly from observed data. The area under the concentration versus time curve (AUC) was calculated for the first 28 days (AUC0-28D) and total AUC, using the linear trapezoidal method. Concentrations below the quantification limit (BQL) were set equal to 0 for the AUC calculations. In Dose Group 3, the predose concentrations between Week 8 and 4 weeks after the last VRC01 dose were summarized as 20 mg/kg steady-state trough concentrations. The target plasma level of VRC01 at 28 days postdose and at all trough measures in Dose Group 3 was 50 mcg/mL because that is the level at which >90% of a multiclade panel of tier-2 viruses are neutralized. It was anticipated that 20 mg/kg would produce the target concentration at day 28 in a portion but not all participants, whereas the 40 mg/kg dose group was expected to exceed 50 mcg/mL for all infants. We enrolled 13 participants for each Dose Group to ensure at least 10 participants with complete PK results and reasonable precision of the PK parameters. Based on expected coefficients of variation of 25%–50% for key PK parameters, a sample size n = 10 was expected to result in normalized 95% confidence intervals for mean PK parameter values of ±15%–30%.
RESULTS
Accrual
Forty infants were enrolled between June 2015 and January 2017 from 11 sites; 13 infants in Dose Group 1 (single dose, 20 mg/kg), 14 infants in Dose Group 2 (single dose, 40 mg/kg), and 13 infants in Dose Group 3 (40 mg/kg followed by 20 mg/kg monthly). Twenty-one infants enrolled in the United States, including Puerto Rico, and 19 enrolled in Africa, including all 13 infants in Dose Group 3.
Baseline demographics are shown in Table 1. All infants received ARV prophylaxis. Most Group 1 and 2 infants received combination ARVs (67%), whereas most in Dose Group 3 received nevirapine alone (92%), the standard of care for infant prophylaxis in most African countries. No infants were HIV-infected. The median days from birth to VRC01 administration was 2 in all Groups. One infant in Dose Group 1 was incorrectly enrolled: absolute neutrophil count of 3150 cells/mm3 was less than the required 4000 cells/mm3. One infant in Dose Group 2 received 40 mg instead of 70 mg and was excluded from the PK analysis. Compliance and retention were excellent. One infant in Dose Group 1 was lost to follow-up after week 25. Three infants in Dose Group 2 discontinued early: 2 for logistical reasons after weeks 1 and 26 and 1 was lost to follow-up after week 4. All Dose Group 1 and 2 participants with complete PK sampling achieved VRC01 concentrations below assay quantification by the end of follow-up. No child in Dose Group 3 discontinued study drug before cessation of breastfeeding.
Table 1.
Baseline Characteristics
| Treatment Group, Total N = 40 | ||||
|---|---|---|---|---|
| Characteristic | Dose Group 1 (20 mg/kg) (N = 13) | Dose Group 2 (40 mg/kg) (N = 14) | Dose Group 3a (40 mg/kg + 20 mg/kg Monthly) (N = 13) | |
| Gender | M | 8 (62%) | 6 (43%) | 8 (62%) |
| Race | Black | 6 (46%) | 11 (79%) | 13 (100%) |
| White | 6 (46%) | 2 (14%) | 0 (0%) | |
| Unknown | 1 (8%) | 1 (7%) | 0 (0%) | |
| Ethnicity | Hispanic or Latino | 5 (38%) | 4 (29%) | 0 (0%) |
| Infant ART | 3TC, ZDV | 1 (8%) | 0 (0%) | 0 (0%) |
| 3TC, ZDV, NFV | 1 (8%) | 0 (0%) | 0 (0%) | |
| 3TC, ZDV, NVP | 2 (15%) | 5 (36%) | 0 (0%) | |
| NVP | 0 (0%) | 1 (7%) | 12 (92%) | |
| ZDV | 4 (31%) | 4 (29%) | 0 (0%) | |
| ZDV, NVP | 5 (38%) | 4 (29%) | 1 (8%) | |
| Age (days) at VRC01 administration | Mean (s.d.) | 1.5 (1.1) | 1.9 (0.9) | 2.7 (1.4) |
| Median (Q1, Q3) | 2 (1, 2) | 2 (1, 3) | 2 (2, 3) | |
| Min, Max | 0, 3 | 0, 3 | 1, 5 | |
| Weight at birth (grams) | Mean (s.d.) | 3185.4 (703.6) | 3134.9 (265.7) | 3055.0 (605.6) |
| Median (Q1, Q3) | 3045 (2676, 3380) | 3160 (2900, 3386) | 2860 (2670, 3380) | |
| Min, Max | 2330, 4675 | 2609, 3580 | 2330, 4320 | |
| Enrollment site | African | 1 (8%) | 5 (36%) | 13 (100%) |
| United States | 12 (92%) | 9 (64%) | 0 (0%) | |
Abbreviations: ART, antiretroviral therapy; Max, maximum; Min, minimum; NFV nelfinavir; NVP nevirapine; s.d., standard deviation; ZDV zidovudine; 3TC lamivudine.
aDose Group 3 inclusion criteria required breastfeeding.
Safety of VRC01
Infants receiving the 20 mg/kg dose primarily received a single SC injection with an average volume per injection of 0.6 mL (range, 0.2–0.9 mL), whereas 18 of 27 (67%) infants receiving a 40 mg/kg dose received the dose split into 2 injections with an average volume per injection site of 0.7 mL (range, 0.6–1.4 mL). Mild, transient local reactions (Table 2) were common, occurring in 46%–86% infants after the first dose; all reactions were Grade 1 or 2 and of short (several hours) duration. Most common was erythema/redness (22 of 40, 55% of infants), ranging in diameter from 0.5 to 4.0 cm. All local reactions in Dose Group 1 resolved by 4 hours. Among those in Dose Groups 2 and 3, 15 of the 21 with a reaction had complete resolution by 4 hours and 20 of 21 had complete resolution by 24 hours. Pain (Grade 1) was reported in 2 infants in Dose Group 2 and after the birth dose in 2 infants in Dose Group 3.
Table 2.
Summary of Local Reactions
| Dose Group 1 | Dose Group 2 | Dose Group 3 First-Dose Only | ||
|---|---|---|---|---|
| Measure | Local Reaction | Dose 20 mg/kg | Dose 40 mg/kg | Dose 40 mg/kg |
| Total no. of children | - | 13 | 14 | 13 |
| Total no. of injection sitesa | - | 14 | 26 | 19 |
| Average volume/injection site (mL) (min/max) | - | 0.6 (0.2/0.9) | 0.7 (0.6/1.2) | 0.8 (0.6/1.4) |
| Number of children with specified reaction (%) | Any local reactionb | 6 (46%) | 12 (86%) | 9 (69%) |
| Edema | 2 (15%) | 5 (36%) | ||
| Erythema/redness | 5 (39%) | 9 (64%) | 8 (62%) | |
| Induration | 2 (15%) | 7 (50%) | 7 (54%) | |
| Pain | 2 (14%) | 2 (15%) | ||
| Bruising | 1 (8%) | 3 (21%) | ||
| Grade average (min/max) | Any local reactionb | 1.2 (1/2) | 1.4 (1/2) | 1.8 (1/2) |
| Edema | 1.0 (1/1) | 1.0 (1/1) | ||
| Erythema/redness | 1.0 (1/1) | 1.4 (1/2) | 1.6 (1/2) | |
| Induration | 1.5 (1/2) | 1.6 (1/2) | 2.0 (2/2) | |
| Tenderness | 1.0 (1/1) | |||
| Pain | 1.0 (1/1) | 1.0 (1/1) | ||
| Bruising | 1.0 (1/1) | 1.0 (1/1) | ||
| Size average in cm (min/max) | Edema | 1.5 (0.9/2.0) | 1.3 (0.5/2.0) | |
| Erythema/redness | 0.7 (0.5/1.0) | 2.3 (1.0/3.3) | 2.8 (0.9/4.0) | |
| Induration | 1.6 (0.5/2.7) | 2.5 (0.2/5.0) | 3.2 (2.8/4.0) | |
| Number resolved by 1 hour (%) | Any local reactionc | 4 (67%) | 2 (17%) | 2 (22%) |
| Edema | 2 (100%) | 5 (100%) | ||
| Erythema/redness | 3 (60%) | 5 (56%) | 7 (88%) | |
| Induration | 1 (50%) | 0 (0%) | 1 (14%) | |
| Number resolved by 4 hours (%) | Any local reactionc | 6 (100%) | 7(58%) | 8 (89%) |
| Edema | 2 (100%) | 5 (100%) | ||
| Erythema/redness | 5 (100%) | 8 (89%) | 8 (100%) | |
| Induration | 2 (100%) | 3 (43%) | 6 (86%) | |
| Number resolved by 24 hours (%) | Any local reaction | 6 (100%) | 11 (92%) | 9 (100%) |
| Edema | 2 (100%) | 5 (100%) | ||
| Erythema/redness | 5 (100%) | 9 (100%) | 8 (100%) | |
| Induration | 2 (100%) | 6 (86%) | 7 (100%) |
Abbreviations: Max, maximum; Min, minimum.
aIn Dose Group 1, 12 children received their dose as a single injection, and 1 child received the dose split into 2 injections. In Dose Group 2, 2 children received their dose as a single injection, and 12 children received the dose split into 2 injections.
bThe values given for grade and size are the largest grade and size of any of the reactions reported (edema, erythema, induration, bruising, etc) within that child or injection site, whichever group is specified.
cThe values given for average duration and percentage resolved by 4 hours are the longest duration among edema, erythema, tenderness, and induration (ie, these calculations exclude bruising and pain).
Dose Group 3 infants received between 3 and 16 monthly doses, for a total of 116 doses administered after the birth dose. A local reaction occurred after 17 of 116 (15%) subsequent injections, most commonly induration (10%), followed by erythema (7%). Two infants had pain (Grade 1) after the 7th injection, and 1 had pain after the 4th injection. Local reactions were less frequent with subsequent doses in Dose Group 3. There were no systemic reactions to VRC01 in the single-dose cohorts. Four participants in Dose Group 3 reported systemic reactions temporally associated with immunization, which included fever, irritability, appetite decrease, and sleeping less than usual, all Grade 1.
All AEs within 30 days of VRC01 injection were reported. Most were mild (Grade 1/2) and were typical of events observed in young infants. Among the infants enrolled in the single-dose cohorts, 6 (22%) experienced a ≥Grade 3 event within 30 days: 4 in the 20 mg/kg dose group and 2 in the 40 mg/kg dose group (Supplemental Table 2). None of the events were considered related to study drug, and each event had alternative explanations (neutropenia or anemia related to ARV agents, normal physiologic jaundice, or dehydration due to formula errors). The one participant with gastrointestinal symptoms had significant symptoms before enrollment, and the participant with respiratory symptoms had respiratory syncytial virus isolated. Beyond day 30, only 3 participants in the single-dose cohorts (all 20 mg/kg dose group) experienced Grade 3 AEs. These included fever in 1 child, bronchiolitis with hypoxia in a second child, and bronchiolitis and pneumonia in a third child. No event was attributed to VRC01 and no Grade 4 AEs occurred. The children in Dose Group 3 received monthly doses of VRC01 for 3–16 months, and during this extended follow-up period 3 (23%) and 1 (8%) infants were reported to experience Grade 3 or 4 events, respectively. Events included elevated bilirubin (N = 2; both day 14), anemia (N = 1; single value of 8.8 on day 194), and weight loss (N = 1; resolved rapidly), all considered not related to study drug.
Pharmacokinetics of VRC01
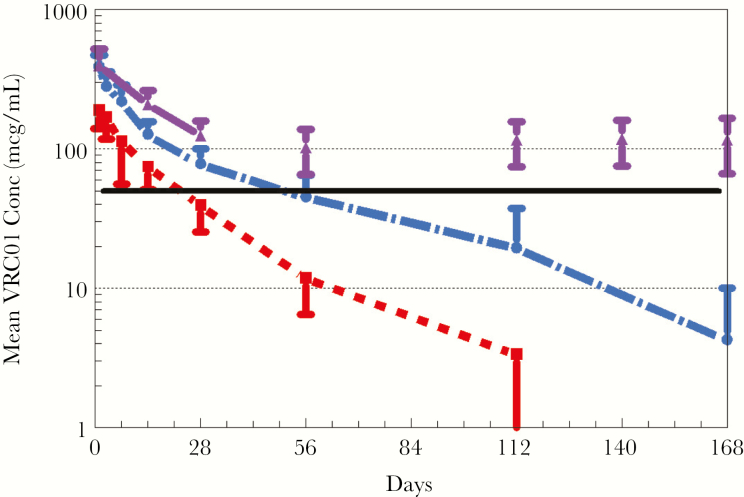
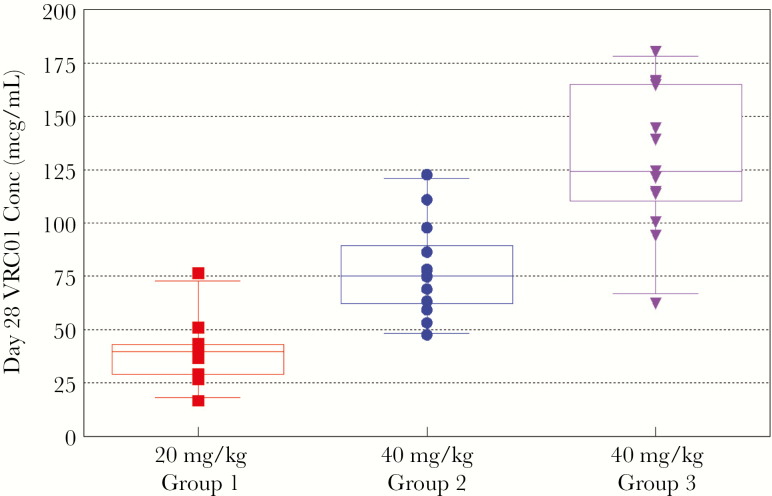
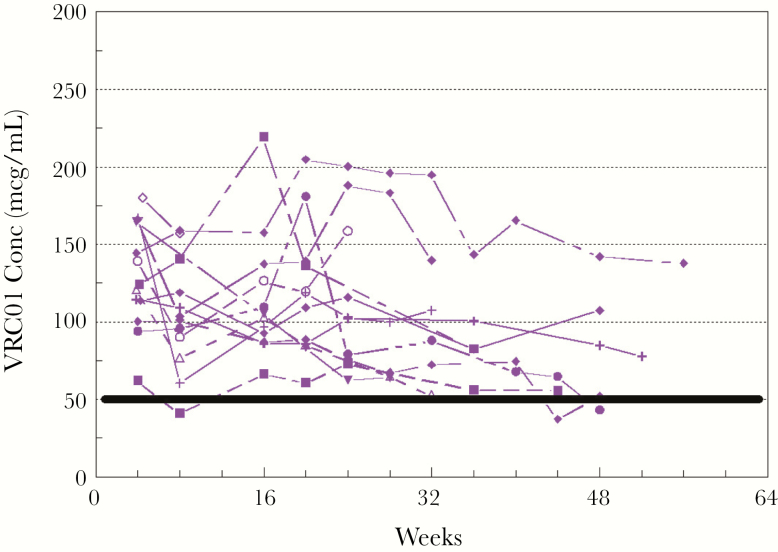
Mean VRC01 levels after single-dose administration are shown in Figure 1. VRC01 was rapidly absorbed after SC administration, with almost all subjects achieving concentrations >100 mcg/mL within 24 hours. Peak concentrations occurred 1–6 days postdose. The 20 mg/kg dose resulted in all but 1 infant maintaining VRC01 concentration >20 mcg/mL at day 28, but most did not reach the target of 50 mcg/mL (Figure 2). The 40 mg/kg dose resulted in all infants but 1 achieving the 50 mcg/mL target at day 28 (range, 47.6–180.2 mcg/mL). Despite similar first doses of 40 mg/kg, the Dose Group 3 VRC01 concentrations were significantly higher than Dose Group 2 at day 14 (P < .005) and day 28 (P < .005). Pharmacokinetic parameters are provided in Table 3. Of note, the infants who received a single dose of 40 mg/kg continued to have measurable levels of antibody with a mean >10 mcg/mL at day 112 (Figure 1). Dose Group 3 infants maintained trough concentrations ≥50 mcg/mL with dosing of 20 mg/kg every 4 weeks (Figure 3), with only 3 samples with VRC01 concentrations slightly below that target across all time points tested. Overall, the Cmax and AUC during the first 28 days after receiving 40 mg/kg were approximately double those after 20 mg/kg. After stopping VRC01 dosing in Dose Group 3, VRC01 levels were below 10 mcg/mL in samples collected 84 days after the last VRC01 dose.
Figure 1.
Pharmacokinetics of VRC01 after a single subcutaneous dose administered within 72 hours of birth. Plasma concentrations of VRC01 (micrograms [mcg]/mL) determined as described in Methods at study visit days 1, 3, 7, 14, 28, 56, 112, and 168 (Dose Group 2 only) for Dose Group 1 (20 mg/kg) in red and for Dose Group 2 (40 mg/kg) in blue. Dose Group 3 (purple bars) received a single dose of 40 mg/kg within 5 days of birth, followed by monthly doses of 20 mg/kg. Levels from day 28 on represent trough levels. Solid black line indicates targeted trough of 50 mcg/mL. Error bars indicate standard deviation
Figure 2.
VRC01 concentrations (Conc) at day 28 in Dose Groups 1 (20 mg/kg), 2 (40 mg/kg), and 3 (40 mg/kg).
Table 3.
VRC01 Pharmacologic Measures When Administered as a Single Subcutaneous Dose to Neonates Born to Women With HIVa
| Pharmacokinetic Parameter | Dose Group | Mean | SD | Median |
|---|---|---|---|---|
| VRC01 C28D (mcg/mL) | 1–20 mg/kg | 39.3 | 14.9 | 39.2 |
| VRC01 C28D (mcg/mL) | 2–40 mg/kg | 78.2 | 21.9 | 75.3 |
| VRC01 C28D (mcg/mL) | 3–40 mg/kg | 130.1 | 34.2 | 124.2 |
| VRC01 C56D (mcg/mL) | 1–20 mg/kg | 12.84 | 4.19 | 13.64 |
| VRC01 C56D (mcg/mL) | 2–40 mg/kg | 45.29 | 19.16 | 47.23 |
| VRC01 C112D (mcg/mL) | 1–20 mg/kg | 3.64 | 2.85 | 3.99 |
| VRC01 C112D (mcg/mL) | 2–40 mg/kg | 19.48 | 17.88 | 10.62 |
| C max (mcg/mL) | 1–20 mg/kg | 226.6 | 30.8 | 233.3 |
| C max (mcg/mL) | 2–40 mg/kg | 387.1 | 82.7 | 385.1 |
| T max (days) | 1–20 mg/kg | 2.33 | 1.85 | 1.58 |
| T max (days) | 2–40 mg/kg | 1.34 | 0.96 | 0.95 |
| AUC (mcg × d/mL) | 1–20 mg/kg | 3971 | 1024 | 3801 |
| AUC (mcg × d/mL) | 2–40 mg/kg | 9250 | 2473 | 9520 |
Abbreviations: AUC, area under the concentration versus time curve; Cmax, maximum concentration; d, day; HIV, human immunodeficiency virus; mcg, micrograms; SD, standard deviation; Tmax, time of maximum concentration.
aVRC01 dose administered within 72 hours of birth for Dose Groups 1 and 2 and within 5 days of birth for Dose Group 3.
Figure 3.
VRC01 trough concentrations for repeated doses: 40 mg/kg subcutaneous (SC) once, then 20 mg/kg SC every 4 weeks. Individual lines represent each Dose Group 3 participant. The solid black line indicates the target VRC01LS trough of 50 micrograms (mcg)/mL.
DISCUSSION
This is the first study of an anti-HIV monoclonal antibody in children of any age. The primary goal of this study was to determine a safe dose that maintained a level >50 mcg/mL through day 28 (Dose Group 1 and 2) and throughout the breastfeeding period (Dose Group 3), which could be administered at birth and at regular intervals during the time of continued risk for breastfed infants. The 40 mg/kg dose consistently achieved that target at day 28, with levels remaining at approximately 50 mcg/mL 8 weeks after a single dose. A single 40 mg/kg dose at birth, followed by repeated 20 mg/kg doses administered every 28 days, resulted in levels consistently at >50 mcg/mL.
The product was well tolerated. VRC01-related AEs were limited to local reactions, which were mild to moderate, self-limited, and consistent with those reported in children with antibody deficiency receiving SC IgG therapy [12]. Most adults have received VRC01 via IV infusion. However, in the small number of adults who received 5 mg/kg VRC01 SC, pain, tenderness, erythema, and induration were relatively common, occurring in 40%–60% [8]. For most adults, the finding was “non-gradable”: not severe enough to be classified as “mild”. Rare infusion reactions have been reported in clinical trials evaluating VRC01 in adults [8, 13]; however, no infusion reactions were observed in VRC01-treated infants. Adverse events ≥Grade 3 did occur, but they had alternative explanations and all resolved.
VRC01 PKs have been described in adult healthy volunteers and adults with HIV demonstrating linear PKs after IV administration of 5–40 mg/kg [9, 14]. The average AUCs and C28D with 5–40 mg/kg IV doses ranged from 1750 to 14 000 mcg × day/mL and 7–65 mcg/mL, respectively. In adults, a SC 5 mg/kg dose resulted in mean AUCs of 800–1250 mcg × day/mL and C28D of 4–7 mcg/mL [9]. After adjusting for dose, SC dosing in infants resulted in AUC similar to adults and C28D approximately 30% higher.
VRC01 elimination in infants is similar to adults, but it is dynamic and complicated by dilutional effects of ongoing growth, tissue redistribution, and maturation of IgG metabolism. Thus, a linear terminal slope could not be characterized with high precision and was dependent on PK sampling duration, because “flattening” of the concentration versus time curve occurred with prolonged sampling. The mean VRC01 concentrations decreased by approximately 50% in the 2 weeks between days 14 and 28 and fell to 57% (equivalent of 1.2 t1/2s) in the 8 weeks between days 56 and 112 in the 40 mg/kg group. The lack of VRC01 trough concentration accumulation with repeated doses and faster washout PK suggest that the functional half-life may change with repeat administration or increasing age. Higher VRC01 concentrations were seen in Dose Group 3 than Dose Group 2 at days 14 and 28. Dose Group 3 had a larger portion of infants from resource-limited settings, were smaller, and were breastfed. These or other factors may have contributed to the modest differences in concentrations observed. Subsequent studies will be necessary to confirm this observation and determine etiology.
The present study is limited by sample size. Given the small number of infants studied, there is limited power to identify infrequent safety events. However, the period of follow-up was extended, and the Dose Group 3 children received multiple doses for up to 16 months with no evidence of systemic toxicity. In addition, there are significant differences in characteristics of infants in the different dosing groups, making it difficult to evaluate the difference in PK in the different Dose Groups. Furthermore, measures of anti-VRC01 antibody are not yet available.
Two Phase 2b studies enrolling adults at high risk of HIV acquisition will assess efficacy of bimonthly VRC01 doses to prevent sexual HIV-1 transmission, with results expected in 2020 (ClinicalTrials.gov: NCT02716675; NCT02568215). A single dose may be beneficial to reduce vertical transmission of HIV for infants born to women who do not have viral suppression at the time of delivery or for infants in whom ARV prophylaxis is inconsistent. Furthermore, a birth dose followed by monthly dosing may be beneficial in breastfeeding infants, especially if the mother is known to have resistant virus or is nonadherent to ART. In settings with high HIV prevalence, where there are high seroconversion rates among postpartum women [15–18] and maternal incidence as high as 16.8/100 person-years [16], bNAb prophylaxis of all infants may be a strategy to prevent infection in infants whose mothers become HIV-infected while they are breastfeeding. In these settings, 1 or 2 doses of bNAb with an extended half-life would be one strategy for protecting this vulnerable population.
Although VRC01 has many desirable characteristics for preventing vertical HIV transmission, there are several approaches for improvement in half-life, potency, and breadth. Fc modifications can alter binding to the neonatal Fc receptor and change antibody half-life [19] and other Fc-mediated functional activities. VRC01 has now been modified by site-directed mutagenesis (M428L/N434S) resulting in VRC01LS, with a 2.5- to 3-fold increase in half-life in adults compared with VRC01 [20] and extended protection in a primate model of simian HIV infection [21]. VRC01LS produces therapeutic levels for prolonged periods and makes passive immunization a practical option for long-term protection after a single dose.
Improving potency and breadth will allow dose reduction, cost saving, and minimize likelihood of breakthrough infection and neutralization escape. VRC01 neutralizes approximately 87% of a panel of 190 env-pseudoviruses with a median IC80 of approximately 1 mcg/mL [5]. Other bNAbs are in clinical development targeting CD4 binding sites [11, 22, 23]: the V3 glycan patch [24, 25]; V1/V2 [26, 27]; and the membrane proximal extracellular region [28]. These alternative bNAbs provide options for optimizing potency and breadth using single or combination bNAbs regimens. Studies of VRC01LS and VRC07-523LS are in progress in HIV-exposed infants (Clinicaltrials.gov: NCT02256631).
CONCLUSIONS
In summary, VRC01 is safe and well tolerated in infants born to women with HIV and demonstrates favorable PK properties. VRC01 or newer bNAbs should be evaluated as an additional strategy to interrupt mother-to-child transmission of HIV.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Presented in part: Conference on Retroviruses and Opportunistic Infections, February 13–16, 2017 Seattle, WA; HIV Research for Prevention (R4P) Conference, October 23, 2018, Madrid, Spain.
Acknowledgments. We thank the VRC for supplying investigational product, scientific and regulatory support, and for conducting key assays for the assessment of the trial. We also especially thank the following personnel: Kevin Carlton, Frank Arnold, Lucio Gama, Jason Gall, Julie Ledgerwood, Emily Coates, Michelle Conan-Cibotti, Robert Bailer, Adrian McDermott, and David Lindsey. J. R. M. is an inventor on an NIH patent for VRC01 (Isolation of Novel Broadly Neutralizing Monoclonal Antibodies Against HIV-1 Using Epitope Specific Glycoprotein Probes to Identify HIV-1 Specific B-Cells, VRC01. US (9,175,070; 8,637,036; 9,738,703; 10,035,845); AU (2010298025); CN (2010800536165)).
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Financial support. Overall fundung for the International Maternal Pediatric Adolescent AIDS Clinical Trials Network (IMPAACT) was provided by the National Institute of Allergy and Infectious Diseases (NIAID) with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Mental Health, all components of the National Institutes of Health (NIH), IMPAACT LOC (UM1AI068632), IMPAACT SDMC (UM1AI068616), and IMPAACT LC (UM1AI106716), and by NICHD (HHSN275201800001I). Funding for this study was also provided by the Intramural Research Program of the Vaccine Research Center, NIAID, NIH. This publication resulted in part from research funded by the Duke University Center for AIDS Research, an NIH-funded program (5P30 AI064518; to C. K. C.), and the Colorado Clinical and Translational Science Award from the National Center for Advancing Translational Science (UL1 TR001082; to E. J. M.).
Potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Members of the protocol team not included on the masthead include the following (in alphabetical order): Grace Aldrovandi, MD, CM; Frederic Bone; Dale Dayton, RN, CCRA; Benjamin Johnston; Patricia Morgan, MS, PA; Kathryn Myers, BA; Nicole Tobin, MD; Bonnie Zimmer.
Individuals at the sites who contributed to the study include the following (sites listed in order according to number of participants enrolled): Family Clinical Research Unit: Magdel Rossouw, MBChB; Lindie Rossouw, MBChB; Jeanne Louw, MSc. Jacobi Medical Center: Joanna Dobroszycki, MD; Marlene Burey, MSP, PNP; Raphaelle Auguste, RN, BSN. South Florida Children’s Diagnostic and Treatment Center Fort Lauderdale: Kathleen K. Graham, PharmD; Hanna Major-Wilson, ARNP, MSN. Harare Family Care: Tsungai Mhembere, MPH, BPharm; Sukunena Maturure, DCHN, Midwifery, RGN; Mutsa Bwakura-Dangarembizi, MBChB, MMed, MSc. University of Colorado Denver: Emily Barr, CPNP, CNM, MSN; Jennifer Dunn, FNP-C, MSN; Carrie Glenny, RN, BSN, MA; Carrie Chambers, RN, BSN. Bronx-Lebanon Hospital Center: Mahboobullah Mirza Baig, MD; Murli Purswani, MD. David Geffen School of Medicine at University of California Los Angeles: Jaime G. Deville, MD; Karin Nielsen-Saines, MD, MPH; Christina Shin, PharmD; Michele F. Carter, RN. Emory University School of Medicine: Ann Chahroudi, MD, PhD; Alexis Ahonen, NP; Martina Badell, MD; Rana Chakraborty, MD, MSc, FRCPCH, FAAP, FPIDS, DPhil (PhD). Johns Hopkins University: Allison Agwu, MD, ScM; W. Christopher Golden, MD; Thuy Anderson, RN, BSN; Aleisha Collinson-Streng, RN, BSN. San Juan City Hospital: Rodrigo Diaz-Velasco MD, FACOG, AAHIVS; Nicolas Rosario MD, MSc; Elvia Pérez, BS, MEd, MA, MPH; Wanda I. Marrero, RN, BSN. University of Puerto Rico Pediatric HIV/AIDS Research Program: Irma Febo, MD; Ruth Santos, RN, MPH; Carmen D. Zorrilla, MD.
Contributor Information
IMPAACT P1112 team:
Grace Aldrovandi, Frederic Bone, Dale Dayton, Benjamin Johnston, Patricia Morgan, Kathryn Myers, Nicole Tobin, Bonnie Zimmer, Magdel Rossouw, Lindie Rossouw, Jeanne Louw, Joanna Dobroszycki, Marlene Burey, Raphaelle Auguste, Kathleen K Graham, Hanna Major-Wilson, Tsungai Mhembere, Sukunena Maturure, Mutsa Bwakura-Dangarembizi, Emily Barr, Jennifer Dunn, Carrie Glenny, Carrie Chambers, Mahboobullah Mirza Baig, Murli Purswani, Jaime G Deville, Karin Nielsen-Saines, Christina Shin, Michele F Carter, Ann Chahroudi, Alexis Ahonen, Martina Badell, Rana Chakraborty, Allison Agwu, W Christopher Golden, Thuy Anderson, Aleisha Collinson-Streng, Rodrigo Diaz-Velasco, Nicolas Rosario, Elvia Pérez, Wanda I Marrero, Irma Febo, Ruth Santos, and Carmen D Zorrilla
References
- 1. Abuogi LL, Humphrey JM, Mpody C, et al. Achieving UNAIDS 90-90-90 targets for pregnant and postpartum women in sub-Saharan Africa: progress, gaps and research needs. J Virus Erad 2018; 4:33–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tippett Barr BA, van Lettow M, van Oosterhout JJ, et al. National estimates and risk factors associated with early mother-to-child transmission of HIV after implementation of option B+: a cross-sectional analysis. Lancet HIV 2018; 5:e688–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anderson DJ, Politch JA, Zeitlin L, et al. Systemic and topical use of monoclonal antibodies to prevent the sexual transmission of HIV. AIDS 2017; 31:1505–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pegu A, Hessell AJ, Mascola JR, Haigwood NL. Use of broadly neutralizing antibodies for HIV-1 prevention. Immunol Rev 2017; 275:296–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wu X, Yang ZY, Li Y, et al. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science 2010; 329:856–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhou T, Georgiev I, Wu X, et al. Structural basis for broad and potent neutralization of HIV-1 by antibody VRC01. Science 2010; 329:811–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pegu A, Yang ZY, Boyington JC, et al. Neutralizing antibodies to HIV-1 envelope protect more effectively in vivo than those to the CD4 receptor. Sci Transl Med 2014; 6:243ra88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mayer KH, Seaton KE, Huang Y, et al. ; HVTN 104 Protocol Team; and the NIAID HIV Vaccine Trials Network Safety, pharmacokinetics, and immunological activities of multiple intravenous or subcutaneous doses of an anti-HIV monoclonal antibody, VRC01, administered to HIV-uninfected adults: results of a phase 1 randomized trial. PLoS Med 2017; 14:e1002435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ledgerwood JE, Coates EE, Yamshchikov G, et al. ; VRC 602 Study Team Safety, pharmacokinetics and neutralization of the broadly neutralizing HIV-1 human monoclonal antibody VRC01 in healthy adults. Clin Exp Immunol 2015; 182:289–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lynch RM, Boritz E, Coates EE, et al. Virologic effects of broadly neutralizing antibody VRC01 administration during chronic HIV-1 infection. Sci Transl Med 2015; 7:319ra206. [DOI] [PubMed] [Google Scholar]
- 11. Rudicell RS, Kwon YD, Ko SY, et al. ; NISC Comparative Sequencing Program Enhanced potency of a broadly neutralizing HIV-1 antibody in vitro improves protection against lentiviral infection in vivo. J Virol 2014; 88:12669–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ochs HD, Gupta S, Kiessling P, Nicolay U, Berger M; Subcutaneous IgG Study Group Safety and efficacy of self-administered subcutaneous immunoglobulin in patients with primary immunodeficiency diseases. J Clin Immunol 2006; 26:265–73. [DOI] [PubMed] [Google Scholar]
- 13. Takuva S, Karuna S. Plenary session. HVTN Sub-Saharan African Regional Meeting (Cape Town, South Africa), 21-22 February 2018.
- 14. Huang Y, Zhang L, Ledgerwood J, et al. Population pharmacokinetics analysis of VRC01, an HIV-1 broadly neutralizing monoclonal antibody, in healthy adults. MAbs 2017; 9:792–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dinh TH, Delaney KP, Goga A, et al. Correction: impact of maternal HIV seroconversion during pregnancy on early mother to child transmission of HIV (MTCT) measured at 4–8 weeks postpartum in South Africa 2011–2012: a national population-based evaluation. PLoS One 2015; 10:e0130321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Drake AL, Wagner A, Richardson B, John-Stewart G. Incident HIV during pregnancy and postpartum and risk of mother-to-child HIV transmission: a systematic review and meta-analysis. PLoS Med 2014; 11:e1001608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Johnson LF, Stinson K, Newell ML, et al. The contribution of maternal HIV seroconversion during late pregnancy and breastfeeding to mother-to-child transmission of HIV. J Acquir Immune Defic Syndr 2012; 59:417–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Thomson KA, Hughes J, Baeten JM, et al. ; Partners in Prevention HSV/HIV Transmission Study and Partners PrEP Study Teams Increased risk of HIV acquisition among women throughout pregnancy and during the postpartum period: a prospective per-coital-act analysis among women with HIV-infected partners. J Infect Dis 2018; 218:16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhu Q, McLellan JS, Kallewaard NL, et al. A highly potent extended half-life antibody as a potential RSV vaccine surrogate for all infants. Sci Transl Med 2017; 9:eaaj1928. [DOI] [PubMed] [Google Scholar]
- 20. Gaudinski MR, Coates EE, Houser KV, et al. ; VRC 606 Study Team Safety and pharmacokinetics of the Fc-modified HIV-1 human monoclonal antibody VRC01LS: a phase 1 open-label clinical trial in healthy adults. PLoS Med 2018; 15:e1002493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ko SY, Pegu A, Rudicell RS, et al. Enhanced neonatal Fc receptor function improves protection against primate SHIV infection. Nature 2014; 514:642–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huang J, Kang BH, Ishida E, et al. Identification of a CD4-binding-site antibody to HIV that evolved near-pan neutralization breadth. Immunity 2016; 45:1108–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Scheid JF, Mouquet H, Ueberheide B, et al. Sequence and structural convergence of broad and potent HIV antibodies that mimic CD4 binding. Science 2011; 333:1633–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Walker LM, Huber M, Doores KJ, et al. ; Protocol G Principal Investigators Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature 2011; 477:466–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mouquet H, Scharf L, Euler Z, et al. Complex-type N-glycan recognition by potent broadly neutralizing HIV antibodies. Proc Natl Acad Sci U S A 2012; 109:E3268–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Doria-Rose NA, Bhiman JN, Roark RS, et al. New member of the V1V2-directed CAP256-VRC26 lineage that shows increased breadth and exceptional potency. J Virol 2016; 90:76–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sok D, van Gils MJ, Pauthner M, et al. Recombinant HIV envelope trimer selects for quaternary-dependent antibodies targeting the trimer apex. Proc Natl Acad Sci U S A 2014; 111:17624–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Huang J, Ofek G, Laub L, et al. Broad and potent neutralization of HIV-1 by a gp41-specific human antibody. Nature 2012; 491:406–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.